- 1UCL School of Pharmacy, University College London, London, United Kingdom
- 2Sciense Ltd., London, United Kingdom
- 3Jenny Walsh Consulting Ltd., Nottingham, United Kingdom
Background: Many medicines for children taste bitter and unpleasant, presenting a significant barrier to effective pharmacotherapy. Anecdotally, this issue is widely recognized; however, empirical evidence on the consequences of unpalatable medicines remains scarce and fragmented. The objective of this scoping review was to investigate the impact of poor tasting pediatric medicines on patient acceptability, medication adherence, and/or treatment outcomes.
Methods: A literature search was performed in MEDLINE/PubMed, EMBASE and CINAHL from inception to June 2023. Eligibility criteria included interventional or observational studies conducted in children aged 0–18 years (population), administered an unpalatable oral medicine (exposure), with any reported impact on patient acceptability, medication adherence, and treatment effects (outcomes). Study screening and data extraction was completed using a standardized form on Covidence.
Results: After searching 2,282 citations and reviewing 429 full-text papers, 225 articles were included in the final analysis. The impact of poor-tasting medicines was observed across 77 diseases or indications, with 156 different unpalatable medicinal products identified. Outcomes were most frequently linked to reduced patient acceptability, with 64% of articles reporting rejection responses, the need for strategies to aid administration (from positive reinforcement to physical restraint and forced administration), and impacts on prescribing practices (e.g., use of non-first line alternative therapies). Medication adherence impacts were reported in 27% of the reviewed studies, where poor taste was reported as a barrier to adherence in chronic diseases and correlated with incomplete dose administration in acute conditions. A small number of studies linked palatability with treatment outcomes, including viral suppression in HIV and seizure control in epilepsy.
Conclusion: This review highlights the widespread adverse impact of poor-tasting pediatric medicines on patient experiences and outcomes, though the true extent of the issue may still be underreported. The problem affects children worldwide, across all age groups, and is frequently noted by parents, caregivers, and healthcare professionals in both clinical and domiciliary settings. These findings emphasize the need for the development and prescription of more palatable medicines for children, as well as the advancement of more universal taste-masking strategies to address this widespread problem.
1 Introduction
Globally, approximately 30% of the population, or around 2.4 billion people, are babies, children, and adolescents under the age of 18 years (United Nations, 2022). Almost all of this pediatric population will need to take medicines at some point during this stage of life, be it over-the-counter (OTC) products for minor, acute ailments to regular prescribed medicines to manage chronic diseases. Two important concepts must be considered when developing and prescribing medicines: patient acceptability, namely, the overall ability and willingness of the patient to use and its care giver to administer the medicine as intended (European Medicines Agency, 2013); and medication adherence, the degree to which use of medication by the patient corresponds with the prescribed regimen (World Health Organization, 2003). Even the most efficacious drug treatments will be futile if patients do not take them as required.
Palatability is a crucial factor that impacts patients’ experiences of taking medicines. Regulatory agencies define this as the overall appreciation of an (often oral) medicinal product in relation to its smell, taste, aftertaste and texture (i.e., feeling in the mouth) (European Medicines Agency, 2013) and the quality of a drug product that makes it pleasant or acceptable in terms of these attributes (Food and Drug Administration, 2018). However, developing palatable medicines is challenging due to a major obstacle: many drugs have bitter and unpleasant tastes (Mennella et al., 2013). This is unsurprising, as most drugs are xenobiotics - chemical substances not naturally produced or expected to be present within the body - that can exert biological, and potentially adverse effects (Schiffman et al., 2002). Thus, bitterness is an innately aversive sensation that serves as an evolutionary, biological warning to prevent the ingestion of potentially toxic and harmful substances (Breslin, 2013; Wooding et al., 2021). Despite this natural aversion, it is crucial that patients are able to take their medications for effective clinical treatment.
Indeed, patients of all ages are more likely to prefer better-tasting medicines with favorable sensory and organoleptic properties, but this issue is particularly challenging in children. While adults are often able to tolerate unpalatable medicines due to their understanding of the health benefits, children’s experiences are shaped by their cognitive development and more primal sense of what is acceptable or not. Young patients may lack the ability to fully understand the importance of taking medication or cooperate in its administration. Taste perception is a complex process, and sensitivity and responses to the same stimuli can vary considerably among individuals. This can be influenced by a multitude of confounding factors including age, sex, genetics, ethnicity, and health status (Shizukuda et al., 2018; Cattaneo et al., 2022). Taste perception first develops in-utero and matures with age, though children have a recognized preference for sweet tastes and dislike for bitter stimuli which stems from their innate biology (Mennella and Bobowski, 2015; Ustun et al., 2022; Mennella, 2014). Studies have shown that children are more sensitive to bitter tastes compared with adults when exposed to the same stimulus (Ranmal et al., 2023; Mennella et al., 2005; Mennella et al., 2010).
In its seminal guideline on the pharmaceutical development of pediatric medicines, the European Medicines Agency (EMA) acknowledges that palatability is one of the main elements of the patient acceptability, and in turn, patient acceptability is likely to have a significant impact on patient adherence and consequently, on the safety and efficacy of a medicinal product (European Medicines Agency, 2013). The United States Food and Drug Administration (FDA) also notes this association between palatability and patient acceptability, and the resulting impact on adherence, a key factor in successful therapeutic intervention (Food and Drug Administration, 2018). This proposed relationship is illustrated in Figure 1, showing in turn how medication taste can facilitate or compromise these related outcomes. This relationship is logically sound and anecdotally, the issue is widely recognized; however, empirical evidence demonstrating the impact of bad-tasting medicines is scarce and fragmented.
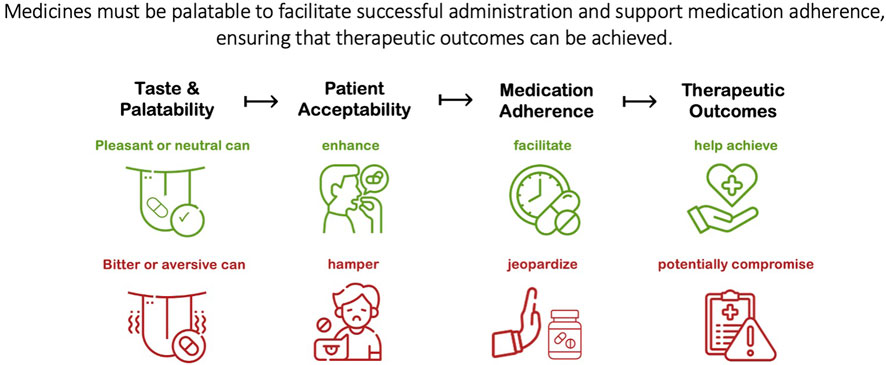
Figure 1. The relationship between medication palatability and patient acceptability, which in turn can impact medication adherence and the safety and efficacy of the treatment.
The objective of this scoping review was to investigate the impact of poor tasting pediatric medicines on three key concerns: patient acceptability, medication adherence, and treatment outcomes. A scoping review allowed this broad research question to be explored by systematically searching and synthesizing existing knowledge, while mapping key concepts and identifying gaps in evidence (Colquhoun et al., 2014). Insights into the real-world experiences of pediatric patients and their caregivers also provides valuable understanding of the practical challenges faced in administering unpalatable medicines. This is complemented by an accompanying article on the experiences of pediatric healthcare providers and caregivers in the United States and Sub-Saharan Africa (El-Sahn et al., 2025).
The conduct and reporting of the review was guided by the JBI Manual for Evidence Synthesis (Peters et al., 2020) and Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews (PRISMA-ScR) (Tricco et al., 2018). In line with the Population-Concept-Context (PCC) framework recommended by JBI, the review focused on: 1) population: children aged 0–18 years; including studies with patients, caregivers, and healthcare professionals (HCPs) involved in medicines administration; 2) concept: exposure to a bad tasting or unpalatable oral medicinal product impacting patient acceptability and administration, medication adherence, and/or therapeutic outcomes; and 3) context: any disease area or ailment and across all geographical locations.
2 Methods
The scoping review protocol was developed a priori and the PRISMA-ScR checklist is included in Supplementary Table S1.
2.1 Eligibility criteria
The review considered primary research from interventional studies (e.g., non-randomized or randomized controlled trials and uncontrolled studies) and observational studies (e.g., cross-sectional, cohort, and case-control). All study designs were eligible, including those using qualitative methodologies (e.g., groups or interviews) or quantitative methods (e.g., surveys, semi-structured questionnaires etc.). Published journal articles, conference abstracts, and letters were included, while reviews, editorials, and commentaries were excluded. When the same data were reported in more than one publication (e.g., a conference abstract and subsequent journal article), only the source reporting the most complete data set was used. No limits on date, subject or type were placed on the database search; however, articles published in languages other than English were excluded.
2.2 Information sources and search strategy
An initial limited search of two electronic databases, MEDLINE and Embase, was undertaken to identify key articles on the topic and optimize the search strategy. The Population-Exposure-Outcome (PEO) framework was used to outline the keywords and index terms for the full search (Supplementary Table S2). The full search was implemented in three electronic databases from inception to 17 June 2023: MEDLINE via PubMed (biomedical sciences, 1946–present), Embase via Ovid (biomedical and pharmaceutical sciences, 1947-present), and Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCO (nursing and allied health; 1981–present). The search strategy was tailored to the specific requirements of each database and was not limited by study design or year (see Supplementary Table S3). The full electronic search strategy used in MEDLINE/PubMed is exemplified below:
((((((pediatric) OR (child)) OR (infant)) OR (adolescent)) AND (((((((palatab*) OR (taste)) OR (smell)) OR (texture)) OR (mouthfeel)) OR (bitter)) OR (avers*))) AND ((((drug therapy) OR (drug formulation)) OR (drug dosage form)) OR (medication))) AND ((((adherence) OR (medication compliance)) OR (accept*)) OR (treatment outcome)) Filters: English.
2.3 Study screening and selection
All citations were collated, screened, and reviewed within Covidence, a web-based collaboration software platform that streamlines the production of systematic and other literature reviews (Covidence, 2023). The relevance of studies as sources of evidence was assessed using a two-stage screening process: 1) title and abstract screening and 2) full text review. Selection was performed against pre-specified inclusion or exclusion criteria based on the PEO framework and study characteristics. The eligibility criteria were uploaded into the software for reference during the screening. During the title and abstract screening, studies were excluded if they were conducted in adult populations, or if the study was in relation to food, beverages, nutritional supplements, or placebo formulations. To meet eligibility criteria, abstracts had to explicitly mention palatability and include an outcome related to the study objectives. The eligibility screening process for full text screening is outlined in Section 2.5. Both stages were completed independently by two reviewers (SRR and JW). Reviewers met throughout the screening process to resolve conflicts and discuss any uncertainties related to study selection. Cohen’s Kappa coefficient (κ) was calculated within Covidence as a statistical measure used to quantify the level of agreement between two main reviewers. Any disagreements were solved by consensus or by the decision of a third reviewer (CT).
2.4 Data extraction and charting
Data from selected studies was extracted independently by the two main reviewers using a data extraction tool developed within Covidence (see Supplementary Table S4). The tool was initially piloted by each reviewer on a random sample of studies and was iteratively modified by consensus as the charting process evolved. Following full data extraction, one reviewer (SRR) verified all charting to ensure accuracy and consistency of data.
Key information collated included 1) study characteristics including location countries categorized by geography and income group based on World Bank Group classifications (World Bank, 2024) and overall classification in relation to palatability report and type of outcome(s); 2) population age range categorized into six pediatric age groups proposed in regulatory guidance (European Medicines Agency, 2006) 3) exposure to medicinal product with disease area categorized by WHO International Classification of Diseases (ICD-11) the international standard for recording and reporting diseases and health related conditions (World Health Organization, 2022) and chemical substance categorized by WHO Anatomical Therapeutic Chemical (ATC) classification (WHO Collaborating Centre for Drug Statistics Methodology, 2023) or as “other” for unclassified interventional products used for treatment or diagnosis.
For studies that included both pediatric and adult participants, the mean or median age of the cohort was considered, and studies were excluded if this exceeded 18 years. In cases where this age information was not reported, the study was only included if the age range of participants started from 12 years old. This cutoff was chosen to account for differences in population definitions, as studies that had been conducted in adults were captured during the screening process when the inclusion criterion was from the age of 16 years.
2.5 Appraisal and synthesis
The process of study appraisal and analysis in relation to palatability and outcome reports is outlined in Supplementary Table S5. Primarily, poor taste or palatability had to be reported either formally (e.g., rated on scale by study participants) or informally (e.g., as a problem reported by authors). Different terms were used to describe these evaluations across studies including “palatability,” “acceptability,” “degree of liking,” “tolerance,” and “satisfaction;” however, the descriptions in the manuscript had to demonstrate that this was in relation to the sensory attributes of the medicinal product. Some studies described bitter taste as an adverse event (AE), and in these cases, further clarity was sought from product literature to determine if this was a taste disturbance related to the systemic effect of the active pharmaceutical ingredient (API) or sensory properties of the drug itself. Since there are no established thresholds or criteria to define whether a medicine is deemed palatable or unpalatable, the review included studies reporting any negative rating (e.g., less than neutral response on self-report scale) or negative response from any single participant in the study.
Impacts on patient acceptability and administration were categorized as rejection responses (e.g., resistance or spitting out), strategies to aid administration (e.g., mixing with food or drink) and any impacts on prescribing (e.g., drug therapy changes). Adherence outcomes had to include formal measures (e.g., pill counts or self-report missed doses) or studies exploring barriers to adherence (e.g., using questionnaire tools or qualitative interviews), where palatability was specifically reported as an obstacle. Studies were excluded if the authors simply remarked that palatability can influence acceptability or adherence, but no quantifiable outcomes were reported. Impact on therapeutic outcomes were included if the authors explicitly linked findings with poor palatability of the medicine and its impact on acceptability and adherence. The synthesis included quantitative analysis (e.g., frequency and proportions) of the PEO criteria and qualitative review of themes and content. The methodological quality or risk of bias of the included articles was not appraised, in accordance with guidelines for conducting scoping reviews (Peters et al., 2020).
3 Results
3.1 Selection of sources of evidence
The PRISMA flow diagram in Figure 2 illustrates the screening and selection process. A total of 2,907 citations (2,889 studies) were identified through the database search. After removing duplicates, 2,282 studies were screened based on titles and abstracts, followed by full-text review of 429 papers. Of these, 204 were excluded for not meeting eligibility criteria, with the most common reasons being incorrect study population, insufficient outcome reporting, or non-oral administration routes. Ultimately, 225 papers were included in the final scoping review analysis (Abdulla et al., 2010; Abu-Khalaf et al., 2018; Adams et al., 2013; Al-Ani et al., 2016; Aljebab et al., 2018; Allen et al., 2003; Almenrader et al., 2007; Ameen et al., 2006; Angelilli et al., 2000; Angwa et al., 2020; Ansah et al., 2001; Bagger-Sjoback and Bondesson, 1989; Baka-Ostrowska et al., 2021; Baker et al., 2021; Barnett and Bhatt, 2020; Bartoli et al., 2006; Berg et al., 2006; Bergene et al., 2019; Bertholet-Thomas et al., 2021; Beuter et al., 2019; Block et al., 2005; Block et al., 2006; Braga et al., 2015; Bryson, 2014; Buchanan et al., 2012; Bullington et al., 2007; Bunupuradah et al., 2006; Caglayan et al., 1989; Cañete et al., 2010; Celebi et al., 2009; Chadwick et al., 2005; Cheng, 2004; Christiansen et al., 2014; Chung et al., 2000; Cifaldi et al., 2004; Claes et al., 2014; Cloyd et al., 1992; Coetzee et al., 2015; Coetzee et al., 2016; Cohen et al., 2005; Cohen et al., 2009; Cote et al., 2002; Cronin et al., 2016; Dagan et al., 1994; Dagnone et al., 2002; Dalton et al., 2019; Davies et al., 2008; Dawson and Sharpe, 1993; DelRosso et al., 2020), (Dillman et al., 2018; Di Pierro et al., 2014; Duniva Inusa et al., 2021; El Edelbi et al., 2015; Elgammal et al., 2022; Escobedo et al., 2008; Escoda et al., 2017; Ewing et al., 2015; Frange et al., 2018; Freedman et al., 2010; Gamston, 2013; Garcia-Prats et al., 2022; Gaskell, 2016; Gibson et al., 2010; Giralt et al., 2019; Goldberg et al., 2013; Goode et al., 2003; Gotesman et al., 2020; Goyanes et al., 2019; Greenley et al., 2018; Greenwood and Vallabhaneni, 2015; Gutierrez-Colina et al., 2018; Gyedu et al., 2023; Hames et al., 2008; Hansen et al., 2008; Haslund-Krog et al., 2022; Hirsch-Moverman et al., 2021; Hoffstedt et al., 2023; Hofmanová et al., 2020; Holas et al., 2005; Holloway et al., 2018; Holmberg, 1994; Horodniceanu et al., 2017; Hou et al., 2022; Ibrahim and Al Ansari, 2016; Imanieh et al., 2022; Isik et al., 2008; Jaffé and Grimshaw, 1983; Jahnsen and Thorn, 1987; Jain et al., 2013; Jamieson et al., 2021; Joanne and Ibrahim, 2018; Kedenge et al., 2013; Kekitiinwa et al., 2016; Kelly et al., 2020; Khalil et al., 2003; Khurana, 1996; Kibleur et al., 2014; Kiruki et al., 2018; Kiryluk and Sobaniec, 2013), (Klingmann et al., 2022; Kokke et al., 2008; Kokki et al., 2000; Kolaczinski et al., 2006; Kolmen et al., 1995; Kumar et al., 2012; Lagos et al., 1999; Lava et al., 2011; Leonard et al., 2014; Liacouras et al., 1993; Lin et al., 2011; Low et al., 2018; Macknin et al., 1998; Maines et al., 2020; Manford Gooch et al., 1999; Marshall et al., 2000; Matsui et al., 1996; Matsumoto et al., 2022; McIntyre and Hull, 1996; Meier et al., 2007; Melvin et al., 1997; Mettey et al., 1990; Milani et al., 2010; Mistry et al., 2018; Moniot-Ville et al., 1998; Mulla et al., 2016; Murnane et al., 2017; Nachman et al., 2014; Nahirya-Ntege et al., 2012; Nasrin et al., 2005; Nathan et al., 2006; Nezam et al., 2022; Nimrouzi et al., 2015; Nizami et al., 1996; Nuzhat et al., 2022; Osterhoudt et al., 2004; Padilla et al., 2000; Paediatric European Network for Treatment of AIDS (PENTA) (1999); Pai et al., 2023; Pajno et al., 2012; Palit et al., 2016; Paller et al., 2020; Paranthaman et al., 2009; Parshuram and McMahon, 1998; Pasipanodya et al., 2018; Patel et al., 2022; Penagini et al., 2017; Phipps and DeCuir-Whalley, 1990; Pichichero et al., 1990; Porteous et al., 1997; Powers, 1996), (Powers et al., 2000; Powers et al., 2020; Prajapati et al., 2022; Pronzini et al., 2008; Purchase et al., 2016; Purchase et al., 2019; Querin et al., 2023; Rasul and Khan, 2001; Reddington et al., 2000; Reich et al., 2000; Rendeli et al., 2006; Rittig et al., 2020; Riva et al., 1997; Rosenthal et al., 2020; Rotsaert et al., 2023; Rouse et al., 2017; Ruperto et al., 2017; Rutebemberwa et al., 2009; Saito et al., 1999; Saneian and Mostofizadeh, 2012; Savino et al., 2012; Scheuern et al., 2017; Schwarz et al., 2020; Shchelochkov et al., 2016; Silva et al., 2022; Sleath et al., 2006; Smith et al., 2013; Sondheimer et al., 1991; Splieth et al., 2000; Steans et al., 1990; Steele et al., 2002; Stevens et al., 1996; Suchismita et al., 2023; Syofyan et al., 2019; Taher et al., 2018; Tannous and Azouz, 1991; Taylor et al., 2016; Thompson et al., 2013; Thomson et al., 2015; Thornburg et al., 2011; Tiengkate et al., 2022; Todd et al., 2023; Toscani et al., 2000; Touchette et al., 1994; Tran et al., 2017; Tsouana et al., 2015; Tucker et al., 2002; Uhari et al., 1986; Unachak et al., 2002; Valayannopoulos et al., 2019), (van der Vossen et al., 2020; Van de Vijver et al., 2011; Van Dyke et al., 2002; Varma et al., 2012; Varnell et al., 2017; Varnell et al., 2021; Venables et al., 2015a; Venables et al., 2015b; Venables et al., 2018; Viprakasit et al., 2023; Voskuijl et al., 2004; Wademan et al., 2019; Warembourg et al., 2020; Warzecha et al., 2022; Wataya, 2017; Wijesekera et al., 2019; Williford et al., 2023; Xin et al., 2022; Yafout et al., 2022; Yeowell et al., 2021; Yoo et al., 2022; Young et al., 2020; Zannikos et al., 2011; Zelikovsky et al., 2011; Zhang et al., 2016). Cohen’s Kappa coefficient was κ = 0.66 for the abstract and title screening and κ = 0.76 for the full-text review, indicating substantial inter-rater reliability and agreement between the two reviewers for study inclusion.
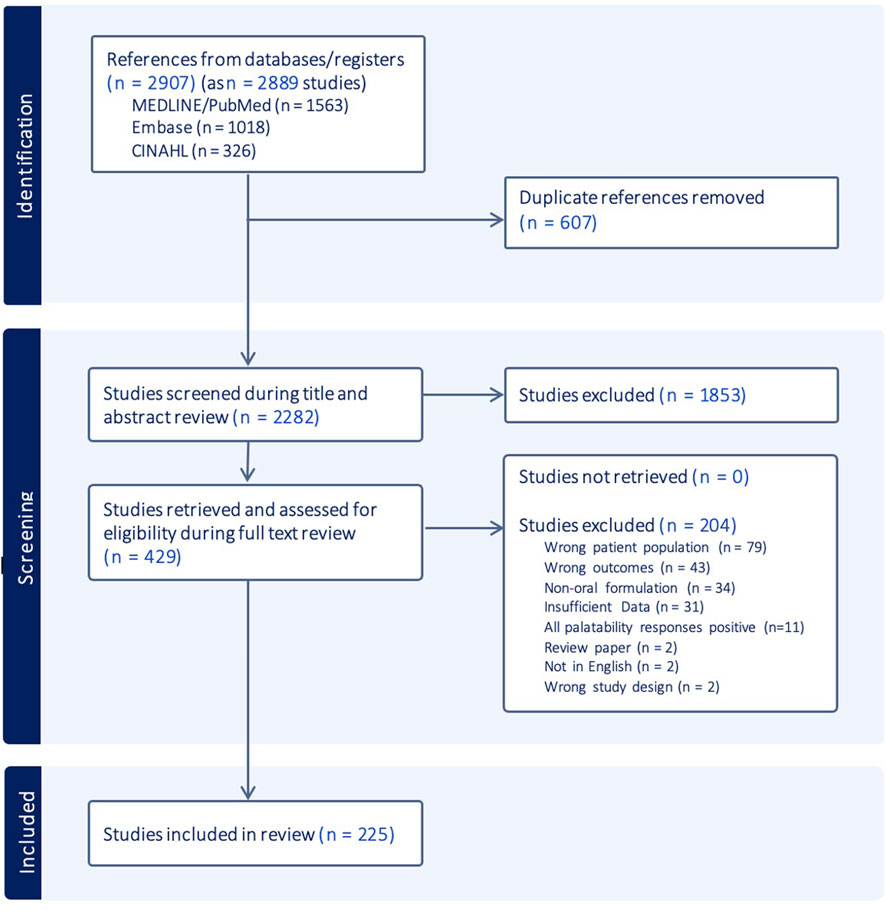
Figure 2. PRISMA flow diagram mapping information flow through the different phases of the scoping review.
3.2 Study characteristics
The characteristics of the 225 studies are summarized in Figure 3, with a complete table of results included in Supplementary Table S6. Studies were mainly journal articles (86%) and published between 1983 and 2023; however, a sharp increase in publications was observed in the last two decades coinciding with the introduction of pediatric legislative changes. A range of study designs were seen including randomized controlled trials (38%) and observational cross-sectional studies (25%). Studies were conducted across all geographical regions, although over half (62%) were conducted exclusively in high income countries in North America and Europe & Central Asia. Around a third (30%) included low- and middle-income countries (LMICs). All pediatric age groups except pre-term newborn infants were included in the studies. Over 70% included pre-school or school children, and over 50% included adolescents.
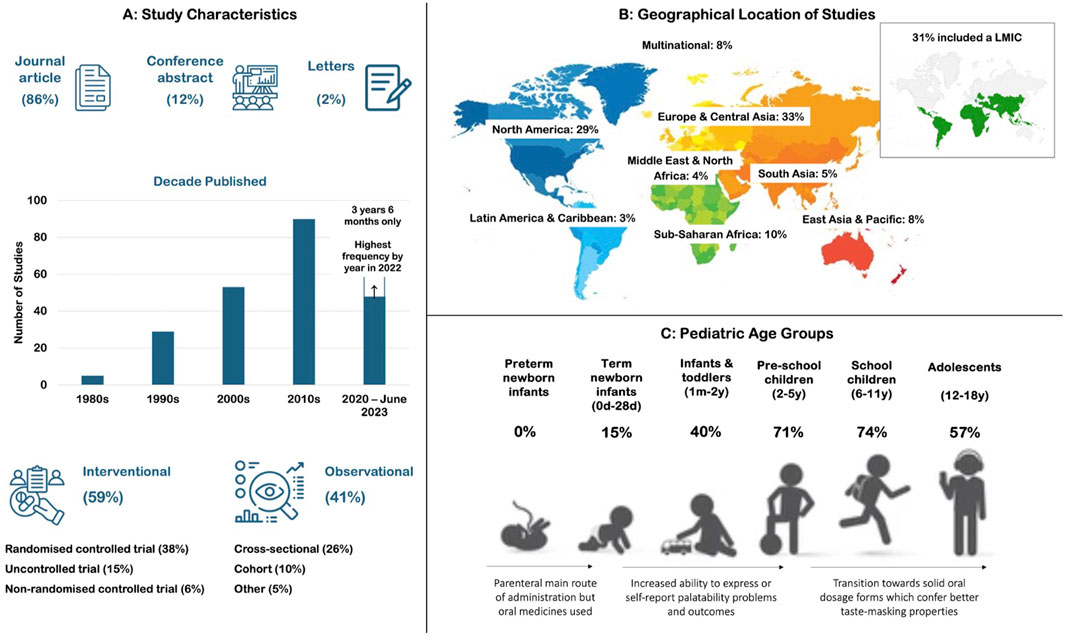
Figure 3. Characteristics of the 225 studies included in the review including (A) publication type, year, and study design; (B) geographical location and economic status; and (C) percentage of studies including each pediatric subset in the participant age range.
Of the studies reviewed, 47% reported palatability issues informally and linked them to outcomes, while 21% formally captured palatability issues and associated them with outcomes. Evaluation of medication palatability or acceptability was the primary objective in 15% of studies, typically for antibiotic drug products. In 5 studies, bitter or bad taste was listed as an AE but deemed to be a sensory characteristic of the API and not a systemic effect. Another 29% of studies formally captured palatability issues, but 21% did not establish a link with outcomes while 8% (n = 19) were thought to have a potential link. The remaining 2% focused solely on the general experiences of participants.
Data was captured from pediatric patients, healthy children, caregivers, and HCPs. A range of methodologies were used to formally capture palatability assessments, including rating products on scales, such as facial hedonic scales (usually with 3-, 5- or 7-point), continuous visual analogue scales (VAS) (10 cm/100 mm), and modified scales using a combination of facial hedonic and VAS. Other approaches included Likert scales, or observations of facial expressions, responses, or observed behavior following administration.
3.3 Overall disease area and drug classifications
The impact of poor tasting medicines was observed across 77 different diseases or indications (Figure 4A). Overall, 156 different medicinal products were reported across 84% (190/225) of the reviewed articles (Figure 4B). Among these products, 93% were APIs with an ATC classification, while the remaining 7% included other unclassified interventional products used for treatment or diagnosis (e.g., herbal medicines, amino acids used in metabolic deficiency states, and products for diagnostic imaging). Poor tasting medicines were found in 12 of the 14 WHO ATC groups, highlighting that the unpleasant taste of medicines is a widespread issue across a broad range of medicinal products. The two ATC groups not covered were dermatologicals (topical preparations) and sensory organs (ophthalmic preparations). A complete list of the categorized diseases and medicinal products, along with their frequencies is included in Supplementary Tables S7, S8.
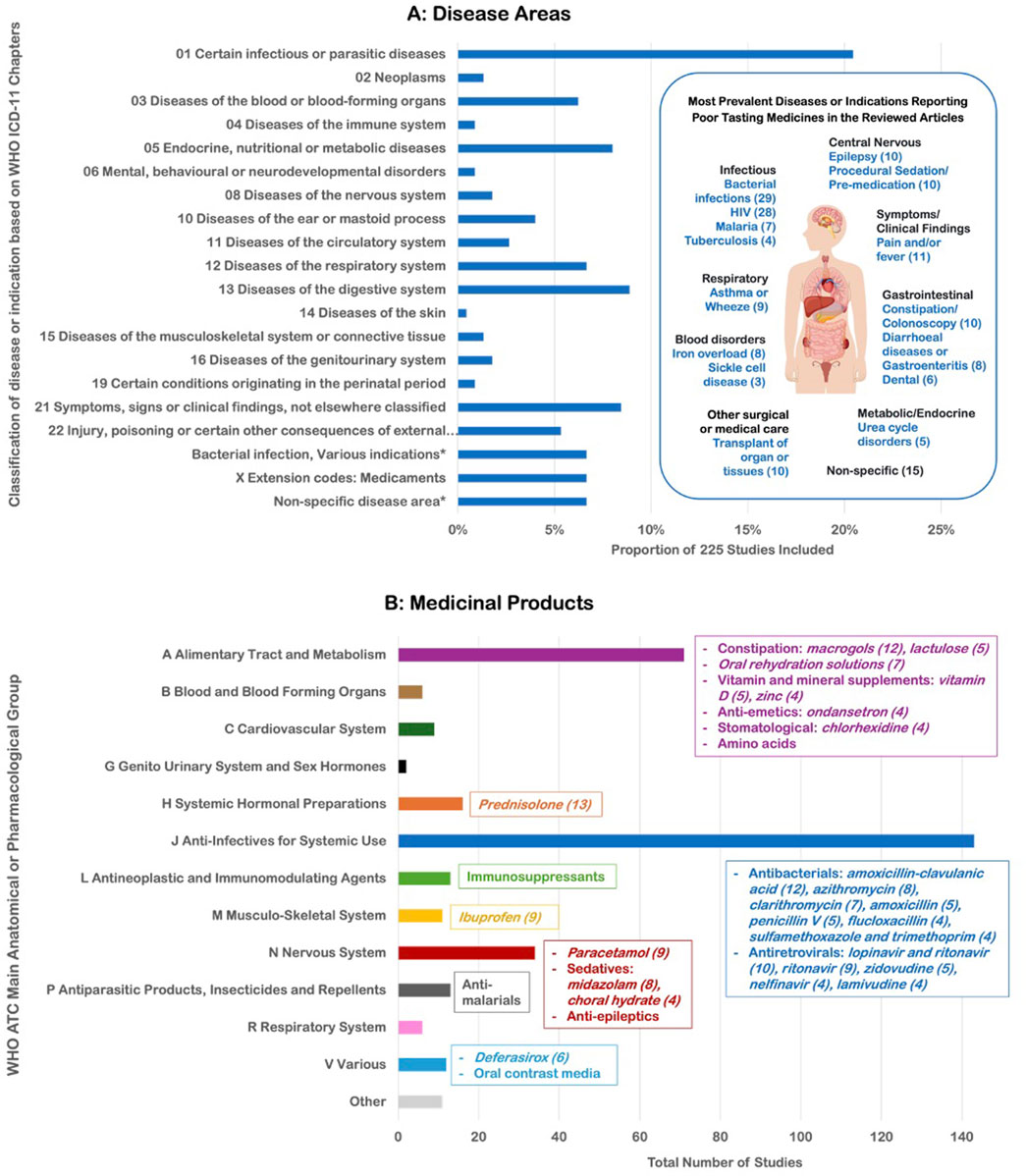
Figure 4. (A) Classification of the 77 different diseases or indications identified in the articles by WHO ICD-11 chapter and (B) Classification of the 156 different medicinal products reported by WHO ATC main anatomical/pharmacological group.
The largest proportion of conditions were notably in the infectious and parasitic diseases (20%), with studies in bacterial infections and human immunodeficiency virus (HIV) predominating. Twenty different antiviral drugs for HIV were identified as poor tasting, with lopinavir/ritonavir, ritonavir, and zidovudine the most frequently reported. Twenty-five different APIs were found across 29 studies for indications related to bacterial infections. Where palatability was formally reported (e.g., self-reported on scales), negative scores were reported for several antibiotics including amoxicillin-clavulanic acid (with 33%–61% of participants rating the taste negatively) (Block et al., 2006; Khurana, 1996; Powers et al., 2000; Steele et al., 2002), clarithromycin (36%–50%) (Manford Gooch et al., 1999; Powers, 1996; Steele et al., 2002), cefuroxime (52%–56%), sulfamethoxazole and trimethoprim (26%) (Dagan et al., 1994) and cloxacillin (mean score of 1.4 on a 10 cm VAS) (Matsui et al., 1996). Palatability reports sometimes differed between children and their caregivers. In a study of phenoxymethylpenicillin formulations, there was a weak correlation between children’s taste ratings and parents’ assessments of acceptability (Ansah et al., 2001), with some children rating the taste negatively while parents reported the medication was easily accepted at home, and vice versa (Bagger-Sjoback and Bondesson, 1989). In other studies, 95.7% of community pharmacists reported poor palatability of oral liquid antibiotics, especially for clarithromycin (31.5%) and flucloxacillin (28.8%) (Elgammal et al., 2022), while prescribers reported palatability issues for flucloxacillin and phenoxymethylpenicillin (Greenwood and Vallabhaneni, 2015). Over a third of caregivers reported more administration difficulties with flucloxacillin than other medicines due to taste (Batchelor et al., 2016).
The second most prevalent ICD-11 chapter encompassed symptoms or clinical findings, notably pain and fever, with the most frequent reports for ibuprofen and paracetamol, respectively. Diseases of the digestive system included constipation, where 12 studies reported poor palatability of macrogols. Prednisolone (most often in studies for asthma, wheeze or croup), oral rehydration salts (for dehydration in diarrhea or burn injury), and midazolam (for pre-procedural sedation or premedication) were the other APIs with the largest frequency of reports for poor taste.
A range of different oral dosage form types were seen across the studies. Liquid formulations included solutions, suspensions, elixirs, slurries, oral rinses, drops, sublingual sprays and topical varnishes (for dental use). Solid oral dosage form types included tablets, capsules, mini-tablets, powders, granules, chewables, dispersible and oro-dispersible preparations and lozenges. During the study screening and selection phase, some ineligible studies were found to report bitter taste sensations being perceived with non-oral dosage forms including nasal drops, eye drops, ear drops, and inhalers (Ahonen et al., 2004; Lenhard et al., 1997; Agro et al., 1998; Naimi et al., 2009); likely due to the anatomical connections between the ear, nose, and throat.
3.4 Impact of medication taste on patient acceptability
The impact of poor taste was most frequently seen in relation to patient acceptability, with 64% of the reviewed articles reporting rejection responses, strategies to aid administration, or effects on prescribing practices. The most frequent types of outcomes are shown in Table 1. Overall, medications used to treat HIV and bacterial infections were most commonly associated with acceptability and administration challenges. Many studies involving administration of prednisolone and midazolam also reported similar issues. Almost a quarter of studies reported incidences of children rejecting or resisting medication, with avoidance behaviors such as crying, covering the mouth, expressing discontent also described. Caregivers resorted to various approaches to overcome these challenges, ranging from positive reinforcement and rewards, to threats, physical restraint, and forced administration. These negative interventions were seen for the treatment of acute infections (Khurana, 1996) and chronic conditions such as HIV (Coetzee et al., 2015), malaria (Ewing et al., 2015), tuberculosis (Wademan et al., 2019), and metabolic disorders (Yeowell et al., 2021).
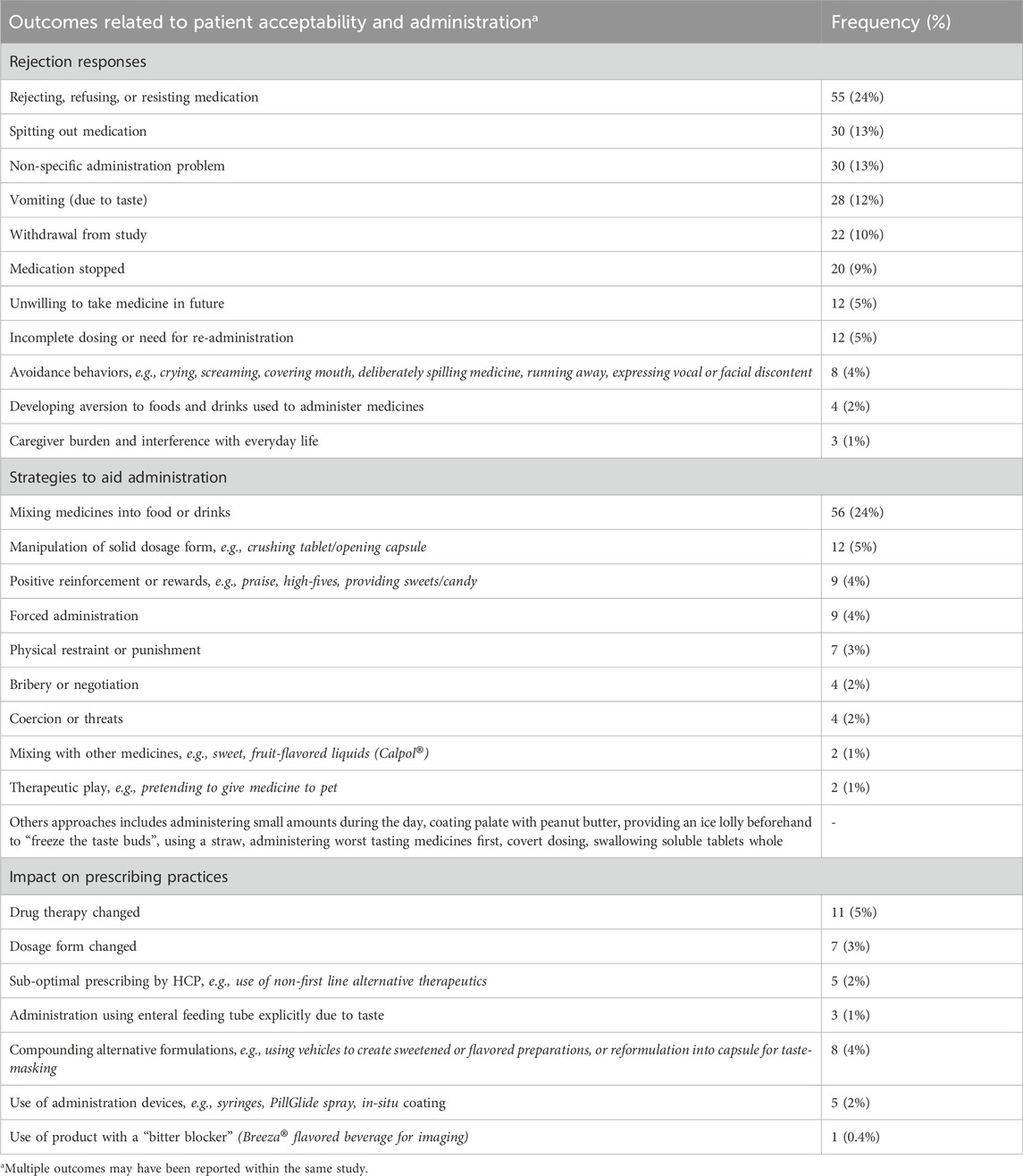
Table 1. Studies reporting outcomes related patient acceptability including rejection responses, administration strategies, and prescribing practices.
The number of subjects withdrawing from studies ranged from single participants to 20% of subjects in a study with the antiretroviral darunavir/cobicistat (Dalton et al., 2019), and 42% in a study with the anti-emetic, metopimazine (Nathan et al., 2006). Taste problems also led to withdrawals over long periods of time, with 21% of patients discontinuing cholestyramine drug therapy within 1 month, and 65% within 3 years; the authors stated that the majority who discontinued the drug complained of foul taste or had difficulty with its ingestion (Liacouras et al., 1993). In 24% of studies, medicines were mixed with foods or drinks to help mitigate taste-related issues. However, when this approach was used with lopinavir/ritonavir (Giralt et al., 2019; Kekitiinwa et al., 2016), naltrexone (Kolmen et al., 1995), and magnesium salts (Mettey et al., 1990), an unintended consequence was the development of aversion to the food in which the medication was mixed. In Uganda, caregivers reported concerns about need to sweeten food with sugar or honey which can be expensive in LMIC settings (Kekitiinwa et al., 2016).
Poor tasting medicines were also found to be a barrier to optimal medical management, with prescribers reporting the need to change drug therapies or use non-first line alternative therapeutics (Bergene et al., 2019; Goode et al., 2003; Jamieson et al., 2021; Lin et al., 2011; Porteous et al., 1997). Lin et al. (2011) identified 15 antiretrovirals with poor taste, which required the need for alternative prescribing (drug change or non-first line therapy) due to patient refusal. Other approaches to overcome taste problems included use of commercial products to create a physical barrier between medications and taste buds in the mouth [e.g., PillGlide (Gaskell, 2016) and in situ coatings (El Edelbi et al., 2015)] and using products with excipients that are marketed to have a “bitter blocking” effect [e.g., Breeza® (Dillman et al., 2018)].
3.5 Impact of medication taste on adherence and outcomes
Medication adherence impacts were reported in 27% (60/225) of the reviewed studies. Of these, 38 identified drug taste as a barrier to adherence, typically in chronic conditions such as organ or tissue transplant (Bullington et al., 2007; Claes et al., 2014; Pai et al., 2023; Phipps and DeCuir-Whalley, 1990; Varnell et al., 2017; Varnell et al., 2021; Wijesekera et al., 2019; Zelikovsky et al., 2011), HIV (Buchanan et al., 2012; Goode et al., 2003; Kiruki et al., 2018; Paranthaman et al., 2009; Purchase et al., 2016; Reddington et al., 2000; Tran et al., 2017), epilepsy (Gutierrez-Colina et al., 2018; Williford et al., 2023), cystic fibrosis (Christiansen et al., 2014), and inflammatory bowel diseases (Greenley et al., 2018). In a study where adherence was formally evaluated, the taste of phenoxymethylpenicillin was positively but weakly correlated with adherence, including taking 100% of the medication (coefficient 0.184, p < 0.01) and taking it at the correct time (coefficient 0.141, p < 0.01) (Cifaldi et al., 2004). Weak correlations between adherence (measured by medication return) and taste scores were also found for cefdinir and amoxicillin-clavulanic acid, with coefficients ranging from 0.1 to 0.2 (Bagger-Sjoback and Bondesson, 1989). In a study by Angwa et al. (2020), objective measures of adherence and dose accuracy were significantly better for amoxicillin dispersible tablets (DT) compared to the oral suspension (OS) (p < 0.01), with more caregivers reporting the DT as tasting better than the OS (51.5% DT vs. 38.5% OS). In another study covering various antibiotics, 21.1% of patients who did not complete their course discontinued due to poor palatability (Patel et al., 2022). In the treatment of acute childhood diarrhoea with dispersible zinc tablets, 55.8% of patients completed the 10-day treatment as advised, and those who rated the taste worse than other medicines were 51% less likely to complete the full course (RR 0.51, 95% CI 0.40–0.66) (Nasrin et al., 2005).
Only 3.5% of studies were identified where unpalatable medicines resulting in poor medication adherence had a reported impact on treatment outcomes. Two studies in HIV used the Pediatric AIDS Clinical Trials Group (PACTG) adherence module, which explicitly includes a question about taste as a drug specific reason for non-adherence (Davies et al., 2008; Van Dyke et al., 2002). Davies et al. (2008) found that taste was the most common barrier to adherence reported by over 20% of respondents, with 21% of participants being non-adherent, defined as an annual medication return <90%. Among adherent children, 78% had an undetectable viral load, compared to 25% of non-adherent children, such that adherent children were over 10 times more likely to achieve viral suppression (OR 10.3 95% CI: 1.92–55.7; p = 0.005) (Davies et al., 2008). Non-adherence was linked to the poor taste of ritonavir. Another study using the same approach found that 30% of children were non-adherent, with 5% missing all doses (Van Dyke et al., 2002). Among those that were non-adherent, 44% had an undetectable RNA viral load compared to 64% who were adherent (p = 0.07). Non-adherence was associated with the taste of ritonavir and nelfinavir. In a chronic hepatitis C virus (HCV) infection study, sustained viral suppression was not achieved in a 4-year-old patient who discontinued treatment due to the poor taste of sofosbuvir (Rosenthal et al., 2020). The drug, formulated as granules in a capsule, was intended to be sprinkled on a spoonful of non-acidic soft food; however, acidic food was mistakenly used, likely causing the granule coating to break and leading to the unpleasant taste of the drug being perceived.
In epilepsy, changes to more palatable formulations of valproic acid were associated with an improved pharmacokinetic profile, positive changes in the EEG trace, and reduction in frequency of epileptic seizures (Cloyd et al., 1992; Kiryluk and Sobaniec, 2013). Gutierrez-Colina et al. (2018) captured taste difficulties within the Pediatric Epilepsy Medication Self-Management Questionnaire (PEMSQ). For children aged 2–5 years, medication taste was the most significant predictor of seizure control and health-related quality of life (HRQOL), while in adolescents aged 13–17 years, disliking the taste of medication was the most important predictor of non-adherence, and second most important predictor of seize control and HRQOL (Gutierrez-Colina et al., 2018). In a study of urea cycle disorder patients, the existing sodium phenylbutyrate (NaPB) product was deemed unacceptable by all 25 patients, with 4 finding it impossible to take. Two patients required NaPB to be reformulated into capsules, 4 needed it to be administered via a nasogastric tube (NGT), and 1 required gastrostomy administration. After switching to a new coated oral pellet formulation (Pheburane) acceptability scores improved, and in 10 patients, the number of hyperammonemia episodes dropped from 20 over 6 months on NaPB to zero over 3–11 months of treatment with Pheburane (Kibleur et al., 2014). The authors observed an inverse relationship between acceptability and bitterness, though this correlation was not formally tested. Unachak et al. (2002) described a case report in neonatal hypomagnesaemia, where poor adherence to magnesium sulfate due to its bitter taste, led to persistent hypomagnesemia, hypocalcemia, and convulsions. Changing to magnesium oxide led to improved compliance and resolution of hypomagnesemia though intermittent symptoms, such as convulsions reappeared due to poor regular adherence with the treatment.
3.6 Other findings related to medication taste
Taste was found to influence both beliefs and treatment preferences in several studies. Over a quarter (26.4%) of Indonesian children aged 10–14 years believed that the taste of a medicine, whether sweet or bitter, impacted its efficacy (Syofyan et al., 2019) while caregivers in Uganda associated bitter taste with the authenticity of medicines, with one participant remarking, “if it wasn’t bitter, it would be fake” (Rutebemberwa et al., 2009). In a discrete choice experiment on pediatric tuberculosis preventive treatment preferences, taste was the most significant factor influencing choice among children, caregivers, and healthcare providers in Eswatini (Hirsch-Moverman et al., 2021). Participants were more than three times likely to choose an alternative treatment if the treatment option presented was described as bitter (OR = 3.51, 95% CI: 2.81–4.38), with this preference for non-bitter formulations outweighing other factors such as treatment duration, dose frequency, pill size, and cost (Hirsch-Moverman et al., 2021).
4 Discussion
4.1 Summary of evidence
This scoping review has highlighted the significant adverse impact of poor-tasting pediatric medicines on patient and stakeholder experiences. The strongest evidence linked poor taste to challenges with medication administration and acceptability, followed by reports that it is a notable barrier to medication adherence, and to a lesser extent, the resulting negative impact on treatment outcomes. The issue of unpleasant drug taste affects a wide range of disease areas and APIs, spanning over 70 different acute and chronic indications and more than 150 treatment products. Poor palatability was experienced with a range of solid and liquid oral dosage forms, and extended to other modes of administration that lead to drug exposure in the ear, nose, and throat. This problem is prevalent globally, impacting almost the entire pediatric population, from newborns to adolescents. The negative effects of poor-tasting medicines have been reported by children, their parents or caregivers, as well as a variety of HCPs, including doctors, nurses, and pharmacists. These issues have been observed in both experimental studies and real-world settings.
4.2 Impact of poor-tasting medicines in pediatric care
Poor-tasting medicines identified in this review include several of the most commonly prescribed treatments for pediatric patients. Analysis of global pharmacy sales data from 75 countries (including 49% LMICs) for 47 oral medicines on the WHO Essential Medicines List for Children (EMLc) revealed the top 10 selling medicines (Tsai et al., 2022). The data focused on child-appropriate formulations, defined as liquid syrups, granules, powders/dry syrups, and dispersible tablets with a standard unit (SU) representing a single dose (one tablet, capsule, ampoule, vial, or 5 mL of liquid). Paracetamol topped the list with 31.1% of total sales, amounting to 11,090 million standard units (SU), followed by amoxicillin (11.8%, 4,206 million SU), ibuprofen (10.8%, 3,870 million SU), and amoxicillin-clavulanic acid (9.9%, 3,549 million SU). Other top-selling medicines included prednisolone (8.4%), cefixime (5.2%), valproic acid (3.0%), sulfamethoxazole-trimethoprim (2.5%), lamotrigine (2.3%), and azithromycin (2.1%). Nine of these top ten medicines were identified in this review, with the exception of lamotrigine. The failure to administer poor-tasting pediatric medicines results in billions of missed doses each year. In a UK study by Mistry et al. (Mistry et al. 2018), researchers observed that refusal, spitting out, or vomiting of liquid medicines occurred most frequently with the top 5 highest selling medicines: in 1% of those taking paracetamol, 13% taking amoxicillin, 2% taking ibuprofen, 6% taking amoxicillin-clavulanic acid, and 9% taking prednisolone. Extrapolating these percentages to the global sales data (Tsai et al., 2022), the number of missed doses globally could reach over 110 million for paracetamol, 546 million for amoxicillin, 77 million for ibuprofen, 212 million for amoxicillin-clavulanic acid, and 268 million for prednisolone.
The issue of poor taste has been recognized in several other studies. In a large survey of nearly 700 European children, the most common reason for difficulty in taking medicines was a dislike of the taste, as reported by 63.7% of respondents (Nordenmalm et al., 2019). Taste issues were reported with 35% (188/542) of all prescribed oral formulations in cross-sectional UK study, with children experiencing a palatability issue to be almost four times more likely to refuse taking the medication compared to those who did not (Venables et al., 2015a). At least 50% of children prescribed ranitidine, prednisolone, trimethoprim, lactulose, macrogol, co-trimoxazole, sodium valproate, levetiracetam, phenoxymethylpenicillin, and ibuprofen reported experiencing taste-related issues, further highlighting the widespread and pervasive nature of this problem (Venables et al., 2015a).
Medicines for the treatment of bacterial infections and HIV were frequently reported to have taste issues, which were linked to adherence challenges and treatment outcomes. In a global survey of HCPs, oral liquid forms of lopinavir/ritonavir, amoxicillin and clavulanic acid, and cefuroxime were most frequently reported as problematic, including for acceptability and palatability issues (Barbieri et al., 2023). Infections remain one of the leading worldwide causes of mortality in children under five (Perin et al., 2022). A narrative review by Baguley et al. (Baguley et al., 2012) discussed how palatability can impact adherence to antibiotics, highlighting flucloxacillin suspension as a particularly challenge drug, consistent with the findings in this review. The authors emphasized the need for doctors to be more aware of the importance of taste when prescribing medications for pediatric patients. Indeed, in a study where HCPs assessed the palatability of twenty-four liquid anti-infectives, participants experienced firsthand how certain formulations were more palatable than others, and many subsequently adjusted their prescribing or counselling practices (Gee and Hagemann, 2007). Failure to complete antibiotic treatment or not taking them as prescribed can lead to prolonged illness, increased risk of complications, and contribute to wider public health threats, including the spread of infectious diseases.
In 2023, an estimated 1.4 million children (0–14 years old) were living with HIV; only around 48% of children living with HIV had suppressed viral loads and there were 76,000 deaths from HIV-related causes in this population (World Health Organization, 2024). The poor taste of ritonavir (alone and in combination with lopinavir) was reported in nineteen studies in the review. These drugs have shown to activate the TAS2R bitter receptors which mediate the perception of this taste sensation in the mouth (Chen et al., 2023). Perceiving the bitter taste of drugs is a key factor contributing to poor palatability. In the accompanying article in this series, between 18%–60% of caregivers reported that their child always or regularly refused medication due to bitter taste (El-Sahn et al., 2025), while over 80% of healthcare providers agreed that bitter taste impacts adherence to both short-term and long-term medications.
Acceptability and adherence are complex, multidimensional phenomena that can be impacted by a myriad of factors. WHO describes five interacting dimensions that can affect adherence: therapy–related factors; patient–related factors; condition–related factors; social and economic factors; and healthcare team and system-related factors (World Health Organization, 2003). These dynamics can also influence patient acceptability of medicines. Caregiver-related factors can also be considered another important dimension in pediatrics, since parents and carers play an important role in medicines administration in this population. A range of administration difficulties and associated coping mechanisms used by caregivers were identified in the review, varying from supportive to detrimental. This highlights the significant burden and psycho-social impact these challenges pose for pediatric patients and their families. Similar to the findings of this review, a qualitative study by Bergene et al. (2017) categorized three strategies parents use to administer oral medicine to children: open administration (involving the child or altering the taste), hidden administration (camouflaging in food or distractions), and forced administration (using restraint), with varying parental attitudes towards the use of force. The use of strategies such as masking the bitter taste of medicine with food or drink, giving rewards or the use of force were reported by caregivers in Sub-Saharan Africa and the United States in the second part of this series (El-Sahn et al., 2025). This emphasizes the need for targeted supportive measures to alleviate these difficulties for all stakeholders.
4.3 Regulatory landscape and equitable global access
The U.S. and EU introduced legislative and regulatory reforms over a decade ago to incentivize and mandate the development of pediatric medicines (Penkov et al., 2017). These advancements have shown a positive impact on the authorization of new medicinal products and pharmaceutical formulations; however, efforts to develop off-patent medicines have been notably less successful, and universal access to these new medicines is not assured (European Medicines Agency, 2017). A limitation of this review was the inability to analyze poor taste at the product-specific level. Notably, other reviews have highlighted that significant variations in palatability exist between different formulations of the same API, particularly when comparing originator versus generic products (Tuleu et al., 2021). There is no regulatory requirement for palatability testing of generic drugs, leading to potential issues with unpalatable formulations entering the market. The findings of this review are important, given the unequal access to various medicinal products worldwide. There remains a critical global need for age-appropriate and accessible medicines, especially in LMICs (Barbieri et al., 2023; Chen et al., 2021). This is pertinent as the proportion of the pediatric population rises in the least developed countries, representing 48% of sub-Saharan Africa versus 21% in North America, and Europe/Central Asia (United Nations, 2022).
The current review was based on an initial screen of titles and abstracts due to the large volume of articles identified, which may have led to the omission of other relevant studies where these outcomes were not considered the main findings. However, despite the widespread impact of poor tasting medicines found in this review, the prevalence of this issue is likely underreported due to several factors. Historically, the taste or palatability of medicines has not been evaluated as a primary or even secondary outcome in clinical trials. Limited data exists on the direct impact of poor taste on patient outcomes, not due to an absence of effect, but because this problem has not been systematically captured or analyzed in research studies to date. Studies on medication adherence are often disease specific and typically focus on patients’ beliefs, knowledge, and behaviors in relation to medicines use (Nguyen et al., 2014). Kardas et al. identified over 700 individual factor items associated with non-adherence to chronic therapies in patients of all ages, with only 3/51 reviews in their analysis mentioning poor taste of medication as a therapy-related factor affecting adherence (Kardas et al., 2013). Incorporating medication taste as a variable in adherence questionnaires and assessment tools is essential to help systematically evaluate its impact on medication adherence. Therapeutic outcomes are typically considered in relation to clinical pharmacology and drug efficacy and even less likely analyzed in relation to medication taste. Notably, regulatory agencies now require the evaluation of patient acceptability as an integral part of the pharmaceutical and clinical development of pediatric medicines, a shift that is expected to help address these evidence gaps and further elucidate the impact of palatability on outcomes (European Medicines Agency, 2013; Food and Drug Administration, 2018).
4.4 Taste masking pediatric formulations
The palatability of a medicines is determined by the characteristics of the API, the excipients used in the formulation, and the finished medicinal product (European Medicines Agency, 2013). Existing reviews provide a detailed exploration of taste-masking strategies and technologies (Hu et al., 2023; Sanjay et al., 2025). These approaches typically involve either blocking taste transmission pathways or preventing drug release in the oral cavity. Examples range from the inclusion of sweeteners and flavors or coating solid oral dosage forms with polymer barriers, to more advanced techniques such as microencapsulation, nanotechnology, ion-exchange resin complexation, and use of bitterness inhibitors. When selecting taste-masking strategies, the goal is to enhance palatability without compromising other critical aspects of the drug product, such as stability, bioavailability, and safety.
The scoping review identified examples where modifications to formulation factors improved the palatability and acceptability of pediatric medicines. A film-coated tablet formulation of deferasirox improved palatability compared to the dispersible tablet by eliminating taste and aftertaste issues (Taher et al., 2018). In this clinical study, patient-reported outcomes were captured using specifically designed questionnaires assessing palatability and satisfaction. A 4-in-1 fixed-dose combination of abacavir/lamivudine/lopinavir/ritonavir was specifically designed for infants and young children with HIV; this strawberry flavored granule in capsule formulation can be taken with liquids or semi-solid food (Rotsaert et al., 2023). In a qualitative study, caregivers generally reported that the formulation had an appealing taste compared with previously used formulations; though, reports of spitting attributable to the new taste were nevertheless also reported (Rotsaert et al., 2023). These recently published studies demonstrate the importance of considering taste as a critical factor when formulating pediatric medicines and the benefits of evaluating this aspect with patients and caregivers. Further robust studies are needed to close the evidence gap, provide insights into the appropriate methods to capture palatability, and confirm that better-tasting medicines are indeed benefiting patients.
4.5 Other limitations
Some limitations of this review have been addressed, including the inability to analyze poor taste of medicines at the product-specific level, and the potential exclusion of relevant articles during the title and abstract screening. Assessment of methodological limitations or risk of bias of the evidence was not conducted as part of the appraisal process, though this acceptable within the remit of a scoping review. The review did not assess the significance of the number of participants reporting taste-related issues. Instead, any mention of palatability or taste issues, including single instances, was included, limiting the ability to evaluate the degree of taste problems. Current regulatory guidelines do not specify a threshold for the proportion of individuals who must find a medicine palatable or acceptable, which restricts the ability to make meaningful comparisons regarding the extent of taste-related challenges across different medications.
4.6 Conclusion and future directions
This scoping review highlights the significant impact of poor-tasting pediatric medicines on medication acceptability, adherence, and ultimately treatment outcomes. Despite the recognition of this challenge, the current body of evidence remains incomplete with regards to the true extent of the problem.
There is a need for more targeted research on palatability and more comprehensive guidance for its evaluation of both new and generic pediatric formulations. Regulatory requirements are playing a pivotal role in driving improvements in palatability assessments; however, the current absence of mandatory assessments for generic formulations creates a gap in ensuring that these medicines meet the same acceptability standards as branded counterparts. Bridging this gap will also support more equitable access to better formulations across different populations, especially in LMICs.
The economic burden of medication wastage due to poor taste is substantial, with millions of missed doses annually. Addressing taste-related challenges could thus have significant cost-saving implications. Raising awareness among HCPs about the impact of medication taste is essential, as these stakeholders are in a key position to engage patients and caregivers and offer education, support, and effective strategies to help overcome administration challenges. Incorporating taste and formulation-related factors into wider research areas, including adherence measures and longitudinal studies, will provide a deeper understanding of the long-term effects of poor-tasting medicines on children’s health and development. This research could also explore broader impacts, such as how medication challenges may influence social behaviors related to healthcare.
Research and development into both existing and innovative taste-masking strategies is critical to help resolve this problem with poor-tasting medicines. The concept of a taste-blocker product is explored in the accompanying article in this series (El-Sahn et al., 2025), as a universal solution that could support the administration of different medications, and be helpful even when taste problems occur in only a few patients. Additionally, developing formulation technologies that allow for more widespread application of taste-masking techniques, without compromising product stability or therapeutic efficacy, would be a significant step forward. Addressing the problem of poor taste is critical to improving the overall experience of taking medicines for children and their caregivers worldwide.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
SRR: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Visualization, Writing–original draft, Writing–review and editing, Validation. JW: Conceptualization, Data curation, Investigation, Methodology, Writing–review and editing, Project administration, Validation. CT: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing–review and editing, Validation.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported in whole by the Bill and Melinda Gates Foundation [Grant Number: INV-054803]. Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission.
Acknowledgments
The authors thank Niya Bowers, Senior Program Officer at the Bill & Melinda Gates Foundation, for her support with this review. We also acknowledge Richard Blackwood, Kim Dozier, and all other collaborators and consultants involved in the wider project for their valuable insights and discussions which contributed to the development of this work.
Conflict of interest
Author SRR was employed by Sciense Ltd. Author JW was employed by Jenny Walsh Consulting Ltd.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fddev.2025.1553286/full#supplementary-material
References
Abdulla, S., Amuri, B., Kabanywanyi, A. M., Ubben, D., Reynolds, C., Pascoe, S., et al. (2010). Early clinical development of artemether-lumefantrine dispersible tablet: palatability of three flavours and bioavailability in healthy subjects. Malar. J. 9 (1), 253. doi:10.1186/1475-2875-9-253
Abu-Khalaf, N., Zaid, A. N., Jaradat, N., AlKilany, A., Abu Rumaila, B., Al Ramahi, R., et al. (2018). The taste of commercially available clarithromycin oral pharmaceutical suspensions in the Palestinian market: electronic tongue and in vivo evaluation. Sensors (Basel) 18 (2), 454. doi:10.3390/s18020454
Adams, L. V., Craig, S. R., Mmbaga, E. J., Naburi, H., Lahey, T., Nutt, C. T., et al. (2013). Children's medicines in Tanzania: a national survey of administration practices and preferences. PLoS ONE 8 (3), e58303. doi:10.1371/journal.pone.0058303
Agro, A. S., Garner, E. T., Wright, J. W., Caballeros de Escobar, I., Villeda, B., and Seidlin, M. (1998). Clinical trial of ototopical ofloxacin for treatment of chronic suppurative otitis media. Clin. Ther. 20 (4), 744–759. doi:10.1016/s0149-2918(98)80137-9
Ahonen, K., Hämäläinen, M. L., Rantala, H., and Hoppu, K. (2004). Nasal sumatriptan is effective in treatment of migraine attacks in children: a randomized trial. Neurology 62 (6), 883–887. doi:10.1212/01.wnl.0000115105.05966.a7
Al-Ani, I. H., Hassan, S. F., Shalan, N. M., Maraqa, A. D., Oriquat, G. A., et al. (2016). Preparation of ibuprofen as pediatric candies. J. Pharm. Sci. Res. 8 (1), 29–34.
Aljebab, F., Alanazi, M., Choonara, I., and Conroy, S. (2018). Observational study on the palatability and tolerability of oral prednisolone and oral dexamethasone in children in Saudi Arabia and the UK. Archives Dis. Child. 103, 83–88. doi:10.1136/archdischild-2017-312697
Allen, U. D., Lapointe, N., Read, S. E., Forbes, J. C., King, S. M., Wasfy, S., et al. (2003). Response to a protease-inhibitor (ritonavir)-containing combination antiretroviral regimen in HIV-infected children. Can. J. Infect. Dis. 14 (2), 89–93. doi:10.1155/2003/891968
Almenrader, N., Passariello, M., Coccetti, B., Haiberger, R., and Pietropaoli, P. (2007). Premedication in children: a comparison of oral midazolam and oral clonidine. Paediatr. Anaesth. 17 (12), 1143–1149. doi:10.1111/j.1460-9592.2007.02332.x
Ameen, V. Z., Pobiner, B. F., Giguere, G. C., and Carter, E. G. (2006). Ranitidine (Zantac) syrup versus ranitidine effervescent tablets (Zantac EFFERdose) in children: a single-center taste preference study. Pediatr. Drugs 8 (4), 265–270. doi:10.2165/00148581-200608040-00005
Angelilli, M. L., Toscani, M., Matsui, D. M., and Rieder, M. J. (2000). Palatability of oral antibiotics among children in an urban primary care center. Arch. Pediatr. Adolesc. Med. 154 (3), 267–270. doi:10.1001/archpedi.154.3.267
Angwa, L. M., Ouma, C., Okoth, P., Nyamai, R., Kamau, N. G., Mutai, K., et al. (2020). Acceptability, adherence, and clinical outcomes, of amoxicillin dispersible tablets versus oral suspension in treatment of children aged 2-59 Months with pneumonia, Kenya: a cluster randomized controlled trial. Heliyon 6 (4), e03786. doi:10.1016/j.heliyon.2020.e03786
Ansah, E. K., Gyapong, J. O., Agyepong, I. A., and Evans, D. B. (2001). Improving adherence to malaria treatment for children: the use of pre-packed chloroquine tablets vs. chloroquine syrup. Trop. Med. Int. Health 6 (7), 496–504. doi:10.1046/j.1365-3156.2001.00740.x
Bagger-Sjoback, D., and Bondesson, G. (1989). Taste evaluation and compliance of two paediatric formulations of phenoxymethylpenicillin in children. Scand. J. Prim. Health Care 7 (2), 87–92. doi:10.3109/02813438909088653
Baguley, D., Lim, E., Bevan, A., Pallet, A., and Faust, S. N. (2012). Prescribing for children - taste and palatability affect adherence to antibiotics: a review. Arch. Dis. Child. 97 (3), 293–297. doi:10.1136/archdischild-2011-300909
Baka-Ostrowska, M., Bolong, D. T., Persu, C., Tøndel, C., Steup, A., Lademacher, C., et al. (2021). Efficacy and safety of mirabegron in children and adolescents with neurogenic detrusor overactivity: an open-label, phase 3, dose-titration study. Neurourol. Urodynamics 40 (6), 1490–1499. doi:10.1002/nau.24657
Baker, S. S., Tesoriero, J., McGowan, J., and Lewis, J. D. (2021). S1281 A safety, efficacy and tolerance study of oral sulfate solution regimens to 4L PEG-ELS in pediatric subjects undergoing colonoscopy. Am. J. Gastroenterology 116 (Suppl. L), S588. doi:10.14309/01.ajg.0000778656.84313.dc
Barbieri, E., Minotti, C., Cavagnis, S., Giaquinto, C., Cappello, B., Penazzato, M., et al. (2023). Paediatric medicine issues and gaps from healthcare workers point of view: survey results and a narrative review from the global accelerator for paediatric formulations project. Front. Pharmacol. 14, 1200848. doi:10.3389/fphar.2023.1200848
Barnett, S., and Bhatt, A. (2020). A chewable pediatric preparation of ibuprofen is palatable and acceptable to children. Paediatr. Neonatal Pain 2 (1), 2–6. doi:10.1002/pne2.12013
Bartoli, F., Martínez, J. M., Ferrarini, A., Recaldini, E., and Bianchetti, M. G. (2006). Poor adherence to the prophylactic use of vitamin D3 in Switzerland. J. Pediatr. Endocrinol. Metabolism 19 (3), 281–282. doi:10.1515/jpem.2006.19.3.281
Batchelor, H., Rayner, O., Nickless, J., Wan, M., Southern, K., and Rose, C. (2016). Children with cystic fibrosis: understanding issues related to oral administration of liquid flucloxacillin. Archives Dis. Child. 101 (9), e2. doi:10.1136/archdischild-2016-311535.33
Berg, J., Riedy, C. A., and Tercero, A. (2006). Patient and parental perception of a new fluoride varnish. Compend. continuing Educ. Dent. (Jamesburg, N. J. 1995) 27 (11), 614–619.
Bergene, E. H., Holst, L., Rø, T. B., and Steinsbekk, A. (2019). Considering formulation characteristics when prescribing and dispensing medicinal products for children: a qualitative study among GPs and pharmacists. Fam. Pract. 36 (3), 351–356. doi:10.1093/fampra/cmy086
Bergene, E. H., Rø, T. B., and Steinsbekk, A. (2017). Strategies parents use to give children oral medicine: a qualitative study of online discussion forums. Scand. J. Prim. Health Care 35 (2), 221–228. doi:10.1080/02813432.2017.1333308
Bertholet-Thomas, A., Guittet, C., Manso-Silván, M. A., Castang, A., Baudouin, V., Cailliez, M., et al. (2021). Efficacy and safety of an innovative prolonged-release combination drug in patients with distal renal tubular acidosis: an open-label comparative trial versus standard of care treatments. Pediatr. Nephrol. 36 (1), 83–91. doi:10.1007/s00467-020-04693-2
Beuter, C., Volkers, G., Radic, T., Goldberg, J., and van den Anker, J. (2019). Efficacy and safety of multiple doses of tapentadol oral solution in the treatment of moderate to severe acute pain in children aged 2 to <18 years - a randomized, double-blind, placebo-controlled trial. J. Pain Res. 12, 3099–3112. doi:10.2147/JPR.S207010
Block, S. L., Cifaldi, M., and Gu, Y. (2005). A comparison of 5 days of therapy with cefdinir or azithromycin in children with acute otitis media: a multicenter, prospective, single-blind study. Clin. Ther. 27 (6), 786–794. doi:10.1016/j.clinthera.2005.06.012
Block, S. L., Schmier, J. K., Notario, G. F., Akinlade, B. K., Busman, T. A., Mackinnon, G. E., et al. (2006). Efficacy, tolerability, and parent reported outcomes for cefdinir vs. high-dose amoxicillin/clavulanate oral suspension for acute otitis media in young children. Curr. Med. Res. Opin. 22 (9), 1839–1847. doi:10.1185/030079906X132406
Braga, C. B. E., Martins, A. C., Cayotopa, A. D. E., Klein, W. W., Schlosser, A. R., da Silva, A. F., et al. (2015). Side effects of chloroquine and primaquine and symptom reduction in malaria endemic area (Mancio lima, Acre, Brazil). Interdiscip. Perspect. Infect. Dis. 2015, 346853. doi:10.1155/2015/346853
Breslin, P. A. (2013). An evolutionary perspective on food and human taste. Curr. Biol. 23 (9), R409–R418. doi:10.1016/j.cub.2013.04.010
Bryson, S. P. (2014). Patient-centred, administration friendly medicines for children - an evaluation of children's preferences and how they impact medication adherence. Int. J. Pharm. 469 (2), 257–259. doi:10.1016/j.ijpharm.2014.04.069
Buchanan, A. L., Montepiedra, G., Sirois, P. A., Kammerer, B., Garvie, P. A., Storm, D. S., et al. (2012). Barriers to medication adherence in HIV-infected children and youth based on self- and caregiver report. Pediatrics 129 (5), e1244–e1251. doi:10.1542/peds.2011-1740
Bullington, P., Pawola, L., Walker, R., Valenta, A., Briars, L., and John, E. (2007). Identification of medication non-adherence factors in adolescent transplant patients: the patient's viewpoint. Pediatr. Transplant. 11 (8), 914–921. doi:10.1111/j.1399-3046.2007.00765.x
Bunupuradah, T., Wannachai, S., Chuamchaitrakool, A., Intasan, J., Nuchapong, T., Neiss, W., et al. (2006). Use of taste-masking product, FLAVORx, to assist Thai children to ingest generic antiretrovirals. AIDS Res. Ther. 3 (1), 30. doi:10.1186/1742-6405-3-30
Caglayan, S., Acar, U., Kasirga, E., and Koloğlu, F. (1989). Diluted yogurt (ayran) versus water in dissolving oral rehydration salts. Turkish J. Pediatr. 31 (1), 25–27.
Cañete, R., Rivas, D. E., Escobedo, A. A., González, M. E., Almirall, P., and Brito, K. (2010). A randomized, controlled, open-label trial evaluating the efficacy and safety of chloroquine in the treatment of giardiasis in children. West Indian Med. J. 59 (6), 607–611.
Cattaneo, C., Mameli, C., D'Auria, E., Zuccotti, G., and Pagliarini, E. (2022). The influence of common noncommunicable diseases on chemosensory perception and clinical implications in children and adolescents. Adv. Nutr. 13 (1), 234–247. doi:10.1093/advances/nmab100
Celebi, S., Hacimustafaoglu, M., Aygun, D., Arisoy, E. S., Karali, Y., Akgoz, S., et al. (2009). Antipyretic effect of ketoprofen. Indian J. Pediatr. 76 (3), 287–291. doi:10.1007/s12098-008-0234-z
Chadwick, E. G., Rodman, J. H., Britto, P., Powell, C., Palumbo, P., Luzuriaga, K., et al. (2005). Ritonavir-based highly active antiretroviral therapy in human immunodeficiency virus type 1-infected infants younger than 24 months of age. Pediatr. Infect. Dis. J. 24 (9), 793–800. doi:10.1097/01.inf.0000177281.93658.df
Chen, S., Zhou, X., Lu, Y., Xu, K., Wen, J., and Cui, M. (2023). Anti-HIV drugs lopinavir/ritonavir activate bitter taste receptors. Chem. Senses 48, bjad035. doi:10.1093/chemse/bjad035
Chen, Z., Li, S., Zeng, L., Liu, Y., Zhang, M., Choonara, I., et al. (2021). Accessibility of medicines for children: a systematic review. Front. Pharmacol. 12, 691606. doi:10.3389/fphar.2021.691606
Cheng, K. K. F. (2004). Children's acceptance and tolerance of chlorhexidine and benzydamine oral rinses in the treatment of chemotherapy-induced oropharyngeal mucositis. Eur. J. Oncol. Nurs. 8 (4), 341–349. doi:10.1016/j.ejon.2004.04.002
Christiansen, N., Gohil, J., Lo, A., and Bishop, S. (2014). Improving medication adherence in children with CF-what a pharmacist can do. Archives Dis. Child. 99 (8), e3. doi:10.1136/archdischild-2014-306798.8
Chung, T., Hoffer, F. A., Connor, L., Zurakowski, D., and Burrows, P. E. (2000). The use of oral pentobarbital sodium (Nembutal) versus oral chloral hydrate in infants undergoing CT and MR imaging--a pilot study. Pediatr. Radiol. 30 (5), 332–335. doi:10.1007/s002470050752
Cifaldi, M. A., Paris, M. M., Devcich, K. J., and Bukofzer, S. (2004). Parent-reported outcomes for treatment of acute otitis media with cefdinir or amoxicillin/clavulanate oral suspensions. Paediatr. Drugs 6 (6), 387–393. doi:10.2165/00148581-200406060-00006
Claes, A., Decorte, A., Levtchenko, E., Knops, N., and Dobbels, F. (2014). Facilitators and barriers of medication adherence in pediatric liver and kidney transplant recipients: a mixed-methods study. Prog. Transplant. (Aliso Viejo, Calif.) 24 (4), 311–321. doi:10.7182/pit2014873
Cloyd, J. C., Kriel, R. L., Jones-Saete, C. M., Ong, B. Y., Jancik, J. T., and Remmel, R. P. (1992). Comparison of sprinkle versus syrup formulations of valproate for bioavailability, tolerance, and preference. J. Pediatr. 120 (4 I), 634–638. doi:10.1016/s0022-3476(05)82496-5
Coetzee, B., Kagee, A., and Bland, R. (2015). Barriers and facilitators to paediatric adherence to antiretroviral therapy in rural South Africa: a multi-stakeholder perspective. AIDS Care - Psychol. Socio-Medical Aspects AIDS/HIV 27 (3), 315–321. doi:10.1080/09540121.2014.967658
Coetzee, B., Kagee, A., and Bland, R. (2016). Video observations of treatment administration to children on antiretroviral therapy in rural KwaZulu-Natal. AIDS Care - Psychol. Socio-Medical Aspects AIDS/HIV 28 (Suppl. 2), 34–41. doi:10.1080/09540121.2016.1176674
Cohen, I. T., Joffe, D., Hummer, K., and Soluri, A. (2005). Ondansetron oral disintegrating tablets: acceptability and efficacy in children undergoing adenotonsillectomy. Anesth. Analgesia 101 (1), 59–63. doi:10.1213/01.ANE.0000154186.03161.35
Cohen, R., de La Rocque, F., Lécuyer, A., Wollner, C., Bodin, M. J., and Wollner, A. (2009). Study of the acceptability of antibiotic syrups, suspensions, and oral solutions prescribed to pediatric outpatients. Eur. J. Pediatr. 168 (7), 851–857. doi:10.1007/s00431-008-0857-0
Colquhoun, H. L., Levac, D., O'Brien, K. K., Straus, S., Tricco, A. C., Perrier, L., et al. (2014). Scoping reviews: time for clarity in definition, methods, and reporting. J. Clin. Epidemiol. 67 (12), 1291–1294. doi:10.1016/j.jclinepi.2014.03.013
Cote, C. J., Cohen, I. T., Suresh, S., Rabb, M., Rose, J. B., Weldon, B. C., et al. (2002). A comparison of three doses of a commercially prepared oral midazolam syrup in children. Anesth. Analgesia 94 (1), 37–43. doi:10.1097/00000539-200201000-00007
Covidence (2023). Covidence systematic review software, Veritas Health Innovation, (Melbourne, Australia). Available online at: www.covidence.org.
Cronin, J. J., McCoy, S., Kennedy, U., An Fhailí, S. N., Wakai, A., Hayden, J., et al. (2016). A randomized trial of single-dose oral dexamethasone versus multidose prednisolone for acute exacerbations of asthma in children who attend the emergency department. Ann. Emerg. Med. 67 (5), 593–601. doi:10.1016/j.annemergmed.2015.08.001
Dagan, R., Shvartzman, P., and Liss, Z. (1994). Variation in acceptance of common oral antibiotic suspensions. Pediatr. Infect. Dis. J. 13 (8), 686–690. doi:10.1097/00006454-199408000-00002
Dagnone, D., Matsui, D., and Rieder, M. J. (2002). Assessment of the palatability of vehicles for activated charcoal in pediatric volunteers. Pediatr. Emerg. Care 18 (1), 19–21. doi:10.1097/00006565-200202000-00006
Dalton, C., Reis-Melo, A., McGarrity, O., Bamford, A., and Foster, C. (2019). An evaluation of the tolerability and efficacy of the offlicence use of Rezolsta and Evotaz in children. HIV Med. 20 (Suppl. 5), 29–30. doi:10.1111/hiv.12739
Davies, M. A., Boulle, A., Fakir, T., Nuttall, J., and Eley, B. (2008). Adherence to antiretroviral therapy in young children in Cape Town, South Africa, measured by medication return and caregiver self-report: a prospective cohort study. BMC Pediatr. 8, 34. doi:10.1186/1471-2431-8-34
Dawson, K. P., and Sharpe, C. (1993). A comparison of the acceptability of prednisolone tablets and prednisolone sodium phosphate solution in childhood acute asthma. Aust. J. Hosp. Pharm. 23 (5), 320–323.
DelRosso, L. M., Yi, T., Chan, J. H. M., Wrede, J. E., Lockhart, C. T., and Ferri, R. (2020). Determinants of ferritin response to oral iron supplementation in children with sleep movement disorders. Sleep 43 (3), 1–5. doi:10.1093/sleep/zsz234
Dillman, J. R., Towbin, A. J., Imbus, R., Young, J., Gates, E., and Trout, A. T. (2018). Comparison of two neutral oral contrast agents in pediatric patients: a prospective randomized study. Radiology 288 (1), 245–251. doi:10.1148/radiol.2018173039
Di Pierro, F., Colombo, M., Zanvit, A., Risso, P., and Rottoli, A. S. (2014). Use of Streptococcus salivarius K12 in the prevention of streptococcal and viral pharyngotonsillitis in children. Drug, Healthc. Patient Saf. 6 (1), 15–20. doi:10.2147/DHPS.S59665
Duniva Inusa, B. P., Inati, A., Maes, P., Githanga, J., Ogutu, B., Abboud, M. R., et al. (2021). Pharmacokinetics and safety of ticagrelor in infants and toddlers with sickle cell disease aged <24 months. Pediatr. Blood Cancer 68 (5), e28977. doi:10.1002/pbc.28977
El Edelbi, R., Eksborg, S., and Lindemalm, S. (2015). In situ coating makes it easier for children to swallow and tolerate tablets and capsules. Acta Paediatr. 104 (9), 956–961. doi:10.1111/apa.13041
Elgammal, A., Ryan, J., Bradley, C., Crean, A., and Bermingham, M. (2022). How patient acceptability influences prescribing and dispensing of oral liquid antibiotics to children: a survey of community pharmacists. Pharmacoepidemiol. Drug Saf. 31 (Suppl. 1), 8. doi:10.1002/pds.5499
El-Sahn, M., Elliott, R., El-Sahn, M., Lucas, I., Kong, K., Walsh, J., and Lucas, J. (2025). Poor-tasting pediatric medicines: Part 2. Exploring caregiver and healthcare provider values and preferences for a novel taste-blocker product to improve acceptability. Front. Drug Deliv. Sec. Drug Delivery for Special Patient Populations. 5. doi:10.3389/fddev.2025.1555522
Escobedo, A. A., Alvarez, G., González, M. E., Almirall, P., Cañete, R., Cimerman, S., et al. (2008). The treatment of giardiasis in children: single-dose tinidazole compared with 3 days of nitazoxanide. Ann. Trop. Med. Parasitol. 102 (3), 199–207. doi:10.1179/136485908X267894
Escoda, A. C., Genestar, J. V., Salvador, H. H., Busquets, F. B., Suárez, J. A., Celma, M. S., et al. (2017). Trametinib safety and acceptability in paediatric oncology patients: experience based on a case series. Eur. J. Hosp. Pharm. 24 (Suppl. 1), A131–A132. doi:10.1136/ejhpharm-2017-000640.289
European Medicines Agency (2013). Guideline on pharmaceutical development of medicines for paediatric use (EMA/CHMP/QWP/805880/2012 Rev. 2). London, UK: European Medicines Agency Available online at: https://www.ema.europa.eu/en/pharmaceutical-development-medicines-paediatric-use-scientific-guideline.
European Medicines Agency (2017). 10-year Report to the European Commission: General report on the experience acquired as a result of the application of the Paediatric Regulation (EMA/231225/2015). London: UK: European Medicines Agency. Available online at: https://health.ec.europa.eu/medicinal-products/medicines-children_en#the-2017-paediatric-report.
European Medicines Agency. (2006). Reflection paper: formulations of choice for the paediatric population. (EMEA/CHMP/PEG/194810/2005). London, UK:European Medicines Agency. Available online at: https://www.ema.europa.eu/en/formulations-choice-paediatric-population-scientific-guideline.
Ewing, V. L., Terlouw, D. J., Kapinda, A., Pace, C., Richards, E., Tolhurst, R., et al. (2015). Perceptions and utilization of the anti-malarials artemether-lumefantrine and dihydroartemisinin-piperaquine in young children in the Chikhwawa District of Malawi: a mixed methods study. Malar. J. 14, 13. doi:10.1186/s12936-014-0528-8
Food and Drug Administration (2018). Use of liquids and/or soft foods as vehicles for drug administration: General considerations for selection and In vitro methods for product quality assessments. Maryland, USA: Center for Drug Evaluation and Research (CDER), Food and Drug Administration. Available online at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-liquids-andor-soft-foods-vehicles-drug-administration-general-considerations-selection-and-vitro.
Frange, P., Avettand-Fenoel, V., and Blanche, S. (2018). Poor palatability of the new ritonavir formulation is a major obstacle to adherence to treatment in young children. J. Antimicrob. Chemother. 73 (5), 1435–1437. doi:10.1093/jac/dky029
Freedman, S. B., Cho, D., Boutis, K., Stephens, D., and Schuh, S. (2010). Assessing the palatability of oral rehydration solutions in school-aged children: a randomized crossover trial. Arch. Pediatr. Adolesc. Med. 164 (8), 696–702. doi:10.1001/archpediatrics.2010.129
Gamston, F. E. (2013). When do paediatric renal transplant recipients convert to solid medicine formulations? Pediatr. Transplant. 17 (Suppl. 1), 88–89. doi:10.1128/aac.02144-21
Garcia-Prats, A. J., Frias, M., van der Laan, L., De Leon, A., Gler, M. T., Schaaf, H. S., et al. (2022). Delamanid added to an optimized background regimen in children with multidrug-resistant tuberculosis: results of a phase I/II clinical trial. Antimicrob. Agents Chemother. 66 (5), e0214421. doi:10.1111/petr.12122
Gaskell, C. (2016). Use of pill glide to encourage transition from liquid to solid dosage formulations. Arch. Dis. Child. 101 (9), e2. doi:10.1136/archdischild-2016-311535.8
Gee, S. C., and Hagemann, T. M. (2007). Palatability of liquid anti-infectives: clinician and student perceptions and practice outcomes. J. Pediatr. Pharmacol. Ther. 12 (4), 216–223. doi:10.5863/1551-6776-12.4.216
Gibson, F., Donachie, P., Blandford, E., Coulson, S., Hemsworth, S., Pizer, B., et al. (2010). Efficacy of gelclair in reducing the pain of oral mucositis. Pediatr. Blood Cancer 55 (5), 818. doi:10.1002/pbc.22779
Giralt, A. N., Nöstlinger, C., Lee, J., Salami, O., Lallemant, M., Onyango-Ouma, W., et al. (2019). Understanding acceptance of and adherence to a new formulation of paediatric antiretroviral treatment in the form of pellets (LPV/r)-A realist evaluation. PLoS ONE 14 (8), e0220408. doi:10.1371/journal.pone.0220408
Goldberg, S. L., Giardina, P. J., Chirnomas, D., Esposito, J., Paley, C., and Vichinsky, E. (2013). The palatability and tolerability of deferasirox taken with different beverages or foods. Pediatr. Blood Cancer 60 (9), 1507–1512. doi:10.1002/pbc.24561
Goode, M., McMaugh, A., Crisp, J., Wales, S., and Ziegler, J. B. (2003). Adherence issues in children and adolescents receiving highly active antiretroviral therapy. AIDS Care - Psychol. Socio-Medical Aspects AIDS/HIV 15 (3), 403–408. doi:10.1080/0954012031000105450
Gotesman, M., Elgar, G., Pak, Y., Hernandez Santiago, L., Alvarez, A., Lin, H., et al. (2020). Pediatric patients with sickle cell disease at harbor-UCLA and early experience of L-glutamine therapy. Blood 136 (Suppl. 1), 6. doi:10.1182/blood-2020-137280
Goyanes, A., Madla, C. M., Umerji, A., Duran Piñeiro, G., Giraldez Montero, J. M., Lamas Diaz, M. J., et al. (2019). Automated therapy preparation of isoleucine formulations using 3D printing for the treatment of MSUD: first single-centre, prospective, crossover study in patients. Int. J. Pharm. 567, 118497. doi:10.1016/j.ijpharm.2019.118497
Greenley, R. N., Reed-Knight, B., Wojtowicz, A. A., Plevinsky, J. M., Lewis, J. D., and Kahn, S. A. (2018). A bitter pill to swallow: medication adherence barriers in adolescents and young adults with inflammatory bowel diseases. Children's Health Care 47 (4), 416–431. doi:10.1080/02739615.2017.1383911
Greenwood, A., and Vallabhaneni, P. (2015). Doc-i don't want to take my medicine. Archives Dis. Child. 100 (Suppl. 3), A135. doi:10.1136/archdischild-2015-308599.295
Gutierrez-Colina, A. M., Smith, A. W., Mara, C. A., and Modi, A. C. (2018). Adherence barriers in pediatric epilepsy: from toddlers to young adults. Epilepsy Behav. 80, 229–234. doi:10.1016/j.yebeh.2018.01.031
Gyedu, A., Mehta, K., Baidoo, H., Addo, D., Abdullah, M., Mesic, A., et al. (2023). Preferences for oral rehydration drinks among healthy individuals in Ghana: a single-blind, cross-sectional survey to inform implementation of an enterally based resuscitation protocol for burn injury. Burns 49 (4), 820–829. doi:10.1016/j.burns.2022.05.016
Hames, H., Seabrook, J. A., Matsui, D., Rieder, M. J., and Joubert, G. I. (2008). A palatability study of a flavored dexamethasone preparation versus prednisolone liquid in children. Can. J. Clin. Pharmacol. = J. Can. de Pharmacol. clinique 15 (1), e95–e98. Available online at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bankcountry-and-lending-groups
Hansen, D. L., Tulinius, D., and Hansen, E. H. (2008). Adolescents' struggles with swallowing tablets: barriers, strategies and learning. Pharm. World Sci. 30 (1), 65–69. doi:10.1007/s11096-007-9142-y
Haslund-Krog, S. S., Jørgensen, I. M., Dalhoff, K., and Holst, H. (2022). Acceptability of prednisolone in an open-label randomised cross-over study-focus on formulation in children. Child. (Basel) 9 (8), 1236. doi:10.3390/children9081236
Hirsch-Moverman, Y., Strauss, M., George, G., Mutiti, A., Mafukidze, A., Shongwe, S., et al. (2021). Paediatric tuberculosis preventive treatment preferences among HIV-positive children, caregivers and healthcare providers in Eswatini: a discrete choice experiment. BMJ Open 11 (10), e048443. doi:10.1136/bmjopen-2020-048443
Hoffstedt, T., Skov Hansen, L. B., Twetman, S., and Sonesson, M. (2023). Effect of an enzyme-containing mouthwash on the dental biofilm and salivary microbiome in patients with fixed orthodontic appliances: a randomized placebo-controlled pilot trial. Eur. J. Orthod. 45 (1), 96–102. doi:10.1093/ejo/cjac062
Hofmanová, J. K., Mason, J., and Batchelor, H. K. (2020). Sensory aspects of acceptability of bitter-flavoured 7.5 mm film-coated tablets in adults, preschool and school children. Int. J. Pharm. 585, 119511. doi:10.1016/j.ijpharm.2020.119511
Holas, C., Chiu, Y. L., Notario, G., and Kapral, D. (2005). A pooled analysis of seven randomized crossover studies of the palatability of cefdinir oral suspension versus amoxicillin/clavulanate potassium, cefprozil, azithromycin, and amoxicillin in children aged 4 to 8 years. Clin. Ther. 27 (12), 1950–1960. doi:10.1016/j.clinthera.2005.11.017
Holloway, J., Okezue, C., and Webb, J. (2018). Retrospective analysis of compliance and efficacy of a novel formulation of deferasirox. Blood 132 (Suppl. 1), 5877. doi:10.1182/blood-2018-99-118810
Holmberg, L. (1994). A comparative taste preference study of loratadine versus terfenadine in children. Curr. Ther. Res. - Clin. Exp. 55 (6), 641–644. doi:10.1016/s0011-393x(05)80797-0
Horodniceanu, E. G., Bal, V., Dhatt, H., Carter, J. A., Huang, V., and Lasch, K. (2017). Qualitative modification and development of patient- and caregiver-reported outcome measures for iron chelation therapy. Health Qual. Life Outcomes 15 (1), 129. doi:10.1186/s12955-017-0702-0
Hou, F., Miao, Y., Wu, X., Gui, X., Wang, Y., Li, H., et al. (2022). Study on masking the bitterness of Chinese medicine decoction-mate. Evidence-based Complementary Altern. Med. 2022, 3701288. doi:10.1155/2022/3701288
Hu, S., Liu, X., Zhang, S., and Quan, D. (2023). An overview of taste-masking technologies: approaches, application, and assessment methods. AAPS PharmSciTech 24 (2), 67. doi:10.1208/s12249-023-02520-z
Ibrahim, K., and Al Ansari, K. (2016). Flavored intravenous ondansetron administered orally for the treatment of persistent vomiting in children. J. Trop. Pediatr. 62 (4), 288–292. doi:10.1093/tropej/fmw009
Imanieh, M. H., Honar, N., Mohagheghzadeh, A., Haghighat, M., Dehghani, S. M., Mosleh, G., et al. (2022). Rosa damascena together with brown sugar mitigate functional constipation in children over 12 months old: a double-blind randomized controlled trial. J. Ethnopharmacol. 298, 115582. doi:10.1016/j.jep.2022.115582
Isik, B., Baygin, O., and Bodur, H. (2008). Effect of drinks that are added as flavoring in oral midazolam premedication on sedation success. Paediatr. Anaesth. 18 (6), 494–500. doi:10.1111/j.1460-9592.2008.02462.x
Jaffé, G., and Grimshaw, J. J. (1983). Randomized single-blind trial in general practice comparing the efficacy and palatability of two cough linctus preparations, 'Pholcolix' and 'Actifed' Compound, in children with acute cough. Curr. Med. Res. Opin. 8 (8), 594–599. doi:10.1185/03007998309109803
Jahnsen, T., and Thorn, P. (1987). An acceptability study of two pivampicillin mixtures in children in general practice. Scand. J. Prim. Health Care 5 (4), 241–243. doi:10.3109/02813438709018102
Jain, E., Pandey, R. K., and Khanna, R. (2013). Liquorice root extracts as potent cariostatic agents in pediatric practice. J. Indian Soc. Pedod. Prev. Dent. 31 (3), 146–152. doi:10.4103/0970-4388.117964
Jamieson, L., Harrop, E., Johnson, M., Liossi, C., Mott, C., Oulton, K., et al. (2021). Healthcare professionals' views of the use of oral morphine and transmucosal diamorphine in the management of paediatric breakthrough pain and the feasibility of a randomised controlled trial: a focus group study (DIPPER). Palliat. Med. 35 (6), 1118–1125. doi:10.1177/02692163211008737
Joanne, C., and Ibrahim, A. (2018). SP1 Medicines optimisation for paediatric patients with learning disability. Archives Dis. Child. 103 (2), e1.1–e1. doi:10.1136/archdischild-2017-314584.1
Kardas, P., Lewek, P., and Matyjaszczyk, M. (2013). Determinants of patient adherence: a review of systematic reviews. Front. Pharmacol. 4, 91. doi:10.3389/fphar.2013.00091
Kedenge, S. V., Kangwana, B. P., Waweru, E. W., Nyandigisi, A. J., Pandit, J., Brooker, S. J., et al. (2013). Understanding the impact of subsidizing artemisinin-based combination therapies (ACTs) in the retail sector--results from focus group discussions in rural Kenya. PLoS One 8 (1), e54371. doi:10.1371/journal.pone.0054371
Kekitiinwa, A., Musiime, V., Thomason, M. J., Mirembe, G., Lallemant, M., Nakalanzi, S., et al. (2016). Acceptability of lopinavir/r pellets (minitabs), tablets and syrups in HIV-infected children. Antivir. Ther. 21 (7), 579–585. doi:10.3851/IMP3054
Kelly, J., Bengry, T., Romanick, M., Jupp, J., and Dersch-Mills, D. (2020). Pediatric pharmacists' perspectives on essential skills and activities for community pharmacists caring for pediatric patients: a mixed-methods study. Can. Pharm. J. 153 (5), 287–293. doi:10.1177/1715163520946079
Khalil, S. N., Vije, H. N., Kee, S. S., Farag, A., Hanna, E., and Chuang, A. Z. (2003). A paediatric trial comparing midazolam/Syrpalta mixture with premixed midazolam syrup (Roche). Paediatr. Anaesth. 13 (3), 205–209. doi:10.1046/j.1460-9592.2003.01062.x
Khurana, C. M. (1996). A multicenter, randomized, open label comparison of azithromycin and amoxicillin/clavulanate in acute otitis media among children attending day care or school. Pediatr. Infect. Dis. J. 15 (9 Suppl. L), S24–S29. doi:10.1097/00006454-199609009-00005
Kibleur, Y., Dobbelaere, D., Barth, M., Brassier, A., and Guffon, N. (2014). Results from a nationwide cohort temporary utilization authorization (ATU) survey of patients in France treated with Pheburane sodium phenylbutyrate taste-masked granules. Pediatr. Drugs 16 (5), 407–415. doi:10.1007/s40272-014-0081-5
Kiruki, M., Otiso, L., Alwar, T., Murira, F., Ikahu, A., Digolo, L., et al. (2018). Factors influencing antiretroviral therapy adherence: voices of adolescents in Kenya. AIDS Res. Hum. Retroviruses 34 (Suppl. 1), 147. doi:10.1089/aid.2018.5000
Kiryluk, B., and Sobaniec, W. (2013). A comparison of efficacy two forms valproic acid (syrup and granules) in children with epilepsy. Pharmacol. Rep. 65 (1), 248–250. doi:10.1016/s1734-1140(13)70992-7
Klingmann, V., Vallet, T., Münch, J., Stegemann, R., Wolters, L., Bosse, H. M., et al. (2022). Dosage forms suitability in pediatrics: acceptability of analgesics and antipyretics in a German hospital. Pharmaceutics 14 (2), 337. doi:10.3390/pharmaceutics14020337
Kokke, F. T., Scholtens, P. A. M. J., Alles, M. S., Decates, T. S., Fiselier, T. J. W., Tolboom, J. J. M., et al. (2008). A dietary fiber mixture versus lactulose in the treatment of childhood constipation: a double-blind randomized controlled trial. J. Pediatr. Gastroenterol. Nutr. 47 (5), 592–597. doi:10.1097/mpg.0b013e318162c43c
Kokki, H., Nikanne, E., and Ahonen, R. (2000). The feasibility of pain treatment at home after adenoidectomy with ketoprofen tablets in small children. Paediatr. Anaesth. 10 (5), 531–535. doi:10.1046/j.1460-9592.2000.00556.x
Kolaczinski, J. H., Ojok, N., Opwonya, J., Meek, S., and Collins, A. (2006). Adherence of community caretakers of children to pre-packaged antimalarial medicines (HOMAPAK) among internally displaced people in Gulu district, Uganda. Malar. J. 5, 40. doi:10.1186/1475-2875-5-40
Kolmen, B. K., Feldman, H. M., Handen, B. L., and Janosky, J. E. (1995). Naltrexone in young autistic children: a double-blind, placebo-controlled crossover study. J. Am. Acad. Child Adolesc. Psychiatry 34 (2), 223–231. doi:10.1097/00004583-199502000-00018
Kumar, N., Sharma, R., Sharma, M., Verma, I., and Sharma, M. (2012). Midazolam pre-medication in paediatrics: comparison of the intranasal and sublingual routes by using an atomizer spray. J. Clin. Diagnostic Res. 6 (1), 65–68. doi:10.7860/JCDR/2012/.1845
Lagos, R., San Martin, O., Wasserman, S. S., Prado, V., Losonsky, G. A., Bustamante, C., et al. (1999). Palatability, reactogenicity and immunogenicity of engineered live oral cholera vaccine CVD 103-HgR in Chilean infants and toddlers. Pediatr. Infect. Dis. J. 18 (7), 624–630. doi:10.1097/00006454-199907000-00011
Lava, S. A. G., Caccia, G., Osmetti-Gianini, S., Simonetti, G. D., Milani, G. P., Falesi, M., et al. (2011). Acceptance of two liquid vitamin D₃ formulations among mothers with newborn infants: a randomized, single-blind trial. Eur. J. Pediatr. 170 (12), 1559–1562. doi:10.1007/s00431-011-1477-7
Lenhard, G., Mivsek-Music, E., Perrin-Fayolle, M., Obtulowicz, K., and Secchi, A. (1997). Double-blind, randomised, placebo-controlled study of two concentrations of azelastine eye drops in seasonal allergic conjunctivitis or rhinoconjunctivitis. Curr. Med. Res. Opin. 14 (1), 21–28. doi:10.1185/03007999709113339
Leonard, S., Jonassaint, J., Anderson, L., and Shah, N. (2014). The use of mobile technology for intensive training in medication management in the pediatric population. Blood 124 (21), 4842. doi:10.1182/blood.v124.21.4842.4842
Liacouras, C. A., Coates, P. M., Gallagher, P. R., and Cortner, J. A. (1993). Use of cholestyramine in the treatment of children with familial combined hyperlipidemia. J. Pediatr. 122 (3), 477–482. doi:10.1016/s0022-3476(05)83444-4
Lin, D., Seabrook, J. A., Matsui, D. M., King, S. M., Rieder, M. J., and Finkelstein, Y. (2011). Palatability, adherence and prescribing patterns of antiretroviral drugs for children with human immunodeficiency virus infection in Canada. Pharmacoepidemiol. Drug Saf. 20 (12), 1246–1252. doi:10.1002/pds.2236
Low, Y. S., Islahudin, F., Razali, K. A. M., and Adnan, S. (2018). Modification of initial highly active antiretroviral therapy (HAART) regimen in paediatric HIV patients. Open AIDS J. 12, 11–19. doi:10.2174/1874613601812010011
Macknin, M. L., Piedmonte, M., Calendine, C., Janosky, J., and Wald, E. (1998). Zinc gluconate lozenges for treating the common cold in children: a randomized controlled trial. JAMA 279 (24), 1962–1967. doi:10.1001/jama.279.24.1962
Maines, E., Urru, S. A. M., Burri, E., Piccoli, G., Pedrolli, A., Pasqualini, A., et al. (2020). Formulation and clinical evaluation of sodium benzoate oral solution for the treatment of urea cycle disorders in pediatric patients. AAPS PharmSciTech 21 (3), 100. doi:10.1208/s12249-020-01642-y
Manford Gooch, I. W., Adelglass, J., Kelsey, D. K., Masica, D., Johns, D., and Weinberg, B. C. (1999). Loracarbef versus clarithromycin in children with acute otitis media with effusion. Clin. Ther. 21 (4), 711–722. doi:10.1016/s0149-2918(00)88322-8
Marshall, J., Rodarte, A., Blumer, J., Khoo, K. C., Akbari, B., and Kearns, G. (2000). Pediatric pharmacodynamics of midazolam oral syrup. Pediatric pharmacology research unit network. J. Clin. Pharmacol. 40 (6), 578–589. doi:10.1002/j.1552-4604.2000.tb05983.x
Matsui, D., Barron, A., and Rieder, M. J. (1996). Assessment of the palatability of antistaphylococcal antibiotics in pediatric volunteers. Ann. Pharmacother. 30 (6), 586–588. doi:10.1177/106002809603000603
Matsumoto, S., Yamaguchi, H., Fukuzawa, M., Kawai, T., and Itoi, T. (2022). Effectiveness of polyethylene glycol for bowel cleansing before colonoscopy in children. Gastrointest. Endosc. 95 (6 Suppl. ment), AB428. doi:10.1016/j.gie.2022.04.1099
McIntyre, J., and Hull, D. (1996). Comparing efficacy and tolerability of ibuprofen and paracetamol in fever. Arch. Dis. Child. 74 (2), 164–167. doi:10.1136/adc.74.2.164
Meier, C. M., Simonetti, G. D., Ghiglia, S., Fossali, E., Salice, P., Limoni, C., et al. (2007). Palatability of angiotensin II antagonists among nephropathic children. Br. J. Clin. Pharmacol. 63 (5), 628–631. doi:10.1111/j.1365-2125.2006.02814.x
Melvin, A. J., Mohan, K. M., Arcuino, L. A., Edelstein, R. E., and Frenkel, L. M. (1997). Clinical, virologic and immunologic responses of children with advanced human immunodeficiency virus type 1 disease treated with protease inhibitors. Pediatr. Infect. Dis. J. 16 (10), 968–974. doi:10.1097/00006454-199710000-00013
Mennella, J. A. (2014). Ontogeny of taste preferences: basic biology and implications for health. Am. J. Clin. Nutr. 99 (3), 704S-11S–11S. doi:10.3945/ajcn.113.067694
Mennella, J. A., and Bobowski, N. K. (2015). The sweetness and bitterness of childhood: insights from basic research on taste preferences. Physiol. Behav. 152 (Pt B), 502–507. doi:10.1016/j.physbeh.2015.05.015
Mennella, J. A., Pepino, M. Y., Duke, F. F., and Reed, D. R. (2010). Age modifies the genotype-phenotype relationship for the bitter receptor TAS2R38. BMC Genet. 11, 60. doi:10.1186/1471-2156-11-60
Mennella, J. A., Pepino, M. Y., and Reed, D. R. (2005). Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics 115 (2), e216–e222. doi:10.1542/peds.2004-1582
Mennella, J. A., Spector, A. C., Reed, D. R., and Coldwell, S. E. (2013). The bad taste of medicines: overview of basic research on bitter taste. Clin. Ther. 35 (8), 1225–1246. doi:10.1016/j.clinthera.2013.06.007
Mettey, R., Guillard, O., Merle, P., and Maillet-Picker, F. (1990). Severe selective magnesium malabsorption: tests of tolerance of oral magnesium supplements. Magnesium Res. official organ Int. Soc. Dev. Res. Magnesium 3 (4), 291–295.
Milani, G., Ragazzi, M., Simonetti, G. D., Ramelli, G. P., Rizzi, M., Bianchetti, M. G., et al. (2010). Superior palatability of crushed lercanidipine compared with amlodipine among children. Br. J. Clin. Pharmacol. 69 (2), 204–206. doi:10.1111/j.1365-2125.2009.03580.x
Mistry, P., Stirling, H., Callens, C., Hodson, J., and Batchelor, H.SPaeDD-UK project (2018). Evaluation of patient-reported outcome measurements as a reliable tool to measure acceptability of the taste of paediatric medicines in an inpatient paediatric population. BMJ Open 8 (7), e021961. doi:10.1136/bmjopen-2018-021961
Moniot-Ville, N., Chelly, M., Consten, L., and Rosenbaum, M. (1998). The acceptability, efficacy and safety of a new paediatric oral suspension of roxithromycin in respiratory tract infections. J. Int. Med. Res. 26 (3), 144–151. doi:10.1177/030006059802600305
Mulla, H., Buck, H., Price, L., Parry, A., Bell, G., and Skinner, R. (2016). Acceptability' of a new oral suspension formulation of mercaptopurine in children with acute lymphoblastic leukaemia. J. Oncol. Pharm. Pract. 22 (3), 387–395. doi:10.1177/1078155215577808
Murnane, P. M., Strehlau, R., Shiau, S., Patel, F., Mbete, N., Hunt, G., et al. (2017). Switching to efavirenz versus remaining on ritonavir-boosted lopinavir in human immunodeficiency virus-infected children exposed to nevirapine: long-term outcomes of a randomized trial. Clin. Infect. Dis. 65 (3), 477–485. doi:10.1093/cid/cix335
Nachman, S., Zheng, N., Acosta, E. P., Teppler, H., Homony, B., Graham, B., et al. (2014). Pharmacokinetics, safety, and 48-week efficacy of oral raltegravir in HIV-1-infected children aged 2 through 18 years. Clin. Infect. Dis. 58 (3), 413–422. doi:10.1093/cid/cit696
Nahirya-Ntege, P., Cook, A., Vhembo, T., Opilo, W., Namuddu, R., Katuramu, R., et al. (2012). Young HIV-infected children and their adult caregivers prefer tablets to syrup antiretroviral medications in Africa. PLoS ONE 7 (5), e36186. doi:10.1371/journal.pone.0036186
Naimi, D. R., Freedman, T. G., Ginsburg, K. R., Bogen, D., Rand, C. S., and Apter, A. J. (2009). Adolescents and asthma: why bother with our meds? J. Allergy Clin. Immunol. 123 (6), 1335–1341. doi:10.1016/j.jaci.2009.02.022
Nasrin, D., Larson, C. P., Sultana, S., and Khan, T. U. (2005). Acceptability of and adherence to dispersible zinc tablet in the treatment of acute childhood diarrhoea. J. Health, Popul. Nutr. 23 (3), 215–221.
Nathan, P. C., Tomlinson, G., Dupuis, L. L., Greenberg, M. L., Ota, S., Bartels, U., et al. (2006). A pilot study of ondansetron plus metopimazine vs. ondansetron monotherapy in children receiving highly emetogenic chemotherapy: a Bayesian randomized serial N-of-1 trials design. Support. Care Cancer 14 (3), 268–276. doi:10.1007/s00520-005-0875-7
Nezam, S., Singh, P., Ojha, R., Khan, S. A., Kumari, N., and Kumari, N. (2022). Evaluation of the antimicrobial activity of magnetized water and its comparison with chlorhexidine 0.2% in young children for 3 weeks. J. Contemp. Dent. Pract. 23 (1), 83–88. doi:10.5005/jp-journals-10024-3237
Nguyen, T. M., La Caze, A., and Cottrell, N. (2014). What are validated self-report adherence scales really measuring? a systematic review. Br. J. Clin. Pharmacol. 77 (3), 427–445. doi:10.1111/bcp.12194
Nimrouzi, M., Sadeghpour, O., Imanieh, M. H., Shams Ardekani, M., Salehi, A., Minaei, M. B., et al. (2015). Flixweed vs. polyethylene glycol in the treatment of childhood functional constipation: a randomized clinical trial. Iran. J. Pediatr. 25 (2), e425. doi:10.5812/ijp.425
Nizami, S. Q., Khan, I. A., and Bhutta, Z. A. (1996). Self-reported concepts about oral rehydration solution, drug prescribing and reasons for prescribing antidiarrhoeals for acute watery diarrhea in children. Trop. Dr. 26 (4), 180–183. doi:10.1177/004947559602600416
Nordenmalm, S., Kimland, E., Ligas, F., Lehmann, B., Claverol, J., Nafria, B., et al. (2019). Children's views on taking medicines and participating in clinical trials. Arch. Dis. Child. 104 (9), 900–905. doi:10.1136/archdischild-2018-316511
Nuzhat, S., Ahmed, T., Alam, J., Billal, S. M., Khan, A. I., and Hossain, M. I. (2022). New formulation zinc sulphate acceptability and adherence in children with acute diarrhoea: a prospective, open-label, interventional study in Bangladesh. J. Paediatr. Child Health 58 (7), 1215–1220. doi:10.1111/jpc.15953
Osterhoudt, K. C., Alpern, E. R., Durbin, D., Nadel, F., and Henretig, F. M. (2004). Activated charcoal administration in a pediatric emergency department. Pediatr. Emerg. Care 20 (8), 493–498. doi:10.1097/01.pec.0000136064.14704.d1
Padilla, N., Diaz, R., and Munoz, M. (2000). Efficacy and safety of quinfamide versus secnidazole in the management of amoebic non-dysenteric colitis in children. Clin. Drug Investig. 20 (2), 89–93. doi:10.2165/00044011-200020020-00003
Paediatric European Network for Treatment of AIDS (PENTA) (1999). Parents' attitudes to their HIV-infected children being enrolled into a placebo-controlled trial: the PENTA 1 trial. Paediatric European Network for Treatment of AIDS. HIV Med. 1 (1), 25–31. doi:10.1046/j.1468-1293.1999.00005.x
Pai, A. L., Deckelman, A., Leone, M., Drake, S., Bills, S., Klink, G., et al. (2023). Adherence barriers in pediatric hematopoietic stem cell transplant in an outpatient setting. Transplant. Cell. Ther. 29 (2 Suppl. ment), S153–S154. doi:10.1016/s2666-6367(23)00263-4
Pajno, G. B., Caminiti, L., Crisafulli, G., Barberi, S., Landi, M., Aversa, T., et al. (2012). Adherence to sublingual immunotherapy in preschool children. Pediatr. Allergy Immunol. 23 (7), 688–689. doi:10.1111/j.1399-3038.2012.01317.x
Palit, M., Hegde, S. K., and Bhat, S. S. (2016). Effectiveness of mouthrinse formulated from aqueous extract of Terminalia chebula on salivary Streptococcus mutans count and pH among 8- to 12-year-old school children of Karnataka: a randomized clinical trial. Int. J. Clin. Pediatr. Dent. 9 (4), 349–354. doi:10.5005/jp-journals-10005-1390
Paller, A. S., Hong, Y., Becker, E. M., de Lucas, R., Paris, M., Zhang, W., et al. (2020). Pharmacokinetics and safety of apremilast in pediatric patients with moderate to severe plaque psoriasis: results from a phase 2 open-label study. J. Am. Acad. Dermatology 82, 389–397. doi:10.1016/j.jaad.2019.08.019
Paranthaman, K., Kumarasamy, N., Bella, D., and Webster, P. (2009). Factors influencing adherence to anti-retroviral treatment in children with human immunodeficiency virus in South India - a qualitative study. AIDS Care - Psychol. Socio-Medical Aspects AIDS/HIV 21 (8), 1025–1031. doi:10.1080/09540120802612857
Parshuram, C. S., and McMahon, K. (1998). Acceptability of flavoured and unflavoured oral prednisolone solution in children with acute asthma. Aust. J. Hosp. Pharm. 28 (4), 290–291. doi:10.1002/jppr1998284290
Pasipanodya, B., Kuwengwa, R., Prust, M. L., Stewart, B., Chakanyuka, C., Murimwa, T., et al. (2018). Assessing the adoption of lopinavir/ritonavir oral pellets for HIV-positive children in Zimbabwe. J. Int. AIDS Soc. 21 (12), e25214. doi:10.1002/jia2.25214
Patel, D. V., Acharya, U. K., Shinde, M. K., and Nimbalkar, S. M. (2022). Compliance to antibiotic therapy at paediatric out-patient clinic. J. Fam. Med. Prim. Care 11 (3), 1012–1018. doi:10.4103/jfmpc.jfmpc_1234_21
Penagini, F., Borsani, B., Maruca, K., Giosia, V., Bova, S., Mastrangelo, M., et al. (2017). Short-term vitamin D3 supplementation in children with neurodisabilities: comparison of two delivery methods. J. Pediatr. Gastroenterology Nutr. 64 (Suppl. 1), 794. doi:10.1097/01.mpg.0000516381.25680.b4
Penkov, D., Tomasi, P., Eichler, I., Murphy, D., Yao, L. P., and Temeck, J. (2017). Pediatric medicine development: an overview and comparison of regulatory processes in the European union and United States. Ther. Innov. Regul. Sci. 51 (3), 360–371. doi:10.1177/2168479017696265
Perin, J., Mulick, A., Yeung, D., Villavicencio, F., Lopez, G., Strong, K. L., et al. (2022). Global, regional, and national causes of under-5 mortality in 2000-19: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child. Adolesc. Health 6 (2), 106–115. doi:10.1016/S2352-4642(21)00311-4
Peters, M.D.J., Godfrey, C., McInerney, P., Munn, Z., Tricco, A.C., and Khalil, H. (2020). in Scoping reviews. Editors E. Aromataris, C. Lockwood, K. Porritt, B. Pilla, and Z. Jordan (Adelaide, Australia: JBI Manual for Evidence Synthesis). doi:10.46658/JBIMES-24-09
Phipps, S., and DeCuir-Whalley, S. (1990). Adherence issues in pediatric bone marrow transplantation. J. Pediatr. Psychol. 15 (4), 459–475. doi:10.1093/jpepsy/15.4.459
Pichichero, M., Aronovitz, G. H., Gooch, W. M., McLinn, S. E., Maddern, B., Johnson, C., et al. (1990). Comparison of cefuroxime axetil, cefaclor, and amoxicillin-clavulanate potassium suspensions in acute otitis media in infants and children. South. Med. J. 83 (10), 1174–1177. doi:10.1097/00007611-199010000-00013
Porteous, J. E., Henry, R. L., O'Loughlin, E. V., Ireland, M., Francis, J. L., and Hankin, R. G. (1997). Management of childhood gastroenteritis in the community. Med. J. Aust. 167 (4), 195–198. doi:10.5694/j.1326-5377.1997.tb138844.x
Powers, J. L. (1996). Properties of azithromycin that enhance the potential for compliance in children with upper respiratory tract infections. Pediatr. Infect. Dis. J. 15 (9 Suppl. L), S30–S37. doi:10.1097/00006454-199609009-00006
Powers, J. L., Gooch, I. W. M., and Oddo, L. P. (2000). Comparison of the palatability of the oral suspension of cefdinir vs. amoxicillin/clavulanate potassium, cefprozil and azithromycin in pediatric patients. Pediatr. Infect. Dis. J. 19 (12 Suppl. L), S174–S180. doi:10.1097/00006454-200012001-00008
Powers, J. M., Nagel, M., Raphael, J. L., Mahoney, D. H., Buchanan, G. R., and Thompson, D. I. (2020). Barriers to and facilitators of iron therapy in children with iron deficiency anemia. J. Pediatr. 219, 202–208. doi:10.1016/j.jpeds.2019.12.040
Prajapati, G. K., Sharma, S., and Shrivastava, J. (2022). Effect of counselling on compliance of hydroxyurea therapy and frequency of hospital admissions among patients with sickle cell disease- A longitudinal study. J. Clin. Diagnostic Res. 16 (9), SC07–SC10. doi:10.7860/jcdr/2022/57120.16922
Pronzini, F., Bartoli, F., Vanoni, F., Corigliano, T., Ragazzi, M., Balice, P., et al. (2008). Palatability of vitamin D3 preparations modulates adherence to the supplementation in infancy. Clin. Pediatr. Endocrinol. 17 (2), 57–60. doi:10.1297/cpe.17.57
Purchase, S., Cunningham, J., Esser, M., and Skinner, D. (2016). Keeping kids in care: virological failure in a paediatric antiretroviral clinic and suggestions for improving treatment outcomes. Afr. J. AIDS Res. 15 (3), 301–309. doi:10.2989/16085906.2016.1210656
Purchase, S. E., Garcia-Prats, A. J., De Koker, P., Draper, H. R., Osman, M., Seddon, J. A., et al. (2019). Acceptability of a novel levofloxacin dispersible tablet formulation in young children exposed to multidrug-resistant tuberculosis. Pediatr. Infect. Dis. J. 38 (6), 608–610. doi:10.1097/INF.0000000000002268
Querin, B., Schweitzer-Chaput, A., Cisternino, S., Auvity, S., Fauqueur, A. S., Negbane, A., et al. (2023). Pharmaceutical oral formulation of methionine as a pediatric treatment in inherited metabolic disease. Pharmaceutics 15 (3), 957. doi:10.3390/pharmaceutics15030957
Ranmal, S. R., Nhouchi, Z., Keeley, A., Adler, L., Lavarde, M., Pensé-Lhéritier, A. M., et al. (2023). Taste assessment for paediatric drug Development: a comparison of bitterness taste aversion in children versus Naïve and expert young adult assessors. Int. J. Pharm. 647, 123494. doi:10.1016/j.ijpharm.2023.123494
Rasul, C. H., and Khan, M. A. (2001). Salbutamol versus theophylline for wheezy infant. J. Pak Med. Assoc. 51 (5), 187–189.
Reddington, C., Cohen, J., Baldillo, A., Toye, M., Smith, D., Kneut, C., et al. (2000). Adherence to medication regimens among children with human immunodeficiency virus infection. Pediatr. Infect. Dis. J. 19 (12), 1148–1153. doi:10.1097/00006454-200012000-00005
Reich, S., Bührer, C., Henze, G., Ohlendorf, D., Mesche, M., Sinha, P., et al. (2000). Oral isobutyramide reduces transfusion requirements in some patients with homozygous beta-thalassemia. Blood 96 (10), 3357–3363. doi:10.1182/blood.v96.10.3357
Rendeli, C., Ausili, E., Tabacco, F., Focarelli, B., Pantanella, A., Di Rocco, C., et al. (2006). Polyethylene glycol 4000 vs. lactulose for the treatment of neurogenic constipation in myelomeningocele children: a randomized-controlled clinical trial. Alimentary Pharmacol. Ther. 23 (8), 1259–1265. doi:10.1111/j.1365-2036.2006.02872.x
Rittig, S., Baka-Ostrowska, M., Tøndel, C., Walle, J. V., Kjaeer, B., Passier, P., et al. (2020). The pharmacokinetics, safety, and tolerability of mirabegron in children and adolescents with neurogenic detrusor overactivity or idiopathic overactive bladder and development of a population pharmacokinetic model-based pediatric dose estimation. J. Pediatr. Urology 16 (1), 31.e1–31. doi:10.1016/j.jpurol.2019.10.009
Riva, J., Lejbusiewicz, G., Papa, M., Lauber, C., Kohn, W., Da Fonte, M., et al. (1997). Oral premedication with midazolam in paediatric anaesthesia. Effects on sedation and gastric contents. Paediatr. Anaesth. 7 (3), 191–196. doi:10.1046/j.1460-9592.1997.d01-75.x
Rosenthal, P., Schwarz, K. B., Gonzalez-Peralta, R. P., Lin, C. H., Kelly, D. A., Nightingale, S., et al. (2020). Sofosbuvir and ribavirin therapy for children aged 3 to <12 Years with hepatitis C virus genotype 2 or 3 infection. Hepatology 71 (1), 31–43. doi:10.1002/hep.30821
Rotsaert, A., Ogara, C., Mwanga-Amumpaire, J., Kekitiinwa, A. R., Musiime, V., Najjingo, E., et al. (2023). Acceptability of a new 4-in-1 Abacavir/Lamivudine/Lopinavir/Ritonavir paediatric fixed-dose combination: the caregiver-child dyads' perspective. Ther. Adv. Infect. Dis. 10, 20499361231159993. doi:10.1177/20499361231159993
Rouse, C., Mistry, P., Rayner, O., Nickless, J., Wan, M., Southern, K. W., et al. (2017). A mixed methods study of the administration of flucloxacillin oral liquid; identifying strategies to overcome administration issues of medicines with poor palatability. Int. J. Pharm. Pract. 25 (5), 326–334. doi:10.1111/ijpp.12308
Ruperto, N., Brunner, H. I., Zuber, Z., Tzaribachev, N., Kingsbury, D. J., Foeldvari, I., et al. (2017). Pharmacokinetic and safety profile of tofacitinib in children with polyarticular course juvenile idiopathic arthritis: results of a phase 1, open-label, multicenter study. Pediatr. Rheumatol. 15 (Suppl. 1), 86. doi:10.1186/s12969-017-0212-y
Rutebemberwa, E., Nsabagasani, X., Pariyo, G., Tomson, G., Peterson, S., and Kallander, K. (2009). Use of drugs, perceived drug efficacy and preferred providers for febrile children: implications for home management of fever. Malar. J. 8 (1), 131. doi:10.1186/1475-2875-8-131
Saito, M., Hoshi, M., Igarashi, A., Ogata, H., and Edo, K. (1999). The marked inhibition of the bitter taste of Polymyxin B sulfate and trimethoprim x sulfamethoxazole by flavored BMI-60 in pediatric patients. Biol. Pharm. Bull. 22 (9), 997–998. doi:10.1248/bpb.22.997
Saneian, H., and Mostofizadeh, N. (2012). Comparing the efficacy of polyethylene glycol (PEG), magnesium hydroxide and lactulosein treatment of functional constipation in children. J. Res. Med. Sci. 17 (1 SPL.1), S145–S149.
Sanjay, L. R., Ashokbhai, M. K., Ghatole, S., Roy, S., Kashinath, K. P., and Kaity, S. (2025). Strategies for beating the bitter taste of pharmaceutical formulations towards better therapeutic outcomes. RSC Pharm. 2 (1), 59–81. doi:10.1039/d4pm00191e
Savino, F., Viola, S., Erasmo, M., Di Nardo, G., Oliva, S., and Cucchiara, S. (2012). Efficacy and tolerability of peg-only laxative on faecal impaction and chronic constipation in children. A controlled double blind randomized study vs a standard peg-electrolyte laxative. BMC Pediatr. 12, 178. doi:10.1186/1471-2431-12-178
Scheuern, A., Tyrrell, P. N., Haas, J. P., and Hügle, B. (2017). Countermeasures against methotrexate intolerance in juvenile idiopathic arthritis instituted by parents show no effect. Rheumatol. Oxf. 56 (6), 901–906. doi:10.1093/rheumatology/kew507
Schiffman, S.S., Zervakis, J., Graham, B.G., and Westall, H.L. (2002). “Age-related chemosensory losses: effect of medications,” in Chemistry of taste (American Chemical Society), 94–108. doi:10.1021/bk-2002-0825.ch008
Schwarz, K. B., Rosenthal, P., Murray, K. F., Honegger, J. R., Hardikar, W., Hague, R., et al. (2020). Ledipasvir-sofosbuvir for 12 Weeks in children 3 to <6 Years old with chronic hepatitis C. Hepatology 71 (2), 422–430. doi:10.1002/hep.30830
Shchelochkov, O. A., Dickinson, K., Scharschmidt, B. F., Lee, B., Marino, M., and Le Mons, C. (2016). Barriers to drug adherence in the treatment of urea cycle disorders: assessment of patient, caregiver and provider perspectives. Mol. Genet. Metabolism Rep. 8, 43–47. doi:10.1016/j.ymgmr.2016.07.003
Shizukuda, S., Marchini, J. S., Adell, A., Santos, M. A., Brandao, C. F. C., Lima, C. M. M., et al. (2018). Influences of weight, age, gender, genetics, diseases, and ethnicity on bitterness perception: a narrative review of current methodological aspects. Nutrire 43 (1), 4. doi:10.1186/s41110-018-0069-y
Silva, C., Law, L., Koochaki, P., Ogg, C., Shah, S., Manso-Silvan, M., et al. (2022). POSB343 living with distal renal tubular acidosis (DRTA): qualitative research with patients, and caregivers, and nephrologists. Value Health 25 (1 Suppl. ment), S227. doi:10.1016/j.jval.2021.11.1110
Sleath, B. L., Jackson, E., Thomas, K. C., Galloway, J., Dumain, L., Thorpe, J., et al. (2006). Literacy and perceived barriers to medication taking among homeless mothers and their children. Am. J. Health Syst. Pharm. 63 (4), 346–351. doi:10.2146/ajhp050070
Smith, C. J., Sammons, H. M., Fakis, A., and Conroy, S. (2013). A prospective study to assess the palatability of analgesic medicines in children. J. Adv. Nurs. John Wiley and Sons, Inc. 69 (3), 655–663. doi:10.1111/j.1365-2648.2012.06050.x
Sondheimer, J. M., Sokol, R. J., Taylor, S. F., Silverman, A., and Zelasney, B. (1991). Safety, efficacy, and tolerance of intestinal lavage in pediatric patients undergoing diagnostic colonoscopy. J. Pediatr. 119 (1 Pt 1), 148–152. doi:10.1016/s0022-3476(05)81056-x
Splieth, C., Steffen, H., Rosin, M., and Welk, A. (2000). Caries prevention with chlorhexidine-thymol varnish in high risk schoolchildren. Community Dent. oral Epidemiol. 28 (6), 419–423. doi:10.1034/j.1600-0528.2000.028006419.x
Steans, A., Manners, P. J., and Robinson, I. G. (1990). A multicentre, long-term evaluation of the safety and efficacy of ibuprofen syrup in children with juvenile chronic arthritis. Br. J. Clin. Pract. 44 (5), 172–175. doi:10.1111/j.1742-1241.1990.tb10774.x
Steele, R. W., Blumer, J. L., and Kalish, G. H. (2002). Patient, physician, and nurse satisfaction with antibiotics. Clin. Pediatr. 41 (5), 285–299. doi:10.1177/000992280204100501
Stevens, R., Votan, B., Lane, R., Schaison, G., Hewitt, M., Kohler, J., et al. (1996). A randomized study of ondansetron syrup in children: evaluation of taste acceptability and tolerance. Pediatr. Hematol. Oncol. 13 (2), 199–202. doi:10.3109/08880019609030816
Suchismita, A., Ashritha, A., Sood, V., Lal, B. B., Khanna, R., Kumar, G., et al. (2023). Study of adherence to medication in pediatric liver diseases “SAMPLD” study in Indian children. J. Clin. Exp. Hepatology 13 (1), 22–30. doi:10.1016/j.jceh.2022.10.006
Syofyan, S., Dachriyanus, D., Masrul, M., and Rasyid, R. (2019). Children's perception and belief about medicines: effectiveness and its autonomy. Open Access Maced. J. Med. Sci. 7 (15), 2556–2562. doi:10.3889/oamjms.2019.662
Taher, A. T., Origa, R., Perrotta, S., Kouraklis, A., Ruffo, G. B., Kattamis, A., et al. (2018). Patient-reported outcomes from a randomized phase II study of the deferasirox film-coated tablet in patients with transfusion-dependent anemias. Health Qual. Life Outcomes 16 (1), 216. doi:10.1186/s12955-018-1041-5
Tannous, W. N., and Azouz, E. M. (1991). Bowel opacification for abdominal computed tomography in children: a clinical trial of oral Telebrix 38. Can. Assoc. Radiol. J. 42 (2), 102–105.
Taylor, F. J., Li, G., Almossawi, O., Dulfeker, H., and Jones, V. (2016). G201 A Really Wheezy Way to Save Money – switching prednisolone formulation to achieve large scale savings in the management of wheeze in children. Archives Dis. Child. 101 (Suppl. 1), A107–A108. doi:10.1136/archdischild-2016-310863.192
Thompson, A., Reader, S., Field, E., and Shephard, A. (2013). Open-label taste-testing study to evaluate the acceptability of both strawberry-flavored and orange-flavored amylmetacresol/2,4-dichlorobenzyl alcohol throat lozenges in healthy children. Drugs R D 13 (2), 101–107. doi:10.1007/s40268-013-0012-x
Thomson, E., Weston, R., and Foster, C. (2015). Gastrostomy administration of antiretroviral therapy across the ages. HIV Med. 16 (Suppl. 2), 15–16. doi:10.1111/hiv.12265
Thornburg, C., Bonner, M., and Puffer, E. (2011). Family perceptions of treatment protocols for secondary stroke prevention in children with sickle cell anemia. Pediatr. Blood Cancer 56 (6), 947. doi:10.1002/pbc.23092
Tiengkate, P., Lallemant, M., Charoenkwan, P., Angkurawaranon, C., Kanjanarat, P., Suwannaprom, P., et al. (2022). Gaps in accessibility of pediatric formulations: a cross-sectional observational study of a teaching hospital in northern Thailand. Child. (Basel) 9 (3), 301. doi:10.3390/children9030301
Todd, K., Luchtman-Jones, L., Blackmore, A., Hennessey, C., and McGrady, M. E. (2023). Barriers to medication adherence in children, adolescents, and young adults prescribed anticoagulation. Pediatr. Blood Cancer 70 (2), e30076. doi:10.1002/pbc.30076
Toscani, M., Drehobl, M., Freed, J., and Stool, S. (2000). A multicenter, randomized, comparative assessment in healthy pediatric volunteers of the palatability of oral antibiotics effective in the therapy of otitis media. Curr. Ther. Res. 61 (5), 278–285. doi:10.1016/s0011-393x(00)80018-1
Touchette, P., Douglass, E., Graeff, J., Monoang, I., Mathe, M., and Duke, L. W. (1994). An analysis of home-based oral rehydration therapy in the Kingdom of Lesotho. Soc. Sci. Med. 39 (3), 425–432. doi:10.1016/0277-9536(94)90140-6
Tran, C. T., Pham, T. H., Tran, K. T., Nguyen, T. K. C., and Larsson, M. (2017). Caretakers' barriers to pediatric antiretroviral therapy adherence in Vietnam - a qualitative and quantitative study. Appl. Nurs. Res. 35, 1–5. doi:10.1016/j.apnr.2017.02.016
Tricco, A. C., Lillie, E., Zarin, W., O'Brien, K. K., Colquhoun, H., Levac, D., et al. (2018). PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann. Intern Med. 169 (7), 467–473. doi:10.7326/M18-0850
Tsai, D., Djukic, F., Martin, J., Cook, A., Hsia, Y., and Sharland, M. (2022). Global accelerator for paediatric formulations network (GAP-f) report. Available online at: https://cdn.who.int/media/docs/default-source/essential-medicines/2023-eml-expert-committee/reviews/r1_emlc-review_attach1-annexb.pdf?sfvrsn=9f99260d_1.
Tucker, C. M., Fennell, R. S., Pedersen, T., Higley, B. P., Wallack, C. E., and Peterson, S. (2002). Associations with medication adherence among ethnically different pediatric patients with renal transplants. Pediatr. Nephrol. 17 (4), 251–256. doi:10.1007/s00467-001-0806-x
Tuleu, C., Hughes, D. A., Clapham, D., Vallet, T., and Ruiz, F. (2021). Acceptability of generic versus innovator oral medicines: not only a matter of taste. Drug Discov. Today 26 (2), 329–343. doi:10.1016/j.drudis.2020.11.008
Uhari, M., Eskelinen, L., and Jokisalo, J. (1986). Acceptance of antibiotic mixtures by infants and children. Eur. J. Clin. Pharmacol. 30 (4), 503–504. doi:10.1007/BF00607970
Unachak, K., Louthrenoo, O., and Katanyuwong, K. (2002). Primary hypomagnesemia in Thai infants: a case report with 7 years follow-up and review of literature. J. Med. Assoc. Thai 85 (11), 1226–1231.
United Nations (2022). World Population Prospects 2022. New York, USA: United Nations Department of Economic and Social Affairs, Population Division. Available online at: https://population.un.org/wpp/.
Ustun, B., Reissland, N., Covey, J., Schaal, B., and Blissett, J. (2022). Flavor sensing in utero and emerging discriminative behaviors in the human fetus. Psychol. Sci. 33 (10), 1651–1663. doi:10.1177/09567976221105460
Valayannopoulos, V., Schiff, M., Guffon, N., Nadjar, Y., García-Cazorla, A., Martinez-Pardo Casanova, M., et al. (2019). Betaine anhydrous in homocystinuria: results from the RoCH registry. Orphanet J. Rare Dis. 14 (1), 66. doi:10.1186/s13023-019-1036-2
van der Vossen, A. C., Cransberg, K., de Winter, B. C. M., Schreuder, M. F., van Rooij-Kouwenhoven, R. W. G., Vulto, A. G., et al. (2020). Use of amlodipine oral solution for the treatment of hypertension in children. Int. J. Clin. Pharm. 42 (3), 848–852. doi:10.1007/s11096-020-01000-9
Van de Vijver, E., Desager, K., Mulberg, A. E., Staelens, S., Verkade, H. J., Bodewes, F. A. J. A., et al. (2011). Treatment of infants and toddlers with cystic fibrosis-related pancreatic insufficiency and fat malabsorption with pancrelipase MT. J. Pediatr. Gastroenterol. Nutr. 53 (1), 61–64. doi:10.1097/MPG.0b013e31820e208e
Van Dyke, R. B., Lee, S., Johnson, G. M., Wiznia, A., Mohan, K., Stanley, K., et al. (2002). Reported adherence as a determinant of response to highly active antiretroviral therapy in children who have human immunodeficiency virus infection. Pediatrics 109 (4), e61. doi:10.1542/peds.109.4.e61
Varma, A. S., Rosicker, R., Noor, T., Chennasamudram, S., and Vasylyeva, T. (2012). Medication adherence among adolescents in pediatric renal clinic. J. Investigative Med. 60 (1), 327–328. doi:10.2310/JIM.0b013e318240d395
Varnell, C., Warmin, A., Barletta, G., Belsha, C., Harshman, L., Kershaw, D., et al. (2021). Reducing rejection in pediatric kidney transplantation: the improving renal outcomes collaborative (IROC). Am. J. Transplant. 21 (Suppl. 4), 411–412. doi:10.1111/ajt.16847
Varnell, C. D., Rich, K. L., Nichols, M., Dahale, D., Goebel, J. W., Pai, A. L. H., et al. (2017). Assessing barriers to adherence in routine clinical care for pediatric kidney transplant patients. Pediatr. Transplant. 21 (7), e13027. doi:10.1111/petr.13027
Venables, R., Batchelor, H., Hodson, J., Stirling, H., and Marriott, J. (2015a). Determination of formulation factors that affect oral medicines acceptability in a domiciliary paediatric population. Int. J. Pharm. 480 (1-2), 55–62. doi:10.1016/j.ijpharm.2015.01.023
Venables, R., Haley, H., Gilchrist, F., and Lenney, W. (2018). P20 Pic study: prednisolone in children: prescribing, dispensing and administering prednisolone formulations to children with acute asthma or wheeze. Archives Dis. Child. 103 (2), e2.22–e2. doi:10.1136/archdischild-2017-314585.29
Venables, R., Stirling, H., Batchelor, H., and Marriott, J. (2015b). Problems with oral formulations prescribed to children: a focus group study of healthcare professionals. Int. J. Clin. Pharm. 37 (6), 1057–1067. doi:10.1007/s11096-015-0152-x
Viprakasit, V., Hamdy, MM., Hassab, HMA., Sherief, LM., Al-Bagshi, M., Khattab, M., et al. (2023). Patient preference for deferasirox film-coated versus dispersible tablet formulation: a sequential-design phase 2 study in patients with thalassemia. Ann Hematol. 102 (8), 2039–2049. doi:10.1007/s00277-023-05240-3
Voskuijl, W., de Lorijn, F., Verwijs, W., Hogeman, P., Heijmans, J., Mäkel, W., et al. (2004). PEG 3350 (Transipeg) versus lactulose in the treatment of childhood functional constipation: a double blind, randomised, controlled, multicentre trial. Gut 53 (11), 1590–1594. doi:10.1136/gut.2004.043620
Wademan, D. T., Busakwe, L., Nicholson, T. J., van der Zalm, M., Palmer, M., Workman, J., et al. (2019). Acceptability of a first-line anti-tuberculosis formulation for children: qualitative data from the SHINE trial. Int. J. Tuberc. Lung Dis. 23 (12), 1263–1268. doi:10.5588/ijtld.19.0115
Warembourg, M., Lonca, N., Filleron, A., Tran, T. A., Knight, M., Janes, A., et al. (2020). Assessment of anti-infective medication adherence in pediatric outpatients. Eur. J. Pediatr. 179 (9), 1343–1351. doi:10.1007/s00431-020-03605-8
Warzecha, J., Dziekiewicz, M., Bieńkowska-Tokarczyk, A., Małecki, M., and Banaszkiewicz, A. (2022). A new viscous budesonide formulation for the treatment of eosinophilic esophagitis in children: a preliminary experience and review of the literature. J. Clin. Med. 11 (22), 6730. doi:10.3390/jcm11226730
Wataya, T. (2017). Fluorescence-guided surgery with 5-aminolevulinic acid for resection of pediatric brain tumors. Neuro-Oncology 19 (Suppl. 6), vi242. doi:10.1093/neuonc/nox168.988
Wijesekera, K., Aralis, H., Sinclair, M., Cosanella, T., Alejos, J., and Lester, P. (2019). Innovative behavioral health screening and resilience building intervention for pediatric heart transplant youth and families: the focus-pedsht program. Pediatr. Transplant. 23 (Suppl. 1). doi:10.1111/petr.13439
Williford, D. N., Guilfoyle, S. M., and Modi, A. C. (2023). Demystifying a family-based epilepsy adherence problem-solving intervention: exploring adherence barriers and solutions. Clin. Pract. Pediatr. Psychol. 11 (1), 66–73. doi:10.1037/cpp0000436
Wooding, S. P., Ramirez, V. A., and Behrens, M. (2021). Bitter taste receptors: genes, evolution and health. Evol. Med. Public Health 9 (1), 431–447. doi:10.1093/emph/eoab031
World Bank (2024). World Bank Country and Lending Groups. Washington DC, USA: World Bank. Available online at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
World Health Organization (2003). Adherence to long-term therapies: evidence for action. Geneva, Switzerland: World Health Organization. Available online at: https://iris.who.int/handle/10665/42682
World Health Organization (2024). HIV statistics, globally and by WHO region, 2024 (WHO/UCN/HHS/SIA/2024.10) Geneva, Switzerland: World Health Organization Available online at: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/strategic-information/hiv-data-and-statistics.
World Health Organization. (2022). International classification of diseases, eleventh revision (ICD-11). World Health Organization. Available online at: https://icd.who.int/browse11.
WHO Collaborating Centre for Drug Statistics Methodology (2023). Guidelines for ATC classification and DDD assignment (2024). Oslo, Norway: WHO Collaborating Centre for Drug Statistics Methodology. Available online at: https://atcddd.fhi.no/atc_ddd_index/
Xin, Z., Wang, H., Li, C., Wan, Q., and Wang, N. (2022). Use of self-made midazolam mixtures in preoperative sedation of children undergoing oral surgery under general anesthesia: a prospective, randomized, control study. Anaesth. Pain Intensive Care 26 (2), 199–204. doi:10.35975/apic.v26i2.1821
Yafout, M., Ousaid, A., Lachguer, K., Khayati, Y., and Ait Haj Said, A. (2022). Medication use in children: a survey among hospital pediatricians in Morocco. Pharm. Clin. 57 (3), 227–233. doi:10.1016/j.phacli.2022.04.008
Yeowell, G., Burns, D. S., and Fatoye, F. (2021). The burden of pharmacological treatment on health-related quality of life in people with a urea cycle disorder: a qualitative study. J. Patient Rep. Outcomes 5 (1), 110. doi:10.1186/s41687-021-00387-x
Yoo, O., Tang, E. K. Y., Salman, S., Nguyen, M. N., Sommerfield, D., Sommerfield, A., et al. (2022). A randomised controlled trial of a novel tramadol chewable tablet: pharmacokinetics and tolerability in children. Anaesthesia 77 (4), 438–448. doi:10.1111/anae.15650
Young, G., Lensing, A. W. A., Monagle, P., Male, C., Thelen, K., Willmann, S., et al. (2020). “Rivaroxaban for treatment of pediatric venous thromboembolism. An Einstein-Jr phase 3 dose-exposure-response evaluation. J. Thromb. Haemost. 18 (7), 1672–1685. doi:10.1111/jth.14813
Zannikos, P. N., Doose, D. R., Leitz, G. J., Rusch, S., Gonzalez, M. D., Solanki, B., et al. (2011). Pharmacokinetics and tolerability of rabeprazole in children 1 to 11 years old with gastroesophageal reflux disease. J. Pediatr. Gastroenterol. Nutr. 52 (6), 691–701. doi:10.1097/MPG.0b013e318207834d
Zelikovsky, N., Dobson, T., and Norman, J. (2011). Medication beliefs and perceived barriers in adolescent renal transplant patients and their parents. Pediatr. Nephrol. 26 (6), 953–959. doi:10.1007/s00467-011-1805-1
Keywords: children, medicines, bitter, taste, palatability, acceptability, adherence, outcomes
Citation: Ranmal SR, Walsh J and Tuleu C (2025) Poor-tasting pediatric medicines: Part 1. A scoping review of their impact on patient acceptability, medication adherence, and treatment outcomes. Front. Drug Deliv. 5:1553286. doi: 10.3389/fddev.2025.1553286
Received: 30 December 2024; Accepted: 11 February 2025;
Published: 22 April 2025.
Edited by:
René Holm, University of Southern Denmark, DenmarkReviewed by:
Daniel Schaufelberger, Schaufelberger Consulting LLC, United StatesSathish Dharani, Texas A and M University, United States
Copyright © 2025 Ranmal, Walsh and Tuleu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sejal R. Ranmal, sejal.ranmal@ucl.ac.uk
 Sejal R. Ranmal
Sejal R. Ranmal