- 1Department of Paediatric Emergency Medicine, Barking Havering and Redbridge University Trust, London, United Kingdom
- 2SAPPHIRE Group, Health Sciences, Leicester University, Leicester, United Kingdom
- 3Paediatric Emergency Medicine Leicester Academic (PEMLA) Group, Children's Emergency Medicine, Leicester Royal Infirmary, Leicester, United Kingdom
- 4Emergency Department, Bristol Royal Hospital for Children, Bristol, United Kingdom
- 5Research in Emergency Care Avon Collaborative Hub (REACH), University of the West of England, Bristol, United Kingdom
- 6Department of Paediatric Emergency Medicine, Children's Health Ireland, Dublin, Ireland
- 7Women's and Children's Health, School of Medicine, University College Dublin, Dublin, Ireland
- 8Department of Paediatric Endocrinology, Evelina London Children's Hospital, Guys and St. Thomas' NHS Foundation Trust, London, United Kingdom
- 9Department of Pediatric Emergency Medicine, Division of Medicine, St. Mary's Hospital - Imperial College NHS Healthcare Trust, London, United Kingdom
- 10Faculty of Medicine, Department of Infectious Diseases, Section of Pediatric Infectious Diseases, Imperial College London, London, United Kingdom
- 11Centre for Pediatrics and Child Health, Imperial College London, London, United Kingdom
Objectives: The objective of this study was to evaluate the characteristics of children presenting with new onset diabetes and diabetic ketoacidosis (DKA) in the first COVID pandemic year, compared to pre-pandemic evidence and identify the factors associated with DKA at diagnosis.
Design: Retrospective medical record review.
Setting: Forty-nine pediatric Emergency Departments (EDs) across the UK and Ireland.
Patients: All children aged 6 months to 16 years presenting to EDs with new onset diabetes and DKA, during the COVID-19 pandemic (1 March 2020–28 February 2021) and the preceding year (1 March 2019–28 February 2020).
Results: There were increases in children presenting with new onset diabetes in DKA (395–566, 43%) and severe DKA (141–252, 79%) in the first COVID pandemic year, with patient characteristics similar to the pre-pandemic period. Healthcare seeking delay did not appear to be the sole contributing factor to DKA during the COVID pandemic. The median duration of symptoms of 14 days for both children who presented with and without DKA and were similar across both years; those in severe DKA had shorter median duration of 7 days (IQR: 5–21 days).
Conclusions: There were significant increases in children with new onset diabetes presenting with DKA in the first COVID pandemic year. Increased DKA rates and severity despite a constant median symptom duration suggest a multifactorial process. Studies to determine checkpoints for intervention between symptom onset and diagnosis of diabetes are vital to mitigate the high incidence of DKA in new onset diabetes.
Introduction
A marked increase in new-onset Type 1 diabetes mellitus was noted in the first COVID-19 pandemic year, there was also a significant rise in diabetic ketoacidosis (DKA) (1–3). The presence of DKA at diagnosis is associated with serious acute and chronic adversity for patients, including cerebral oedema, shock, recurrent episodes of DKA, altered brain growth and negative impact on long-term spatial memory and cognitive scores. DKA at diagnosis of Type 1 diabetes predicts poor long-term glycaemic control, independent of demographic and socioeconomic factors (4). Current awareness campaigns have not reduced DKA rates over the past decade (1). We present an a priori planned secondary analysis of children presenting in DKA from the Diabetes Mellitus in Children and Young People in the SARS-CoV-2 Pandemic study (DIMPLES) (3).
Methods
In this retrospective chart review study conducted in 49 UK & Ireland pediatric EDs (3) we evaluated the characteristics of children with new onset diabetes presenting with DKA in the first COVID pandemic year compared to pre-pandemic evidence. All children aged 6 months to 16 years presenting to EDs with (1) new onset diabetes and DKA, during the COVID-19 pandemic (1 March 2020–28 February 2021) and the preceding year (1 March 2019–28 February 2020) were included. Here we discuss the factors contributing to the marked increase in DKA in the COVID pandemic, the prevalence of excess DKA in the pre-pandemic period and strategies to reduce DKA in new onset diabetes. DKA was defined as a pH <7.3, or bicarbonate <15 mmol/ and severe DKA as a pH <7.1 or serum bicarbonate <5 mmol/ and ketones of >3.0 mmol/L in line with national and international guidance.
Results
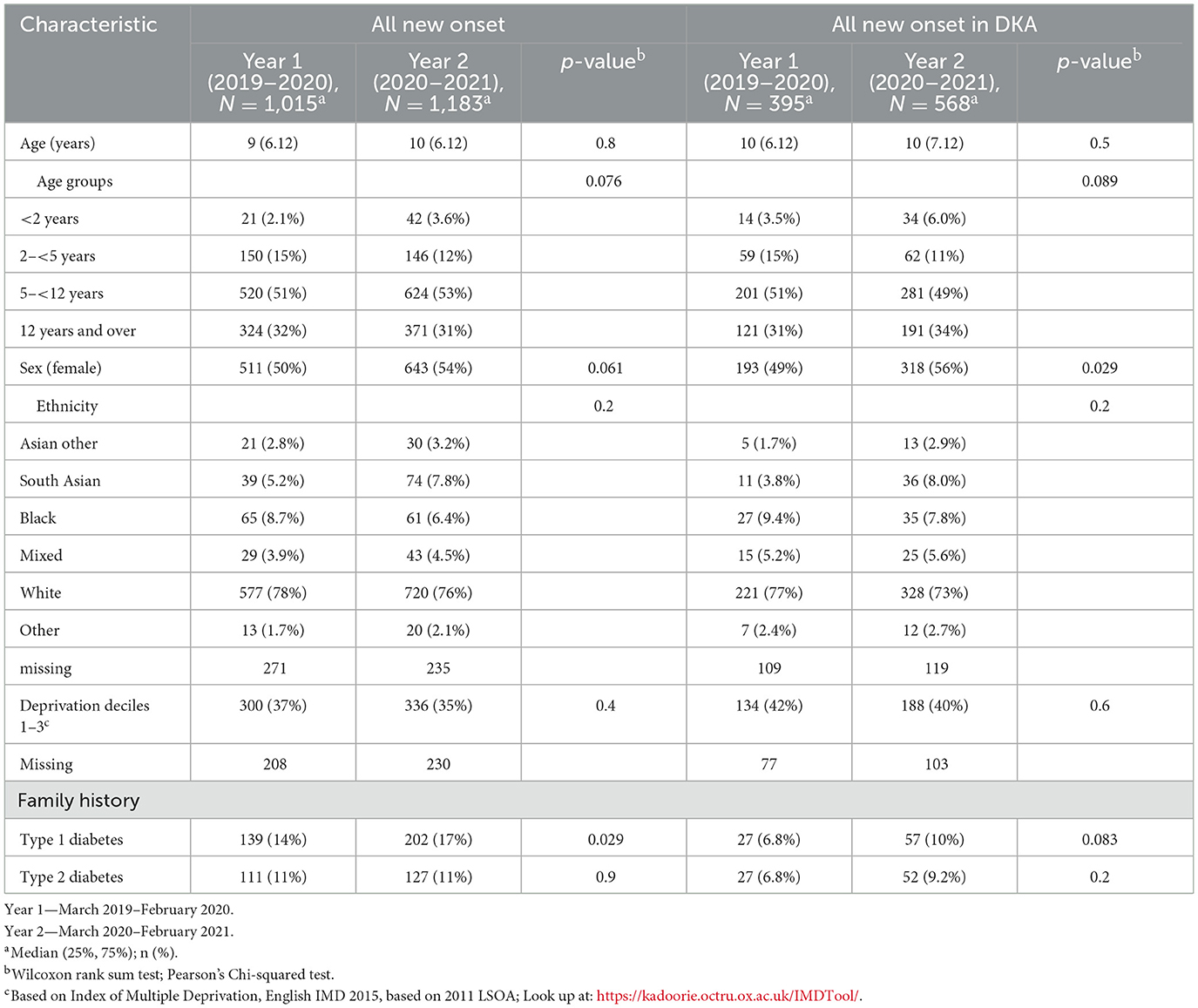
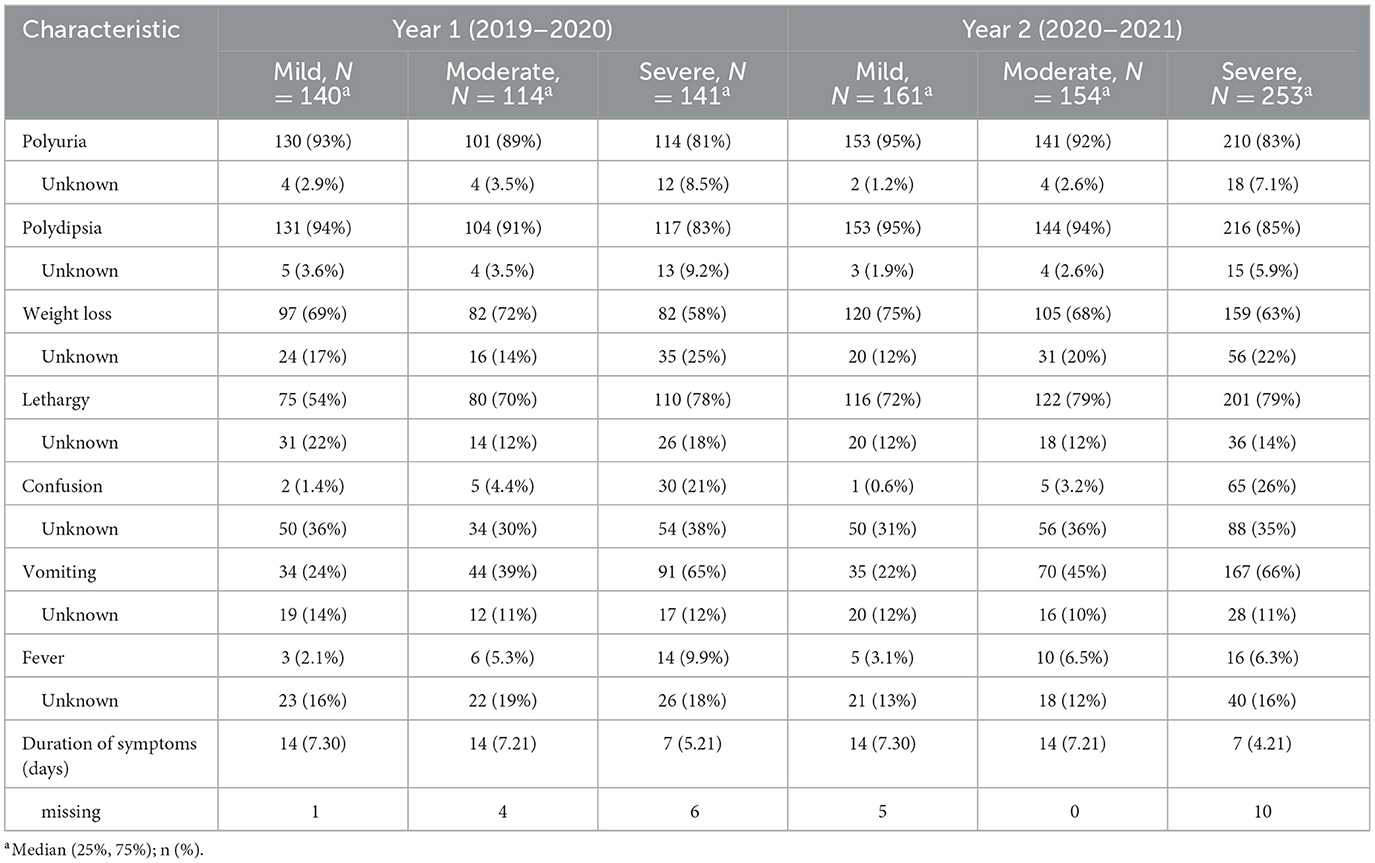
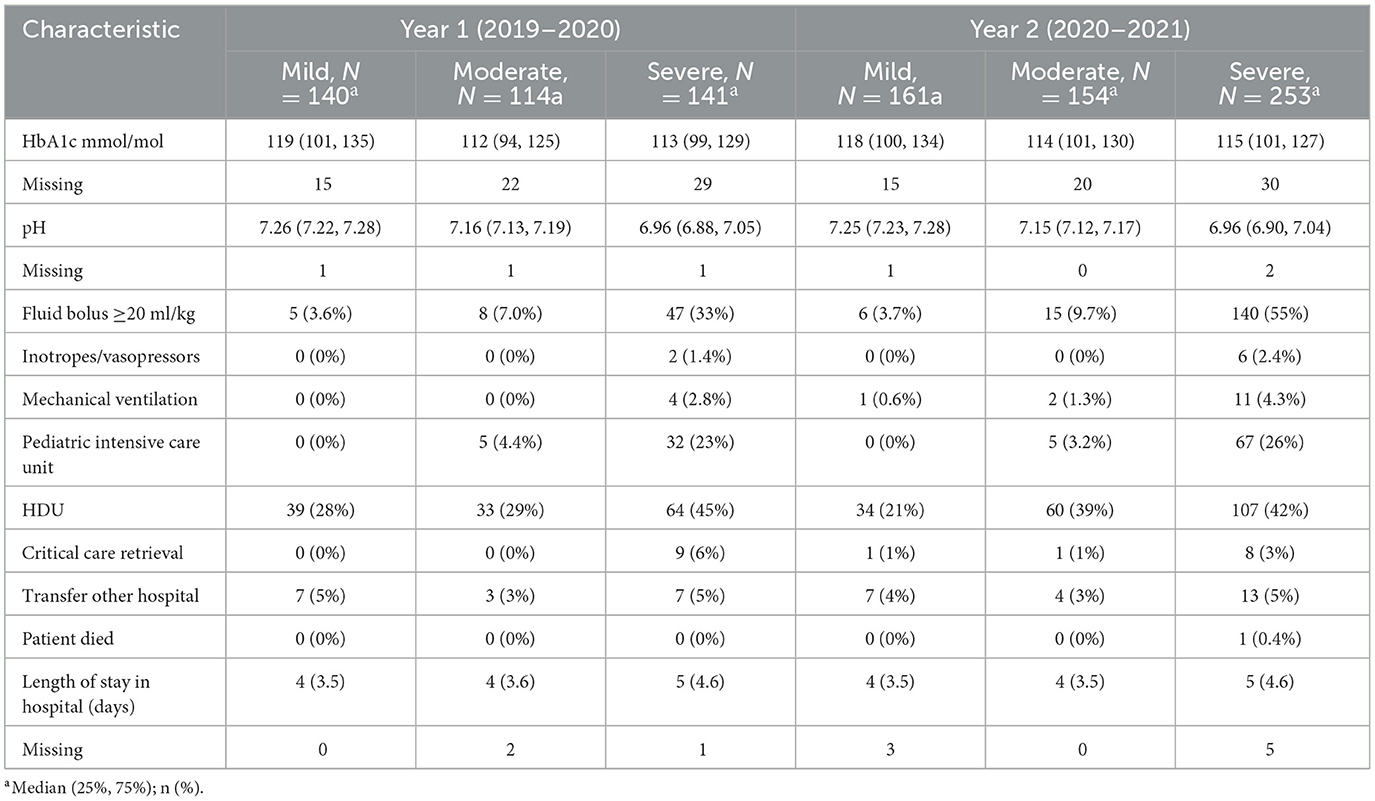
A total of 963 children presenting with new onset diabetes and DKA were included in the final analysis. Ninety-seven percent of all children with new onset diabetes were diagnosed with Type 1 diabetes mellitus (T1DM) and 2% were diagnosed with Type 2 diabetes (T2DM); for those presenting in DKA all had T1DM. During the pre-pandemic year (“year 1”) 395/1,015 children with new-onset diabetes presented with DKA (38.9%), rising to 568/1,183 (48%) in the pandemic year (“year 2”) (Table 1). This upswing was incremental across across all age ranges and driven mostly by a rise of 79% in cases of severe DKA (141–252, p < 0.001; Table 2, Figure 1). Notably, 51% of new onset diabetes over 12 years of age presented with DKA in the COVID pandemic year compared to 37% in the previous year (Tables 2, 3). Deprivation was not significantly associated with DKA at diagnosis and ethnicity characteristics were unchanged. There was an increase in admissions to intensive care (38–72, p < 0.001; Table 3).
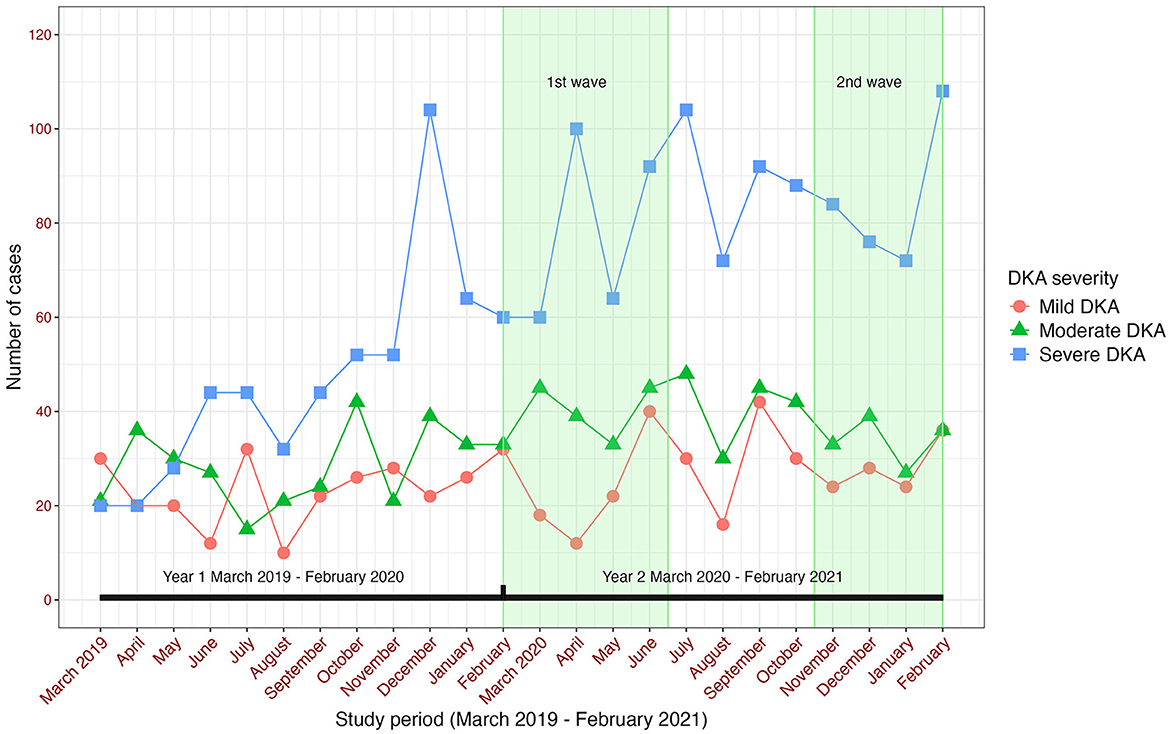
Figure 1. Number of cases of diabetic ketoacidosis in children with new onset diabetes in the Diabetes Mellitus in Children and Young People in the SARS-CoV-2 Pandemic study.
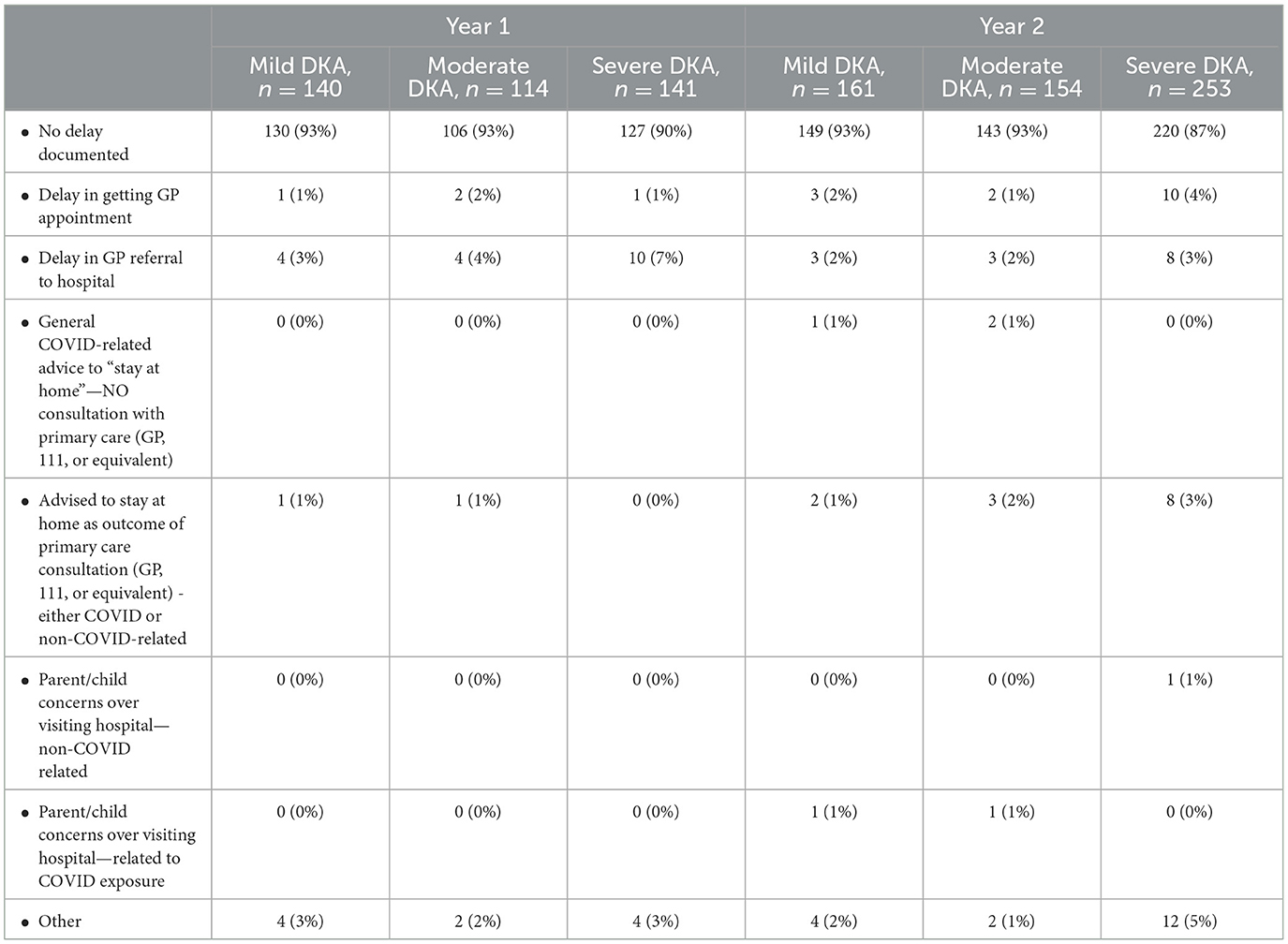
Median diagnostic intervals (days from symptom onset to diagnosis) were similar across years, at 14 days overall (IQR 7–30), and seven days for severe DKA (IQR 5–21); this was similar to those presenting without DKA for both years (median 14; IQR 7–30) (Table 4). Reported healthcare seeking delays were infrequent (Appendix A).
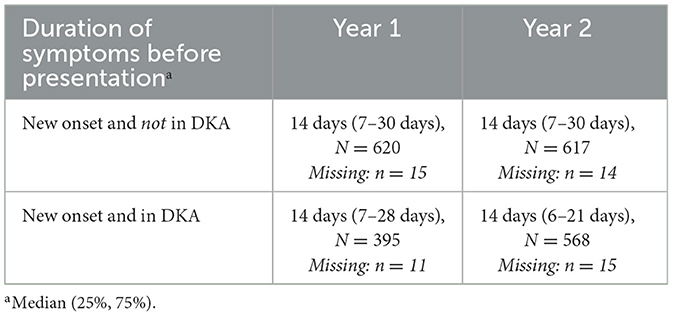
Table 4. Symptom duration of children presenting with new onset Type 1 Diabetes Mellitus in the COVID-19 pandemic (Year 2) and the pre-pandemic year (Year 1).
Discussion
The significant change in DKA volume and severity in the first pandemic year are best interpreted in the context of the high incidence of new onset T1DM, the presence of a ubiquitous diabetogenic environmental trigger (4, 5) and system changes, framed in the background of excess DKA prevalence in the pre-pandemic period (1, 3). In this DIMPLES study, 16 children tested positive for SARS-CoV-2 on NPA RT- PCR of which 13 presented in DKA. This is a small sample size therefore it is difficult to determine whether an acute illness with SARS-CoV-2 infection makes decompensation more likely. However, it is possible that the inflammatory cascades of the COVID-19 and DKA may have acted synergistically contributing to the severity of the clinical manifestations in these children. Increased susceptibility of the endocrine pancreas to SARS-CoV-2 is suggested by pancreatic tissue, post-mortem and animal studies proposing mechanisms for beta cell injury (6, 7).
The characteristics of children presenting with new onset diabetes and DKA were similar across both years. Our data do not support the hypothesis that healthcare interruption was the main driver for the increased DKA in the pandemic. Whilst we found some evidence of reported delays in accessing healthcare, the rates were low and mostly unchanged across both years, intervals to diagnosis were largely unchanged. Furthermore, the marked increase in DKA were sustained beyond the first year of the pandemic when containment measures were reduced (1, 2).
Children with longer symptom duration (more than 6 weeks) presented without DKA in our study, whereas others with <2 weeks of reported symptoms presented in severe DKA, suggesting that individual host factors and the heterogeneity of T1DM contributes to DKA susceptibility in new onset diabetes. The shorter duration of reported symptoms in some children presenting with DKA indicate that children can tip the balance quickly from being symptomatic to being metabolically unstable. We previously reported that 72% of children with new onset T1DM had one or more pancreatic autoantibodies (4), indicating the existence of cohort of children with autoimmunity. As a main strength, we collected standardized data from consecutive children with new onset diabetes presenting to a large number of representative UK and Irish EDs; however, for some, but not all, clinical and biochemical variables data appeared to be missing or unknown more frequently in those with severe DKA, potentially reflecting variation in clinical documentation, history taking and data collection, possibly linked to site practice variability.
Our study confirms known risk factors for DKA at diagnosis such as younger age (<2 years). However, the observed pattern of increased DKA, and significantly severe DKA at diagnosis in older children highlights the challenges surrounding timely recognition of T1DM despite the presence and frequency of symptoms of diabetes in our cohort. Publicly stressing the high incidence of DKA since the pandemic, involving the community and schools may help facilitate earlier diagnosis of new onset diabetes. Pancreatic autoantibodies screening to detect at-risk children offers the prospect of preventing DKA at presentation (8). Our study indicates that DKA at diagnosis may reflect a complex multifactorial etiology, prospective studies to understand the events and timeline preceding presentation of a child with new onset diabetes are required.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
CP: Conceptualization, Project administration, Writing – original draft, Writing – review & editing. DR: Conceptualization, Methodology, Project administration, Writing – review & editing. ML: Conceptualization, Writing – review & editing. MB: Conceptualization, Project administration, Writing – review & editing. TH: Conceptualization, Writing – review & editing. RN: Conceptualization, Data curation, Formal analyses, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/femer.2024.1385450/full#supplementary-material
References
1. Birkebaek NH, Kamrath C, Grimsmann JM, Aakesson K, Cherubini V, Dovc K, et al. Impact of the COVID-19 pandemic on long-term trends in the prevalence of diabetic ketoacidosis at diagnosis of paediatric type 1 diabetes: an international multicentre study based on data from 13 national diabetes registries. Lancet Diabetes Endocrinol. (2022) 10:786–94. doi: 10.1016/S2213-8587(22)00246-7
2. D'Souza D, Empringham J, Pechlivanoglou P, Uleryk EM, Cohen E, Shulman R. Incidence of diabetes in children and adolescents during the COVID-19 pandemic: a systematic review and meta-analysis. JAMA Netw Open. (2023) 6:e2321281. doi: 10.1001/jamanetworkopen.2023.21281
3. Ponmani C, Nijman RG, Roland D, Barrett M, Hulse T, Whittle V, et al. Children presenting with diabetes and diabetic ketoacidosis to Emergency Departments during the COVID-19 pandemic in the UK and Ireland: an international retrospective observational study. Arch Dis Child. (2023) 108:799–807. doi: 10.1136/archdischild-2022-325280
4. Ponmani C, Lyttle MD, Barrett M, Hulse T, Nijman RG, Roland D, et al. What does SARS-CoV-2 tell us about the aetiology of environmental triggers for diabetes in children and young people? Acta Paediatr. (2023) 112:2462–4. doi: 10.1111/apa.16971
5. Duca LM, Wang B, Rewers M, Rewers A. Diabetic ketoacidosis at diagnosis of type 1 diabetes predicts poor long-term glycemic control. Diabetes Care. (2017) 40:1249–55. doi: 10.2337/dc17-0558
6. Albrecht L, Bishop E, Jay B, Lafoux B, Minoves M, Passaes C. COVID-19 research: lessons from non-human primate models. Vaccines. (2021) 9:886. doi: 10.3390/vaccines9080886
7. Fignani D, Licata G, Brusco N, Nigi L, Grieco GE, Marselli L, et al. SARS-CoV-2 receptor angiotensin i-converting enzyme type 2 (ACE2) is expressed in human pancreatic β-cells and in the human pancreas microvasculature. Front Endocrinol. (2020) 11:596898. doi: 10.3389/fendo.2020.596898
8. Besser REJ, Bell KJ, Couper JJ, Ziegler AG, Wherrett DK, Knip M, et al. Clinical practice consensus guidelines 2022: stages of type 1 diabetes in children and adolescents. Pediatr Diabetes. (2022) 23:1175–87. doi: 10.1111/pedi.13410
Appendix
Keywords: children, diabetes, COVID-19, DKA, Emergency medicine
Citation: Ponmani C, Roland D, Lyttle MD, Barrett M, Hulse T and Nijman RG (2024) Characteristics of children presenting with new onset diabetes and DKA in the COVID-19 pandemic: a national cohort study. Front. Disaster Emerg. Med. 2:1385450. doi: 10.3389/femer.2024.1385450
Received: 12 February 2024; Accepted: 29 July 2024;
Published: 22 August 2024.
Edited by:
Shane George, Gold Coast University Hospital, AustraliaReviewed by:
Joseph Pompa, University of Florida, United StatesAman Bhakti Pulungan, University of Indonesia, Indonesia
Copyright © 2024 Ponmani, Roland, Lyttle, Barrett, Hulse and Nijman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caroline Ponmani, Y2Fyb2xpbmUucG9ubWFuaUBuaHMubmV0
 Caroline Ponmani
Caroline Ponmani Damian Roland
Damian Roland Mark D. Lyttle4,5
Mark D. Lyttle4,5 Michael Barrett
Michael Barrett Ruud G. Nijman
Ruud G. Nijman