- 1Division of Cardiovascular Medicine, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, MA, United States
- 2Division of Cardiovascular Medicine, Department of Medicine, Saint Vincent Hospital, Worcester, MA, United States
- 3Department of Internal Medicine, Central Michigan University, Mount Pleasant, MI, United States
- 4Department of Population and Quantitative Health Sciences, University of Massachusetts Chan Medical School, Worcester, MA, United States
- 5Department of Biomedical Engineering, University of Connecticut, Storrs, CT, United States
- 6Department of Pharmacy and Health Systems Sciences, School of Pharmacy, Northeastern University, Boston, MA, United States
Background: Increasing ownership of smartphones among Americans provides an opportunity to use these technologies to manage medical conditions. We examine the influence of baseline smartwatch ownership on changes in self-reported anxiety, patient engagement, and health-related quality of life when prescribed smartwatch for AF detection.
Method: We performed a post-hoc secondary analysis of the Pulsewatch study (NCT03761394), a clinical trial in which 120 participants were randomized to receive a smartwatch-smartphone app dyad and ECG patch monitor compared to an ECG patch monitor alone to establish the accuracy of the smartwatch-smartphone app dyad for detection of AF. At baseline, 14 days, and 44 days, participants completed the Generalized Anxiety Disorder-7 survey, the Health Survey SF-12, and the Consumer Health Activation Index. Mixed-effects linear regression models using repeated measures with anxiety, patient activation, physical and mental health status as outcomes were used to examine their association with smartwatch ownership at baseline.
Results: Ninety-six participants, primarily White with high income and tertiary education, were randomized to receive a study smartwatch-smartphone dyad. Twenty-four (25%) participants previously owned a smartwatch. Compared to those who did not previously own a smartwatch, smartwatch owners reported significant greater increase in their self-reported physical health (β = 5.07, P < 0.05), no differences in anxiety (β = 0.92, P = 0.33), mental health (β = −2.42, P = 0.16), or patient activation (β = 1.86, P = 0.54).
Conclusions: Participants who own a smartwatch at baseline reported a greater positive change in self-reported physical health, but not in anxiety, patient activation, or self-reported mental health over the study period.
Introduction
Atrial fibrillation (AF) is associated with a more than 3-fold higher stroke risk among US adults (1). An estimated 1 out of 5 patients who suffer a stroke ultimately have AF diagnosed (2). Stroke has significant adverse effects on patients' actions and quality of life (3, 4). Timely diagnosis of AF and subsequent initiation of anticoagulation therapy can prevent incident and recurrent strokes among older adults (2, 5). Prompt diagnosis of AF, however, remains challenging because of the often clinically silent and intermittent nature of this arrhythmia (2).
Extended ECG monitoring is often utilized in stroke survivors to capture this rhythm abnormality (5). Traditional methods to detect AF such as Holter monitors or implantable loop recorders are limited by their relative low diagnostic yield and invasive nature, respectively (6–8). The development of algorithms using photoplethysmography (PPG) recordings from smartwatches has been shown to be an accurate way to detect heart rhythm abnormalities (9–15). Recently, several wearable devices have been cleared by the FDA for incident AF detection (10). Smartphone and smartwatch ownership and familiarity among US adults over the age of 50 has steadily increased, with about 42% reporting almost daily use (16). As reported by Nuvvula et al. in the NexUS-Heart study, which included 327 AF and 895 older adults at risk for AF, around a third of participants in both groups reported owning a commercial wearable device and the majority had used one for longer than a year (17). About 46% of participants with AF and prior smartwatch ownership used these devices to share information from commercial wearables with their medical team (17). Additionally, a randomized trial of healthy young adults by Yen showed that use of wearables might have positive effects on physical health and quality of life (QoL) (18). A study by Filippaios et al. including older populations suggest a more complex relationship between smart device use and QoL, including potential adverse effects of rhythm alert notifications from a smartwatch on QoL (19).
Familiarity with technology used for AF detection and higher technology engagement at baseline may influence users' experiences with smartphones and wearables. For such devices to be deployed clinically in high-risk older populations, more information is needed. Using data from the randomized trial of older stroke survivors randomized to receive a smartwatch-smartphone dyad vs. conventional monitoring, we examined the potential influence of baseline smartwatch ownership on changes in self-reported anxiety, patient engagement, and health-related quality of life.
Methods
Study population
We analyzed data from the Pulsewatch study (NCT03761394) (12), a prospective, randomized controlled, multiphase study to assess the accuracy, feasibility, and compliance of a smartphone-smartwatch dyad for AF detection in patients who suffered from a stroke or transient ischemic attack (TIA). Individuals who meet the criteria to be included in the study were (1) more than 50 years of age; (2) had a history of stroke or TIA, and (3) were able to travel to the clinic at the University of Massachusetts Memorial Medical Center. Those with a serious physical illness that prevents interactions with the smart device, a contraindication for anticoagulation treatment, an incapacity to sign consent, an inability to read and write in English, an unwillingness to complete all study procedures, or plans to move from the area during the study period were excluded (12).
Study design
Phase I of the Pulsewatch study focused on accuracy and usability of the Pulsewatch system, whereas Phase II focused on adherence to smartwatch prescription for AF detection. At baseline, participants completed a questionnaire assessing their demographic and psychosocial factors. In Phase I, participants were randomized 3:1 into intervention or control groups. Participants in the intervention group were provided with a smartwatch (either Samsung Gear S3 or Galaxy Watch 3) and a Samsung smartphone with our Pulsewatch apps downloaded on them. Participants were asked to wear watches continuously and charge them daily over a 14-day period. In addition, they were given gold-standard FDA-approved Cardiac Insight Cardea Solo cardiac patch monitors (Seattle, WA) and they were asked to wear the patches continuously over a 14-day period. Participants in the control group were provided with only the cardiac patch monitor. At the end of the initial 14-day study period, participants completed a follow-up questionnaire and were once again randomized into either intervention or control groups (1:1). Participants in the intervention group were offered continuous use of the Pulsewatch system (Samsung smartwatch + Samsung Smartphone with study apps) for 30 days, as well as an FDA-approved AliveCor KardiaMobile device (Mountain View, CA) for AF detection, whereas participants from the control group did not receive any devices. University of Massachusetts Chan Medical School Institutional Review Board approved the study protocols (H00016067).
Self-reported outcomes
Participant information, including demographics, medical history, and psychosocial characteristics was collected by trained research staff (12). All participants completed validated study questionnaires at baseline, 14 days, and 44 days (if randomized to long-term use) to assess various psychosocial measures and health behaviors. We used the Generalized Anxiety Disorder-7 scale (GAD-7), a standardized 7-item questionnaire to assess generalized anxiety (20). The GAD-7 scale was scored from 0 to 3 with overall assessment scores ranging in severity from 0 to 21 (20). Higher scores of GAD-7 indicate higher anxiety levels (20). The Physical Component Summary (PCS) and Mental Component Summary (MCS) of the 12-Item validated Short Form Survey (SF-12) was administered to evaluate physical and mental health-related quality of life, respectively (21). With scores ranging from 0 to 100, higher SF-12 score demonstrates a better quality of life and general health perception (21). The Consumer participants' ability and willingness to manage his or her own health (22). App usability was captured through the Mobile Application Rating Scale (MARS) App Classification.
Statistical analysis
In this analysis, we included participants who received the smartwatch/smartphone dyad across both phases of the RCT and grouped them into those who owned a smartwatch at baseline vs. those who did not. Differences in baseline characteristics by smartwatch ownership were examined using Fisher's Exact Test and Wilcox Signed Rank Test for categorical and continuous variables respectively. Longitudinal mixed-effects linear regression was used to examine the relationship between psychosocial changes over time and smartwatch ownership prior to enrollment. Due to modest sample size, we did not adjust for any confounding variables. The psychosocial changes were quantified by the standard questionnaires completed by participants at baseline, 14 days, and 44 days. All statistical analyses were completed using SAS 9.4 (SAS Institute Inc. Cary, NC, USA).
Results
A total of 96 participants were randomized to receive a smartwatch-smartphone dyad for AF detection and completed 45 days of follow-up. The mean age of the participants was 65 ± 9 years, forty-one participants were women (43%), most of the study population was white (88%), over half had completed at least a bachelor's degree, and 68% were married (Table 1). Twenty-four participants owned smartwatches at baseline, whereas 72 participants did not report ownership of a smartwatch. We did not find statistically significant differences in the characteristics of those who owned smartwatches and those who did not with regards to demographic, medical history, and psychosocial characteristics at baseline (Tables 1, 2).
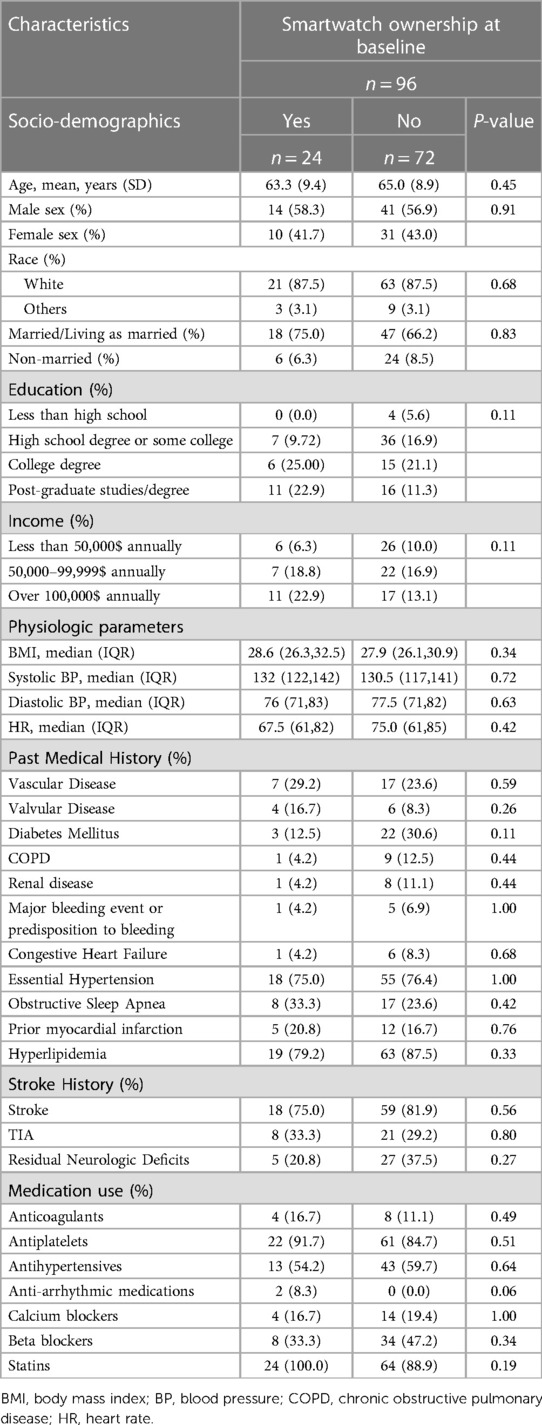
Table 1. Baseline demographics and medical history of participants stratified by smartwatch ownership.
Over the 44 day study period, we observed that self-reported physical health (SF-12 PCS, β = 5.07, P < 0.05) increased over time in participants who previously owned smartwatches as compared with those who did not, while anxiety (GAD-7, β = 0.87, P = 0.29), self-reported mental health (SF-12 MCS, β = −2.42, P = 0.16), and patient activation (CHAI, β = 1.86, P = 0.54) were not different (Table 3).
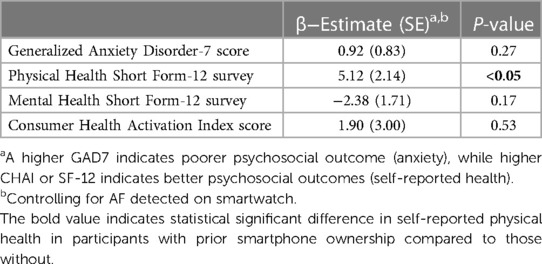
Table 3. Psychosocial changes after smartwatch prescription in participants with prior smartwatch ownership compared to those without.
Discussion
In this multiphase RCT of older stroke survivors randomized to receive a smartwatch for AF detection, we found that participants who previously owned smartwatches reported increased perceived physical health over the study period. No effect was detected on anxiety level, mental health status, or activation towards self-care.
Participants who previously owned smartwatches appear to report better overall physical health as measured by the SF-12 questionnaire after being prescribed a smartwatch for AF detection. It could be that familiarity with wearable devices empowered participants to monitor and engage in physical activities that leads to better perceived physical health. This change in perceived physical health is perhaps not mediated by anxiety or mental health as we did not observe differences reported in GAD-7 or SF12 MCS score over the study period with respect to prior smartwatch ownership. Consistent with our findings, Yen's analysis on another RCT comparing the use of smartwatches to traditional mobile applications in healthy young adults demonstrated better physical quality of life (QOL) in the smartwatch arm (Table 4) (18). Our results are further supported by findings of Macridis et al. demonstrating that consumer wearable ownership and use among Canadian adults is correlated with meeting physical activity guidelines (23). Additionally, awareness of how these wearable devices operate by these smartwatch owners also increased their comfort with using the study's devices which may have led to higher perceived physical health activity and quality of life.
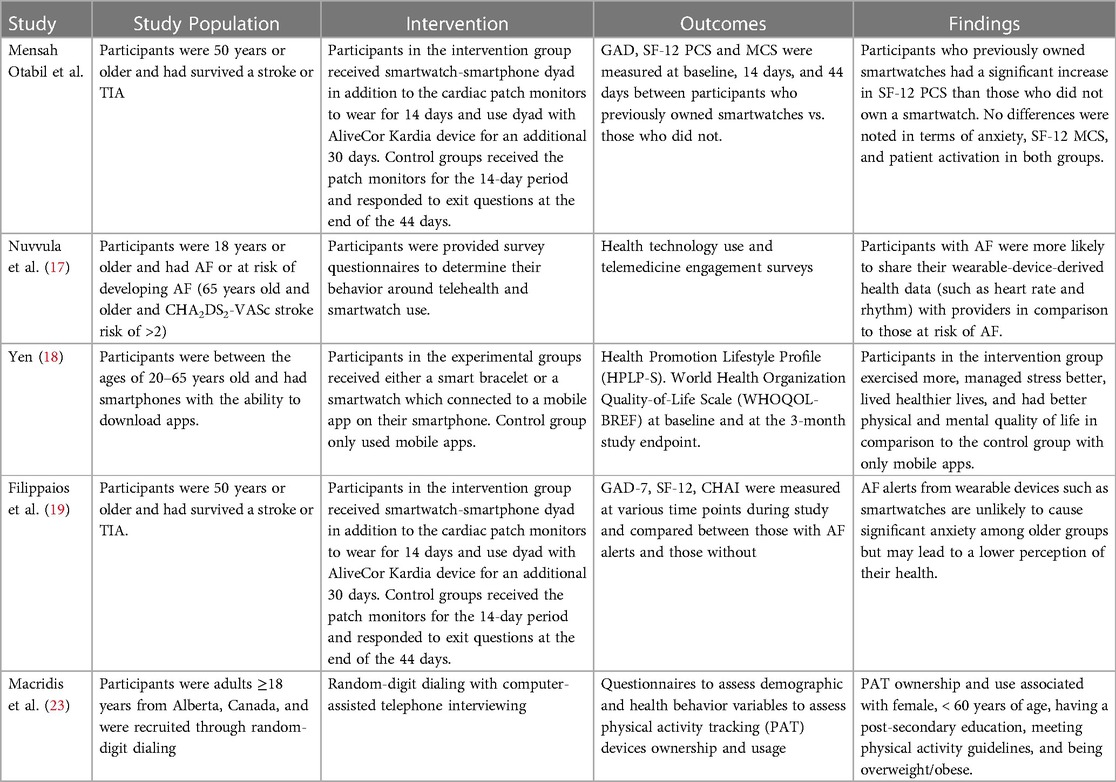
Table 4. Comparison of the current study design with other smartwatch atrial fibrillation (AF) and heart arrhythmia detection studies.
Despite the positive perception on physical health by prior owners of a smartwatch, their mental health status was not significantly different from that of participants who did not own a smartwatch. The mental health component of SF-12 questionnaire is a useful tool for monitoring mental status for targeted treatment (20). As a new treatment with a new device, it is important to evaluate whether such intervention would affect that individual's mental health. Continuous, instant monitoring was found to bring unnecessary anxiety to people who were using wearable devices for health purposes (23). Studies have shown that a repeated alert and notification system in wearable devices can trigger anxiety and other mental health issues in older populations. In our study, however, we used two validated assessments to evaluate psychological status change: mental health component of SF-12 and GAD-7 score. Both showed no difference between participants who previously owned a smart device and those who did not. Our results suggest that clinicians should not shy from prescribing smartwatch for AF screening in those with less exposure to digital technology as we find no significant differences in change in self-reported anxiety and mental health with respect to prior ownership of smartwatch.
Our work provides evidence of feasibility in using smartwatch devices in an elderly population and showed the potential positive health effects of their use. In our study, participants with prior use of smartwatches, reported higher perceived physical health when prescribed smartwatches for AF detection post stroke, in comparison to participants who had never used a smartwatch before. In this context, it might be helpful for providers to consider familiarity and baseline technology engagement of participants, when applying modern technologies in the routine monitoring and screening for atrial fibrillation. Prior ownership of smartwatches had no effect on changes in anxiety levels, mental health status, or participant activation during the time of smartwatch monitoring. Albeit further studies are necessary to investigate this association, a smartwatch-naïve population, adequately trained and educated by their medical teams, can likely benefit from rhythm monitoring via smartwatches. Lack of familiarity with smartwatch technology at baseline did not seem to have a negative impact on anxiety, self-perceived mental health and patient activation while using smartwatches for rhythm monitoring.
Strengths and limitations
Our cohort has several strengths. First, the cohort's participants had rigorous assessments of sociodemographic, clinical, and psychosocial characteristics. We utilized standardized questionnaires including GAD-7, CHAI, and SF-12 at several time-points to examine changes in, anxiety, patient activation, and health-related quality of life among participants in a randomized trial, increasing the generalizability and potential reproducibility of our study findings. However, participants were recruited from a single tertiary medical center in central Massachusetts. Most of our participants are white stroke survivors with some level of college education; this may limit generalization of our findings to other populations. Further, this is a post-hoc secondary analysis of a modestly sized cohort and may lack the power to detect potential changes of self-reported outcomes over a relatively short period of observation.
Conclusion
In our study of elderly stroke survivors randomized to receive a smartwatch and smartphone for AF monitoring and followed for 45 days, participants who owned a smartwatch at baseline reported better physical health than participants who did not own smartwatches. No differences were noted between smartwatch owners and non-owners as it relates to anxiety, mental health status and activation. Our study suggests that technology familiarity at baseline strongly influences patient experience and quality of life among those prescribed technologies for AF monitoring. Large studies are needed to further understand how best to improve health and well-being among older adults who are not familiar with digital devices. Studies evaluating mobile health approaches to AF detection should assess baseline technology familiarity and ownership.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by University of Massachusetts Chan Medical School Institutional Review Board protocol H00016067. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
DM and KC conceived the approach and designed the study. EMO, KN, AH, DD and ED recruited participants and collected the data. K-VT, AF, KN, ED, ZW, DL, DH, FM, JM, TP, AS, HL, JS, BB, KC, DM analyzed the data. All authors discussed the results. EMO, K-VT, ED, PA, QD, AF, JM, JS, DM wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The Pulsewatch Study is funded by R01HL137734 from the National Heart, Lung, and Blood Institute. Ding's time is supported by F30HL149335 from the National Heart, Lung, and Blood Institute. Filippaios's time is supported by NIH grant T32HL120823. Tran's time is supported by K23HL161432 from the National Heart, Lung, and Blood Institute. Mehawej's time is supported by NIH grant T32HL120823. McManus's time is supported by R01HL126911, R01HL137734, R01HL137794, R01HL135219, R01HL136660, U54HL143541, and 1U01HL146382 from the National Heart, Lung, and Blood Institute and Chon's time was supported by R01 HL137734.
Conflict of interest
DM reports receiving honorary fees, speaking/consulting fees, or research grants from FLEXcon, Heart Rhythm Society, Rose Consulting, Bristol-Myers Squibb, Pfizer, Boston Biomedical Associates, Avania, VentureWell, Samsung, Phillips, CareEvolution, Boehringer-Ingelheim, Biotronik, Otsuka Pharmaceuticals, and Sanofi; he also declares financial support for serving on the Steering Committee for the GUARD-AF study (ClinicalTrials.gov identifier NCT04126486) and Advisory Committee for the Fitbit Heart Study (ClinicalTrials.gov identifier NCT04176926). He reports non-financial research support from Apple Computer and Fitbit. The other authors have no competing interests.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yaghi S, Kamel H. Stratifying stroke risk in atrial fibrillation: beyond clinical risk scores. Stroke. (2017) 48(10):2665–70. doi: 10.1161/STROKEAHA.117.017084
2. Bernstein RA, Kamel H, Granger CB, Piccini JP, Sethi PP, Katz JM, et al. Effect of long-term continuous cardiac monitoring vs usual care on detection of atrial fibrillation in patients with stroke attributed to large- or small-vessel disease: the STROKE-AF randomized clinical trial. JAMA. (2021) 325(21):2169–77. doi: 10.1001/jama.2021.6470
3. Fatema Z, Sigamani A, Vikneswaran G, Manuel D. Quality of life at 90 days after stroke and its correlation to activities of daily living: a prospective cohort study. J Stroke Cerebrovasc Dis. (2022) 31(11):106806. doi: 10.1016/j.jstrokecerebrovasdis.2022.106806
4. Bártlová S, Šedová L, Havierniková L, Hudácková A, Dolák F, Sadílek P. Quality of life of post-stroke patients. Zdr Varst. (2022) 61(2):101–8. doi: 10.2478/sjph-2022-0014
5. Buck BH, Hill MD, Quinn FR, Butcher KS, Menon BK, Gulamhusein S, et al. Effect of implantable vs prolonged external electrocardiographic monitoring on atrial fibrillation detection in patients with ischemic stroke: the PER DIEM randomized clinical trial. JAMA. (2021) 325(21):2160–8. doi: 10.1001/jama.2021.6128
6. Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. (2014) 370(26):2478–86. doi: 10.1056/NEJMoa1313600
7. Ko D, Dai Q, Flynn DB, Bosch NA, Helm RH, Monahan KM, et al. Meta-analysis of randomized clinical trials comparing the impact of implantable loop recorder versus usual care after ischemic stroke for detection of atrial fibrillation and stroke risk. Am J Cardiol. (2022) 162:100–4. doi: 10.1016/j.amjcard.2021.09.013
8. Lee R, Mittal S. Utility and limitations of long-term monitoring of atrial fibrillation using an implantable loop recorder. Heart Rhythm. (2018) 15(2):287–95. doi: 10.1016/j.hrthm.2017.09.009
9. Strain T, Wijndaele K, Brage S. Physical activity surveillance through smartphone apps and wearable trackers: examining the UK potential for nationally representative sampling. JMIR Mhealth Uhealth. (2019) 7(1):e11898. doi: 10.2196/11898
10. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. (2019) 381(20):1909–17. doi: 10.1056/NEJMoa1901183
11. Rosenberg MA, Samuel M, Thosani A, Zimetbaum PJ. Use of a noninvasive continuous monitoring device in the management of atrial fibrillation: a pilot study. Pacing Clin Electrophysiol. (2013) 36(3):328–33. doi: 10.1111/pace.12053
12. Dickson EL, Ding EY, Saczynski JS, Han D, Moonis M, Fitzgibbons TP, et al. Smartwatch monitoring for atrial fibrillation after stroke-the pulsewatch study: protocol for a multiphase randomized controlled trial. Cardiovasc Digit Health J. (2021) 2(4):231–41. doi: 10.1016/j.cvdhj.2021.07.002
13. Bumgarner JM, Lambert CT, Hussein AA, Cantillon DJ, Baranowski B, Wolski K, et al. Smartwatch algorithm for automated detection of atrial fibrillation. J Am Coll Cardiol. (2018) 71(21):2381–8. doi: 10.1016/j.jacc.2018.03.003
14. White RD, Flaker G. Smartphone-based arrhythmia detection: should we encourage patients to use the ECG in their pocket? J Atr Fibrillation. (2017) 9(6):1605. doi: 10.4022/jafib.1605
15. Shah CH, Brown JD. Reliability and validity of the short-form 12 item version 2 (SF-12v2) health-related quality of life survey and disutilities associated with relevant conditions in the U. S. Older Adult Population. J Clin Med. (2020) 9(3):661. doi: 10.3390/jcm9030661
16. American Association of Retired Persons (AARP). 2022 Tech Trends and the 50-Plus. December 2021. Available at: https://www.aarp.org/content/dam/aarp/research/surveys_statistics/technology/2021/2022-technology-trends-older-amer icans.doi.10.26419-2Fres.00493.001.pdf (Accessed March 8, 2023).
17. Nuvvula S, Ding EY, Saleeba C, Shi Q, Wang Z, Kapoor A, et al. NExUS-heart: novel examinations using smart technologies for heart health-data sharing from commercial wearable devices and telehealth engagement in participants with or at risk of atrial fibrillation. Cardiovasc Digit Health J. (2021) 2(5):256–63. doi: 10.1016/j.cvdhj.2021.08.001
18. Yen HY. Smart wearable devices as a psychological intervention for healthy lifestyle and quality of life: a randomized controlled trial. Qual Life Res. (2021) 30(3):791–802. doi: 10.1007/s11136-020-02680-6
19. Filippaios A, Tran KT, Mehawej J, Ding E, Paul T, Lessard D, et al. Psychosocial measures in relation to smartwatch alerts for atrial fibrillation detection. Cardiovasc Digit Health J. (2022) 3(5):198–200. doi: 10.1016/j.cvdhj.2022.07.069
20. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. (2006) 166(10):1092–7. doi: 10.1001/archinte.166.10.1092
21. Huo T, Guo Y, Shenkman E, Muller K. Assessing the reliability of the short form 12 (SF-12) health survey in adults with mental health conditions: a report from the wellness incentive and navigation (WIN) study. Health Qual Life Outcomes. (2018) 16(1):34. doi: 10.1186/s12955-018-0858-2
22. Wolf MS, Smith SG, Pandit AU, Condon DM, Curtis LM, Griffith J, et al. Development and validation of the consumer health activation index. Med Decis Making. (2018) 38(3):334–43. doi: 10.1177/0272989X17753392
Keywords: atrial fibrillation detection, smartwatch, Pulsewatch, self-reported physical health, anxiety, patient activation, self-reported mental health
Citation: Mensah Otabil E, Dai Q, Anzenberg P, Filippaios A, Ding E, Mehawej J, Mathew JE, Lessard D, Wang Z, Noorishirazi K, Hamel A, Paul T, DiMezza D, Han D, Mohagheghian F, Soni A, Lin H, Barton B, Saczynski J, Chon KH, Tran K-V and McManus DD (2023) Technology engagement is associated with higher perceived physical well-being in stroke patients prescribed smartwatches for atrial fibrillation detection. Front. Digit. Health 5:1243959. doi: 10.3389/fdgth.2023.1243959
Received: 21 June 2023; Accepted: 20 November 2023;
Published: 6 December 2023.
Edited by:
Andreas Guenter Franke, University of Applied Labour Studies of the Federal Employment Agency, GermanyReviewed by:
Ivan Miguel Pires, Universidade da Beira Interior, PortugalMariana Alves, Universidade de Lisboa, Portugal
© 2023 Mensah Otabil, Dai, Anzenberg, Filippaios, Ding, Mehawej, Mathew, Lessard, Wang, Noorishirazi, Hamel, Paul, DiMezza, Han, Mohagheghian, Soni, Lin, Barton, Saczynski, Chon, Tran and McManus. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khanh-Van Tran a2hhbmgtdmFuLnRyYW5AdW1hc3NtZW1vcmlhbC5vcmc=
 Edith Mensah Otabil
Edith Mensah Otabil Qiying Dai2
Qiying Dai2 Eric Ding
Eric Ding Joanne E. Mathew
Joanne E. Mathew Darleen Lessard
Darleen Lessard Alexander Hamel
Alexander Hamel Tenes Paul
Tenes Paul Dong Han
Dong Han Fahimeh Mohagheghian
Fahimeh Mohagheghian Apurv Soni
Apurv Soni Honghuang Lin
Honghuang Lin Bruce Barton
Bruce Barton Jane Saczynski
Jane Saczynski Ki H. Chon
Ki H. Chon Khanh-Van Tran
Khanh-Van Tran