- 1Department of Critical Care Medicine, Hejin People's Hospital, Yuncheng, Shanxi, China
- 2Department of Rehabilitation, Hejin People's Hospital, Yuncheng, Shanxi, China
- 3Department of Emergency Medicine, Hejin People's Hospital, Yuncheng, Shanxi, China
- 4Department of Emergency Medicine, Ninth Hospital of Xi'an, Xi'an, Shaanxi, China
In recent years, there has been an increase in complications related to heat stroke (HS), especially those affecting the neurological system. The co-occurrence of HS and Guillain–Barré syndrome (GBS) is exceptionally rare, with fewer than 15 documented cases. This case report describes a patient who developed GBS following an HS and includes a literature review that highlights the rarity of this neurological complication. This study aimed to increase awareness and aid in clinical decision-making regarding the management of classic HS.
1 Introduction
In recent decades, extreme climate change has contributed to a continuous rise in global sea surface temperatures. In the last decade, this trend has led to an increased global incidence of heat stroke (HS) (1, 2), which carries a high mortality rate despite ongoing efforts to improve its prognosis through various treatment modalities (3, 4). Hyperthermia, resulting from HS, is a type of neuropathy that can lead to severe neurological complications, including hypothermia, altered mental state, agitation, combativeness, seizures, and significant changes in consciousness levels, primarily due to the heightened sensitivity of human neuronal cells to heat. Neuroimaging studies of HS patients have revealed that the cerebellum is the most commonly affected region, followed by the hippocampus, the midbrain, and the thalamus (5). Injuries to the peripheral nervous system, particularly the combination of HS and Guillain–Barré syndrome (GBS), are extremely rare. GBS is an acute, immune-mediated inflammatory peripheral neuropathy that typically presents with a sudden onset, with the majority of symptoms intensifying within approximately 2 weeks. It causes damage to multiple nerve roots and peripheral nerves, leads to cerebrospinal fluid (CSF) protein–cell dissociation, and is self-limiting (6). GBS is recognized as a rare potential consequence of HS, with Fewer than 15 documented cases (7, 8). This report describes a patient who developed GBS following HS and provides a literature review to highlight this unusual neurological complication. This study aimed to raise awareness of the condition and support clinical decision-making regarding the management of classic HS (CHS).
2 Case report
A 71-year-old man with a history of hypertension and a cerebral infarction 6 months earlier, which resulted in right upper and lower limb dysfunction (manual muscle strength score of 4), was admitted to the emergency department. He had no family history of genetic diseases. The patient was found consciousness in an unventilated space for an unknown duration on 13 July 2023.
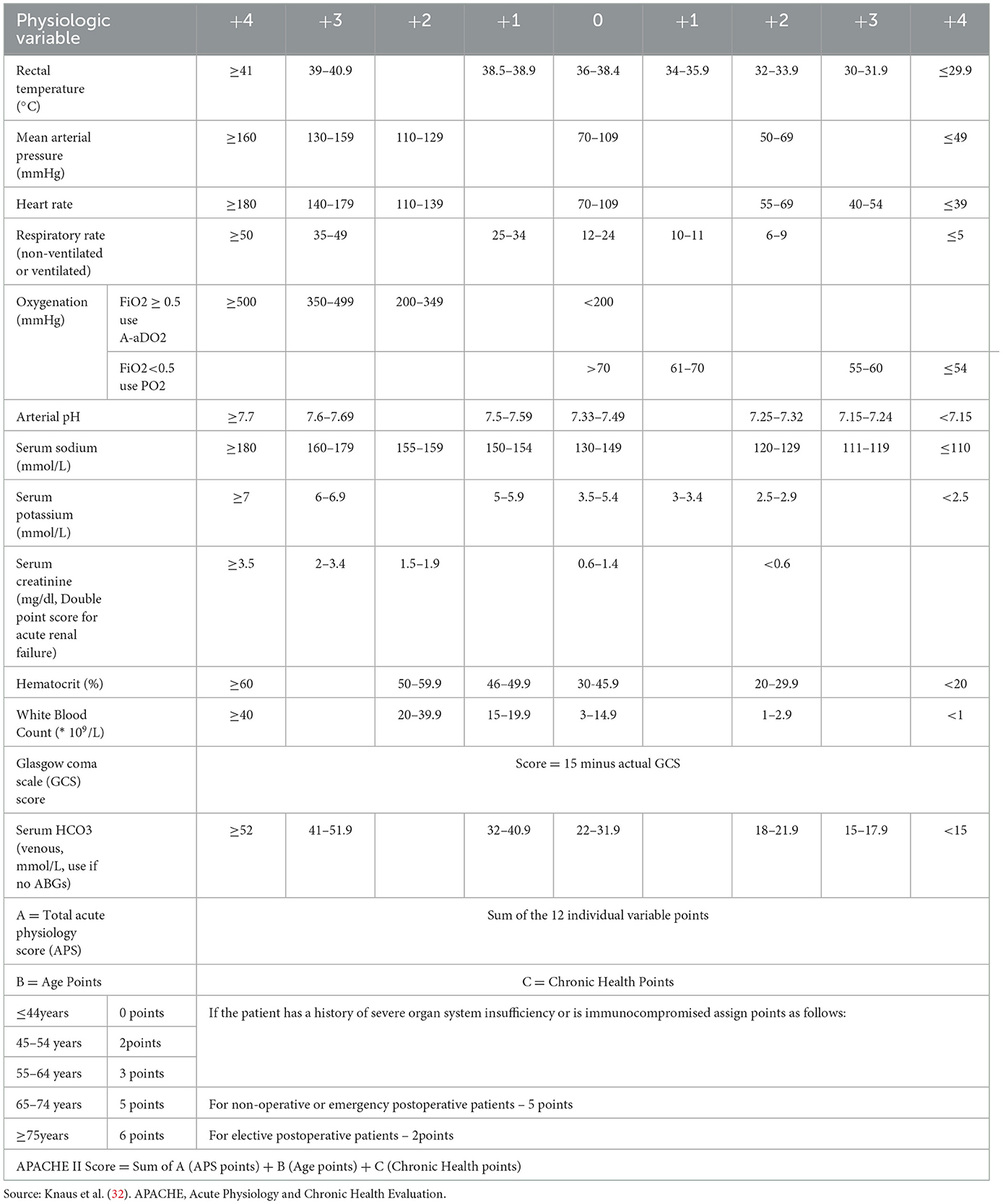
Upon admission, his core body temperature was >41°C. Vital signs included blood pressure of 139/69 mmHg, pulse rate of 149 beats/min, respiratory rate of 33 breaths/min, oxygen saturation of 88%, and blood glucose level of 11.13 mmol/L. He was comatose, with a Glasgow Coma Scale score of E2V2M4, absent light reflexes, and distant heart sounds. Initial blood tests showed respiratory alkalosis and metabolic acidosis (pH 7.610, pCO2 18 mmHg, HCO3− 18.1 mmol/L, Lac 4.2 mmol/L), renal and hepatic dysfunction (serum creatinine 165 μmol/L), elevated inflammatory markers (white blood cell [WBC] count 16.5 × 109/L; procalcitonin 23.09 ng/mL), myocardial damage (serum troponin I 2.5 ng/mL; creatine kinase-MB isoenzymes 105.19 ng/mL), rhabdomyolysis (methaemoglobin > 1,000 ng/mL), thrombocytopenia (platelet count 46 × 109/L), and disseminated intravascular coagulation (DIC) with an International Society of Thrombosis and Haemostasis (ISTH) score of 7. His electrocardiogram showed nodal tachycardia, and a computed tomography (CT) scan of the brain revealed no abnormalities. His Acute Physiology and Chronic Health Evaluation (APACHE) II score was 33 (Appendix 1).
He was diagnosed with severe HS, multi-organ dysfunction syndrome, and a lung infection. His treatment included cooling measures, such as using an ice blanket, administering fluid infusions, providing oxygen through a mask. He underwent plasma transfusions and received treatment for dehydration to help lower intracranial pressure. Additionally, he was given anticoagulant therapy, support for his liver and kidney functions, prophylactic antibiotics, and both enteral and parenteral nutrition. These interventions also aimed to address internal environmental disturbances. The patient began hyperbaric oxygen therapy after 1 week. By the 10th day of treatment, his level of consciousness improved, and he was able to eat and move independently. After 5 days of hyperbaric oxygen therapy and communicating his condition with the family, he was transferred to the rehabilitation department for further treatment.
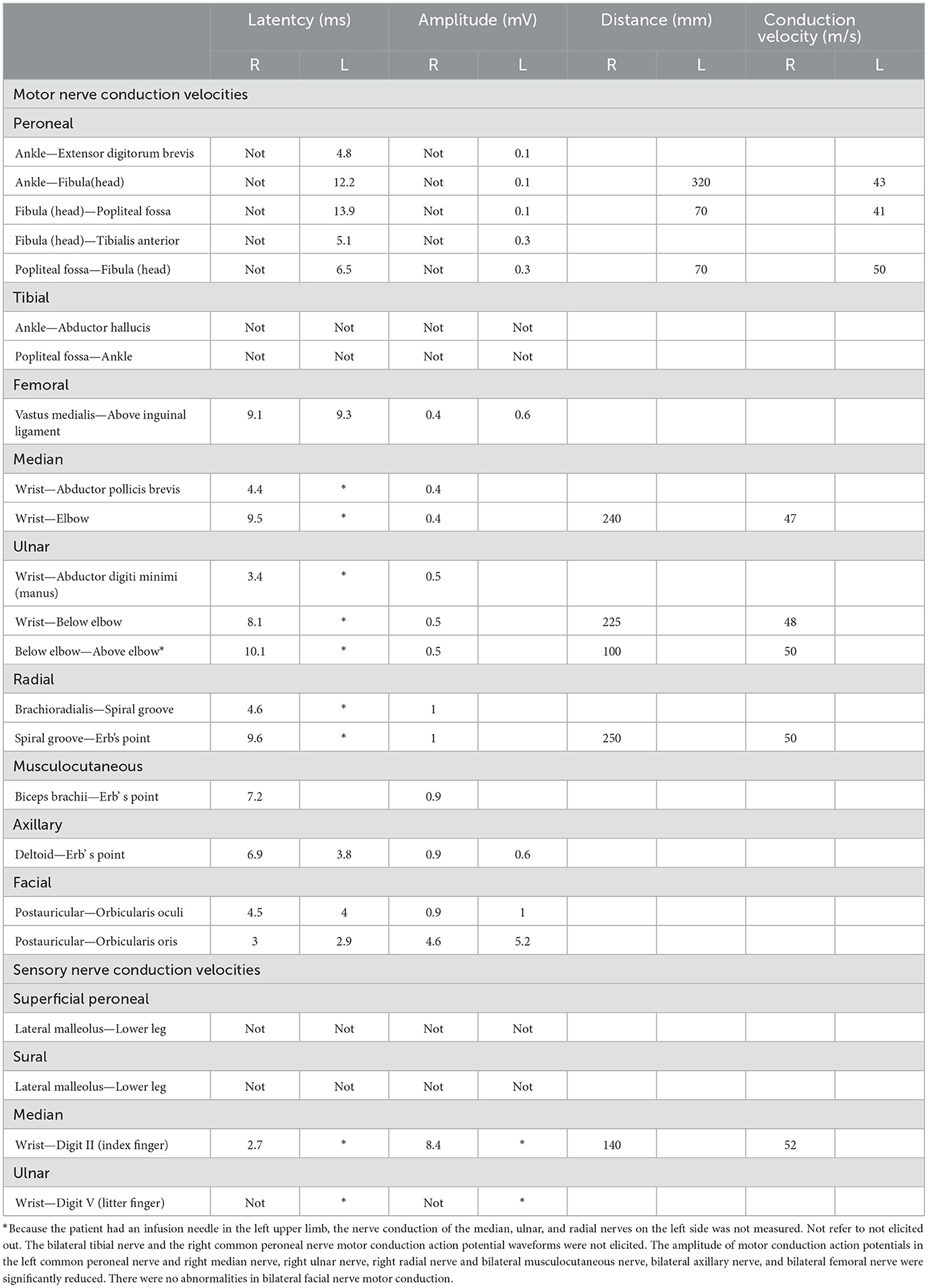
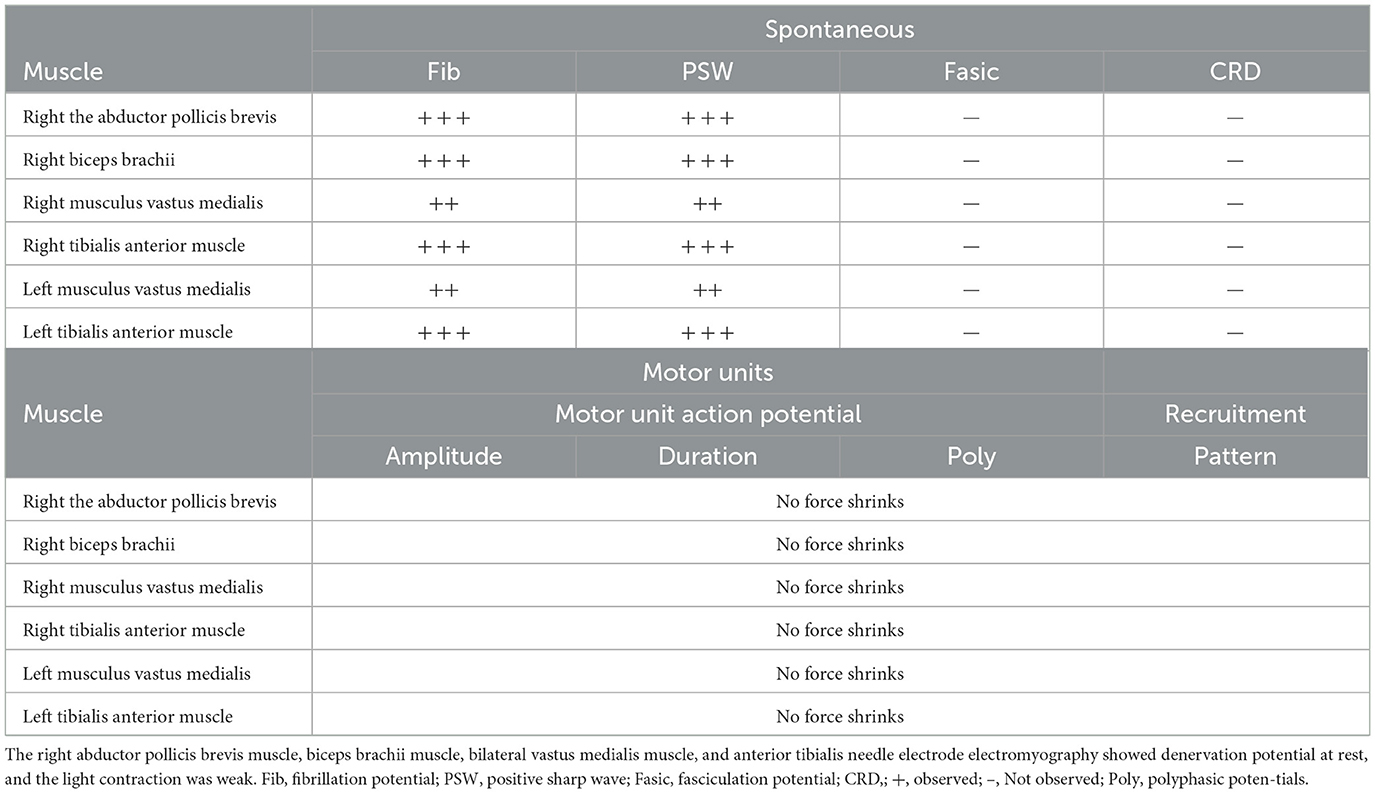
Two weeks after his initial recovery, while still in the rehabilitation department, the patient developed a low-grade fever and showed progressively decreasing muscle strength, along with absent deep tendon reflexes. He did not experience diarrhea and was unable to respond to questions, although his swallowing function was preserved. His serum potassium levels were normal, and inflammatory markers, including procalcitonin and C-reactive protein, were within the normal range. Etiological cultures yielded negative results. Despite physical rehabilitation of the limbs and electromyographic stimulation therapy, his symptoms did not improve. After 1 month, he was transferred to the neurology department for further diagnosis and treatment. Contrast-enhanced magnetic resonance imaging ruled out myelopathy, and CSF analysis showed albuminocytologic dissociation, with a white cell count of < 10 cells/μL and elevated protein levels. Electromyography and nerve conduction velocity studies revealed symmetrical peripheral nerve injury in the extremities, affecting both motor and sensory functions (Tables 1, 2). The patient was diagnosed with GBS and treated with intravenous immunoglobulin at a dosage of 400 mg/kg per day for 5 days. He was drowsy, his speech was slurred, his limb strength was rated level 1, his muscle tone was reduced, and his bilateral pathological signs were negative.
One and a half months later, the patient was transferred back to the rehabilitation department of our hospital. His treatment involved acupuncture at six points on both sides of the limbs: the anterior shoulder, scapula, shou san li, wai guan, he gu, and xue hai. He also continued to receive limb function training and electromyographic stimulation rehabilitation (Figure 1).
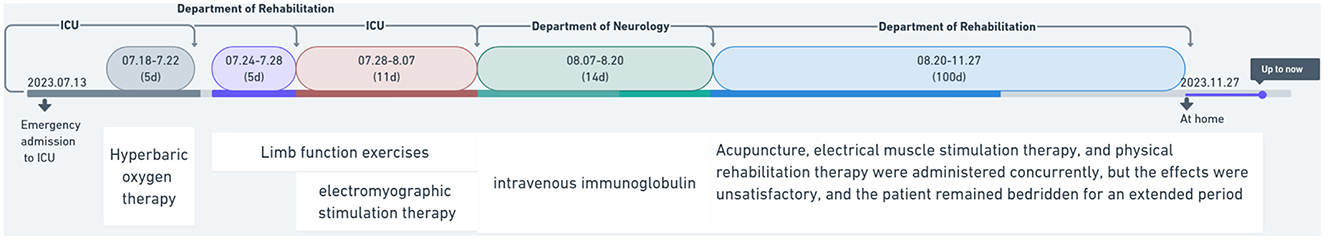
Figure 1. Treatment timing and department transfer flowchart for heat stroke patients complicated with Guillain–Barré syndrome.
After 3 months, he exhibited neurological sequelae, including upper limb dysfunction characterized by a manual muscle strength score of 2 and absent deep tendon reflexes, along with non-fluent speech. After a year, his condition improved only minimally; he remained bedridden and was unable to engage in self-care activities. This case demonstrates that HS-induced GBS progresses rapidly and has a poor prognosis. The impact on patients' quality of life is significant, with most patients unable to care for themselves.
3 Discussion
3.1 Impact of HS and GBS on the elderly, young adults, and children
HS is characterized by neurological symptoms resulting from an excessively high core body temperature, such as seizures and altered consciousness. It may occur with or without persistent multi-organ failure, and it has the potential to impair various organs and tissues, including the brain, myocardium, liver, and muscles. Notably, the brain is the most vulnerable organ to damage during HS and may suffer permanent neurological damage, which can be fatal. HS results primarily from an imbalance between heat generation and heat dissipation in the body due to passive exposure to a hot environment. It can be categorized as CHS or exertional HS (EHS). Notably, elderly patients with chronic illnesses or compromised immune function have a greater risk of CHS, whereas most often, healthy adults, typically athletes, firefighters, and sanitation workers, are at risk of EHS. Children, especially preschool children, are at a higher risk of developing HS than adults because their immature physiology prevents an appropriate response to a high environmental temperature and humidity. The prognosis also differs with age. Elderly patients suffering from HS have a mortality rate of 70%−80%, as their poor thermoregulatory ability and the presence of multiple chronic disorders increase the risk of multi-organ dysfunction. By contrast, young patients generally have better reserves and, in most cases, fully recover through timely treatment; however, long-term cognitive and neurological dysfunctions are possible. Children who recover from HS are at an increased risk of experiencing it again, and those with severe HS may have prolonged physiological responses to physical exertion in hot environments (2, 5, 9, 10).
Delgado et al. (11) reported a case of CHS that severely affected the central nervous system, particularly the spinal cord, despite there being no damage to the cerebrum. This case highlights that, in addition to the cerebellum, the spinal cord is also particularly sensitive to high temperatures. The mortality rate of HS can reach up to 20%, and the central nervous system's high sensitivity to severe fever can lead to various neurological complications, including reversible encephalopathy syndrome, cerebellar symptoms, transverse myelopathy, central pontine myelinolysis, and long-term central nervous system damage such as cognitive impairments and speech disorders. However, cases of HS affecting the peripheral nerves are rarely reported, particularly in the context of GBS (12–15). Therefore, HS primarily affects the central nervous system in its early stages, with peripheral nerve involvement being less common.
GBS is an immune-mediated polyradiculoneuropathy characterized by an acute or subacute onset, with more than 100,000 new cases reported globally each year (6). Initially described by Guillain et al. (33), GBS often occurs following upper respiratory tract infections and gastrointestinal illnesses, such as diarrhea, caused by various pathogens. In addition, vaccinations (including the H1N1 influenza vaccine), immune checkpoint inhibitors, surgical procedures, and organ transplants can also trigger GBS (6, 16, 17). The diagnosis of GBS primarily relies on clinical symptoms, such as rapidly progressive symmetrical limb weakness and the absence or reduction of tendon reflexes, electromyographic findings indicative of peripheral neuropathy, and the presence of protein–cell dissociation in CSF (6). The complex causes of limb weakness following HS, coupled with early symptoms that are often masked, complicate the diagnosis of GBS triggered by HS (18). The condition typically progresses faster, with longer recovery times for neurological function and poorer prognoses in patients with HS and concurrent GBS compared to those with classic GBS (19). Early recognition and diagnosis of limb weakness are crucial for improving the outcomes in patients who have experienced HS.
3.2 Treatment of GBS induced by HS
Treatment for patients with HS-induced GBS includes immunotherapy, mainly intravenous immunoglobulin and plasma exchange (20). Although recent research suggests that both are effective treatments for GBS, no reported cases or studies provide high-quality evidence of their therapeutic benefits in patients with HS-induced GBS. Immunotherapy should be initiated based on the course of the patient's treatment, the severity of the disease, its progression and associated risks, and the patient's preferences. The use of glucocorticoids is not supported by evidence-based medicine and should thus be considered on a case-by-case basis (20).
The latest relevant guidelines for diagnosing and treating GBS (20) emphasize the significance of comprehensive treatment. Once the patient's condition stabilizes, neurological rehabilitation exercises should be carried out promptly to prevent disuse amyotrophy and joint contractures. Additionally, these exercises may be beneficial in alleviating symptoms of limb fatigue. Physical rehabilitation may include physical therapy, myoelectric stimulation, ultrasound therapy, laser, acupuncture, brace, and limb function training. Physical therapy can improve muscle contraction, range of motion, flexibility, and muscle strength through various training methods, including, active, passive, boosted active, and resistance training, adjusted according to the patient's muscle strength. Additionally, transcranial magnetic and neuromuscular electrical stimulation can target the meridians and improve the circulation of qi and blood in the limbs. The specific treatment plan should be tailored according to the patient's symptoms.
In addition, studies examining the predictors of inability to walk independently at 3 months or 6 months have found that several factors do not play a significant role, and these factors include age at admission, progression of muscle weakness assessed during the emergency episode, mechanical ventilation after admission, axonal electrophysiological subtypes, modified Erasmus GBS Outcome Score predictors, and GBS disability score with the worst total Medical Research Council score at admission or within 2 weeks (21). The severity of electromyography findings may indicate potential muscle strength recovery, but there is a lack of relevant case reports and studies. In addition, no studies or case reports detail the post-rehabilitation treatment of HS-induced muscle weakness. The prognosis of our patient after rehabilitation, hormone therapy, and other comprehensive treatments was poor, and at this writing, he remains bedridden.
3.3 Mechanisms, potential prognostic biomarkers, and future research directions related to HS-induced GBS
The pathogenic mechanisms underlying HS-induced GBS remain unclear, as the majority of available information is obtained from case reports. The literature suggests that the majority of the reported GBS cases are triggered by EHS and occur in the elderly, while the incidence among adolescents is negligible. To date, all documented patients diagnosed with HS and GBS were the elderly. Currently, only Kalita and Misra (7) and Wen (8) have reported cases of CHS combined with GBS. In these reports, the patients were administered intravenous immunoglobulin early, which improved GBS symptoms. Previous studies have shown that reducing core body temperature to < 38.9°C within 30 min of presentation increases survival rates. However, in this case, despite receiving the same treatment, the patient showed no significant improvement. We suspect that peripheral nerve damage associated with HS-induced GBS may be more severe than that observed in classic GBS. In addition, the patient's history of cerebral infarction and prolonged elevated core body temperature likely contributed to extended hypoxia, worsening the prognosis. The exact etiology and pathophysiology of HS-induced GBS remain unclear. Some research has indicated elevated levels of heat shock protein 70 (HSP-70) antibodies in both the serum and CSF of patients with GBS (22, 23). However, previous studies did not find a change in HSP-70 concentrations in patients with HS-induced GBS, possibly due to the absence of serum IgG–level measurements, and no evaluations of serum HSP-70 antibody concentrations were reported in any cases.
The pathophysiology of HS is complex, particularly in elderly patients. Recently, the “dual-channel mechanism” has been gaining recognition; the first channel involves direct heat exposure (24), while the second pertains to the physiological response to heat stress (25). The etiology and pathophysiology of GBS also remain unclear, but some research (5) suggests that the thermosensitivity of cells changes due to increased HSP-70 expression during hyperthermic episodes (22, 23), which is primarily regulated at the transcriptional level by heat shock transcription factor (HSF) (26). Notably, HSP-70 expression tends to decline with age, correlating with reduced HSF-binding activity (27, 28). Heat shock protein 72 (HSP-72) is found to be significantly elevated in HS cases and may serve as a prognostic marker in the brain (29). Severe HS, indicated by a core temperature >40°C, often indicates higher serum levels of HSP-70 autoantibodies (30).
A recent study (31) found that the loss of Z-DNA binding protein 1 (ZBP1) during heat stress may help prevent conditions such as HS-induced DIC, systemic inflammatory response syndrome (SIRS), circulatory failure, multiple organ injury, and death. Additionally, Yuan et al. demonstrated that heat stress could be mitigated through the genetic deletion of receptor-interacting protein kinase 3 (RIPK3) (24, 25). After 1 year of follow-up, the patient in this study continues to be in a long-term bedridden state. The HS-induced GBS progresses rapidly and has a poor prognosis, significantly impacting patients' quality of life, with the majority of individuals being unable to care for themselves. Therefore, there is an urgent need for further clinical and basic research to explore its pathogenesis and clinical biomarkers, which could lay the groundwork for early diagnosis and treatment.
4 Limitations
The relationship between HS and GBS has yet to be fully elucidated. As a result, the prognosis of patients with HS-induced GBS is often poor. The specific pathological mechanism behind this condition is unclear, and treatment options remain suboptimal.
5 Conclusion
In conclusion, the peripheral nervous system may be particularly sensitive to hyperthermia and heat waves. HS is a rare but serious cause of GBS that progresses rapidly and has a poor prognosis, severely affecting the patient's quality of life. If a patient exhibits limb weakness following HS, it is important to remain vigilant for signs of GBS. Early diagnosis should be sought through lumbar puncture and electromyography, and timely immunotherapy and supportive care are essential. Further extensive research is needed to verify whether HSP-70 serves as a marker for its onset.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving humans were approved by the Hejin People's Hospital, Yuncheng, Shanxi, China. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
YP: Data curation, Investigation, Validation, Writing – original draft, Writing – review & editing. FL: Data curation, Investigation, Writing – review & editing. SL: Data curation, Investigation, Writing – review & editing. JY: Data curation, Investigation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors are very thankful to all personnel who contributed to this research in any capacity. This case report follows the case report guideline (CARE) guidelines for case reports. All authors are clinicians.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Tobías A, Madaniyazi L, Gasparrini A, Armstrong B. High summer temperatures and heat stroke mortality in Spain. Epidemiology. (2023) 34:892–6. doi: 10.1097/EDE.0000000000001661
3. Misset B, De Jonghe B, Bastuji-Garin S, Gattolliat O, Boughrara E, Annane D, et al. Mortality of patients with heatstroke admitted to intensive care units during the 2003 heat wave in France: a national multiple-center risk-factor study. Crit Care Med. (2006) 34:1087–92. doi: 10.1097/01.CCM.0000206469.33615.02
4. Argaud L, Ferry T, Le QH, Marfisi A, Ciorba D, Achache P, et al. Short- and long-term outcomes of heatstroke following the 2003 heat wave in Lyon, France. Arch Intern Med. (2007) 167:2177–83. doi: 10.1001/archinte.167.20.ioi70147
5. Bouchama A, Abuyassin B, Lehe C, Laitano O, Jay O, O'Connor FG, et al. Classic and exertional heatstroke. Nat Rev Dis Primers. (2022) 8:8. doi: 10.1038/s41572-021-00334-6
6. Shahrizaila N, Lehmann HC, Kuwabara S. Guillain-Barré syndrome. Lancet. (2021) 397:1214–28. doi: 10.1016/S0140-6736(21)00517-1
7. Kalita J, Misra UK. Neurophysiological studies in a patient with heat stroke. J Neurol. (2001) 248:993–5. doi: 10.1007/s004150170056
8. Wen H. Guillain-Barre syndrome induced by heat stroke: a case report and literature review. Clin Lab. (2017) 63:1507–11. doi: 10.7754/Clin.Lab.2017.170231
9. Liu SY, Song JC, Mao HD, Zhao JB, Song Q. Expert consensus on the diagnosis and treatment of heat stroke in China. Mil Med Res. (2020) 7:1. doi: 10.1186/s40779-019-0229-2
10. Dematte JE, O'Mara K, Buescher J, Whitney CG, Forsythe S, McNamee T, et al. Near-fatal heat stroke during the 1995 heat wave in Chicago. Ann Intern Med. (1998) 129:173–81. doi: 10.7326/0003-4819-129-3-199808010-00001
11. Delgado GA, Tunon T, Gallego J, Villanueva JA. Spinal cord lesions in heat stroke. J Neurol Neurosur Psychiat. (1985) 48:1065–7. doi: 10.1136/jnnp.48.10.1065
12. Shimada T, Miyamoto N, Shimada Y, Watanabe M, Shimura H, Ueno Y, et al. Analysis of clinical symptoms and brain MRI of heat stroke: 2 case reports and a literature review. J Stroke Cerebrovasc Dis. (2020) 29:104511. doi: 10.1016/j.jstrokecerebrovasdis.2019.104511
13. Lin JJ, Chang MK, Sheu YD, Ting KS, Sung SC, Lin TQ. Permanent neurologic deficits in heat stroke. Zhonghua Yi Xue Za Zhi. (1991) 47:133–8.
14. McNamee T, Forsythe S, Wollmann R, Ndukwu IM. Central pontine myelinolysis in a patient with classic heat stroke. Arch Neurol. (1997) 54:935–6. doi: 10.1001/archneur.1997.00550200005002
15. Biary N, Madkour MM, Sharif H. Post-heatstroke parkinsonism and cerebellar dysfunction. Clin Neurol Neurosurg. (1995) 97:55–7. doi: 10.1016/0303-8467(94)00065-E
16. de Andrade R, da Silva RC, Rebello Pinho JR, de Oliveira JB, Coelho FM. Guillain-Barré syndrome-the challenge of unrecognized triggers. Neurol Sci. (2019) 40:2403–4. doi: 10.1007/s10072-019-03926-z
17. Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain-Barré syndrome: a systematic review and meta-analysis. Neuroepidemiology. (2011) 36:123–33. doi: 10.1159/000324710
18. Yang Y, Quan Z, Shun-cheng X, Jianfeng S, Ben-gang W, Wen-bo W. Report on a case of severe heatstroke combined with Guillain-Barré syndrome. Chin Med Case Reposit. (2023) 05:01554. doi: 10.3760/cma.j.cmcr.2023.01554
19. Ping L, Yan-fang X, Shuai H, Xiao-xia Z, Juan-juan H, Xue-liang Q. Guillain-Barré syndrome caused by heat stroke: three cases report and literature review. Chin J Contemp Neurol Neurosurg. (2024) 24:352–8. doi: 10.3969/j.issn.1672-6731.2024.05.008
20. Chinese Chinese Society of Neurology and Peripheral Neuropathy Collaboration Group Neurology Branch Chinese Medical Association. Guidelines for the diagnosis and treatment of Guillain-Barré syndrome in China 2024. Chin J Neurol. (2024) 57:936–944. doi: 10.3760/cma.j.cn113694-20240220-00099
21. Busl KM, Fried H, Muehlschlegel S, Wartenberg KE, Rajajee V, Alexander SA, et al. Guidelines for Neuroprognostication in Adults with Guillain-Barré Syndrome. Neurocrit Care. (2023) 38:564–83. doi: 10.1007/s12028-023-01707-3
22. Helgeland G, Petzold A, Hoff JM, Gilhus NE, Plant GT, Romi FR. Anti-Heat Shock Protein 70 antibody levels are increased in myasthenia gravis and Guillain-Barré syndrome. J Neuroimmunol. (2010) 225:180–3. doi: 10.1016/j.jneuroim.2010.04.024
23. Yonekura K, Yokota S, Tanaka S, Kubota H, Fujii N, Matsumoto H, et al. Prevalence of anti-heat shock protein antibodies in cerebrospinal fluids of patients with Guillain-Barré syndrome. J Neuroimmunol. (2004) 156:204–9. doi: 10.1016/j.jneuroim.2004.07.017
24. Elinder CG. Heat stroke - the tip of an iceberg. J Intern Med. (2023) 294:4–6. doi: 10.1111/joim.13645
25. Expert Expert panel on the prevention and treatment of Heat Stroke Expert Expert consensus group on the emergency diagnosis and treatment of Heat Stroke. Consensus of experts on emergency diagnosis and treatment of Heat Stroke (2021version). Chin J Emerg Med. (2021) 30:1290–1299. doi: 10.3760/cma.j.issn.1671-0282.2021.11.002
26. Sorger PK. Heat shock factor and the heat shock response. Cell. (1991) 65:363–6. doi: 10.1016/0092-8674(91)90452-5
27. Gutsmann-Conrad A, Heydari AR, You S, Richardson A. The expression of heat shock protein 70 decreases with cellular senescence in vitro and in cells derived from young and old human subjects. Exp Cell Res. (1998) 241:404–13. doi: 10.1006/excr.1998.4069
28. Moore SA, Lopez A, Richardson A, Pahlavani MA. Effect of age and dietary restriction on expression of heat shock protein 70 in rat alveolar macrophages. Mech Ageing Dev. (1998) 104:59–73. doi: 10.1016/S0047-6374(98)00052-9
29. Dehbi M, Baturcam E, Eldali A, Ahmed M, Kwaasi A, Chishti MA, et al. Hsp-72, a candidate prognostic indicator of heatstroke. Cell Stress Chaperones. (2010) 15:593–603. doi: 10.1007/s12192-010-0172-3
30. Lee WC, Wen HC, Chang CP, Chen MY, Lin MT. Heat shock protein 72 overexpression protects against hyperthermia, circulatory shock, and cerebral ischemia during heatstroke. J Appl Physiol. (2006) 100:2073–82. doi: 10.1152/japplphysiol.01433.2005
31. Yuan F, Cai J, Wu J, Tang Y, Zhao K, Liang F, et al. Z-DNA binding protein 1 promotes heatstroke-induced cell death. Science. (2022) 376:609–15. doi: 10.1126/science.abg5251
32. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. (1985) 13:818–29.
33. Guillain G, Barré JA, Strohl A. Sur un syndrome de radiculo-névrite avec hyperalbuminose du liquide céphalo-rachidien sans réaction cellulaire. Remarques sur les caractères cliniques et graphiques des réflexes tendineux [Radiculoneuritis syndrome with hyperalbuminosis of cerebrospinal fluid without cellular reaction. Notes on clinical features and graphs of tendon reflexes. 1916]. Ann Med Interne (Paris). (1999) 150:24–32.
Appendix
Keywords: heat stroke (HS), classic heat stroke (CHS), exertional heat stroke (EHS), Guillain–Barré syndrome (GBS), heat shock protein 70 (HSP-70)
Citation: Pang Y, Li F, Li S and Yuan J (2025) Heat stroke-induced Guillain–Barré syndrome: a case report and literature review. Front. Disaster Emerg. Med. 2:1457466. doi: 10.3389/femer.2024.1457466
Received: 30 June 2024; Accepted: 04 December 2024;
Published: 06 January 2025.
Edited by:
Theodore Chan, University of California, San Diego, United StatesReviewed by:
Lorna Galleguillos, Clínica Alemana, ChileMaria Mermiri, University Hospital of Larissa, Greece
Copyright © 2025 Pang, Li, Li and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Pang, cHlhbmcyMDE4MTIyMUAxNjMuY29t; Jiaojiao Yuan, eXVhbmpqMThAMTYzLmNvbQ==
 Yang Pang
Yang Pang Feijie Li2
Feijie Li2 Jiaojiao Yuan
Jiaojiao Yuan