- 1Aboca SpA, Sansepolcro, Italy
- 2Department of Biomedical, Surgical and Dental Sciences, University of Milan, Milan, Italy
- 3P.O. Giugliano ASL Napoli2 Nord, Napoli, Italy
- 4ASP6 Palermo, Palermo, Italy
Background: Gastroesophageal reflux disease (GERD) and functional dyspepsia (FD) are very common in the general population. GERD prevalence is considerably high in pregnant women, and it increases at a young age, alongside obesity. Mucosal protective agents (MPAs) are over-the-counter (OTC) treatments for FD and GERD commonly used alone or as add-on therapy to proton pump inhibitors (PPIs). Real-world data through surveys allow a clinical evaluation of marketed products that also complies with the new regulation on substance-based medical devices (SBMDs).
Aim: The study aimed to evaluate perceived effectiveness, safety, and pattern of usage among patients, physicians, and pharmacists of the natural MPA Poliprotect, as assessed by a validated survey methodology.
Methods: Questionnaire repeatability was first assessed, resulting in the intraclass correlation coefficient agreement level >0.9 in the three validation cohorts of physicians, pharmacists, and patients. All questions were closed multiple-choice, allowing measuring variations in frequency, quality, or magnitude of effect on a 5-point Likert-like verbal scale.
Results: Three different surveys were performed in Italy and Spain on a total of 3,471 physicians, including 77 gastroenterologists, 848 patients, and 146 pharmacists who had an experience with Poliprotect in the previous year. Over 90% of general practitioners (GPs) rated Poliprotect effectiveness as good/excellent in controlling pyrosis, 80% for epigastric pain, and approximately 70% for digestion difficulties. GPs reported Poliprotect as very or extremely useful as an alternative to PPIs (73%) and for pregnancy-associated GERD symptoms (61%), almost unanimously (99.5%) reporting an excellent to good tolerability; 79% of the gastroenterologists answered to be extremely or very satisfied with the improvement of typical GERD symptoms, whereas improvement of dyspepsia and pregnancy- and breast-feeding-associated GERD symptoms was rated as highly satisfactory for 69, 52, and 62%, respectively, among GI specialists. Its use because of painful dyspeptic symptoms was reported by over 80% of patients, who rated symptom relief as excellent/good, and reported a marked quality-of-life improvement in 73% and in 65% of their answers, respectively. The product was used as monotherapy by 63% of patients. Conclusion: Large-scale, validated surveys support the safety and effectiveness of Poliprotect in the treatment of common functional upper GI disorders.
1 Introduction
Gastroesophageal reflux disease (GERD) and functional dyspepsia (FD) are gastrointestinal (GI) disorders very common in developing countries, with an estimated prevalence of up to 40% in the general population (Enck et al., 2017).
Heartburn (also referred to as pyrosis) and regurgitation are typical symptoms of GERD, reported in approximately 20% of the adult population of western countries (Sweis and Fox, 2020). However, those estimates could be higher because several acid-reducing medications are over-the-counter (OTC) drugs and, therefore, are easily accessible as self-medication.
Dyspepsia encompasses a range of painful and painless upper GI symptoms, often with no identifiable organic cause, thus classified as FD (Ford et al., 2010) that comprises two types of syndromes: the epigastric pain syndrome (EPS) and the postprandial distress syndrome (PDS: the painless subtype), commonly described by patients as “difficulties in digestion” (Black et al., 2018). GERD and FD can be clinically difficult to distinguish. Consistently, in both disorders proton pump inhibitors (PPIs) are recommended as first-line therapy (Karamanolis et al., 2006; Lee et al., 2006; Vanheel et al., 2017; Wauters et al., 2021).
GERD is very often associated with obesity, a growing phenomenon in western countries, and pregnancy due to the physiological hormonal and anatomical changes which can influence gastric and esophageal functions in both conditions (Keller et al., 2008; Alimi and Azagury, 2021).
The treatment of these upper GI disorders can be quite challenging and unsuccessful. It includes acid-reducing drugs such as PPIs, prokinetics, and neuromodulators, as well as herbal substances, together with psychological and alternative medicine approaches (Harer and Hasler, 2020). Mucosal protective agents (MPAs) are also commonly used, alone or as an add-on to PPIs, that have proven effective in reducing symptoms (Scally et al., 2018). However, PPIs can be associated with relevant side effects, including enteric infections (Hafiz et al., 2018). In addition, young adults, children, and pregnant women often with mild symptoms may be over-treated by those drugs, with risks outweighing the benefits.
Poliprotect (Neobioanacid, Aboca S. p.A, Sansepolcro, Italy), is a therapeutical substance-based medical device (SBMD), classified as class 2 according to the Medical Device Directive since it is used in the GI tract in contact with the gastric mucosa (Official Journal of the European Union, 2007). It is a natural complex in which constituents of medicinal plants and natural minerals are gathered and selected on the basis of its emerging behavior (i.e., the behavior acquired by the final complex when components are pooled together) to confer mucosal-adhesive and buffering properties, thus acting as a surface protective agent on the gastro-esophageal mucosa. Poliprotect has been developed for symptoms of GERD and FD at single or repeated daily doses, as needed, in children, adults, and pregnant women. Assessing real-world data (RWD) on the safety and effectiveness of SBMD is becoming central since this complex is easily accessible OTC and often used as self-medication. Importantly, this assessment is required by the new European Union (EU) Regulation on MD, which has revised MD approval, classification, and post-market surveillance of safety and performance, and has promoted transparency and post-market oversight in the EU (Official Journal of the European Union, 2017).
This study was aimed at the following: 1) reporting the generation and implementation of a validated methodology of surveys to comply with the EU regulation on SBMD in a real-world setting; 2) assessing Poliprotect's perceived effectiveness, tolerability, safety, and pattern of usage among different categories of users and prescribers (general practitioners [GPs] and specialist physicians, patients, and pharmacists).
2 Methods
2.1 Product
Neobianacid is a complex system composed of Poliprotect (made of a polysaccharide fraction from Aloe vera, Malva sylvestris, Althaea officinalis, and the natural minerals limestone and nahcolite) and a flavonoid fraction from Glycyrrhiza glabra and Matricaria recutita. One tablet of 1.55 g contains Poliprotect (polysaccharides and minerals from aloe vera 43 mg, mallow 41 mg, marshmallow 21 mg, limestone 346 mg, and nacholite 78 mg), chamomille 23 mg, licorice 23 mg, sugar 934 mg, arabic gum 18 mg, and spearmint natural flavor 23 mg. Given its emerging behavior, a multiples approach is needed to standardize and control the production process and the final quality of the product in its complexity. The product is controlled according to the following chemical, physical, and biological parameters: NIRS (near-infrared spectroscopy) technology through which the standardization of the production process is confirmed, adhesion test through which the barrier and protective performance on gastric mucosa is confirmed, and polysaccharide content ≥1.8% as the representative class of compound of the product.
Its suggested dosage for GERD and functional GI symptoms is one tablet after the main meals and one in the evening at bedtime, with the possibility of repeating daily dosing as needed, at shorter intervals.
2.2 Design of the study
This study consists of cross-sectional observational surveys on Poliprotect, performed between 2017 and 2021 in Italy and Spain on three different cohorts: 3,471 physicians, 848 patients, and 146 pharmacists. Different questionnaires were used for the surveys; however, for this work, we included only questions addressing aspects common to the three participating cohorts, reported in Supplementary Material S1. The common questions were related to the perceived effectiveness with respect to typical GERD and dyspeptic symptoms, patient tolerability, and safety and explored the clinical settings in whom the substance complex was prescribed the most. All questions were closed multiple choice questions (MCQs), with one or sometimes multiple possible answers to each statement or question, as specified, allowing measuring variations in frequency, quality, or magnitude of effect on a 5-point Likert verbal scale. Most MCQs had a single answer only, and some questions allowed more than one answer, as indicated. Open notes for physicians and pharmacists were also provided to further detail information on treated disorders/symptoms, treatment regimen, and tolerability. The survey’s participants were also requested to report unwanted side effects or possible product interactions with concomitant treatments addressing safety issues directly to the manufacturer's vigilance department at a specific mailbox.
2.2.1 Physicians
Four surveys were conducted among five cohorts of physicians practicing in Italy and Spain; physicians were in part recruited through Aboca Group Scientific informants who performed computer-assisted interviews through the Aboca Group validated web platform. Physicians who filled out the questionnaire had an experience with the product over at least the previous year.
2.2.2 Patients
Patients were recruited and invited to complete an online questionnaire through public and online advertisements. All responders who completed the online questionnaire had used the product over the previous year.
2.2.3 Pharmacists
Pharmacists were recruited through the Aboca Group’s validated web platform. All pharmacists had the experience of suggesting the natural complex over the previous year.
2.3 Ethical aspects
Requirements for ethical review for surveys differ in different countries, being often exempted from ethical review; since surveys are not considered de facto, a clinical study as protocol, endpoints, inclusion, or exclusion criteria is lacking, and no risk for participants derived from an intervention, which is lacking as well, is posed (Whicher and Wu, 2015). Even in the case of observational studies with drugs, the Italian National Regulatory Agency for Medicinal Products (AIFA) states that ethical evaluation is required only for prospective studies (AIFA, 2008). According to the non-anonymous nature of the data, starting from 2018, information privacy was provided to each participant in compliance with laws and regulations of the privacy and management of personal information of Spain and Italy and that of the EU General Data Protection Regulation, and each participant declared to have read and accept this information.
All voluntary participants were aged at least 14 years, data from patient’s questionnaires were pseudo-anonymized, and aggregated data were analyzed.
2.4 Assessment of questionnaire reproducibility
To assess the precision of the current survey, a preliminary repeatability and reproducibility study was performed to measure the precision and as an indirect measure of the survey questionnaire's potential validity (Lundell et al., 2022). This preliminary assessment was performed for a product other than Poliprotect; however, questions were designed in the same method, and therefore, we considered it appropriate to infer that those repeatability results could be extended to the present surveys.
2.5 Sample size and statistical analysis
A sample size of at least 583 responders to the questionnaires was calculated on patients considering questions on the degree of satisfaction with safety and tolerability, quality of life, and improvement of symptoms, assuming a margin of error equal to 4%, a 95% confidence level, and a conservative estimate of the response rate to the question of 50%. The calculation was performed with an online sample size calculator tool available at http://www.raosoft.com/samplesize.html.
In analyzing survey data obtained from physicians, responder cohorts were put together so as to perform an aggregate analysis of over 3,000 responses to important questions addressing patterns of usage, effectiveness, safety, and tolerability.
Within the physician’s category, data from gastroenterologists, as specialists, were analyzed separately.
We conducted a descriptive analysis for each question; the answers were expressed as percentages of the total answers, as indicated.
3 Results
3.1 Questionnaire’s reproducibility
For the assessment of questionnaire reproducibility, 72 patients, 43 physicians, and 68 pharmacists answered the same questionnaire twice over a 20-day interval. The statistical analysis of the repeated questionnaires showed excellent repeatability in the answers to all questionnaires in each cohort (patients, physicians, and pharmacists). The level of agreement expressed by the intraclass correlation coefficient (ICC) was 0.95 (confidential interval-CI 0.85–0.96) for the 72 patients, 0.89 (CI 0.81–0.94) for the 68 pharmacists, and 0.92 for the 43 physicians, indicating a high level of repeatability of the answers, and thus, a high precision of the investigational tool was used for the present study.
3.2 Physicians
A total of 3,394 physicians, largely (>90%) GPs, and 77 gastroenterology specialists were included in the present analysis. All the physicians who completed the questionnaire declared to have had the experience of prescribing Poliprotect to their patients over the previous year.
The answers related to the perceived effectiveness on typical symptoms of GERD (i.e., pyrosis) and FD clinical sub-groups (i.e., gastric pain and difficulties in digestion) from 3,394 GPs are shown in Figure 1A. Overall, over 90% of the answers rated Poliprotect as good/excellent in controlling pyrosis, 80% for gastric pain, and approximately 70% of the answers rated Poliprotect as good/excellent in ameliorating difficulties in digestion.
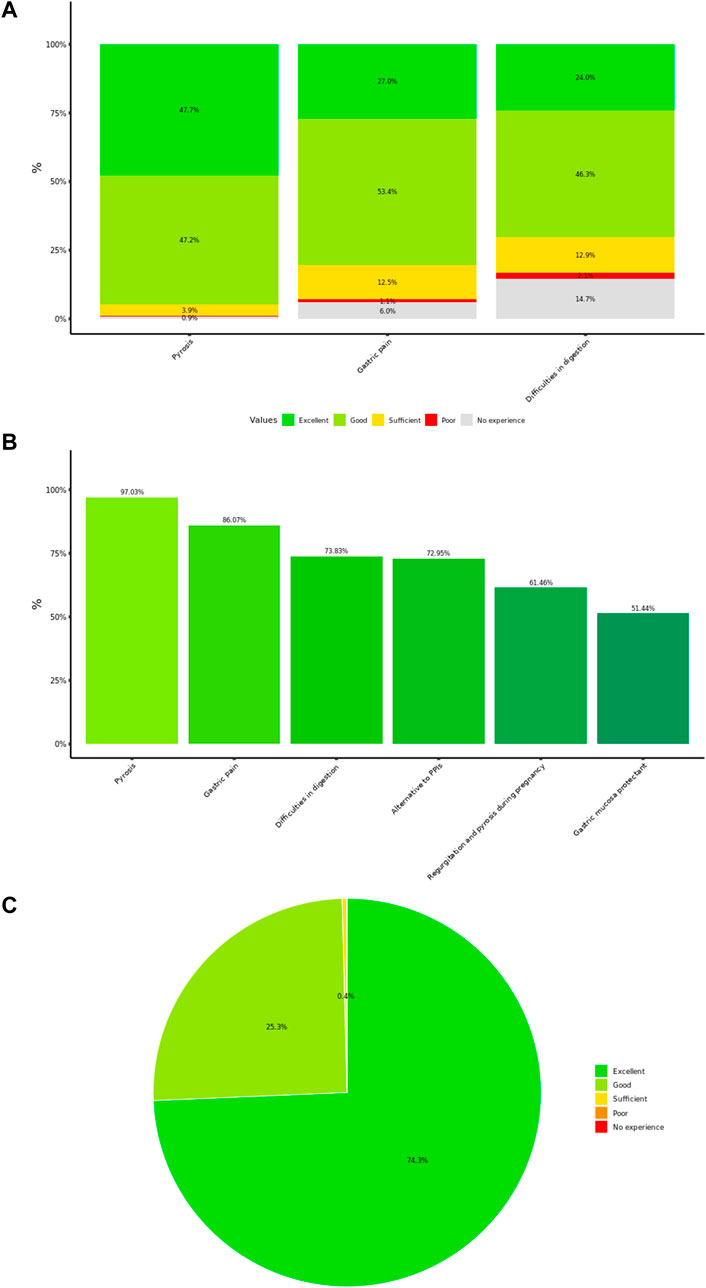
Figure 1. (A) Physicians’ rating of product effectiveness. The plot reports the distribution (%) of the answers from 3,394 physicians, largely general practitioners, who rated the perceived overall effectiveness of the product on typical symptoms of gastroesophageal reflux disease and dyspepsia. (B) General practitioners’ rating of product perceived usefulness in diverse symptom’s settings. Rating of 3,394 general practitioners regarding product perceived usefulness rated as extremely/very useful, in different clinical settings. (C) General practitioners perceived patient’s tolerability. Distribution of the answers from 3,394 general practitioners to the question on the perceived patient’s tolerability is represented.
Physicians answered that Poliprotect in their clinical practice was very or extremely useful mostly for pyrosis (97%) and EPS (i.e., gastric pain; 86%) (Figure 1B). Moreover, this same rating of perceived usefulness of the product was indicated for treating PDS (i.e., difficulties in digestion; 74%), as an alternative to PPIs (73%) when the latter could not be used, for pregnancy-associated GERD symptoms (61%) and as a protective agent in association to drugs known to be able to damage the gastric mucosa (non-steroidal anti-inflammatory drugs, NSAIDs, and corticosteroids) (51%) (Figure 1B).
The patient’s tolerability was considered excellent/good by 99.5% of the GPs (Figure 1C). Overall, three non-serious adverse events were reported by GPs.
We analyzed separately the cohort of 77 gastroenterology specialists as the confirmatory cohort of experts in GI disorders. The answers from this cohort were largely consistent with the GP’s opinions and clinical management choices. In particular, 79% of the gastroenterologists answered to be extremely or very satisfied with the improvements of typical GERD symptoms by Poliprotect, whereas improvement of dyspepsia both painful and painless and pregnancy- and breast-feeding associated GERD symptoms was considered highly satisfactory by 69, 52, and 62% of the specialists, respectively. Gastroenterologists recommended the product mostly for typical GERD ed EPS symptoms (i.e., pyrosis and gastric pain; Figure 2) and reported a good/excellent improvement of the quality of life (QoL) in 88% of their patients. Interestingly, they also used the product in patients below 18 years of age (2.6% of the patient population). Moreover, the most used pattern of usage of Poliprotect among gastroenterology specialists was largely in association with anti-secretory drugs, antacids, or other mucosal protectors (>95%), over prolonged periods (weeks to months).
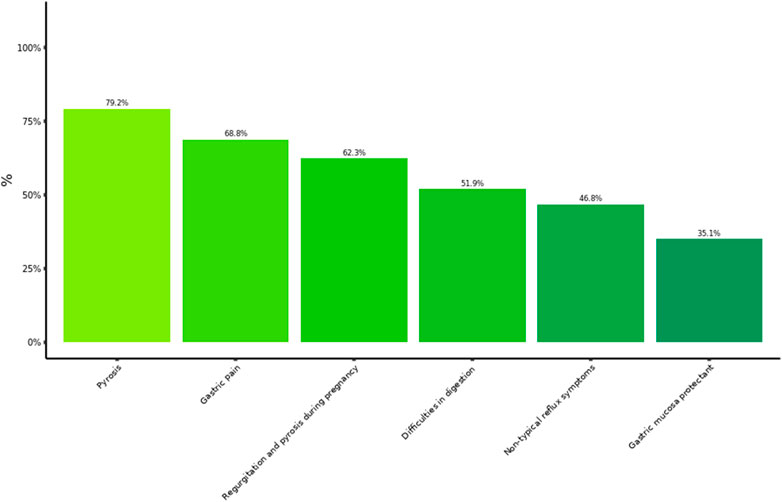
Figure 2. Symptoms for whom gastroenterologists recommended MPA. Frequency of recommendation by 77 gastroenterologists within the range from very much to extremely useful, for use in each of different clinical settings.
3.3 Patients
A total of 848 patients answered the questionnaires, >50% aged between 30 and 50 years, 80% women. The vast majority of the patients were self-medicated (82%), with no advice from any GP or GI specialist (98% of the cohort) for their GI symptoms, and all had used the product within the previous year.
The distribution of symptoms for which Poliprotect was used by patients was coherent with the use described in the product leaflet. Intake because of painful dyspeptic symptoms was reported by over 80% of the patients, followed by painless dyspeptic symptoms (i.e., difficulties in digestion, 51%) and heartburn (42%) (Figure 3A). We could not detect any difference after excluding those patients (37%) who used the product in association with therapies with similar intended use (Figure 3B). The product was used alone, not in association with therapies with similar indications by 63% of patients.
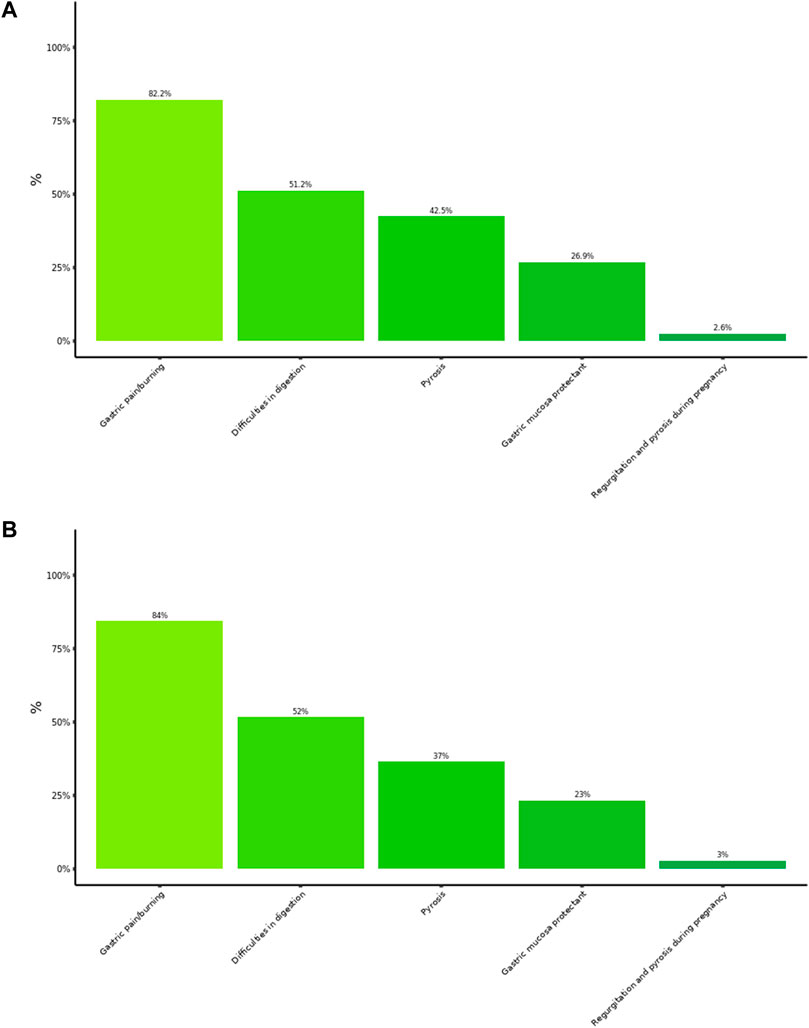
Figure 3. Patient’s pattern of usage. Each column represents the rating (%) of use of the product in the patient cohort. Multiple answers were possible: a total of 2,210 answers were collected and analyzed. (A) Results in the entire patient cohort (N = 848), irrespective of whether the product was used alone or in association with therapies with similar indications. (B) Results in the subgroup of patients (N = 523) who used the product in monotherapy.
Patients reported a symptom relief rated as excellent/good in 73% of the answers (Figure 4A). No difference was observed after excluding patients who used the product in association with other drugs (Figure 4B). Furthermore, patients reported a marked QoL improvement in 65% of the answers. Determinants of this improvement seem to be largely related to the possibility of being able to eat more freely (38%), sleep better (27.6%), and live with a more positive mood (19.4%). Consistently, the self-reported tolerability was ranked between excellent and good in 93% of the answers.
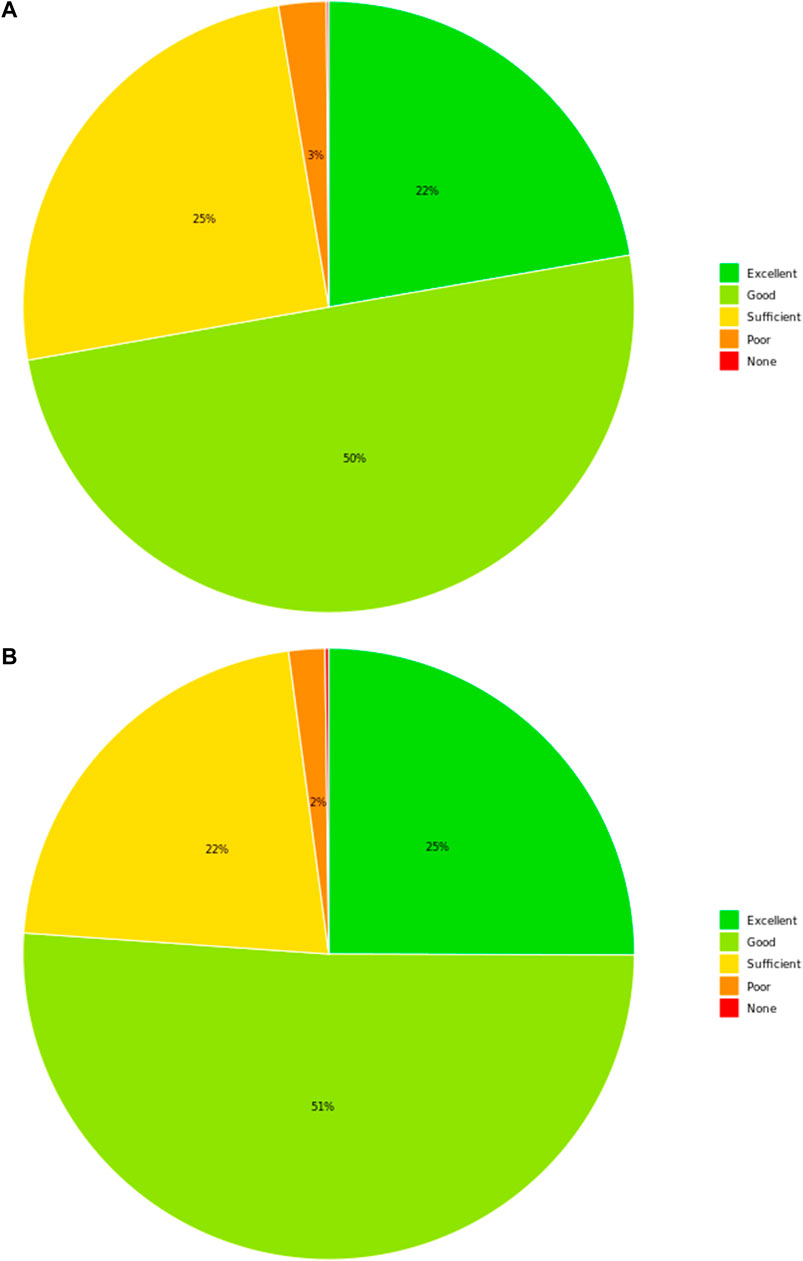
Figure 4. Patient’s perceived effectiveness. Overall symptoms’ amelioration rated by patients is represented. (A) Results in the entire patient cohort (N = 848), irrespective of whether the product was used alone or in association with therapies with similar indications. (B) Results in the subgroup of patients (N = 523) who used the product in monotherapy.
3.4 Pharmacists
Questionnaires were collected from 146 pharmacists, who had experience with the product over the previous year. The most prevalent age among their customers was between 31 and 50 years, with 5.5% below the age of 18 years. The pattern of suggested use of Poliprotect by the pharmacists was strictly adherent to the leaflet. Similarly, to the other professional cohorts, pharmacists recommended the product mostly for painful dyspeptic symptoms, largely for typical GERD symptoms (Figure 4). Notably, pharmacists suggested the use in pregnancy in the same proportion as gastroenterology specialists. Pharmacists suggested Poliprotect as a front-line compound for upper GI disturbances in 76% of the customers.
The proportion of pharmacists who replied to be extremely or very satisfied with improvements achieved with Poliprotect was 75% in typical GERD symptoms, 74% for gastric pain, and 66% for pregnancy- and breastfeeding-associated GERD symptoms.
Pharmacists reported an excellent/good impact on the QoL of their customers in 74% of the answers, and safety and tolerability were rated as excellent/good in 97% of the answers.
4 Discussion
This study reports a large survey that included 4,467 subjects recruited among medical doctors (both GPs and specialists), patients, and pharmacists, focusing on the perceived effectiveness, tolerability, safety, and on the pattern of usage of a natural MPA, Poliprotect, approved for the treatment of GERD and dyspepsia. This study also describes a new methodology of validated surveys that have been developed to fulfill the requirements of the new EU regulation for SBMD.
The surveys from specialists and pharmacists consistently reported a largely prevalent usage of this product to treat typical GERD and EPS symptoms (i.e., pyrosis and gastric pain) (Figures 2, 5), although with an inverse preference. Moreover, use in painful dyspeptic symptoms was the most suggested one by pharmacists (97%) and the most prevalent in patients (82%) (Figures 3, 5). This pattern may be related to the OTC availability of the product to subjects with symptoms, and it is consistent with the notion of self-medication in these subjects. A large fraction of patients used the product for digestive difficulties as well (Figure 3), a condition that was rated less by physicians and pharmacists (Figures 2, 5). However, for this condition, the proportion of those who suggested/recommended Poliprotect was equal to that of patients (Figure 3). This may reflect a tendency of patients suffering from chronic post-prandial distress symptoms to seek the advice of healthcare professionals, probably also because of a substantial lack of OTC treatments with this indication.
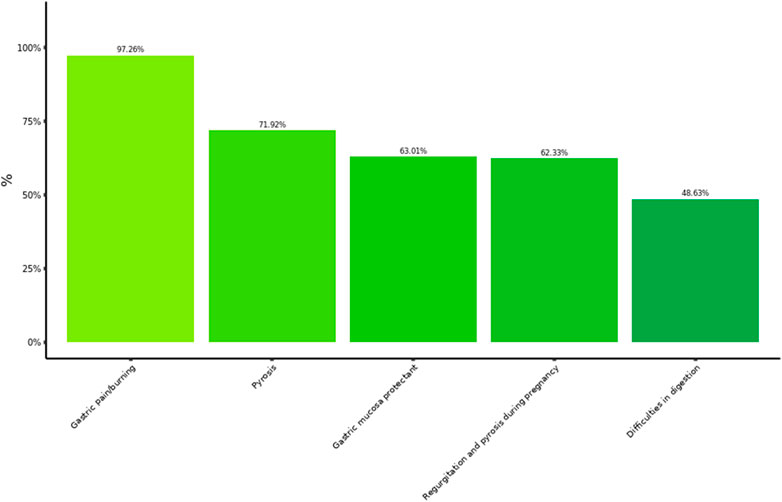
Figure 5. Pharmacist’s pattern of usage. Each column represents the rating (%) of 146 pharmacists on the suggested use of the product. Multiple answers were possible: a total of 630 answers were collected and analyzed.
The specialist’s data on perceived effectiveness showed the highest rank for improving both GERD and dyspepsia, with a very high patient tolerability and acceptance and no concerns about safety.
Patients used Poliprotect largely alone and without a doctor’s prescription; gastroenterologists used this SBMD mostly associated with other gastroprotective drugs; pharmacists largely suggested the use of the product alone; GPs used Poliprotect both alone and in combination. Independently of the single or combined usage, all categories reported high-perceived effectiveness on the specific pattern of usage.
Ex vivo data on biopsies of the normal human esophageal mucosa showed that the damage of the barrier induced by an acidic-peptic solution was reduced by Poliprotect similarly to sodium alginate solution (Liguori et al., 2018). These ex vivo data provide a pathophysiological context for explaining the protective effect on the GI mucosa reported by these surveys.
Functional upper GI tract disorders have a high prevalence in the general population worldwide, especially in western countries in association with the lifestyle and the type of diet (Oshima and Miwa, 2015). Treatment can rely on medical and non-medical approaches (Oshima and Miwa, 2015; Harer and Hasler, 2020) which document the complexity of achieving a successful management strategy for these disorders.
Moreover, the rising incidence of obesity worldwide, the so-called “globesity” (Murray and Lopez, 2013), especially among younger adults, adolescents, and children, is expected to further increase disorders such as GERD and FD, which can share common pathogenetic mechanisms (Alimi and Azagury, 2021). Interestingly, GI specialists report using Poliprotect also in children and adolescents, likely reflecting a growing unmet therapeutic need in these special groups of subjects, in whom long-term treatment with drugs is usually not the preferred choice considering that PPIs for instance may increase the risk of severe enteric infections (Vandenplas, 2014; Hafiz et al., 2018). However, the current management of children older than 10 years is similar to that in adults, and the risks of prokinetics largely outweigh their potential benefits. Thus, Poliprotect can be an important option in children, or at least this SBMD can be tested in future pediatric randomized, intervention studies assessing benefit/risk balance versus pharmacological agents with the same indications (Vandenplas, 2014).
Pregnancy is also frequently associated with GERD, affecting up to 80% of pregnant women (Thelin and Richter, 2020). Interventions used to relieve symptoms in pregnant women begin with lifestyle modifications, followed by pharmacological interventions in case of failure of lifestyle changes, which include antacids, alginates, and sucralfate as first-line agents, while H2 receptor antagonists or PPIs are reserved for intractable symptoms or complicated GERD (Thelin and Richter, 2020). Poliprotect can be a valuable first-line treatment option in association with/or in alternative to lifestyle changes or even to other MPAs. Thus, future studies might explore this special population as well. Interestingly, pregnancy was consistently reported to a sizeable extent among the patterns of usage of all the studied cohorts.
The usage of Poliprotect has been reported as a stand-alone agent by patients, pharmacists, and in part of the GPs, or as an agent in the combination of other gastro-protectant drugs by most of the gastroenterologists. These data suggest that both patterns of usage can be potentially effective, as a function of the background disease and therapy, justifying a single or combined strategy. Importantly, no Poliprotect–drug interactions were reported by physicians, even though more data may be needed in this respect.
Out of 4,467 responders, only three non-serious adverse events were reported, for whom a causal relationship with the natural compound was not established. Thus, the safety profile is reassuring in pediatric or pregnant subjects as well.
The current study, being observational allows conclusions on effectiveness rather than efficacy. However, a real-life context allows evaluation of the extent to which expected clinical benefits and safety are accomplished in large, unselected populations, thus obtaining a more generalizable estimate of the benefit-risk profile of treatment. To this aim, the large sample of the present research and the different cohorts in which it was performed, are of value. The choice of providing only descriptive statistics was made considering the limited value of performing a multivariate analysis where the dependent variable, for example, “symptom improvement,” would have been dichotomized due to a very small number, if any, of responses belonging to the last two classes, i.e., “poor” and “none,” thus generating de facto a comparison between subjects who were “markedly improved” vs. “moderately improved”, and not between “improved” vs. “not improved”.
The present work has some limitations: since most of the participants were invited to complete an online questionnaire, the population was somehow self-selected and not unbiased, if we consider, for instance, the exclusion of individuals without internet access, or with poor internet skills (i.e., elderly subjects). In addition, this is not a comparison versus drugs with similar approved use, alone or as add-on therapy, or placebo; thus, conclusion on the comparative effectiveness or efficacy cannot be drawn. For the QoL assessment, participants were asked to rate the effect of treatment (as described by patients/consumers) on the quality of life considering the following set of components: mood, social life, work, dietary options, and sleep (Supplementary Material S2). Validated QoL assessment tools, which include at least 15 questions, were not used to avoid low-completion rates due to a high number of total questions.
In conclusion, the present surveys indicate that Poliprotect can be considered safe and effective in the treatment of a variety of upper GI symptoms and in a large array of subjects, including special populations with poor available evidence and feasibility of randomized trials, such as children and pregnant women, in which unmet therapeutic need may be more present.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
EG: conceptualization, supervision, review, and editing. PM and MR: formal analysis, data curation, and writing—original draft preparation. LM and FS: investigation, review, and editing. RC and AC: review and editing, and project management.
Funding
This study has been funded by Aboca S.p.A., the manufacturer of the medical device.
Conflict of interest
Three of the authors, RC, AC, and EG, are employees of the sponsor and worked on this project as part of their employment.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer LB declared a shared affiliation, with one of the authors PM, MR to the handling editor at the time of the review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdsfr.2022.969831/full#supplementary-material
References
AIFA (2008). Guidelines for the classification and conduct of observational studies with medicinal products [Online]. on line: UFFICIO PUBBLICAZIONE LEGGI E DECRETI. Available: https://www.gazzettaufficiale.it/eli/gu/2008/03/31/76/sg/pdf (Accessed July, 2022).
Alimi, Y., and Azagury, D. E. (2021). Gastroesophageal reflux disease and the patient with obesity. Gastroenterol. Clin. North Am. 50 (4), 859–870. doi:10.1016/j.gtc.2021.08.010
Black, C. J., Houghton, L. A., and Ford, A. C. (2018). Insights into the evaluation and management of dyspepsia: Recent developments and new guidelines. Ther. Adv. Gastroenterol. 11. 1–17. doi:10.1177/1756284818805597
Enck, P., Azpiroz, F., Boeckxstaens, G., Elsenbruch, S., Feinle-Bisset, C., Holtmann, G., et al. (2017). Functional dyspepsia. Nat. Rev. Dis. Prim. 3, 17081. doi:10.1038/nrdp.2017.81
Ford, A. C., Marwaha, A., Lim, A., and Moayyedi, P. (2010). What is the prevalence of clinically significant endoscopic findings in subjects with dyspepsia? Systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 8 (10), 830–837. doi:10.1016/j.cgh.2010.05.031
Hafiz, R. A., Wong, C., Paynter, S., David, M., and Peeters, G. (2018). The risk of community-acquired enteric infection in proton pump inhibitor therapy: Systematic review and meta-analysis. Ann. Pharmacother. 52 (7), 613–622. doi:10.1177/1060028018760569
Harer, K. N., and Hasler, W. L. (2020). Functional dyspepsia: A review of the symptoms, evaluation, and treatment options. Gastroenterol. Hepatol. 16 (2), 66–74.
Karamanolis, G., Caenepeel, P., Arts, J., and Tack, J. (2006). Association of the predominant symptom with clinical characteristics and pathophysiological mechanisms in functional dyspepsia. Gastroenterology 130 (2), 296–303. doi:10.1053/j.gastro.2005.10.019
Keller, J., Frederking, D., and Layer, P. (2008). The spectrum and treatment of gastrointestinal disorders during pregnancy. Nat. Clin. Pract. Gastroenterol. Hepatol. 5 (8), 430–443. doi:10.1038/ncpgasthep1197
Lee, K. J., Kim, J. H., and Cho, S. W. (2006). Dyspeptic symptoms associated with hypersensitivity to gastric distension induced by duodenal acidification. J. Gastroenterol. Hepatol. 21 (3), 515–520. doi:10.1111/j.1440-1746.2005.03976.x
Liguori, G., Baldi, F., and Di Simone, M. P. (2018). Mo1175 - topical protection of esophageal mucosa: In vitro evaluation of a medical device made of natural substances in comparison with sodium alginate. Gastroenterology 154 (6), 696. doi:10.1016/s0016-5085(18)32456-9
Lundell, S., Toots, A., Sönnerfors, P., Halvarsson, A., and Wadell, K. (2022). Participatory methods in a digital setting: Experiences from the co-creation of an eHealth tool for people with chronic obstructive pulmonary disease. BMC Med. Inf. Decis. Mak. 22 (1), 68. doi:10.1186/s12911-022-01806-9
Murray, C. J., and Lopez, A. D. (2013). Measuring the global burden of disease. N. Engl. J. Med. 369 (5), 448–457. doi:10.1056/NEJMra1201534
Official Journal of the European Union (2007). Directive 2007/47/EC of the European Parliament and of the Council of 5 September 2007 amending Council Directive 90/385/EEC on the approximation of the laws of the Member States relating to active implantable medical devices, Council Directive 93/42/EEC concerning medical devices and Directive 98/8/EC concerning the placing of biocidal products on the market [Online]. on line: European Union. Available: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32007L0047&from=EN (Accessed July, 2022).
Official Journal of the European Union (2017). Regulation (EU) 2017/745 of the European parliament and of the council of 5 april 2017 on medical devices, amending directive 2001/83/EC, regulation (EC) No 178/2002 and regulation (EC) No 1223/2009 and repealing council directives 90/385/EEC and 93/42/EEC [online]. on line: European Union. Available: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32017R0745 (Accessed April, 2022).
Oshima, T., and Miwa, H. (2015). Epidemiology of functional gastrointestinal disorders in Japan and in the world. J. Neurogastroenterol. Motil. 21 (3), 320–329. doi:10.5056/jnm14165
Scally, B., Emberson, J. R., Spata, E., Reith, C., Davies, K., Halls, H., et al. (2018). Effects of gastroprotectant drugs for the prevention and treatment of peptic ulcer disease and its complications: A meta-analysis of randomised trials. Lancet. Gastroenterol. Hepatol. 3 (4), 231–241. doi:10.1016/S2468-1253(18)30037-2
Sweis, R., and Fox, M. (2020). The global burden of gastro-oesophageal reflux disease: More than just heartburn and regurgitation. Lancet. Gastroenterol. Hepatol. 5 (6), 519–521. doi:10.1016/s2468-1253(20)30002-9
Thelin, C. S., and Richter, J. E. (2020). Review article: The management of heartburn during pregnancy and lactation. Aliment. Pharmacol. Ther. 51 (4), 421–434. doi:10.1111/apt.15611
Vandenplas, Y. (2014). Management of paediatric GERD. Nat. Rev. Gastroenterol. Hepatol. 11 (3), 147–157. doi:10.1038/nrgastro.2013.199
Vanheel, H., Carbone, F., Valvekens, L., Simren, M., Tornblom, H., Vanuytsel, T., et al. (2017). Pathophysiological abnormalities in functional dyspepsia subgroups according to the rome III criteria. Am. J. Gastroenterol. 112 (1), 132–140. doi:10.1038/ajg.2016.499
Wauters, L., Dickman, R., Drug, V., Mulak, A., Serra, J., Enck, P., et al. (2021). United European gastroenterology (UEG) and European society for neurogastroenterology and motility (ESNM) consensus on functional dyspepsia. United Eur. Gastroenterol. J. 9 (3), 307–331. doi:10.1002/ueg2.12061
Keywords: substance-based medical device, gastroesophageal reflux disease, functional dyspepsia, gastroprotective agents, real-world evidence, safety, effectiveness
Citation: Cioeta R, Muti P, Rigoni M, Morlando L, Siragusa F, Cossu A and Giovagnoni E (2022) Effectiveness and tolerability of Poliprotect, a natural mucosal protective agent for gastroesophageal reflux disease and dyspepsia: Surveys from patients, physicians, and pharmacists. Front. Drug Saf. Regul. 2:969831. doi: 10.3389/fdsfr.2022.969831
Received: 15 June 2022; Accepted: 19 August 2022;
Published: 10 October 2022.
Edited by:
Elisabetta Bigagli, University of Florence, ItalyReviewed by:
Luigi Bonavina, University of Milan, ItalyAnna Rita Bilia, University of Florence, Italy
Salvatore Crisafulli, University of Verona, Italy
Sara De Martin, University of Padua, Italy
Copyright © 2022 Cioeta, Muti, Rigoni, Morlando, Siragusa, Cossu and Giovagnoni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roberto Cioeta, cmNpb2V0YUBhYm9jYS5pdA==
 Roberto Cioeta
Roberto Cioeta Paola Muti2
Paola Muti2 Emiliano Giovagnoni
Emiliano Giovagnoni