
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Digit. Health, 15 November 2023
Sec. Health Technology Implementation
Volume 5 - 2023 | https://doi.org/10.3389/fdgth.2023.1274355
This article is part of the Research TopicDigital Health and Virtual Health Care for Adults and Older Adults: Innovative Technological Solutions for Diagnosis, Management, and RehabilitationView all 9 articles
 Jessica Chapman-Goetz1
Jessica Chapman-Goetz1 Nerida Packham2
Nerida Packham2 Kitty Yu3
Kitty Yu3 Genevieve Gabb4
Genevieve Gabb4 Cassandra Potts5
Cassandra Potts5 Adaire Prosser5
Adaire Prosser5 Margaret A. Arstall6
Margaret A. Arstall6 Christine Burdeniuk7
Christine Burdeniuk7 Alicia Chan8
Alicia Chan8 Teena Wilson9
Teena Wilson9 Elizabeth Hotham1
Elizabeth Hotham1 Vijayaprakash Suppiah1,10*
Vijayaprakash Suppiah1,10*
Introduction: Heart failure (HF) is an increasing global concern. Despite evidence-based pharmacotherapy, associated morbidity and mortality remain high. This study aimed to assess the acceptability, feasibility, and value of the NPS MedicineWise dose reminder app in a tiered, pharmacist-led intervention to address medication non-adherence in patients with HF.
Methods: This prospective, single-blinded, randomised controlled trial recruited 55 patients with HF between September 2019 and October 2020. Participants were randomly assigned to either the intervention or control arms. Intervention participants used the app which prompted medication administration at each dosing interval. Control participants received standard care and remained blinded to the app throughout the study. Treatment non-adherence prompted a tiered, pharmacist-led intervention. Comparison of the Self-Efficacy for Appropriate Medication Use Scale (SEAMS) at baseline and 6-months measured the app's value in supporting medication adherence. Secondary outcome measures included self-reported medication knowledge, health-related quality of life, psychological wellbeing, and signs and symptoms of HF. Data were analysed using standard statistical tests with significance set at α 0.05.
Results: Approximately half of respondents reported managing HF and medications better by using the MedicineWise app (Tier 1). Most respondents expressed satisfaction with the in-app messages (Tier 2) and pharmacists' phone calls (Tier 3). The intervention participants demonstrated a significant improvement in the SEAMS between baseline and 6-months follow-up.
Discussion: It is feasible and potentially of value to use the MedicineWise app with a tiered, pharmacist-led intervention to support medication adherence in patients with HF. Our findings provide clinicians with “real-world” information on the practicality and potential value of using mobile health to support treatment adherence in patients with HF.
Trial registration number: Australian New Zealand Clinical Trials Registry Clinical trial registration number: ACTRN12619000289112p (http://www.ANZCTR.org.au/ACTRN12619000289112p.aspx)
The prevalence of heart failure (HF) in Australia has been estimated to be around 1%–2%, rising significantly to 10% in those aged 75 years and above (1, 2). Significant morbidity in this population contributes to frequent and prolonged hospitalizations with approximately four-fifths being hospitalized at least once and up to three-quarters dying within five years of diagnosis (1, 3).
Pharmacotherapy is the foundation of HF management with evidence supporting improvement in morbidity and mortality (4–6). Despite implementing polypharmacy as strongly advocated by several guidelines (4, 5, 7), clinical outcomes remain suboptimal (8).
As therapeutic efficacy is dependent on routine medication administration, non-adherence has been shown to increase both frequency of hospitalizations and relative risk of all-cause mortality (8). Medication non-adherence in HF can be further complicated by co-morbidities like dementia and depression, disease, progression and severity, and inherent regimen complexity and high “pill burden” (9–12). Studies have shown that approximately one-fifth to one-third of HF patients may not consistently take their medications as prescribed (13, 14).
The exponential growth in mobile/smartphone ownership has driven interest in mobile health (mHealth) as a strategy to address medication non-adherence (15). Relative ease of use, accessibility and low-cost of mobile devices make mHealth a particularly desirable modality for patient-centered services (15–17). Since the emergence of smartphones, mHealth has evolved from telephone call and text message-based supports to include applications (apps), social media platforms and health information systems (18, 19). These can better promote interventions that support health-related behaviors including medication adherence via dose reminders/alerts, dose tracking, prescription refill reminders and/or storing medication-related information (20, 21).
The NPS MedicineWise dose reminder app is a free medicine and health management app funded by the Australian government to help patients and their carers, keep track of their medicines and other important health information with the aim, among others, of improving medication adherence, being a source of reliable medical information and storing patients' health and medicine information in one place (22, 23).
The primary aim of this pilot study was to determine the acceptability, feasibility, and value of incorporating the NPS MedicineWise dose reminder app in a tiered, pharmacist-led intervention to address medication non-adherence in patients with HF. The Self-Efficacy for Appropriate Medication Use Scale (SEAMS) (24) will be used to determine the app's value in supporting medication adherence. The secondary aim was to determine the impact of the app on signs and symptoms of HF and quality of life at 6-months.
This was a prospective, single-blinded observational randomized controlled trial. The full study protocol has been published elsewhere (25). Australian New Zealand Clinical Trials Registry Clinical trial number: ACTRN12619000289112p (http://www.ANZCTR.org.au/ACTRN12619000289112p.aspx). Briefly, the study was conducted at six South Australian investigator centers between September 2019 and October 2020. Recruitment of study participants took place from 1st November 2019 to 30th April 2020 and the last patients were followed till October 2020. Rural participants were recruited through the Integrated Cardiovascular Clinical Network (ICCNet) and interstate participants via an NPS MedicineWise social media advertisement. Eligible participants were briefed about the study by the treating physician or cardiology nurse either in person or by phone during routine clinic visits or, for rural patients, during their scheduled tele-health consultation/ appointments. Potential participants were provided with a study summary and referred to the study team for eligibility screening (Supplementary Table S1) prior to a preliminary interview - face-to-face, at any participating hospital, or via phone. At this initial visit, written informed consent was obtained from those who agreed to participate, and a baseline assessment was completed. Interstate participants reached via the NPS social media advertisement were directed to an eligibility survey. Upon its completion, a preliminary interview occurred as above. Written informed consent was obtained via post or email and a baseline assessment was completed. The eligibility criteria for this study were broadly designed (Supplementary Table S1) to include older individuals and those with comorbidities like depression as they can be underrepresented in mHealth research (9, 26).
Sample size calculation for this study was based upon a medium (d = 0.5) to large (d = 0.8) effect size as measured by Cohen's d (27). While the upper limit of real-world adherence to cardiovascular medications was estimated to be 60% (28), a patient is considered to have good adherence when ≥80% of doses had been taken (29). To detect an increase in adherence from 60% to 80%, a minimum total sample size of 52 participants was needed to provide 80% power, with a one tail 0.05 significance level with an effect size of 0.7 (30). To allow for dropouts, a total of 55 participants will be recruited for this study as previously described (25).
Participants were randomly allocated to intervention or control. Control participants did not have access to any medication reminder apps (including the MedicineWise app) and remained blinded to the existence of an intervention arm for study duration. This was facilitated by using a different patient information sheet and consent form for the recruitment of control and intervention arm participants. Participation in the control arm involved regular follow ups of functional assessments (questionnaires), medication adherence and knowledge questionnaire and the collection of laboratory results. This was intended to minimize recognized limitations of earlier research where blinding was not present (19). However, researchers undertaking data collection were not blinded due to the personalized and tiered nature of the intervention.
At baseline, intervention participants were instructed in using the MedicineWise app. Following set-up, a reminder (Tier 1) prompted medication administration at each dosing interval. If non-adherence was suggested from 24 h reports (critical medications) or 72 h reports (non-critical medications), the participant was escalated through a tiered, pharmacist-led intervention which consisted of in-app messages (Tier 2) and phone calls from the NPS MedicineWise Medicines Line Pharmacists (Tier 3). Critical medications were those that form the foundation of pharmacotherapy due to established morbidity or mortality benefits (5). Medicines Line is a national consumer medicines information telephone service, delivered by NPS MedicineWise. The NPS MedicineWise Medicines Line (Medicines Line) – was a “real world” national consumer medicine information service delivered by pharmacists and operated between Mondays to Fridays 9 am to 5 pm AEST (excluding New South Wales public holidays). The Medicines Line service and the MedicineWise app were funded by federal Department of Health and Aged Care to 31 December 2022.
The study protocol was approved by the respective institutional Human Research Ethics Committees (Protocol numbers R20190302 and 202450).
The primary outcome was the acceptability, feasibility, and value of this approach in supporting medication adherence. Comparison of the SEAMS (24) at baseline and 6-months was used to measure the app's value in supporting medication adherence. Secondary outcome measures included health-related quality of life, psychological wellbeing, and signs and symptoms of HF.
Trial data were collected via face-to-face or phone interviews as determined by participant preference, geographical location, or local governmental health guidelines for COVID-19 infection control. Baseline data were collected at the time of recruitment and repeated at each follow-up with details of any changes to participants' medication recorded. Part A of the Self-care of Heart Failure Index (SCHFI) questionnaire (27) was utilized to determine whether control participants had used any drug administration aids, medication reminder apps or other electronic reminder systems for daily medication administration, since the previous interview. The satisfaction survey was completed with intervention participants upon study completion by another researcher not associated with data collection.
Continuous variables were presented as the mean ± standard deviation (SD), or the median and interquartile range (IQR) for data that were not normally distributed (skewed). Categorical (or discrete) variables were expressed as the number and percentage of frequency. Thematic analysis was performed on qualitative data to generate emerging and overarching themes (31, 32).
The paired t-test was used within each arm to examine continuous data collected at baseline and 6-month follow-up. Differences in categorical variables were assessed using the McNemar test. Between group analysis was conducted with the two-sample t-test (continuous variables) or the χ2 test (categorical variables). The Wilcoxon Signed Rank test or Mann–Whitney U test were used for continuous data that did not display a normal distribution. Analysis of the SEAMS, medication adherence and knowledge, EQ-5D-5l (33), Short Form 36 Health Survey version 2 (SF-36v2) 34, Depression Anxiety and Stress Scales (DASS-21) (35) and SCHFI (36) questionnaires were performed with SPSS software, with statistical significance set at α 0.05.
Of the referred 80 patients with HF, 55 agreed to participate in the study (Figure 1). Time constraints and changing social circumstances (such as moving into an aged care facility or interstate) were reasons for declining to participate. Forty-nine individuals (89%) were followed until study completion (Figure 1). Three intervention arm participants were lost by the 3-month and one by 6-month follow-ups, without explanation. Time constraints prompted one intervention arm participant to withdraw prior to the last interview and hospitalization prevented one control arm participant from undergoing her 6-month follow-up.
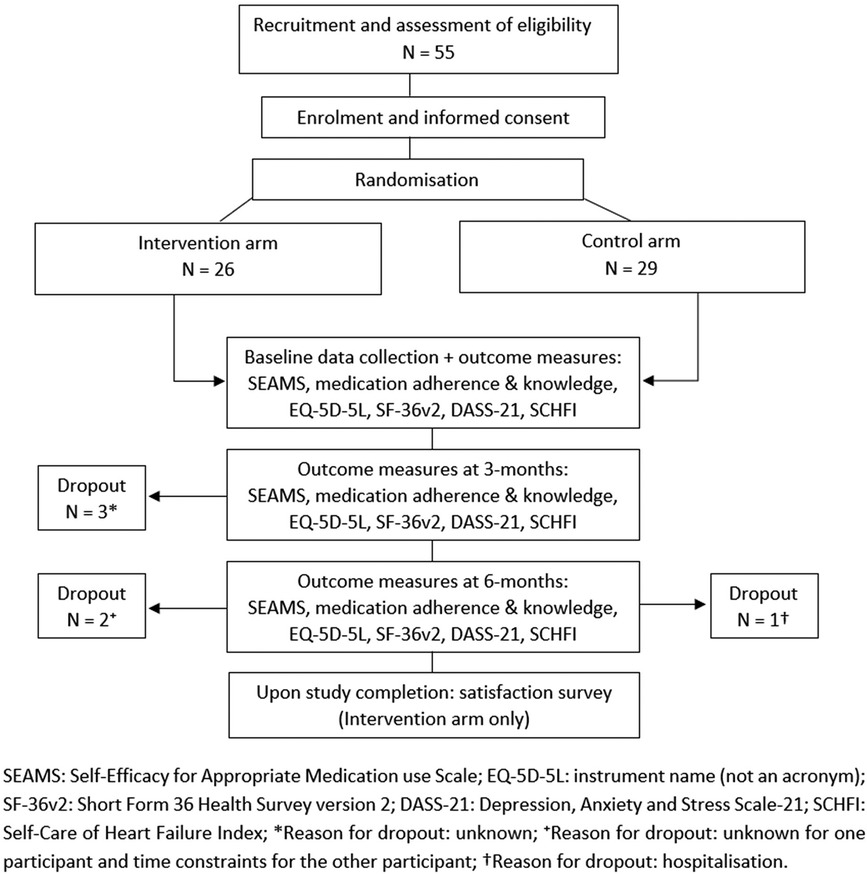
Figure 1. Schematic diagram of trial design with representation of sample size and loss follow-up (dropout).
The study cohort characteristics are presented as a whole cohort as there were no statistically significant differences between the two groups (Table 1). While participants were predominantly Caucasian (95%) and male (67%), a near equal number lived either with a partner (married or de facto) or alone (divorced, widowed or single). The mean age and body mass index (BMI) were 64 years (SD: 13 years) and 33 kg/m2 (SD: 8 kg/m2), respectively. Most participants (65%) were unemployed or retired and had no regular daytime activity. Only seven individuals (13%) in the cohort identified as current smokers. Commonly documented comorbidities included type 2 diabetes mellitus (38%), hypertension (35%), atrial fibrillation (22%) and cardiovascular disease (20%). Only five individuals (9%) had a history of depression. Nearly all participants (85%) were classified as having either “no” symptoms of HF or limitations in ordinary physical activity [NYHA class I (42%)], or “mild” symptoms of HF including fatigue and shortness of breath with ordinary physical activity [NYHA class II (43%)]. On average, participants took 4 (± 1) “critical” and 3 (± 3) “non-critical” medications (Supplementary Table S2) which were mostly administered from an organizer such as a dosette or Webster® pack (58%).
A total of 2,679 “missed” doses were logged in the app's database between December 2019 and October 2020 (Table 2). However, 779 (29%) of these were incorrectly recorded as “missed”. Investigation by the NPS MedicineWise team revealed that the automated “batch-sync” had intermittently malfunctioned between March-April 2020 and not all data were transferring to the cloud within 24 h. A series of “hotfixes” was released in June, July, and September 2020 to assist in diagnosing, monitoring and subsequent resolution of the malfunction. In addition, functional improvements to the app were implemented via the last “hotfix,” allowing users to visualize successful recording of their “TAKEN” doses in the cloud as a time stamp on their device. Despite the “batch-sync” difficulty, a total of 1,900 (71%) “missed” doses were correctly recorded over the study.
A total of 749 “push” notification messages were sent between December 2019 and October 2020 (Table 3). Of these, 426 (57%) were in response to true (correctly recorded) “missed” doses. The remainder were erroneously sent due to the false (incorrectly recorded) “missed” doses incurred by the “batch-sync” malfunction. This malfunction only affected participants using Android devices (n = 20).
Over the study period, 17 (71%) intervention arm participants were escalated to Tier 3 and received on average 3 ± 2 phone calls from a Medicines Line pharmacist. The remaining 7 (29%) participants did not require a Tier 3 intervention for the entire duration of the study.
Due to the unforeseen technical issues like the “batch-sync” malfunction the nominated cap of 3 phone calls per participant was removed. In all, a total of 96 Tier 3 interventions were conducted between December 2019 and October 2020. However, no instances of medication non-adherence were identified. The main issues were technical difficulties with the MedicineWise app (39%) and non-adherence with the app itself (43%). Common explanations for the loss of engagement included: hospitalization, temporary cessation of medication (primarily prior to surgery), inconsistent smartphone use and delay in downloading the app onto an upgraded device. Accidentally entering a new medication (furosemide) as daily, rather than ‘when required,’ prompted intervention on another 2 occasions (2%) and contact could not be made for the remaining 16 (17%) Tier 3 interventions.
Only half of the initial 26 participants in the intervention arm took part in the satisfaction survey. Although all respondents felt “very” or “somewhat” confident in their ability to use the MedicineWise app after initial training, about half (54%) stated that they “would not have been confident” to use the app without the training. Although there were other app functions available, only 22% had used the app to record blood pressure, weight, and the contact details of their medical specialists.
Over the study period, the app prompted medication administration “all of the time” for 5 (38%) respondents, “some of the time” for another 5 (38%) and “none of the time” for the rest 3 (24%). Only 3 (24%) individuals felt that these reminders were “useful”. Most respondents 9 (69%) tapped the “TAKEN” icon in less than 15 min while the rest did so within 15 to 30 min after taking their medication. The reassurance provided by tapping “TAKEN” was deemed to be “very useful” by 4 (31%) respondents. Three (24%) respondents did not find tapping “TAKEN” useful as visual evidence of dose administration was already provided by their medication organizer.
Nine (90%) of the ten individuals who received Tier 2 interventions expressed satisfaction with the messages and did not suggest any improvement. Tier 3 interventions were received by 8 (62%) respondents, of whom, five (62.5%) considered the conversation with the Medicines Line pharmacists to be “very helpful” or “good”. Most respondents (75%) felt “very comfortable” in asking these pharmacists additional medicine-related questions. The rest (25%) felt that this was “not needed” as they already had good relationships with existing healthcare providers. Three participants (23%) were not contacted by a Medicines Line pharmacist for the entire duration of the study.
The MedicineWise app helped 6 (46%) respondents to manage their HF and medications by acting as a prompt or additional reminder. Of the 7 (54%) respondents who did not feel that the app helped them, 2 (29%) explained that they would have taken their medications regardless. Five (38%) respondents stated that they were “very likely” to continue using the app as they found it helpful and well-integrated into their daily routines. Of the reminder, 2 (15%) said “unlikely” to continue using the app without further elaboration and the rest (46%) chose not to answer that question. Nevertheless, approximately half of respondents (54%) would recommend the app to other patients with HF.
Self-efficacy with medication adherence was subjectively measured with the SEAMS (24). Both control and intervention arm participants had high self-reported medication adherence across all time points (Supplementary Table S5). While there was no difference between groups at baseline or 6-months follow-up, the intervention arm had a marginally significant (p = 0.049) increase in the median SEAMS score over this period (Table 4).
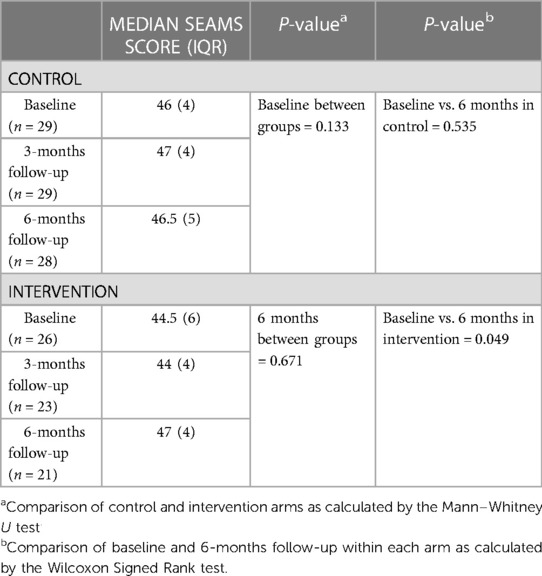
Table 4. Median SEAMS scores for the control and intervention arms at baseline, 3- and 6-months follow-up.
Even though nearly all control and intervention arm participants (93% vs. 88% respectively, p = 0.549) believed that their medications were improving their health, most participants (79% vs. 85% respectively, p = 0.61) were able to recall a time when they did not take all prescribed medication doses. Recurring themes were forgetfulness, running out of medication, unpleasant side effects (including dizziness or frequent urination from diuretics), “pill burden”, distraction, and loss of routine.
Previous research has shown that individuals with a chronic health condition may become “non-adherent” with medication after 6-months of treatment (37, 38). When asked if this scenario applied, significantly fewer control than intervention arm participants (17% vs. 42% respectively, p = 0.041) confirmed that it did. Whilst most participants (90% vs. 96% respectively, p = 0.354) stated that they would tell their health professionals if non-adherent, approximately half (52% vs. 58% respectively, p = 0.657) reported never being asked if they were taking or using their medications. Due to the infrequent or sporadic nature of non-adherence, 14 (48%) control and 9 (35%) intervention arm participants considered that volunteering this information to their health professional was irrelevant.
Although only 52% of control and 62% of intervention participants knew the names of all of their medications at baseline (p = 0.463), most (83% vs. 96% respectively, p = 0.111) participants could visually identify them (Supplementary Table S3 and S4) at baseline, without change at 6 months. Knowledge of actual or potential drug interactions was lacking for participants in both arms at baseline and 6-months follow-up (Supplementary Table S3 and S4). While the knowledge deficit at each interval was more prominent for controls, the difference between groups was not significant.
Apart from a significant improvement in pain or discomfort at 6 months in the control arm (p = 0.039), all the other aspects of health domains tested in the EQ-5D-5l were not significant within and between the groups at the three time points. Results from the SF-36v2 indicating control arm participants experienced significantly less physical health (p = 0.003) and emotional health (p = 0.006) problems by 6-months follow-up is concordant with their substantially greater limitations at baseline when compared with intervention arm participants (Supplementary Table S5 and S6). Further, both measures were not significantly different by 6 months for the participants in the intervention arm. Additionally, unlike control arm participants, the intervention arm had a statistically significant (p = 0.032) improvement in the median score of part A of the SCHFI between baseline and 6 months (Supplementary Table S7). Emotional states measured by the DASS-21 and parts B and C of the SCHFI showed no significant changes in either group by the end of the study period.
This is the first study to assess the acceptability, feasibility, and value of the MedicineWise app by extending the app's capabilities by including a tiered, pharmacist-led intervention to address medication non-adherence in patients with HF. Our findings suggest that this approach was acceptable to HF patients. Although delivery of Tier 1 intervention was feasible due to automation, Tiers 2 and 3 interventions required more input from the Medicines Line pharmacists' team. An improvement in the SEAMS between baseline and 6-months follow-up in the intervention participants shows the potential value of the app in supporting medication adherence of these patients. However, the secondary outcome measures remained unchanged for the duration of the study.
As shown in previous studies, it is possible that with adequate training, older patients can successfully incorporate technology into their daily lives (17, 26, 39, 40). Even though the app training was well received by the majority (77%), about half (54%) of survey respondents stated that they would have lacked confidence to use the app without training. The subsequent ease with which the MedicineWise app was integrated into the participants' daily lives showed that an older population can be adequately trained to use mHealth apps and to receive meaningful outcomes while achieving satisfaction from ongoing engagement (26, 39).
The alarm reminders of Tier 1 intervention acted as a safeguard against known contributors to non-adherence, including distraction and forgetfulness. Park et al. (2019) (20) similarly concluded that mHealth apps could overcome these barriers and support medication-taking behavior in older individuals with cardiovascular disease. In this study, tapping “TAKEN” provided visual reassurance for some participants that medication had been administered, which eased reliance upon memory and concern of potential non-adherence. However, as shown by Andre et al. (2019) (16), these prompts lost their usefulness in participants who have already established a daily routine for taking their medications.
Intervention participants who “missed” a dose of a critical medication for 24 h or a non-critical medication for 3 days received a Tier 2 “push” notification message within the MedicineWise app. Even though, 43% of the total 749 Tier 2 messages were sent because of technical issues, most survey respondents (90%) who received the messages were satisfied and did not suggest further improvement. This is consistent with prior research where text message-based interventions to support medication adherence were well received by patients with chronic diseases (41). Reassuringly, none of the 96 Tier 3 interventions identified medication non-adherence. Instead, non-adherence with the app was responsible for 41 (43%) of these phone calls. Telephone follow-ups have been successfully used by clinicians, particularly nurses, to support community-dwelling patients with chronic diseases (20, 42, 43).
Intervention participants, who received the tiered intervention, confirmed the value of the Medicine Line pharmacists when surveyed. They demonstrated an improvement in SEAMS-measured medication adherence between baseline and 6-months, in contrast to control participants who did not demonstrate a similar change, reinforcing the usefulness of health professional involvement as previously reported.
Most of the secondary measures remained unchanged across the study. While this study did not feature an educational component to improve health literacy, neither group was better able to name or visually identify their medications, verify that correct medications were supplied, or state understanding of actual or potential drug interactions, when compared to baseline. This suggests that clinicians may need to utilize a multi-modal approach including individualized education to improve patients' medication knowledge as suggested by Sheilini et al. (2019) (44). The intervention did not have a significant effect on health-related “quality of life” across timepoints. Depression scores were maintained within the “normal” range, although reduction in median scores was greater for the intervention group. However, this was not observed in the other two domains of the DASS-21. Up to a third of patients with HF experience depression and even more exhibit depressive symptoms (45). Either the prevalence of mental illness in this cohort was too low to demonstrate a significant change or improved medication adherence alone may not be sufficient to improve emotional states of depression, anxiety, or stress in patients with HF.
The MedicineWise app assumes that medications had been administered when the “TAKEN” icon was tapped. This may have caused reporting biases as intervention-arm participants could have acted in accordance with socially desired behavior by tapping “TAKEN” even if doses of medication were missed. The reverse might also have been true whereby medications were administered but the “TAKEN” icon not tapped. The possible discordance between medication administration and tapping “TAKEN” was a limitation when interpreting study results and determining implications for clinical practice. These assumptions are a recognized drawback of objective adherence assessment methods (46). However, this was mitigated if the participant was escalated to a Tier 3 intervention because a Medicines Line pharmacist could ascertain reasons for app non-adherence (e.g., forgetfulness or technical difficulties) and confirm whether the patient was taking their medications.
Inclusion of participants with multiple device models, different operating systems, and in particular older device models and operating systems, may have contributed to the technical issues observed during the study. The rapid change in smartphone technology “forces” app development teams to use “digital languages” that are compatible with both older and newer device models and operating systems. This has been highlighted to be a common challenge in the consumer app space contributing to high user attrition rates and unreliable engagement with mHealth (11, 12). Technical issues encountered during this study highlight the vital importance of ongoing app maintenance and user support. The Medicines Line pharmacists provided a valuable link between participants and Consumer Support to resolve app-related issues, by a series of updates, “hotfixes,” and troubleshooting provided by the app development team, Consumer Support service and the research team.
The COVID-19 pandemic posed a significant recruitment challenge for this study. Prior to the implementation of local health guidelines for COVID-19 infection control, study participants were referred from HF clinics and exercise classes. With the conversion of the former to telehealth and the closure of the latter, patients with HF were comparatively “out of sight.” As clinicians reported an increase in workload from changes in healthcare delivery, the challenges of usual roles and added responsibilities likely impeded their capacity to identify and refer potentially eligible patients with HF, impacting on participant recruitment.
The relatively small sample size may have resulted in potential Type II errors and limited the strength of these findings. Ideally, replicating this study with a larger sample size could strengthen the power of between group analyses to detect changes in secondary outcome measures. Extending the follow-up period beyond 6-months may provide added insight into longer-term acceptability, feasibility, and value of the tiered intervention in supporting medication adherence in patients with HF.
Additionally, interventions that support medication-taking behavior may prove more beneficial for patients with a prior history of non-adherence. As there was already a high level of adherence in this cohort, most of the secondary aims remained unchanged. Future research could add the criterion of including only those with a history of non-adherence. However, non-adherence is notoriously difficult to quantify and the feasibility of busy clinicians being able to screen and refer patients must be considered.
The rise in smartphone ownership has piqued interest in mHealth as a strategy to address medication non-adherence. While evidence supports its use, mHealth alone may not be the solution to such a complex problem. Although the use of mHealth and mobile phone app became more critical during the pandemic lockdowns and restrictions, they have an established value to aiding patients with all aspects of their health and medications (47). Mobile phone apps are another tool in a community pharmacy environment where pharmaceutical care has become a broader, more demanding, and holistic endeavor (48). The value of mobile phone apps could be enhanced within a more complex, structured program as shown previously (49) and here. They would be more effective with strong pharmacist support, but this support should be balanced with workload and perceived financial disadvantages (50).
A major finding of this study was that the utilization of the MedicineWise app in a tiered, pharmacist-led intervention was acceptable to patients with HF. However, initial training was necessary to enhance confidence in navigating the app, particularly for anyone with lower baseline technology proficiency. Nevertheless, technical issues could limit ongoing app engagement without access to Consumer Support services. Tier 1 interventions were easily delivered, but Tiers 2 and 3 interventions, although valuable, required additional resources. In conclusion, this pilot study provides evidence that incorporating a smartphone medication reminder app into a tiered intervention in a real-world situation is acceptable, feasible, and is able to support the medication adherence of patients with HF.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservations.
The studies involving humans were approved by CALHN Human Research Ethics Committee (R20190302) and UniSA Human Research Ethics Committee (202450). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
VS: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing – review & editing. JC-G: Formal Analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. NP: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Supervision, Writing – review & editing. KY: Data curation, Formal Analysis, Writing – review & editing. GG: Conceptualization, Resources, Writing – review & editing. CP: Conceptualization, Writing – review & editing. AP: Conceptualization, Writing – review & editing. MA: Resources, Writing – review & editing. CB: Resources, Writing – review & editing. AC: Resources, Writing – review & editing. TW: Resources, Writing – review & editing. EH: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
Funding for this study was independently provided to NPS MedicineWise and the University of South Australia by the Innovation Connections Grant scheme by the Department of Industry, Innovation and Science and NPS VentureWise Pty Ltd. (grant no. ICG000775 and ICG000776). NPS MedicineWise would like to acknowledge funding from the Australian Government Department of Health to develop and maintain the MedicineWise app. JC-G was supported by the Australian Government Research Training Program (RTP) fee offset scholarship to conduct this research as part of her Masters by Research degree at the University of South Australia. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
The authors would like to acknowledge the support of the following clinical staff: Dale Ashby, Shirley Chui, Cassandra Potts, Susan Edwards, Dale Thompson, Gregory Roberts, Tim Pearson, Lyn Chan, Ning He, Jeff Briggs, Hayley Surman and Natalie Simpson for sharing our vision and assisting with the design and/or implementation of this study. In addition, Emily Aldridge and Greer Dymmott assisted in getting site-specific approvals. We would also like to acknowledge the continuous support of the NPS MedicineWise/Medicines Line pharmacists, the NPS MedicineWise Consumer Support service and technical support team for delivering the tiered intervention, supporting the study participants, and assisting with technical issues.
NP and KY were employees of NPS MedicineWise at the time of the study.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdgth.2023.1274355/full#supplementary-material
1. Sahle BW, Owen AJ, Mutowo MP, Krum H, Reid CM. Prevalence of heart failure in Australia: a systematic review. BMC Cardiovasc Disord. (2016) 16:32. doi: 10.1186/s12872-016-0208-4
2. Nadrian H, Shojafard J, Mahmoodi H, Rouhi Z, Rezaeipandari H. Cognitive determinants of self-care behaviors among patients with heart failure: a path analysis. Health Promot Perspect. (2018) 8(4):275–82. doi: 10.15171/hpp.2018.39
3. Talmor G, Nguyen B, Keibel A, Temelkovska T, Saxon L. Use of software applications to improve medication adherence and achieve more integrated disease management in heart failure. Trends Cardiovasc Med. (2018) 28(7):483–8. doi: 10.1016/j.tcm.2018.04.001
4. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC) with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. (2021) 42(48):4901. doi: 10.1093/eurheartj/ehab670
5. Atherton JJ, Sindone A, De Pasquale CG, Driscoll A, MacDonald PS, Hopper I, et al. National heart foundation of Australia and cardiac society of Australia and New Zealand: guidelines for the prevention, detection, and management of heart failure in Australia 2018. Heart Lung Circ. (2018) 27(10):1123–208. doi: 10.1016/j.hlc.2018.06.1042
6. Shah A, Gandhi D, Srivastava S, Shah KJ, Mansukhani R. Heart failure: a class review of pharmacotherapy. Pharm Ther. (2017) 42(7):464–72.
7. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. J Card Fail. (2017) 23(8):628–51. doi: 10.1016/j.cardfail.2017.04.014
8. Fitzgerald AA, Powers JD, Ho PM, Maddox TM, Peterson PN, Allen LA, et al. Impact of medication nonadherence on hospitalizations and mortality in heart failure. J Card Fail. (2011) 17(8):664–9. doi: 10.1016/j.cardfail.2011.04.011
9. Unverzagt S, Meyer G, Mittmann S, Samos FA, Unverzagt M, Prondzinsky R. Improving treatment adherence in heart failure. Dtsch Arztebl Int. (2016) 113(25):423–30. doi: 10.3238/arztebl.2016.0423
10. Riles EM, Jain AV, Fendrick AM. Medication adherence and heart failure. Curr Cardiol Rep. (2014) 16(3):458. doi: 10.1007/s11886-013-0458-z
11. Hincapie AL, Gupta V, Brown SA, Metzger AH. Exploring perceived barriers to medication adherence and the use of mobile technology in underserved patients with chronic conditions. J Pharm Pract. (2019) 32(2):147–53. doi: 10.1177/0897190017744953
12. Davies MJ, Kotadia A, Mughal H, Hannan A, Alqarni H. The attitudes of pharmacists, students and the general public on mHealth applications for medication adherence. Pharm Pract. (2015) 13(4):644. doi: 10.18549/PharmPract.2015.04.644
13. Viana M, Laszczynska O, Mendes S, Friões F, Lourenço P, Bettencourt P, et al. Medication adherence to specific drug classes in chronic heart failure. J Manag Care Spec Pharm. (2014) 20(10):1018–26. doi: 10.18553/jmcp.2014.20.10.1018
14. Calvin JE, Shanbhag S, Avery E, Kane J, Richardson D, Powell L. Adherence to evidence-based guidelines for heart failure in physicians and their patients: lessons from the heart failure adherence retention trial (HART). Congest Heart Fail. (2012) 18(2):73–8. doi: 10.1111/j.1751-7133.2011.00263.x
15. Gandapur Y, Kianoush S, Kelli HM, Misra S, Urrea B, Blaha MJ, et al. The role of mHealth for improving medication adherence in patients with cardiovascular disease: a systematic review. Eur Heart J Qual Care Clin Outcomes. (2016) 2(4):237–44. doi: 10.1093/ehjqcco/qcw018
16. Andre N, Wibawanti R, Siswanto BB. Mobile phone-based intervention in hypertension management. Int J Hypertens. (2019) 2019:1–7. doi: 10.1155/2019/9021017
17. Dayer L, Heldenbrand S, Anderson P, Gubbins PO, Martin BC. Smartphone medication adherence apps: potential benefits to patients and providers. J Am Pharm Assoc (2003). (2013) 53(2):172–81. doi: 10.1331/JAPhA.2013.12202
18. Godinho MA, Jonnagaddala J, Gudi N, Islam R, Narasimhan P, Liaw S-T. Mhealth for integrated people-centred health services in the western pacific: a systematic review. Int J Med Inform. (2020) 142:104259. doi: 10.1016/j.ijmedinf.2020.104259
19. Pérez-Jover V, Sala-González M, Guilabert M, Mira JJ. Mobile apps for increasing treatment adherence: systematic review. J Med Internet Res. (2019) 21(6):e12505. doi: 10.2196/12505
20. Park JYE, Li J, Howren A, Tsao NW, De Vera M. Mobile phone apps targeting medication adherence: quality assessment and content analysis of user reviews. JMIR Mhealth Uhealth. (2019) 7(1):e11919. doi: 10.2196/11919
21. Tripoliti EE, Karanasiou GS, Kalatzis FG, Naka KK, Fotiadis DI. The evolution of mHealth solutions for heart failure management. Adv Exp Med Biol. (2018) 1067:353–71. doi: 10.1007/5584_2017_99
22. MedicineWise app. Available at: https://www.nps.org.au/medicinewiseapp (Accessed April 14, 2023) (2023).
23. Introducing dose tracker on the MedicineWise app. Available at: https://www.nps.org.au/medicinewiseapp-dose-tracker#it%E2%80%99s-time-to-test-drive-medicinewise-app (Accessed April 14 2023) (2023).
24. Risser J, Jacobson TA, Kripalani S. Development and psychometric evaluation of the self-efficacy for appropriate medication use scale (SEAMS) in low-literacy patients with chronic disease. J Nurs Meas. (2007) 15(3):203–19. doi: 10.1891/106137407783095757
25. Chapman-Goetz J, Packham N, Gabb G, Potts C, Yu K, Prosser A, et al. Acceptability and feasibility of the NPS MedicineWise mobile phone application in supporting medication adherence in patients with chronic heart failure: protocol for a pilot study. PLoS One. (2022) 17(2):e0263284. doi: 10.1371/journal.pone.0263284
26. Searcy RP, Summapund J, Estrin D, Pollak JP, Schoenthaler A, Troxel AB, et al. Mobile health technologies for older adults with cardiovascular disease: current evidence and future directions. Curr Geriatr Rep. (2019) 8(1):31–42. doi: 10.1007/s13670-019-0270-8
27. Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. (2013) 4:863. doi: 10.3389/fpsyg.2013.00863
28. Bowry AD, Shrank WH, Lee JL, Stedman M, Choudhry NK. A systematic review of adherence to cardio-vascular medications in resource-limited settings. J Gen Intern Med. (2011) 26:1479–91. doi: 10.1007/s11606-011-1825-3
29. Granger BB, Bosworth HB. Medication adherence: emerging use of technology. Curr Opin Cardiol. (2011) 26:279–87. doi: 10.1097/HCO.0b013e328347c150
30. Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. (2009) 41:1149–60. doi: 10.3758/BRM.41.4.1149
31. Nowell LS, Norris JM, White DE, Moules NJ. Thematic analysis: striving to meet the trustworthiness criteria. Int J Qual Methods. (2017) 16:1–13. doi: 10.1177/1609406917733847
32. Braun E, Baidusi A, Alroy G, Azzam ZS. Telephone follow-up improves patients satisfaction following hospital discharge. Eur J Intern Med. (2009) 20(2):221–5. doi: 10.1016/j.ejim.2008.07.021
33. Herdman M, Gudex C, Lloyd A, Janssen MF, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5l). Qual Life Res. (2011) 20(10):1727–36. doi: 10.1007/s11136-011-9903-x
34. Rand Corporation. 36-item short form survey instrument Available at: https://www.rand.org/health/surveys_tools/mos/mos_core_36item_survey.html (Accessed April 10, 2019) (2015).
35. Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the depression anxiety stress scales (DASS) with the beck depression and anxiety inventories. Behav Res Ther. (1995) 33(3):335–43. doi: 10.1016/0005-7967(94)00075-u
36. Riegel B, Lee CS, Dickson VV, Carlson B. An update on the self-care of heart failure index. J Cardiovasc Nurs. (2009) 24(6):485–97. doi: 10.1097/JCN.0b013e3181b4baa0
37. Wood B. Medication adherence: the real problem when treating chronic conditions. US Pharm. (2012) 373:3–6.
38. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Prac. (2011) 86:304–14. doi: 10.4065/mcp.2010.0575
39. Mortara A, Vaira L, Palmieri V, Iacoviello M, Battistoni I, Iacovoni A, et al. Would you prescribe mobile health apps for heart failure self-care? An integrated review of commercially available mobile technology for heart failure patients. Card Fail Rev. (2020) 6:e13. doi: 10.15420/cfr.2019.11
40. Portz JD, Vehovec A, Dolansky MA, Levin JB, Bull S, Boxer R. The development and acceptability of a mobile application for tracking symptoms of heart failure among older adults. Telemed J E Health. (2018) 24(2):161–5. doi: 10.1089/tmj.2017.0036
41. Thakkar J, Kurup R, Laba T-L, Santo K, Thiagalingam A, Rodgers A, et al. Mobile telephone text messaging for medication adherence in chronic disease: a meta-analysis. JAMA Intern Med. (2016) 176(3):340–9. doi: 10.1001/jamainternmed.2015.7667
42. Ng R, Carter SR, El-Den S. The impact of mobile applications on medication adherence: a systematic review. Transl Behav Med. (2020) 10(6):1419–35. doi: 10.1093/tbm/ibz125
43. Ruppar TM, Cooper PS, Mehr DR, Delgado JM, Dunbar-Jacob JM. Medication adherence interventions improve heart failure mortality and readmission rates: systematic review and meta-analysis of controlled trials. J Am Heart Assoc. (2016) 5(6):e002606. doi: 10.1161/JAHA.115.002606
44. Sheilini M, Hande HM, Prabhu MM, Pai MS, George A. Impact of multimodal interventions on medication nonadherence among elderly hypertensives: a randomized controlled study. Patient Prefer Adherence. (2019) 13:549–59. doi: 10.2147/PPA.S195446
45. Celano CM, Villegas AC, Albanese AM, Gaggin HK, Huffman JC. Depression and anxiety in heart failure: a review. Har Rev Psychiatry. (2018) 26(4):175–84. doi: 10.1097/HRP.0000000000000162
46. Nguyen TM, La Caze A, Cottrell N. What are validated self-report adherence scales really measuring? A systematic review. Br J Clin Pharmacol. (2014) 77(3):427–45. doi: 10.1111/bcp.12194
47. Cen ZF, Tang PK, Hu H, Cavaco A, Zeng L, Lei SL, et al. Systematic literature review of adopting eHealth in pharmaceutical care during COVID-19 pandemic: recommendations for strengthening pharmacy services. BMJ Open. (2022) 12:e066246. doi: 10.1136/bmjopen-2022-066246
48. Contemporary Community Pharmacy Practice: White Paper. Available at: https://my.psa.org.au/s/article/CSI-CCPP-white-paper (Accessed April 14, 2023) (2023).
49. Spanakis M, Sfakianakis S, Kallergis G, Spanakis EG, Sakkalis V. Pharmacta: personalized pharmaceutical care eHealth platform for patients and pharmacists. J Biomed Inform. (2019) 100:103336. doi: 10.1016/j.jbi.2019.103336
Keywords: mobile health (mHealth), mobile phone application, heart failure, medication adherence, mobile technology
Citation: Chapman-Goetz J, Packham N, Yu K, Gabb G, Potts C, Prosser A, Arstall MA, Burdeniuk C, Chan A, Wilson T, Hotham E and Suppiah V (2023) NPS MedicineWise application in supporting medication adherence in chronic heart failure: an acceptability and feasibility pilot study. Front. Digit. Health 5:1274355. doi: 10.3389/fdgth.2023.1274355
Received: 8 August 2023; Accepted: 27 October 2023;
Published: 15 November 2023.
Edited by:
Davide Maria Cammisuli, Catholic University of the Sacred Heart, ItalyReviewed by:
Katherine Blondon, Hôpitaux universitaires de Genève (HUG), Switzerland© 2023 Chapman-Goetz, Packham, Yu, Gabb, Potts, Prosser, Arstall, Burdeniuk, Chan, Wilson, Hotham and Suppiah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vijayaprakash Suppiah dmlqYXkuc3VwcGlhaEB1bmlzYS5lZHUuYXU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.