
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Digit. Health, 27 March 2025
Sec. Health Informatics
Volume 7 - 2025 | https://doi.org/10.3389/fdgth.2025.1528882
 Rania Kousovista1
Rania Kousovista1 Georgina Cosma1*
Georgina Cosma1* Emeka Abakasanga1
Emeka Abakasanga1 Ashley Akbari2
Ashley Akbari2 Francesco Zaccardi3
Francesco Zaccardi3 Gyuchan Thomas Jun4
Gyuchan Thomas Jun4 Reza Kiani5
Reza Kiani5 Satheesh Gangadharan5
Satheesh Gangadharan5
Introduction: Identifying and understanding the co-occurrence of multiple long-term conditions (MLTCs) in individuals with intellectual disability (ID) is crucial for effective healthcare management. Individuals with ID often experience earlier onset and higher prevalence of MLTCs compared to the general population, however, the specific patterns of co-occurrence and temporal progression of these conditions remain largely unexplored. This study presents an innovative unsupervised approach for examining and characterising clusters of MLTC in individuals with ID, based on their shared disease trajectories.
Methods: Using a dataset of electronic health records (EHRs) from 13,069 individuals with ID, encompassing primary and secondary care data in Wales from 2000 to 2021, this study analysed the time sequences of disease diagnoses. Significant pairwise disease associations were identified, and their temporal directionality assessed. Subsequently, an unsupervised clustering algorithm—spectral clustering—was applied to the shared disease trajectories, grouping them based on common temporal patterns.
Results: The study population comprised 52.3% males and 47.7% females, with a mean of 4.5 3 long-term conditions (LTCs) per patient. Distinct MLTC clusters were identified in both males and females, stratified by age groups (<45 and 45 years). For males under 45, a single cluster dominated by neurological conditions (32.4%), while three clusters were identified for older males, with the largest characterised by circulatory (51.8%). In females under 45, one cluster was found with digestive system conditions (24.6%) being most prevalent. For females 45 years, two clusters were identified: the first cluster was predominantly defined by circulatory (34.1%), while the second cluster by digestive (25.9%) and musculoskeletal (21.9%) system conditions. Mental illness, epilepsy, and reflux disorders were prevalent across all groups.
Discussion: This study reveals complex multimorbidity patterns in individuals with ID, highlighting age and sex differences. The identified clusters provide new insights into disease progression and co-occurrence in this population. These findings can inform the development of targeted interventions and risk stratification strategies, potentially improving personalised healthcare for individuals with ID and MLTCs with the aim of improving health outcome for this vulnerable group of patients i.e. reducing frequency and length of hospital admissions and premature mortality.
People with intellectual disability (ID) face a significantly higher risk of developing a range of physical and mental health conditions compared to the general population. These conditions often occur at a younger age and lead to poorer outcomes, owing to a combination of genetic, behavioural, and social factors (1–3). Studies show a much higher occurrence of multiple long-term conditions (MLTCs) in this population (2). MLTCs, defined as two or more conditions in addition to ID, is linked to premature death and poorer quality of life (4). Despite this, there appear to be only a few studies reporting the prevalence of MLTCs conducted on a large scale (2, 5), but no studies were found to reveal patterns of MLTCs and conditions more likely to co-occur together in this population.
The growing use of electronic health records (EHRs) has enabled significant advances in addressing clinical challenges, enhancing diagnostic capabilities, and improving patient outcomes (6–8). In addition to enabling studies on co-occurring conditions, the longitudinal nature of EHR provides a unique opportunity to uncover temporal associations and trajectories between conditions. Importantly, chronic health conditions frequently co-occur more than expected by chance, often as a consequence of shared risk factors, pathogenicity, or their treatment (9). However, most prior studies have not incorporated the time dimension due to the short time span of the available data (10, 11).
Only recently have a few large-scale analyses assessed disease trajectories by evaluating temporal ordering of co-morbidity pairs over time in general population (12–14). Many studies further developed the framework initially proposed by Jensen et al. (12), who described general principles for temporal trajectory analysis using Danish national data. For instance, Siggaard et al. (15) published a browser of these results, while Jørgensen and Brunak (16) focused on chronic obstructive pulmonary disease (COPD) trajectories. Hu et al. (17) linked the data to a cancer registry to investigate pre-diagnosis trajectories. Jensen et al.’s (12) approach has been applied, with modifications, to other populations including post-depression trajectories in UK Biobank (18), and end-of-life trajectories in California (19). Furthermore, Giannoula et al. (13, 20) proposed a framework to detect and cluster co-morbidity pairs and shared trajectories over time using a dynamic time warping (DTW)-based unsupervised algorithm in EHRs, and later extended this to include genetic information in the clustering step. Unsupervised algorithms discover natural patterns in data without learning predefined outcomes or classifications. Trajectory analyses can reveal complex, time-ordered condition associations, as well as MLTCs patterns to enable better understanding of disease progression for improved prediction outcomes.
In this study, we propose a computational framework for the analysis of temporal MLTCs on EHRs in 13,069 adults diagnosed with ID in Wales, incorporating 40 long-term conditions (LTCs) from both primary and secondary care data. While most prior studies have applied temporal trajectory analysis to secondary care data and ICD-9 or -10 codes (12, 13, 15–20), with only one study using primary care data (21), our approach utilises both to fully capture MLTCs, as most chronic conditions are treated in general practice. This approach highlights several differences in MLTC patterns between male and female sub-populations across different age groups, acknowledging sex and age as crucial factors in understanding MLTCs. The primary contributions of this research include statistical analysis to identify significant temporal condition pairs, identification of shared MLTCs trajectories, construction of a network of all shared trajectories, and identification of trajectory clusters using an unsupervised machine learning algorithm.
This study focused on Welsh residents aged 18 and older with intellectual disability and at least one long-term condition (LTC) between 1st January 2000, and 31st December 2021. This population-based study utilised the Secure Anonymised Information Linkage (SAIL) Databank, a Welsh data repository that enables individual-based data linkage across datasets (22). We identified eligible individuals who were registered with a general practitioner (GP) at the study start date (Supplementary Figure S1). For inclusion in the cohort, individuals required key identifying information as defined within Wales, including a unique anonymised patient identifier, age (or date of birth), sex, residential (WIMD) and GP registration information. Primary and secondary care electronic health records (EHRs) securely stored within SAIL were used to capture LTC diagnoses. Demographic data were used from the Welsh Demographic Service Dataset (WDSD) that contains information relating to people who are resident in Wales and registered with a Welsh GP. Data collected by GPs is captured via Read v2 codes (5-digit codes related to diagnosis, medication, and process of care codes). Hospital in- and out- patient data are collected in the Patient Episode Database for Wales, which contains clinical information regarding patients’ hospital admissions, discharges, diagnoses and operations utilising the International Classification of Diseases (ICD-10) clinical coding system. The Annual District Death Extract (ADDE) from the Office for National Statistics (ONS) was used to capture all deaths and dates of death that occurred over the study period for all Welsh residents, contains information regarding the dates and causes of deaths (also ICD-10).
In this study, a LTC is defined as a condition that cannot, at present, be cured but is controlled by medication and/or other treatment/therapies (23). For conditions that do not always fall into the chronic category, we applied duration-based criteria to define them as long-term or chronic (Supplementary Figure S2). For the purpose of this study, multiple long-term conditions (MLTCs) were defined as two or more chronic conditions. We selected 40 LTCs for this study (Supplementary Table S1), based on consensus from a multidisciplinary professional advisory panel. The professional advisory panel (PAP) comprising a team of experts, including General Practitioners, a consultant Psychiatrist, nurses, pharmacists, and data analysts. The full details of condition merging, grouping, and the comprehensive list of Read v2 and ICD-10 codes for each condition can be found in our study protocol (24).
We introduced a novel methodology for identifying and analysing shared disease trajectories in patients with ID. Our approach comprises three main stages: (1) identify pairwise condition associations and their temporal directions; (2) construct shared MLTC trajectories; and (3) utilise a network-based technique to cluster these trajectories into meaningful clusters of similar disease trajectories.
The extracted primary (i.e., GP) and secondary (i.e., hospital) data for all patients were harmonised to a single table, where each row represents a unique patient and each unique variable column represents a binary indicator of the patient’s diagnosis of one of the LTCs. For every patient, we extracted the date of first diagnosis for each LTC, creating a chronological sequence of LTC diagnoses. The analysis was stratified according to sex and age. Age was categorised into two groups, under 45 years old (<45) and 45 years old and above (), taking into account the median observed age per patient’s clinical history. The age stratification threshold of 45 years was selected based on epidemiological literature indicating significant increases in chronic condition prevalence after this age (25, 26), and evidence that individuals with intellectual disabilities experience earlier onset of aging-related health conditions around this age (27).
Thereafter, we derived all possible pairwise combinations of LTCs from our dataset, considering only those where at least 10 patients shared both conditions and had a minimum temporal separation of six months between the diagnoses. The threshold of at least 10 patients sharing both conditions was selected to provide statistical robustness and minimise the risk of identifying incidental associations without clinical meaning, consistent with similar multimorbidity studies (12, 13, 15–17, 20, 28). The minimum six-month interval between diagnoses was established after consultation with our professional advisory panel to capture temporal progression rather than simultaneous diagnoses. The six-month separation criterion was established to distinguish true disease progression from conditions diagnosed during a single clinical evaluation period, allowing us to identify meaningful temporal relationships. Fisher’s exact test was then implemented on contingency tables constructed for each qualifying pair of conditions. The resulting p-values were then corrected using the Bonferroni correction for multiple testing, with a threshold of = 0.001. For all co-morbidity pairs that demonstrated a significant association, we assessed whether a statistically significant temporal order (direction) existed between condition 1 () and condition 2 (). Specifically, a Binomial test was used to evaluate the temporal direction of diagnoses, comparing the number of patients for whom condition follows condition against those where follows ( vs. ). If the p-value < 0.05 from the Binomial test, a preferred direction was assigned to that pair of conditions, based on the more frequently occurring sequence.
Shared disease trajectories were developed by combining significant temporally ordered condition pairs into longer sequences of MLTCs. These pairs were combined to form all possible longer trajectories. For example, if the pairs and were found to be significant, they were combined to form the trajectory . Pairs with statistically significant (preferred) directionality were included (p-values < 0.05 in the Binomial test for directionality), while in the case of no preferred directionality (p-values 0.05), both directions were considered. The actual occurrences of these trajectories in the patient population were then counted. These trajectories could contain other intermediate conditions, as long as the conditions maintained their significant chronological order. Consequently, a list of all identified trajectories was obtained, along with their respective occurrence counts in the patient cohort. These trajectories varied in length, with the longer ones representing sequences of conditions where two or more patients shared the exact same chronological order. All trajectories with a length of three conditions shared in more than ten patients used for clustering. For trajectories containing the same three conditions, only the most frequent unique sequence was retained, ensuring each set of three conditions was represented by its most common temporal order.
We propose a network-based clustering technique that employs a novel approach to quantify and analyse the associations between conditions across multiple disease trajectories. This method consists of four key steps: constructing a trajectory condition network, developing a condition similarity metric based on shortest paths, creating a trajectory similarity matrix, and applying spectral clustering. These steps are described as follows.
Step 1. The first step is about constructing a trajectory condition network. A network of all trajectories was constructed to explore the associations between conditions across multiple trajectories and define a similarity metric among them (Figure 1). Let be an undirected graph (or network graph) where is the set of nodes (conditions) and is the set of edges. Each edge connects two conditions and , where , where is the total number of unique conditions across all trajectories.
Step 2. To quantify similarity between trajectory conditions we developed a condition similarity metric based on the shortest path problem in graph theory. The weight of an edge , where , is defined as shown in Equation 1:
where , , is the frequency of edges between conditions and in the network. This weight function assigns lower weights to more frequent edges, effectively making frequently co-occurring conditions closer in the network graph.Let be a path from to in network graph . The shortest path length is calculated using Equation 2:
where the minimum is taken over all possible paths from to in the graph, and is the weight of the edge between consecutive conditions and in the path. We compute this shortest path using Dijkstra’s algorithm (29). To convert the shortest path lengths into similarity scores ranging from 0 to 1, we take the inverse as shown in Equation 3:
This transformation ensures that conditions with shorter path lengths between them (i.e., more closely related) have higher similarity scores.
Step 3. To assess the overall similarity between trajectories, we constructed an symmetric similarity matrix, where is the total number of trajectories (Figure 1). The similarity between two trajectories, and , is calculated as the mean of the shortest path similarities between their respective condition, as formulated in Equation 4:
where and are the numbers of conditions in trajectories and respectively, where is the total number of trajectories, and is the shortest path similarity between conditions and as defined in Equation 3.
Step 4. To identify clusters of highly similar trajectories based on their condition similarities, we applied spectral clustering to the precomputed trajectory similarity matrix (). We employed spectral clustering for trajectory analysis due to its proven efficacy with complex network structures. Unlike traditional clustering methods that assume spherical or convex cluster shapes, spectral clustering operates by transforming the similarity matrix into a lower-dimensional space using eigenvectors of the Laplacian matrix, making it particularly suitable for our disease trajectory network where relationships are defined by shortest-path distances. This graph-theoretic approach (30) enables detection of meaningful patterns in multimorbidity progression, even when trajectories exhibit nonlinear or overlapping relationships. We implemented spectral clustering using the Scikit-Learn library with default parameter settings (31). The algorithm treats the trajectory similarity matrix as a weighted graph adjacency matrix, performs a spectral embedding of the data points into a lower-dimensional space, and then clusters the embedded points using the k-means algorithm. The optimal number of clusters was determined using the Calinski-Harabasz score.
Table 1 summarises the counts and percentages of patient demographic characteristics included in the study and reports the mean number of LTCs, prevalence of MLTCs (%), and prevalence of physical-mental MLTCs (%) stratified by sex, age group, ethnic group, and Welsh Index of Multiple Deprivation (WIMD) quintiles. The study population comprised 13,069 patients, with 52.3% being male. The mean number of LTCs for all patients was 4.5 (3), with 85.9% having MLTCs and 31.8% having physical-mental MLTCs. Subgroup analysis revealed notable trends across demographic categories. Females showed a slightly higher mean number of LTCs (4.9 3.3) and prevalence of MLTCs (87.7%) compared to males (4.2 2.8 and 84.3% respectively) (Tables 1, Supplementary Table S2). Patients aged 45 years and older had a higher mean number of LTCs (5.2 3.2) and prevalence of MLTCs (91%) compared to those under 45 (3.5 2.4 and 79%). Socioeconomic factors, indicated patients from the most deprived areas had a higher mean number of LTCs (4.9 3.2) and prevalence of MLTCs (88.9%) compared to those from the least deprived areas (4.5 2.9 and 85.6%).
Supplementary Table S3 shows the total number of patients after stratification. A comparison of the prevalence of several conditions among males and females, as well as between age groups, can be found in Supplementary Tables S4, S5. The statistically significant condition pairs are provided in Table 2.
Mental illness was the most prevalent condition affecting 32.8% of all males (Supplementary Table S4). A key finding was that its prevalence was higher in those under 45 years (35%) compared to those 45 and above (31%). Epilepsy followed closely, present in 31% of all males, again with a higher prevalence in the younger group (35.3% vs. 27.7%). Reflux disorders showed a consistent prevalence of 29.7% across both age groups. Hypertension demonstrated a marked increase with age, affecting 15.5% of males under 45 and 29.7% of those 45 and above. Additionally, chronic kidney disease (CKD) showed a significant increase from 10.4% in the younger group to 26.9% in the older group. Similarly, diabetes increased from 13.8% in the younger group to 25.3% in the older group.
The most frequent condition pair association in males under 45 (encountered in 385 patients) was found to be between mental illness and reflux disorders (Table 2). Other significant co-morbidities include pairs of different neuropsychiatric conditions (e.g., epilepsy mental illness in 287 patients, mental illness insomnia in 267 patients, and cerebral palsy epilepsy in 205 patients), combinations of neuropsychiatric and haematological conditions (e.g., mental illness anaemia, 267 patients) or associations between endocrine and circulatory with renal conditions (e.g., diabetes CKD and hypertension CKD in 82 patients). In males aged 45 and above, hypertension CKD emerged as the most prevalent pair, affecting 447 patients, followed by mental illness reflux disorders (422 patients) and hypertension diabetes (411 patients).
Mental illness was also the most common condition among females, affecting 35.1% overall (Supplementary Table S5). Thyroid conditions were prevalent in 30.8% of all females, with 33.3% in the 45 and above age group and 27.1% in those under 45. Reflux disorders affected 30.1% of all females, with 30.9% in the under 45 group and 29.5% in those 45 and above. Hypertension showed a significant age-related increase, from 11.9% in those under 45 to 31.9% in those 45 and above. As expected, menopausal and perimenopausal conditions were more prevalent in the older female group, affecting 25.9% of those 45 and above compared to 8.8% in those under 45.
The most frequent condition pair in females under 45 was mental illness reflux disorders, shared by 375 patients (Table 2). Chronic airway conditions featured prominently in this group, with chronic airway diseases mental illness and chronic airway diseases reflux disorders affecting 357 and 293 patients, respectively. In the older age groups, we noted significant associations between circulatory and musculoskeletal conditions (e.g., hypertension chronic arthritis, 484 patients), followed by mental illness reflux disorders (473 patients) and mental illness chronic arthritis (470 patients).
In general, the younger age groups, particularly those under 45, showed a higher prevalence of co-morbidities involving neuropsychiatric conditions, often in combination with other system conditions (Table 2). This was evident in both males and females, with mental illness frequently co-occurring with conditions from various other physiological systems. While mental illness and epilepsy were among the top five most prevalent conditions in both sexes, thyroid disorders ranked second in females but did not appear in the top five for males (Supplementary Tables S4, S5). Hypertension was among the top five conditions in males across all ages, but in females, it only ranked in the top five for the 45 and above age group.
In the male sub-population, one cluster was identified for males under 45 years (<45) and three clusters for males 45 years and older (45) (Supplementary Table S6). Thirty-seven shared trajectories were considered for clustering in males <45 years, and 229 shared trajectories in males 45 years (Supplementary Table S7). Only trajectories with a minimum of ten patients were included. Table 3 presents an overview of the clusters identified within the male population.
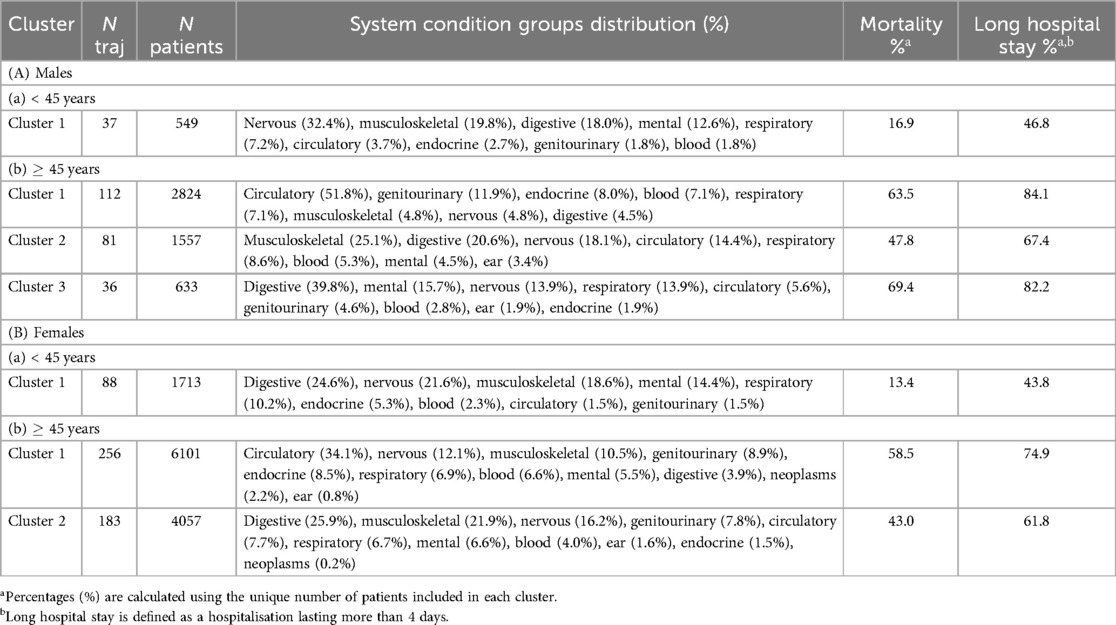
Table 3. Long-term condition clusters for (A) males and (B) females categorised by age groups. Each cluster presents the most frequent system condition categories included in the trajectories alongside the count of trajectories (N traj) and total patient numbers (N patients). System percentages (%) are calculated based on the total number of trajectories in each cluster. Clusters are presented in descending order of patient count within each age category.
The analysis of shared trajectories in males revealed distinct patterns across different age groups (Figure 2). For males under 45 years, neurological conditions were the most common affecting 32.4% of this subpopulation. This was followed by conditions of the musculoskeletal (19.8%) and digestive system (18.0%). Mental health conditions were also notable, present in 12.6% of cases. Looking at more specific conditions of this younger cohort revealed an equal prevalence (37.8%) of chronic pain conditions, mental illness, and insomnia. Other significant conditions included reflux disorders (35.1%), epilepsy (27.0%), and chronic airway diseases (21.6%) (Supplementary Tables S8, S10).
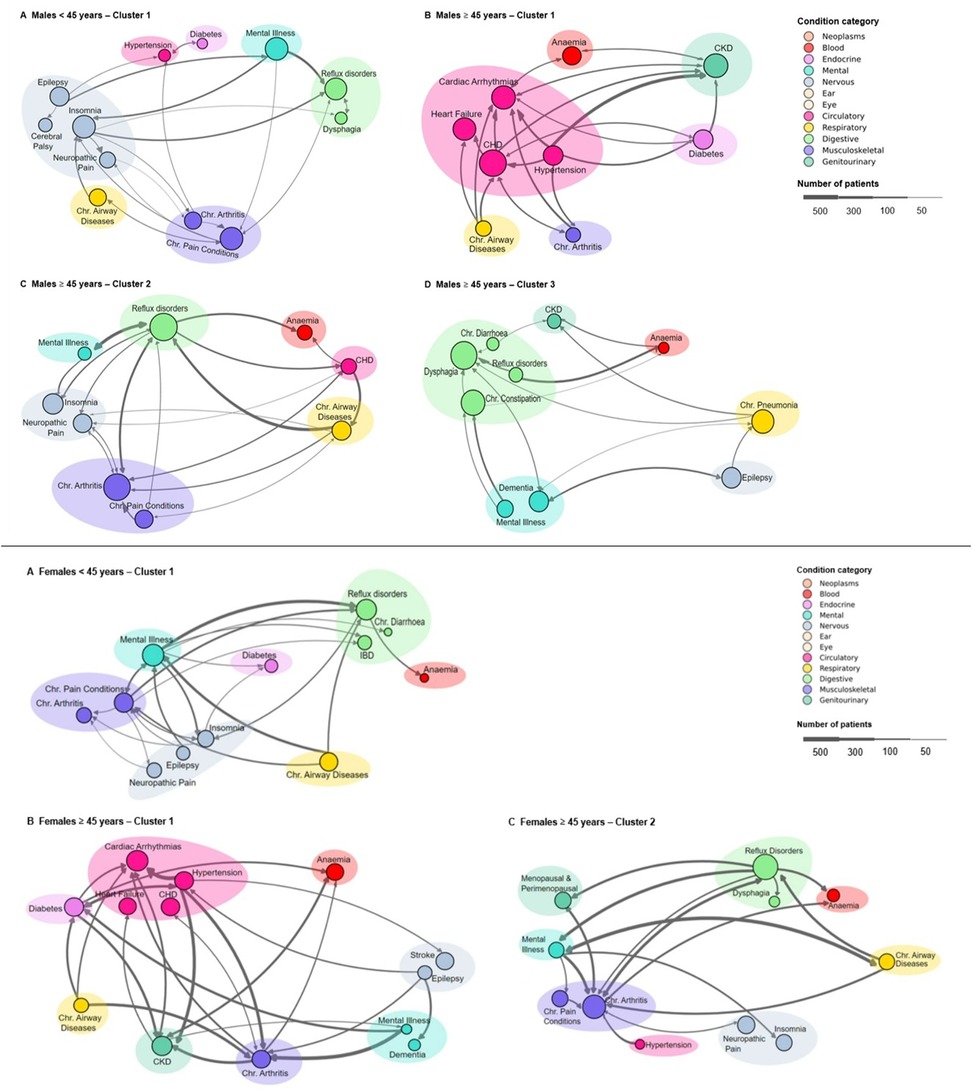
Figure 2. Disease trajectory clusters across age groups and genders. Top: Males – (A) <45 years cluster 1, (B) 45 years cluster 1, (C) 45 years cluster 2, and (D) 45 years cluster 3. Bottom: Females – (A) <45 years single cluster; (B) 45 years cluster 1, and (C) 45 years cluster 2. Node size indicates condition prevalence, with larger nodes representing more frequent occurrences. Conditions in each cluster represent more than 5% of the total conditions. Edges show associations between conditions, with edge thickness corresponding to the frequency of condition pair occurrences (minimum edge frequency of 1%). Coloured shaded areas group related conditions within the same category as defined in the legend. The legend specifies condition categories and provides a scale for patient numbers.
The analysis of health trajectories in males aged 45 years and older revealed three distinct clusters, each characterised by unique patterns of multimorbidity. The largest cluster, encompassing 112 shared trajectories and 2,824 patients, was predominantly defined by circulatory system conditions (51.8%) (Table 3). Coronary heart disease (CHD) emerged as the most prevalent condition, affecting 44.6% of patients, closely followed by CKD (35.7%) and cardiac arrhythmias (34.8%) (Supplementary Tables S8, S11). Heart failure was also significantly present, occurring in 32.1% of cases. This cluster highlighted a strong interplay between cardiovascular and renal health, further complicated by metabolic conditions such as diabetes and hypertension, each present in 24.1% of patients. The presence of peripheral vascular disease (19.6%) and chronic airway diseases (15.2%) in this cluster underscores the complex associations between cardiovascular, respiratory, and metabolic systems in ageing men.
The second cluster, comprising 81 trajectories and 1,557 patients, was dominated by musculoskeletal (25.1%) and digestive system (20.6%) conditions. Reflux disorders were most prevalent, affecting 53.1% of patients, closely followed by chronic arthritis at 50.6% (Supplementary Table S8). This cluster was prominently associated with the high incidence of chronic pain conditions (23.5%) and neuropathic pain (24.7%), indicating that chronic pain significantly affects many older men. Cardiovascular conditions remained relevant, with CHD affecting 17.3% of patients. The presence of mental illness (12.3%) and hearing loss (8.6%) in this cluster points to the diverse health challenges faced by this subgroup.
The third cluster, though smaller with 36 trajectories and 633 patients was characterised by a high prevalence of digestive (39.8%) and neurological (13.9%) conditions. Dysphagia was the most common condition, present in 50.0% of patients, followed closely by chronic constipation at 41.7% (Supplementary Table S8). Neurological conditions were prominent, with both epilepsy and dementia affecting 27.8% of patients each. The significant presence of chronic pneumonia (36.1%) in this cluster highlights the persistent relevance of respiratory conditions in older males, while the occurrence of CKD (13.9%) further emphasises the complex interplay of multiple organ systems in ageing.
In the female sub-population, three clusters were obtained, one for females under 45 years (<45) and two for females 45 years and older (45) (Supplementary Table S6). Eighty-eight shared trajectories were considered for clustering in females <45 years, and 439 shared trajectories in females 45 years (Supplementary Table S7). Table 3, Figure 2 illustrates the distinct patterns of health trajectory clusters identified within the female population across different age groups.
The analysis of shared trajectories in females revealed distinct patterns across different age groups. For females under 45 years, digestive system conditions emerged as the predominant health concern, affecting 24.6% of this subpopulation. This was closely followed by neurological conditions (21.6%) and musculoskeletal conditions(18.6%). Mental health conditions were also prominent, present in 14.4% of cases. Looking at more specific conditions of this younger cohort revealed a high prevalence of mental illness (43.2%), followed by reflux disorders (38.6%), and chronic pain conditions (34.1%). Other significant conditions included chronic airway diseases (30.7%), insomnia (25.0%), and chronic arthritis (21.6%) (Supplementary Tables S9, S12).
The analysis of health trajectories in females aged 45 years and older revealed two distinct clusters. The larger of these clusters, encompassing 256 shared trajectories and 6,101 patients, was predominantly defined by circulatory system conditions (34.1%) (Table 3). Cardiac arrhythmias emerged as the most prevalent condition, affecting 30.5% of patients, closely followed by CKD (26.6%) and diabetes (24.2%) (Supplementary Table S9). Hypertension was also significantly present, occurring in 23.4% of cases. This cluster highlighted a strong interplay between cardiovascular and metabolic health, further complicated by musculoskeletal conditions such as chronic arthritis, present in 22.7% of patients. The presence of CHD (22.3%) and chronic airway diseases (14.5%) in this group underscores the complex associations between cardiovascular, respiratory, and metabolic systems in ageing women.
A distinct health profile emerged in the other cluster, comprising 183 trajectories and 4,057 patients, dominated by digestive (25.9%) and musculoskeletal (21.9%) conditions. Reflux disorders were most prevalent, affecting 48.1% of patients, closely followed by chronic arthritis at 41.5% (Supplementary Tables S9, S13). This group was significant for its high incidence of neuropathic pain (23.5%) and chronic pain conditions (20.2%), indicating that chronic pain significantly affects many older women. Menopausal and perimenopausal conditions were also prominent, affecting 20.8% of patients.
Figure 3, Supplementary Table S14 present the mortality and long hospital stay ( 4 days) rates observed across the identified clusters, stratified by sex and age groups. In the younger age group (<45 years), both males and females exhibited single clusters with similar mean ages (35.6 5.8 and 35.9 6.1 years, respectively). Mortality percentages for clusters <45 years were comparably low, with males at 16.9% and females at 13.4%. Long hospital stays were less common in this age group, with rates of 46.8% for males and 43.8% for females, significantly lower than in 45 year populations.
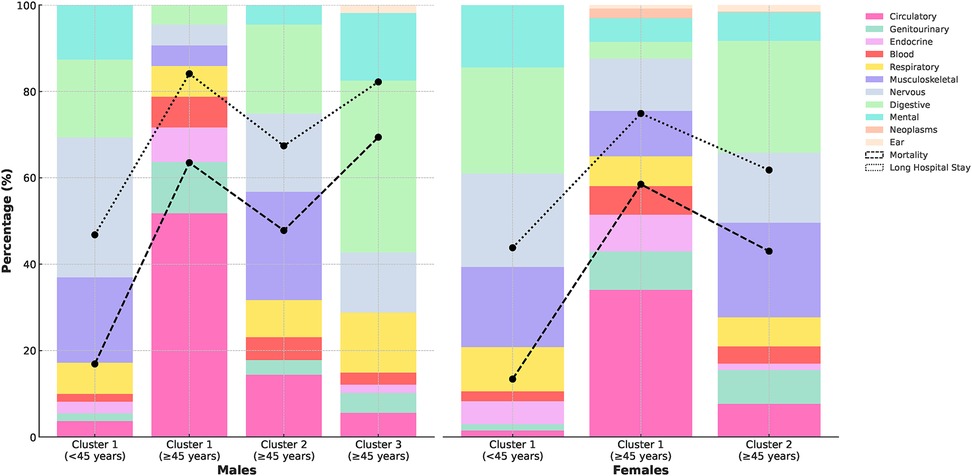
Figure 3. Distribution of long-term conditions and rates of mortality and long hospital stays across patient clusters.
For the age group 45 years, we observed more distinct patterns in mortality and long hospital stays. In males, clusters 1 and 3 showed particularly high mortality percentages (63.5% and 69.4%). These clusters also had the highest rates of long hospital stays (84.1% and 82.2%, respectively). Cluster 1 was predominantly characterised by circulatory system conditions (51.8%), while cluster 3 was notable for digestive system conditions (39.8%), suggesting a strong association between these condition categories and both increased mortality risk and extended hospitalisations. Similarly, in females 45 years, we observed a similar pattern, with the larger cluster (cluster 1) showing a higher mortality rate (3.5 per 100 patient-years) and percentage (58.5%) compared to cluster 2 (2.4 per 100 patient-years and 43.0%). Cluster 1 also had a higher rate of long hospital stays (74.9% vs 61.8% in cluster 2) and was primarily affected by circulatory system conditions (34.1%).
Figure 4 presents the distribution of the five leading causes of death among individuals with ID in our study population, which we can compare to the findings from the most recent LeDeR (learning from lives and deaths-people with a learning disability and autistic people) annual report (32). Across all groups, circulatory system conditions consistently appear as a major cause of death, particularly prominent in the older age groups (45 years). Notably, in the <45 years age groups, neoplasms and respiratory system conditions feature more prominently as causes of death compared to the older groups. In the older age groups, we observe some variations between clusters, with cluster 1 for both males and females showing a higher proportion of deaths due to circulatory system conditions compared to other clusters. This aligns with our earlier observation of cluster 1 being characterised by a higher prevalence of circulatory system conditions. These findings underscore the complex interplay between age, sex, and specific health conditions in determining mortality patterns among individuals with ID.
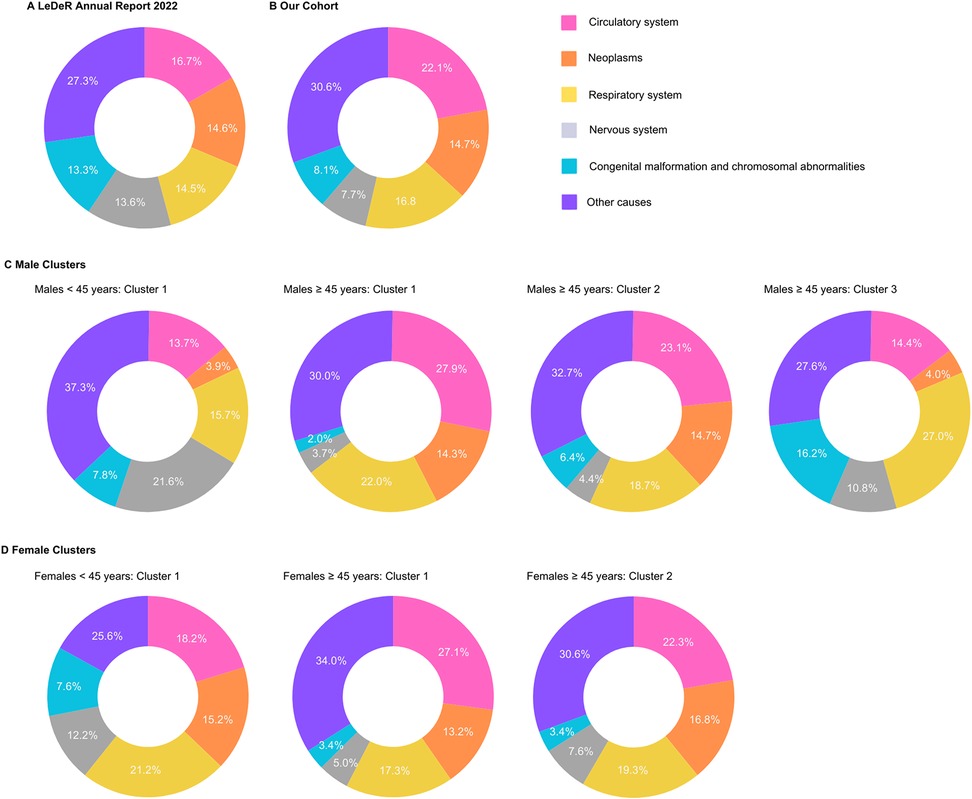
Figure 4. Comparison of the five leading causes of death among individuals with intellectual disabilities (ID), grouped based on ICD-10 chapters. Data presented includes findings from the (A) LeDeR Annual Report 2022 (32), (B) our cohort study using the SAIL (Secure Anonymised Information Linkage) databank and stratification by clusters based on sex and age groups for (C) male cluster (<45 years: cluster 1 and 45 years: clusters 1, 2, 3) and (D) females (<45 years: cluster 1; 45 years: clusters 1, 2). Values represent percentages for each cause of death.
This study presents a novel approach for the identification and temporal analysis of LTC trajectories in adults with ID using both primary and secondary care data. By employing an unsupervised clustering method on trajectories of a large dataset of electronic health records, we have uncovered distinct patterns of co-morbidity and disease trajectories that vary significantly across sex and age groups. Our findings reveal a high prevalence of MLTCs in the ID population, with 85.9% of individuals experiencing two or more long-term conditions. This aligns with previous research highlighting the complex health needs of this population (1, 33). On average this group has 4.5 long-term conditions per patient.
Our analysis demonstrated significant sex differences in the prevalence of LTCs among adults with ID, while also identifying common patterns. Mental illness and epilepsy were the most prevalent conditions across both sexes, with mental illness affecting more than 30% and epilepsy affecting approximately 30% of both males and females. This prevalence aligns with previous findings in the literature (5). However, clear sex differences were apparent in other conditions. Females exhibited a higher prevalence of thyroid disorders (30.8%) compared to males (14.1%), and anaemia affected 20% of females compared to 14% of males. Females also showed increased prevalence of endocrine, skeletal, and digestive conditions, a finding consistent with previous research (34). Our investigation of temporal co-morbidities revealed that some conditions displayed opposite temporal associations in males and females for certain pairs of conditions. For instance, we observed that in females under 45 years, epilepsy tended to precede thyroid disorders, while this association was not significant in males of any age group. This finding highlights the importance of investigating sex-stratified disease trajectories, as the temporal direction between conditions can vary depending on sex (28).
Age-related differences were apparent in condition patterns between <45 years and 45 years adults with ID. Under 45 groups showed a predominance of neurological (such as epilepsy and dementia), digestive (including dysphagia and chronic constipation), and mental health conditions, consistent with previous research in the ID population (35, 36). In males under 45 years, neurological conditions accounted for 32.4% of the identified cluster, while digestive system conditions were most prevalent (24.6%) in females of the same age group.
In the over 45 groups, circulatory system conditions emerged as a primary concern for both sexes with ID. We found a higher prevalence of CHD, cardiac arrhythmias, heart failure, hypertension, kidney disease, and diabetes in older adults with ID. The co-occurrence of these conditions is particularly noteworthy, as it mirrors the complex interplay of cardiovascular and renal health observed in the general population (37). Furthermore, the strong association between hypertension and various other cardiovascular complications, including heart failure, arrhythmias, and CHD, aligns with patterns seen in the broader population (38).
Across all age groups and clusters, males generally exhibited slightly higher mortality rates and percentages compared to females—a pattern observed in the general population but appears to be more pronounced in individuals with ID (39). Our analysis revealed a strong association between circulatory system conditions and both increased mortality and extended hospitalisations, particularly in older adults, highlighting these conditions as the most prevalent cause of death in the identified clusters. While mental health and neurological conditions are prevalent across all groups, their impact on mortality appears less direct compared to circulatory and certain digestive system conditions. However, mental conditions significantly contribute to extended hospital care needs and overall quality of life, especially in younger adults with ID (40). We observed a clear association between higher rates of long hospital stays and increased mortality in specific clusters of the older population. For instance, in males 45 years, cluster 1 exhibited both the highest rate of long stays (84.1%) and a high mortality rate (3.9 per 100 patient-years). Lastly, our study underscores the value of temporality in predicting both co-morbidity and mortality (41).
The reliance on clinical records may result in underdiagnosis of certain conditions, particularly those with subtle presentations in the ID population. Moreover, our analysis was constrained by limited ethnic diversity in the dataset. Additionally, the lack of a general population comparison group limits our ability to determine whether observed multimorbidity patterns are unique to individuals with intellectual disabilities or reflect common disease associations. While our clusters demonstrate statistically significant disease groupings and have been reviewed by healthcare professionals, further validation against external clinical benchmarks would strengthen their clinical utility. Future work could involve clinical validation studies to examine how these clusters relate to treatment responses and patient outcomes, potentially transforming these statistical findings into actionable clinical insights.
In conclusion, this study presents a data-driven overview of MLTC trajectory patterns in adults with ID. By revealing distinct clusters of conditions and their progression over time, our findings underscore the complex interplay of age, sex, and health conditions in adults with ID and provide a foundation for more targeted, personalised healthcare strategies. Our work advances understanding of how MLTCs manifest and progress in people with ID, revealing distinct patterns of disease development and complex interactions between conditions in this population. Future research aims to validate these clusters in diverse populations and investigate the underlying mechanisms of the observed progression patterns.
The datasets presented in this article are not readily available as all proposals to use SAIL data are subject to review by the independent IGRP. The anonymised individual-level data sources used in this study are available in the SAIL Databank at Swansea University, Swansea, UK, Before any data can be accessed, approval must be given by the IGRP. The IGRP gives careful consideration to each project to ensure proper and appropriate use of SAIL data. When access has been granted, it is gained through a privacy-protecting safe haven and remote access system referred to as the SAIL Gateway. SAIL has established an application process to be followed by anyone who would like to access data via SAIL at: https://www.saildatabank.com/application-process.
The studies involving humans were approved by the SAIL Databank’s independent Information Governance Review Panel (IGRP Project: 1375), ensuring compliance with ethical standards and data protection regulations. Individual written patient consent was not required for this study. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Individual written patient consent was not required for this study.
RK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. GC: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. EA: Data curation, Writing – review & editing. AA: Data curation, Funding acquisition, Resources, Writing – review & editing. FZ: Data curation, Funding acquisition, Writing – review & editing. GTJ: Funding acquisition, Writing – review & editing. RK: Writing – review & editing. SG: Data curation, Funding acquisition, Writing – review & editing, Resources.
The author(s) declare that financial support was received for the research and/or publication of this article. The work was funded by National Institute for Health and Care Research. The project is entitled “DECODE: Data-driven machinE-learning aided stratification and management of multiple long-term COnditions in adults with intellectual disabilitiEs.” Grant no. NIHR203981.
We extend our deepest gratitude to our Public and Patient Involvement and Engagement (PPIE) partners including people with intellectual disabilities, family and paid carers. Their invaluable contributions, rooted in their lived personal experience of intellectual disabilities, were instrumental in the research. Without their expertise, insight, and collaborative spirit, this work would not have been possible. We also want to thank Dr. Alison Drewett for her insights regarding the selection of colours for the visualisations. This decision-making supports wider aims pertaining to accessibility for people with intellectual disabilities.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdgth.2025.1528882/full#supplementary-material
1. Cooper SA, Allan L, Greenlaw N, McSkimming P, Jasilek A, Henderson A, et al. Rates, causes, place and predictors of mortality in adults with intellectual disabilities with and without down syndrome: cohort study with record linkage. BMJ Open. (2020) 10:e036465. doi: 10.1136/bmjopen-2019-036465
2. Kinnear D, Morrison J, Allan L, Henderson A, Smiley E, Cooper SA. Prevalence of physical conditions and multimorbidity in a cohort of adults with intellectual disabilities with and without down syndrome: cross-sectional study. BMJ Open. (2018) 8:e018292. doi: 10.1136/bmjopen-2017-018292
3. Emerson E, Hatton C. Health Inequalities and People with Intellectual Disabilities. Cambridge: Cambridge University Press (2014).
4. Heslop P, Hoghton M. The learning disabilities mortality review (leder) programme. Br J Gen Pract. (2018) 68:bjgp18X697313. doi: 10.3399/bjgp18X697313
5. Carey IM, Shah SM, Hosking FJ, DeWilde S, Harris T, Beighton C, et al. Health characteristics and consultation patterns of people with intellectual disability: a cross-sectional database study in english general practice. Br J Gen Pract. (2016) 66:e264–70. doi: 10.3399/bjgp16X684301
6. Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. New Engl J Med. (2010) 363:501–4. doi: 10.1056/NEJMp1006114
7. Solares JRA, Raimondi FED, Zhu Y, Rahimian F, Canoy D, Tran J, et al. Deep learning for electronic health records: a comparative review of multiple deep neural architectures. J Biomed Inform. (2020) 101:103337. doi: 10.1016/j.jbi.2019.103337
8. Pham T, Tran T, Phung D, Venkatesh S. Deepcare: a deep dynamic memory model for predictive medicine. In: Advances in Knowledge Discovery and Data Mining: 20th Pacific-Asia Conference, PAKDD 2016, Auckland, New Zealand, April 19–22, 2016, Proceedings, Part II 20. Springer (2016). p. 30–41.
9. Johnston MC, Crilly M, Black C, Prescott GJ, Mercer SW. Defining and measuring multimorbidity: a systematic review of systematic reviews. Eur J Public Health. (2019) 29:182–9. doi: 10.1093/eurpub/cky098
10. Chmiel A, Klimek P, Thurner S. Spreading of diseases through comorbidity networks across life and gender. New J Phys. (2014) 16:115013. doi: 10.1088/1367-2630/16/11/115013
11. Teno JM, Weitzen S, Fennell ML, Mor V. Dying trajectory in the last year of life: does cancer trajectory fit other diseases? J Palliat Med. (2001) 4:457–64. doi: 10.1089/109662101753381593
12. Jensen AB, Moseley PL, Oprea TI, Ellesøe SG, Eriksson R, Schmock H, et al. Temporal disease trajectories condensed from population-wide registry data covering 6.2 million patients. Nat Commun. (2014) 5:4022. doi: 10.1038/ncomms5022
13. Giannoula A, Gutierrez-Sacristán A, Furlong LI. Identifying temporal patterns in patient disease trajectories using dynamic time warping: a population-based study. Sci Rep. (2018) 8:4216. doi: 10.1038/s41598-018-22578-1
14. Lyons J, Akbari A, Abrams KR, Lorenzo AA, Dhafari TB, Chess J, et al. Trajectories in chronic disease accrual and mortality across the lifespan in Wales, UK (2005–2019), by area deprivation profile: linked electronic health records cohort study on 965,905 individuals. Lancet Reg Health Eur. (2023) 32:100687. doi: 10.1016/j.lanepe.2023.100687
15. Siggaard T, Reguant R, Jørgensen IF, Haue AD, Lademann M, Aguayo-Orozco A, et al. Disease trajectory browser for exploring temporal, population-wide disease progression patterns in 7.2 million danish patients. Nat Commun. (2020) 11:4952. doi: 10.1038/s41467-020-18682-4
16. Jørgensen IF, Brunak S. Time-ordered comorbidity correlations identify patients at risk of mis- and overdiagnosis. NPJ Digit Med. (2021) 4:12. doi: 10.1038/s41746-021-00382-y
17. Hu JX, Helleberg M, Jensen AB, Brunak S, Lundgren J. A large-cohort, longitudinal study determines precancer disease routes across different cancer types. Cancer Res. (2019) 79:864–72. doi: 10.1158/0008-5472.CAN-18-1677
18. Han X, Hou C, Yang H, Chen W, Ying Z, Hu Y, et al. Disease trajectories and mortality among individuals diagnosed with depression: a community-based cohort study in UK Biobank. Mol Psychiatry. (2021) 26:6736–46. doi: 10.1038/s41380-021-01170-6
19. Paik H, Kim J. Condensed trajectory of the temporal correlation of diseases and mortality extracted from over 300,000 patients in hospitals. PLoS One. (2021) 16:e0257894. doi: 10.1371/journal.pone.0257894
20. Giannoula A, Centeno E, Mayer MA, Sanz F, Furlong LI. A system-level analysis of patient disease trajectories based on clinical, phenotypic and molecular similarities. Bioinformatics. (2021) 37:1435–43. doi: 10.1093/bioinformatics/btaa964
21. Planell-Morell P, Bajekal M, Denaxas S, Raine R, Alexander DC. Trajectories of disease accumulation using electronic health records. Stud Health Technol Inform. (2020) 270:469–73. doi: 10.3233/SHTI200204
22. Lyons RA, Jones KH, John G, Brooks CJ, Verplancke JP, Ford DV, et al. The sail databank: linking multiple health and social care datasets. BMC Med Inform Decis Mak. (2009) 9:1–8. doi: 10.1186/1472-6947-9-3
23. Department of Health and Social Care. Long term conditions compendium of information. Report. NHS (2012).
24. Shabnam S, Kousovista R, Abakasanga E, Kaur N, Cosma G, Akbari A, et al. Data from: Data preparation and epidemiological plan—decode (2024). doi: 10.17605/OSF.IO/KT5FY
25. Zheng DD, McCollister KE, Christ SL, Lam BL, Feaster DJ, Lee DJ. Chronic condition patterns in the US population and their association with health related quality of life. Prev Med. (2020) 136:106102. doi: 10.1016/j.ypmed.2020.106102
26. Buttorff C, Ruder T, Bauman M. Data from: Multiple chronic conditions in the United States (2017). Vol. 10. Santa Monica (CA): RAND.
27. García-Domínguez L, Navas P. Chronic health conditions in aging individuals with intellectual disabilities. Int J Environ Res Public Health. (2020) 17:3126. doi: 10.3390/ijerph17093126
28. Westergaard D, Moseley P, Sørup FKH, Baldi P, Brunak S. Population-wide analysis of differences in disease progression patterns in men and women. Nat Commun. (2019) 10:666. doi: 10.1038/s41467-019-08475-9
29. Dijkstra EW. A note on two problems in connexion with graphs. In: Hamilton K, Liskov B, Myers BA, editors. Edsger Wybe Dijkstra: His Life, Work, and Legacy. New York, NY: Association for Computing Machinery (2022). p. 287–90.
30. Ding L, Li C, Jin D, Ding S. Survey of spectral clustering based on graph theory. Pattern Recognit. (2024) 147:110366. doi: 10.1016/j.patcog.2024.110366
31. Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al. Scikit-learn: machine learning in python. J Mach Learn Res. (2011) 12:2825–30. doi: 10.48550/arXiv.1201.0490
32. White A, Sheehan R, Ding J, Roberts C, Magill N, Keagan-Bull R, et al. Learning from Lives and Deaths-People with a Learning Disability and Autistic People (Leder) Report for 2022. London: King’s College London (2023).
33. Mann C, Jun GT, Tyrer F, Kiani R, Lewin G, Gangadharan SK. A scoping review of clusters of multiple long-term conditions in people with intellectual disabilities and factors impacting on outcomes for this patient group. J Intellect Disabil. (2023) 27:1045–61. doi: 10.1177/17446295221107275
34. Yang H, Pawitan Y, Fang F, Czene K, Ye W. Biomarkers and disease trajectories influencing women’s health: results from the UK Biobank cohort. Phenomics. (2022) 2:184–93. doi: 10.1007/s43657-022-00054-1
35. Tyrer F, Dunkley A, Singh J, Kristunas C, Khunti K, Bhaumik S, et al. Multimorbidity and lifestyle factors among adults with intellectual disabilities: a cross-sectional analysis of a UK cohort. J Intellect Disabil Res. (2019) 63:255–65. doi: 10.1111/jir.12571
36. Van Timmeren E, Waninge A, Van Schrojenstein Lantman-de H, Van der Putten A, Van der Schans C. Patterns of multimorbidity in people with severe or profound intellectual and motor disabilities. Res Dev Disabil. (2017) 67:28–33. doi: 10.1016/j.ridd.2017.05.002
37. De Bhailis ÁM, Kalra PA. Hypertension and the kidneys. Br J Hosp Med. (2022) 83:1–11. doi: 10.12968/hmed.2021.0440
38. Masenga SK, Kirabo A. Hypertensive heart disease: risk factors, complications and mechanisms. Front Cardiovasc Med. (2023) 10:1205475. doi: 10.3389/fcvm.2023.1205475
39. Tyrer F, Morriss R, Kiani R, Gangadharan SK, Kundaje H, Rutherford MJ. Health needs and their relationship with life expectancy in people with and without intellectual disabilities in England. Int J Environ Res Public Health. (2022) 19:6602. doi: 10.3390/ijerph19116602
40. Siddiqui N, Dwyer M, Stankovich J, Peterson G, Greenfield D, Si L, et al. Hospital length of stay variation and comorbidity of mental illness: a retrospective study of five common chronic medical conditions. BMC Health Serv Res. (2018) 18:1–10. doi: 10.1186/s12913-018-3316-2
Keywords: disease trajectories, chronic disease, co-morbidity, clustering, intellectual disability, multimorbidity
Citation: Kousovista R, Cosma G, Abakasanga E, Akbari A, Zaccardi F, Jun GT, Kiani R and Gangadharan S (2025) Temporal patterns of multiple long-term conditions in individuals with intellectual disability living in Wales: an unsupervised clustering approach to disease trajectories. Front. Digit. Health 7:1528882. doi: 10.3389/fdgth.2025.1528882
Received: 15 November 2024; Accepted: 12 March 2025;
Published: 27 March 2025.
Edited by:
Elena Casiraghi, Università Degli Studi di Milano, ItalyReviewed by:
Luca Lalli, National Cancer Institute Foundation (IRCCS), ItalyCopyright: © 2025 Kousovista, Cosma, Abakasanga, Akbari, Zaccardi, Jun, Kiani and Gangadharan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Georgina Cosma, Zy5jb3NtYUBsYm9yby5hYy51aw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.