- 1Laboratory of Human Pathologies Biology, Department of Biology, Faculty of Sciences, Faculty of Medicine and Pharmacy, Genomic Center of Human Pathologies, University Mohammed V, Rabat, Morocco
- 2Laboratory of Genomics and Bioinformatics, School of Pharmacy, Mohammed VI University of Health Sciences, Casablanca, Morocco
- 3Department of Surgery, School of Medicine, Mohammed VI University of Health Sciences, Casablanca, Morocco
- 4Environmental Health Laboratory, Institut Pasteur Du Maroc, Casablanca, Morocco
- 5Virology Unit, Immunovirology Laboratory, Institut Pasteur Du Maroc, Casablanca, Morocco
- 6Department of Epidemiology and Biostatistics, International School of Public Health, Mohammed VI University of Health Sciences, Casablanca, Morocco
- 7Department of Epidemiology and Public Health, Faculty of Medicine, University Sidi Mohammed Ben Abdellah, Fez, Morocco
- 8Moroccan Health Competencies Abroad Network (C3M), Brussels, Belgium
- 9National Center for Scientific and Technical Research, Rabat, Morocco
During the unprecedented COVID-19 pandemic, the primary goal of many countries has been to achieve herd immunity through the organization of massive vaccination campaigns. Nevertheless, developing countries, including Africans, have been facing limited vaccine supply. Conventional inactivated or subunit vaccines are widely used across the world; however, their production is costly and could be limited by the supply chain during a pandemic such as COVID-19. Genetic vaccines, such as mRNA- or adenovirus-based vaccines, have been developed as alternatives but are still costly and require low-temperature storage. The plant-based vaccine concept has attracted increasing attention in recent years due to its potential advantages, such as low cost, high production volume, and thermostability. In this review, we propose plant-based vaccines as an attractive alternative for massive and rapid vaccination protocols against COVID-19 in African countries by exploiting local crops. In addition, we discuss the mechanisms of action, required standards, benefits, challenges, and prospects for the application of this novel biotechnological tool in the African continent.
Introduction
Infectious diseases are responsible for the deaths of over a million people each year around the world (Giddings et al., 2000). Many new or previously unrecognized bacterial, fungal, viral, and parasitic diseases have emerged in recent years. Simultaneously, however, many previously controlled infections have re-emerged or become resistant to antimicrobial therapies. Re-emerging infectious diseases and the emergence of new viral strains are worsening the global health situation and pose a continuous threat (Szymanski et al., 2016); the latest example is the widespread novel coronavirus SARS-CoV-2 causing the COVID-19 disease. This outbreak underlines the need to find an expedient vaccine capable of preventing the disease and ideally limiting the spread of the virus (Rosales-Mendoza et al., 2020).
Vaccines are biological preparations that are designed to trigger an immune response to a given pathogen (Kurup and Thomas, 2020). They can be prepared from live-attenuated or inactivated disease-causing organisms, recombinant vectors, protein subunits, or DNA/RNA nucleic acids (D’Amico et al., 2021). During the vaccination process, the immune system is primed, thus allowing the body to face and combat new infectious agents (Govea-Alonso et al., 2014b).
However, a limitation of conventional vaccines is the complex and costly industrial production process, limiting access to vaccines, particularly in developing countries such as Africa (Johansen et al., 1999). In recent years, a novel vaccine concept has been tested that has the potential to overcome the drawbacks of traditional vaccines (Kurup and Thomas, 2020). This new concept relates to the use of genetically modified plants as antigen biofactories. These plant-made pharmaceuticals are known as plant-based vaccines (Márquez-Escobar et al., 2017). Recently, plant-based vaccines, as a neoteric technology, have gained wide recognition and have attracted significant attention from both academia and industry (Sohrab et al., 2017). This promising technique aims to induce specific immune responses within a short period of time (Shakoor et al., 2019) following the oral administration and absorption of the plant-based vaccine (Sohrab et al., 2017). One of the interesting merits of plant-based vaccines is that the odds of contamination by plant pathogens are very weak or even negligible given that plant pathogens are not known to infect humans (Ulmer et al., 2006).
Since plants can be produced in a rapid and convenient manner, plant-based production platforms are seen as exemplary alternatives to conventional vaccines (Márquez-Escobar et al., 2017; Gunasekaran and Gothandam, 2020). In addition to the manufacturing process and cost-effectiveness, plant-based vaccines are also a suitable choice that can pave the way for bulk production to meet market demands with reduced processing times (Dhama et al., 2020). These specific advantages make plant-based vaccines an attractive concept for rapidly developing potent vaccines for COVID-19, a disease that has experienced a sudden and unpredictable outbreak across the entire globe (Esqueda and Chen, 2021; LeBlanc et al., 2020). At present, many companies around the globe are racing to use plants as plant-based vaccines or to produce vaccines in engineered plants or plant culture systems (Uthaya Kumar et al., 2021). In this article, we present plant-based vaccines as an option for massive vaccination against COVID-19 in Africa and discuss the mechanisms of action, required standards, benefits, challenges and prospects for this powerful biotechnological tool on the African continent.
An Overview of Plants as Vaccine Biofactories
The most common method of administering vaccines is by injection (subcutaneous or intramuscular), however, some vaccines can be delivered mucosally (nasal or oral). Injected vaccines trigger protective immunity by stimulating the production of IgG antibodies and are therefore particularly suitable against systemic or respiratory infections. Nonetheless, purification of the target antigen is required before administration. Injectable vaccines are often produced in mammalian cell cultures using stable expression systems (Cerovska et al., 2012; Ortega-Berlanga and Pniewski, 2022). Purified vaccines are generally unsuitable for the oral route as opposed to plant-based vaccines, which have the potential to preserve antigen immunogenicity and biological activities in the gastrointestinal tract throughout their natural bioencapsulation in plant cell organelles (Takeyama et al., 2015).
There are several target diseases for which plant-based vaccine technology has been applied, including measles, cholera, foot and mouth diseases, and hepatitis B, C, and E (Muynck et al., 2010). Thus far, several plant species, including Arabidopsis, alfalfa, potato, soybean, lupine, lettuce, tomato, wheat, cowpea, apple, rice, black-eyed bean, corn, banana, canola, carrot, clover, papaya, peanut, spinach, and tobacco, have been widely used to express foreign antigens in their plant-based parts (Korban, 2002; Sohrab et al., 2017). Some of these have reached advanced stages of preclinical and clinical evaluation (e.g., potato, spinach, and lettuce have reached phase 1, tobacco and maize reached phase 2, while carrot cell suspension has reached phase 3) (Sohrab, 2020).
The mucosal immune system is the first line of defense where disease-causing organisms begin to infect the human body. Mucosal surfaces are found lining the urinary-reproductive, digestive, and respiratory tracts (Zeitlin et al., 1999). The leading mechanism of action of plant-based vaccines is to stimulate systemic and mucosal immune systems in the fight against a targeted foreign pathogen by expressing a transgene in a selected plant cell. However, integration of the transgene into the plant host genome is not always needed. Rapid and high-level gene expression is possible when using plant viruses (e.g., Tobacco Mosaic Virus (TMV)) or by transient expression via the infiltration of tissues with Agrobacterium or by direct gene delivery methodology without combination with a vector (Kurup and Thomas, 2020).
The formulation of a vaccine of plant origin is intended to serve as a source of a recombinant antigen produced in a host, biomass, or purified fractions of which are intended to serve as elicitors of protective immunity throughout administration by different routes. This represents a promising method for the production of mucosally administered vaccines, particularly oral vaccines, which require minimal raw plant biomass processing and administration training. The mucosa is the primary site of entry for many pathogenic agents, which pervade the host through the respiratory, genital, or gastrointestinal tracts, triggering a secretory immunoglobulin A (IgA) response to provide the first line of defense against these pathogens. Mucosal immune cells are connected to an organized group of lymphoid tissue structures known as mucosa-associated lymphoid tissue (MALT) (Govea-Alonso et al., 2013). According to anatomical localization, these can be classified into distinct terms, including nasopharynx-associated lymphoid tissue (NALT), gut-associated lymphoid tissue (GALT), and bronchi-associated lymphoid tissue (BALT). Peyer’s patches (PP), a large group of lymphoid follicles, are a primary mucosal-inducing location in GALT. Specialized antigen-sampling epithelial cells, the microfold (M) cells, are found in the follicle-associated epithelium (FAE) overlying the PPs. These cells have a folded luminal surface and do not release digestive enzymes or mucus. They also have a thin glycocalyx surface that inhibits particles larger than 1 µm from entering. The functions of M cells include the transfer of intact macromolecules and microbes over epithelial barriers to subepithelial dendritic cells (DCs), which can then deliver these antigens to neighboring mucosal B- and T-cell areas. M cells also exhibit a pocket in the basolateral membrane, which is closely associated with DCs and T and B cells. As a result, these pockets play roles in the initiation of mucosal immune responses. After antigen presentation, B cells migrate to distant effector sites, such as the respiratory and gastrointestinal lamina propria (LP). As a result, dimeric IgAs are produced and secreted, with the potential to either prevent the pathogen from interacting with host receptors or neutralize pathogenic toxins, resulting in protective immunity. Because PPs contain serum IgG-producing cells, mucosal vaccination can also induce local IgG synthesis. One key feature of mucosal vaccines is their ability to stimulate both mucosal and systemic immune responses, providing two relevant arms for immunoprotection (Govea-Alonso et al., 2014a). With respect to this, mucosal IgA secretion has been observed with several orally administered plant-based vaccines, and these antibodies have been recorded at both the mucosal site of antigen presentation and other mucosal sites. The detection of antibody-secreting cells in peripheral blood after oral administration of plant-based vaccines provides additional evidence for the induction of mucosal responses (Streatfield and Howard, 2003a; Streatfield, 2005).
The demonstration of immune response induction is an important step in the development of new vaccines. Candidate subunit vaccines derived from plants or plant viruses have been tested on laboratory animals, domestic animals, and humans. Immune responses to antigens spiked from plants and then administered intraperitoneally, subcutaneously, intramuscularly, intranasally, or orally have been recorded with several of these vaccine candidates, as well as antigens delivered orally into raw plant tissues or transformed used for expression. To determine immunogenicity, serum IgG and gut mucosal IgA levels are typically measured. Other mucosal site responses were also monitored (Streatfield and Howard, 2003b).
The main technical challenges for subunit vaccines, including plant-based vaccines, when administered orally are to survive digestive processes in the gut, cross the mucus barrier, interact with epithelial and professional sampling cells (e.g., M-cells) and ultimately be taken up in such a way to induce an immune response, and confer protection. Antigens have been protected from digestive enzymes using various methods. There are two main categories: the use of attenuated bacterial strains and the encapsulation of the antigen in a protective layer. Attenuated strains have the advantage of directing the antigen to the surface of the intestine, where it can be taken up by the mucosal immune system. This approach, however, has the same potential safety disadvantage as any vaccine based on an attenuated strain. Encapsulation is a naturally safer method of antigen protection that can be accomplished with biodegradable polymers, liposomes, proteosomes, or a product derived from transgenic plants expressing the antigen. In contrast to other methods of antigen encapsulation, which require additional processing steps, providing protection for these antigens through bioencapsulation in seeds is at no extra cost (Streatfield and Howard, 2003a).
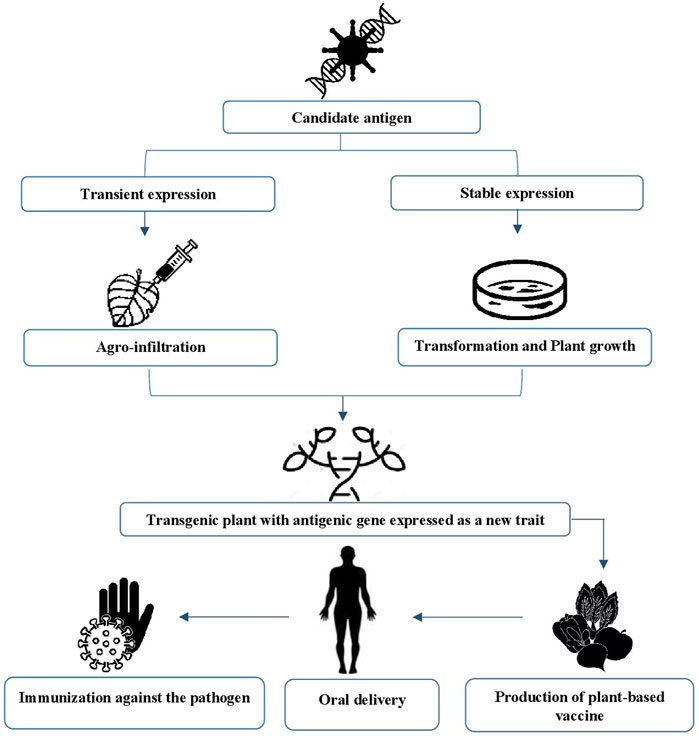
At present, two methods are used to generate plant-based antigens from genetically modified plants (Sohrab et al., 2017): stable transformation and transient expression transformation systems (Figure 1) (Kurup and Thomas, 2020). The choice of method depends on the location at which the transgene has been inserted into the cells. The stable transformation system, also known as nuclear and plastid transformation, is characterized by its ability to induce permanent modifications within the genetic material of the recipient cell, given that the desired transgene is inserted into the host’s genome (Laere et al., 2016).
The most commonly used approach to express heterologous proteins in plants is the stable transformation system. However, the long period of time needed to generate transformed plant lines is disadvantageous (Márquez-Escobar et al., 2017). To produce large amounts of recombinant proteins in a short span of time, transient expression systems are the preferred choice. These systems can either be based on the infection of plants with a modified plant virus, the infiltration of plants with Agrobacterium, or a combination of both methods. Compared with stable transformation, all variants of the transient expression system are much faster (Redkiewicz et al., 2014). Agrobacteria (carriers of the transgenic vector system) can be incorporated into all aerial parts of the plant by vacuum infiltration technology (Wirz et al., 2012) and can transform their own T-DNA into plant cells (Sohrab et al.,2017). The Agrobacterium delivery-based method is characterized by robust yields, easy scalability, and the availability of industrial processes (Márquez-Escobar et al., 2017). These criteria make it possible to overcome the pitfalls associated with stable integration (Laere et al., 2016).
Posttranslational modifications (PTMs) can be performed evenly in this system due to the presence of an endomembrane that is similar to that of the animal cell (Esqueda and Chen, 2021). Indeed, plants have demonstrated a high degree of tolerance to changes in posttranslational modifications pathways allowing recombinant proteins to be modified in a specific and controlled manner, frequently resulting in a homogeneity of products by alternative expression platforms. In addition, plants have been engineered that feature a humanized glycosylation system (Webster and Thomas, 2012; Schähs et al., 2007). By growing plants over many generations, this approach allows for continuous vaccine manufacture and availability, making this strategy highly efficient in terms of large-scale production in only a matter of weeks (Dhama et al., 2020). However, it is not possible to create a seed bank when viral-mediated expression is performed (Márquez-Escobar et al., 2017; Kumar et al., 2021). Furthermore, transient expression typically requires antigen purification, while stable transformation allows for the implementation of oral immunization schemes without purification, particularly in plant-based crops (Rosales-Mendoza et al., 2020).
Transient expression is also restricted to the specific tissue that has been infiltrated with a suspension of Agrobacterium. This limits the quantity of tissue available and thus maximizes the expression levels of proteins. One means of attaining this outcome necessitates the use of replicating virus-based vectors such as pEAQ vectors, which are a series of binary vectors designed for the controlled expression of multiple proteins in plants. However, these vectors are limited by the genetic stability of the constructs during replication of the viral genome, the complexity, and size of the proteins that can be expressed, and concerns related to biocontainment (Sainsbury et al., 2009).
Required Standards and Challenges Facing the Development and Manufacture of Plant-based Vaccines
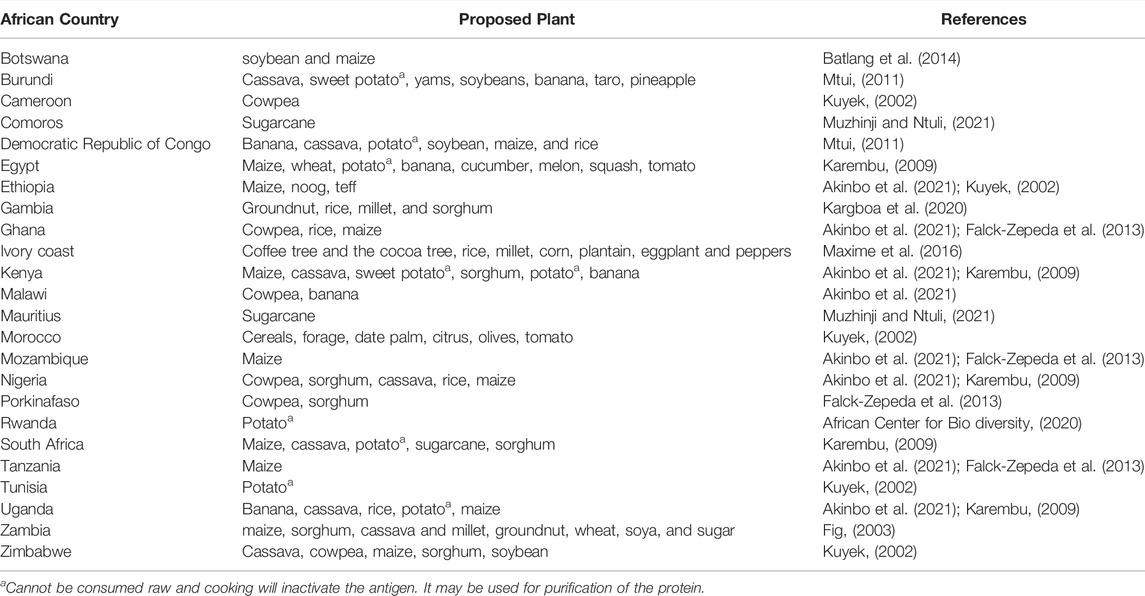
There are still no plant-based vaccines that have been approved for human use, and significant research is required for plant vaccines to be approved for public consumption (Uthaya Kumar et al., 2021). Despite the intriguing potential of plant-based vaccines, significant challenges and limitations persist. Particular attention should be given to the selection of candidate plants that are most suitable for the expression of antigens (Shakoor et al., 2019); there are distinct determinants that render a plant useful as a plant-based vaccine. In this regard, the consumable part of the plant must have a long shelf life and be safe from degradation even after long periods of storage; it must also be able to grow and mature quickly and undergo transformation readily (Gunasekaran and Gothandam, 2020). Such plants must also be palatable, native, have good taste, and be easily accessible. In Table 1, we suggest candidate plants for certain African countries.
When selecting appropriate plants, the site of expression is also an important factor to consider. For example, whether the antigen is to be expressed in the chloroplasts or leaves of germinating seedlings as a final vaccine product or in dry tissues such as the seeds needs to be considered. (Shakoor et al., 2019). Seeds are advantageous in that proteins remain stable in dry seeds over long periods of time at room temperature. This is an important advantage in the African context because the seeds will not require any special storage conditions. Improved storage possibilities for plant-based vaccines become possible as the seeds of genetically modified plants can quickly be dried, are heat-stable and have a lower moisture content. In addition, the seeds of plants have a higher protein content and can express high levels of foreign proteins (Sahoo et al., 2020). Seeds can also provide a platform for antibody production and can be stored at high concentrations in a closed space and are also appropriate for oral administration. More significantly, a crushed seed matrix can protect antibodies from gastric digestion by supplanting proteases (Virdi et al., 2013; Vanmarsenille et al., 2018).
To fulfill the objectives of vaccine production, we need to consider both the candidate plant and the selected antigen; the latter is a substantial aspect because not all antigens will be compatible with the chosen host plant. This factor, in addition to safety considerations, affects the thermal stability of the final vaccine. The application of genomic and proteomic approaches can help to identify appropriate antigen candidates (Laere et al., 2016). Another major factor to consider is dosage consistency, particularly when dealing with a pandemic and associated large-scale vaccine distribution (Uthaya Kumar et al., 2021). Due to differences in the size and maturity of the plants used, the concentration of the final vaccine produced will inevitably vary from fruit to fruit and from generation to generation because transgenic candidates exhibit intrinsic variability in terms of antigen expression. Furthermore, numerous elements make it quite difficult to determine the required dosage for each patient, such as differences in age, body weight, and individual immune responses. If close monitoring of these factors fails, then an underdose will result in a reduction in antibody production, while immunological tolerance may occur if a patient is overdosed (Laere et al., 2016). This is a good argument for extracting and dosing the antigen rather than administering transgenic material as food. This challenge must be addressed carefully, particularly when public health is at stake (Uthaya Kumar et al., 2021). In some cases, certain protein antigens expressed in plants can be harvested, purified, and then administered by injection to humans; this may avoid the potential immunotolerance arising from oral administration.
The plant-based purified antigen will likely need adjuvants to potentiate immunogenicity and tailor the immune response to the desired arm depending on the pathogen being considered. For example, historical aluminum-based adjuvants could be suitable for bacterial and some viral pathogens requiring neutralizing antibody-mediated immune arms for protection. Other pathogens involving chronic intracellular infection, such as HIV and tuberculosis, may require an additional strong T-cell mediated response, cytotoxic CD8 T cells, to clear infected cells. The pro-adjuvant T-cell response may involve more elaborate strategies, such as TLR-agonist ligands or saponins.
Compared to traditional vaccines, orally administered vaccines require higher doses of antigens to provoke a sufficient immune response. This poses challenges relating to the formulation used and increases the risk of immunotolerance if the body is unable to recognize the formulation as pathogenic. Therefore, powerful adjuvants must be added to the vaccine to boost the immune system (Vela Ramirez et al., 2017). The administration of an antigen in conjunction with an additional oral boost is pivotal for the generation of significant long-term mucosal and systemic immunity. Thanks to these immunopotentiating agents, the body raises its baseline immunity, and in some cases, immunostimulants or cytokines are combined with the antigen and are secreted in parallel (New, 2019).
The development and use of adjuvants has certainly improved the efficacy of oral vaccines in animal models. Nevertheless, this knowledge has yet to be extrapolated to humans. There are many differences between humans and animals, such as genetic diversity; these differences may influence the efficacy and safety of a vaccine (De Smet et al., 2014). Over the last few years, several techniques have been developed for the successful oral delivery of vaccines, including chemical modifications, enzyme inhibitors, macromolecular conjugation, targeted delivery to the colon, and the encapsulation of vaccines into particulate delivery systems. However, none of these strategies can be considered a breakthrough (Jazayeri et al., 2021).
Another concern related to the production of plant-based vaccines is controlling the stability of the transgenes, as this may cause allergic reactions during posttranslational modifications as well as breaking oral tolerance when coadministered with oral adjuvants to enhance the mucosal response. This type of immune disorder may lead to hypersensitivity to other proteins in everyday food (Kurup and Thomas, 2020). These two contradictory phenomena, tolerance and allergenicity, are the main disadvantages of plant-based pharmaceuticals. N-glycosylation, a form of posttranslational modification, could be responsible for the onset of an allergic response, and the frequent oral intake of these vaccines could activate regulatory T cells against the vaccine antigen (Takeyama et al., 2015). Therefore, the stability of the candidate plants must be carefully monitored. This stability is dependent on several determinants that significantly influence expression and can differ within groups of plants generated in the same gene transfer experiment. The main factors affecting the stability of genetically modified plants include the site of transgene integration, the number of transgene copies, their relative arrangement, and intactness; these factors can influence the induction of silencing via DNA methylation and/or the production of aberrant RNA species (Kohli et al., 2010).
In this same context, the rigorous control of plants during their growth is fundamental in view of the likelihood of cross-contamination between genetically and non genetically modified plants during pollination; thus, transgenic plants can become invasive. Moreover, there is an increased risk of fortuitous integration of these products into the human food chain which may exert an influence on wildlife (Kurup and Thomas, 2020).
Although oral delivery is the most patient-accepted and desirable route of administration, this technique is highly challenging because it must avoid the induction of tolerance and requires appropriate formulations to overcome the harsh gastrointestinal environment to achieve potent protection (Vela Ramirez et al., 2017). The stimulation of an effective oral immune response entails transport across the mucosal barrier, delivery of the active and intact antigen to the intestine and the subsequent activation of antigen-presenting cells. However, the gastrointestinal tract poses many obstacles, including the requirement for suitable doses to initiate immunity instead of tolerance and the possibility of antigen degradation due to the harsh environment of the stomach (Vela Ramirez et al., 2017). In addition, the gastrointestinal tract is characterized by an acidic gastric environment, a considerable pH range along its entire length, the presence of proteolytic enzymes for protein denaturation and the mucus layer covering the epithelial surface and severely limiting macromolecular antigen access to the cell surface. Collectively, these factors may lead to the degradation of fragile biomolecules, including antigenic proteins (Vela Ramirez et al., 2017).
Oral plant-based vaccines have the potential to activate regulatory T cells (Tregs) and decrease the antigen-specific immune response through oral tolerance (Kostrzak et al., 2009). Changes in Tregs and antigen-specific immune responses produced by oral administration of Nicotiana tabacum expressing hepatitis B surface antigen (HBsAg) were measured in mice humanized for two human leukocyte antigen (HLA) alleles (HLA-A2.1 and HLA-DR1). Activation of antigen-specific CD8+ T cells was not detected, but oral plant-based immunization without adjuvant elicited humoral responses comparable to those obtained by DNA immunization with adjuvant. While DNA immunization had no effect on Treg titers plant immunization, in contrast, raised Tregs in a linear fashion, reaching a plateau at high antigen doses. The lowest plant antigen dose was associated with the highest humoral IgA and IgG responses, whereas the best antibody responses for DNA immunization were achieved at higher DNA doses. These findings indicate that plant-based oral vaccinations could be adapted to reduce tolerance while still eliciting an immunological response. Obviously, further research is needed to completely understand the link between the immune response and the oral administration of plant-derived antigens (Kostrzak et al., 2009).
Many plant-derived vaccines have been studied for their ability to provoke oral tolerance. Transgenic rice plants that accumulate mouse T-cell epitope peptides corresponding to Japanese cedar pollen allergens, for example, have been developed as a proof-of-concept study of the ability of plant-derived antigens to induce oral tolerance. Mice that consumed transgenic rice before systemic challenge exhibited allergen-induced oral tolerance following the challenge. Although the absence of a systemic response correlates to a reduction in Th2-mediated IgE responses specific to pollen allergens and histamine release, the proliferative response of CD4+ T cells was not affected (Takagi et al., 2006). In the research conducted by Beyer et al. (2007), corn-derived heat-labile enterotoxin B (LTB) was utilized as a model system to estimate the maximal nonstimulant dose in mice. This study identified a threshold level of orally administered plant-derived LTB that did not stimulate detectable levels of antibodies but could nevertheless induce immune priming. These results demonstrate that LTB derived from transgenic maize is immunogenic at the nanogram level when administered orally to mice (Beyer et al., 2007).
Transgenic tobacco plants expressing an HIV-1 polyepitope linked to hepatitis B (HBV) virus-like particles were also studied. Guetard et al. (2008) found that oral administration of these transgenic plants to humanized high sugar and butter (HSB) mice to enhance DNA priming can result in CD8+ T-cell activation specific for HIV-1 detectable in the mesenteric lymph nodes. The vaccination methods did, however, induce considerable activation of regulatory T cells in vivo. In the proof of concept of a plant-based vaccine, the balance between tolerance and immunogenicity remains a major challenge (Guetard et al., 2008).
Compared with the natural pathogen in plants, a transgenic product may undergo various posttranslational modifications, which may lead to allergenic responses in the host during vaccination. Suzuki et al. (2011) investigated the feasibility of oral immunotherapy for bronchial asthma using a newly invented subunit vaccine in which a house dust mite (HDM) (Dermatophagoides pteronyssinus (Der p) and Dermatophagoides farinae (Der f)) allergen fragment comprising immunodominant human and mouse T-cell epitopes was encapsulated in protein bodies extracted from the endoplasmic reticulum of transgenic rice seeds (Tg). Allergen-specific serum immunoglobulin responses, bronchial hyperresponsiveness (BHR), airway inflammatory cell infiltration, lung histology, and Th1/Th2 cytokines production were investigated in allergen-immunized and challenged mice. Prophylactic oral vaccination with Tg rice seeds significantly minimized the serum levels of allergen-specific IgE and IgG. Allergen-induced CD4 (+) T-cell proliferation and production of Th2 cytokines in vitro, neutrophils and mononuclear cells into the airways, infiltration of eosinophils, and BHR were also restrained by oral vaccination. The vaccine effects were an antigen-specific immune response, as specific IgE and IgG levels in mice immunized with Der f 2 or ovalbumin were not highly suppressed by oral vaccination with Der p 1 expressing Tg rice. Therefore, the vaccine does not induce nonspecific bystander suppression, which has been a serious issue with many oral tolerance regimens. These results highlight that this vaccine strategy is promising for allergen-specific oral immunotherapy against allergic diseases, including bronchial asthma (Suzuki et al., 2011).
In addition, a major challenge confronting this form of the vaccine is its acceptance by the population, as there are some judgments that prevail in different parts of the world relating to the assumption that transgenic plants are injurious to society and the environment (Wurm, 2004). Therefore, it is necessary to counter such beliefs and educate the community with regard to the use and benefits of plant-based vaccines (Kurup and Thomas, 2020).
Regardless of the considerable challenges facing the implementation of this potential technology, efforts by researchers to develop an acceptable plant-based vaccine are still ongoing (Laere et al., 2016). Nonetheless, the benefits of plant-based vaccines are prominent enough to overcome their side effects (Kurup and Thomas, 2020). In addition, as technology develops, genetically modified plants are becoming far safer than ever before (Gunasekaran and Gothandam, 2020). As such, African researchers must make an extra effort to respect and consider the sensitive manufacturing requirements associated with these third-generation vaccines and try to overcome the challenges that they may encounter during or after the production process.
Advantages of Plant-Based Vaccines
Different systems (i.e., bacteria and mammalian cell lines) are currently available to express therapeutic proteins as vaccines; however, these systems have many limitations, including but not limited to target integrity, high cost, time consumption, safety, purification and refrigeration requirements, and potential for administration by injection, which requires trained personnel, the addition of an adjuvant or multiple doses (Kurup and Thomas, 2020), and the induction of a modest mucosal response (Sohrab, 2020). Collectively, these factors can ultimately cause the rapid emergence or re-emergence of pandemics (Sohrab et al., 2017).
Yeasts or unicellular fungi represent highly efficient heterologous gene expression systems. Since these organisms can grow on a simple mineral medium and secrete low levels of endogenous proteins, their purification process is very simple. The use of candidate yeasts to produce a vaccine offers many advantages that make these organisms a model of choice, including the increased stability of antigens in the cellular environment, the reduced costs of protein purification, the nonrequirement of a specific adjuvant, ease of use and the cost-effectiveness associated with the mass culture of recombinant yeasts. Moreover, yeast-based vaccines help in the rapid identification of pathogen antigens and the inactivation of T cells via the interaction and recognition of antigen peptides (Kumar and Kumar, 2019). Pichia pastoris is a methylotrophic yeast capable of performing posttranslational modifications that are similar to the modifications of human proteins (Kumar and Kumar, 2019). Pichia pastoris is a strong model for vaccine development due to the availability of a well-established genetic and a complete genome sequence, its nonpathogenic nature, inherent natural adjuvants, high yields of secreted proteins, and the high density of cell growth. Nonetheless, further improvements related to either process engineering (e.g., continuous processing alternative induction systems) or strain engineering (e.g., engineering of glycosylation) will strongly enhance the productivity of the Pichia pastoris system and extend the range of target proteins, including those that are only achieved by other expression systems (de Sá Magalhães and Keshavarz-Moore 2021).
Plants are known for their low risk of harboring pathogenic agents, which minimizes the probability of unintentional contamination of the final pharmaceutical products. Plants also feature a eukaryotic endomembrane that allows them to perform posttranslational modifications that are important for protein function, structure, and stability (Esqueda and Chen, 2021). The expression of recombinant proteins in plants (stable nuclear or plastid expression) can be hereditary, occur in seed form, and take place from generation to generation, thus making it possible to cultivate plants on an agricultural scale. Furthermore, a large amount of product can accumulate and gather in a small volume, thus considerably reducing processing costs (Virupaksh, 2006). Moreover, plants can grow easily, and their production does not require any special buildings or sterilization techniques (Sohrab et al., 2017). Plants are also characterized by their potential for large-scale biomass production at low cost using cheap bioreactors and greenhouses that use inexpensive and unsophisticated equipment that overcomes the complexity of conventional systems based on insect cells, mammalian cells, or bacteria (Márquez Escobar et al., 2017). However, we should also be aware that the production of a plant line that expresses a transgene in a stable manner is arduous and that biosafety directives exist to restrict the growth of transgenic plants in the open field (Esqueda and Chen, 2021).
The oral delivery of these vaccines is the most advantageous factor because there is no requirement for trained personnel. Furthermore, no pain is generated during administration. Moreover, oral immunization can trigger immune responses in the mucous membrane of the respiratory tract, which will provide good levels of protection against respiratory conditions (Márquez-Escobar et al., 2017). Oral delivery can also protect the vaccine from degradation by intestinal and stomach fluids if it maintains antigen integrity while passing through the acidic environment of the stomach (Aboul-Ata et al., 2014). The use of transgenic seeds as antigen vehicles would probably be the best route to avoid protein degradation (Virdi et al., 2013).
Oral plant-based vaccines can also be considered in a prime boost manner. Appropriate plant expression systems, as well as optimization of vaccine formulations and administration methods, are required for effective vaccination against hepatitis B virus (HBV) and other diseases with oral plant-based vaccines. The goal of Pniewski et al. (2011)’s research was to develop a prototype oral vaccination formula appropriate for human immunization. Herbicide-resistant lettuce was genetically modified, stably expressing through progeny generation micrograms of S-HBsAg per g of fresh weight, and formed virus-like particles (VLPs). Lyophilized tissue comprising a relatively low, VLP-assembled antigen dose, delivered only orally to mice with a long, 60-days interval between prime and boost immunizations and without exogenous adjuvant, generated systemic and mucosal humoral anti-HBs responses at the nominally protective level (Pniewski et al., 2011). In another study, the cholera toxin B (CTB) subunit of Vibrio cholerae fused to malaria vaccine antigens apical membrane antigen-1 (AMA1) and merozoite surface protein-1 (MSP1) was expressed in lettuce and tobacco chloroplasts. Nine groups of mice were immunized subcutaneously or orally with purified antigens or transplastomic tobacco leaves. High levels of antigen-specific antibody titers from immunized mice completely inhibited malaria parasite proliferation and cross-reacted with native parasite proteins. Subcutaneous injection of the purified antigen with a strong adjuvant ensures antigen presentation during priming. This set up an immune response that was further enhanced by twelve oral boosters and six subcutaneous boosters. Thus, the oral booster vaccine is effective when the systemic response has been established by priming with antigen in presence of an adjuvant (Davoodi-Semiromi et al., 2010).
Long-term immunological memory was assessed in mice injected with a primary dose of Recombivax and boosted with orally administered HBsAg wafers made from maize germ expressing HBsAg. Mice boosted with orally administered HBsAg wafers showed large increases in fecal mucosal IgA titers and large increases in serum IgA, while mice boosted with Recombivax showed no detectable levels of IgA in fecal or serum samples after four booster treatments. Long-term memory in orally treated mice was evidenced by fecal IgA and serum IgA, IgG, and total serum Ig titers (mIU/ml) sustained over 1 year, while Recombivax-treated mice showed sustained serum IgG and mIU/mL. Moreover, strong increases in these same antibodies were induced after a new boost at 47 and 50 weeks after the primary injection. Therefore, orally administered vaccines can provide long-term mucosal and systemic immune responses (Hayden et al., 2015).
The high cost and restricted supply of inactivated polio vaccine (IPV), as well as the persistent transmission of circulating vaccine-derived polioviruses and the necessity for booster shots, are all issues that have yet to be resolved. To address this critical need, a low-cost novel strategy, cold-chain-free viral protein 1 (VP1) oral subunit booster vaccine after a single dose of IPV is reported. Codon optimization of the VP1 gene augmented expression 50-fold in tobacco and lettuce chloroplasts. Oral boosting of VP1 expressed in plant cells with plant-derived adjuvants after a single IPV prime considerably increased VP1-IgG1 and VP1-IgA titers compared to lower or negligible IgG1 titers of IgA with IPV injections. Neutralizing antibody titers and seropositivity against all three Sabin poliovirus serotypes were revealed with two doses of IPV and plant cell-based oral boosters, but a single dose of IPV resulted in weak neutralization (Chan et al., 2016). Daniell et al. (2019) reported a novel, low-cost, cold-chain and poliovirus-free booster vaccine using the poliovirus capsid protein (VP1, conserved in all serotypes) fused with cholera nontoxic B subunit (CTB) expressed in lettuce chloroplasts. Mice primed with IPV and boosted three times with freeze-dried plant cells expressing CTB-VP1co, formulated with oral plant-derived adjuvants, improved VP1-specific IgG1 titers, VP1-IgA, and neutralization. In contrast, a single dose of IPV resulted in VP1-IgG1 titers < 50% and negligible VP1-IgA titers, weak neutralization and seropositivity. Mice boosted orally with CTB-VP1co, without IPV priming, produced no protective neutralizing antibodies. Since the world’s population receives a single dose of IPV, the booster vaccine without poliovirus or cold chain offers a timely and inexpensive solution to eradicate poliomyelitis (Daniell et al., 2019).
In addition to oral administration, which is characteristic of plant-based vaccines, the sublingual route can also be used for transmucosal drug administration and the treatment of allergies (Paris et al., 2021). The latter can induce immune responses against a variety of antigens, including inter-particulate antigens, live-attenuated viruses, and soluble protein administration (Shim et al., 2013). Sublingual vaccines have been shown to be very effective and safe in generating robust immune responses against administered antigens and can also represent an alternative to the traditional parental route of vaccine administration (Shim et al., 2013).
Since plants can grow in several environmental conditions, manufacturing operations can consider regional and local infrastructure (Dhama et al., 2020). Sufficient time for preclinical and clinical trials is required to evaluate the practical efficacy of a plant-based vaccine before commercialization (Dhama et al., 2020).
In the developing world, plant-based vaccines offer an exciting option to reduce disease burden, where vaccine storage and administration are often a major complication (Kurup and Thomas, 2020). Plant vaccines are a milestone in the creation of inexpensive vaccines that may be particularly useful in massive vaccination campaigns for the populations of developing countries, including Africa, where logistical issues and high costs can compromise effective vaccine programs (Virupaksh, 2006).
In addition, it is noteworthy that transient transgene expression in cells grown in culture may not be useful for plant-based vaccines. This technique largely relies on the introduction of Agrobacterium into plant tissues shortly before harvesting. In such cases, thorough purification of the vaccine will be required to completely remove the bacteria and their components (e.g., DNA, LPS, peptidoglycan) for oral delivery. This is not an issue if protein antigens are purified prior to administration.
Preclinical and Clinical Trials of Plant Based Vaccines
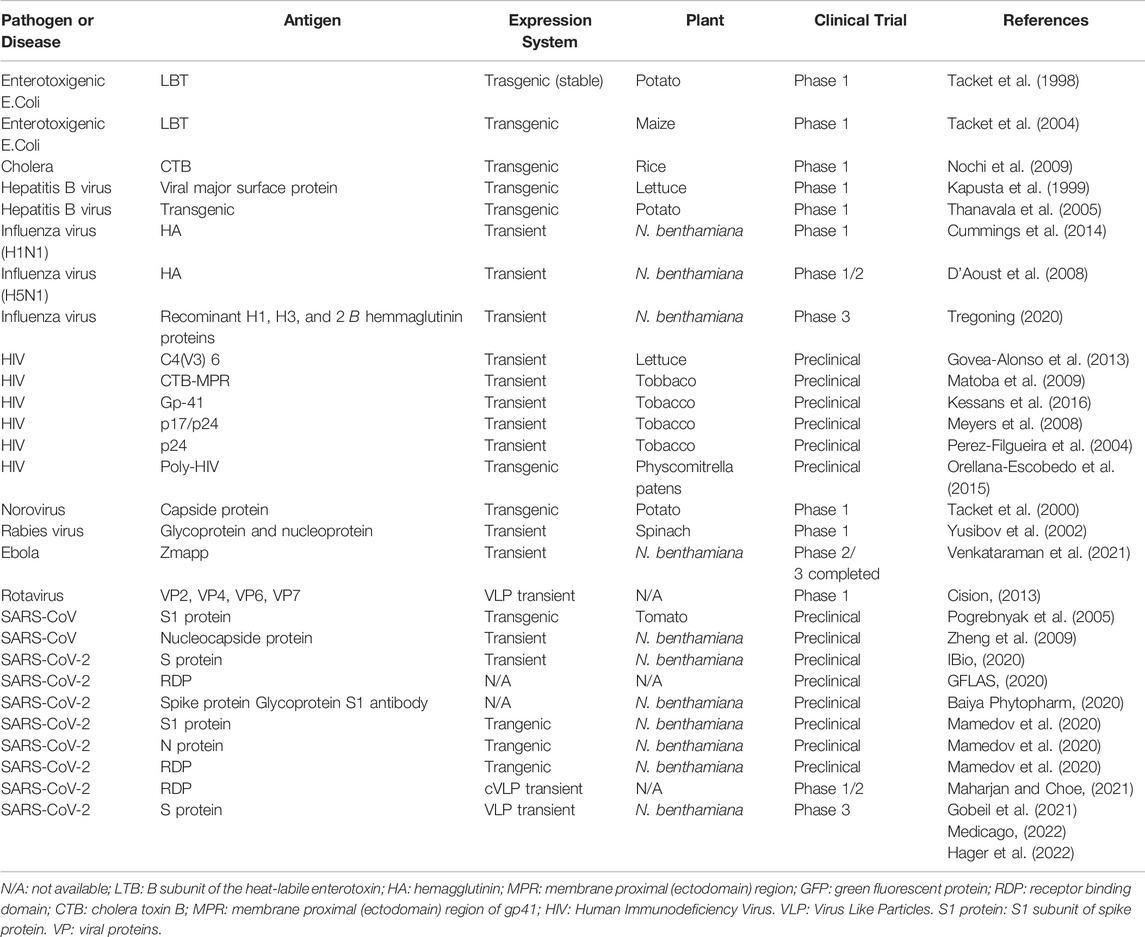
The only plant-based vaccine currently approved by the United States Department of Agriculture (USDA) is a Newcastle disease vaccine for poultry made from suspended-cultured tobacco cells (Floss et al., 2007). Although progress toward the commercialization of plant-based vaccines takes a long time and requires much effort, several candidate vaccines for humans use are being tested in clinical trials (Takeyama et al., 2015). Preclinical evaluation of the immune response and protection induced by these vaccines is critical, however the availability of appropriate animal models is limited due to differences in innate and adaptive immunities between humans and animal models as well as the often observed species-specific disease pathogenesis (Sharma et al., 2021).
The nontoxic B subunit of the heat-labile enterotoxin (LTB) of enterotoxigenic E. coli produced in potato or maize was orally administered to healthy participants to test its immunogenicity and safety. The serological survey revealed that the vaccinated volunteers had increased levels of serum IgG and IgA specific for LTB and that no adverse events due to vaccination were observed (Tacket et al., 1998; Tacket et al., 2004). The norovirus capsid protein VP1 was produced in potato plants in the same way as LTB. Volunteers were vaccinated with recombinant VP1 and twenty percent developed significant anti-VP1-specific serum IgG titers (Tacket et al., 2000).
Many plant-based vaccines have been developed to fight against the human immunodeficiency virus (HIV). For instance, a C4(V3)6 multiepitope protein has been designed with the aim of pursuing broad immunization against HIV. This C4(V3)6 chimeric protein is based on gp120 sequences (a glycoprotein protruding from the outer surface of the HIV virion), comprising epitopes of the conserved fourth domain (C4) and six tandem repeats of the third variable domain (V3), which represent different HIV isolates. The expression of C4(V3)6 in lettuce and tobacco was obtained without toxic effects on the growth of transgenic plants (Govea-Alonso et al., 2013).
Likewise, hepatitis B virus (HBV) surface antigen (HBsAg) has been produced in plants. HBsAg transgenic lettuce leaves were given orally to three adult volunteers. Two out of three vaccinated volunteers showed transient protective levels of HBsAg-specific IgG 2 weeks after the second dose, but no serum HBsAg-specific IgA was revealed (Kapusta et al., 1999). Another clinical study was conducted with oral HBsAg produced in transgenic potato plants. A total of 52.9% of the participants enrolled in the study from the two-dose group and 62.5% of participants from the three-dose group had elevated serum HBsAg antibody titers over the 70-days follow-up period after the first immunization (Thanavala et al., 2005).
Current human rabies vaccines are effective both before and after exposure to the rabies virus. Yusibov et al. (2002) fused chimeric protein-coding fragments of protein G and protein N with that of alfalfa mosaic virus coat protein binding RNA (AMV CPB) and introduced this fusion construct into tobacco mosaic virus (TMV) genetic materials. Spinach was infected with a recombinant virus to obtain transient expression of the chimeric rabies peptide. Three of the five volunteers previously vaccinated with an injectable commercial vaccine showed an elevation of rabies-specific IgG after oral administration of three doses of transduced spinach. In another protocol involving volunteers with no history of rabies vaccination, five of nine volunteers responded to spinach rabies antigen. Regardless of the order of vaccination, oral spinach rabies vaccine in combination with currently available vaccines can boost immunity against the rabies virus (Yusibov et al., 2002).
The influenza virus has 16 hemagglutinin (HA) subtypes, and in 2009, the H1N1 type influenza virus became pandemic. To control its propagation through vaccination, the rapid production of a new HA antigen was necessary. Medicago has developed technology to produce influenza virus HA virus-like particles (VLPs) in Nicotiana benthamiana with a transient expression system based on Agrobacterium tumefaciens. Phase I and II clinical trials of VLPs composed of the HA protein of the H5N1 influenza virus have been accomplished. After 6 months of vaccination with H5-VLP, the volunteers group indicated cross-protective CD4+ T-cell responses, which were not noticed in the placebo group, showing a strong induction of cell-mediated immunity to long-term by plant-derived H5-VLP. Thirty-four percent of the volunteers developed transient IgG and, in some cases, IgE to plant glyco-epitopes. After 6 months, most subjects’ antibody levels returned to baseline (D’Aoust et al., 2008). Another plant-based influenza vaccine has completed a phase I clinical trial. HA was also produced in N. benthamiana using an infiltration method of A. tumefaciens. HAC1 and HAI-05 were injected twice into mice and rabbits at 3-week intervals in preclinical experiments. In mice, the seropositivity rate of serum anti-HA antibody responses was 100%. Rabbits were also revealed to be HA seropositive. A phase I clinical trial for HAC1 and HAI-05 was conducted as a randomized, double-blind, placebo-controlled study with healthy volunteers between the ages of 18 and 49. Almost all adverse events were mild to moderate; the highest responses were spotted by hemagglutination inhibition and viral neutralizing antibody titers and were detected in the group immunized with the highest dose without adjuvant (Cummings et al., 2014).
A plant-based vaccine against SARS-CoV stably expressed protein S (S1) in low-nicotine tobacco and tomato plants. Preclinical studies have shown that the plant-derived vaccine generated an antibody response in mice. Another study analyzed the immunogenicity of the SARS-CoV N recombinant protein produced transiently in N. benthamiana. Tobacco-produced recombinant N protein induced a significant humoral immune response after the third parental injection (Shanmugaraj et al., 2021).
Recently, Medicago, a Canadian biopharmaceutical company in Quebec, has been involved in the development and production of a plant-based vaccine against COVID-19 infection. The technology used to manufacture the vaccines was based on the synthesis of virus-like particles (VLPs) in plant cells by transient expression. VLPs without genetic material mimic the native structure of the virus, allowing the body’s immune system to mount a strong immune response. This COVID-19 VLP (Co-VLP) developed by Medicago company utilizes a full-length spike protein from the SARS-CoV-2 viral genome that trimerizes and assembles into VLPs inside plant cells. The vaccine combined the VLP strategy as an antigen and the squalen-tocopherol based emulsion (AS03) as an adjuvant and has recently completed phase 3 clinical trials (Hager et al., 2022). Results showed an efficacy of 69.5% against any symptomatic Covid-19 caused by five variants that were identified by sequencing. In a post hoc analysis, vaccine efficacy was 78.8% against moderate-to-severe disease and 74.0% among the participants who were seronegative at baseline. No severe cases of Covid-19 occurred in the vaccine group, in which the median viral load for breakthrough cases was lower than that in the placebo group by a factor of more than 100. Solicited adverse events were mostly mild or moderate and transient and were more frequent in the vaccine group than in the placebo group. No adverse effects related to vaccine-associated disease aggravation were observed (Hager et al., 2022). This is, to the best of our knowledge, the first plant-based vaccine against COVID-19 to successfully complete phase 3.
Another company, Kentucky BioProcessing (KBP), located in Owensboro, Kentucky, United States, is also competing to market its innovative, fast-growing plant-based COVID-19 vaccine KBP-201, which uses N. benthamiana as an expression host. KBP-201 adjuvanted with CpG oligonucleotide is currently in phase I/II clinical trials in the United States (Shanmugaraj et al., 2021). Likewise, iBio, based in Bryan, Texas, is developing the plant-based vaccine candidates IBIO-200, a VLP-based vaccine, and IBIO-201, a SARS-CoV-2 spike-based subunit vaccine targeting the SARS-CoV-2 nucleocapsid protein (IBio, 2020). Baiya Phytopharm, a Thai start-up, is using its BaiyaPharming™ protein expression platform to manufacture a subunit vaccine against SARS-CoV-2 in N. benthamiana. Six candidate vaccines were studied for efficacy, and based on the findings, a candidate was selected that reported better immunogenicity in monkeys and mice (Baiya Phytopharm, 2020).
In summary, researchers around the world are making tremendous efforts using various available platforms to develop effective and safe plant-derived vaccines to control many diseases, including COVID-19 (Table 2). With the emergence of transient production technology, the plant expression system is considered a viable approach. It has attracted increasing interest among pharmaceutical companies for production of recombinant biopharmaceutical (for both therapeutic and vaccine applications) due to its potential streamlined scalability (Shanmugaraj et al., 2021).
Plant-Based Vaccines Against COVID-19 in Africa: Challenges and Prospects
The COVID-19 pandemic has given the world, especially developing countries, a valuable lesson. While most African countries are still struggling with the pandemic, developed countries are racing against time to provide vaccines to their populations and achieve herd immunity as quickly as possible to overcome a pandemic that is throwing a shadow over all walks of life. However, we should also praise the African government, healthcare professionals, and the public who have made extraordinary efforts to avoid the worst-case scenarios of COVID-19 in Africa. These countries must also be vigilant and continue to expand their ability to diagnose and act according to precautionary measures.
At present, the battle against COVID-19 in Africa has reached a new level. If the African continent does not want to be left at the back of the queue and return to normal life, massive vaccination campaigns should be initiated immediately or in the very near future. Any unwanted delay in immunization programs could lead to a significant increase in death rates and negatively affect the economic sector of these developing countries and worsen their already ailing economies. The limited capacity of African countries to manufacture their own vaccines for COVID-19 is neither new nor surprising because implementing vaccine manufacturing is a heavy investment that necessitates tens to hundreds of millions of dollars. Therefore, these countries should strive to secure a vaccine in the required quantities; at present, the importation of a suitable vaccine is the only possible option. Africa must identify other solutions and alternatives so that a robust and satisfactory vaccination program can be initiated to attain the required objectives quickly and efficiently. Biotechnology has a pivotal role to play in making this a reality, potentially via plant-based vaccines. Biotechnology could move the continent from dependency to increased self-sufficiency and security with regard to supply. However, making this aspiration a reality is not easy due to a variety of constraints.
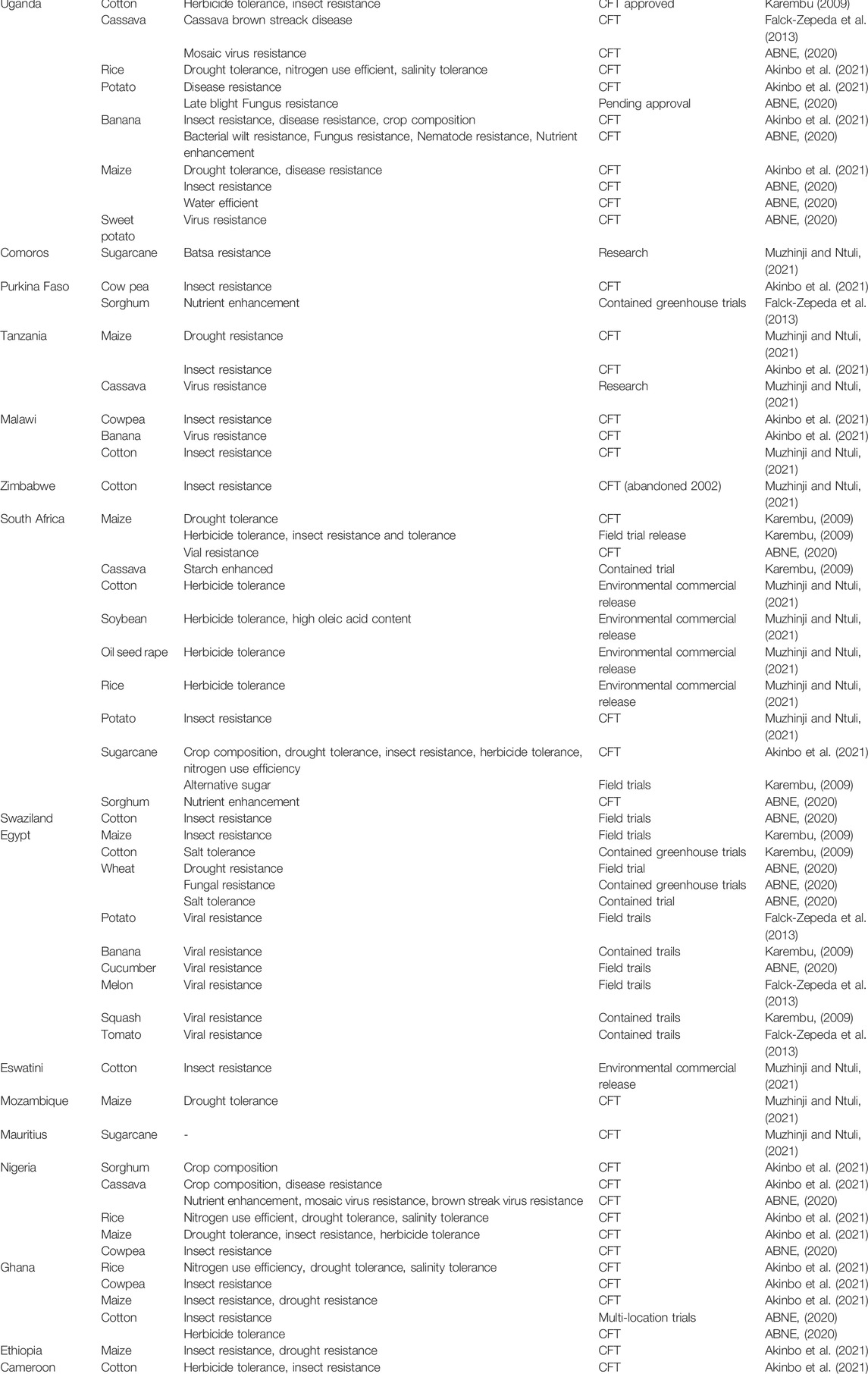
The state of biotechnology in Africa, at this time, is seriously flawed and weak. The current status of agricultural biotechnology adoption on the African continent is presented in Table 3. Improving and upgrading technical capabilities, legal regulations, infrastructure, human, and financial resources is the first step toward a new era of biotechnology in Africa, thus creating a strong basis for the development of vaccines. One of the most injurious factors affecting the progression of biotechnology on the continent is that only a limited number of countries are legally authorized to use genetically modified crops (only seven African countries have functional regulatory frameworks (Chambers et al., 2014)). Subsequently, considerable effort should be made by governmental and nongovernmental agencies to invest in this field so that scientific research can provide scientific proof of the advantages of biotechnology and genetic engineering, thus gaining regulatory and public acceptance of these products. Moreover, launching new Afro-African or Afro-international initiatives and collaborations would be fruitful in terms of the exchange of expertise through training programs or capacity-building initiatives, and related efforts are also needed to build capacity in agricultural stewardship. To succeed in any endeavor, both public and private sector activities should be encouraged.
Plant-based vaccines, with their significant advantages and benefits, are a reasonable option for use in third world countries, including Africa. Following the mass production of plant-based vaccines in each African country and the performance of successful clinical trials, it will be necessary to promote partnerships and cooperation with pharmaceutical companies before we can move to commercialization and the implementation of mass vaccination protocols in Africa. Although these third-generation vaccines have proven their potential to revolutionize the field of vaccinology, as we highlighted above, regulations and laws in many African countries still restrict the marketing of genetically modified plants for human use. With this regard, legal obstacles must be overcome to provide the population of Africa with an opportunity to benefit from these pharmaceuticals as soon as possible. We should not avoid the fact that more research needs to be carried out in this nascent field in Africa where candidate plants need to be studied and clinical trials need to be conducted on African land with the overall aim of obtaining legal and public acceptance for the use of a plant-based vaccine on the continent. It is important to note that this ambitious strategy will not only be effective in confronting the emerging COVID-19 but will also create a strategy to rapidly and accurately tackle any future pandemic, highly infectious pathogens, or life-threatening diseases affecting larger masses of the population. These possibilities may be more likely if plant-produced pure proteins are marketed instead of GM plants. Such proteins can also be injected if this is more appropriate.
It is important to perform a large clinical trial of plant-based vaccines in Africa to test their efficacy, verify their safety, and confirm their ability to meet the regulatory requirements. The production of a plant-based vaccine for COVID-19 in Africa could also benefit from the achievements made thus far, as some vaccines have been evaluated in clinical and pilot trials. Furthermore, the increasing levels of attention given by the scientific community, as demonstrated in the increasing number of published reports, can provide a useful foundation to initiate the successful manufacturing of these products.
The next few months will be very challenging for Africa and the world in general to prove their ability to harness this technology to save lives. Interestingly, plant-based pharmaceuticals have the potential to positively affect immunization targeting respiratory illnesses. The clinical trials carried out on candidate plant-based vaccines thus far have strengthened the realistic possibility of a publicly marketed African COVID-19 plant-based vaccine in the short term. However, for this to occur, a range of technical, infrastructural, regulatory, public, and financial challenges need to be overcome.
Conclusion
COVID-19 is a potentially life-threatening disease that has affected the lives of millions of people around the world and can only be controlled by the development of suitable vaccines. The discovery of plant-based vaccines is one of the prime breakthroughs in the biotechnology discipline, represents a worthy alternative for the alleviation and prevention of infectious disease outbreaks and shows undeniable potential to counteract COVID-19. Plant-based vaccination is considered a milestone for the creation of vaccines and could be particularly applicable for immunizing the populations of developing countries, including Africa, where high costs and logistical problems can constrain massive vaccine programs. In addition to their scalability, the exploitation of plant-based vaccines provides the potential for generating low-cost, easy-to-administer, safe and effective vaccines to combat COVID19 and other infections.
Although plant-based COVID-19 vaccines are easily accessible and stable, there are some limitations that limit the development of such vaccines in Africa. Perhaps the main challenge will be testing the efficacy of such vaccines in large clinical trials to validate their safety and confirm their ability to meet regulatory requirements. United efforts by conventional African vaccine developers and immunologists could be of great value to produce an alternative form of vaccines. Adequate research and advancement in this area are vital, as such activities could lead to an era in which infectious diseases can be controlled more effectively, including COVID-19. The benefits of plant-based plant-based pharmaceuticals must outweigh the challenges faced by this interesting product. Thus, it is expected that regulatory approval will eventually be given after positive clinical trials to help control outbreaks of infectious diseases in Africa. Once plant-based vaccines have been deployed and distributed, COVID-19 could be eradicated or controlled.
Plant-based vaccines are less likely to be available in the near future, as developing and testing these medicines remains a significant challenge and could take time. However, accelerating the industrial level development of these types of vaccines will be crucial if we are to face the current COVID-19 pandemic and future outbreaks of other infectious diseases.
Author Contributions
IE wrote the manuscript. NA contributed to writing the manuscript. NA, SH, LW, SA, CN, AE, and YB reviewed the manuscript. HG conceived and designed the study and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
HG is a recipient of a grant from NIH through the h3abionet/H3Africa. The Article Processing Charge (APC) is paid by Mohammed VI University of Health Sciences (UM6SS).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are very thankful to Prof. Jean-Pierre Hernalsteens (Department of biology, VUB, Brussels, Belgium) for the critical readings of this article.
References
ABNE (African Biosafety Network of Expertise) (2020). Status of Crop Biotechnology in Africa. Available online: http://nepad-abne.net/biotechnology/status-of-crop-biotechnology-in-africa/.
Aboul-Ata, A.-A. E., Vitti, A., Nuzzaci, M., El-Attar, A. K., Piazzolla, G., Tortorella, C., et al. (2014). Plant-Based Vaccines. Adv. Virus Res. 89, 1–37. doi:10.1016/b978-0-12-800172-1.00001-x
African center for bio diversity (2020). African Center for Biodiversity – the GM Potato Push in Rwanda: Key Issues and Concerns. Nairobi, Kenya: Biotech Crops in Africa. Available online: https://www.isaaa.org/resources/publications/biotech_crops_in_africa/download/default.asp (Accessed September 1, 2021).
Akinbo, O., Obukosia, S., Ouedraogo, J., Sinebo, W., Savadogo, M., Timpo, S., et al. (2021). Commercial Release of Genetically Modified Crops in Africa: Interface between Biosafety Regulatory Systems and Varietal Release Systems. Front. Plant Sci. 12, 605937. doi:10.3389/fpls.2021.605937
Baiya Phytopharm (2020). COVID-19 Vaccine Development. Available online: https://baiyaphytopharm.com/covid-19.
Batlang, U., Tsurupe, G., Segwagwe, A., and Obopile, M. (2014). Development and Application of Modern Agricultural Biotechnology in Botswana: the Potentials, Opportunities and Challenges. GM Crops Food 5 (3), 183–194. doi:10.4161/21645698.2014.945887
Beyer, A. J., Wang, K., Umble, A. N., Wolt, J. D., and Cunnick, J. E. (2007). Low-dose Exposure and Immunogenicity of Transgenic Maize Expressing the Escherichia coli Heat-Labile Toxin B Subunit. Environ. Health Perspect. 115 (3), 354–360. doi:10.1289/ehp.9687
Cerovska, N., Hoffmeisterova, H., Moravec, T., Plchova, H., Folwarczna, J., Synkova, H., et al. (2012). Transient Expression of Human Papillomavirus Type 16 L2 Epitope Fused to N- and C-Terminus of Coat Protein of Potato Virus X in Plants. J. Biosci. 37 (1), 125–133. doi:10.1007/s12038-011-9177-z
Chambers, J. A., Zambrano, P., Falck-Zepeda, J., Gruère, G., Sengupta, D., and Hokanson, K. (2014). GM Agricultural Technologies for Africa a State of Affairs. Washington, DC: International Food Policy Research Institute and African Development Bank. http://ebrary.ifpri.org/cdm/ref/collection/p15738coll2/id/128215.
Chan, H. T., Xiao, Y., Weldon, W. C., Oberste, S. M., Chumakov, K., and Daniell, H. (2016). Cold Chain and Virus‐free Chloroplast‐made Booster Vaccine to Confer Immunity against Different Poliovirus Serotypes. Plant Biotechnol. J. 14 (11), 2190–2200. doi:10.1111/pbi.12575
Cision (2013). Medicago Successfully Produces Plant-Based Rotavirus VLP Vaccine Candidate. Available online: https://www.prnewswire.com/news-releases/medicago-successfully-produces-plant-based-rotavirus-vlp-vaccine-candidate-212290651.html.
Cummings, J. F., Guerrero, M. L., Moon, J. E., Waterman, P., Nielsen, R. K., Jefferson, S., et al. (2014). Safety and Immunogenicity of a Plant-Produced Recombinant Monomer Hemagglutinin-Based Influenza Vaccine Derived from Influenza A (H1N1)pdm09 Virus: a Phase 1 Dose-Escalation Study in Healthy Adults. Vaccine 32, 2251–2259. doi:10.1016/j.vaccine.2013.10.017
D’Amico, C., Fontana, F., Cheng, R., and Santos, H. A. (2021). Development of Vaccine Formulations: Past, Present, and Future. Drug Deliv. Transl. Res. 11 (2), 353–372. doi:10.1007/s13346-021-00924-7
Daniell, H., Rai, V., and Xiao, Y. (2019). Cold Chain and Virus‐free Oral Polio Booster Vaccine Made in Lettuce Chloroplasts Confers Protection against All Three Poliovirus Serotypes. Plant Biotechnol. J. 17 (7), 1357–1368. doi:10.1111/pbi.13060
D’Aoust, M.-A., Lavoie, P.-O., Couture, M. M.-J., Trépanier, S., Guay, J.-M., Dargis, M., et al. (2008). Influenza Virus-like Particles Produced by Transient Expression inNicotiana Benthamianainduce a Protective Immune Response against a Lethal Viral Challenge in Mice. Plant Biotechnol. J. 6, 930–940. doi:10.1111/j.1467-7652.2008.00384.x
Davoodi-Semiromi, A., Schreiber, M., Nalapalli, S., Verma, D., Singh, N. D., Banks, R. K., et al. (2010). Chloroplast-derived Vaccine Antigens Confer Dual Immunity against Cholera and Malaria by Oral or Injectable Delivery. Plant Biotechnol. J. 8 (2), 223–242. doi:10.1111/j.1467-7652.2009.00479.x
De Muynck, B., Navarre, C., and Boutry, M. (2010). Production of Antibodies in Plants: Status after Twenty Years. Plant Biotechnol. J. 8, 529–563. doi:10.1111/j.1467-7652.2009.00494.x
de Sá Magalhães, S., and Keshavarz-Moore, E. (2021). Pichia pastoris (Komagataella Phaffii) as a Cost-Effective Tool for Vaccine Production for Low- and Middle-Income Countries (LMICs). Bioengineering 8, 119. doi:10.3390/bioengineering8090119
De Smet, R., Allais, L., Cuvelier, C. A., Natesan, S., Iqbal, Y. M., Patel, S. K., et al. (2014). Recent Advances in Oral Vaccine developmentPlant-Based Vaccines and Antibodies to Combat COVID-19: Current Status and Prospects. Hum. Vaccines Immunother. 10 (5), 1309–1318. doi:10.4161/hv.28166
Dharma, K., Natesan, S., Yatoo, M. I., Patel, S. K., Tiwari, R., and Saxena, S. A. (2020). Plant-based Vaccines and Antibodies to Combat COVID-19: Current Status and Prospects. Vaccin Immunother. 16, 2913–2920. doi:10.1080/21645515.2020.1842034
Esqueda, A., and Chen, Q. (2021). Development and Expression of against Viruses in. Methods Mol. Biol. 2225, 25–38. doi:10.1007/978-1-0716-1012-1_2
Falck-Zepeda, J. B., Gruère, G., and Sithole-Niang, I. (2013). Genetically Modified Crops in Africa Economic and Policy Lessons from Countries South of the Sahara. Washington, DC: International Food Policy Research Institute. doi:10.2499/9780896297951
Floss, D. M., Falkenburg, D., and Conrad, U. (2007). Production of Vaccines and Therapeutic Antibodies for Veterinary Applications in Transgenic Plants: an Overview. Transgenic Res. 16 (3), 315–332. doi:10.1007/s11248-007-9095-x
GFLAS (2020). Life Sciences Succeeds in Expressing COVID-19 Recombinant Vaccine Candidates with Plant Based Platform. Available online: http://gflas.com/about/press_view.php?idx=165.
Giddings, G., Allison, G., Brooks, D., and Carter, A. (2000). Transgenic Plants as Factories for Biopharmaceuticals. Nat. Biotechnol. 18, 1151–1155. doi:10.1038/81132
Gobeil, P., Pillet, S., Boulay, I., Séguin, A., Makarkov, A., Heizer, G., et al. (2021). Phase 2 Randomized Trial of an AS03 Adjuvanted Plant-Based Virus-like Particle Vaccine for Covid-19 in Healthy Adults, Older Adults and Adults with Comorbidities. medRxiv. doi:10.1101/2021.05.14.21257248
Govea-Alonso, D. O., Cardineau, G. A., and Rosales-Mendoza, S. (2014a). Principles of Plant-Based Vaccines. Spread Dis., 1–14. doi:10.1007/978-1-4939-0850-9_1
Govea-Alonso, D. O., Gómez-Cardona, E. E., Rubio-Infante, N., García-Hernández, A. L., Varona-Santos, J. T., Salgado-Bustamante, M., et al. (2013). Production of an Antigenic C4(V3)6 Multiepitopic HIV Protein in Bacterial and Plant Systems. Plant Cell. Tiss. Organ Cult. 113, 73–79. doi:10.1007/s11240-012-0252-4
Govea-Alonso, D. O., Rybicki, E., and Rosales-Mendoza, S. (2014b). Plant-Based Vaccines as a Global Vaccination Approach: Current Perspectives. Genet. Eng. Plants as a Source Vaccines Against Wide Spread Dis. Integr. View, 265–280. doi:10.1007/978-1-4939-0850-9_13
Guetard, D., Greco, R., Cervantes Gonzalez, M., Celli, S., Kostrzak, A., Langlade-Demoyen, P., et al. (2008). Immunogenicity and Tolerance Following HIV-1/HBV Plant-Based Oral Vaccine Administration. Vaccine 26 (35), 4477–4485. doi:10.1016/j.vaccine.2008.06.059
Gunasekaran, B., and Gothandam, K. M. (2020). A Review on Edible Vaccines and Their Prospects. Braz J. Med. Biol. Res. 53 (2), e8749. doi:10.1590/1414-431X20198749
Hager, K. J., Pérez Marc, G., Gobeil, P., Diaz, R. S., Heizer, G., Llapur, C., et al. CoVLP Study Team (2022). Efficacy and Safety of a Recombinant Plant-Based Adjuvanted Covid-19 Vaccine. N. Engl. J. Med. 386 (22), 2084–2096. doi:10.1056/NEJMoa2201300
Hayden, C. A., Fischer, M. E., Andrews, B. L., Chilton, H. C., Turner, D. D., Walker, J. H., et al. (2015). Oral Delivery of Wafers Made from HBsAg-Expressing Maize Germ Induces Long-Term Immunological Systemic and Mucosal Responses. Vaccine 33 (25), 2881–2886. doi:10.1016/j.vaccine.2015.04.080
IBio (2020). IBIO-201 Demonstrates Ability to Elicit Anti-SARS-CoV-2 Immune Response in Preclinical Studies. Available online: https://www.ibioinc.com/ibio-provides-update-on-ibio-201-covid-19-vaccine-program/.
Jazayeri, S. D., Lim, H. X., Shameli, K., Yeap, S. K., and Poh, C. L. (2021). Nano and Microparticles as Potential Oral Vaccine Carriers and Adjuvants against Infectious Diseases. Front. Pharmacol. 12, 682286. doi:10.3389/fphar.2021.682286
Johansen, F.-E., Pekna, M., Norderhaug, I. N., Haneberg, B., Krajci, M. A., et al. (1999). Absence of Epithelial Immunoglobulin a Transport, with Increased Mucosal Leakiness, in Polymeric Immunoglobulin Receptor/secretory Component-Deficient Mice. J. Exp. Med. 190, 915–922. doi:10.1084/jem.190.7.915
Kapusta, J., Modelska, A., Figlerowicz, M., Pniewski, T., Letellier, M., Lisowa, O., et al. (1999). A Plant‐derived Edible Vaccine against Hepatitis B Virus. FASEB J. 13 (13), 1796–1799. doi:10.1096/fasebj.13.13.1796
Karembu, M. (2009). Nguthi F and Ismail H. Biotech Crops in Africa: The Final Frontier. AfriCenter, Nairobi, Kenya: ISAAA.
Kargboa, A., Fattyb, L. K. M., Mendyc, A., Yahayad, J., and Dibbae, J. (2020). Biotechnology as a Change Agent for National Development: Review in the Gambia. Int. J. Sci. Basic Appl. Res. (IJSBAR) 49 (2), 43–55.
Kessans, S. A., Linhart, M. D., Meador, L. R., Kilbourne, J., Hogue, B. G., Fromme, P., et al. (2016). Immunological Characterization of Plant-Based HIV-1 Gag/Dgp41 Virus-like Particles. PLoS One 11, e0151842. doi:10.1371/journal.pone.0151842
Kohli, A., Miro, B., and Twyman, R. M. (2010). “Transgene Integration, Expression and Stability in Plants: Strategies for Improvements,” in Transgenic Crop Plants (Berlin, Heidelberg: Springer), 201–237. doi:10.1007/978-3-642-04809-8_7
Korban, S. S. (2002). Targeting and Expression of Antigenic Proteins in Transgenic Plants for Production of Edible Oral Vaccines. Vitro Cell. Dev. Biol. - Plant 38 (3), 231–236. doi:10.1079/IVP2002292
Kostrzak, A., Cervantes Gonzalez, M., Guetard, D., Nagaraju, D. B., Wain-Hobson, S., Tepfer, D., et al. (2009). Oral Administration of Low Doses of Plant-Based HBsAg Induced Antigen-specific IgAs and IgGs in Mice, without Increasing Levels of Regulatory T Cells. Vaccine 27 (35), 4798–4807. doi:10.1016/j.vaccine.2009.05.092
Kumar, M., Kumari, N., Thakur, N., Bhatia, S. K., Saratale, G. D., Ghodake, G., et al. (2021). A Comprehensive Overview on the Production of Vaccines in Plant-Based Expression Systems and the Scope of Plant Biotechnology to Combat against SARS-CoV-2 Virus Pandemics. Plants 106, 1213. doi:10.3390/plants10061213
Kumar, R., and Kumar, P. (2019). Yeast-based Vaccines: New Perspective in Vaccine Development and Application. FEMS Yeast Res. 19, foz007. doi:10.1093/femsyr/foz007
Kurup, V. M., and Thomas, J. (2020). Edible Vaccines: Promises and Challenges. Mol. Biotechnol. 62 (2), 79–90. doi:10.1007/s12033-019-00222-1
Kuyek, K. (2002). Genetically Modifi Ed Crops in Africa: Implications for Small Farmers. Genetic Resources Action International (GRAIN). Available online: www.grain.org (Accessed August 25, 2021).
Laere, E., Ling, A. P. K., Wong, Y. P., Koh, R. Y., Mohd Lila, M. A., and Hussein, S. (2016). 1–11. doi:10.1155/2016/4928637Plant-Based Vaccines: Production and ChallengesJ. Bot.
LeBlanc, Z., Waterhouse, P., and Bally, J. (2020). Plant-Based Vaccines: The Way Ahead? Viruses 13, 5. doi:10.3390/v13010005
Maharjan, P. M., and Choe, S. (2021). Plant-Based COVID-19 Vaccines: Current Status, Design, and Development Strategies of Candidate Vaccines. Vaccines 9, 992. doi:10.3390/vaccines9090992
Mamedov, T., Yuksel, D., Ilgın, M., Gürbüzaslan, İ., Gulec, B., Mammadova, G., et al. (2020). Engineering, Production and Characterization of Spike and Nucleocapsid Structural Proteins of SARS-CoV-2 in Nicotiana Benthamiana as Vaccine Candidates against COVID-19. BioRxiv. doi:10.1101/2020.12.29.424779
Márquez-Escobar, V. A., Rosales-Mendoza, S., Beltrán-López, J. I., and González-Ortega, O. (2017). Plant-based Vaccines against Respiratory Diseases: Current Status and Future Prospects. Expert Rev. Vaccines 16 (2), 137–149. doi:10.1080/14760584.2017.1232167
Matoba, N., Kajiura, H., Cherni, I., Doran, J. D., Bomsel, M., Fujiyama, K., et al. (2009). Biochemical and Immunological Characterization of the Plant-Derived Candidate Human Immunodeficiency Virus Type 1 Mucosal Vaccine CTB-Mpr649-684. Plant Biotechnol. J. 7, 129–145. doi:10.1111/j.1467-7652.2008.00381.x
Maxime, B. N. t. K., Séverin, A., and Gasquez, J. (2016). Introduction Des Organismes Génétiquement Modifiés(OGM) Dans L'agriculture En Côte d'Ivoire: Etat De Connaissance Et Souhaits Des Populations De Six (6) Localités Du Sud Du Pays. Esj 1230, 112. doi:10.19044/esj.2016.v12n30p112
Medicago (2022). Medicago Announces Publication of Phase 3 COVID-19 Vaccine Study Results in New England Journal of Medicine. Available online: https://medicago.com/en/press-release/medicago-announces-publication-of-phase-3-covid-19-vaccine-study-results-in-new-england-journal-of-medicine/.
Meyers, A., Chakauya, E., Shephard, E., Tanzer, F. L., Maclean, J., Lynch, A., et al. (2008). Expression of HIV-1 Antigens in Plants as Potential Subunit Vaccines. BMC Biotechnol. 8, 53. doi:10.1186/1472-6750-8-53
Mtui, G. Y. S. (2011). Status of Biotechnology in Eastern and Central Africa. Biotechnol. Mol. Biol. Rev. 6 (9), 183–198. doi:10.5897/BMBR11.021
Muzhinji, N., and Ntuli, V. (2021). Genetically Modified Organisms and Food Security in Southern Africa: Conundrum and Discourse. GM Crops Food 12 (1), 25–35. doi:10.1080/21645698.2020.1794489
New, R. R. C. (2019). Formulation Technologies for Oral Vaccines. Clin. Exp. Immunol. 198 (2), 153–169. doi:10.1111/cei.13352
Nochi, T., Yuki, Y., Katakai, Y., Shibata, H., Tokuhara, D., Mejima, M., et al. (2009). A Rice-Based Oral Cholera Vaccine Induces Macaque-Specific Systemic Neutralizing Antibodies but does not Influence Pre-Existing Intestinal Immunity. J. Immunol. 183 (10), 6538–6544. doi:10.4049/jimmunol.0901480
Orellana-Escobedo, L., Rosales-Mendoza, S., Romero-Maldonado, A., Parsons, J., Decker, E. L., Monreal-Escalante, E., et al. (2015). An Env-Derived Multi-Epitope HIV Chimeric Protein Produced in the Moss Physcomitrella Patens Is Immunogenic in Mice. Plant Cell. Rep. 34, 425–433. doi:10.1007/s00299-014-1720-6
Ortega-Berlanga, B., and Pniewski, T. (2022). Plant-Based Vaccines in Combat against Coronavirus Diseases. Vaccines 102, 138. doi:10.3390/vaccines10020138
Paris, A. L., Colomb, E., Verrier, B., Anjuère, F., and Monge, C. (2021). Sublingual Vaccination and Delivery Systems. J. Control. Release 332, 553–562. doi:10.1016/j.jconrel.2021.03.017
Pérez-Filgueira, D. M., Brayfield, B. P., Phiri, S., Borca, M. V., Wood, C., and Morris, T. J. (2004). Preserved Antigenicity of HIV-1 P24 Produced and Purified in High Yields from Plants Inoculated with a Tobacco Mosaic Virus (TMV)-derived Vector. J. Virological Methods 121, 201–208. doi:10.1016/j.jviromet.2004.06.022
Pniewski, T., Kapusta, J., Bociąg, P., Wojciechowicz, J., Kostrzak, A., Gdula, M., et al. (2011). Low-dose Oral Immunization with Lyophilized Tissue of Herbicide-Resistant Lettuce Expressing Hepatitis B Surface Antigen for Prototype Plant-Derived Vaccine Tablet Formulation. J. Appl. Genet. 52 (2), 125–136. doi:10.1007/s13353-010-0001-5
Pogrebnyak, N., Golovkin, M., Andrianov, V., Spitsin, S., Smirnov, Y., Egolf, R., et al. (2005). Severe Acute Respiratory Syndrome (SARS) S Protein Production in Plants: Development of Recombinant Vaccine. Proc. Natl. Acad. Sci. U.S.A. 102, 9062–9067. doi:10.1073/pnas.0503760102
Redkiewicz, P., Sirko, A., Kamel, K. A., and Góra-Sochacka, A. (2014). Plant Expression Systems for Production of Hemagglutinin as a Vaccine against Influenza Virus. Acta Biochim. Pol. 61 (3), 551–560. doi:10.18388/abp.2014_1877
Rosales-Mendoza, S., Márquez-Escobar, V. A., González-Ortega, O., Nieto-Gómez, R., and Arévalo-Villalobos, J. I. (2020). What Does Plant-Based Vaccine Technology Offer to the Fight against COVID-19? Vaccines 8 (2), 183. doi:10.3390/vaccines8020183
Sahoo, A., Mandal, A. K., Dwivedi, K., and Kumar, V. (2020). A Cross Talk between the Immunization and Edible Vaccine: Current Challenges and Future Prospects. Life Sci. 261, 118343. doi:10.1016/j.lfs.2020.118343
Sainsbury, F., Thuenemann, E. C., and Lomonossoff, G. P. (2009). pEAQ: Versatile Expression Vectors for Easy and Quick Transient Expression of Heterologous Proteins in Plants. Plant Biotechnol. J. 7 (7), 682–693. doi:10.1111/j.1467-7652.2009.00434.x
Schähs, M., Strasser, R., Stadlmann, J., Kunert, R., Rademacher, T., and Steinkellner, H. (2007). Production of a Monoclonal Antibody in Plants with a Humanized N-Glycosylation Pattern. Plant Biotechnol. J. 5 (5), 657–663. doi:10.1111/j.1467-7652.2007.00273.x
Shakoor, S., Rao, A. Q., Shahid, N., Yaqoob, A., Samiullah, T. R., Shakoor, S., et al. (2019). Role of Oral Vaccines as an Edible Tool to Prevent Infectious Diseases. av 63 (3), 245–252. doi:10.4149/av_2019_301
Shanmugaraj, B., Siriwattananon, K., Malla, A., and Phoolcharoen, W. (2021). Potential for Developing Plant-Derived Candidate Vaccines and Biologics against Emerging Coronavirus Infections. Pathogens 108, 1051. doi:10.3390/pathogens10081051
Sharma, P., Majumder, R., Mondal, H., and Mondal, S. (2021). Plant-based Vaccines: Potentiality against Severe Acute Respiratory Syndrome Coronavirus 2. Biomed. Biotechnol. Res. J. 5, 366–373. doi:10.4103/bbrj.bbrj_185_21
Shim, B.-S., Choi, Y., Cheon, I. S., and Song, M. K. (2013). Sublingual Delivery of Vaccines for the Induction of Mucosal Immunity. Immune Netw. 133, 81–85. doi:10.4110/in.2013.13.3.81
Sohrab, S. S. (2020). An Edible Vaccine Development for Coronavirus Disease 2019: the Concept. Clin. Exp. Vaccine Res. 9 (2), 164–168. doi:10.7774/cevr.2020.9.2.164
Sohrab, S. S., Suhail, M., Kamal, M. A., Husen, A., and Azhar, E. I. (2017). Recent Development and Future Prospects of Plant-Based Vaccines. Cdm 18 (9), 831–841. doi:10.2174/1389200218666170711121810
Streatfield, S. J., and Howard, J. A. (2003b). Plant-based Vaccines. Int. J. Parasitol. 33 (5–6), 479–493. doi:10.1016/S0020-7519(03)00052-3
Streatfield, S. J. (2005). Delivery of Plant-Derived Vaccines. Expert Opin. Drug Deliv. 2 (4), 719–728. doi:10.1517/17425247.2.4.719
Streatfield, S. J., and Howard, J. A. (2003a). Plant Production Systems for Vaccines. Expert Rev. Vaccines 2 (6), 763–775. doi:10.1586/14760584.2.6.763
Suzuki, K., Kaminuma, O., Yang, L., Takai, T., Mori, A., Umezu-Goto, M., et al. (2011). Prevention of Allergic Asthma by Vaccination with Transgenic Rice Seed Expressing Mite Allergen: Induction of Allergen-specific Oral Tolerance without Bystander Suppression. Plant Biotechnol. J. 9 (9), 982–990. doi:10.1111/j.1467-7652.2011.00613.x
Szymanski, C. M., Schnaar, R. L., and Aebi, M. (2016). “Bacterial and Viral Infections,” in Essentials of Glycobiology (Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press).
Tacket, C. O., Mason, H. S., Losonsky, G., Clements, J. D., Levine, M. M., and Arntzen, C. J. (1998). Immunogenicity in Humans of a Recombinant Bacterial Antigen Delivered in a Transgenic Potato. Nat. Med. 4, 607–609. doi:10.1038/nm0598-607
Tacket, C. O., Mason, H. S., Losonsky, G., Estes, M. K., Levine, M. M., and Arntzen, C. J. (2000). Human Immune Responses to a Novel Norwalk Virus Vaccine Delivered in Transgenic Potatoes. J. Infect. Dis. 182, 302–305. doi:10.1086/315653
Tacket, C. O., Pasetti, M. F., Edelman, R., Howard, J. A., and Streatfield, S. (2004). Immunogenicity of Recombinant LT-B Delivered Orally to Humans in Transgenic Corn. Vaccine 22, 4385–4389. doi:10.1016/j.vaccine.2004.01.073
Takagi, H., Hirose, S., Yasuda, H., and Takaiwa, F. (2006). Biochemical Safety Evaluation of Transgenic Rice Seeds Expressing T Cell Epitopes of Japanese Cedar Pollen Allergens. J. Agric. Food Chem. 54 (26), 9901–9905. doi:10.1021/jf061848v
Takeyama, N., Kiyono, H., and Yuki, Y. (2015). Plant-based Vaccines for Animals and Humans: Recent Advances in Technology and Clinical Trials. Ther. Adv. Vaccines 3 (5-6), 139–154. doi:10.1177/2051013615613272
Thanavala, Y., Mahoney, M., Pal, S., Scott, A., Richter, L., Natarajan, N., et al. (2005). Immunogenicity in Humans of an Edible Vaccine for Hepatitis B. Proc. Natl. Acad. Sci. U.S.A. 102, 3378–3382. doi:10.1073/pnas.0409899102
Tregoning, J. S. (2020). First Human Efficacy Study Of A Plant-Derived Influenza Vaccine. Lancet 396 (10261), 1464–1465. doi:10.1016/S0140-6736(20)32010-9
Ulmer, J. B., Valley, U., and Rappuoli, R. (2006). Vaccine Manufacturing: Challenges and Solutions. Nat. Biotechnol. 24, 1377–1383. doi:10.1038/nbt1261
Uthaya Kumar, A., Kadiresen, K., Gan, W. C., and Ling, A. P. K. (2021). Current Updates and Research on Plant-Based Vaccines for Coronavirus Disease 2019. Clin. Exp. Vaccine Res. 10 (1), 13–23. doi:10.7774/cevr.2021.10.1.13
Vanmarsenille, C., Elseviers, J., Yvanoff, C., Hassanzadeh-Ghassabeh, G., Garcia Rodriguez, G., Martens, E., et al. (2018). In Planta Expression of Nanobody-Based Designer Chicken Antibodies Targeting Campylobacter. PLoS One 13 (9), e0204222. doi:10.1371/journal.pone.0204222
Vela Ramirez, J. E., Sharpe, L. A., and Peppas, N. A. (2017). Current State and Challenges in Developing Oral Vaccines. Adv. Drug Deliv. Rev. 114, 116–131. doi:10.1016/j.addr.2017.04.008
Venkataraman, S., Hefferon, K., Makhzoum, A., and Abouhaidar, M. (2021). Combating Human Viral Diseases: Will Plant-Based Vaccines Be the Answer? Vaccines 97, 761. doi:10.3390/vaccines9070761
Virdi, V., Coddens, A., De Buck, S., Millet, S., Goddeeris, B. M., Cox, E., et al. (2013). Orally Fed Seeds Producing Designer IgAs Protect Weaned Piglets against Enterotoxigenic Escherichia coli Infection. Proc. Natl. Acad. Sci. U.S.A. 110 (29), 11809–11814. doi:10.1073/pnas.1301975110
Virupaksh, P. (2006). Edible Vaccines from Transgenic Plants. Res. J. Biotechnol. 1, 1. doi:10.1007/978-81-322-1554-7_12
Webster, D. E., and Thomas, M. C. (2012). Post-Translational Modification of Plant-Made Foreign Proteins; Glycosylation and beyond. Biotechnol. Adv. 30 (2), 410–418. doi:10.1016/j.biotechadv.2011.07.015
Wirz, H., Sauer-Budge, A. F., Briggs, J., Sharpe, A., Shu, S., and Sharon, A. (2012). Automated Production of Plant-Based Vaccines and Pharmaceuticals. SLAS Technol. 17 (6), 449–457. doi:10.1177/2211068212460037
Wurm, F. M. (2004). Production of Recombinant Protein Therapeutics in Cultivated Mammalian Cells. Nat. Biotechnol. 22, 1393–1398. doi:10.1038/nbt1026
Yusibov, V., Hooper, D., Spitsin, S., Fleysh, N., Kean, R., Mikheeva, T., et al. (2002). Expression in Plants and Immunogenicity of Plant Virus-Based Experimental Rabies Vaccine. Vaccine 20, 3155–3164. doi:10.1016/s0264-410x(02)00260-8
Zeitlin, L., Cone, R. A., and Whaley, K. J. (1999). Using Monoclonal Antibodies to Prevent Mucosal Transmission of Epidemic Infectious Diseases. Emerg. Infect. Dis. 5, 54–64. doi:10.3201/eid0501.990107
Keywords: vaccine, plant-based, COVID-19, massive vaccination, Africa
Citation: El Jaddaoui I, Al Idrissi N, Hamdi S, Wakrim L, Nejjari C, Amzazi S, Elouahabi A, Bakri Y and Ghazal H (2022) Plant-Based Vaccines Against COVID-19 for Massive Vaccination in Africa. Front. Drug. Deliv. 2:909958. doi: 10.3389/fddev.2022.909958
Received: 31 March 2022; Accepted: 23 June 2022;
Published: 03 August 2022.
Edited by:
Rein Verbeke, Ghent University, BelgiumReviewed by:
Thorunn Asta Olafsdottir, deCODE Genetics, IcelandDavid A. Muller, The University of Queensland, Australia
Copyright © 2022 El Jaddaoui, Al Idrissi, Hamdi, Wakrim, Nejjari, Amzazi, Elouahabi, Bakri and Ghazal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hassan Ghazal, aGFzc2FuLmdoYXphbEBmdWxicmlnaHRtYWlsLm9yZw==
 Islam El Jaddaoui
Islam El Jaddaoui Najib Al Idrissi
Najib Al Idrissi Salsabil Hamdi
Salsabil Hamdi Lahcen Wakrim
Lahcen Wakrim Chakib Nejjari
Chakib Nejjari Saaïd Amzazi
Saaïd Amzazi Abdelatif Elouahabi8
Abdelatif Elouahabi8 Youssef Bakri
Youssef Bakri Hassan Ghazal
Hassan Ghazal