- 1Department of Neurology, Veterans Affairs Puget Sound Health Care System, Seattle, WA, United States
- 2Department of Neurology, University of Washington, Seattle, WA, United States
Background: Interest in wearable device use in Parkinson's disease (PD) has grown rapidly with many compelling studies supporting diagnostic and therapeutic uses. Concurrently, consumer devices have proliferated and their role in health and wellness has expanded. However, incorporation of consumer and medical wearable devices into medical care has in our experience been limited.
Objective: We sought to assess the current state of consumer and medical wearable device use among those with PD and to understand the factors impacting their rate of use.
Methods: An anonymous online survey of individuals with PD in the US was conducted from July 9th, 2023, to Jan 8th, 2024, with 298 completed responses collected.
Results: Greater than 90% of respondents were interested in new technologies with 67% having had experiences with consumer wearable devices. Only 24% were using consumer devices for disease management and many functions were not fully utilized. Medical wearable device use was very limited with only 8% having used a device. Users of both consumer and medical wearables generally reported low barriers to use despite continued strong perceptions on the importance of cost, impact on care, comfort, and other factors.
Conclusion: This study demonstrates that for the clinical management of PD there is limited use of wearable devices even among individuals who are motivated and experienced with consumer wearable device use. Additionally, it is suggested that substantial barriers to medical wearable use are likely originating from the provider and/or systemic level.
Introduction
Parkinson's disease (PD) is a neurodegenerative condition which results in abnormal movements, cognitive changes, and autonomic dysfunction. Diagnosis and management are made challenging due to inherent fluctuations in the severity of disease manifestations as well as dynamic changes that are induced by treatment. Standard clinical practice can only capture brief snapshots of the patient's experience and relies heavily on subjective report and quasi-objective exams, thus ability to optimally intervene is limited (1–4). Recently, there has been considerable interest in addressing these challenges through the creation of objective and continuous measures which hope to allow for better understanding of each person's unique disease state and thereby improve treatment and reduce disability (5).
Wearable devices have been of great interest in healthcare due to their ability to contain imbedded sensors that help track various physiologic signals. Research in wearables in Parkinson's disease has been focused on the evaluation of motor features, and various devices have data supporting their ability to detect sub-clinical motor features, disease state fluctuations, disease progression, and to assist with therapeutic adjustments (6–9). However, various physiologic signals such as sleep (10), heart rate variability (11), cerebral oxygenation (12), and many others have been studied in PD with wearables. Studies in this area have rapidly increased over time with PubMed entries for “wearable” AND “Parkinson's Disease” going from a mere seven in 2012 to 170 in 2022. To help researchers and clinicians understand this landscape, many excellent reviews are available (13–18).
However, collection of valid data alone is not sufficient to change practice. The patient's perspective on device usability and utility is critical, and this has not been forgotten. Many studies of specific devices have included patient perspectives on features of interest, usability, and barriers to use (19–21). Additionally, more conceptually focused studies using surveys and focused groups have identified key features of interest such as wearability, ability to provide feedback, and clinical accuracy (22, 23). All this research has ultimately culminated in several medical wearable devices that are validated, designed with the patient in mind, and cleared for clinical use.
Concurrent with this explosion of research and approval of medical wearable use, there has been substantial adoption of consumer wearable devices for health tracking and lifestyle management. Devices such as the Apple Watch and Fitbit were in 2020 estimated to be used by around 25%–30% of the US population for health monitoring (24). These consumer devices also appear to have merits in PD as they offer the ability to potentially improve medication adherence, encourage and track physical activity, document symptoms, monitor sleep, and collect various other forms of information.
However, despite the extensive research on validation, the understanding of the factors important to patient users available to device manufacturers, the widespread use of consumer wearable devices, and the availability of approved medical devices for clinical use, real-world clinical data appears to be scant. In our clinical experience and after discussion with colleagues, few individuals are using these devices. While issues with the payor model for device use, lack of clinical impact, poor tolerability for patient and clinician users, and general disinterest in new technologies are commonly mentioned as factors playing a role, the evaluation of these barriers has not been extensively evaluated in routine clinical care.
We therefore sought to conduct a comprehensive evaluation of the current usage of wearable devices in PD, and to go beyond the controlled research setting to understand the real-world usage of wearable devices both consumer and medical. Additionally, we wanted to understand what factors were currently playing a role in current device usage and whether these were the same as those reported previously.
Methods
An anonymous online survey was conducted from July 9th, 2023, to Jan 8th, 2024. Respondents were self-identified individuals with PD and were requested to be at least 18 years of age.
Wearables were defined in this survey as any technological accessory which is affixed to the surface of an individual and which provides information on their movements (monitoring devices). A medical wearable device definition was not supplied, but options were explicitly listed (Apple Watch with StrivePD, PKG, KinesiaU, PDMonitor). Of note, given that StrivePD is an application that functions on a consumer device, we asked users of StrivePD to answer both as consumer wearable device Apple Watch users and as medical wearable device users.
The survey was designed by the study team with input from other specialist clinicians. Question topics included basic demographics, disease state, understanding and use of wearable devices (divided into consumer and medical device categories), general perceptions as related to theoretical devices, and general barriers to use. Survey questions were generally multiple-choice questions, but free response sections were provided in many cases to allow participants to provide answers that were not accounted for by the survey developers (Supplementary Survey Document).
Recruitment was conducted by collaborating groups who distributed study-related information and a link to the survey. The American Parkinson Disease Association (APDA) and the Washington State Parkinson Disease Registry participated in participant outreach (25).
Interested individuals followed the link and were brought to the online REDCap electronic data capture tool. The first page of the survey provided potential participants with information about the study and associated risks and benefits. Interested individuals would electronically confirm that they consented to participate, which would then allow them to proceed to the survey content (26, 27).
After survey completion, PD disease status nor any other characteristics of participants were verified. It was determined that verification would have limited the response rate and would have introduced more risk of identification and more bias as the systems available for use would tie individuals to specific medical systems. Additionally, it was believed that false representation was unlikely, as the survey was targeted, a response required substantial effort, and no notable financial incentive was present for respondents.
After survey closure, data processing and statistical analysis were performed using the R statistical analysis platform. Targeted sub-group analysis evaluating the effects of demographic and disease features on perceptions and experiences was performed post-hoc.
Results
Survey response, demographics, and disease state
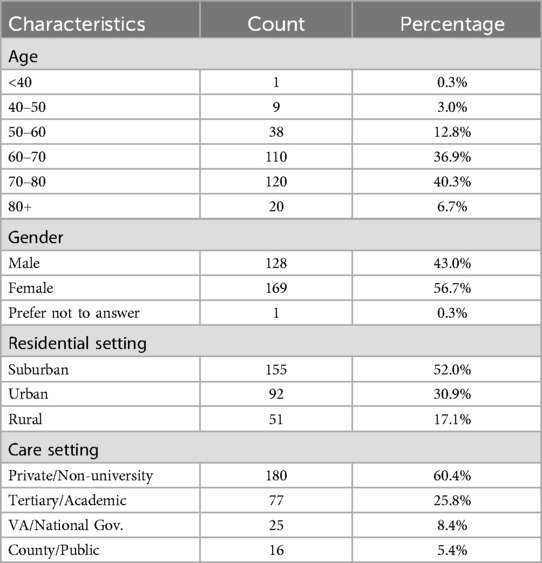
A total of 346 responses were collected with 298 completed surveys (86%). The response rate was unknown but was suspected to be very low given the size of the APDA distribution network. Only completed surveys were included in the analysis. Responses came from individuals living in 28 states with the greatest number coming from Washington State (63%) (Supplementary Table S1). A limited set of demographic features were recorded (Table 1). Disease related symptoms and characteristics varied encompassing both early and late stages of disease (Supplementary Tables S2, S3).
Technology and consumer device experiences
Regarding technology and wearable device use, there was a high degree of interest in new technologies with 91% of individuals either very or somewhat interested. Knowledge about and use of wearable devices was also high with 87% knowing about wearable devices, 67% having used a device, and 56% currently using one (Figure 1). Most respondents knew about smart watches and fitness trackers (Supplementary Table S4); the Apple Watch was the most used and most preferred consumer device among respondents (Supplementary Table S5). Device use retention rates were also high with 84% of those with experience with wearable devices continuing to use a device. Among all device users, device usage time was very high with 90% using their preferred device nearly always or at least all the time while awake (Supplementary Table S6).
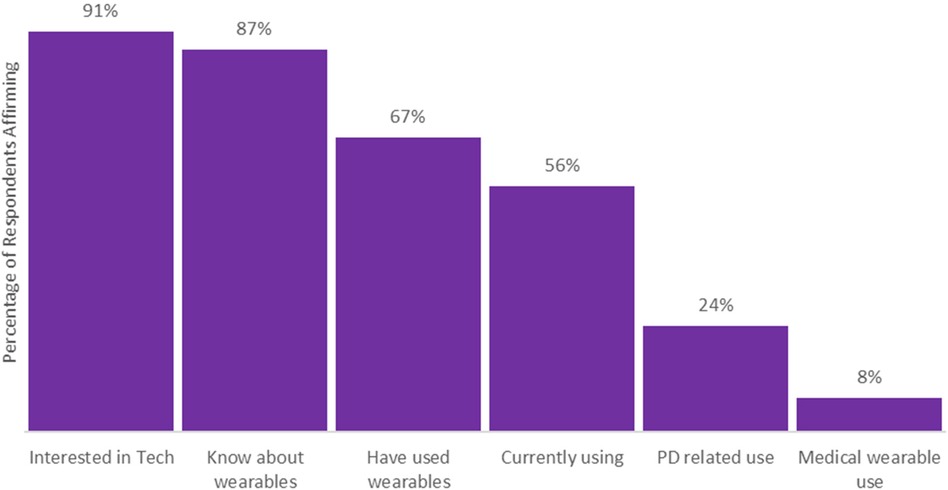
Figure 1. Respondent interest, knowledge, or experiences with wearable device use. For the interested in technologies category answers of moderately or very interested were combined, neutral or below were not incorporated. All other questions were yes or no responses.
Consumer wearable device feature use
However, use of wearable devices for the management of PD was low at only 24% of respondents. Among those using wearable devices, individuals were using them most to track physical activity, medication timings, and sleep (Figure 2). Post-hoc subgroup analysis of Apple Watch wearable device users was performed to evaluate whether device capability limitations were playing a large role in lack of management related use, however findings mirrored those seen among all device users (Supplementary Figure S1).
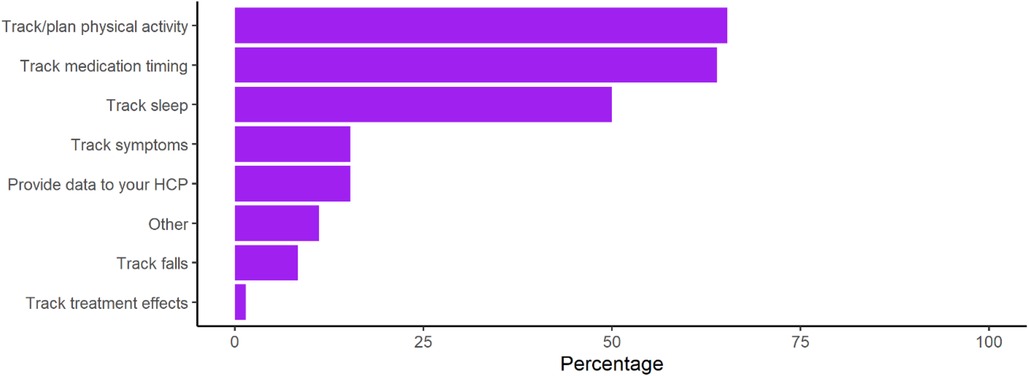
Figure 2. Use of specific consumer device functions for the management of PD. Calculated as the percentage of respondents using each function from the subset of respondents who affirmed that they were using consumer wearables to manage PD (n = 72).
Consumer wearable devices effect on disease management
Among those using wearables to manage PD, 76% reported a positive impact on personal management of PD (38% substantially, 39% somewhat), 24% reported that use was not particularly impactful, and no negative responses were recorded. Impact on medical team management of PD was felt to be not particularly impactful by 57% of respondents, though substantially positive (10%) or somewhat positive (33%) responses were reported, and no negative responses were reported.
Consumer wearable device barrier to use
Among survey respondents, current device use was limited most frequently because of lack of knowledge about the abilities of wearable devices and by cost (Supplementary Table S7). More generally, individuals were also surveyed on the use of smartphones and other applications for PD monitoring with 26% reporting the use of an application.
Medical wearable device usage and barrier to use
Medical wearable device use among respondents was 8% (n = 23). There were 19 StrivePD, 3 PD Monitor, 1 PKG, and 0 Kinesia users. Device use frequency was variable and many limitations on use were noted (Table 2). Impact on personal management was 26% positive (9% substantially, 17% somewhat) and impact on medical team management was 30% positive (4% substantially, 26% somewhat) (Supplementary Table S8).
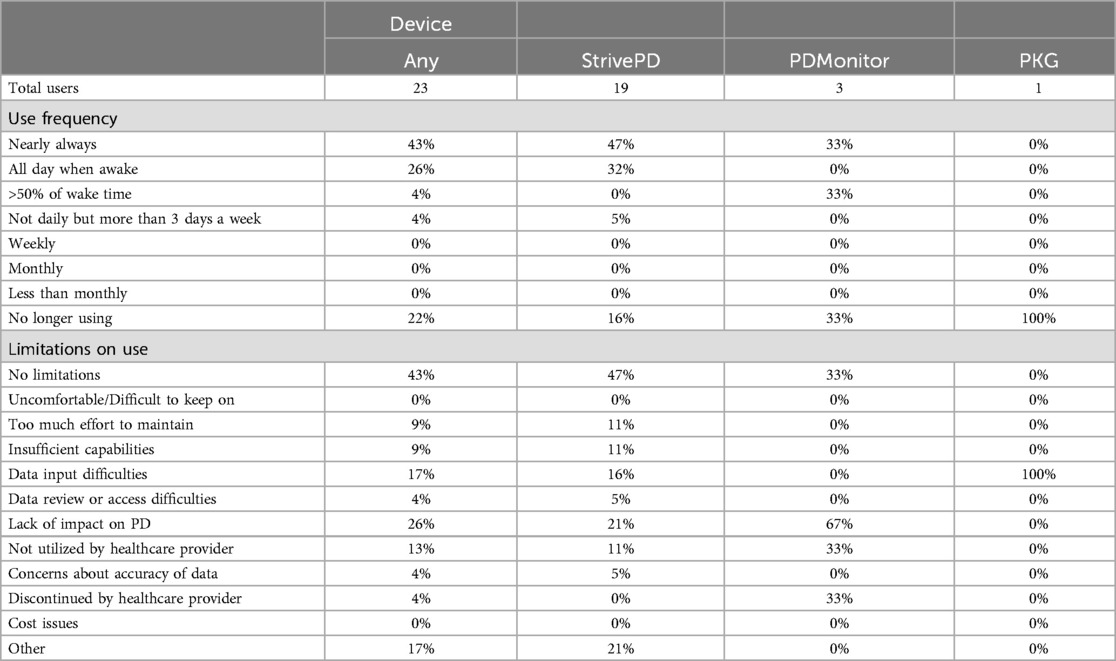
Table 2. Respondent medical wearable device use, frequency of use, and limitations on use. Other limitations reported for Strive PD were that the device was in the process of being setup (1), difficulties logging in (1), geographic limitations on use (1), and an allergic response (1).
General wearable interest and limitations
To separate perceptions and experiences tied to current consumer or medical devices, we asked about two similar theoretical devices. We described a version which provided information to the patient but did not directly provide it to the healthcare team (Type A) and one that provided information directly to both (Type B). For both versions, individuals were interested in using such a device (Supplementary Figure S2). Additionally, among those who would consider using the devices greater than 90% of respondents were willing to use either device at least all day while awake (Supplementary Figure S3). However, only 49% of those interested were willing to pay for a device if not covered by insurance. Of those who were willing to pay for such a device, the median one-time payment was $200 for both and the mean $252 (Type A) and $259 (Type B). Alternatively, we also asked about subscription pricing and what individuals would be willing to pay with the median being $10 for both devices and mean being $14 (Type A) and $15 dollars (Type B). There was no difference between the cost individuals were willing to pay for device [p = 0.6 (lump sum) and p = 0.2 (subscription), Wilcoxon paired signed rank test in the setting of non-normality of data shown by Shapiro-Wilk testing].
Finally, individuals were surveyed on barriers to their use of wearable devices for PD (Figure 3). Respondents reported concerns about all surveyed barriers with most respondents reporting at least a moderate level of concern for 4 out of the 5 surveyed barriers. Respondents reported the largest proportion of extreme concern for cost and impact on care. Additionally, we compared perceptions between individuals with different levels of wearable device experience (Supplementary Figure S4).
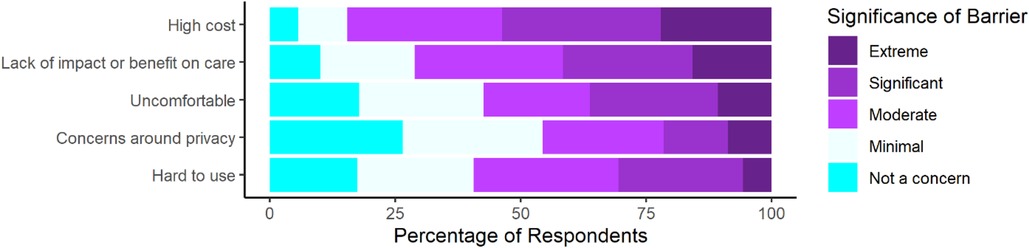
Figure 3. Perceived significance of certain barriers to respondents’ personal use of wearable devices for management of PD as rated on a 5-point scale from not a concern to an extremely significant barrier (n = 298).
Discussion
This study captured wearable device experiences and perceptions among individuals with PD. Respondents were very technologically inclined (91% reporting interest) and were more likely to use wearable devices than the general US population, 67% vs. 35% (Morning Consult, Survey, 2023). Consumer devices among respondents also appeared to be well tolerated as discontinuation of wearables was rare and users also wore the devices most of the time.
However, despite the barriers to general consumer use being overcome, consumer wearable use for the specific purpose of managing PD was strikingly low at 24% and use of commonly available devices functions was low. This incomplete pattern of usage remained true even after removing ambiguity in the definition of “management” and ensuring that all functions queried were possible. In this subset, despite previous reported interest (28) and the importance placed on these features in PD, less than three quarters reported using the device to track physical activity, less than half tracked sleep, and less than a quarter tracked symptoms. Participants reported the lack of PD related use to be most often due to knowledge of functionality (26.2%). Features previously noted as important to address such as difficulties with wearability or comfort (4.7%), data input (9.4%), and data review (5.0%) were not prominently reported (23). These findings are supportive of the acceptability and usability of current consumer devices in PD.
While consumer wearable device use for PD was sub-optimal, medical wearable device use was marginal. Only 8% of respondents had any experience with them, which was less than 1/8th the number of consumer wearable device users. However, there did not appear to be marked barriers to use once implemented, given 43% of respondents reported no limitations. The most noted barrier to use was lack of impact on care 26% and this was additionally supported by most medical device users indicating that their devices had negligible impact on their healthcare providers' management of PD and even on their own management. However, other issues appear to be reasonably addressed with less than 20% reporting difficulties with data input, less than 10% issues with wearability, and less than 5% issues with data review.
To better understand what factors were limiting wearable use and to compare current perceptions to prior research, many questions were directed to assess their perceptions on the significance of certain barriers, and it was again seen that individuals with PD were concerned about comfort, usability, and impact on care, as well as cost and privacy (23). However, these results seemingly conflict with the results obtained from direct questioning about their personal wearable use. Due to this conflict we sought to assess whether this was due to differences between users with more and less experience. However, consumer and medical wearable users still appeared to endorse similar levels of concern. These findings therefore suggest that while certain factors are still of high importance to people with PD they have generally been addressed by the current generation of devices.
Ultimately, it appears that the barriers to medical wearable device use and to a lesser degree consumer wearable device use in Parkinson's disease do not stem from individuals with PD. Even when an individual with PD is motivated, experienced with wearable use, and interested in theoretical medical wearable devices there is still a high likelihood that they will not be a wearable device user. We believe that this reflects difficulties with technology and device integration at the level of the provider user and/or the healthcare system.
The main strengths of this study were the number of respondents, the diversity of disease stages included, the inclusion of multiple wearables, and the level of detail obtained about perceptions and experiences. As with most survey studies, there were meaningful limitations. Foremost among them was sampling bias which was likely substantial given the online format and low estimated respondent rate. Respondents were likely highly motivated and technologically inclined. The use patterns and perceptions noted in this study do not directly reflect those of the population. However, they retain value as they are almost certainly a reflection of the upper bound of device use and their perceptions likely reflect the most positive reflections of the population, as such one can reasonably infer that the population rate of experience is lower and that perceptions are likely to be less positive than were seen in this sample. Additionally, the survey was heavily biased towards the Pacific Northwest region of the United States despite the goal of having a national distribution, demographic and socioeconomic data was limited, and the number of respondents for questions relating to consumer wearable device use in PD and medical wearables was relatively small.
Future studies should work to better understand the true perceptions of the PD population by expanding distribution, reducing respondent barriers, collecting more demographic and socioeconomic data, and engaging the community. Furthermore, future longitudinal studies should be performed to assess the evolution of individual perceptions of wearables as they evolve in relation to exposure to wearable devices and disease progression.
In conclusion, this study confirms the existence of a highly motivated subpopulation of individuals with PD who have a strong interest in wearable devices and confirms the feasibility of high levels of wearable device use in real-world use. Novelly, it identifies and partially quantifies large gaps in the use of consumer wearable device health tracking features and integration of wearable devices into PD related health management. Additionally, it confirms that medical wearable device use is low, but suggests that this isn't an issue with patient usability, thereby implicating provider and/or systemic barriers as the bottleneck to medical device use. We believe that these results call for further investigation into understanding the barriers affecting real-world use among clinician users and healthcare systems, as well as studies targeted at enhancing the utility and understanding of all forms of wearable device use in PD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by University of Washington, Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin because human subjects could not be readily ascertained and data was not of a highly sensitive nature.
Author contributions
SH: Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Writing – original draft, Writing – review & editing. CZ: Conceptualization, Methodology, Resources, Supervision, Visualization, Writing – review & editing. YL: Conceptualization, Methodology, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Center for Advancing Translational Sciences, National Institutes of Health through a block grant (UL1 TR002319). Dr. Hirczy was supported by the Veterans Affairs Advanced Fellowship Program in Parkinson's Disease.
Acknowledgments
We wish to acknowledge our collaborators who made this survey possible, Dr. Rebecca Gilbert and the American Parkinson Disease Association (APDA) and the Washington State Parkinson Disease Registry. We also wish to acknowledge Dr. Alberto Espay for his assistance with the development of the survey.
Conflict of interest
YL reports a relationship with Boston Scientific Corporation, CereGate GmBH, and Bial, all of which provide funding grants.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdgth.2024.1472691/full#supplementary-material
Supplementary Figure S1 | Use of specific consumer device functions for the management of PD. Calculated as the percentage of respondents using each function from the subset of respondents who affirmed that they were using consumer wearables to manage PD and who were using the Apple Watch as their preferred device (n = 41).
Supplementary Figure S2 | Respondent degree of interest in using theoretical wearable devices in PD. Device A was defined as a device with the ability to help individuals better understand their disease and responses to medical interventions, but without ability to directly provide information to healthcare providers. Device B was similarly defined but with the added benefit of healthcare provider integration (n = 192).
Supplementary Figure S3 | Amount of time respondents were willing to wear theoretical devices A and B if there were no issues with comfort or usability. (Device A n = 293, Device B n = 292).
Supplementary Figure S4 | Significance of barriers to wearable device use among individuals with different degrees of experience with wearable device use. MWU = medical wearable device user, CWU + PD = consumer wearable device user who manages PD with the device. CWU-PD = consumer wearable device user not managing PD with the device. Ex CWU = participants who have used consumer wearable devices but no longer are. Non-User = no experience with wearable devices (n = 298).
Supplementary Table S1 | States in which survey respondents reside (n = 298).
Supplementary Table S2 | Respondent PD related characteristics and symptom characteristics (n = 298).
Supplementary Table S3 | Respondent assistive gait device utilization frequencies among those who reported using an assistive device (n = 58).
Supplementary Table S4 | Respondent awareness of and experiences with wearable devices (n = 298).
Supplementary Table S5 | Respondent wearable use preferences among those with experience with wearable devices (n = 193). Other responses for preferred device: apollo neuro (1), iphone (1), kospet (1), and Samsung watch (1).
Supplementary Table S6 | Preferred consumer wearable device use time among individuals still using thei preferred device (n = 160).
Supplementary Table S7 | Respondent reported current barriers to the use of consumer wearable devices for the management of PD. Individual respondents could affirm the presence of multiple barriers.
Supplementary Table S8 | Medical wearable device user perceptions on impact of devices on management of PD (n = 23).
References
1. Evers LJW, Krijthe JH, Meinders MJ, Bloem BR, Heskes TM. Measuring Parkinson’s disease over time: the real-world within-subject reliability of the mds-updrs. Mov Disord. (2019) 34(10):1480–7. doi: 10.1002/mds.27790
2. Regnault A, Boroojerdi B, Meunier J, Bani M, Morel T, Cano S. Does the Mds-updrs provide the precision to assess progression in early Parkinson’s disease? Learnings from the Parkinson’s progression marker initiative cohort. J Neurol. (2019) 266(8):1927–36. doi: 10.1007/s00415-019-09348-3
3. Papapetropoulos SS. Patient diaries as a clinical endpoint in Parkinson’s disease clinical trials. CNS Neurosci Ther. (2012) 18(5):380–7. doi: 10.1111/j.1755-5949.2011.00253.x
4. Shulman LM, Pretzer-Aboff I, Anderson KE, Stevenson R, Vaughan CG, Gruber-Baldini AL, et al. Subjective report versus objective measurement of activities of daily living in Parkinson’s disease. Mov Disord. (2006) 21(6):794–9. doi: 10.1002/mds.20803
5. Ymeri G, Salvi D, Olsson CM, Wassenburg MV, Tsanas A, Svenningsson P. Quantifying Parkinson’s disease severity using mobile wearable devices and machine learning: the parkapp pilot study protocol. BMJ Open. (2023) 13(12):e077766. doi: 10.1136/bmjopen-2023-077766
6. Farzanehfar P, Woodrow H, Horne M. Sensor measurements can characterize fluctuations and wearing off in Parkinson’s disease and guide therapy to improve motor, non-motor and quality of life scores. Front Aging Neurosci. (2022) 14:852992. doi: 10.3389/fnagi.2022.852992
7. Schalkamp A-K, Peall KJ, Harrison NA, Sandor C. Wearable movement-tracking data identify Parkinson’s disease years before clinical diagnosis. Nat Med. (2023) 29(8):2048–56. doi: 10.1038/s41591-023-02440-2
8. Powers R, Etezadi-Amoli M, Arnold EM, Kianian S, Mance I, Gibiansky M, et al. Smartwatch inertial sensors continuously monitor real-world motor fluctuations in Parkinson’s disease. Sci Transl Med. (2021) 13(579):eabd7865. doi: 10.1126/scitranslmed.abd7865
9. Sotirakis C, Su Z, Brzezicki MA, Conway N, Tarassenko L, FitzGerald JJ, et al. Identification of motor progression in Parkinson’s disease using wearable sensors and machine learning. NPJ Parkinson’s Dis. (2023) 9(1):142. doi: 10.1038/s41531-023-00581-2
10. Ko YF, Kuo PH, Wang CF, Chen YJ, Chuang PC, Li SZ, et al. Quantification analysis of sleep based on smartwatch sensors for Parkinson’s disease. Biosensors. (2022) 12(2):74. doi: 10.3390/bios12020074
11. Suzuki M, Nakamura T, Hirayama M, Ueda M, Hatanaka M, Harada Y, et al. Wearable sensor device-based detection of decreased heart rate variability in Parkinson’s disease. J Neural Transm. (2022) 129(10):1299–306. doi: 10.1007/s00702-022-02528-y
12. Joo JY, Yoo D, Kim JM, Shin C, Ahn TB. Effect of positional changes on cerebral perfusion in Parkinson’s disease patients with orthostatic hypotension. J Mov Disord. (2024) 17(4):408–15. doi: 10.14802/jmd.24104
13. Moreau C, Rouaud T, Grabli D, Benatru I, Remy P, Marques A-R, et al. Overview on wearable sensors for the management of Parkinson’s disease. NPJ Parkinson’s Dis. (2023) 9(1):153. doi: 10.1038/s41531-023-00585-y
14. Mughal H, Javed AR, Rizwan M, Almadhor AS, Kryvinska N. Parkinson’s disease management via wearable sensors: a systematic review. IEEE Access. (2022) 10:35219–37. doi: 10.1109/ACCESS.2022.3162844
15. Ancona S, Faraci FD, Khatab E, Fiorillo L, Gnarra O, Nef T, et al. Wearables in the home-based assessment of abnormal movements in Parkinson’s disease: a systematic review of the literature. J Neurol. (2022) 269(1):100–10. doi: 10.1007/s00415-020-10350-3
16. Deb R, An S, Bhat G, Shill H, Ogras UY. A systematic survey of research trends in technology usage for Parkinson’s disease. Sensors. (2022) 22(15):5491. doi: 10.3390/s22155491
17. Li P, van Wezel R, He F, Zhao Y, Wang Y. The role of wrist-worn technology in the management of Parkinson’s disease in daily life: a narrative review. Front Neuroinform. (2023) 17:1135300. doi: 10.3389/fninf.2023.1135300
18. Sigcha L, Borzì L, Amato F, Rechichi I, Ramos-Romero C, Cárdenas A, et al. Deep learning and wearable sensors for the diagnosis and monitoring of Parkinson’s disease: a systematic review. Expert Syst Appl. (2023) 229:120541. doi: 10.1016/j.eswa.2023.120541
19. Botros A, Schutz N, Camenzind M, Urwyler P, Bolliger D, Vanbellingen T, et al. Long-term home-monitoring sensor technology in patients with Parkinson’s disease-acceptance and adherence. Sensors. (2019) 19(23):5169. doi: 10.3390/s19235169
20. Zhao Y, Heida T, van Wegen EE, Bloem BR, van Wezel RJ. E-Health support in people with Parkinson’s disease with smart glasses: a survey of user requirements and expectations in The Netherlands. J Parkinsons Dis. (2015) 5(2):369–78. doi: 10.3233/JPD-150568
21. van den Bergh R, Evers LJW, de Vries NM, Silva de Lima AL, Bloem BR, Valenti G, et al. Usability and utility of a remote monitoring system to support physiotherapy for people with Parkinson’s disease. Front Neurol. (2023) 14:1251395. doi: 10.3389/fneur.2023.1251395
22. Ozanne A, Johansson D, Hallgren Graneheim U, Malmgren K, Bergquist F, Alt Murphy M. Wearables in epilepsy and Parkinson’s disease-a focus group study. Acta Neurol Scand. (2018) 137(2):188–94. doi: 10.1111/ane.12798
23. Kenny L, Moore K, O'Riordan C, Fox S, Barton J, Tedesco S, et al. The views and needs of people with Parkinson disease regarding wearable devices for disease monitoring: mixed methods exploration. JMIR Form Res. (2022) 6(1):e27418. doi: 10.2196/27418
24. Dhingra LS, Aminorroaya A, Oikonomou EK, Nargesi AA, Wilson FP, Krumholz HM, et al. Use of wearable devices in individuals with or at risk for cardiovascular disease in the us, 2019 to 2020. JAMA Network Open. (2023) 6(6):e2316634-e. doi: 10.1001/jamanetworkopen.2023.16634
25. Kim HM, Leverenz JB, Burdick DJ, Srivatsal S, Pate J, Hu S-C, et al. Diagnostic validation for participants in the Washington state Parkinson disease registry. Parkinson’s Disease. (2018) 2018:3719578. doi: 10.1155/2018/3719578
26. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The redcap consortium: building an international community of software platform partners. J Biomed Inform. (2019) 95:103208. doi: 10.1016/j.jbi.2019.103208
27. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (redcap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42(2):377–81. doi: 10.1016/j.jbi.2008.08.010
Keywords: Parkinson's disease, wearable, clinical practice, survey, barriers to use, consumer devices, medical devices
Citation: Hirczy S, Zabetian C and Lin Y-H (2024) The current state of wearable device use in Parkinson's disease: a survey of individuals with Parkinson's. Front. Digit. Health 6:1472691. doi: 10.3389/fdgth.2024.1472691
Received: 29 July 2024; Accepted: 12 December 2024;
Published: 23 December 2024.
Edited by:
Graham Jones, Tufts Medical Center, United StatesReviewed by:
Brant Chapman, Bergen Community College, United StatesTommaso Di Libero, University of Cassino, Italy
Copyright: © 2024 Hirczy, Zabetian and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Siegfried Hirczy, c2llZ2ZyaWVkLmhpcmN6eUB2YS5nb3Y=
 Siegfried Hirczy
Siegfried Hirczy Cyrus Zabetian
Cyrus Zabetian Yi-Han Lin
Yi-Han Lin