
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Digit. Health, 25 February 2025
Sec. Digital Mental Health
Volume 7 - 2025 | https://doi.org/10.3389/fdgth.2025.1552396
This article is part of the Research TopicAdvanced interventions for self-regulation and neuroplasticityView all articles
Mental health disorders and cognitive decline are pressing global concerns, increasing the demand for non-pharmacological interventions targeting emotional dysregulation, memory deficits, and neural dysfunction. This review systematically examines three promising methodologies—music therapy, brainwave entrainment (binaural beats, isochronic tones, multisensory stimulation), and their integration into a unified therapeutic paradigm. Emerging evidence indicates that music therapy modulates affect, reduces stress, and enhances cognition by engaging limbic, prefrontal, and reward circuits. Brainwave entrainment, particularly within the gamma frequency range (30–100 Hz), facilitates neural oscillatory patterns linked to relaxation, concentration, and memory, with 40 Hz showing promise for cognitive enhancement, albeit with individual variability. Synchronized multisensory stimulation, combining auditory and visual inputs at gamma frequencies, has demonstrated potential in enhancing memory and supporting neural integrity, particularly in Alzheimer's disease. However, challenges such as patient response variability, lack of standardization, and scalability hinder widespread implementation. Recent research suggests that a synergistic application of these modalities may optimize therapeutic outcomes by leveraging complementary mechanisms. To actualize this, AI-driven biofeedback, enabling real-time physiological assessment and individualized adjustments—such as tailoring musical complexity, entrainment frequencies, and multisensory components—emerges as a promising solution. This adaptive model enhances treatment accessibility and consistency while maximizing long-term efficacy. Although in early stages, preliminary evidence highlights its transformative potential in reshaping non-pharmacological therapeutic strategies. Advancing this field requires interdisciplinary research, rigorous evaluation, and ethical data stewardship to develop innovative, patient-centered solutions for mental health and cognitive rehabilitation.
Mental health disorders and cognitive deterioration exert considerable pressures on both individuals and healthcare infrastructures. Conventional pharmacological treatments frequently alleviate symptoms yet inadequately address the fundamental neural impairments, with adverse effects and restricted long-term effectiveness remaining ongoing obstacles (1). Consequently, researchers have increasingly investigated comprehensive, safer, and more sustainable methodologies that capitalize on the brain's plasticity, emotional regulation, and integrative sensory processing capabilities.
Music therapy is particularly noteworthy for its capacity to activate limbic, prefrontal, and sensory pathways, influencing mood and cognitive function through dopaminergic reward circuits and stress-related hormonal responses (2–5). In contrast, brainwave entrainment methods—including binaural beats, isochronic tones, and multisensory stimulation—seek to synchronize neural oscillations with frequencies correlated to desired mental states, though their effectiveness is inconsistent among individuals due to inherent biological and psychological variances (6–11). Particularly within the gamma frequency spectrum (30–100 Hz), these approaches have attracted scholarly interest for their potential to enhance memory and cognitive abilities while mitigating neurodegenerative indicators (12–14).
Notwithstanding their potential, these modalities have often been examined independently, with minimal investigation into the synergistic and enduring benefits that might arise from their integration. Obstacles to wider implementation encompass variability in patient responses, a lack of standardized protocols, and logistical impediments related to accessibility and scalability. Overcoming these challenges necessitates a paradigm shift: the incorporation of emotional-cognitive support (music therapy) and brainwave entrainment methods under adaptive AI-driven biofeedback platforms capable of dynamically customizing interventions to individual physiological, cognitive, and emotional conditions (15).
This review consolidates the prevailing evidence regarding music therapy, binaural beats, isochronic tones, and multisensory stimulation while critically assessing their underlying mechanisms, therapeutic applications, and limitations. Additionally, it proposes an integrative framework that harnesses AI-driven personalization to unify these modalities and optimize their complementary strengths. By bridging the divide between theoretical constructs, empirical investigations, and clinical practice, this approach aspires to foster patient-centered, scalable solutions for mental health treatment and cognitive rehabilitation.
A systematic and structured methodology was employed to identify, select, and synthesize relevant literature, ensuring methodological rigor, thematic relevance, and inclusion of high-quality studies. This approach spans diverse disciplines, including neuroscience, psychology, rehabilitation medicine, digital health, and investigations into brainwave entrainment methods, to comprehensively examine music therapy, binaural beats, isochronic tones, multisensory stimulation, and their potential AI-driven integrative applications.
The review utilized multiple electronic databases, including PubMed, Scopus, Web of Science, and Google Scholar. The searches were confined to peer-reviewed journal articles, conference proceedings, and reputable academic sources published in English. To capture foundational theories along with cutting-edge advancements, no fixed start date was imposed, although most studies have been conducted over the last two decades. Keyword combinations reflected the review's core themes: “music therapy,” “binaural beats,” “isochronic tones,” “multisensory stimulation,” “brainwave entrainment,” “gamma oscillations,” “cognitive enhancement,” “mental health,” “neuroplasticity,” and “non-pharmacological interventions,” along with terms related to “Artificial Intelligence” or “AI,” “Machine learning” or “ML”, “Artificial General Intelligence” or “AGI”, “real-time feedback,” and “adaptive therapy.”
The initial search generated several hundred sets of results. Titles and abstracts were screened systematically to exclude studies that did not report measurable cognitive, emotional, or neurological outcomes or those focusing solely on pharmacological treatments. Empirical research demonstrating quantifiable impacts on mood, anxiety, depression, attention, memory, and cognitive performance was prioritized in this study. Intervention studies, quasi-experimental designs, case series, and systematic reviews that provided mechanistic insights or clinical relevance were included. Articles that lacked methodological clarity, empirical evidence, or those addressing unrelated topics were excluded.
A standardized data extraction process was used to ensure transparency and reproducibility. For each study, details such as authorship, publication year, participant demographics, clinical characteristics, study design, interventions, outcome measures, and key findings were systematically recorded. Special emphasis was placed on studies examining neural mechanisms using techniques like fMRI, PET, EEG, or established biomarkers of emotional and cognitive states. This process enabled cross-study comparisons, the identification of knowledge gaps, and the recognition of patterns relevant to integrative strategies.
Quality assessment further refined the selected corpus. Preference was given to studies employing randomized controlled trials (RCTs) or robust quasi-experimental designs. Exploratory and pilot studies were included only if they offered valuable mechanistic insights or novel perspectives. The evaluation criteria included the presence of control conditions, statistical rigor, sample size adequacy, and clarity of reporting. Limitations such as small sample sizes, brief intervention durations, and reliance on subjective measures were noted to contextualize the findings critically.
Given the heterogeneity in study designs, populations, and intervention protocols, narrative synthesis was adopted instead of a meta-analytic structure. Evidence was integrated to highlight convergent trends, persistent challenges, and complementary strengths across music therapy, brainwave entrainment methods, and multisensory stimulation. Key themes emerged, such as the engagement of emotional circuits, the potential for frequency-based modulation of brainwave activity, enhancement of memory and attention, and scalability through digital platforms and AI adaptation. Conceptual models and emerging empirical findings proposing integrative strategies are also incorporated to bridge theoretical and practical considerations.
This methodology balanced breadth by capturing the interdisciplinary scope of the topic and depth by rigorously scrutinizing the quality and relevance of the included studies. The synthesis situates each modality within its empirical and theoretical context, with particular attention paid to their interplay under AI-driven systems. This foundation supports the detailed analyses and forward-looking propositions explored in the subsequent sections, advancing the discussion on innovative, non-pharmacological interventions for mental health and cognitive enhancement.
As an emotional-cognitive support modality, music therapy encompasses a multifaceted interaction of neural circuits, utilizing the auditory, emotional, and cognitive pathways of the brain to influence mood, diminish stress, and augment cognitive performance (4, 5, 16, 17). Employing sophisticated neuroimaging modalities, such as functional magnetic resonance imaging (fMRI) and electroencephalography (EEG), scholars have demonstrated that musical experiences engage a variety of cerebral regions, including the auditory cortex for sound analysis, the amygdala and hippocampus for emotional relevance, and the prefrontal cortex for executive functioning (2, 3). In contrast to passive auditory engagement, music therapy actively modulates neural processes, fostering neuroplasticity, emotional regulation, and the encoding of memories (17, 18).
The modulation of neurochemicals constitutes a fundamental mechanism that underlies these therapeutic effects. Enjoyable or significant musical stimuli incite the release of dopamine within the mesolimbic pathway, thereby reinforcing affirmative emotional states and encouraging active participation (4, 17). Concurrently, music therapy diminishes cortisol levels, the principal hormone associated with stress, while elevating serotonin levels, which together promote relaxation and psychological resilience (2, 3). These neurochemical alterations facilitate long-term stabilization of mood, rendering music therapy particularly efficacious for individuals grappling with emotional dysregulation or mood disorders (18).
Beyond its neurochemical influences, music therapy also affects neural connectivity and oscillatory dynamics. By engaging both sensory and cognitive faculties, it reorganizes synaptic connections and fortifies networks associated with memory, attention, and executive functions (16, 19, 20). For instance, structured musical rhythms may assist stroke survivors by entraining motor networks, thereby enhancing motor recovery and improving sensorimotor integration. In geriatric populations or those with mild cognitive impairment, musical interventions activate preserved neural pathways, potentially decelerating cognitive decline and enhancing life quality (17).
A further notable advantage is the enhancement of emotional regulation. Music therapy recalibrates emotional processing by stimulating the amygdala and hippocampus, which are integral to the contextualization of emotions and memory. Individuals suffering from anxiety, depression, or post-traumatic stress may experience alleviation as music restores equilibrium between limbic reactivity and prefrontal inhibitory mechanisms (3–5). Group-based interventions, such as choral singing or drumming, amplify these therapeutic effects by synchronizing the brain rhythms of participants and fostering social connectedness and collective emotional experiences (21–23). This collective engagement not only enhances individual wellness but also bolsters social resilience.
The therapeutic capacity of music therapy aligns well with other non-pharmacological interventions. By providing a stable emotional and cognitive baseline, music therapy can complement brainwave entrainment methods (e.g., binaural beats, isochronic tones) and multisensory stimulation, potentially enhancing their efficacy (15, 20). Innovations in digital technology have further augmented this potential by facilitating real-time personalization grounded in physiological feedback. Adaptive systems have the capability to customize musical complexity, rhythm, and style to align with the specific needs of patients, thereby sustaining neuroplastic transformations while accommodating cultural and individual preferences (24, 25). Furthermore, the ability of music therapy to engage dopaminergic reward systems, modulate stress hormones, and strengthen neural connectivity provides a robust foundation for non-pharmacological mental health and cognitive interventions (2, 3, 17, 23). Its integration with AI-driven biofeedback holds promise for the development of innovative and highly individualized therapeutic frameworks (5, 16, 17).
Binaural beats offer a subtle yet compelling auditory intervention, where slightly different frequencies presented to each ear create the perception of an illusory “beat” absent in the actual sound waves (7, 8, 11). Similarly, isochronic tones employ a single pulsed tone that switches on and off at precise intervals, likewise intended to entrain brain activity. These methods are thought to modulate neural oscillations toward frequency bands associated with specific mental states. For instance, alpha frequencies (8–12 Hz) support relaxed alertness and stress reduction; theta ranges (4–7 Hz) are linked to meditative states and memory consolidation; and gamma frequencies (30–100 Hz) are associated with attention and higher-order cognitive processing (6, 19, 20).
Preliminary findings suggest that these brainwave entrainment techniques may influence mental states and enhance mental health and cognitive rehabilitation outcomes. In some studies, participants exposed to targeted frequencies reported reduced anxiety and improved sustained attention and working memory (6, 9). By modulating neural oscillatory activity, binaural beats and isochronic tones can complement or amplify other interventions—such as preparing the brain for relaxation before music therapy or enhancing the integration of multisensory stimulation (7, 8).
Despite these potential benefits, several significant challenges remain. The chief factor among these is the variability in individual responses. Baseline neural states, differences in auditory processing, and levels of engagement or expectancy significantly affect the effectiveness of neural entrainment (8, 10). Moreover, the lack of standardized protocols complicates generalization. Questions remain regarding the most effective frequency ranges for addressing specific conditions, optimal session durations, and whether these methods are more effective as standalone stimuli or when embedded within music or natural soundscapes (7, 11).
Methodological limitations also constrain the evidence base. Many studies have relied on small samples or pilot designs that lack control groups and robust biomarkers. For example, while EEG measures can confirm genuine shifts in alpha, theta, or gamma power, such data are rarely incorporated, leaving causal links between brainwave entrainment and cognitive-emotional benefits largely speculative (8, 26). Cultural and personal preferences add another layer of complexity. Pure tones may feel uncomfortable for some individuals, underscoring the need for personalization. Embedding beats into music or tailoring session lengths to align with user comfort can enhance adherence and effectiveness (9, 10).
By addressing these challenges through standardizing protocols, identifying target populations more clearly, and refining intervention parameters, binaural beats and isochronic tones may become powerful adjuncts to music therapy and multisensory stimulation in personalized treatment models (18, 27). Building on their conceptual framework, these auditory entrainment approaches have the potential to transition from niche interventions to widely accessible, technology-driven strategies for mental health care.
Although music therapy and previously discussed brainwave entrainment methods (e.g., binaural beats, isochronic tones) primarily rely on auditory inputs and have been shown to modulate emotional processing (2, 3), multisensory stimulation (MSS) expands these principles by integrating auditory, visual, and sometimes tactile or proprioceptive inputs (12). Importantly, this modality capitalizes on the brain's inherent ability to synthesize multiple inputs, thereby enhancing cognitive outcomes through synchronized stimuli. For instance, rhythmic light flickers paired with corresponding auditory signals can reinforce or entrain neural oscillations in the gamma frequency range (approximately 30–100 Hz), with particular emphasis on 40 Hz, which research has linked to memory consolidation, attention regulation, and integrative perceptual processing (13, 19, 20, 28–30).
Recent empirical studies underscore the therapeutic potential of this expanded approach, demonstrating improvements in memory performance and possible mitigation of neuropathological changes observed in Alzheimer's disease (12, 13, 30, 31). Unlike pharmacological interventions, which often produce systemic side effects, MSS attempts to directly modulate brain activity in a noninvasive manner. By aligning external sensory inputs with intrinsic oscillatory dynamics, this strategy aims to reorganize neural connectivity and processing patterns toward healthier states. Beyond memory enhancement, multisensory interventions could potentially address attentional deficits, motor recovery, and emotional regulation, thereby broadening their role in neurorehabilitation (32).
Nevertheless, MSS presents notable implementation challenges. Precise temporal coordination of auditory and visual stimuli is critical because even minimal desynchronizations can weaken entrainment effects (33). Moreover, the specialized equipment and controlled environments required to deliver synchronized stimuli pose logistical and financial barriers to large-scale adoption. At the same time, individual variability adds another layer of complexity, as factors such as baseline cognitive function, sensory acuity, cultural background, and personal preferences shape outcomes (34, 35).
Despite these obstacles, MSS aligns with fundamental principles of neural integration in the brain. Humans naturally merge multiple sensory inputs to interpret their surroundings, making this modality especially resonant with day-to-day functioning. Future research could explore tailored approaches that adapt sensory combinations to specific user needs. For example, auditory and visual cues might enhance memory training for certain individuals, whereas tactile inputs can better address motor learning or emotional grounding (36, 37).
It is important to note that MSS also dovetails with music therapy and auditory entrainment methods in potentially synergistic ways. For instance, familiar music paired with 40 Hz light flickers could heighten emotional engagement, priming neural circuits for enhanced plasticity (4, 17). Likewise, embedding binaural beats in a multisensory setting may help sustain attention and achieve targeted neural states more consistently (7, 9). In essence, MSS is a holistic and innovative approach for cognitive enhancement. By emphasizing the interplay among diverse sensory pathways, it broadens the therapeutic landscape beyond purely auditory or affective channels. While early-stage research highlights technical, logistical, and individual variability challenges, the potential to combine MSS with music therapy and other brainwave entrainment methods offers an exciting frontier for neurorehabilitation, cognitive training, and emotional support (27, 38, 39).
Despite a growing body of evidence supporting music therapy, binaural beats, and multisensory stimulation, significant challenges limit their effectiveness, scalability, and clinical integration. One of the most prominent obstacles is marked variability in patient responses. Baseline neural states, sensory processing abilities, cultural influences, and personal preferences shape how individuals perceive and respond to these interventions (8, 34). For example, music therapy often requires careful personalization, as musical elements must resonate emotionally and cognitively with each patient, making it resource-intensive and difficult to standardize or scale (17). Similarly, binaural beats show heterogeneous effects, with some individuals experiencing relaxation or enhanced focus, whereas others report negligible changes (9, 10). Multisensory stimulation further compounds this variability, as outcomes depend on individual sensory acuity and cognitive status, along with the precise alignment of auditory, visual, and other inputs (33, 40).
Another critical limitation is the absence of standardized protocols. Few universally accepted frameworks exist to guide practitioners on matching specific musical elements, binaural frequencies, or multisensory stimuli to desired therapeutic outcomes (18). This lack of consistency hampers reproducibility complicates evidence-based practice and creates barriers for large-scale implementation. Without robust clinical guidelines, practitioners may struggle to adopt these modalities confidently, and researchers may find it challenging to compare findings across studies (15, 24).
In addition, scalability and accessibility pose substantial challenges. Delivering these therapies often requires skilled practitioners, advanced equipment, and controlled environments, which limits their reach to underserved or remote populations (39, 41). Music therapy depends on trained professionals to tailor sessions; binaural beats require high-quality audio systems and distraction-free environments; and multisensory stimulation involves specialized apparatus for precise synchronization. While digital health technologies may address some barriers, they introduce further considerations, such as user-friendliness, data privacy protection, and technical support requirements (15).
Ethical considerations regarding data governance are equally pressing. As AI-driven systems increasingly support these modalities, collecting physiological data, such as EEG signals or heart rate variability, raises concerns about informed consent, data security, and algorithmic transparency (15, 42). Notably, users must understand how their data are utilized, who has access, and whether algorithmic decisions are free from bias or hidden errors. While the prospect of dynamically adjusting interventions based on real-time neural or emotional states is promising, rigorous oversight is required to ensure fairness, accountability, and patient autonomy (41).
Moreover, theoretical gaps undermine the potential of these modalities. Although supported by empirical work—such as neural plasticity mechanisms in music therapy, frequency-specific entrainment in binaural beats, and gamma coherence in multisensory stimulation—many studies lack sufficient depth to establish causal pathways (28, 29). Research is often constrained by small sample sizes, short durations, and reliance on subjective measures, limiting conclusions about long-term safety, efficacy, or dose-response relationships (8, 26). Translational gaps further exacerbate this issue, as findings from controlled laboratory studies may not replicate real-world conditions with distractions and user fatigue.
Cost and economic barriers also complicate scalability. Developing AI-driven platforms, acquiring reliable hardware, and training clinicians or users to operate complex systems require significant investment (15). These expenses may restrict access to affluent institutions or well-resourced users, thereby perpetuating existing health disparities. Ensuring cost-effective, durable, and user-friendly solutions in diverse socioeconomic settings is critical for equitable access (18).
Finally, there is a lack of high-quality comparative research evaluating the synergy of these modalities. Few studies have systematically examined combinations such as music therapy paired with binaural beats or multisensory stimulation enhanced by AI-guided adjustments, leaving their interactive benefits largely speculative (8, 10). Hence, future interdisciplinary collaborations could address this gap by designing protocols that vary and combine modalities systematically, clarifying their neural and behavioral interactions across different conditions (15).
Addressing these challenges requires coordinated efforts across disciplines. By developing standardized guidelines, refining technological infrastructure, and investing in longitudinal studies, the field can overcome current limitations and pave the way for integrative, AI-powered therapeutic frameworks. Such advancements will enable more reliable, scalable, and equitable interventions, ultimately broadening access to nonpharmacological mental health and cognitive rehabilitation strategies (15, 18).
Having explored the mechanisms, evidence, and limitations of music therapy, brainwave entrainment methods (including binaural beats, isochronic tones and multisensory stimulation), it is logical to propose an integrative framework that leverages machine learning–based artificial intelligence (AI) and continuous biofeedback to create adaptive, personalized interventions. While fully realized AI remains speculative, current AI algorithms can already process physiological data in real time, recognizing that mental health conditions and cognitive deficits vary widely across individuals and evolve over time (43, 44). Such machine learning–driven systems enable dynamic adjustments to therapeutic parameters, aligning with a user's changing needs and contexts.
Additionally, distinguishing machine learning (ML) from artificial general intelligence (AGI).
To begin with, ML refers to a subset of AI that focuses on algorithms trained to identify patterns in data, thereby enabling task-specific predictions or decisions without explicit reprogramming (45, 46). By contrast, AGI aims to emulate the broad cognitive flexibility of humans, encompassing complex reasoning, problem-solving, and adaptive learning across diverse domains (45). Although ML systems are already extensively applied in fields ranging from healthcare to finance, AGI remains largely theoretical and would require significant breakthroughs in our understanding of human cognition (46). Therefore, the personalized digital therapeutics proposed here rely on machine learning–based methods that can operate effectively within well-defined parameters. Ultimately, the possibility of AGI might broaden these real-time adaptive therapies, but it is not yet feasible for immediate clinical application. In light of these constraints, the present framework leverages machine learning capabilities that are already attainable, particularly in the realm of real-time monitoring and adaptive feedback.
Furthermore, at the heart of this vision lies AI-empowered real-time monitoring. Continuous data streams collected from wearable sensors, EEG recordings, heart rate variability monitors, or self-report apps provide a detailed, moment-to-moment understanding of an individual's physiological and emotional state (47, 48). These data inform AI algorithms to detect patterns—such as elevated stress markers, lapses in attention, or mood shifts—and adapt interventions accordingly. For example, if binaural beats fail to achieve alpha-wave relaxation, the system can modify the frequency range or transition into a soothing musical sequence. Similarly, if memory outcomes during multisensory stimulation remain suboptimal, adjusting gamma-frequency alignment or stimulus intensity may enhance neural entrainment (49, 50).
By integrating multiple modalities, AI-driven systems can unlock new opportunities for synergy. The ability of music therapy to foster emotional engagement primes neural circuits for enhanced attentional states, potentially amplified by binaural beats or isochronic tones. Meanwhile, multisensory stimulation reinforces neural oscillations such as gamma rhythms, thereby consolidating cognitive gains (43, 44). As summarized in Table 1, these approaches differ in mechanisms, required engagement, and adaptability, but AI-driven biofeedback system can dynamically tailor each to individual needs, thus overcoming the limitations of static, one-size-fits-all interventions.
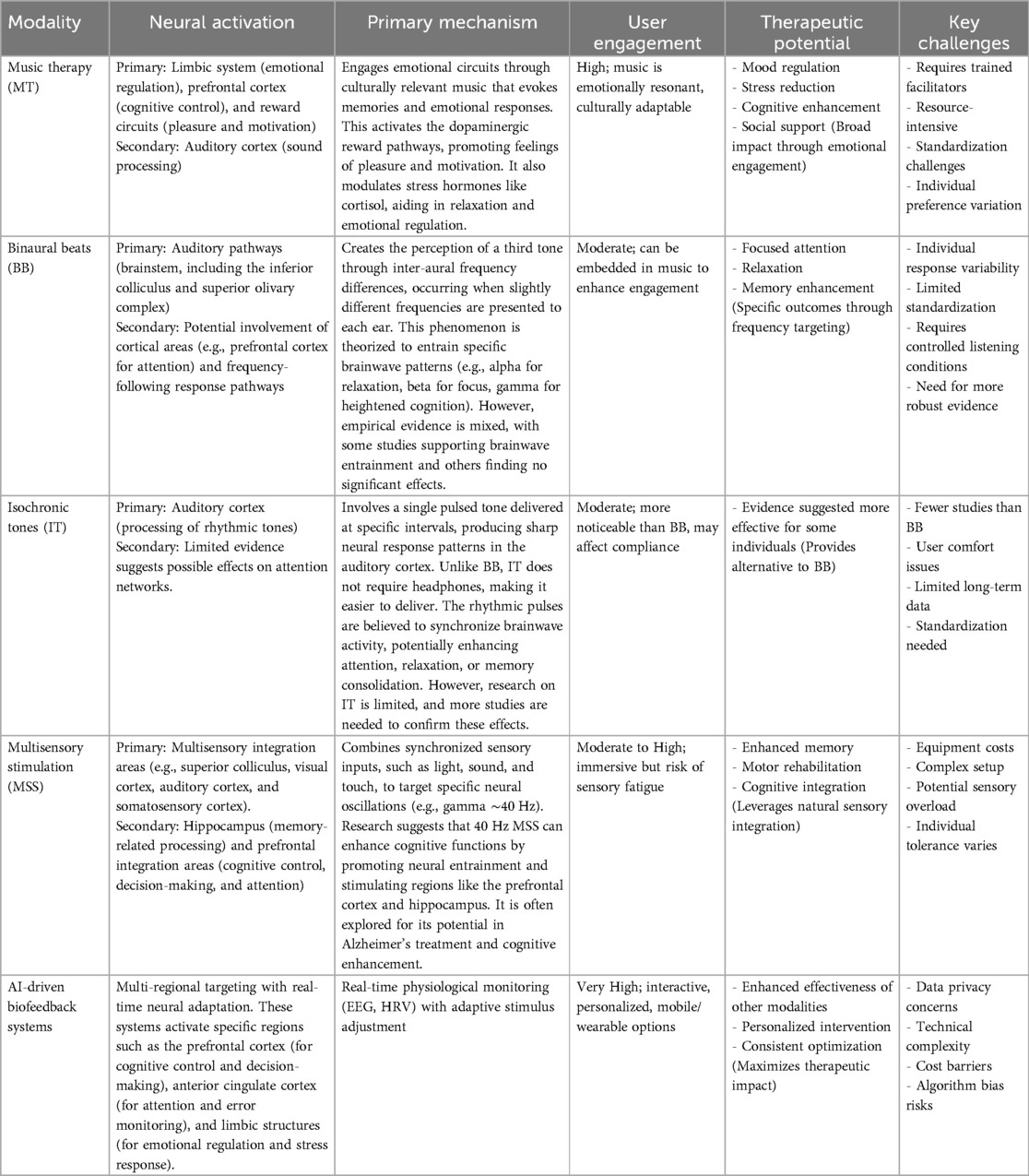
Table 1. Comparative overview of music therapy, brainwave entrainment methods, and AI-driven biofeedback systems.
The adaptability of AI-enhanced systems is especially effective in overcoming current challenges. By leveraging real-time feedback, these systems address variability in user responses, ensuring greater consistency across sessions. Additionally, AI integration with mobile and wearable devices significantly boosts accessibility and scalability. This innovation allows interventions to extend beyond clinical environments, diminishing reliance on trained professionals and controlled settings, thus making personalized therapies more widely available (43, 51, 52).
Furthermore, AI integration facilitates large-scale data collection of therapeutic protocols (Figure 1). As anonymized datasets expand, machine learning models can discern which combinations of interventions work best for specific populations under various conditions (47, 53). This iterative refinement process—deploying interventions, analyzing outcomes, and updating algorithms—can establish evidence-based guidelines, enhance reproducibility, and create standardized therapeutic frameworks (44, 48).
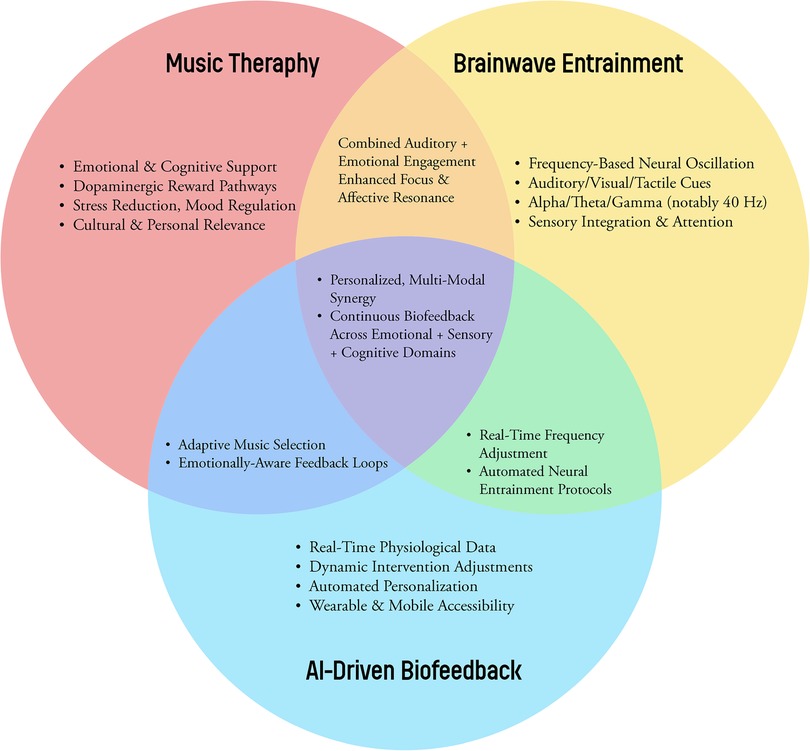
Figure 1. A conceptual Venn diagram illustrating the synergy among music therapy, brainwave entrainment methods (binaural beats, isochronic tones, multisensory stimulation), and AI-driven biofeedback.
Nevertheless, ethical considerations must remain paramount. Individuals must consent to data collection and comprehend its usage, while developers ensure secure handling of sensitive physiological and emotional data (52, 54). Bias in AI systems poses additional risks, as underrepresentation in training datasets can yield unequal benefits across demographic groups (42). Transparency in algorithm design, routine audits, and stakeholder engagement are vital for equitable implementation. Regulatory bodies must establish guidelines that balance innovation with safety, autonomy, and fairness (44).
Technical challenges also warrant further attention. Developing robust biofeedback loops that respond promptly to changing signals requires precise engineering to prevent overstimulation or user discomfort. For instance, abruptly shifting modalities may confuse users, highlighting the need for seamless transitions and gradual adjustments (55). The reliability and stability of hardware and software are equally crucial, as interruptions can undermine trust and engagement. Moreover, user-centered design ensures that platforms remain intuitive and accessible to diverse populations (52, 56).
A further benefit is the interdisciplinary scope of AI-driven integrative therapies. Neuroscientists can identify neural signatures that predict optimal responses to specific interventions, while psychologists contribute insights into motivational strategies that enhance user engagement (48, 57). Engineers and data scientists refine algorithms, clinicians align protocols with established practices, and ethicists guide responsible development (58). This collaborative approach enables cohesive advancement across research and practice (59, 60).
Initial efforts toward these integrative models have already demonstrated feasibility. For example, pilot studies have used EEG signals to adjust cognitive training tasks or integrated heart rate variability feedback into relaxation exercises (61). In the next phase, scaling these initiatives—combining them with music therapy, binaural beats, isochronic tones, and multisensory stimulation—and testing them in rigorous clinical trials are logical steps forward. Multisite, long-term studies involving heterogeneous populations and robust control conditions are especially important for validating efficacy, user satisfaction, and real-world functionality (53, 62).
Ultimately, the future of non-pharmacological mental health and cognitive interventions lies in dynamic, AI-driven systems. By merging music therapy's emotional richness, the oscillatory precision of binaural beats and isochronic tones, and the integrative scope of multisensory stimulation, personalized, adaptive solutions can be crafted (43, 44, 50). Although these concepts remain aspirational, their foundations are rapidly taking shape, offering a transformative shift in how mental health and cognitive challenges are addressed, optimized, and overcome.
The integration of music therapy, brainwave entrainment methods such as binaural beats, isochronic tones, and multisensory engagement, enhanced by adaptive artificial intelligence technologies, holds transformative potential for non-pharmacological approaches to mental health and cognitive rehabilitation. Each modality offers distinct advantages: music therapy engages emotional and cognitive pathways to alleviate mood disorders and build resilience; auditory entrainment guides neural oscillations toward frequencies associated with relaxation, focus, and memory retention; and multisensory stimulation harmonizes multiple inputs, fostering gamma oscillations linked to higher-order cognitive functions. Within a unified therapeutic framework, these modalities can create synergistic effects that surpass the efficacy of isolated interventions.
However, achieving this vision requires overcoming persistent barriers. Notably, variability in patient responses, the lack of standardized protocols, and the logistical challenges of scaling these interventions to diverse populations and real-world contexts limit their current applicability. Targeted interdisciplinary research is essential to address these limitations. Efforts must focus on refining methodologies, developing validated guidelines, and conducting rigorous longitudinal studies to establish a robust evidence base. The incorporation of ethically designed AI-driven biofeedback systems offers a promising solution. Such systems can adapt interventions dynamically, leveraging real-time physiological feedback, cultural considerations, individual preferences, and evolving clinical needs. This adaptability enhances therapeutic efficacy and patient satisfaction while also improving accessibility, particularly as mobile and wearable technologies reduce infrastructural barriers.
The successful implementation of these integrative, AI-enabled therapies depends on establishing strong ethical and regulatory frameworks. Transparent data governance, equitable algorithmic design, and safeguards for patient privacy, autonomy, and safety are critical to ensure these innovations align with the highest standards of care. Collaborative efforts among neuroscientists, clinicians, psychologists, engineers, music therapists, ethicists, and policymakers will be essential to anchor these advances in clinical realities while addressing economic and infrastructural challenges. By prioritizing equity, these solutions can democratize access to effective mental health and cognitive rehabilitation interventions, rather than exacerbating existing health disparities.
The next phase of research must focus on systematically evaluating combined interventions. Key priorities include identifying optimal modality combinations, determining patient characteristics that predict responsiveness, and validating these approaches through large-scale, multi-site clinical trials. Moving beyond proof-of-concept studies, community-based implementations can help establish the real-world feasibility and sustainability of these interventions. By consolidating disparate yet promising therapies into an evidence-based and scalable paradigm, their therapeutic potential can be maximized.
Ultimately, embracing the synergy between music therapy, binaural beats, isochronic tones, and multisensory stimulation—augmented by AI-driven personalization—represents a paradigm shift in non-pharmacological treatments. This patient-centered framework not only has the potential to enhance mental health and cognitive resilience but also stands to revolutionize neurorehabilitation and preventive healthcare. By refining theoretical models, building comprehensive datasets, and advancing adaptive technologies, the field moves closer to a future where interventions are scientifically rigorous, ethically managed, and tailored to the complexities of individual needs. Parallel to these advancements, emerging initiatives in biofeedback art—where real-time physiological signals drive artistic or musical outputs—further underscore the creative potential of integrating binaural beats, music therapy, and visual elements. Beyond conventional clinical applications, such approaches can foster deeper engagement, emotional resonance, and heightened self-awareness. The incorporation of biofeedback art into future research protocols holds promise not only for enhancing treatment adherence but also for enabling the exploration of novel, immersive therapeutic paradigms that capitalize on the synergy between multisensory stimulation and artistic expression. In this way, the evolving landscape of neurotherapeutic interventions seeks to honor the diversity of human experiences while advancing the universal goal of enhancing well-being.
DJ: Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mok VCT, Cai Y, Markus HS. Vascular cognitive impairment and dementia: mechanisms, treatment, and future directions. Int J Stroke. (2024) 19(8):838–56. doi: 10.1177/17474930241279888
2. Black DS, Cole SW, Irwin MR, Breen E, St. Cyr NM, Nazarian N, et al. Yogic meditation reverses NF-κB and IRF-related transcriptome dynamics in leukocytes of family dementia caregivers in a randomized controlled trial. Psychoneuroendocrinology. (2013) 38(3):348–55. doi: 10.1016/j.psyneuen.2012.06.011
3. Davidson RJ, Kabat-Zinn J, Schumacher J, Rosenkranz M, Muller D, Santorelli SF, et al. Alterations in brain and immune function produced by mindfulness meditation. Psychosom Med. (2003) 65(4):564–70. doi: 10.1097/01.PSY.0000077505.67574.E3
4. Fell J, Axmacher N, Haupt S. From alpha to gamma: electrophysiological correlates of meditation-related states of consciousness. Med Hypotheses. (2010) 75(2):218–24. doi: 10.1016/j.mehy.2010.02.025
5. Lomas T, Ivtzan I, Fu CHY. A systematic review of the neurophysiology of mindfulness on EEG oscillations. Neurosci Biobehav Rev. (2015) 57:401–10. doi: 10.1016/j.neubiorev.2015.09.018
6. Borges LR, Britto Arantes APB, Naves ELM. Influence of binaural beats stimulation of gamma frequency over memory performance and EEG spectral density. Healthcare (Switzerland). (2023) 11(6):801. doi: 10.3390/healthcare11060801
7. Gao X, Cao H, Ming D, Qi H, Wang X, Wang X, et al. Analysis of EEG activity in response to binaural beats with different frequencies. Int J Psychophysiol. (2014) 94(3):399–406. doi: 10.1016/j.ijpsycho.2014.10.010
8. Ingendoh RM, Posny ES, Heine A. Binaural beats to entrain the brain? A systematic review of the effects of binaural beat stimulation on brain oscillatory activity, and the implications for psychological research and intervention. PLoS One. (2023) 18(5):e0286023. doi: 10.1371/journal.pone.0286023
9. Leistiko NM, Madanat L, Yeung WKA, Stone JM. Effects of gamma frequency binaural beats on attention and anxiety. Curr Psychol. (2023) 43(6):5032–9. doi: 10.1007/s12144-023-04681-3
10. Robison MK, Brewer GA, Obulasetty M, Blais C, Wingert K. The effect of binaural auditory beat stimulation on sustained attention. Center for Open Science. (2020). doi: 10.31234/osf.io/u5cja
11. Sudre S, Kronland-Martinet R, Petit L, Rozé J, Ystad S, Aramaki M. A new perspective on binaural beats: investigating the effects of spatially moving sounds on human mental states. PLoS One. (2024) 19(7):e0306427. doi: 10.1371/journal.pone.0306427
12. Blanco-Duque C, Chan D, Kahn MC, Murdock MH, Tsai LH. Audiovisual gamma stimulation for the treatment of neurodegeneration. J Intern Med. (2024) 295(2):146–70. doi: 10.1111/joim.13755
13. Chan D, Suk HJ, Jackson BL, Milman NP, Stark D, Klerman EB, et al. Gamma frequency sensory stimulation in mild probable Alzheimer’s dementia patients: results of feasibility and pilot studies. PLoS One. (2022) 17(12):e0278412. doi: 10.1371/journal.pone.0278412
14. Yan H, Hou S-T, Bartlett P, Liu B, Wang Y, Jun D, et al. Enhancement of spatial learning by 40 hz visual stimulation requires parvalbumin interneuron-dependent hippocampal neurogenesis. Cold Spring Harbor Laboratory. (2024) :2024.04.28.591481. doi: 10.1101/2024.04.28.591481
15. Tuckute G, Hansen LK, Kjaer TW, Hansen ST. Real-time decoding of attentional states using closed-loop EEG neurofeedback. Neural Comput. (2021) 33(4):967–1004. doi: 10.1162/neco_a_01363
16. Braboszcz C, Cahn BR, Levy J, Fernandez M, Delorme A. Increased gamma brainwave amplitude compared to control in three different meditation traditions. PLoS One. (2017) 12(1). doi: 10.1371/journal.pone.0170647
17. Ferrarelli F, Smith R, Dentico D, Riedner BA, Zennig C, Benca RM, et al. Experienced mindfulness meditators exhibit higher parietal-occipital EEG gamma activity during NREM sleep. PLoS One. (2013) 8(8):e73417. doi: 10.1371/journal.pone.0073417
18. González-Valero G, Ubago-Jiménez JL, Zurita-Ortega F, Puertas-Molero P. Use of meditation and cognitive behavioral therapies for the treatment of stress, depression and anxiety in students. A systematic review and meta-analysis. Int J Environ Res Public Health. (2019) 16(22):4394. doi: 10.3390/ijerph16224394
19. Craig MT, Bielska MH, Jeffery K. Mechanisms and implications of gamma oscillation plasticity. Trends Neurosci. (2024) 47(6):398–9. doi: 10.1016/j.tins.2024.05.002
20. Han C, Shapley R, Xing D. Gamma rhythms in the visual cortex: functions and mechanisms. Cogn Neurodyn. (2022) 16(4):745–56. doi: 10.1007/s11571-021-09767-x
21. Gordon I, Cohen S, Haimovich N, Siegman S, Pinhasi S, Gilboa A, et al. Physiological and behavioral synchrony predict group cohesion and performance. Sci Rep. (2020) 10(1). doi: 10.1038/s41598-020-65670-1
22. Hill NTM, Mowszowski L, Naismith SL, Chadwick VL, Valenzuela M, Lampit A. Computerized cognitive training in older adults with mild cognitive impairment or dementia: a systematic review and meta-analysis. Am J Psychiatry. (2017) 174(4):329–40. doi: 10.1176/appi.ajp.2016.16030360
23. Williams E, Dingle GA, Clift S. A systematic review of mental health and wellbeing outcomes of group singing for adults with a mental health condition. Eur J Public Health. (2018) 28(6):1035–42. doi: 10.1093/eurpub/cky115
24. Boto E, Hill RM, Rea M, Holmes N, Seedat ZA, Leggett J, et al. Measuring functional connectivity with wearable MEG. NeuroImage. (2021) 230:117815. doi: 10.1016/j.neuroimage.2021.117815
25. Sengupta S, Konidena DA. Generating music therapy using deep learning and reinforcement learning. Int J Eng Appl Sci Technol. (2020) 04(12):502–5. doi: 10.33564/IJEAST.2020.v04i12.089
26. Colzato LS, Sellaro R, Steenbergen L. Retraction note to: the effect of gamma-enhancing binaural beats on the control of feature bindings. Exp Brain Res. (2021) 239(2):363. doi: 10.1007/s00221-020-06018-z
27. Li J, Yang H, Xiao Y, Liu X, Ma B, Ma K, et al. The analgesic effects and neural oscillatory mechanisms of virtual reality scenes based on distraction and mindfulness strategies in human volunteers. Br J Anaesth. (2023) 131(6):1082–92. doi: 10.1016/j.bja.2023.09.001
28. Buzsáki G, Wang X-J. Mechanisms of gamma oscillations. Annu Rev Neurosci. (2012) 35(1):203–25. doi: 10.1146/annurev-neuro-062111-150444
29. Cardin JA. Snapshots of the brain in action: local circuit operations through the lens of γ oscillations. J Neurosci. (2016) 36(41):10496–504. doi: 10.1523/JNEUROSCI.1021-16.2016
30. Islam MR, Jackson B, Schatz B, Murdock M, Tan N, Park DS, et al. Multisensory gamma stimulation enhances adult neurogenesis and improves cognitive function in a mouse model of Down syndrome. Cold Spring Harbor Laboratory (2024). doi: 10.1101/2024.10.03.616486
31. Murdock MH, Yang CY, Sun N, Pao PC, Blanco-Duque C, Kahn MC, et al. Multisensory gamma stimulation promotes glymphatic clearance of amyloid. Nature. (2024) 627(8002):149–56. doi: 10.1038/s41586-024-07132-6
32. Ju J, Liu B, Zhong H, Pan Y, Zhang Z, Hou S-T, et al. Adenosine mediates the amelioration of social novelty deficits during rhythmic light treatment of 16p11.2 deletion female mice. Mol Psychiatry. (2024) 29:3381–94. doi: 10.1038/s41380-024-02596-4
33. Soula M, Gan W-B, Dhingra A, Martín-Ávila A, Sadowski MJ, Nitzan N, et al. Forty-hertz light stimulation does not entrain native gamma oscillations in Alzheimer’s disease model mice. Nat Neurosci. (2023) 26(4):570–8. doi: 10.1038/s41593-023-01270-2
34. Judd N, Aristodemou M, Kievit R, Klingberg T. Interindividual differences in cognitive variability are ubiquitous and distinct from mean performance in a battery of eleven tasks. J Cogn. (2024) 7(1):45. doi: 10.5334/joc.371
35. Katz B, Jones MR, Shah P, Buschkuehl M, Jaeggi SM. Individual differences in cognitive training research. In Strobach T, Karbach J editors. Cognitive Training. Cham, Switzerland: Springer (2021). p. 115–34. doi: 10.1007/978-3-030-39292-5_8
36. Beldzik E, Domagalik A, Beres A, Marek T. Linking visual gamma to task-related brain networks-a simultaneous EEG-fMRI study. Psychophysiology. (2019) 56(12):e13462. doi: 10.1111/psyp.13462
37. Dos Anjos T, Di Rienzo F, Benoit CE, Daligault S, Guillot A. Brain wave modulation and EEG power changes during auditory beats stimulation. Neuroscience. (2024) 554:156–66.
38. Kinreich S, Djalovski A, Kraus L, Louzoun Y, Feldman R. Brain-to-brain synchrony during naturalistic social interactions. Sci Rep. (2017) 7(1):17060. doi: 10.1038/s41598-017-17339-5
39. Lahijanian M, Aghajan H, Vahabi Z. Auditory gamma-band entrainment enhances default mode network connectivity in dementia patients. Sci Rep. (2024) 14:63727. doi: 10.1038/s41598-024-63727-z
40. Hadler MD, Tzilivaki A, Schmitz D, Alle H, Geiger JRP. Gamma oscillation plasticity is mediated via parvalbumin interneurons. Sci Adv. (2024) 10(5):eadj7427. doi: 10.1126/sciadv.adj7427
41. Mukhopadhaya S, Bag A, Panwar P, Malagi V. Navigating the quandaries of artificial intelligence-driven mental health decision support in healthcare. In: Ara A, Ara A, editors. Exploring the Ethical Implications of Generative AI. Advances in Computational Intelligence and Robotics Book Series. Hershey, PA: IGI Global (2024). p. 211–36. doi: 10.4018/979-8-3693-1565-1.ch012
42. Tejani AS, Rayan JC, Ng YS, Xi Y. Understanding and mitigating bias in imaging artificial intelligence. Radiographics. (2024) 44(5). doi: 10.1148/rg.230067
43. Maggio MG, Luca A, Calabrò RS, Drago F, Nicoletti A. Can mobile health apps with smartphones and tablets be the new frontier of cognitive rehabilitation in older individuals? A narrative review of a growing field. Neurol Sci. (2024) 45(1):37–45. doi: 10.1007/s10072-023-07045-8
44. Patterson MD, Ng KP, bin Jabar S, Leonardo J, Chiew HJ, Su A, et al. NG-001: a novel multi-domain digital cognitive intervention for cognitive symptoms in mild cognitive impairment. Alzheimer’s & Dementia. (2023) 19(S19):e076452. doi: 10.1002/ALZ.076452
45. Jones L, Golan D, Hanna S, Ramachandran M. Artificial intelligence, machine learning and the evolution of healthcare. Bone Joint Res. (2018) 7:223–5. doi: 10.1302/2046-3758.73.BJR-2017-0147.R1
46. Stahl B, Andreou A, Brey P, Hatzakis T, Kirichenko A, Macnish K, et al. Artificial intelligence for human flourishing – beyond principles for machine learning. J Bus Res. (2020) 124:374–88. doi: 10.1016/j.jbusres.2020.11.030
47. Lee J, Yang H, Xiao Y, Liu X, Ma B, Ma K, et al. Efficacy of a Mobile-based multi-domain intervention to improve cognitive function and health-related outcomes among older Korean adults with subjective cognitive decline. J Alzheimers Dis. (2023) 93(4):1551–62. doi: 10.3233/JAD-221299
48. Trinh TT, Tsai CF, Hsiao YT, Lee CY, te Wu C, Liu YH. Identifying individuals with mild cognitive impairment using working memory-induced intra-subject variability of resting-state EEGs. Front Comput Neurosci. (2021) 15. doi: 10.3389/fncom.2021.700467
49. Cavallo M, Hunter EM, van der Hiele K, Angilletta C. Computerized structured cognitive training in patients affected by early-stage Alzheimer’s disease is feasible and effective: a randomized controlled study. Arch Clin Neuropsychol. (2016) 31(8):868–76. doi: 10.1093/ARCLIN/ACW072
50. Brugada-Ramentol V, Bozorgzadeh A, Jalali H. Enhance VR: a multisensory approach to cognitive training and monitoring. Front Digit Health. (2022) 4. doi: 10.3389/fdgth.2022.916052
51. Loriette C, Ziane C, ben Hamed S. Neurofeedback for cognitive enhancement and intervention and brain plasticity. Rev Neurol (Paris). (2021) 177(9):1133–44. doi: 10.1016/j.neurol.2021.08.004
52. Kunkel T, Hayduk T, Lock D. Push it real good: the effects of push notifications promoting motivational affordances on consumer behavior in a gamified mobile app. Eur J Mark. (2023) 57(9):2592–618. doi: 10.1108/EJM-06-2021-0388
53. Irazoki E, Toribio-Guzmán JM, Contreras-Somoza LM, Franco-Martín MA, Jenaro-Río C, Van Der Roest H. Technologies for cognitive training and cognitive rehabilitation for people with mild cognitive impairment and dementia. A systematic review. Front Psychol. (2020) 11. doi: 10.3389/fpsyg.2020.00648
54. Freeman D, Reeve S, Robinson A, Ehlers A, Clark D, Spanlang B, et al. Virtual reality in the assessment, understanding, and treatment of mental health disorders. Psychol Med. (2017) 47(14):2393–400. doi: 10.1017/S003329171700040X
55. Al-Alshaikh HA, Kumar A, Saudagar AKJ, Poonia RC. Design and development of cognitive improvement through virtual reality-based treatment using mathematical model. J Interdiscip Math. (2024) 27:6. doi: 10.47974/jim-1993
56. Fantozzi C, Zanella A, Simoni M, Gollin D, Ruaro C, Casa M, et al. Towards digital therapy for Alzheimer’s disease and other forms of neurocognitive disorder: the INFORMA software platform. ACM International Conference Proceeding Series (2022). p. 68–74. doi: 10.1145/3524458.3547238
57. Goghari VM, Krzyzanowski D, Yoon S, Dai Y, Toews D. Attitudes and beliefs towards computerized cognitive training in the general population. Front Psychol. (2020) 11. doi: 10.3389/fpsyg.2020.00503
58. Zhu Y, Jiang H, Su H, Zhong N, Li R, Li X, et al. A newly designed Mobile-based computerized cognitive addiction therapy app for the improvement of cognition impairments and risk decision making in methamphetamine use disorder: randomized controlled trial. JMIR Mhealth Uhealth. (2018) 6(6):e10292. doi: 10.2196/10292
59. Lee U, Kim Y, Kim J, Choi B, Lee J. Data-driven digital therapeutics analytics. 2023 IEEE International Conference on Big Data and Smart Computing (BigComp); IEEE (2023). p. 386–8. doi: 10.1109/BigComp57234.2023.00093
60. Abd N, Abou-Shady E, Omara T, Mousa K, El A, Soliman H, et al. Influence of Mobile application based brain training program on cognitive function and quality of life in patients post-stroke. J Adv Zool. (2023) 44(S3):204–10. doi: 10.17762/JAZ.V44IS-3.568
61. Lavy Y, Dwolatzky T, Kaplan Z, Guez J, Todder D. Mild cognitive impairment and neurofeedback: a randomized controlled trial. Front Aging Neurosci. (2021) 13. doi: 10.3389/fnagi.2021.657646
Keywords: music therapy, binaural beats, multisensory stimulation, brainwave entrainment, neuroplasticity, cognitive rehabilitation, artificial intelligence, non-pharmacological interventions
Citation: Jiao D (2025) Advancing personalized digital therapeutics: integrating music therapy, brainwave entrainment methods, and AI-driven biofeedback. Front. Digit. Health 7:1552396. doi: 10.3389/fdgth.2025.1552396
Received: 28 December 2024; Accepted: 13 February 2025;
Published: 25 February 2025.
Edited by:
Naama Schwartz, Reichman University, IsraelReviewed by:
Krisztián Hofstädter, University of York, United KingdomCopyright: © 2025 Jiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dian Jiao, ZGlhbi5qaWFvQGtjbC5hYy51aw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.