- 1Department of Cardiology, University Hospital of Patras, Patras, Greece
- 2First Department of Cardiology, Hippocration General Hospital, National and Kapodistrian University of Athens, Athens, Greece
- 3Department of Radiology, Brigham and Women's Hospital, Boston, MA, United States
- 4First Department of Neurology, Eginition Hospital, Medical School, National and Kapodistrian University of Athens, Athens, Greece
- 5Department of Neurology, Tallaght University Hospital, Dublin, Ireland
Cardiogenic Shock represents a life-threatening condition characterized by high mortality and a spectrum of clinical presentations, complicating ~5%−10% of patients presenting with Acute Coronary Syndromes. Despite advances in interventional cardiology and emergency medicine, mortality rates remain extremely high and evidence concerning its management is scarce. Consequently, the decision making relies heavily on a single operator's experience. This comprehensive review aims to provide a thorough update on the latest proof regarding mechanical circulatory support devices of the left ventricle and examines the role of the classification scores on the selection of the appropriate patient and timing for the initiation of the device. The five necessary steps to a successful mechanical circulatory support device's insertion. The picture was made by Pixlr AI Image Generator.
1 Introduction
Cardiogenic shock (CS) is a medical emergency with high mortality and morbidity, which consists of a wide spectrum of clinical presentations, ranging from an early hemodynamic compromise, shock to even full-blown multi-organ failure (1).
Despite the state-of-the-art advances in the field of cardiology and emergency medicine, its mortality rate as a complication of acute myocardial infraction (AMI) remains extremely high (2), complicating about 5%−10% of ST-elevation and non-ST elevation myocardial infarction cases.
Apart from the urgent revascularization of the culprit lesion, key components of CS management, are pharmacotherapeutic regimens concerning volume management, inotropes, vasopressors and the use of Mechanical Circulation Support (MCS) devices, if necessary. The current ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure recommend the use of MCS for patients with cardiogenic shock as a bridge to bridge, a bridge to recovery or a bridge to decision (IIa), including treatment of the cause, long-term support, or transplantation (3, 4).
Although the timely introduction of the optimal device is of utmost importance, due to the emergent nature of this medical entity, the inherent difficulty in patient allocation and ethical issues surrounding the type of the scientific hypothesis, there is a lack of Randomized Control Trials (RCTs) increasing the definitive evidence. For this reason, the decision of whether, when and which circulatory support device to use is based mainly on observational data from specialized centers with conflicting results (5).
Herein, we aim to summarize the existing evidence regarding the use of MCS devices during AMICS in the literature and their current role in contemporary clinical practice.
2 Mechanical circulation support devices in cardiogenic shock
The temporary circulatory support devices are mainly introduced as a bridge to the heart's recovery and to limit the patient's dependency on inotropes and/or vasopressors. The utilization of these medications, apart from a beneficial role in improving the cardiac output and the vascular tone, could be possibly connected with severe adverse events. In more detail, due to their effect on the left ventricle's afterload, increased oxygen demand from the myocardium and arrhythmias could be provoked (6, 7). On the other hand, the temporary MCS devices unload the left ventricle, thereby intracardiac filling pressures are reduced, which contributes to a decline in myocardial stress and oxygen consumption; however, they are also connected to vascular and non-vascular adverse events (8).
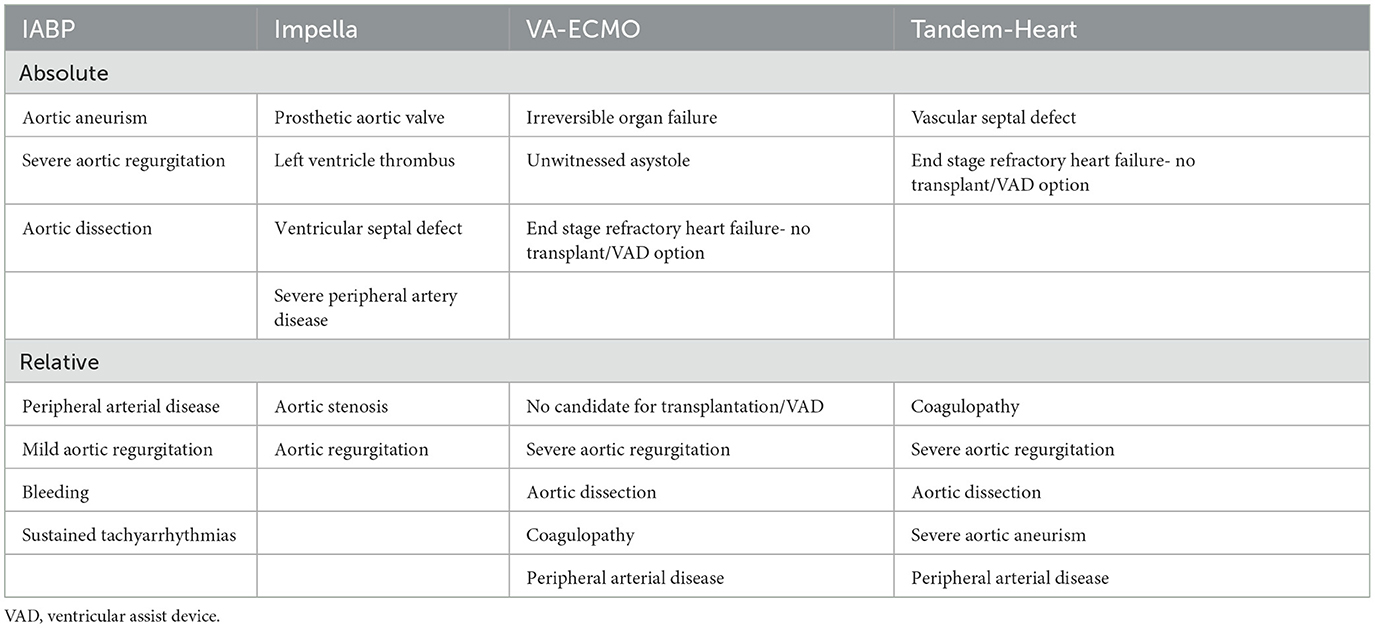
Currently, the percutaneous devices that are mainly used to support the failing left ventricle are the Intra-aortic Balloon Pump (IABP), the Impella pumps (Abiomed Europe GmbH, Aachen, Germany), the Tandem Heart (LivaNova, London, United Kingdom) and the Veno-Arterial Extracorporeal Membrane Oxygenation (VA-ECMO). However, sufficient randomized data regarding them is still limited (Tables 1, 2).
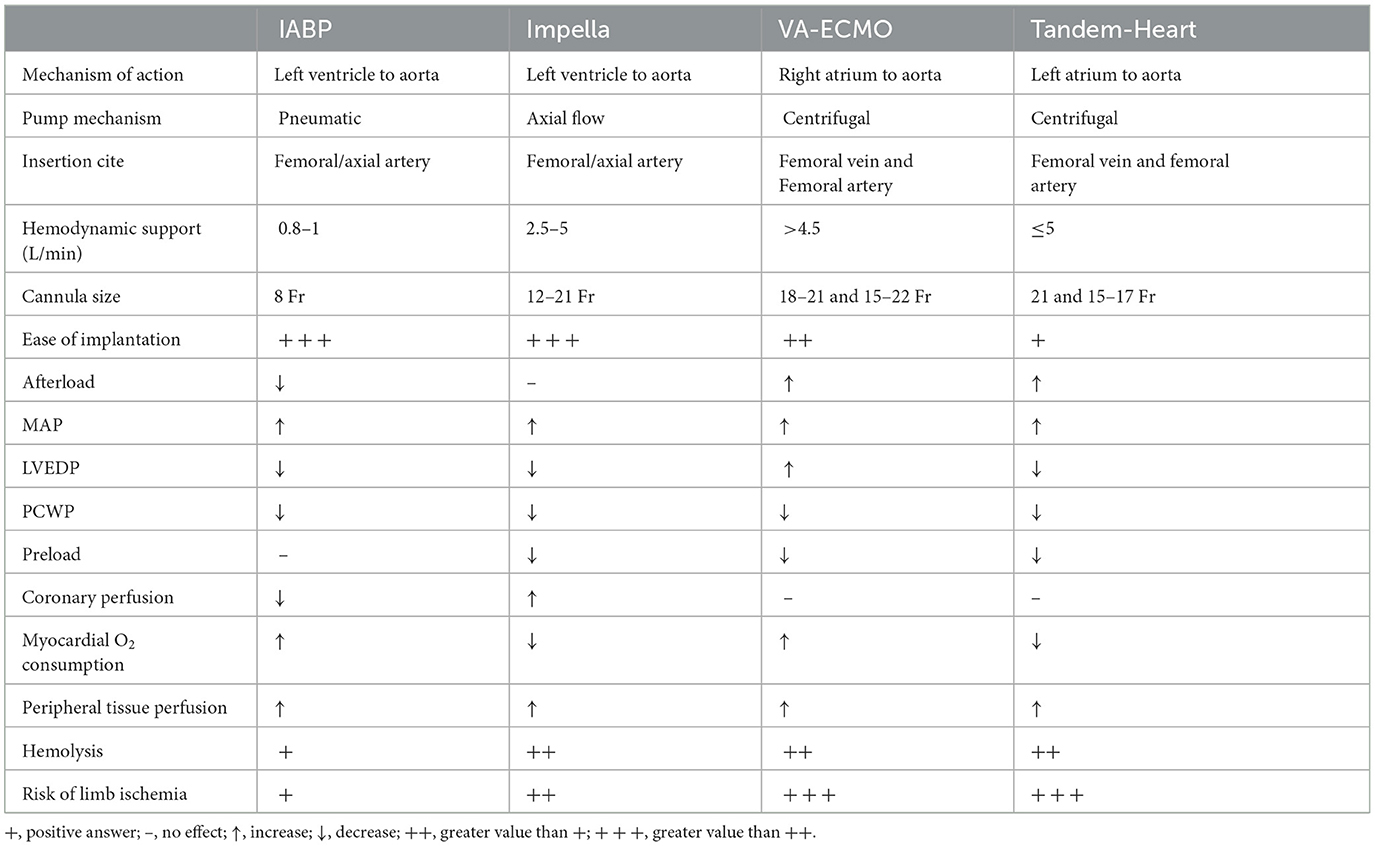
Table 1. Implantation characteristics, hemodynamic effects and adverse events of the temporary mechanical circulation support devices.
2.1 Intra-aortic balloon pump
The IABP has undergone extensive utilization and research over the past decades. Nevertheless, recent guidelines have diminished its recommended use, with a significant rise in the critical application of supplementary percutaneous devices in the latest years, prompted by findings from the IABP SHOCK II Trial (9). This study aimed to examine the impact of initiating IABP in patients experiencing cardiogenic shock complicating acute myocardial infarction on 30-day mortality (39.7 vs. 41.3%; RR 0.96; 95% CI 0.79–1.17; p = 0.69). Furthermore, the long-term follow-up of the trial reaffirmed the absence of superiority in IABP usage concerning long-term mortality (66.3 vs. 67.0%; RR 0.99; 95% CI 0.88–1.11; p = 0.98) (10).
2.2 Veno-arterial extracorporeal membrane oxygenation
The VA-ECMO or extracorporeal life support (ECLS) exhibits the dual ability to initiate both circulatory and respiratory support, traits that make it appropriate for patients suffering concomitantly from cardiac and respiratory failure.
Compared to IABP, VA-ECMO in a metanalysis of observational studies demonstrated a 33% higher 30-day survival rate (95% CI, 14%−52%; p < 0.001). In contrast, no significance was observed when comparing it with Tandem Heart/Impella (−3%; 95% CI −21% to 14%; p = 0.70) (11). Nevertheless, this 30-day mortality benefit was contradicted in the recently published EURO-SHOCK (12), ECMO CS (13), and ECLS-SHOCK (14) trials.
More specifically, in the EURO-SHOCK trial, which was held during the COVID-19 period and consequently the recruitment was limited, a superiority of the VA-ECMO group in terms of 30-day all-cause mortality (43.8 vs. 61.1%; HR 0.56, 95% CI 0.21–1.45; p = 0.22) and 1-year mortality (51.8 vs. 81.5%; HR 0.52, 95% CI 0.21–1.26; p = 0.14) was reported. At the same time, an increase of vascular (21.4 vs. 0%) and bleeding complications (35.7 vs. 5.6%) was documented. Due to the power failure, this trial could not provide sufficient data to draw definite conclusions. Moreover, the ECMO CS investigated its immediate implementation in patients with rapidly deteriorating or severe cardiogenic shock in comparison to early conservative pharmacological therapy. In that trial, even though greater clinical outcomes with the use of VA-ECMO were not depicted (63.8% early VA-ECMO vs. 71.2% no VA-ECMO; HR 0.72, 95% CI 0.46–1.12; p = 0.21), the cross-over rate from the conservative arm to ECLS or other pMCS device was notably high (39%), because of the rapidly deteriorating clinical situation of these patients. Additionally, the ECLS trial, a multicenter trial that included 420 patients proved that not only was there no decrease in 30-day mortality with early VA-ECMO introduction (47.8 vs. 49%; RR 0.98; 95% CI 0.80–1.19; p = 0.81), but there was also an increase in the complications (23.4 vs. 9.6%; RR 2.44; 95% CI, 1.50–3.95).
One notable limitation of employing the VA-ECMO is the resultant increase in left ventricular afterload due to the retrograde aortic flow. This, in the context of cardiogenic shock could potentially lead to myocardial ischemia, delayed ventricular recovery, ventricular arrhythmias, pulmonary edema, thrombotic events, and multiorgan dysfunction. Therefore, venting VA-ECMO with an IABP or other percutaneous ventricular assist device (pVAD) may be considered to address this challenge (15). This subject was recently approached in a meta-analysis of observational studies that included almost 4,000 patients (16). The venting technique seemed to provide lower mortality rate (54%) in comparison to (65%) the use of VA-ECMO without unloading (RR: 0.79; 95% CI: 0.72–0.87; p < 0.00001). On top of that, apart from a higher hemolysis rate, the double technique did not cause more adverse events. Nevertheless, in the absence of prospective randomized data, the consideration of left ventricular unloading may be appropriate for patients undergoing VA-ECMO support, provided they are carefully selected.
2.3 Impella devices
As far as the Impella pumps are concerned, their mechanism of action relies on pulling blood from the left ventricle and consequently delivering it to the systematic circulation through the aorta. Thus, the cardiac output is increased, while the myocardial oxygen consumption and the pulmonary capillary wedge pressure are diminished. Based on the type of the pump, the Impella can provide a contribution to the circulation of 2.5–5.5 L/min. In some RCTs (17, 18), which included a small number of patients, the Impella 2.5 device, in comparison to the IABP exhibited superior hemodynamic support (19, 20). Nonetheless, in the IMPRESS study (21), a multicenter trial which evaluated the use of Impella CP vs. IABP in 48 patients, the investigators reported similar results concerning the circulatory support of the two devices and the mortality, both on the short-term (50 vs. 46% at 30 days; HR 0.96; 95% CI 0.42–2.18; p = 0.92) and the long-term (50 vs. 63% at 5 years; RR 0.87, 95% CI 0.47–1.59, p = 0.65) follow-up (22). Of note, this came at the cost of increased vascular and bleeding complications for the Impella cohort, with the authors commenting on its wider sheath size (14 vs. 7.5 Fr).
The results of the ongoing STEMI-DTU (23) and DanGer shock (24) trials may shed light to whether the use of Impella CP could improve survival in Acute Myocardial Infarct complicated by Cardiogenic Shock (AMICS).
2.4 Tandem Heart
With the Tandem Heart's assistance, the left ventricle is unloaded by redirecting blood from the left atrium to systemic circulation, therefore is the preload reduced and systematic perfusion with a maximal flow of 5 L/min is achieved. Interestingly, this device functions by creating a left atrium to femoral artery bypass. This is succeeded by access through the femoral vein with a 21 Fr cannula and a septostomy, which offers entrance to the left atrium. In this way, oxygenated blood is withdrawn from the left atrium and is reinstated in the femoral artery via a 15 or 17 Fr cannula. The requirement for a transseptal puncture may pose a challenge for operators, who are not proficient in this technique. Additionally, the limited adoption of this approach may be attributed to its intricate nature and the considerable amount of time that its placement requires.
In early reports, a mean flow of 3.2 ± 0.6 L/min, an improvement to the cardiac index of 0.7 ± 0.3 L/min and to the mean blood pressure of averagely 17 mmHg were documented. Furthermore, the pulmonary capillary wedge pressure, central venous pressure, and pulmonary artery pressure were reduced in average by 7, 5, and 8 mmHg respectively. Finally, the 30-day mortality rate was reported to be 44% (25).
Even though the initiation of the TandemHeart in comparison to the IABP showed promising data concerning hemodynamic and metabolic variables, contemporary outcomes remain scarce and until recently have not been reported (26). Lately, a retrospective analysis of the THEME registry, a multicenter, prospective, observational cohort (27), reported a significant amelioration in cardiac index 1.0 (0.5–2.25 L/min/m2) and lactate clearance −2.3 (−5.0 to −0.7 mmol/L). Furthermore, it is important to note that the 30- and 180-day survival were 74% (95% confidence interval: 60%−85%) and 66% (95% confidence interval: 51%−79%), respectively.
3 Mechanical circulation support devices in out-of-hospital cardiac arrest
The use of MCS devices has also been investigated and may be beneficial in the case of refractory out-of-hospital cardiac arrest. Currently, continued conventional cardiopulmonary resuscitation (CCPR) and defibrillation constitute the standard of care for such patients. However, a significant number of patients fail to achieve return of spontaneous circulation (ROSC), restraining physicians to implement necessary intervention measures (28).
Extracorporeal cardiopulmonary resuscitation (ECPR) presents a potential solution by restoring circulation. This approach could help minimize or even reverse organ damage, prevent re-arrest due to ischemia-induced myocardial dysfunction, and provide time for the identification and treatment of the underlying cause (29).
Several studies have indicated the feasibility and potential advantages of ECPR in terms of both survival rates and neurological outcomes compared to conventional methods (30). Recently, the INCEPTION and Prague OHCA trials randomized patients (31, 32) who experienced out-of-hospital cardiac arrest (OHCA) to receive either ECPR or CCPR, with the primary outcome being survival with a favorable neurological outcome at 30 and 180 days, respectively. Although both studies showed promising results and positive neurological outcomes with the initiation of ECPR, neither met the primary endpoint. Only in the ARREST trial (33) was it demonstrated that for patients with OHCA and refractory ventricular fibrillation, survival to hospital discharge and functional status were significantly improved with the use of extracorporeal life support (ECLS) compared with standard ACLS treatment. This finding not only has paved the way for multicenter phase 3 trials, but also underscores the importance of a well-organized and experienced emergency system.
4 Risk stratification
As it was stated earlier in this paper, the CS is a medical entity that could be presented with a wide variety of clinical presentations. Therefore, the immediate risk stratification and the correct patient selection for each therapy type is critical. The conflicting results of the up to this point published trials, have been attributed to the high complication rates of the pMCS devices but also non-personalized patient selection (34). Subsequently, the identification of the mortality predictors and the introduction of a unanimous risk assessment score may be beneficial to the treatment planning and the optimal patient enrollment in upcoming clinical trials.
4.1 Biomarker-based predictive scores
In the past few years, various biochemical factors were accused of predicting CS complicated AMI mortality. More specifically, cytokines such as INF-γ, TNF-α, MIP-1β, G-CSF, MCP-1β and IL6-10 have been accused of reflecting the inflammatory response that is initiated after an acute coronary syndrome (ACS) and consequently the CS triggered multiorgan dysfunction syndrome (MODS), which is combined with poor clinical outcome and high mortality rates (35, 36).
The CLIP score (37) is a biomarker-based predictive model that evaluates the levels of cystatin C, lactate, IL-6 and N-terminal pro-B-type natriuretic peptide (NT-proBNP), biomarkers that represent the neurohormonal stress and the inflammatory response of the cardiogenic shock. In the internal and external validation, it was proven to outperform the prediction of the 30-day mortality risk of previously established scores. Even so, it is argued that the mortality in cardiogenic shock is stronger connected to clinical factors than biomarkers reflecting its pathophysiology (38). In addition to that, its administration in the current clinical practice could be challenging, because even though it consists of four biochemical values, such parameters are not routinely obtained in acute settings.
4.2 Clinical classification scores
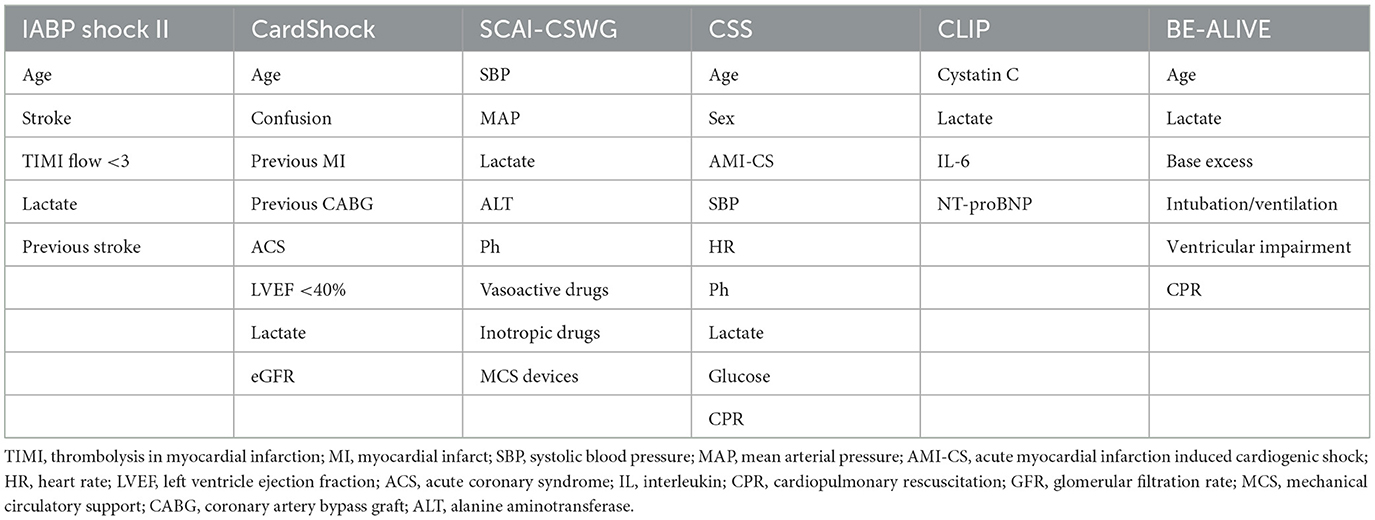
Several studies have proposed a practical risk classification score. Firstly, a risk stratification in three levels (low, intermediate, and high risk) of patients suffering from Cardiogenic Shock of all causes has been proposed by CardShock score. In this score, age, confusion, ACS as a cause, previous myocardial infract, Coronary Artery Bypass Graft, Left Ventricle Ejection Fraction, eGFR and lactate levels are estimated to allocate the patients to the appropriate treatment. In the same context, the IABP-SHOCK II score (39) applies only for ACS patients, incorporates age, history of stroke, TIMI flow after Percutaneous Coronary Intervention, glucose, creatinine, and lactate values at first presentation. Although these prediction models have been externally validated regarding the 30-day mortality prediction, in everyday clinical practice, their use remains limited owing to the acute nature of this clinical setting. Indeed, patient information regarding past medical history may even be impossible to retrieve, especially when the patient is intubated or in an impaired mental condition, and unaccompanied.
Recently, the Society for Cardiovascular Angiography and Interventions (SCAI) shock stage classification has been introduced (40). Since its publication, it has been widely cited, validated, and incorporated in multiple clinical studies (41, 42). Nevertheless, it is argued that it lacks uniform criteria defining each stage. For this reason, it was modified to the SCAI-CSWG Classification (43, 44) that was based on objective parameters estimated on admission and throughout hospitalization. More specifically, five stages (1–5) from hemodynamic stability to refractory shock, depending on the hypoperfusion, the hypotension and the treatment intensity have been established.
Another recently developed score system that tried to help pursue a targeted treatment approach in CS, irrespective of the underlying cause is the CSS (45). According to it, age, sex, acute myocardial infarction as a cause, cardiac arrest and the measurement of systolic blood pressure, heart rate, pH, lactate and glucose are the most important predictors of the CS induced mortality.
Finally, the BE-ALIVE Classification (46), is a newly developed score that includes parameters that are always available at the first contact with the patient. It assesses laboratory parameters like the base excess, lactate levels, as well as the patient's age, ventricular impairment by echocardiography, whether the patient is intubated and if the patient experienced a cardiac arrest (Table 3).
5 Discussion
Although hospitalizations attributed to CS have tripled between 2004 and 2018, in-hospital and short-term mortality due to CS has remained relatively same (47). On top of that, the lack of sufficiently powered randomized controlled trials in this emergent field leaves clinical decision-making largely reliant on the experience of medical practitioners.
Except for the treatment of the culprit lesion, the use of vasopressors, inotropes and temporary circulatory support devices is important. The use of such devices could be demanding, as the initial improvements in cardiac output may be counterbalanced by significant complications such as limb ischemia, bleeding, embolization of material, stroke, infection, and hemolysis (48).
One major limitation of the lack of robust data stemming from the available studies is the selection bias that was introduced since patients opted for circulatory support may be either in preliminary or in very advanced shock stages (49). In a recent editorial (50), the initiation of extracorporeal circulatory support was compared to parachute opening. Therefore, the incorporation of risk stratification tools may be a solution to guiding treatment decisions in a timely manner, as well as facilitate patient selection for enrolment to the new randomized control trials.
In addition, the miniaturization of the circulatory support systems could serve as a valuable aid for physicians, contributing to simplified insertion procedures and simultaneously reducing the risk of complications. In recent developments, a miniaturized catheter-mounted axial flow pump, which incorporates a self-expanding impeller and pump head has been introduced for providing mechanical circulatory support to the left ventricle. This pioneering high- output, low-French size device, currently undergoing examination in the EFS study (51), aims to achieve mean flows exceeding 5 L/min through insertion via a 10 Fr arterial sheath, accessed from the femoral artery. Preliminary results of the study were presented at the TCT 2023 in San Fransisco and reported positive outcomes regarding the delivery of the device and the device-related adverse events.
Promising results could be also claimed through the combination of the percutaneous circulatory support devices. For example, the combination of VA-ECMO and Impella, referred as ECMELLA is currently widely used and has already showed improving clinical outcomes in selected VA-ECMO patients, which needs to be further validated (52, 53).
In conclusion, the role of temporary mechanical circulatory support devices is limited because of the conflicting results of the available data. However, the incorporation of prediction scores to provide a personalized treatment approach selection has recently been linked with encouraging results. Apart from that, the administration of such devices is important to be held by experienced staff and in a manner to prevent possible complications. Nonetheless, all these should be sufficiently investigated and validated by upcoming clinical trials.
Author contributions
AP: Conceptualization, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. DC: Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing. AA: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing. TM: Methodology, Supervision, Validation, Writing – review & editing. GT: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chioncel O, Parissis J, Mebazaa A, Thiele H, Desch S, Bauersachs J, et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock - a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. (2020) 22:1315–41. doi: 10.1002/ejhf.1922
2. Goldberg RJ, Spencer FA, Gore JM, Lessard D, Yarzebski J. Thirty-year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population-based perspective. Circulation. (2009) 119:1211–9. doi: 10.1161/CIRCULATIONAHA.108.814947
3. Wayangankar SA, Bangalore S, McCoy LA, Jneid H, Latif F, Karrowni W, et al. Temporal trends and outcomes of patients undergoing percutaneous coronary interventions for cardiogenic shock in the setting of acute myocardial infarction: a report from the CathPCI Registry. JACC Cardiovasc Interv. (2016) 9:341–35. doi: 10.1016/j.jcin.2015.10.039
4. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. (2021) 42:3599–726. doi: 10.1093/eurheartj/ehab368
5. Hochman JS, Buller CE, Sleeper LA, Boland J, Dzavik V, Sanborn TA, et al. Cardiogenic shock complicating acute myocardial infarction–etiologies, management and outcome: a report from the SHOCK Trial Registry. Should we emergently revascularize occluded coronaries for cardiogenic shock? J Am Coll Cardiol. (2000) 36(3 Suppl A):1063–70. doi: 10.1016/S0735-1097(00)00879-2
6. Thiele H, de Waha-Thiele S, Freund A, Zeymer U, Desch S, Fitzgerald S. Management of cardiogenic shock. EuroIntervention. (2021) 17:451–65. doi: 10.4244/EIJ-D-20-01296
7. Basir MB, Lemor A, Gorgis S, Taylor AM, Tehrani B, Truesdell AG, et al. Vasopressors independently associated with mortality in acute myocardial infarction and cardiogenic shock. Catheter Cardiovasc Interv. (2022) 99:650–7. doi: 10.1002/ccd.29895
8. Ni hIci T, Boardman HM, Baig K, Stafford JL, Cernei C, Bodger O, et al. Mechanical assist devices for acute cardiogenic shock. Cochrane Database Syst Rev. (2020) 6:CD013002. doi: 10.1002/14651858.CD013002.pub2
9. Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. (2012) 367:1287–96. doi: 10.1056/NEJMoa1208410
10. Thiele H, Zeymer U, Thelemann N, Neumann FJ, Hausleiter J, Abdel-Wahab M, et al. Intraaortic balloon pump in cardiogenic shock complicating acute myocardial infarction: long-term 6-year outcome of the randomized IABP-SHOCK II Trial. Circulation. (2019) 139:395–403. doi: 10.1161/CIRCULATIONAHA.118.038201
11. Ouweneel DM, Schotborgh JV, Limpens J, Sjauw KD, Engström AE, Lagrand WK, et al. Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis. Intensive Care Med. (2016) 42:1922–34. doi: 10.1007/s00134-016-4536-8
12. Banning AS, Sabaté M, Orban M, Gracey J, López-Sobrino T, Massberg S, et al. Venoarterial extracorporeal membrane oxygenation or standard care in patients with cardiogenic shock complicating acute myocardial infarction: the multicentre, randomised EURO SHOCK trial. EuroIntervention. (2023) 19:482–92. doi: 10.4244/EIJ-D-23-00204
13. Thiele H, Zeymer U, Akin I, Behnes M, Rassaf T, Mahabadi AA, et al. Extracorporeal life support in infarct-related cardiogenic shock. N Engl J Med. (2023) 389:1286–97. doi: 10.1056/NEJMoa2307227
14. Ostadal P, Rokyta R, Karasek J, Kruger A, Vondrakova D, Janotka M, et al. Extracorporeal membrane oxygenation in the therapy of cardiogenic shock: results of the ECMO-CS randomized clinical trial. Circulation. (2023) 147:454–64. doi: 10.1161/CIRCULATIONAHA.122.062949
15. Lüsebrink E, Binzenhöfer L, Kellnar A, Müller C, Scherer C, Schrage B, et al. Venting during venoarterial extracorporeal membrane oxygenation. Clin Res Cardiol. (2023) 112:464–505. doi: 10.1007/s00392-022-02069-0
16. Russo JJ, Aleksova N, Pitcher I, Couture E, Parlow S, Faraz M, et al. Left ventricular unloading during extracorporeal membrane oxygenation in patients with cardiogenic shock. J Am Coll Cardiol. (2019) 73:654–62. doi: 10.1016/j.jacc.2018.10.085
17. O'Neill WW, Kleiman NS, Moses J, Henriques JP, Dixon S, Massaro J, et al. A prospective, randomized clinical trial of hemodynamic support with Impella 25 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. (2012) 126:1717–27. doi: 10.1161/CIRCULATIONAHA.112.098194
18. Alushi B, Douedari A, Froehlig G, Knie W, Wurster TH, Leistner DM, et al. Impella versus IABP in acute myocardial infarction complicated by cardiogenic shock. Open Heart. (2019) 6:e000987. doi: 10.1136/openhrt-2018-000987
19. Seyfarth M, Sibbing D, Bauer I, Fröhlich G, Bott-Flügel L, Byrne R, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. (2008) 52:1584–8. doi: 10.1016/j.jacc.2008.05.065
20. Bochaton T, Huot L, Elbaz M, Delmas C, Aissaoui N, Farhat F, et al. Mechanical circulatory support with the Impella® LP50 pump and an intra-aortic balloon pump for cardiogenic shock in acute myocardial infarction: the IMPELLA-STIC randomized study. Arch Cardiovasc Dis. (2020) 113:237–43. doi: 10.1016/j.acvd.2019.10.005
21. Ouweneel DM, Eriksen E, Sjauw KD, van Dongen IM, Hirsch A, Packer EJ, et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. (2017) 69:278–87. doi: 10.1016/j.jacc.2016.10.022
22. Karami M, Eriksen E, Ouweneel DM, Claessen BE, Vis MM, Baan J, et al. Long-term 5-year outcome of the randomized IMPRESS in severe shock trial: percutaneous mechanical circulatory support vs. intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. Eur Heart J Acute Cardiovasc Care. (2021) 10:1009–15. doi: 10.1093/ehjacc/zuab060
23. Kapur NK, Kim RJ, Moses JW, Stone GW, Udelson JE, Ben-Yehuda O, et al. Primary left ventricular unloading with delayed reperfusion in patients with anterior ST-elevation myocardial infarction: rationale and design of the STEMI-DTU randomized pivotal trial. Am Heart J. (2022) 254:122–32. doi: 10.1016/j.ahj.2022.08.011
24. Udesen NJ, Møller JE, Lindholm MG, Eiskjær H, Schäfer A, Werner N, et al. Rationale and design of DanGer shock: Danish-German cardiogenic shock trial. Am Heart J. (2019) 214:60–8. doi: 10.1016/j.ahj.2019.04.019
25. Thiele H, Lauer B, Hambrecht R, Boudriot E, Cohen HA, Schuler G. Reversal of cardiogenic shock by percutaneous left atrial-to-femoral arterial bypass assistance. Circulation. (2001) 104:2917–22. doi: 10.1161/hc4901.100361
26. Thiele H, Sick P, Boudriot E, Diederich KW, Hambrecht R, Niebauer J, et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. (2005) 26:1276–83. doi: 10.1093/eurheartj/ehi161
27. Megaly M, Gandolfo C, Zakhour S, Jiang M, Burgess K, Chetcuti S, et al. Utilization of TandemHeart in cardiogenic shock: insights from the THEME registry. Catheter Cardiovasc Interv. (2023) 101:756–63. doi: 10.1002/ccd.30582
28. Grunau B, Kime N, Leroux B, Rea T, Van Belle G, Menegazzi JJ, et al. Association of intra-arrest transport vs continued on-scene resuscitation with survival to hospital discharge among patients with out-of-hospital cardiac arrest. JAMA. (2020) 324:1058–67. doi: 10.1001/jama.2020.14185
29. Lott C, Truhlár A, Alfonzo A, Barelli A, González-Salvado V, Hinkelbein J, et al. European Resuscitation Council Guidelines 2021: cardiac arrest in special circumstances. Resuscitation. (2021) 161:152–219. doi: 10.1016/j.resuscitation.2021.02.011
30. Mørk SR, Stengaard C, Linde L, Møller JE, Jensen LO, Schmidt H, et al. Mechanical circulatory support for refractory out-of-hospital cardiac arrest: a Danish nationwide multicenter study. Crit Care. (2021) 25:174. doi: 10.1186/s13054-021-03606-5
31. Belohlavek J, Smalcova J, Rob D, Franek O, Smid O, Pokorna M, et al. Effect of intra-arrest transport, extracorporeal cardiopulmonary resuscitation, and immediate invasive assessment and treatment on functional neurologic outcome in refractory out-of-hospital cardiac arrest: a randomized clinical trial. JAMA. (2022) 327:737–47. doi: 10.1001/jama.2022.6548
32. Suverein MM, Delnoij TSR, Lorusso R, Brandon Bravo Bruinsma GJ, Otterspoor L, Elzo Kraemer CV, et al. Early extracorporeal CPR for refractory out-of-hospital cardiac arrest. N Engl J Med. (2023) 388:299–309. doi: 10.1056/NEJMoa2204511
33. Yannopoulos D, Bartos J, Raveendran G, Walser E, Connett J, Murray TA, et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet. (2020) 396:1807–16. doi: 10.1016/S0140-6736(20)32338-2
34. Harjola VP, Lassus J, Sionis A, Køber L, Tarvasmäki T, Spinar J, et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail. (2015) 17:501–9. doi: 10.1002/ejhf.260
35. Prondzinsky R, Unverzagt S, Lemm H, Wegener N, Heinroth K, Buerke U, et al. Acute myocardial infarction and cardiogenic shock: prognostic impact of cytokines: INF-γ, TNF-α, MIP-1β, G-CSF, and MCP-1β. Med Klin Intensivmed Notfmed. (2012) 107:476–84. doi: 10.1007/s00063-012-0117-y
36. Prondzinsky R, Unverzagt S, Lemm H, Wegener NA, Schlitt A, Heinroth KM, et al. Interleukin-6,−7,−8 and−10 predict outcome in acute myocardial infarction complicated by cardiogenic shock. Clin Res Cardiol. (2012) 101:375–84. doi: 10.1007/s00392-011-0403-3
37. Ceglarek U, Schellong P, Rosolowski M, Scholz M, Willenberg A, Kratzsch J, et al. The novel cystatin C, lactate, interleukin-6, and N-terminal pro-B-type natriuretic peptide (CLIP)-based mortality risk score in cardiogenic shock after acute myocardial infarction. Eur Heart J. (2021) 42:2344–52. doi: 10.1093/eurheartj/ehab110
38. Josiassen J, Frydland M, Holmvang L, Lerche Helgestad OK, Okkels Jensen L, Goetze JP, et al. Mortality in cardiogenic shock is stronger associated to clinical factors than contemporary biomarkers reflecting neurohormonal stress and inflammatory activation. Biomarkers. (2020) 25:506–12. doi: 10.1080/1354750X.2020.1795265
39. Pöss J, Köster J, Fuernau G, Eitel I, de Waha S, Ouarrak T, et al. Risk stratification for patients in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. (2017) 69:1913–20. doi: 10.1016/j.jacc.2017.02.027
40. Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. (2019) 94:29–37. doi: 10.1002/ccd.28329
41. Hanson ID, Tagami T, Mando R, Kara Balla A, Dixon SR, Timmis S, et al. SCAI shock classification in acute myocardial infarction: insights from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv. (2020) 96:1137–42. doi: 10.1002/ccd.29139
42. Schrage B, Dabboura S, Yan I, Hilal R, Neumann JT, Sörensen NA, et al. Application of the SCAI classification in a cohort of patients with cardiogenic shock. Catheter Cardiovasc Interv. (2020) 96:E213–9. doi: 10.1002/ccd.28707
43. Naidu SS, Baran DA, Jentzer JC, Hollenberg SM, van Diepen S, Basir MB, et al. SCAI SHOCK stage classification expert consensus update: a review and incorporation of validation studies: this statement was endorsed by the American College of Cardiology (ACC), American College of Emergency Physicians (ACEP), American Heart Association (AHA), European Society of Cardiology (ESC) Association for Acute Cardiovascular Care (ACVC), International Society for Heart and Lung Transplantation (ISHLT), Society of Critical Care Medicine (SCCM), and Society of Thoracic Surgeons (STS) in December 2021. J Am Coll Cardiol. (2022) 79:933–46. doi: 10.1016/j.jacc.2022.01.018
44. Kapur NK, Kanwar M, Sinha SS, Thayer KL, Garan AR, Hernandez-Montfort J, et al. Criteria for defining stages of cardiogenic shock severity. J Am Coll Cardiol. (2022) 80:185–98. doi: 10.1016/j.jacc.2022.04.049
45. Beer BN, Jentzer JC, Weimann J, Dabboura S, Yan I, Sundermeyer J, et al. Early risk stratification in patients with cardiogenic shock irrespective of the underlying cause - the Cardiogenic Shock Score. Eur J Heart Fail. (2022) 24:657–67. doi: 10.1002/ejhf.2449
46. Tindale A, Panoulas V. The BE-ALIVE score: assessing 30-day mortality risk in patients presenting with acute coronary syndromes. Open Heart. (2023) 10:e002313. doi: 10.1136/openhrt-2023-002313
47. Osman M, Syed M, Patibandla S, Sulaiman S, Kheiri B, Shah MK, et al. Fifteen-year trends in incidence of cardiogenic shock hospitalization and in-hospital mortality in the United States. J Am Heart Assoc. (2021) 10:e021061. doi: 10.1161/JAHA.121.021061
48. Fernando SM, Price S, Mathew R, Slutsky AS, Combes A, Brodie D. Mechanical circulatory support in the treatment of cardiogenic shock. Curr Opin Crit Care. (2022) 28:434–41. doi: 10.1097/MCC.0000000000000956
49. Zeymer U, Thiele H. Mechanical support for cardiogenic shock: lost in translation? J Am Coll Cardiol. (2017) 69:288–90. doi: 10.1016/j.jacc.2016.10.025
50. Henry TD, Yannopoulos D, van Diepen S. Extracorporeal membrane oxygenation for cardiogenic shock: when to open the parachute? Circulation. (2023) 147:465–8. doi: 10.1161/CIRCULATIONAHA.122.063190
51. ClinicalTrials.gov. Identifier: NCT05727059. Magenta Elevate™ EFS in High-Risk PCI Patients sponsored by Magenta Medical Ltd. Available online at: https://clinicaltrials.gov/ct2/show/NCT05727059
52. Radakovic D, Zittermann A, Rojas SV, Opacic D, Razumov A, Prashovikj E, et al. Left ventricular unloading in patients on venoarterial extracorporeal membrane oxygenation therapy in cardiogenic shock: prophylactic versus bail-out strategy. Life. (2023) 13:582. doi: 10.3390/life13020582
53. Schrage B, Becher PM, Bernhardt A, Bezerra H, Blankenberg S, Brunner S, et al. Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: results from an international, multicenter cohort study. Circulation. (2020) 142:2095–106. doi: 10.1161/CIRCULATIONAHA.121.053992
Keywords: cardiogenic shock, mechanical circulation support, CS, Impella, VA-ECMO
Citation: Papanikolaou A, Chlorogiannis DD, Apostolos A, Mavridis T and Tsigkas G (2024) Advances and challenges in mechanical support for cardiogenic shock complicating acute myocardial infarct: a comprehensive review of the latest data. Front. Disaster Emerg. Med. 2:1374291. doi: 10.3389/femer.2024.1374291
Received: 21 January 2024; Accepted: 13 May 2024;
Published: 14 June 2024.
Edited by:
Patrick Van De Voorde, Ghent University Hospital, BelgiumReviewed by:
Casey Carr, University of Florida, United StatesCopyright © 2024 Papanikolaou, Chlorogiannis, Apostolos, Mavridis and Tsigkas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Dimitris Chlorogiannis, ZGR4bG9yb2dpYUBnbWFpbC5jb20=
 Amalia Papanikolaou
Amalia Papanikolaou David Dimitris Chlorogiannis
David Dimitris Chlorogiannis Anastasios Apostolos
Anastasios Apostolos Theodoros Mavridis
Theodoros Mavridis Grigorios Tsigkas
Grigorios Tsigkas