
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Drug Discov. , 29 November 2022
Sec. Anti-Cancer Drugs
Volume 2 - 2022 | https://doi.org/10.3389/fddsv.2022.1050687
This article is part of the Research Topic Discovery of EGFR Tyrosine Kinase Inhibitors for Cancer Treatment View all 6 articles
 Tao Zhang1†
Tao Zhang1† Fang Feng1†
Fang Feng1† Linjiang Tong1
Linjiang Tong1 Shingpan Chan2
Shingpan Chan2 Yi Chen1
Yi Chen1 Yan Li1
Yan Li1 Peiran Song3
Peiran Song3 Yingqiang Liu1
Yingqiang Liu1 Gang Bai1
Gang Bai1 Mengzhen Lai1
Mengzhen Lai1 Yi Ning1
Yi Ning1 Yanan Wang1
Yanan Wang1 Yan Fang1
Yan Fang1 Zilu Pan1
Zilu Pan1 Meiyu Geng1
Meiyu Geng1 Ke Ding2
Ke Ding2 Jian Ding1,4*
Jian Ding1,4* Hua Xie1,3,4*
Hua Xie1,3,4*Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are classic strategies for the individualized treatment for patients with non-small cell lung cancer (NSCLC). However, EGFR exon20 insertion (EGFR 20ins) mutations, accounting for 6%–12% of all EGFR mutant cases in NSCLC, are generally resistant to the reversible EGFR TKIs (such as gefitinib and erlotinib), which makes them challenging drug-targets in lung cancer. In our previous study, we identified ASK120067 (limertinib) as a novel 3rd-generation EGFR TKI targeting EGFR T790M mutation with promising clinical activities. Here, we accessed the potency of ASK120067 on EGFR 20ins activation and evaluated its in vitro and in vivo anti-tumor activity against EGFR 20ins driven tumor models. We found that ASK120067 showed potent inhibitory activity on TKI-resistant EGFR 20ins kinase. In TKI-resistant EGFR 20ins-dependent BaF3 cells, it dose-dependently suppressed EGFR phosphorylation, impeded cell proliferation, and induced cell apoptosis with much superior efficacy to gefitinib and erlotinib. Moreover, oral administration of ASK120067 decreased the level of phospho-EGFR 20ins and caused significant tumor regression in EGFR 20ins BaF3 xenograft model. These results presented the pre-clinical anti-tumor efficacy of ASK120067 in EGFR 20ins models and highlighted the potential value of ASK120067 for the treatment of NSCLC patients harboring EGFR 20ins mutations.
Epidermal growth factor receptor (EGFR) mutations exist in almost 50% of Asian patients and 20% of Caucasians patients with non-small cell lung cancer (NSCLC) (Rosell et al., 2009; Chang et al., 2019). The classic EGFR activating mutations (exon19 deletions and L858R point mutation) are the most common subtypes (80%–90%) in NSCLC, and have been used as clinical sensitive biomarkers of the-generation EGFR tyrosine kinase inhibitors (TKIs) (e.g., gefitinib, erlotinib). However, drug-resistance limits the clinical application of those EGFR TKIs. EGFR T790M is found to be the leading cause of resistance (∼50%) (Murtuza et al., 2019; Tan et al., 2018), and new generation EGFR TKIs targeting T790M have been developed. Among them, mutant-selective 3rd generation EGFR TKI osimertinib is the only one approved by the Food and Drug Administration (FDA) for the treatment of NSCLC patients harboring EGFR T790M. Other 3rd-generation EGFR TKIs, almonertinib and alflutinib also have been approved by the National Medical Products Administration (NMPA) of China for the second-line treatment T790M mutation-positive NSCLC patients (Pao and Chmielecki, 2010; Rosell et al., 2012; Cooper et al., 2022).
Besides T790M, EGFR exon20 insertions (EGFR 20ins), which are the third most frequent EGFR mutations in NSCLC (6%–12%), are also important resistant reasons to EGFR TKIs. Though few subtypes of EGFR 20ins, such as A763_Y764insX (4.6%) and D761-E762 insX (1.7%) remain sensitive to EGFR TKIs, the common EGFR 20ins mutations including D770-N771insX (25.5%), V769-D770 insX (24.6%), and H773-V774 insX (22.6%) are associated with intrinsical resistance to EGFR TKIs, with clinical response rates less than 10% for gefitinib, erlotinib and afatinib (Li and Chen, 2017; Cardona et al., 2018; Syahruddin et al., 2018; Vyse and Huang, 2019; Harrison et al., 2020; Qin et al., 2020; Meador et al., 2021). Due to the poor clinical performance of EGFR TKIs, several researches have been carried out for discovering new effective approaches to overcome EGFR 20ins resistant mutations over the past years.
Among those approaches, EGFR/Met bispecific antibody amivantamab (JNJ-61186372) is the first targeted agent approved by the FDA, for the second-line therapy of previously treated advanced NSCLC harboring EGFR 20ins (Vyse and Huang, 2022; Roskoski, 2022). An EGFR/HER2 TKI, mobocertinib (TAK-788), has been approved as an oral available second-line therapy for EGFR 20ins NSCLC patients, recently (Gonzalvez et al., 2011; Riely et al., 2021). Right behind amivantamab and mobocertinib, pan-ErbB TKI poziotinib also showed impressive pre-clinical effects and promising efficacy on partial EGFR 20ins positive NSCLC patients in clinical trials. In a multi-cohort phase II study, poziotinib demonstrated clinically meaningful activity (ORR:27.8%) in treatment-naïve patients, while caused non-negligible toxicity due to the lack of selectivity for EGFR WT at current dose schedule (Le et al., 2020; Sacher et al., 2021). More recently, a novel EGFR 20ins selective TKI sunvozertinib was reported with superior efficacy (ORR:37.5%) and tolerability in the phase I trial 3 (Wang et al., 2022). Meanwhile, the launched 3rd-generation EGFR TKI osimertinib shows certain therapeutic potency (ORR:25%) against 20ins mutant NSCLC patients in a phase I/II clinical trial (NCT03191149) (Yasuda et al., 2021). As osimertinib has been widely used in the first-line treatment of EGFR mutant NSCLC and has demonstrated good safety with lower rates of serious adverse effects than other 20ins-targeted TKIs (Passaro et al., 2021), it is also considered as a promising agent for EGFR 20ins NSCLC treatment. Thus, several agents have been developed to overcome EGFR 20ins mediated resistance in NSCLC, however, some disadvantages still limit their widely clinical application, such as the high price and non-oral bioavailability of amivantamab, and higher treatment emergent adverse events (TEAEs) of mobocertinib and poziotinib, raised by their unsatisfied selectivity over EGFR WT or other kinases. Thus, new strategies targeting EGFR 20ins, especially mutant selective TKIs, are still unmet clinical needs.
We previously identified ASK120067 (limertinib) as a novel 3rd-generation EGFR TKI with comparable preclinical and clinical anti-tumor effects to osimertinib against EGFR T790M positive NSCLC, and its chemical structure was shown in Figure 1A (Zhang et al., 2020; Shi et al., 2022). Now a New Drug Application (NDA) of ASK120067 has been submitted in China. Meanwhile, inspired by the promising efficacy of the osimertinib on EGFR 20ins mutant NSCLC, we sought to investigate the potential value of ASK120067 as a new strategy for the treatment of EGFR 20ins mutant NSCLC. In this study, ASK120067 was proven to be an effective EGFR 20ins TKI. It showed potent kinase inhibitory activity on EGFR 20ins protein, robustly inhibited EGFR phosphorylation, and showed much higher anti-tumor activity than the 1st-generation EGFR TKIs in both EGFR 20ins-driven BaF3 cells and xenograft model. These findings confirmed the in vitro and in vivo efficacy of ASK120067 against EGFR 20ins and supported ASK120067 as a novel oral agent for EGFR 20ins mutant malignancy.
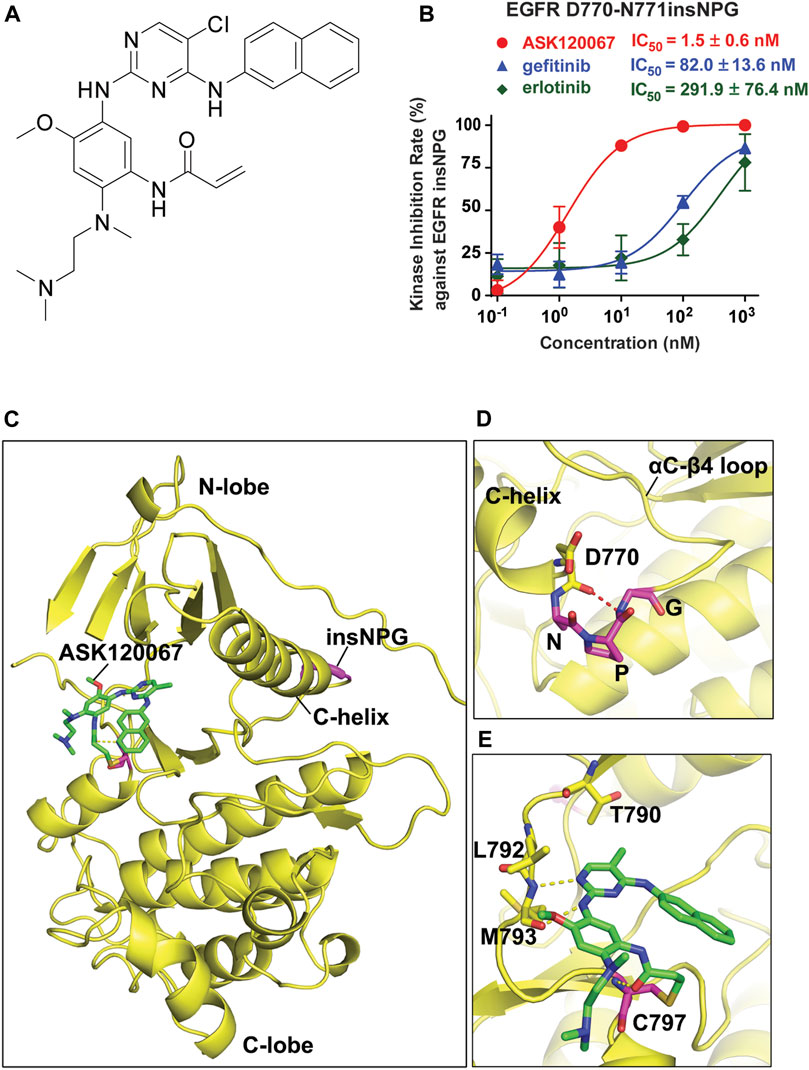
FIGURE 1. ASK120067 exhibited potent kinase-inhibitory activity against EGFR insNPG. (A) The chemical structure of ASK120067. (B) The inhibition activities of ASK120067, gefitinib and erlotinib against EGFR D770_N771insNPG (insNPG) kinase were evaluated by ELISA assay. Experiments were performed in triplicate or quadruplicate and shown as mean ± SD. (C) Computational modeling shows the binding model of ASK120067 with EGFR insNPG protein (PDB: 4LRM). The compound ASK120067 is shown in stick form with carbon atoms colored green. The EGFR insNPG structure is shown in yellow with the inserted NPG sequence highlighted in magenta. (D) Local map confirms the D770-N771 NPG insertion. The inserted NPG residues form a tight turn (magenta stick form). (E) Detailed view of the binding mode of ASK120067 with EGFR insNPG mutant. Structural model shows the covalent binding of ASK120067 with EGFR C797. The aminopyrimidine of ASK120067 binds to the hinge residues Met 793 through hydrogen bonds (indicated by yellow dashed lines).
EGFR D770_N771insNPG (#E10-132GG) kinase protein was purchased from signalchem. Kinase activity was evaluated with the enzyme-linked immunosorbent assay (ELISA). Briefly, 20 μg/ml Poly (Glu, Tyr) 4:1 (Sigma, #P0275) was pre-coated in 96-well ELISA plates as substrate. Added 50 μl of 10 μM/L ATP solution diluted in kinase reaction buffer, then the indicated concentrations of compounds were added per well. Reaction was initiated by adding enzyme and maintained for 60 min at 37°C, then the experiment was performed according to the standard ELISA procedures described previously (Chen et al., 2019). Absorbance was measured at 492 nm using a multi-well spectrophotometer (Molecular Devices Spectra max plus 384). The inhibitory rate (%) was calculated with the formula [1-(A492 treated/A492 control)] ×100%. IC50 values were calculated by SoftMax Pro from inhibitory curves.
A published structure of the EGFR D770_N771insNPG (PDB:4LRM) was used for the modeling of potential binding modes of ASK120067 with CovDock, a covalent docking program from Schrodinger (Schrodinger, LLC: New York, NY, 2019). The ligand was prepared by the LigPrep module in Schrodinger software, and the protein structures were optimized by the module of Protein Preparation Wizard module in the Maestro program. Choosing C797 as the reactive residue of the EGFR D770_N771insNPG, centroid of PD168393 was confined to the enclosing box, and the reaction type was set as a Michael Addition. Other parameters were left at the default settings.
The parental IL3-dependent BaF3 cells were obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ), and cultured in 1,640 with 10% FBS and 10 ng/mL IL3. BaF3 cells overexpressing EGFR D770_N771insNPG (BaF3-EGFR insNPG) were constructed by retrovirus transduction using pBABE-puro-EGFR insNPG, and the positive clones were screened by 3 μg/ml puromycin for 1–2 weeks. BaF3-EGFR insNPG were cultured in 1,640 with 10% FBS independent of IL3.
ASK120067 was provided by Jiangsu Aosaikang Pharmaceutical Co., Ltd. Gefitinib (#S1025) and erlotinib (#S7786) were obtained from Selleck.
Cells were seeded in 6-well plates, and starved in serum-free medium for 24 h. Then cells were treated with indicated concentrations of compounds for 2 h and EGFR signaling was stimulated with 50 ng/mL EGF (Life Technologies, #PHG0315) during the last 15 min of compound treatment. After washed with cold PBS for 3 times, cells were lysed in SDS lysis buffer and heated for 20 min at 100°C.
Tumor tissues were lysed with RIPA Lysis Buffer (Beyotime) with protease and phosphatase inhibitor cocktail (Roche). Protein content was determined by BCA Protein Assay Kit (Beyotime) and normalized with SDS loading buffer. Samples were heated for 20 min at 100°C for the following immunoblotting analysis.
Cell lysis- or tumor lysis samples were loaded on SDS-PAGE gels and transferred to nitrocellulose membranes. After blocked with 5% milk-TBST for 30 min, the membranes were blotted with primary antibodies against phospho-EGFR (Tyr1068; Cell Signaling Technologies, #3777), EGFR (Cell Signaling Technologies, #4267), caspase-3 (Cell Signaling Technologies, #9662), cleaved caspase-3 (Asp175) (Cell Signaling Technologies, #9664), PARP (Cell Signaling Technologies, #9532), β-actin (Abgent, #P60709) or GAPDH (Proteintech, #60004-1-lg) overnight at 4°C. The subsequent steps of Western blot analysis were performed according to the standard procedures.
Cell viability was evaluated using Cell Counting Kit-8 (CCK-8). Cells were seeded in 96-well plates. After cultured for at least 2 h, cells were treated with a dilution series of test compounds for 48 h in CO2 incubator at 37°C. 10 μl of CCK-8 solution was added to each well of the plate using a repeating pipettor, and the plate was incubated for 1–2 h in the incubator. The absorbance was measured in a multi-well spectrophotometer (Molecular Devices Spectra max plus 384) at 450 nm. The growth inhibitory rate of compounds was calculated as [1-(A450treated/A450 control)] ×100%. The IC50 values were determined using Logit method.
Cells were seeded in 12-well plates and cultured for 2 h. After treated with test compounds for 24, 48 or 72 h, cells were collected and the apoptosis rate was evaluated using the Annexin V-FITC/PI Apoptosis Detection Kit (Vazyme, #A211-02). Signals were detected using FACS-Calibur flow cytometer (Becton Dickinson).
BaF3-EGFR insNPG cells (5 × 106) were injected subcutaneously into the right flank of BALB/cA nude mice. When tumor volume reached 60–100 mm3, mice were randomly assigned into control and treatment groups (N ≥ 5 animals/group), and oral administered with vehicle or compounds. Tumor volume (TV) was measured every 2 or 3 days, and was calculated with the formula TV = (length × width2)/2. The relative tumor volume (RTV) was calculated as RTV = TV(t)/TV(0), where TV(t) represented the tumor volume on each day, and TV(0) was the tumor volume at the beginning of drug treatment. Tumor growth inhibition (TGI) rate was determined as TGI% = [1 − ΔRTVtreated/ΔRTVvehicle] × 100%, where ΔRTV was calculated as ΔRTV = RTV(t) − RTV(0). Animal experiments were performed according to the guidelines approved by Institutional Animal Care and Use Committee following the guidance of Association for Assessment and Accreditation of Laboratory Animal Care.
Data were presented as mean ± SD or mean ± SEM from repeated experiments. Statistical analysis was performed using student’s t-test and p < 0.05 was considered significant.
We firstly evaluated the kinase inhibitory activity of ASK120067 against EGFR 20ins mutations using an established ELISA-based kinase activity assay. As shown in Figure 1B, ASK120067 potently and dose-dependently suppressed the kinase activity of D770_N771insNPG (EGFR insNPG) protein, a representative TKI-resistant EGFR 20ins kinase, with IC50 value of 1.5 nM. While the 1st-generation EGFR TKIs gefitinib and erlotinib, as expected, showed 54-fold or 194-fold weaker kinase inhibitory activity on EGFR insNPG than ASK120067, with IC50 values of 82.0 or 291.9 nM, respectively.
Then the molecular docking was conducted to simulate the binding model of ASK120067 and EGFR 20ins protein (Figure 1C). The structure of EGFR insNPG was used for the modeling. As shown in Figure 1D, EGFR insNPG is inserted at the C-terminal end of the C-helix, followed by D770. The three inserted residues form a tight turn with the D770, and the main-chain carbonyl group of D770 forms a hydrogen bond to the amide group of the inserted glycine. Two hydrogen bonds are formed between the 2-aminopyrimidine core of ASK120067 and the hinge residue M793, while the acrylamide group forms a covalent bond with the conserved C797 residue in the ATP-binding pocket. In addition, the C5-Cl group is found to interact with the gatekeeper M790 residue. Both C2 and C4 substitutions adapt a “U” shaped binding mode. The amine moiety is pointed to the solvent-accessible region without any special interaction with the protein (Figure 1E). Similar to osimeritinib and mobocertinib [17], ASK120067 occupies the ATP binding pocket and forms a covalent bond with C797 in EGFR. However, the Cl group of ASK120067 is designed to interact with the gatekeeper M790 residue while the similar effect is found on the isopropyl ester in Mobocertinib. These results indicated that ASK120067 could bind to EGFR 20ins kinase through both covalent and hydrogen bond, and thus suppress its kinase activity.
To access the cellular activity of ASK120067 against EGFR 20ins NPG, we constructed BaF3 cell line exogenously over-expressing EGFR insNPG, named BaF3-EGFR insNPG cells. Then the effect of ASK120067 on EGFR insNPG activation was explored using Western Blot analysis. As shown in Figure 2A, ASK120067 treatment dose-dependently blocked the EGF-induced activation of EGFR 20ins NPG, which was consistent with the data from kinase assay. It significantly suppressed the phosphorylation of EGFR insNPG at a concentration as low as 30 nM, with more than 60% inhibition on p-EGFR expression compared to that under EGF stimulation alone, and caused even more complete inhibition (inhibition rate > 80%) as the dose increased. In contrast, gefitinib or erlotinib treatment at up to 300 nM failed to inhibit EGFR activation in BaF3-EGFR insNPG cells (Figures 2B,C).
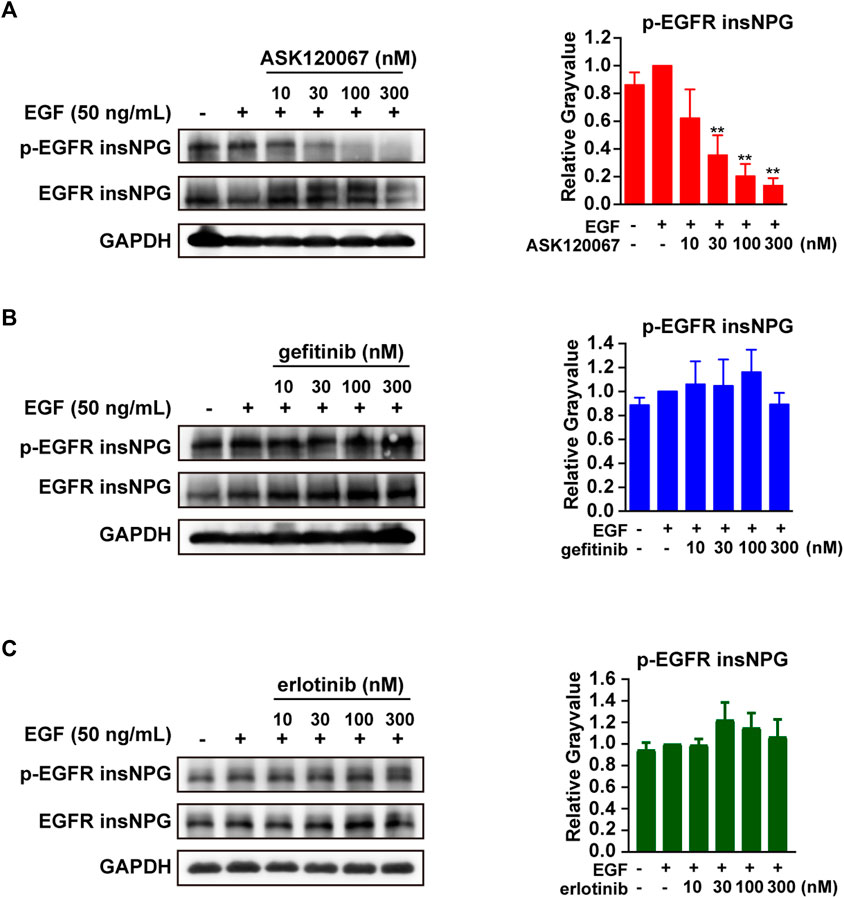
FIGURE 2. ASK120067 dose-dependently inhibited the phosphorylation of EGFR in BaF3-EGFR insNPG cells. The anti-target effects of ASK120067 (A), gefitinib (B) or erlotinib (C) in BaF3-EGFR insNPG cells were detected. Cells were treated with indicated compounds for 2 h and stimulated with EGF for 15 min, then the expression of p-EGFR and EGFR were detected by Western Blot analysis and presented as representative pictures and quantitative analysis. Relative levels of p-EGFR were normalized with EGFR levels. Data were presented as mean ± SEM from no less than four independent experiments, and significance of difference compared with EGF treated control was determined by student’s t-test (*p < 0.05, **p < 0.01).
The anti-proliferative effect of ASK120067 in BaF3-EGFR insNPG cells was further examined using Counting Kit-8 (CCK-8). As expected, ASK120067 potently inhibited the proliferation of BaF3-EGFR insNPG cells with an IC50 value of 0.15 μM, which showed more than 20-fold superior efficacy than gefitinib (IC50 = 3.51 μM) or erlotinib (IC50 = 4.11 μM) (Figure 3A).
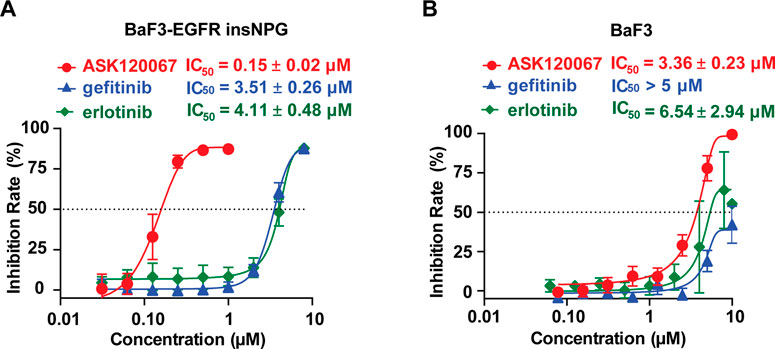
FIGURE 3. ASK120067 effectively inhibited proliferation of EGFR insNPG-dependent BaF3 cells. Anti-proliferation activities of ASK120067, gefitinib and erlotinib against BaF3-EGFR insNPG (A) or the parental BaF3 cells (B) were determined. Cells were treated with indicated compounds for 48 h, and then cell viability was determined using CCK8 (Cell Counting Kit-8) assay. Experiments were performed no less than three times, each time in triplicate, and were shown as mean ± SD.
To further rule out potential off-target effects contributing to the anti-growth activity, we synchronously detected the potency of these compounds on the parental BaF3 cells without mutant EGFR. As shown in Figure 3B, ASK120067 was much less efficacious in the BaF3 parental cells with a higher IC50 value of 3.36 μM, similar to that of gefitinib (IC50 > 5 μM) and erlotinib (IC50 = 6.54 μM). Thus, we concluded that ASK120067 exerted robust anti-proliferative potency through its EGFR 20ins-target inhibition activity.
Considering that inducing cell apoptosis is one of the major mechanisms that EGFR TKIs achieved the anti-tumor effects, we explored the apoptosis-inducing efficacy of ASK120067 in BaF3-EGFR insNPG cells. The proportion of apoptotic cells was determined by Annexin-V/FITC staining after cells treated with an increasing concentration of ASK120067, gefitinib or erlotinib for gradient time. As shown in Figure 4A, ASK120067 induced remarkable cells apoptosis in BaF3-EGFR insNPG cells in both dose-dependent and time-dependent manner. Specifically, compared to DMSO control group, cell apoptosis rate has been increased from 8.0% to 68.0, 84.6% or 88.8% under 24 h ASK120067 treatment at 0.1, 0.3 and 1 μM, respectively. And with longer treatment time of 48 or 72 h, ASK120067, at a low concentration of 0.3 μM, could induced 76.3% or 83.6% more cell apoptosis than DMSO control, and led to even more cell apoptosis as dose increased. In contrast, gefitinib and erlotinib, as expected, hardly caused significant cell apoptosis at the same tested concentration and treatment time (Figures 4B,C).
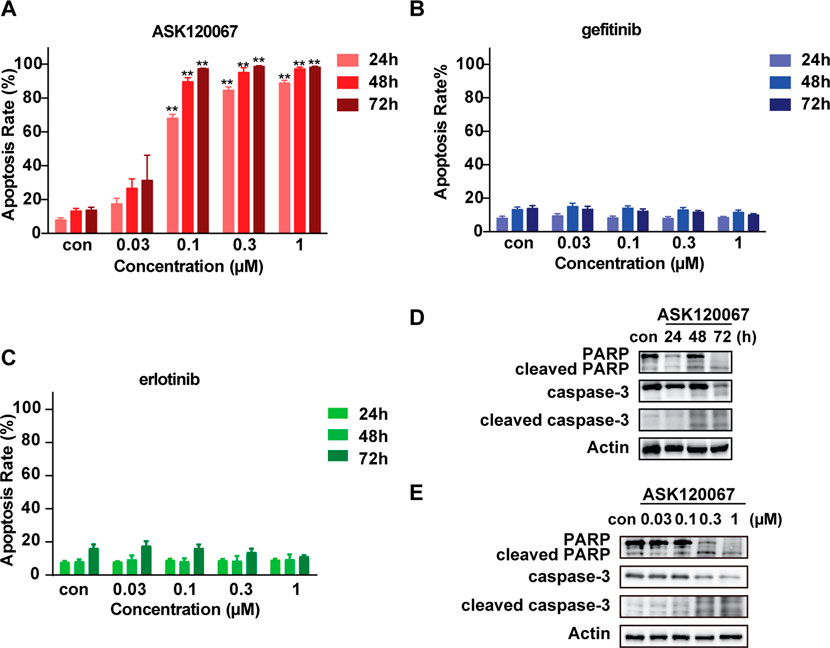
FIGURE 4. ASK120067 treatment caused significant cell apoptosis in EGFR insNPG mutant BaF3 cells. Cell apoptosis in BaF3-EGFR insNPG cells upon ASK120067 (A), gefitinib (B) or erlotinib (C) treatment was evaluated. Cells were treated with increasing concentrations of compounds for 24, 48 or 72 h, and the apoptosis rate were determined using flow cytometry and showed as quantitative analysis. Data were presented as mean ± SEM from no less than three independent experiments. Significance of differences compared with the control group was determined by Student’s t test (*p < 0.05, **p < 0.01). ASK120067 treatment caused increased cleavage of PARP and caspase-3 in a time-dependent and a dose-dependent manner. BaF3-EGFR insNPG cells were treated with 0.3 μM ASK120067 for 24, 48 or 72 h (D), or treated with increasing concentrations of ASK120067 for 48 h (E). The expression of cleaved PARP and cleaved caspase-3 was evaluated by no less than three independent Western Blot analysis.
As the activation of caspase-3, a main executioner of apoptosis, and the cleavage of its major substrate PARP-1 are important markers of apoptosis (Bressenot et al., 2009), we also evaluated the impact of ASK120067 on the cleavage of caspase-3 and PARP in EGFR 20ins mutant cells. Consistent with previous results from flow cytometry detection, time-dependent (Figure 4D) and dose-dependent (Figure 4E) increase of the cleaved caspase-3 and cleaved PARP were observed upon ASK120067 treatment. Thus, these results demonstrated that ASK120067 effectively suppressed EGFR 20ins activation, inhibited cell proliferation and induced apoptosis in malignant cells driven by 20ins mutant EGFR.
Prompted by the significant in vitro efficacy of ASK120067 on EGFR 20ins, we further evaluated its in vivo anti-tumor activity. BaF3-EGFR insNPG subcutaneous xenograft model was established, and tumor-bearing mice were orally treated with ASK120067 at doses of 15, 25, 50 mg/kg, erlotinib 50 mg/kg or vehicle control once daily. As shown in Figures 5A,B, ASK120067 yielded dose-dependent tumor regression, with tumor growth inhibition (TGI) rates of 32.5%, 48.5% and 65.9% at 15, 25, 50 mg/kg, respectively. However, erlotinib treatment did not cause significant anti-tumor activity even administrated at a high dose of 50 mg/kg.
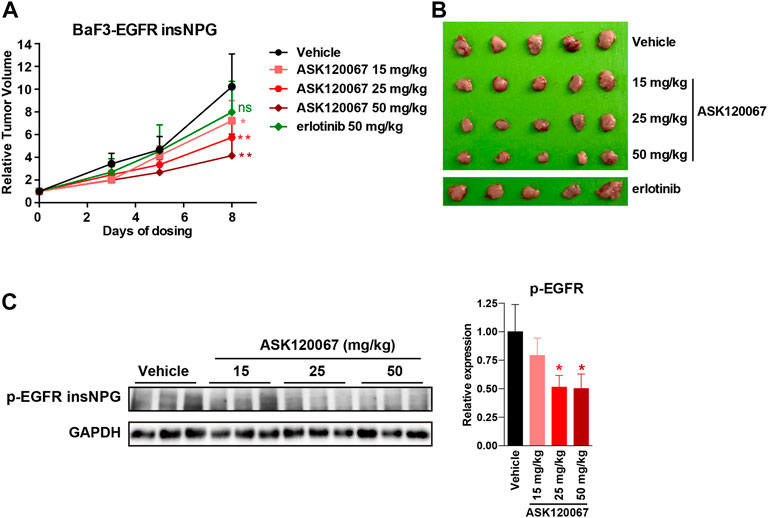
FIGURE 5. ASK120067 exhibited in vivo anti-tumor potency against EGFR 20ins mutant tumor xenograft model. (A,B) Oral administration of ASK120067 caused significant tumor regression in BaF3-EGFR insNPG xenograft model. Nude mice bearing BaF3-EGFR insNPG subcutaneous xenograft were oral administrated with ASK120067 at doses of 15, 25, 50 mg/kg/qd, erlotinib 50 mg/kg/qd, or vehicle control for 8 days. Tumor volume was monitored every 2 or 3 days. At the end of the experiment, mice were sacrificed to collect tumors. (C) ASK120067 showed target-inhibition effects in BaF3-EGFR insNPG xenograft model. The phosphorylation levels of EGFR in BaF3-EGFR insNPG tumor tissues were detected by immunoblotting analysis after 8 days treatment of ASK120067 or vehicle control and presented as representative picture and quantitative analysis. Data were presented as mean ± SD, and significance of differences compared with the vehicle control group was calculated by Student’s t test (*p < 0.05, **p < 0.01, ns, not significant).
To confirm that the in vivo tumor regression was accompanied by EGFR target-inhibition, we detected the activation of EGFR 20ins in tumor tissues at the endpoint of drug administration. As shown in Figure 5C, ASK120067 dose-dependently inhibited EGFR insNPG phosphorylation in tumor tissues, which is consistent with its anti-tumor effects. Taken together, the above results demonstrated both the in vitro and in vivo potency of ASK120067 to overcome drug-resistance caused by EGFR 20ins mutation.
Lung cancer is the leading cause of mortality of malignant tumors worldwide (Siegel et al., 2022). Among all lung cancer cases, NSCLC is the most common type (∼80%) whose pathogenesis and therapy are best studied (Chen et al., 2014). EGFR is a classic drug-target of personalized treatments for NSCLC, and EGFR TKIs have become the first-line therapy for NSCLC patients with classic EGFR activating mutations (L858R and 19del), which provide significantly superior clinical benefit to chemotherapy alone (Herbst et al., 2018; Ke and Wu, 2016). However, EGFR 20ins, the third frequent common type of EGFR mutations, are generally insensitive to the approved EGFR TKIs with limited therapeutic effects (Harrison et al., 2020; Santos et al., 2011). ASK120067 is a novel 3rd-generation EGFR inhibitor developed by our research group. Similar to osimertinib, it showed potent activity against activating EGFR mutations and EGFR T790M, with selectivity over the wild-type EGFR. In this study, we validated its pre-clinical target-inhibition and anti-tumor activities on EGFR 20ins mutant tumor models.
ASK120067 treatment decreased the phosphorylation of EGFR 20ins, induced cell apoptosis and abrogated tumor growth in EGFR 20ins-dependent BaF3 cells or xenograft. Although most of EGFR 20ins mutations are exclusive with other mutation (Remon et al., 2020), the concurrence of EGFR 20ins with EGFR activating mutations (19del or L858R) or EGFR T790M resistance mutation was identified in some cases (Fang et al., 2019). Moreover, the emergence of secondary mutation of T790M mutation was reported to account for 10% of acquired resistance to poziotinib in EGFR 20ins mutant NSCLC (Elamin et al., 2019; Pacini et al., 2021). Thus, ASK120067 might have superior clinical efficacy on those patients intrinsically harboring both EGFR 20ins and T790M, and might have the potential to reduce or delay the acquired resistance mediated by acquired T790M mutation in EGFR 20ins mutant NSCLC patients. Also, the less clinical side effects due to its selectivity over EGFR WT, and the relatively extensive clinical safety data in EGFR mutant NSCLC could provide competitive advantages for ASK120067 on treating EGFR 20ins mutant lung cancer patients. Currently, an NDA application of ASK120067 has been submitted in China for the second-line therapy of locally advanced or metastatic EGFR T790M-positive NSCLC, and a phase Ⅲ clinical trial is undergoing to evaluate its the safety and effectiveness for first-line treatment in locally advanced or metastatic EGFR-mutant NSCLC compared with gefitinib (NCT04143607). Here, we elucidated the anti-tumor efficacy of ASK120067 in EGFR 20ins mutant preclinical models, and this study would support the further clinical evaluation of ASK120067 as a new drug candidate for NSCLC patients driven by EGFR 20ins mutations.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee, Shanghai Institute of Materia Medica.
Conceptualization, HX, TZ, and FF; methodology, FF, LT, and SC; validation, FF and TZ; formal analysis, TZ and FF; resources, JD, HX, and MG; writing—original draft preparation, TZ and SC; writing—review and editing, TZ, FF, YC, and HX; supervision, HX and JD; project administration, FF, LT, YC, YaL, PS, YiL, GB, ML, YN, YW, YF, and ZP; funding acquisition, HX, TZ, and JD; All authors have read and agreed to the published version of the manuscript.
This work was supported by the National Science & Technology Major Project “Key New Drug Creation and Manufacturing Program,” China (2019ZX09301157-004), National Natural Science Foundation of China (81903638), the Special Research Assistant Program of the Chinese Academy of Sciences and the project sponsored by the development fund for Shanghai talents, Foundation SIMM2205KF-09 from State Key Laboratory of Drug Research, and Lingang Laboratory Grant (LG202103-02-02).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bressenot, A., Marchal, S., Bezdetnaya, L., Garrier, J., Guillemin, F., and Plénat, F. (2009). Assessment of apoptosis by immunohistochemistry to active caspase-3, active caspase-7, or cleaved PARP in monolayer cells and spheroid and subcutaneous xenografts of human carcinoma. J. Histochem. Cytochem. 57 (4), 289–300. doi:10.1369/jhc.2008.952044
Cardona, A. F., Rojas, L., Zatarain-Barrón, Z. L., Freitas, H. C., Granados, S. T., Castillo, O., et al. (2018). EGFR exon 20 insertion in lung adenocarcinomas among Hispanics (geno1.2-CLICaP). Lung Cancer 125, 265–272. doi:10.1016/j.lungcan.2018.10.007
Chang, L. C., Lim, C. K., Chang, L. Y., Chen, K. Y., Shih, J. Y., and Yu, C. J. (2019). Non-small cell lung cancer harbouring non-resistant uncommon EGFR mutations: Mutation patterns, effectiveness of epidermal growth factor receptor-tyrosine kinase inhibitors and prognostic factors. Eur. J. Cancer 119, 77–86. doi:10.1016/j.ejca.2019.06.025
Chen, Z., Fillmore, C. M., Hammerman, P. S., Kim, C. F., and Wong, K. K. (2014). Non-small-cell lung cancers: A heterogeneous set of diseases. Nat. Rev. Cancer 14 (8), 535–546. doi:10.1038/nrc3775
Chen, Z., Tong, L. J., Tang, B. Y., Liu, H. Y., Wang, X., Zhang, T., et al. (2019). C11, a novel fibroblast growth factor receptor 1 (FGFR1) inhibitor, suppresses breast cancer metastasis and angiogenesis. Acta Pharmacol. Sin. 40 (6), 823–832. doi:10.1038/s41401-018-0191-7
Cooper, A. J., Sequist, L. V., and Lin, J. J. (2022). Third-generation EGFR and ALK inhibitors: Mechanisms of resistance and management. Nat. Rev. Clin. Oncol. 19, 499–514. doi:10.1038/s41571-022-00639-9
Elamin, Y., Robichaux, J., Carter, B., Altan, M., Gibbons, D., Fossella, F., et al. (2019). MA09.03 identification of mechanisms of acquired resistance to poziotinib in EGFR exon 20 mutant non-small cell lung cancer (NSCLC). J. Thorac. Oncol. 14, S282–S283. doi:10.1016/j.jtho.2019.08.567
Fang, W., Huang, Y., Hong, S., Zhang, Z., Wang, M., Gan, J., et al. (2019). EGFR exon 20 insertion mutations and response to osimertinib in non-small-cell lung cancer. BMC Cancer 1719 (1), 595. doi:10.1186/s12885-019-5820-0
Gonzalvez, F., Vincent, S., Baker, T. E., Gould, A. E., Li, S., Wardwell, S. D., et al. (2011). Mobocertinib (TAK-788): A targeted inhibitor of EGFR exon 20 insertion mutants in non-small cell lung cancer. Cancer Discov. 11 (7), 1672–1687. doi:10.1158/2159-8290.CD-20-1683
Harrison, P. T., Vyse, S., and Huang, P. H. (2020). Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin. Cancer Biol. 61, 167–179. doi:10.1016/j.semcancer.2019.09.015
Herbst, R. S., Morgensztern, D., and Boshoff, C. (2018). The biology and management of non-small cell lung cancer. Nature 24553 (7689), 446–454. doi:10.1038/nature25183
Ke, E. E., and Wu, Y. L. (2016). EGFR as a pharmacological target in EGFR-mutant non-small-cell lung cancer: Where do we stand now? Trends Pharmacol. Sci. 37 (11), 887–903. doi:10.1016/j.tips.2016.09.003
Le, X. N., Goldman, J. W., Clarke, J. M., Tchekmedyian, N., Piotrowska, Z., Chu, D., et al. (2020). Poziotinib shows activity and durability of responses in subgroups of previously treated EGFR exon 20 NSCLC patients. J. Clin. Oncol. 38, 9514. doi:10.1200/jco.2020.38.15_suppl.9514
Li, Y., and Chen, L. A. (2017). Precise therapy for lung cancer patients with rare sensitive mutations of epidermal growth factor receptor. Zhonghua Zhong Liu Za Zhi 2339 (12), 881–884. doi:10.3760/cma.j.issn.0253-3766.2017.12.001
Meador, C. B., Sequist, L. V., and Piotrowska, Z. (2021). Targeting EGFR exon 20 insertions in non-small cell lung cancer: Recent advances and clinical updates. Cancer Discov. 11 (9), 2145–2157. doi:10.1158/2159-8290.CD-21-0226
Murtuza, A., Bulbul, A., Shen, J. P., Keshavarzian, P., Woodward, B. D., Lopez-Diaz, F. J., et al. (2019). Novel third-generation EGFR tyrosine kinase inhibitors and strategies to overcome therapeutic resistance in lung cancer. Cancer Res. 1579 (4), 689–698. doi:10.1158/0008-5472.CAN-18-1281
Pacini, L., Jenks, A. D., Vyse, S., Wilding, C. P., Arthur, A., and Huang, P. H. (2021). Tackling drug resistance in EGFR exon 20 insertion mutant lung cancer. Pharmgenomics. Pers. Med. 9 (14), 301–317. doi:10.2147/PGPM.S242045
Pao, W., and Chmielecki, J. (2010). Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat. Rev. Cancer 10 (11), 760–774. doi:10.1038/nrc2947
Passaro, A., Mok, T., Peters, S., Popat, S., Ahn, M. J., and de Marinis, F. (2021). Recent advances on the role of EGFR tyrosine kinase inhibitors in the management of NSCLC with uncommon, non exon 20 insertions, EGFR mutations. J. Thorac. Oncol. 16 (5), 764–773. doi:10.1016/j.jtho.2020.12.002
Qin, Y., Jian, H., Tong, X., Wu, X., Wang, F., Shao, Y. W., et al. (2020). Variability of EGFR exon 20 insertions in 24 468 Chinese lung cancer patients and their divergent responses to EGFR inhibitors. Mol. Oncol. 14 (8), 1695–1704. doi:10.1002/1878-0261.12710
Remon, J., Hendriks, L. E. L., Cardona, A. F., and Besse, B. (2020). EGFR exon 20 insertions in advanced non-small cell lung cancer: A new history begins. Cancer Treat. Rev. 90, 102105. doi:10.1016/j.ctrv.2020.102105
Riely, G. J., Neal, J. W., Camidge, D. R., Spira, A. I., Piotrowska, Z., Costa, D. B., et al. (2021). Activity and safety of mobocertinib (TAK-788) in previously treated non-small cell lung cancer with EGFR exon 20 insertion mutations from a phase I/II trial. Cancer Discov. 11 (7), 1688–1699. doi:10.1158/2159-8290.CD-20-1598
Rosell, R., Carcereny, E., Gervais, R., Vergnenegre, A., Massuti, B., Felip, E., et al. (2012). Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet. Oncol. 13 (3), 239–246. doi:10.1016/S1470-2045(11)70393-X
Rosell, R., Moran, T., Queralt, C., Porta, R., Cardenal, F., Camps, C., et al. (2009). Screening for epidermal growth factor receptor mutations in lung cancer. N. Engl. J. Med. 3361 (10), 958–967. doi:10.1056/NEJMoa0904554
Roskoski, R. (2022). Properties of FDA-approved small molecule protein kinase inhibitors: A 2020 update. Pharmacol. Res. 175, 104609. doi:10.1016/j.phrs.2019.104609
Sacher, A., Le, X., Cornelissen, R., Shum, E., Suga, J., Socinski, M., et al. (2021). 36MO Safety, tolerability and preliminary efficacy of poziotinib with twice daily strategy in EGFR/HER2 Exon 20 mutant non-small cell lung cancer. Ann. Oncol. 32, S15. doi:10.1016/j.annonc.2021.01.051
Santos, G. C., Shepherd, F. A., and Tsao, M. S. (2011). EGFR mutations and lung cancer. Annu. Rev. Pathol. 6, 49–69. doi:10.1146/annurev-pathol-011110-130206
Shi, Y., Li, B., Wu, L., Pan, Y., Pan, Z., Liu, Y., et al. (2022). Efficacy and safety of limertinib (ASK120067) in patients with locally advanced or metastatic EGFR Thr790Met-mutated NSCLC: A multicenter, single-arm, phase 2b study. J. Thorac. Oncol. S1556-0864 (22), 1205–1215. doi:10.1016/j.jtho.2022.05.011
Siegel, R. L., Miller, K. D., Fuchs, H. E., and Jemal, A. (2022). Cancer statistics, 2016. Ca. Cancer J. Clin. 72 (1), 7–30. doi:10.3322/caac.21332
Syahruddin, E., Wulandari, L., Sri Muktiati, N., Rima, A., Soeroso, N., Ermayanti, S., et al. (2018). Un-common EGFR mutations in cytological specimens of 1, 874 newly diagnosed Indonesian lung cancer patients. Lung Cancer (Auckl) 23 (9), 25–34. doi:10.2147/LCTT.S154116
Tan, C. S., Kumarakulasinghe, N. B., Huang, Y. Q., Ang, Y. L. E., Choo, J. R., Goh, B. C., et al. (2018). Third generation EGFR TKIs: Current data and future directions. Mol. Cancer 1917 (1), 29. doi:10.1186/s12943-018-0778-0
Vyse, S., and Huang, P. H. (2022). Amivantamab for the treatment of EGFR exon 20 insertion mutant non-small cell lung cancer. Expert Rev. Anticancer Ther. 22 (1), 3–16. doi:10.1080/14737140.2022.2016397
Vyse, S., and Huang, P. H. (2019). Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduct. Target. Ther. 8 (4), 5. doi:10.1038/s41392-019-0038-9
Wang, M., Yang, J. C-H., Mitchell, P. L., Fang, J., Camidge, D. R., Nian, W., et al. (2022). Sunvozertinib, a selective EGFR inhibitor for previously treated non–small cell lung cancer with EGFR exon 20 insertion mutations. Cancer Discov. 12, 1676–1689. doi:10.1158/2159-8290.CD-21-1615
Yasuda, H., Ichihara, E., Sakakibara-Konishi, J., Zenke, Y., Takeuchi, S., Morise, M., et al. (2021). A phase I/II study of osimertinib in EGFR exon 20 insertion mutation-positive non-small cell lung cancer. Lung Cancer 162, 140–146. doi:10.1016/j.lungcan.2021.10.006
Keywords: ASK120067, limertinib, EGFR TKIs, EGFR exon20 insertion, drug resistance
Citation: Zhang T, Feng F, Tong L, Chan S, Chen Y, Li Y, Song P, Liu Y, Bai G, Lai M, Ning Y, Wang Y, Fang Y, Pan Z, Geng M, Ding K, Ding J and Xie H (2022) ASK120067 (limertinib) exerts pre-clinical anti-tumor activity by inhibiting EGFR exon20 insertion. Front. Drug. Discov. 2:1050687. doi: 10.3389/fddsv.2022.1050687
Received: 22 September 2022; Accepted: 15 November 2022;
Published: 29 November 2022.
Edited by:
Panupong Mahalapbutr, Khon Kaen University, ThailandReviewed by:
Yihui Song, Zhengzhou University, ChinaCopyright © 2022 Zhang, Feng, Tong, Chan, Chen, Li, Song, Liu, Bai, Lai, Ning, Wang, Fang, Pan, Geng, Ding, Ding and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Ding, amRpbmdAc2ltbS5hYy5jbg==; Hua Xie, aHhpZUBzaW1tLmFjLmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.