
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Drug Saf. Regul. , 26 October 2022
Sec. Substance-Based Medical Devices
Volume 2 - 2022 | https://doi.org/10.3389/fdsfr.2022.1054972
This article is part of the Research Topic Medical Devices made of substances for human health: a challenge in terms of efficacy, safety and sustainability View all 9 articles
This article is a correction to:
Substance-based medical devices made of natural substances: An opportunity for therapeutic innovation
A Corrigendum on
Substance-based medical devices made of natural substances: An opportunity for therapeutic innovation
by Giovagnoni E (2022). Front. Drug. Saf. Regul. 2:998114. doi: 10.3389/fdsfr.2022.998114
In the published article, there are some errors in Table 1 as published. One error is the percentage in the second column third line and the other one is the price in the second column last line (1.50 €), both referred to Italy. The third error is the price in the third column last line (3.97 €) referred to Poland. Other numerous errors were found in the “France” column, the entire column. The last error is in the caption where the word “total” is missing.
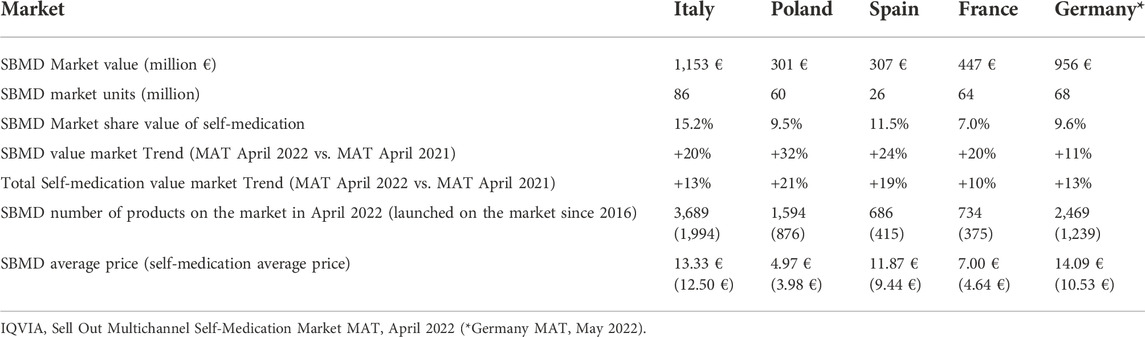
Table 1. Summary of sales data showing the importance of SBMDs in the total self-medication sector, including food supplements, in some European Union Member States. Source: IQVIA, Sell Out Multichannel Self-Medication Market MAT, April 2022 (* Germany MAT, May 2022).
The corrected Table 1 and its caption appear below.
In the published article, there were some errors in the following paragraph that contains data related to Table 1.
The corrections have been made to Introduction in the paragraph Substance-based medical device: Market share, page 03. These sentences previously stated:
“Aggregate data for these five markets indicate that the SBMD sector is worth 2.9 billion euros, equivalent to 270 million units (MAT May 2022 for Germany, MAT April 2022 for the other countries), and has grown +19% vs. +13.5% of the total self-medication sector (including OTC drugs, medical devices, food supplements and homeopathic medicines).
The number of products registered as SBMDs is greater than 8,700, of which over 4,600 have been placed on the market since 2016. This means that the companies involved in the self-medication sector are investing a lot in the development of SBMDs. This is due to the degree of innovation being delivered by these non-pharmacologically acting products as well as the approval of Regulation 2017/745, which has clarified the EU regulatory framework.
SBMDs currently represent 10% of the total self-medication market, with an average price of € 10.86 vs. € 7.47 for the total self-medication sector.”
The corrected sentences appear below
“Aggregate data for these five markets indicate that the SBMD sector is worth 3.2 billion euros, equivalent to 304 million units (MAT May 2022 for Germany, MAT April 2022 for the other countries), and has grown +18% vs. +13.5% of the total self-medication sector (including OTC drugs, medical devices, food supplements and homeopathic medicines). The number of products registered as SBMDs is greater than 9,100, of which about 4,900 have been placed on the market since 2016. This means that the companies involved in the self-medication sector are investing a lot in the development of SBMDs. This is due to the degree of innovation being delivered by these non-pharmacologically acting products as well as the approval of Regulation 2017/745, which has clarified the EU regulatory framework. SBMDs currently represent 11% of the total self-medication market, with an average price of € 10.41 vs. € 7.47 for the total self-medication sector.”
The author apologizes for this errors and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: substance-based medical device, medical device made of substances, natural substances, innovation, herbal medicines, medical device regulation, mechanism of action, therapeutic effect
Citation: Giovagnoni E (2022) Corrigendum: Substance-based medical devices made of natural substances: An opportunity for therapeutic innovation. Front. Drug Saf. Regul. 2:1054972. doi: 10.3389/fdsfr.2022.1054972
Received: 27 September 2022; Accepted: 05 October 2022;
Published: 26 October 2022.
Edited and reviewed by:
Juan L. Tamargo, Complutense University of Madrid, SpainCopyright © 2022 Giovagnoni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emiliano Giovagnoni, ZWdpb3ZhZ25vbmlAYWJvY2EuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.