Abstract
People with HIV (PWH), even when well-controlled on antiretroviral therapy (ART), are at an increased risk for cardiovascular disease (CVD) and CVD events including sudden cardiac death and acute myocardial infarction (MI). While PWH may appear virally suppressed in peripheral blood, viral reservoirs persist in gut-associated lymphoid tissue (GALT) and have been shown to be associated with CVD-related morbidity and mortality. Effective treatments exist for CVD in HIV seronegative persons, but there is an unmet clinical need to address CVD in PWH. Novel therapies are needed to target the drivers of CVD in PWH. This literature review focuses on the role of GALT in HIV infection, inflammatory pathways in HIV-related CVD, and novel therapeutics with potential to address this problem.
Introduction
Most of the morbidity and mortality amongst persons with HIV (PWH) on antiretroviral therapy (ART) is due to non-AIDS related conditions, including cardiovascular disease (CVD). In a study, CVD was the cause of death in 23 percent of PWH less than 40 years of age, and 20 percent in those who were 50 years of age (Miller et al., 2014). Such evidence has made it increasingly clear that PWH are at a greater risk for experiencing CVD-related events than HIV seronegative persons, and for having a more severe outcome from such events. In fact, PWH are 4.5 times more likely to experience sudden cardiac death (Tseng et al., 2012), 2 times more likely to experience acute myocardial infarction (MI) (Rao et al., 2019), and 1.6 times more likely to have new carotid plaque (Hanna et al., 2015). The presence of HIV establishes CVD risk factors far beyond common external and behavioral ones, such as dietary habits and smoking. Carotid intima-media thickness and carotid plaque is greater in PWH, which can double to triple the likelihood of CVD-related events. Moreover, aortic pulse wave velocity — indicative of arterial stiffness (Schillaci et al., 2008), a precursor of clinical hypertension — was increased in PWH who were on ART (Guadalupe et al., 2003). The objective of this systematic review is to elucidate the relationship between HIV persistence in the gut and development of CVD. Understanding these mechanistic interactions can provide insight into proactive care for PWH and build towards customized therapeutic options to mitigate CVD in PWH by exploiting mechanistic paradigms across the gut and the cardiovascular system.
Methods
PubMed searches were utilized to complete this review. Key words were searched, including HIV reservoir, gut, HIV persistence, HIV latency, HIV and CVD, and inflammatory markers of HIV. The searches were completed between August of 2022 and December of 2022. The paper was completed in accordance with the PRISMA guidelines for systematic reviews.
The data collected was reviewed by a team of six individuals who worked independently of each other. Data collected included both quantitative and qualitative data collected from preexisting peer reviewed studies and meta-analyses. Papers that discussed CVD were limited to those that also discussed.
HIV, while all HIV papers were included, regardless of if they discussed CVD. Database searches encompassed adult subjects of all sexes and ages but were restricted to studies completed in English. Study limitations include papers that were published over 10 years ago, contained conflicting results, and were geographically limited to mainly the United States. In the presence of conflicting results, both sides were discussed in the paper. These criteria were used throughout the paper, as well as in completion of the table.
Inflammatory biomarkers of CVD among PWH
Existing research and standard HIV-tracking practices focus on peripheral blood, which contains only 2 to 5 percent of the body’s lymphocytes. In contrast, gut-associated lymphoid tissue (GALT) houses over 80 percent of lymphocytes. Biopsies from intestinal tissues can still be readily collected, further highlighting the need to shift attention toward GALT for monitoring of PWH. Viruses have been found to accumulate in GALT, making it a hotspot for sudden infection, and thus immune activation. Primary HIV-1 infection significantly diminished concentrations of CD4+ and CD4+CD8 T-cell concentrations in GALT but not in peripheral blood. In one study, participants lost 60 percent of their CD4+ T cells of GALT within 3 weeks of infection. These low cellular counts did not rebound following ART with levels remaining lower in GALT compared to peripheral blood (Figure 1). CD4+ T cells vulnerability to HIV infections stems from their expression of CCR5, a receptor to which HIV is reactive. Depleted CD4+ T cell concentration in GALT compared to peripheral blood persisted in asymptomatic PWH. Primary HIV-1 infection also spiked cell proliferation rates in GALT, pointing to the tissue’s relevance in HIV infection. Furthermore, CD4+ T cell levels in peripheral blood returned to normal following viral suppression. This rebound in cellular concentration was not evident in GALT for people with chronic infection, albeit it was evident in people who received ART early in infection (Guadalupe et al., 2003; Mehandru et al., 2006; Costiniuk and Angel, 2012).
FIGURE 1
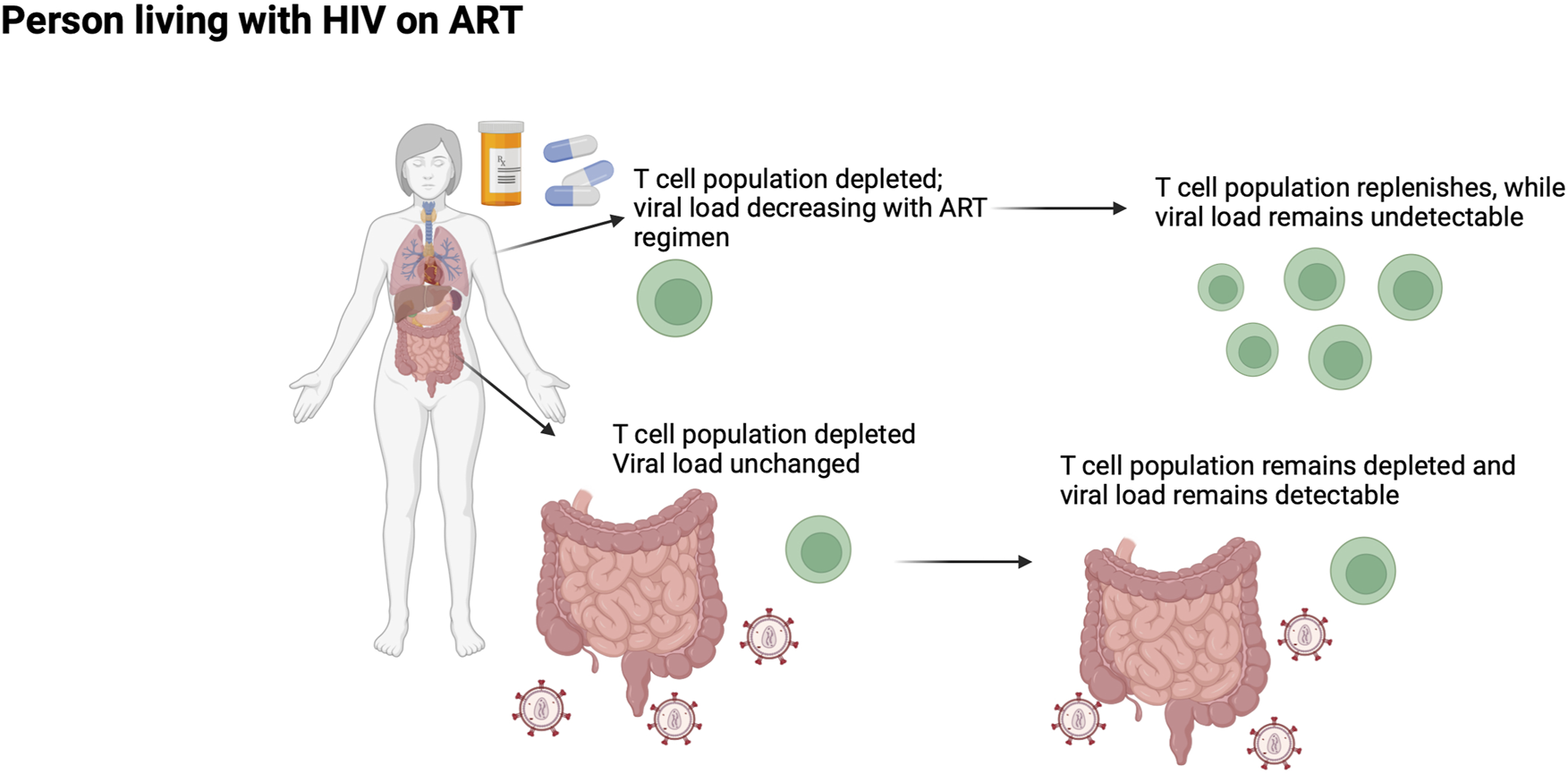
The gut as a sanctuary site for ongoing HIV-1 replication in PWH receiving suppressive ART.
Failure of T cell concentration to return in GALT even after peripheral viral suppression is thought to majorly contribute to the existence of a viral reservoir (Chun et al., 2008). Also of possible contribution, though less conclusively, is the ability of ART medications to penetrate GALT. Dolutegravir, an HIV integrase inhibitor given in ART, was found in lower concentrations in rectal tissue than in plasma. This is due to dolutegravir having characteristics that make it not optimally absorbed by GALT, such as a lower molecular weight and log octanol water partition coefficient. Moreover, HIV infection and ART impacts expression of intestinal drug transporters, complicating optimal absorption (Lahiri et al., 2018).
Specifically, the cytokine Interleukin 6 (IL-6) is recognized for its role in regulating the Th17-Treg cells in GALT (Bargieł et al., 2021). IL-6 is an inflammatory marker, that is pathologically stimulated by increased heart rate, through exercise of traumatic incidences (Bargieł et al., 2021). Its disrupted homeostasis often leads to inflammation in CVD. In response to high concentration of IL-6, C-Reactive protein (CRP) is produced (Brown and Bittner, 2008). CRP levels in the plasma exponentially increase following inflammatory events. These levels pose a risk of higher mortality due to CVD and non-CVD incidences (Baker and Duprez, 2010).
Among PWH, elevated levels of D-dimer and fibrinogen have been recognized to increase the risk of mortality (Ridker et al., 2018). Fibrinogen is a glycoprotein that been associated with atherosclerosis and CVD, at elevated levels (Madden et al., 2008; Ridker et al., 2018). Fibrinogen, an acute phase reactant, facilitates platelet aggregation, increases viscosity, and advances smooth muscle proliferation, leading to vascular dysregulation (Madden et al., 2008). D-dimer is a degradation product of a split fibrin, and elevated levels have been associated with higher cardiac mortality, compared to populations that survived (Zhao et al., 2020). These four biomarkers are frequently used to predict CVD incidence or outcome among PWH or associated inflammation.
Immune cell imbalance and GALT
HIV infection has also shown to have an impact on regulatory T cells (Tregs), which maintain the activity of effector lymphocytes, including TH17 cells. However, there is conflicting literature about whether this impact is net positive or negative. Some studies have found Tregs to be helpful in PWH by suppressing chronic inflammation, while others have found them to be harmful by suppressing immune activity during infection. Despite conflicting findings, there is overwhelming support that Treg levels are impacted by viral infection, and that they consequently impact viral pathogenesis. This dysregulation of Tregs has been demonstrated to impact the development of CVD by leading to an increase in atherosclerosis. Atherosclerosis, a disease in which lipids and other fibrous elements accumulate under the endothelial lining of arterial tissue, is a leading cause of CVD (Albany et al., 2019; Lusis, 2000). Impaired Treg function has been associated with the worsening of atherosclerosis (Baardman and Lutgens, 2020), and subsequently an increase in incidence of CVD in PWH.
Research has found elite controllers, or PWH who naturally control their viral replication, to have lower levels of Tregs. Elite controllers also naturally maintain a proper ratio between Tregs and the TH17 cells they suppress (Brandt et al., 2011). The imbalanced ratios of such T cells in PWH without viral control has an impact on the occurrence of leaky gut. Homeostatic T cells, including TH17, are involved in the maintenance of the epithelial barrier. As these cells get depleted during HIV infection, the epithelial barrier becomes less robust. This increases permeability of the barrier, making microbes of the GALT more susceptible to entering circulation. This phenomenon, known as microbial translocation, increases chronic inflammation, decreases proper immune function, and is associated with HIV pathogenesis (Figure 1). As mentioned before, ART does not eliminate the HIV reservoir in the GALT, leaving PWH vulnerable to microbial translocation even after peripheral viral suppression (Zevin et al., 2016).
Immunological biomarkers of CVD events in GALT
T cell activation is triggered by many immunological biomarkers. Interestingly, and regardless of a CVD history, these biomarkers have been shown to predict cardiac related events in PWH. Levels of CRP, IL-6, and D-Dimer are elevated in PWH who develop CVD as opposed to PWH who do not develop CVD. Moreover, the concentrations of these three biomarkers are higher in the plasma of PWH who experience fatal CVD in comparison to PWH who experience nonfatal CVD (Nordell et al., 2014). HIV patients who had high levels of CRP were also at an increased risk of acute myocardial infarction (Triant et al., 2009). Immunological biomarkers are not eradicated through ART for PWH. In women whose HIV had been treated with ART, CD4+ and CD8+ T cell activity remained high. CD4+ and CD8+ T cell activations are more elevated in women with HIV who have carotid lesions compared to women with HIV who do not have these lesions. Furthermore, T cell activation was not higher in women without HIV who had carotid lesions. The presence of carotid lesions was further impacted by immunosenescence of T cells. Immunosenescence is a cellular aging process, and is expedited by HIV infection, even after completion of ART (Kaplan et al., 2011).
Inflammatory pathways and treatments
As discussed, HIV is frequently undetectable in peripheral blood despite viral loads existing throughout the body. Available anti-retroviral therapies (ART), which are the standard of care for PWH, target HIV in peripheral blood cells. Patients with undetectable levels of plasma HIV are still left susceptible to non-AIDS comorbidities, such as CVD. Gut reservoirs of HIV infection result in chronic inflammation for PWH. This inflammation is indicated by elevated levels of inflammatory markers and markers of endothelial dysfunction and hypercoagulability, including but not limited to IL-6, IFN-a/b, IL-1a/b, TNF-a, CRP, D-dimer (Hsue and Waters, 2018; Duprez et al., 2012; Subramanya et al., 2019). Activation of the Jak-STAT pathway, which is both reported during initial HIV infection and associated with HIV persistence, has been shown to increase levels of inflammation throughout the body (Reece et al., 2021; Marconi et al., 2022; de Armas et al., 2021; Bovolenta et al., 1999; Cecile et al., 2022).
While there are effective treatment options available to treat CVD in HIV seronegative individuals, there is a clear unmet clinical need for therapies that target the underlying drivers of CVD in PWH (Table 1). Colchicine, lamivudine, and atorvastatin are commonly prescribed to target inflammation. Our group has found that colchicine does have some utility in reducing pro-inflammatory cytokines. However, it has demonstrated a narrow therapeutic window of safety in vivo and is not FDA approved for use in the United States. Neither Lamivudine nor Atorvastatin have conferred efficacy in inhibiting HIV-induced inflammation despite their abilities to block viral replication. This underscores the fact that neither ART nor statins (common therapeutic intervention for CVD) can mitigate harmful inflammation in PWH (Titanji B. et al., 2021; Gavegnano et al., 2017). It is imperative to recognize that the 2015 Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE) found a significant reduction in cardiovascular events in PWH. Study participants were randomized to receive 4 mg of pitavastatin or a placebo daily during their enrollment that ranged between three to 8 years (clinicaltrials.gov NCT02344290). While there are therapeutics that are effective in reducing CVD, including statins, novel therapeutics are needed to target the elevated inflammation levels among PWH.
TABLE 1
| Agent class | Example drug | FDA approval indication | Admin route | Challenges/Limitations | Benefits |
|---|---|---|---|---|---|
| HMG-reductase inhibitor | Pitavastatin Saito (2011) | Reduced levels of LDL cholesterol Saito (2011) | Oral Saito (2011) | -Associated with possible complications, including myalgia, diabetes mellitus, hepatic and renal dysfunction Bansal and Cassagnol (2023) | -Higher bioavailability than other statins -Anti-inflammatory and anti-oxidant -Improvements in endothelial function and coronary plaque conditions Saito (2011) |
| IL-1R antagonist | Anakira Titanji et al. (2020) | Rheumatoid arthritis (RA) Thaler et al. (2009) | Subcutaneous injection Thaler et al. (2009) | -Reactions at injection site -Infection and administration route Thaler et al. (2009) |
-Improves RA caused endothelial dysfunction -Lowers inflammation caused by acute myocardial dysfunction Titanji et al. (2020) |
| TNF-Alpha inhibitors | Infliximab Titanji et al. (2020) | Rheumatoid arthritis (RA) Titanji et al. (2020) | Subcutaneous injection Titanji et al. (2020) | -Increased infection rates -Administration route Titanji et al. (2020) |
-Enhanced HDL cholesterol and endothelial function Titanji et al. (2020) |
| Anti-CD20 antibody | Rituximab Titanji et al. (2020) | -Certain cancers (e.g., non-Hodgkin’s lymphoma and chronic lymphocytic leukemia) and rheumatoid arthritis Hanif and Anwer (2023) | Intravenous infusion Titanji et al. (2020) | -Higher susceptibility to infection and adverse transfusion reactions Titanji et al. (2020) -Administration mode (intravenous infusion) |
-Potential reduction in HIV reservoir -Improved atherosclerotic lesions Titanji et al. (2020) |
| Inhibition of ATP citrate lyase and activation of AMP activated protein kinase in liver | “Bempedoic Acid” Titanji et al. (2020) | Atherosclerotic CVD and high cholesterol levels, particularly in patients with statin intolerance Marrs and Anderson (2020) | Oral Marrs and Anderson (2020) | -Higher susceptibility to development of diabetes mellitus, urinary tract infection, and nasopharyngitis -Novel -Minimal tracking in HIV-negative population Titanji et al. (2020) |
-Convenient means of administration (once daily) Titanji et al. (2020) |
| Factor IIa inhibitor | Dabigatran Titanji et al. (2020) | Anticoagulation Titanji et al. (2020) | Oral Titanji et al. (2020) | -Higher risk of hemorrhage Titanji et al. (2020) | -Experiments done in mice improve endothelial function and atherosclerosis Titanji et al. (2020) |
| JAK 1/2 inhibitor | Baricitinib Gavegnano et al. (2019) | Rheumatoid arthritis (RA) Gavegnano et al. (2019) | Oral Ahmad et al. (2023) | -Lack of extensive data due to novelty | -Data shows decrease in HIV reservoir and related inflammation Gavegnano et al. (2019) -Oral administration Ahmad et al. (2023) -Renal clearance Ahmad et al. (2023) |
Holistic analysis of agents and novel therapeutics.
Agents that inhibit the Jak-STAT pathway can address this unmet clinical need for safe, potent, and specific inhibitors of chronic inflammation in ART-peripherally suppressed PWH. Such an agent could prevent CVD and other end-organ diseases, as well as decay the HIV reservoir found in the gut and brain. Baricitinib is a promising candidate. A second-generation Janus kinase (Jak) 1/2 inhibitor, the drug works by blocking IFN-a/b, IL-1a/b, TNF-α, CRP, D-dimer, IL-6, IL-7 and IL-15. It is currently FDA approved for the indications of COVID-19, rheumatoid arthritis, and alopecia areata. It is the first and only immunomodulator fully FDA approved for COVID-19 and has monotherapy status. Baricitinib is approved for chronic long-term use in both adults and children with once daily dosing and renal clearance. Renal clearance is critically advantageous as drug-drug reactions with hepatically cleared agents (such as ART) are unlikely. Jak inhibitors have been extensively studied by our group in vitro, ex vivo, and in vivo. The safety profile of baricitinib is significantly improved compared to ruxolitinib, a first-generation Jak inhibitor. With improved safety, baricitinib has greater clinical potential in treating the same conditions as ruxolitinib. Our group has demonstrated that ruxolitinib confers significant anti-HIV-1 effects in macrophages and primary CD4+ T cells (Gavegnano et al., 2019; Gavegnano et al., 2014; Haile et al., 2016; Titanji B. K.et al., 2021; Dougados et al., 2017; Emery et al., 2017; Genovese et al., 2016). We have observed that ruxolitinib reduces the concentration of cells with integrated HIV-1 DNA, blocks reactivation from latent reservoirs, and reduces bystander cell activation and immune dysfunction (Fleischmann et al., 2017; Taylor et al., 2017; Weinblatt et al., 2016; Saito, 2011; Bansal and Cassagnol, 2023; Titanji et al., 2020).
A multi-site phase 2a study funded by the AIDS Clinical Trials Group (ACTG) conducted in large part by our group, studied the efficacy of ruxolitinib in patients with well-controlled HIV-1 on suppressive ART (A5336; n = 60). Data showed that those randomized to ruxolitinib did not experience more adverse safety events than those in the control group but did demonstrate a significant decrease in key markers of HIV-1 persistence such as CD25, HLA-DR/CD38, and cell survival marker bcl-2 (Taylor et al., 2017).
Baricitinib shows great promise for HIV-1 eradication and therapeutic intervention. Baricitinib has a less toxic side effect profile than ruxolitinib and is dosed only once daily. Emerging data from Phase 3 studies suggest that severe side effects are very uncommon and its existing FDA approval for COVID-19, rheumatoid arthritis, and alopecia areata further underscore the safety profile of the agent (Weinblatt et al., 2016; Saito, 2011; Bansal and Cassagnol, 2023; Titanji et al., 2020; Thaler et al., 2009; Hanif and Anwer, 2023). Baricitinib displays direct-acting antiviral and immunomodulatory effects that target HIV reservoirs. Consequently, the novel drug can likely combat CVD and other HIV-associated comorbidities.
Conclusion
Among PWH, there is a greater prevalence of CVD that leads to mortality and related risks. There is an association between HIV persistency in the gut and incidence of CVD. Furthermore, GALT is the recommended form of monitoring CD4+ T cells concentrations in addition to assessing the function of Tregs, as they contextualize the pathology of CVD. This review recommends the incorporation of agents that inhibit the Jak-STAT Pathway, namely, Barcitinib. Improving our understanding of the relationship between the inflammatory pathway in the gut and CVD will help inform the development of novel therapeutics or care options for PWH.
Statements
Author contributions
KK: Writing–review and editing, Writing–original draft, Resources, Investigation, Conceptualization. AM: Writing–review and editing, Writing–original draft, Resources, Investigation, Conceptualization. GQ: Writing–review and editing. SeR: Writing–review and editing. SuR: Writing–review and editing, Supervision. CL: Writing–review and editing, Supervision. CG: Writing–original draft, Supervision, Project administration, Funding acquisition, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Open access funding provided by National Institutes of Health, 5R01HL166004-02 and the Adiuvo Award.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Ahmad A. Zaheer M. Balis F. J. (2023). Baricitinib. Tampa, Florida: StatPearls Publishing LLC. Available at: https://www.ncbi.nlm.nih.gov/books/NBK572064/.
2
Albany C. J. Trevelin S. C. Giganti G. Lombardi G. Scottà C. (2019). Getting to the heart of the matter: the role of regulatory T-cells (Tregs) in cardiovascular disease (CVD) and atherosclerosis. Front. Immunol.10, 2795. 10.3389/fimmu.2019.02795
3
Baardman J. Lutgens E. (2020). Regulatory T cell metabolism in atherosclerosis. Metabolites10 (7), 279. 10.3390/metabo10070279
4
Baker J. V. Duprez D. (2010). Biomarkers and HIV-associated cardiovascular disease. Curr. Opin. HIV AIDS5 (6), 511–516. 10.1097/COH.0b013e32833ed7ec
5
Bansal A. B. Cassagnol M. (2023). HMG-CoA reductase inhibitors. Tampa, Florida: StatPearls Publishing LLC.
6
Bargieł W. Cierpiszewska K. Maruszczak K. Pakuła A. Szwankowska D. Wrzesińska A. et al (2021). Recognized and potentially new biomarkers-their role in diagnosis and prognosis of cardiovascular disease. Med. Kaunas.57 (7), 701. 10.3390/medicina57070701
7
Bovolenta C. Camorali L. Lorini A. L. Ghezzi S. Vicenzi E. Lazzarin A. et al (1999). Constitutive activation of STATs upon in vivo human immunodeficiency virus infection. Blood94 (12), 4202–4209. 10.1182/blood.v94.12.4202
8
Brandt L. Benfield T. Mens H. Clausen L. N. Katzenstein T. L. Fomsgaard A. et al (2011). Low level of regulatory T cells and maintenance of balance between regulatory T cells and TH17 cells in HIV-1-infected elite controllers cells in HIV-1-infected elite controllers. J. Acquir Immune Defic. Syndr.57 (2), 101–108. 10.1097/QAI.0b013e318215a991
9
Brown T. M. Bittner V. (2008). Biomarkers of atherosclerosis: clinical applications. Curr. Cardiol. Rep.10 (6), 497–504. 10.1007/s11886-008-0078-1
10
Cecile D. Lahiri K. T. M. D. R. Taylor R. K. Shaw R. M. Collins S. Mehta C. C. et al (Editors) (2022). Rectal tissue hiv rna is associated with systemic inflammation in persons with hiv. Boston, MA, USA: Conference of Retroviruses and Opportunistic Infections.
11
Chun T. W. Nickle D. C. Justement J. S. Meyers J. H. Roby G. Hallahan C. W. et al (2008). Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J. Infect. Dis.197 (5), 714–720. 10.1086/527324
12
Costiniuk C. T. Angel J. B. (2012). Human immunodeficiency virus and the gastrointestinal immune system: does highly active antiretroviral therapy restore gut immunity?. Mucosal Immunol.5 (6), 596–604. 10.1038/mi.2012.82
13
de Armas L. R. Gavegnano C. Pallikkuth S. Rinaldi S. Pan L. Battivelli E. et al (2021). The effect of JAK1/2 inhibitors on HIV reservoir using primary lymphoid cell model of HIV latency. Front. Immunol.12, 720697. 10.3389/fimmu.2021.720697
14
Dougados M. van der Heijde D. Chen Y.-C. Greenwald M. Drescher E. Liu J. et al (2017). Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann. Rheumatic Dis.76 (1), 88–95. 10.1136/annrheumdis-2016-210094
15
Duprez D. A. Neuhaus J. Kuller L. H. Tracy R. Belloso W. De Wit S. et al (2012). Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLOS ONE7 (9), e44454. 10.1371/journal.pone.0044454
16
Emery P. Blanco R. Maldonado Cocco J. Chen Y. C. Gaich C. L. DeLozier A. M. et al (2017). Patient-reported outcomes from a phase III study of baricitinib in patients with conventional synthetic DMARD-refractory rheumatoid arthritis. RMD Open3 (1), e000410. 10.1136/rmdopen-2016-000410
17
Fleischmann R. Schiff M. van der Heijde D. Ramos-Remus C. Spindler A. Stanislav M. et al (2017). Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and No or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol.69 (3), 506–517. 10.1002/art.39953
18
Gavegnano C. Brehm J. H. Dupuy F. P. Talla A. Ribeiro S. P. Kulpa D. A. et al (2017). Novel mechanisms to inhibit HIV reservoir seeding using Jak inhibitors. PLoS Pathog.13 (12), e1006740. 10.1371/journal.ppat.1006740
19
Gavegnano C. Detorio M. Montero C. Bosque A. Planelles V. Schinazi R. F. (2014). Ruxolitinib and tofacitinib are potent and selective inhibitors of HIV-1 replication and virus reactivation in vitro. Antimicrob. Agents Chemother.58 (4), 1977–1986. 10.1128/AAC.02496-13
20
Gavegnano C. Haile W. B. Hurwitz S. Tao S. Jiang Y. Schinazi R. F. et al (2019). Baricitinib reverses HIV-associated neurocognitive disorders in a SCID mouse model and reservoir seeding in vitro. J. Neuroinflammation16 (1), 182. 10.1186/s12974-019-1565-6
21
Genovese M. C. Kremer J. Zamani O. Ludivico C. Krogulec M. Xie L. et al (2016). Baricitinib in patients with refractory rheumatoid arthritis. N. Engl. J. Med.374 (13), 1243–1252. 10.1056/NEJMoa1507247
22
Guadalupe M. Reay E. Sankaran S. Prindiville T. Flamm J. McNeil A. et al (2003). Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol.77 (21), 11708–11717. 10.1128/jvi.77.21.11708-11717.2003
23
Haile W. B. Gavegnano C. Tao S. Jiang Y. Schinazi R. F. Tyor W. R. (2016). The Janus kinase inhibitor ruxolitinib reduces HIV replication in human macrophages and ameliorates HIV encephalitis in a murine model. Neurobiol. Dis.92, 137–143. 10.1016/j.nbd.2016.02.007
24
Hanif N. Anwer F. (2023). Rituximab. Tampa, Florida: StatPearls Publishing LLC. Available at: https://www.ncbi.nlm.nih.gov/books/NBK564374/.
25
Hanna D. B. Post W. S. Deal J. A. Hodis H. N. Jacobson L. P. Mack W. J. et al (2015). HIV infection is associated with progression of subclinical carotid atherosclerosis. Clin. Infect. Dis.61 (4), 640–650. 10.1093/cid/civ325
26
Hsue P. Y. Waters D. D. (2018). Time to recognize HIV infection as a major cardiovascular risk factor. Circulation138 (11), 1113–1115. 10.1161/circulationaha.118.036211
27
Kaplan R. C. Sinclair E. Landay A. L. Lurain N. Sharrett A. R. Gange S. J. et al (2011). T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J. Infect. Dis.203 (4), 452–463. 10.1093/infdis/jiq071
28
Lahiri C. D. Brown N. L. Ryan K. J. Acosta E. P. Sheth A. N. Mehta C. C. et al (2018). HIV RNA persists in rectal tissue despite rapid plasma virologic suppression with dolutegravir-based therapy. AIDS32 (15), 2151–2159. 10.1097/QAD.0000000000001945
29
Lusis A. J. (2000). Atherosclerosis. Nature407 (6801), 233–241. 10.1038/35025203
30
Madden E. Lee G. Kotler D. P. Wanke C. Lewis C. E. Tracy R. et al (2008). Association of antiretroviral therapy with fibrinogen levels in HIV-infection. Aids22 (6), 707–715. 10.1097/QAD.0b013e3282f560d9
31
Marconi V. C. Moser C. Gavegnano C. Deeks S. G. Lederman M. M. Overton E. T. et al (2022). Randomized trial of ruxolitinib in antiretroviral-treated adults with human immunodeficiency virus. Clin. Infect. Dis.74 (1), 95–104. 10.1093/cid/ciab212
32
Marrs J. C. Anderson S. L. (2020). Bempedoic acid for the treatment of dyslipidemia. Drugs Context9, 1–9. 10.7573/dic.2020-6-5
33
Mehandru S. Poles M. A. Tenner-Racz K. Jean-Pierre P. Manuelli V. Lopez P. et al (2006). Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med.3 (12), e484. 10.1371/journal.pmed.0030484
34
Miller C. J. Baker J. V. Bormann A. M. Erlandson K. M. Huppler Hullsiek K. Justice A. C. et al (2014). Adjudicated morbidity and mortality outcomes by age among individuals with HIV infection on suppressive antiretroviral therapy. PLoS One9 (4), e95061. 10.1371/journal.pone.0095061
35
Nordell A. D. McKenna M. Borges Á. H. Duprez D. Neuhaus J. Neaton J. D. et al (2014). Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J. Am. Heart Assoc.3 (3), e000844. 10.1161/jaha.114.000844
36
Rao S. G. Galaviz K. I. Gay H. C. Wei J. Armstrong W. S. Del Rio C. et al (2019). Factors associated with excess myocardial infarction risk in HIV-infected adults: a systematic review and meta-analysis. J. Acquir Immune Defic. Syndr.81 (2), 224–230. 10.1097/qai.0000000000001996
37
Reece M. D. Taylor R. R. Song C. Gavegnano C. (2021). Targeting macrophage dysregulation for viral infections: novel targets for immunomodulators. Front. Immunol.12, 768695. 10.3389/fimmu.2021.768695
38
Ridker P. M. MacFadyen J. G. Everett B. M. Libby P. Thuren T. Glynn R. J. CANTOS Trial Group (2018). Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet391 (10118), 319–328. 10.1016/S0140-6736(17)32814-3
39
Saito Y. (2011). Pitavastatin: an overview. Atheroscler. Suppl.12 (3), 271–276. 10.1016/s1567-5688(11)70886-8
40
Schillaci G. De Socio G. V. Pucci G. Mannarino M. R. Helou J. Pirro M. et al (2008). Aortic stiffness in untreated adult patients with human immunodeficiency virus infection. Hypertension52 (2), 308–313. 10.1161/hypertensionaha.108.114660
41
Subramanya V. McKay H. S. Brusca R. M. Palella F. J. Kingsley L. A. Witt M. D. et al (2019). Inflammatory biomarkers and subclinical carotid atherosclerosis in HIV-infected and HIV-uninfected men in the Multicenter AIDS Cohort Study. PLOS ONE14 (4), e0214735. 10.1371/journal.pone.0214735
42
Taylor P. C. Keystone E. C. van der Heijde D. Weinblatt M. E. Del Carmen Morales L. Reyes Gonzaga J. et al (2017). Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N. Engl. J. Med.376 (7), 652–662. 10.1056/NEJMoa1608345
43
Thaler K. Chandiramani D. V. Hansen R. A. Gartlehner G. (2009). Efficacy and safety of anakinra for the treatment of rheumatoid arthritis: an update of the Oregon Drug Effectiveness Review Project. Biologics3, 485–498. 10.2147/btt.2009.3755
44
Titanji B. Gavegnano C. Hsue P. Schinazi R. Marconi V. C. (2020). Targeting inflammation to reduce atherosclerotic cardiovascular risk in people with HIV infection. J. Am. Heart Assoc.9 (3), e014873. 10.1161/jaha.119.014873
45
Titanji B. K. Farley M. M. Mehta A. Connor-Schuler R. Moanna A. Cribbs S. K. et al (2021). Use of baricitinib in patients with moderate to severe coronavirus disease 2019. Clin. Infect. Dis.72 (7), 1247–1250. 10.1093/cid/ciaa879
46
Titanji B. Schinazi R. Marconi V. (2021). “Baricitinib reduces macrophage-mediated inflammation in HIV infection,” in International AIDS Society, Virtual Symposium, July 18-22, 2021.
47
Triant V. A. Meigs J. B. Grinspoon S. K. (2009). Association of C-reactive protein and HIV infection with acute myocardial infarction. J. Acquir Immune Defic. Syndr.51 (3), 268–273. 10.1097/QAI.0b013e3181a9992c
48
Tseng Z. H. Secemsky E. A. Dowdy D. Vittinghoff E. Moyers B. Wong J. K. et al (2012). Sudden cardiac death in patients with human immunodeficiency virus infection. J. Am. Coll. Cardiol.59 (21), 1891–1896. 10.1016/j.jacc.2012.02.024
49
Weinblatt M. Taylor P. Tanaka Y. Keystone E. Schiff M. Fleischmann R. et al (2016). THU0193 response to baricitinib at 4 Weeks predicts response at 12 and 24 Weeks in patients with rheumatoid arthritis: results from two phase 3 studies: table 1. Ann. Rheumatic Dis.75, 255–256. 10.1136/annrheumdis-2016-eular.1597
50
Zevin A. S. McKinnon L. Burgener A. Klatt N. R. (2016). Microbial translocation and microbiome dysbiosis in HIV-associated immune activation. Curr. Opin. HIV AIDS11 (2), 182–190. 10.1097/coh.0000000000000234
51
Zhao X. Li J. Tang X. Jiang L. Chen J. Qiao S. et al (2020). D-dimer as a thrombus biomarker for predicting 2-year mortality after percutaneous coronary intervention. Ther. Adv. Chronic Dis.11, 2040622320904302. 10.1177/2040622320904302
Summary
Keywords
HIV-1, inflammation, cardiovascular disease, therapeutics, comorbidities
Citation
Kramer K, Michael A, Quiñones G, Roa S, Ribeiro SP, Lahiri CD and Gavegnano C (2024) The role of the HIV-1 gut reservoir in driving early cardiovascular events in people living with HIV. Front. Drug Discov. 4:1334307. doi: 10.3389/fddsv.2024.1334307
Received
06 November 2023
Accepted
27 August 2024
Published
02 October 2024
Volume
4 - 2024
Edited by
Ahmed Sheriff, Charité University Medicine Berlin, Germany
Reviewed by
Atefe Ghamar Talepoor, Shiraz University of Medical Sciences, Iran
Updates
Copyright
© 2024 Kramer, Michael, Quiñones, Roa, Ribeiro, Lahiri and Gavegnano.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christina Gavegnano, christina.gavegnano@emory.edu
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.