
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Disaster Emerg. Med. , 22 January 2024
Sec. Resuscitation and Cardiac Emergency Medicine
Volume 2 - 2024 | https://doi.org/10.3389/femer.2024.1328502
This article is part of the Research Topic Advancements and Challenges in Resuscitation and Cardiac Emergency Medicine View all 5 articles
Current guidelines suggest considering extracorporeal cardiopulmonary resuscitation (ECPR) in refractory cardiac arrest and provide some guidance for favorable prognostic factors. However, inclusion and exclusion criteria are not strictly defined. We describe a 60-year-old male who underwent extracorporeal life support (ECLS) following refractory out-of-hospital cardiac arrest and made a full neurological recovery despite severe metabolic derangements, including a pH of 6.6 and lactate of 29 mmol/l. The aim is to present a favorable neurological outcome after ECPR despite severe significant physiologic derangements and put relative contraindications for ECPR into perspective.
The use of extracorporeal cardiopulmonary resuscitation (ECPR) increased globally, and the European Resuscitation Council Guidelines suggest considering it as a rescue therapy. It involves rapid deployment of veno-arterial extracorporeal membrane oxygenation (VA-ECMO) to provide circulatory support in order to achieve sustained return of spontaneous circulation (ROSC) when conventional CPR is unsuccessful (1, 2). Nevertheless, its benefit on survival and favorable neurological outcomes following out-of-hospital cardiac arrest is yet to be demonstrated in clinical trials (3). Studies comparing ECPR to conventional CPR show conflicting results (4–6). In the absence of randomized controlled trials, current guidelines do not define absolute indications, contraindications or standardized algorithms, rendering clinical decision making for ECPR in an emergency situation highly challenging (7, 8).
We report a case of a 60-year-old male with full neurological recovery despite severe metabolic disturbances with an initial pH of 6.6 and a lactate of 29 mmol/l following out-of-hospital cardiac arrest due to an ST-elevation myocardial infarction (STEMI) and subsequent ventricular fibrillation, who underwent prolonged CPR and eventually, extracorporeal life support (ECLS). The aim is to present a favorable neurological outcome after ECPR despite severe significant physiologic derangements and put relative contraindications for ECPR into perspective.
After finishing his cardiovascular workout, a 60-year-old male experienced sudden sharp chest pain and called an ambulance. He reported no similar episodes in the past and no relevant medical history. An initial electrocardiogram (ECG) done by paramedics showed an ST-segment elevation in the anterior leads consistent with myocardial infarction. A loading dose of oral acetylsalicylic acid 250 mg and intravenous unfractionated heparin 5,000 units were administered. Additionally, a few minutes later, the patient lost consciousness and became hemodynamically unstable with no palpable pulse. The paramedics on the site started cardiopulmonary resuscitation immediately. Due to recurrent ventricular fibrillation, defibrillation with 200 Joules was performed three times, and a cumulative dose of 2 mg epinephrine was administrated intravenously according to the Advanced Cardiac Life Support algorithm before reaching our emergency department where handoff was done between the paramedics and the emergency medicine, anesthesiology, cardiology, and cardiac surgery teams. Following handoff, endotracheal intubation was performed. Based on the very good prognostic factors being the STEMI as a reversible cause of the witnessed cardiac arrest, no-flow time of 0 min, the absence of any relevant comorbidities, as well as ventricular fibrillation refractory to conventional ACLS, a multidisciplinary decision was made to initiate ECLS immediately after the handoff. During CPR the patient showed some “signs of life” such as gasps and small uncoordinated movements of the upper extremities. After admission, an ECG obtained during a brief period of ROSC demonstrated an accelerated idioventricular rhythm with a heart rate of 50/min and absent P waves, QRS complexes > 120 milliseconds (Figure 1).
During percutaneous cannulation using the Seldinger technique for the Vf-Af ECMO device (right femoral vein and left common femoral artery with distal perfusion cannula), mechanical resuscitation was continued reaching a total time of approximately 60 min before commencing Vf-Af ECMO. It included a brief ROSC after 45 min and 10 defibrillations with 200 Joules as well as intravenous administration of 8 x 1 mg epinephrine and cumulative 450 mg amiodarone. Moreover, there was no need for further vasopressor therapy. The first arterial blood gas analysis (ABL825 Flex) obtained from the left femoral artery immediately after cannulation and shortly before starting Vf-Af ECMO revealed severe metabolic acidosis with a pH of 6.6 and a lactate level of 29 mmol/l, therefore 100 mL of sodium bicarbonate 8.4% were administered intravenously. Despite this severe metabolic derangement, we agreed to proceed with the ECLS because of good prognostic factors and we did not use the low pH and high lactate in the decision-making process.
After initiating Vf-Af ECMO the patient was taken directly to coronary angiography, which showed 70% stenosis of the left main coronary artery (LMCA) and 70% stenosis of the left anterior descending artery (LAD) (Figure 2) because of a ruptured plaque and thrombosis, which were successfully treated by thrombus aspiration and implantation of a drug-eluting stent displaying a prompt thrombolysis in myocardial infarction (TIMI) 3-flow and a low ejection fraction of 20% in the ventriculography.
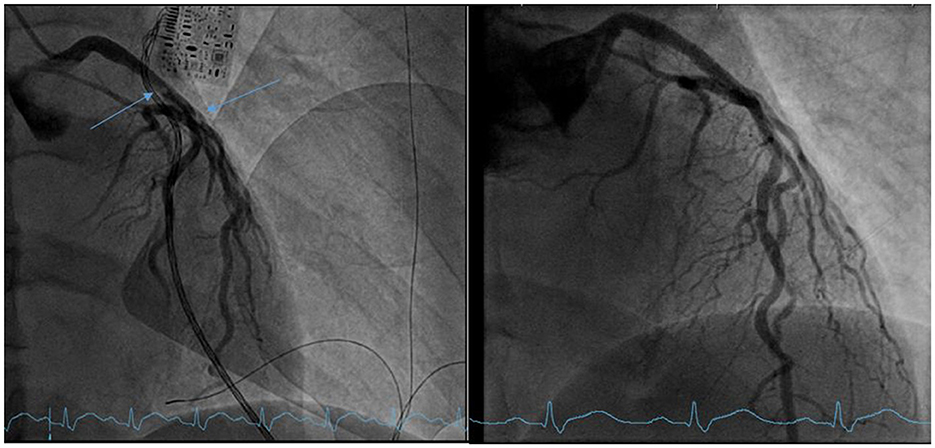
Figure 2. Left: coronary angiography before stenting showing a 70% distal stenosis of the left main coronary artery (LMCA) (blue arrows) and a 70% stenosis of the left anterior descending artery (LAD). Right: coronary angiography after stenting.
Standard dual antiplatelet therapy was initiated. After the intervention, the patient was transferred to the intensive care unit where targeted temperature management (TTM) was applied for 72 h as part of post-resuscitation care. As a documented complication of the Vf-Af ECMO therapy, there was prolonged bleeding at the cannula insertion site, which extended into the right proximal thigh and right scrotum, which ceased spontaneously without intervention. Trans-thoracic echocardiography 72 h after the event showed an improvement of the left ventricular ejection fraction to 40% and the patient was weaned from Vf-Af ECMO. On the fourth day after cardiac arrest, the patient was extubated successfully. On day seven, apart from mild memory impairment, reflected in the Rapid Evaluation of Cognitive Function (ERFC) test, used for neuropsychological impairment screening, no other deficits were detected (48/50 points). Ten days after cardiac arrest, cranial Magnetic Resonance Imaging (cMRI) showed a small linear subacute infarction of the left frontal lobe (Figure 3), but was otherwise unremarkable. After 17 days of admission, the patient was discharged and completed 3 weeks in a cardiac rehabilitation clinic. He was able to return to his normal daily activities without any impairments. The case timeline is presented in Figure 4.
One major challenge of cardiac arrest is to initially identify those who would benefit from ECPR with favorable neurological outcomes and avoid bridging patients with no predicted benefits or poor prognosis to nowhere. The current literature is conflicting; a systematic review and meta-analysis by Ouweneel et al. showed that ECLS was associated with better survival rates as well as better neurological outcomes in the setting of refractory cardiac arrest (9), although more recent studies found no superiority in comparison to conventional CPR (4–6, 10), possibly due to inconsistent inclusion and exclusion criteria. Current recommendations suggest locally determined inclusion criteria, and list as examples age < 70 years, witnessed cardiac arrest, an initial shockable rhythm or pulseless electrical activity, a no-flow time < 5 min, a low-flow time < 60 min, consistent high-quality CPR, intermittent ROSC, and the presence of a reversible cause of the cardiac arrest (e.g., myocardial infarction). Suggested exclusion criteria are age over 70 years, non-observed cardiac arrest, no-flow time more than 10 min, signs of poor neurological prognosis, comorbidities reducing life expectancy, prolonged CPR, known DNR status, severe aortic valve incompetence, contraindications to full anticoagulation, and pH < 6.8 or high lactate > 20 mmol/L (8, 11). In clinical practice, decision making regarding initiation of ECLS is usually accompanied by incomplete clinical information and ambiguous guidelines. For a long time, severe metabolic disturbances were believed to be not compatible with life. Brunet et al. showed that no patient with an arterial pH < 6.95 survived a cardiac arrest regardless of the cause (12). In a study by Shin et al., no patient with pH < 6.8 had favorable neurological outcomes after out-of-hospital cardiac arrest (13). Furthermore, corresponding to our case, we found more recent reports demonstrating favorable neurological ECLS outcome despite severe metabolic acidosis after cardiac arrest due to drowning or ethylene glycol poisoning (14, 15). These two examples and our case report share rapid decision making in favor of ECLS initiation as well as a reversible cause of cardiovascular system compromise. This suggests the etiology of arrest may be of higher relevance than metabolic disturbances when deciding for ECLS. Some studies demonstrate better neurological outcomes when combining TTM with ECPR, which, in concordance with immediate CPR, may have also contributed to the favorable outcome in our patient (16, 17).
Our case fulfilled the inclusion ECLS criteria of age, witnessed cardiac arrest due to a treatable cause, and no known comorbidities. As CPR was immediately initiated, a no-flow time < 5 min was also achieved. Though, the favorable neurological outcome of our patient is remarkable despite the severe metabolic acidosis (pH 6.6, lactate 29 mmol/l). Hyperlactatemia representing anaerobic metabolism during resuscitation is considered a strong predictive factor for poor neurological outcome, as brain tissue is highly vulnerable to hypoxia (18). Notwithstanding severe metabolic disturbance, our patient showed “signs of life” during resuscitation, which in contrary suggest at least some brain perfusion, known to strongly correlate with survival (19). This discordance between laboratory results and clinical presentation of the patient shows that thresholds for certain criteria like metabolic acidosis may be too restrictive, considering that if current ECLS exclusion criteria were to have been followed, this patient may have been excluded given the pH and lactate values.
This case report highlights the importance of patient-centered decision making and challenges metabolic acidosis as a contraindication for ECPR in the absence of evidence-based guidelines. Further studies to review current cut-off values for metabolic disturbances are urgently needed in order to increase survival using proven methods for ROSC and improved neurological outcomes.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethical approval was not required for the studies involving humans because it was not a study, but a case report. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from it was not a study, but a case report. Written informed consent of the patient was acquired. Written informed consent to participate in this study was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
LK: Writing—original draft, Writing—review & editing. A-ID: Writing—review & editing. DE: Supervision, Writing—review & editing. KS: Supervision, Writing—review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Soar J, Böttiger BW, Carli P, Couper K, Deakin CD, Djärv T, et al. European Resuscitation Council Guidelines 2021: adult advanced life support. Resuscitation. (2021) 161:115–51. doi: 10.1016/j.resuscitation.2021.02.010
2. Inoue A, Hifumi T, Sakamoto T, Kuroda Y. Extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest in adult patients. J Am Heart Assoc. (2020) 9:e015291. doi: 10.1161/JAHA.119.015291
3. Abrams D, MacLaren G, Lorusso R, Price S, Yannopoulos D, Vercaemst L, et al. Extracorporeal cardiopulmonary resuscitation in adults: evidence and implications. Intensive Care Med. (2022) 48:1–15. doi: 10.1007/s00134-021-06514-y
4. Bougouin W, Dumas F, Lamhaut L, Marijon E, Carli P, Combes A, et al. Extracorporeal cardiopulmonary resuscitation in out-of-hospital cardiac arrest: a registry study. Eur Heart J. (2020) 41:1961–71. doi: 10.1093/eurheartj/ehz753
5. Suverein MM, Delnoij TSR, Lorusso R, Bravo Bruinsma BGJ, Otterspoor L, Elzo Kraemer CV, et al. Early extracorporeal CPR for refractory out-of-hospital cardiac arrest. N Engl J Med. (2023) 388:299–309. doi: 10.1056/NEJMoa2204511
6. Belohlavek J, Smalcova J, Rob D, Franek O, Smid O, Pokorna M, et al. Effect of intra-arrest transport, extracorporeal cardiopulmonary resuscitation, and immediate invasive assessment and treatment on functional neurologic outcome in refractory out-of-hospital cardiac arrest: a randomized clinical trial. JAMA. (2022) 327:7377–47. doi: 10.1001/jama.2022.1025
7. Brandi G, Drewniak D, Buehler PK, Budilivschi A, Steiger P, Krones T. Indications and contraindications for extracorporeal life support for severe heart or lung failure: a systematic review. Minerva Anestesiol. (2021) 87:199–209. doi: 10.23736/S0375-9393.20.14513-9
8. Richardson ASC, Tonna JE, Nanjayya V, Nixon P, Abrams DC, Raman L, et al. Extracorporeal cardiopulmonary resuscitation in adults. Interim guideline consensus statement from the extracorporeal life support organization. ASAIO J. (2021) 67:221. doi: 10.1097/MAT.0000000000001344
9. Ouweneel DM, Schotborgh JV, Limpens J, Sjauw KD, Engström AE, Lagrand WK, et al. Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis. Intensive Care Med. (2016) 42:1922–34. doi: 10.1007/s00134-016-4536-8
10. Jeong D, Lee GT, Park JE, Chang H, Kim T, Cha WC, et al. Extracorporeal life-support for out-of-hospital cardiac arrest: a nationwide multicenter study. Shock. (2022) 57:680–6. doi: 10.1097/SHK.0000000000001924
11. Michels G, Wengenmayer T, Hagl C, Dohmen C, Böttiger BW, Bauersachs J, et al. Recommendations for extracorporeal cardiopulmonary resuscitation (eCPR): consensus statement of DGIIN, DGK, DGTHG, DGfK, DGNI, DGAI, DIVI and GRC. Clin Res Cardiol. (2019) 108:455–64. doi: 10.1007/s00392-018-1366-4
12. Brunet J, Valette X, Ivascau C, Lehoux P, Sauneuf B, Dalibert Y, et al. Extracorporeal life support for refractory cardiac arrest or shock: a 10-year study. ASAIO J. (2015) 61:676–81. doi: 10.1097/MAT.0000000000000282
13. Shin J, Lim YS, Kim K, Lee HJ, Lee SJ, Jung E, et al. Initial blood pH during cardiopulmonary resuscitation in out-of-hospital cardiac arrest patients: a multicenter observational registry-based study. Crit Care. (2017) 21:322. doi: 10.1186/s13054-017-1893-9
14. Chai Y, Zhang X, Liu H. Veno-venous extracorporeal membrane oxygenation support in the resuscitation from extreme metabolic acidosis (pH < 65) after drowning cardiac arrest: a case report. Int J Emerg Med. (2023) 16:24. doi: 10.1186/s12245-023-00501-4
15. Orlando A, Sciutti F, Colombo CN, Fiocco E, Ambrosini E, Coccolo M, et al. Ethylene glycol poisoning requiring veno-arterial ECMO: a case report. Perfusion. (2022). doi: 10.1177/02676591221141327
16. Pang PYK, Wee GHL, Huang MJ, Hoo AEE, Sheriff IMT, Lim SL, et al. Therapeutic hypothermia may improve neurological outcomes in extracorporeal life support for adult cardiac arrest. Heart Lung Circ. (2017) 26:817–24. doi: 10.1016/j.hlc.2016.11.022
17. Duan J, Ma Q, Zhu C, Shi Y, Duan B. eCPR combined with therapeutic hypothermia could improve survival and neurologic outcomes for patients with cardiac arrest: a meta-analysis. Front Cardiovasc Med. (2021) 8:703567. doi: 10.3389/fcvm.2021.703567
18. Zhou BC, Zhang Z, Zhu JJ, Liu LJ, Liu CF. Blood lactate or lactate clearance: which is robust to predict the neurological outcomes after cardiac arrest? A systematic review and meta-analysis. Biomed Res Int. (2018) 2018:8014213. doi: 10.1155/2018/8014213
19. Lamhaut L, Hutin A, Puymirat E, Jouan J, Raphalen JH, Jouffroy R, et al. A pre-hospital extracorporeal cardio pulmonary resuscitation (ECPR) strategy for treatment of refractory out hospital cardiac arrest: an observational study and propensity analysis. Resuscitation. (2017) 117:109–17. doi: 10.1016/j.resuscitation.2017.04.014
Keywords: out-of-hospital cardiac arrest, ECLS, ECPR, metabolic acidosis, neurological outcome
Citation: Kavaliukaite L, Diaconescu A-I, Eis D and Slankamenac K (2024) Case report: Successful extracorporeal cardiopulmonary resuscitation despite severe metabolic acidosis after refractory out-of-hospital cardiac arrest. Front. Disaster Emerg. Med. 2:1328502. doi: 10.3389/femer.2024.1328502
Received: 26 October 2023; Accepted: 09 January 2024;
Published: 22 January 2024.
Edited by:
Torben K. Becker, University of Florida, United StatesReviewed by:
Charles Hwang, University of Florida, United StatesCopyright © 2024 Kavaliukaite, Diaconescu, Eis and Slankamenac. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Loreta Kavaliukaite, bG9yZXRhLmthdmFsaXVrYWl0ZUB1c3ouY2g=; Anca-Isabela Diaconescu, YW5jYS1pc2FiZWxhLmRpYWNvbmVzY3VAdXN6LmNo; YW5jYS5kaWFjb25lc2N1QHlhaG9vLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.