
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Drug Discov., 09 September 2022
Sec. Anti-Cancer Drugs
Volume 2 - 2022 | https://doi.org/10.3389/fddsv.2022.963045
A correction has been applied to this article in:
Corrigendum: Effect of itraconazole on the safety and pharmacokinetics of antitumor SHR6390
The experimental drug SHR6390 has anti-tumor activity as a cyclin dependent kinase 4/6 inhibitor and is metabolized primarily by the cytochrome P450 3A4 enzyme. Therefore, The purpose of this trial was to evaluate the safety and pharmacokinetics of SHR6390, a potent cytochrome P450 3A4 inhibitor, in healthy Chinese subjects. In this trial study, 18 subjects received a single oral dose of SHR6390 50 mg on day 1, multiple doses of 200 mg itraconazole on days 12–24 for 13 days, and a single oral dose of SHR6390 50 mg on day 15. After coadministration with itraconazole, the maximum plasma concentration (Cmax) of SHR6390 increased by 70.7% (from 14.3 ng/ml to 24.5 ng/ml), and the area under the time curve from 0 to T (AUC0-T) increased by 110.8% from 468 h∙ng/mL to 988 h∙ng/mL. The area under the concentration-time curve extrapolated to ∞(AUC0-∞) increases from 509 H∙ng/mL to 1,040 h∙ng/mL, an increase of 105.1%. Oral gap (CL/F) decreased (47.9 L/h and 98.3 L/h) and apparent volume of distribution (Vz/F) decreased (4190 L and 5890 L). According to common terminology criteria, 15 32 adverse events were reported in 18 subjects (AEs) (27 SHR6390-related AEs and 15 Itraconazole-related AEs), AEs were all Class 1 adverse events. Overall, co-administration of Itraconazole increased the plasma exposure of SHR6390 in healthy subjects. Both SHR6390 alone and co-administration of Itraconazole showed acceptable safety profiles, which warrants further investigation. The experimental drug SHR-6390 of this clinical trial has been applied for registration, which is classified as Chemical drugs Class 1. The study drug SHR6390 registration number:ClinicalTrials.gov Identifier: NCT04423601 (https://clinicaltrials.gov/)
Cyclins and cyclin-dependent kinases (CDK) mainly regulate and process cyclins. Early in G1 phase, CDK4/6 interacts with cyclin D, phosphorylates retinoblastoma protein (Rb), releases transcription factor E2F, and accelerates transcription of downstream genes. Transitioning the cell cycle from G1 phase to S Phase Although Rb loss typically occurs in some types of tumors, increased ACTIVATION of CDK4/6 occurs in most tumors, suggesting unwinding of the Cyclin D1-CDK4/6-Rb pathway in cancer suggests that acceleration of the G1 phase occurs and leads to uncontrolled cell proliferation. According to the significance of CDK4/6 activity in cancer cells, selective CDK4/6 inhibitors has been utilized as a therapeutic strategy for cancer. Among them, Palbociclib, Abemaciclib and Ribociclib have been approved by the US Food and Drug Administration and are mainly used for hormone receptor-positive (HR+) and human epidermal growth factor receptor-2 negative (HER2-) advanced breast cancer in combination with endocrine therapy. Abemaciclib is approved as a post-endocrine monotherapy for the treatment of advanced HR+/HER2- breast cancer.
SHR6390, an oral, highly selective, small molecule CDK4/6 inhibitor, reduced Rb phosphorylation in PDX models in vivo, and had similar or better anti-tumor efficacy than Palbociclib in xenografts in vivo. Within an in vivo study, SHR6390 was a P-glycoprotein (P-gp) substrate which was metabolized in the liver by Cytochrome P450 (CYP) enzymes, primarily metabolized by CYP3A4, and partly metabolized by CYP2C9 and CYP2C8. Considering that inhibitors or inducers of CYP3A4 might have influence on the exposure of SHR6390, this study evaluated the effect of Itraconazole, one of the potent strong CYP3A4 inhibitors, on the pharmacokinetics and safety of SHR6390 in Chinese healthy subjects, and the results will provide reference for concomitant clinical medications.
This study was reviewed and approved by the Clinical Trial Ethics Committee of Sun Yat-sen Memorial Hospital, Sun Yat-sen University, and conducted in the Phase I Clinical Research Center of Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China. The study was conducted in accordance with the Clinical Trial Quality Management Practice and the Declaration of Helsinki, and all subjects were taught and signed informed consent.
SHR6390 was provided by Jiangsu Hengrui Pharmaceuticals Co., Ltd. with a specification of 50 mg per tablet, batch number of 190215KF, storage below 25°C. Itraconazole was provided by Xian Janssen Pharmaceutical Ltd. with a specification of 100 mg per capsule, batch number of 190512179, storage at cool and dry place.
This open-label, single-dose, self-controlled study (ClinicalTrials.gov Identifier: NCT04423601) was designed to investigate the effects of itraconazole on the safety and tolerability of SHR6390 and drug metabolism in healthy Chinese subjects. On day 1 after one night of fasting, enrolled subjects received a single oral dose of 50 mg SHR6390 (Figure 1), with no food allowed for 4 h before and after administration and no water allowed for 1 h. On days 12–14 and 16–24, 200 mg itraconazole was orally administered daily after breakfast, with no food and no water allowed for 1 h before and 4 h after administration. On day 15 after the night fast, a single oral dose of 50 mg SHR6390 and 200 mg itraconazole was given, with no food and no water allowed for 4 h before and after administration. All subjects were checked out on day 25 after the trial and followed up on day 31–33.
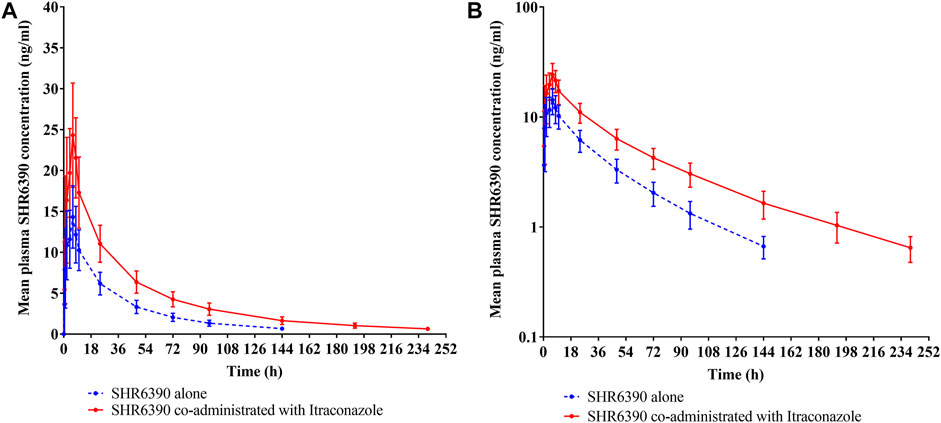
Figure 1. Mean plasma concentration-time profile of SHR6390 alone and co-administrated with Itraconazole in healthy subjects: (A) linear scale and (B) semi-log scale.
The main inclusion criteria are as follows: (Malumbres and Barbacid, 2009) the age requirement is 18–45 years old (including boundary values), (Hamilton and Infante, 2016) the subject is willing to use effective contraception, The serum human chorionic gonadotropin test in women must be negative from the time of signing the informed consent until the final dose, after 6 months and pre-drug Fertility enabled, (Gao et al., 2020) weight ≥50 kg for men and ≥45 kg for women, Body mass index (BMI) 19–26 kg/m2 (including boundary value), (Dickler et al., 2017) general examination, laboratory examination, 12-lead electrocardiogram, chest radiography and other examinations showed no significant clinical abnormalities.
The main exclusion criteria were: (Malumbres and Barbacid, 2009) blood donation ≥400 ml (examination before receiving blood transfusion within 3 months), (Hamilton and Infante, 2016) allergy or known allergy to SHR6390, itraconazole, (Gao et al., 2020) drug abuse, (Dickler et al., 2017) alcohol and non-smoking (Wang et al., 2017) excessive smoking or active testing, (Long et al., 2019) male or female QTcF value ≥ 450 ms ≥ 470 Msec, (Zhang et al., 2021) clear history of major organ disease, (U. S. Food and Drug Administration, 2020) examination within 6 months prior to any surgery, (Liu et al., 2016) long-term examination of hep drugs prior to 6 months, (Yu et al., 2017) participation of any clinical trial subjects within 3 months, (Hoffman et al., 2014) administration of any drugs that alter the activity of liver enzymes within 28 days prior to administration, (Vermeer et al., 2016) use of any medication Or health products, or herbal medicine within 14 days of dose prior to (13) positive hepatitis C antibodies, HIV antibodies, HEPATITIS B surface antigen, syphilis antibodies.
The 18 subjects were all Han nationality, including 10 male subjects and 8 female subjects, with an average age (Standard deviation) 27.6 (±4.90) years (median age 28.0 years, range 20–36 years), mean height (± standard deviation),165.83 (±8,351) cm, mean weight (± SD) 58.89 (± 8.258) kg, mean body mass index (soil SD) 21.37 kgm2 (+/-2.233).
The drug was administered once in the morning after intensive blood collection. Water was forbidden within 1 h before and after administration, and fasting within 4 h after administration. For the rest of the time, the standard breakfast was taken 30mi before Dl2 to D14 administration (half of the meal was finished within 25 min), and itraconazole 200 mg was taken orally 30 mn (±lmin) postprandial. In this study, food was forbidden during the intensive blood collection time, while breakfast was available during the non-intensive blood collection before drug administration, so the effect of food on blood drug concentration was extremely small.
Blood samples for pharmacokinetic assessments of SHR6390 were collected on day 1 (13 collection points:1 h pre-dose, 0.5, 1, 2, 4, 6, 8, 10, 24, 48, 72, 96, and 144 h post-dose), and on day 15 (15 collection points:1 h pre-dose, 0.5, 1, 2, 4, 6, 8, 10, 24, 48, 72, 96, 144, 192, and 240 h post-dose), 4 ml blood samples were collected from each sample. For determination of plasma concentrations of SHR6390, bloods were centrifugated at 4°C, 2000 g for 10 min within 1 h after sampling, the upper layer of blood plasma was separated and stored at -80°C. PK samples were sent to Shanghai Frontage Biotechnology Co., Ltd (Shanghai, China) for analysis of SHR6390 by The method verified by liquid chromatography-mass spectrometry (LC-MS/MS technology), the calibration range was 0.390–390 ng/ml, linear regression was applied, and the lower limit of quantification (LLOQ) was 0.390 ng/ml.
PK parameters for SHR6390 were calculated by the compartment method using Phoenix WinNonlin software (Version 8.0:Pharsight Corporation): maximum plasma concentration (Cmax), elimination half-time (t1/2), time to reach Cmax (Tmax), area under the concentration-time curve extrapolated to infinity (AUC0-∞), area under the concentration-time curve from time zero to time oral clearance (CL/F),t (AUC0-t), and apparent volume of distribution (Vz/F).
Safety was assessed by monitoring AEs, vital sign values and clinical laboratory parameters, physical examination, 12-lead electrocardiogram, and blood pregnancy tests. AEs is graded for each event according to the Common Terminology standard for Adverse Events (Version 5.0:CTCAE). Severe adverse events (SAEs) are defined as medical events resulting in hospitalization or extension of existing hospital stay, incapacity, disability, life-threatening, death, or congenital abnormalities after administration.
The sample size was not determined by hypothesis testing, in which 18 subjects were planned to be recruited and enrolled to allow for drop-off or drop-out. PK concentration analysis set: includes all subjects who received at least 1 dose of the test drug and had at least 1 dose of the post-administration PK concentration. PK parameter analysis set: includes all subjects who received at least 1 dose of the test drug and had at least 1 dose of the PK parameter after administration. In addition, safety analysis set: includes all subjects who received at least 1 dose of the investigational drug.
PK concentrations, PK parameters, and safety data of SHR6390 treated with itraconazole alone or in combination were summarized by descriptive statistics. Mean and median plasma concentration-time profiles for SHR6390 were obtained under both treatments. AUC0-∞, AUC0-T and Cmax after log transformation were analyzed by using mixed effects model, which were treated as fixed effects and subjects as random effects. The least-squares mean difference of the model between the two treatments and the corresponding 90% CI(credibility interval) limit values are reverted to the original scale. Use SAS, version 9.4 (SAS Institute, Cary, NC) for organization, statistics, processing, and analysis.
A total of 18 subjects (10 males, 8 females) were enrolled, completed the trial, and were included in the full pharmacokinetic and safety analysis (Table 1). The mean age was 27.6 (range, 20.0–36.0) years, mean height was 165.8 (range, 149.0–181.0) cm, mean body weight was 58.9 (range, 45.0–71.2) kg and median BMI was 21.4 (range, 19.0–25.3) kg/m2.
The mean plasma concentration-time profile of SHR6390 alone or combination with Itraconazole was shown in Figure 2, and the statistical comparison of PK parameters was shown in Table 2.
Combination with Itraconazole had no significant effect on the median Tmax of SHR6390 (6 h versus 6 h), however, SHR6390 plasma concentrations demonstrated a slower decline in the presence of Itraconazole after attainment of Cmax (geometric mean 24.5 ng/ml versus 14.3 ng/ml). Mean t½ was longer in SHR6390 with Itraconazole than SHR6390 alone (61.1 versus 42.2 h), CL/F and Vz/F were reduced in SHR6390 with Itraconazole compared with SHR6390 alone (47.9 L/h versus 98.3 L/h, 4190 L versus 5890 L, respectively), AUC0-t and AUC0-∞ were increased in SHR6390 with Itraconazole compared with SHR6390 alone (988 h∙ng/mL versus 468 h∙ng/mL, 1,040 h∙ng/mL versus 509 h∙ng/mL, respectively). The geometric least-square mean treatment ratio of AUC0-t was 2.108 (90% CI, 1.954, 2.274), the ratio of AUC0-∞ was 2.051 (90% CI, 1.903, 2.211) and the ratio of Cmax was 1.707 (90% CI, 1.541, 1.890), when SHR6390 with Itraconazole compared with SHR6390 alone. The overall exposure of SHR6390 was increased in the presence of Itraconazole.
The safety evaluable population was comprised of all 18 subjects. A summary of AEs was listed in Table 3. A total of 32 AEs were reported for 15 subjects (83.3%), of which AEs were recorded for 8 subjects (44.4%, 16 times) after treatment with SH6390 alone, 1 subject (5.6%, 1 times) after treatment with Itraconazole alone, and 10 subjects (55.6%, 15 times) after co-administration of SH6390 with Itraconazole. The most common AEs were constipation (4 subjects, 4 times), increased alanine aminotransferase (2 subjects, 3 times), shortened PR interval on electrocardiogram (2 subjects, 2 times), increased serum creatine phosphokinase (2 subjects, 2 times), reduced fibrinogen (2 subjects, 2 times), diarrhea (2 subjects, 2 times), and urinary tract infection (2 subjects, 2 times).
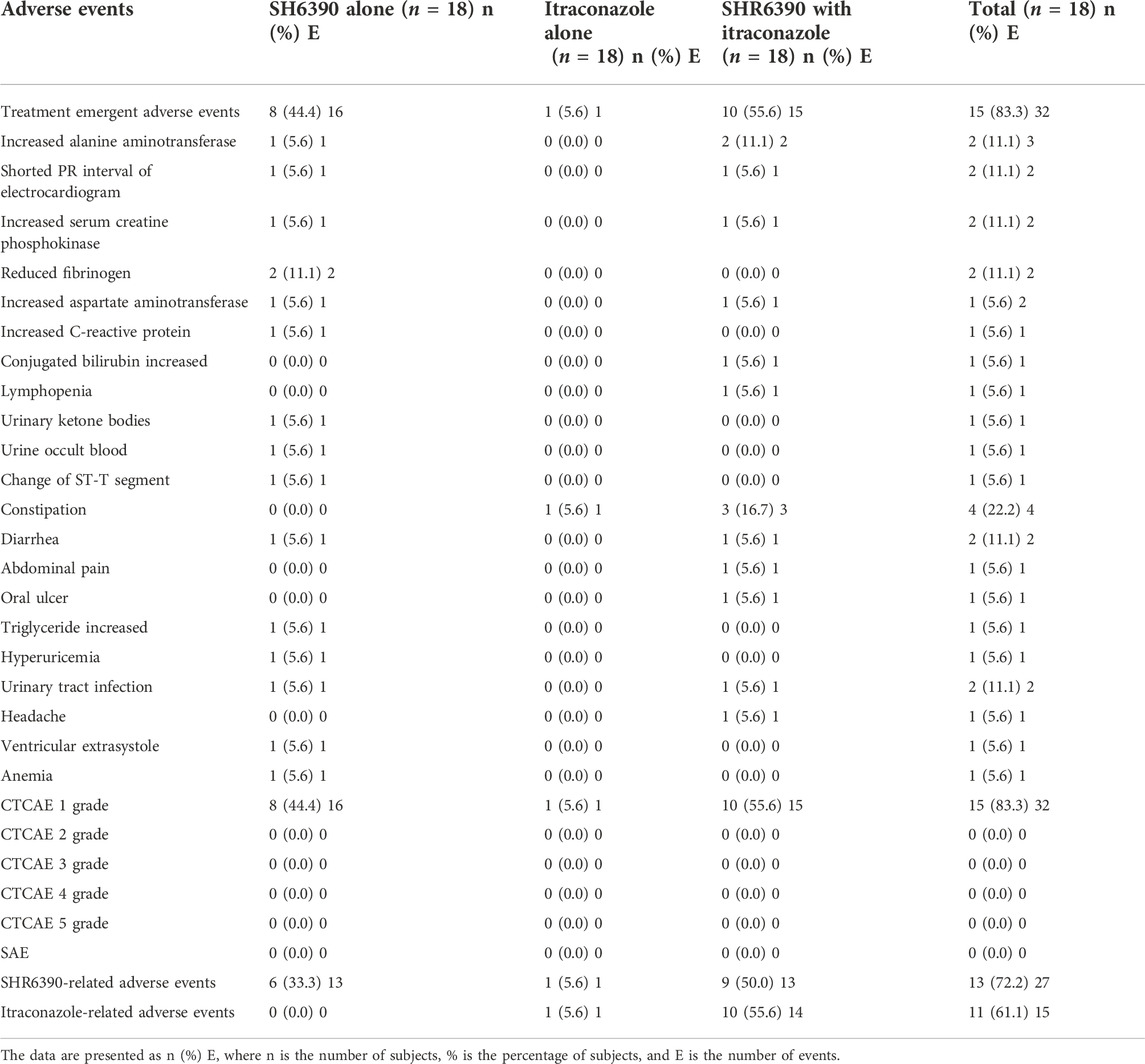
Table 3. Summary of adverse events in subjects treated with SH6390 alone or Itraconazole alone or SH6390 co-administrated with Itraconazole.
A total of 27 SHR6390-related AEs were reported for 13 subjects (72.2%), which mainly included constipation (4 subjects, 4 times), increased alanine aminotransferase (2 subjects, 3 times), shortened PR interval on electrocardiogram (2 subjects, 2 times), increased serum creatine phosphokinase (2 subjects, 2 times), reduced fibrinogen (2 subjects, 2 times), and diarrhea (2 subjects, 2 times), whereas 15 Itraconazole-related AEs were reported for 11 subjects (61.1%), which mainly included constipation (4 subjects, 4 times) and increased alanine aminotransferase (2 subjects, 2 times). All reported AEs were treatment-emergent adverse events and were considered as CTCAE grade I. There were no SAEs reported and no subjects discontinued treatment, required dose reductions, or withdrew due to AEs during this study. Overall, SHR6390 was well tolerated with no safety profile concerns with a single dose of 50 mg or in combination with 200 mg Itraconazole.
SHR6390 is mainly metabolized by CYP3A4. According to the drug-drug interaction guidelines, itraconazole is recommended to be included as a potent inhibitor of CYP3A4, and drug-drug interaction is studied through the metabolism of CYP3A4. Therefore, itraconazole is adopted as a co-administration drug in this study. The selected 50 mg dose of the investigational drug SHR6390 was safe and well tolerated. The usual daily dose of itraconazole is 200–400 mg, and a single dose of 400 mg has a limited increase in inhibition of CYP3A4 compared with 200 mg. Therefore, we hope that a daily dose of 200 mg of itraconazole (day 15–24) has a longer and comprehensive inhibition of CYP3A4.
Tmax of 6 h and t1/2 of 42.2 h for SHR6390 supported the once-daily administration. A previous tissue distribution study of rats showed that SHR6390 was widely distributed in tissues except the brain, SHR6390 with a large Vz/F of 4190 L suggested that SHR6390 was widely distributed in the body. SHR6390 with a large Vz/F of 4190 L in the study suggested that SHR6390 distributed widely in the body. Another CDK4/6 inhibitor, palbociclib was mainly metabolized by CYP3A, in physiologically based pharmacokinetic modeling, the predicted ratios of geometric mean AUC0-∞ and Cmax for palbociclib with itraconazole compared with palbociclib alone were 2.10 and 1.48. In the study of palbociclib pharmacokinetics in healthy volunteers, compared with a single 125 mg palbociclib, coadministration of multiple 200 mg itraconazole with palbociclib increased AUC0-∞ and Cmax of palbociclib by 87 and 34%. In the study, the geometric least-square mean treatment ratios of AUC0-t, AUC0-∞ and Cmax were 2.108, 2.051 and 1.707 for SHR6390 with itraconazole compared with SHR6390 alone, indicating CYP3A4 inhibition was the probable mechanism on the interactions between itraconazole and SHR6390. In addition, SHR6390 was a P-glycoprotein substrate, inhibition of itraconazole on the transport protein P-glycoprotein might contribute to interaction, but it required further investigation.
Previous studies on SHR6390 showed that the most common hematological AEs included neutropenia and leukopenia, and the most frequent non-hematological AEs included increased alanine aminotransferase and aspartate aminotransferase. No SAEs were reported during the study, and all AEs were grade 1 with no treatment discontinuation, where the most common AEs included constipation, increased alanine aminotransferase, shortened PR interval on electrocardiogram, increased serum creatine phosphokinase, reduced fibrinogen, diarrhea, and urinary tract infection. Overall, the incidence of AEs was similar when SHR6390 was co-administered with Itraconazole (55.6%) compared with SHR6390 alone (44.4%). Therefore, SHR6390 was well tolerated when administered alone or co-administered with Itraconazole.
In summary, the pharmacokinetics of SHR6390 were affected by the co-administration of Itraconazole, where co-administration of Itraconazole resulted in the increase of SHR6390 exposure, where the Cmax, AUC0-t and AUC0-∞ increased by 70.7, 110.8, 105.1%, respectively. A single 50 mg dose of SHR6390 alone and co-administration with 200 mg Itraconazole were well tolerated according to the presence of grade 1 AEs and the absence of SAEs in healthy subjects. The results provide information on potential pharmacokinetic interactions and reference for the co-administration of a potent CYP3A4 inhibitor with SHR6390.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Sun Yat-sen Memorial Hospital of Sun Yat-sen University. The patients/participants provided their written informed consent to participate in this study.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that financial support was received for the research, authorship, and/or publication of this article. Funding went to principal investigator JWfor the name of the clinical trial sponsor—itraconazole against SHR6390 in healthy subjects trial. This study was funded by Jiangsu Hengrui Pharmaceutical Co., Ltd. The involvement of funder in the study is as follows: the funders only participated in the design of national clinical trials, and the subsequent data collection, analysis and interpretation were all completed by the researchers of our center. The funders did not participate in the writing of this paper or the decision of whether to publish it.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Dickler, M. N., Tolaney, S. M., Rugo, H. S., Cortes, J., Dieras, V., Patt, D., et al. (2017). MONARCH 1, A phase II study of Abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory hr+/HER2(-) metastatic breast cancer. Clin. Cancer Res. 23 (17), 5218–5224. doi:10.1158/1078-0432.CCR-17-0754
Gao, X., Leone, G. W., and Wang, H. (2020). Cyclin D-CDK4/6 functions in cancer. Adv. Cancer Res. 148, 147–169. doi:10.1016/bs.acr.2020.02.002
Hamilton, E., and Infante, J. R. (2016). Targeting CDK4/6 in patients with cancer. Cancer Treat. Rev. 45, 129–138. doi:10.1016/j.ctrv.2016.03.002
Hoffman, J. T., O'Gorman, M., Loi, C., et al. (2014). 76p A phase 1 open-label fixed-sequence two-period crossover study of the effect of multiple doses of tamoxifen on palbociclib (Pd-0332991) pharmacokinetics in healthy male volunteers. Ann. Oncol. 25 (1), i25.
Liu, L., Bello, A., Dresser, M. J., Heald, D., Komjathy, S. F., O'Mara, E., et al. (2016). Best practices for the use of itraconazole as a replacement for ketoconazole in drug-drug interaction studies. J. Clin. Pharmacol. 56 (2), 143–151. doi:10.1002/jcph.562
Long, F., He, Y., Fu, H., Li, Y., Bao, X., Wang, Q., et al. (2019). Preclinical characterization of SHR6390, a novel CDK 4/6 inhibitor, invitro and in human tumor xenograft models. Cancer Sci. 110 (4), 1420–1430. doi:10.1111/cas.13957
Malumbres, M., and Barbacid, M. (2009). Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer 9 (3), 153–166. doi:10.1038/nrc2602
U. S. Food and Drug Administration. Guidance for industry; clinical drug interaction studies - cytochrome P450 enzyme and transportermediated drug interactions. 2020. Available at: https://www.fda.gov/media/134581/download. [Accessed January 2020].
Vermeer, L., Isringhausen, C. D., Ogilvie, B. W., and Buckley, D. B. (2016). Evaluation of ketoconazole and its alternative clinical CYP3A4/5 inhibitors as inhibitors of drug transporters: The in vitro effects of ketoconazole, ritonavir, clarithromycin, and itraconazole on 13 clinically-relevant drug transporters. Drug Metab. Dispos. 44 (3), 453–459. doi:10.1124/dmd.115.067744
Wang, J., Li, Q., Yuan, J., Chen, Z., Liu, Z., et al. (2017). CDK4/6 inhibitor-SHR6390 exerts potent antitumor activity in esophageal squamous cell carcinoma by inhibiting phosphorylated Rb and inducing G1 cell cycle arrest. J. Transl. Med. 15, 127–138. doi:10.1186/s12967-017-1231-7
Yu, Y., Loi, C. M., Hoffman, J., and Wang, D. (2017). Physiologically based pharmacokinetic modeling of palbociclib. J. Clin. Pharmacol. 57 (2), 173–184. doi:10.1002/jcph.792
Keywords: SHR6390, itraconazole, pharmacokinetic, safety, drug-drug interaction, healthy subjects
Citation: Lv X, Wu J, Yao H, Ye S and Zhang N (2022) Effect of itraconazole on the safety and pharmacokinetics of antitumor SHR6390. Front. Drug. Discov. 2:963045. doi: 10.3389/fddsv.2022.963045
Received: 07 June 2022; Accepted: 05 August 2022;
Published: 09 September 2022.
Edited by:
Greco Hernández, National Institute of Cancerology (INCAN), MexicoReviewed by:
Chunlin Zhuang, Second Military Medical University, ChinaCopyright © 2022 Lv, Wu, Yao, Ye and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junyan Wu, d3VqdW55YW5AbWFpbC5zeXN1LmVkdS5jbg==; Herui Yao, eWFvaGVydWlAbWFpbC5zeXN1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.