
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Digit. Health , 02 September 2022
Sec. Health Informatics
Volume 4 - 2022 | https://doi.org/10.3389/fdgth.2022.958284
This article is part of the Research Topic Surfacing Best Practices for AI Software Development and Integration in Healthcare View all 10 articles
As the implementation of artificial intelligence (AI)-enabled tools is realized across diverse clinical environments, there is a growing understanding of the need for ongoing monitoring and updating of prediction models. Dataset shift—temporal changes in clinical practice, patient populations, and information systems—is now well-documented as a source of deteriorating model accuracy and a challenge to the sustainability of AI-enabled tools in clinical care. While best practices are well-established for training and validating new models, there has been limited work developing best practices for prospective validation and model maintenance. In this paper, we highlight the need for updating clinical prediction models and discuss open questions regarding this critical aspect of the AI modeling lifecycle in three focus areas: model maintenance policies, performance monitoring perspectives, and model updating strategies. With the increasing adoption of AI-enabled tools, the need for such best practices must be addressed and incorporated into new and existing implementations. This commentary aims to encourage conversation and motivate additional research across clinical and data science stakeholders.
As the implementation of artificial intelligence (AI)-enabled tools is realized across diverse clinical environments, there is a growing understanding of the need for ongoing monitoring and updating of prediction models (1–5). Beyond initial validation and local tailoring of models transported across settings, temporal deterioration in model accuracy after development has been documented across clinical domains and settings (6–10). Neither regression nor advanced machine learning algorithms are exempt from these temporal changes in performance (8, 11). Such performance drift degrades the clinical utility of AI-enabled tools, jeopardizes user trust, and poses safety concerns when insufficiently accurate predictions are used in decision-making (1, 6, 12, 13).
Dataset shift (14)—temporal changes in clinical practice, patient populations, and information systems—is well-documented as a source of performance drift and recognized as a challenge to the sustainability of AI-enabled tools in clinical care (6–8, 15–17). Model developers and system managers have access to a variety of approaches to address performance drift and underlying dataset shift in order to restore model performance to clinically acceptable levels. In some cases, model performance may be restored by correcting technical errors introduced by structural changes in information systems, such as implementation of revised data standards. However, in many cases where dataset shift is more nuanced and multifaceted, model updating through recalibration, retraining, or revision will be required. While best practices are well-established for training and validating new AI models (18), there is limited guidance on prospective validation and few best practices for model monitoring and updating.
In this paper, we highlight the need for maintaining clinical prediction models and discuss open questions regarding this critical aspect of the AI modeling lifecycle. First, we illustrate performance drift across models implemented in the production electronic health record (EHR) system at an academic medical center. Second, we discuss several open research questions and describe the nuances required for best practice guidance. Despite advances in continuous learning algorithms that evolve models as data accrue, such algorithms are subject to additional challenges and healthcare applications still predominantly rely on static models that will require periodic updating (19). Although we focus our discussion on updating static models, similar questions may arise around surveillance practices for continuous learning models.
Most studies documenting temporal model performance have been conducted in registry or research datasets rather than with operational data from models running in real-time clinical settings (7–9, 16). However, the transition from a retrospective research frame to real-time operational implementation may impact performance as input mappings change and the timing data availability shifts (20–22). To explore performance drift in an operational setting, we evaluated the performance of two models currently implemented in the production EHR system at Vanderbilt University Medical Center (VUMC): a non-proprietary, externally developed model predicting readmission (LACE+) (23) and a locally developed model predicting suicidal behaviors (Vanderbilt Suicide Attempt and Ideation Likelihood model, VSAIL) (24).
Table 1 provides an overview of each model, highlighting differences in modeling methods, training cohorts, and intended use. We extracted stored predictions calculated in real-time and outcomes associated with each prediction using data available in VUMC's EHR. For the LACE+ model, we note that this approach may undercount readmissions if patients were readmitted to a different medical facility. Monthly performance was evaluated using metrics relevant to each model's intended use. We measured the mean calibration of the LACE+ readmission model with the observed to expected outcome ratio (O:E) and clinical utility of the VSAIL suicidality model with the number needed to screen (NNS; the inverse of positive predictive value).
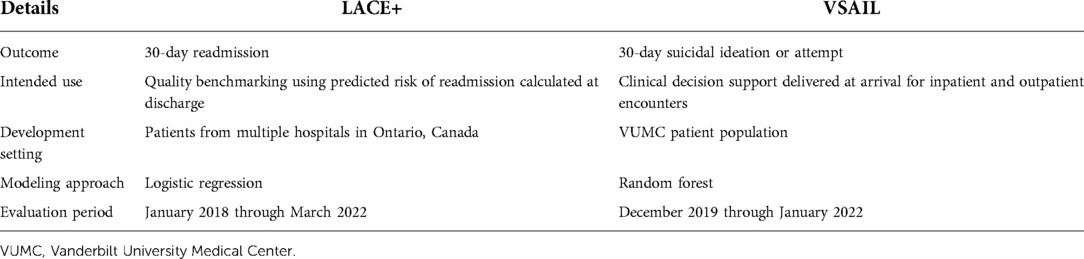
Table 1. Prediction models evaluated for temporal validation of real-time scores generated within a production electronic medical record system.
The LACE+ model, locally calibrated to the VUMC population, sustained performance over the evaluation period (Figure 1A). Monitoring highlighted the importance of distinguishing noise from both informative local change in performance and true model deterioration. Over the first 2.5 years, variability in observed O:Es did not follow a significant trend. In the last year of evaluation, however, there may be a trend toward lower O:Es. Depending on the use case, this declining O:E could be seen as indicating improved local quality (i.e., reducing readmissions) or increasing miscalibration. We note that O:E, a crude measure of calibration, may conceal calibration drift within clinically important risk ranges (25). VSAIL maintained a relatively stable NNS during the first year of implementation (median monthly NNS = 19), with the NNS abruptly increasing in February 2021 (median monthly NNS = 136); Figure 1B). This shift corresponds to operational changes in implementation, with the model being applied to a much broader patient population. Within the original population, VSAIL's NNS remained stable (median monthly NNS = 22). The higher NNS in the broader population may still be feasible but should be considered in the implementation team's cost-benefit analysis and may warrant further investigation of performance in select clinical settings or subpopulations. These findings illustrate performance drift in a single health system's EHR and contribute to the mounting evidence that AI-enabled tools require long-term strategies to understand performance trajectories and maintain utility.
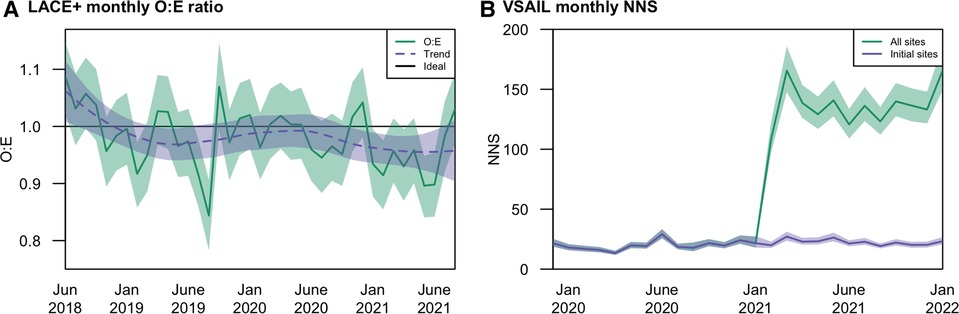
Figure 1. Temporal performance at Vanderbilt University Medical Center of the (A) LACE+ readmission model in terms of mean calibration (O:E); and (B) VSAIL suicidality model in terms of number needed to screen (NNS).
Despite concerns over the long-term stability of model performance, health systems lack generalizable guidance for operationalizing post-implementation maintenance strategies. To develop guidance and establish best practices, additional research and debate are needed in three focus areas: model maintenance policies, performance monitoring perspectives, and model updating strategies (Table 2).
Oversight policies at the health system level could facilitate the maintenance of a portfolio of models by defining a consistent, systematic groundwork for sustaining both new and existing AI-enabled tools. System-level policies can also inform use case parameters to consider when establishing model-specific maintenance plans.
Health systems certainly have a right and duty to monitor the local performance of the AI models they implement, regardless of where those models originated. However, how to address deteriorations in performance is complicated by model ownership and licensing restrictions. For models developed in-house and local implementations of models in the public domain, health systems have full control over maintenance approaches and may consider the full spectrum of updating methods. At VUMC, the local VSAIL model will be retrained using more recent data and subsequently maintained through a tailored data-driven surveillance approach. When models are developed in collaboration across health systems, best practices could guide collaborative updating and establishment of parameters for local model adjustments.
Updating proprietary models is particularly challenging, despite locally documented drift having required the deactivation of proprietary AI-enabled clinical tools (6). Licenses may restrict updating by not permitting local model recalibration or retraining. Updating options may be further limited by inadequate documentation of training methods (26). Proactive updating of proprietary models by model owners, such as semi-annual updates of the National Surgical Quality Improvement Program (ACS NSQIP) risk models, may alleviate some, but not all, of the need for local updating options. Health systems, national organizations, and policymakers should advocate for more complete documentation of proprietary models and increased access to updating options. This may include the relaxation of local updating restrictions; clear documentation of owner-driven maintenance plans; and proactive, transparent dissemination of updated models to all customers. Enabling such expectations of model owners will require more detailed and consistent guidance on model updating practices covering the concerns described throughout this paper.
Model fairness is now recognized as a critical element of clinical AI models (27, 28). While model fairness comprises a broad set of concerns regarding implementation practices, user uptake and application, and sociotechnical contexts of use (29), fairness also requires models to perform similarly across demographic groups. Establishing initial comparable model performance across subpopulations and subsequently maintaining comparable performance within these groups is thus critical to ensuring model fairness. Novel metrics for evaluating algorithmic fairness across subpopulations are providing insight during model validation and selection of models for implementation in clinical tools (30, 31). A clear next step is to incorporate these new metrics and performance within subpopulations into model monitoring to evaluate fairness over time. This poses new questions regarding how to handle the potential for fairness drift, defined as differential performance drift across subpopulations. Researchers and policymakers will need to address tests for temporal changes in fairness metrics; methods for updating models experiencing fairness drift that prioritize equitable utility for all patients; and whether and when changes in model fairness warrant pausing AI-enabled tools to avoid creating or exacerbating disparities.
Open communication between modeling teams and clinical end users is essential to the monitoring and maintenance phase of the AI lifecycle. End users may identify failing AI-enabled tools before performance monitors detect changes in accuracy. They may also provide insight when models are no longer useful from a clinical perspective even with sustained performance, allowing tools to be de-implemented or revised as needed. At the same time, modeling teams should establish policies for disseminating information about model updates to end-users, whether updating is driven by end-user concerns, local model maintenance efforts, or new releases of proprietary models. Such communication, while particularly important for reestablishing trust in models updated in response to end-user concerns, is relevant for all updates. Model maintenance programs need to include specific strategies for this bidirectional communication. Such engagement and transparency regarding model maintenance may also increase acceptance of AI more broadly by assuring users that models are actively being curated, monitored, and assessed with an eye to promoting utility and safety.
The appropriate mode of communication and level of detail provided about model updates are likely to use case-dependent. The ACS NSQIP surgical risk calculator, for example, displays a banner message highlighting recent updates, setting expectations for any noticeable changes in predictions, and eliciting feedback if concerns arise (32). Extensive model revisions or reimplementation of a paused model with restored performance may require more explanation than a banner message can effectively convey. Workflow and communication experts will be key collaborators in designing best practices for disseminating information on model updates. These best practices will likely need to evolve as the health care workforce becomes better trained in AI.
Ongoing monitoring provides necessary insight into model stability and can alert model managers to concerning performance trends in need of intervention (3, 33, 34). However, insights from monitoring require careful determinations of how model performance is defined and evaluated.
AI models, even when operationalized to meet the needs of a specific health system, may need to be monitored and updated locally, regionally, or nationally. Key features to consider in determining the appropriate level of model maintenance include use case goals, model ownership, and data and analysis resources.
Our understanding of best practices is well-defined in terms of use case and the level of model maintenance. For benchmarking models in quality evaluations, maintenance should be centralized at the largest relevant scope. Stabilizing the performance of quality-oriented models at higher levels imbues local performance deviations with information about variations in care and allows facilities to validly interpret performance trends as indicating improvement or deterioration of local performance over time. For AI-enabled tools aimed at clinical decision-making and population management, individual predictions should be well-calibrated to ensure utility and benefit to patients (13). As a result, more localized monitoring and maintenance are appropriate.
Unfortunately, practical considerations may require centralized monitoring and updating at regional or national scales even when local performance would typically be prioritized. Ownership and licensing requirements of proprietary models may preclude updating models to optimize local performance. Guidance on how to assess and handle local drift in light of such restrictions is necessary to trigger pauses in model implementations when local monitoring efforts reveal concerning performance drift; facilitate communication with end users about paused models and support end users’ information needs during such pauses, and promote timely reporting of issues to model owners.
When local updating is permissible, monitoring and updating remain a challenge for small organizations where data volumes and analytic resources may be limited. Insufficient sample sizes can lead to highly variable performance during monitoring and limit the ability to distinguish performance drift from noise. Smaller organizations, as well as their larger peers, should leverage recent studies by Riley et al. to assess whether sufficient sample sizes are available to validate binary (35), time-to-event (36), and continuous models (35). Recalibration, retraining, and model revision also require sufficient sample sizes (37) and dedicated data science teams that may not be feasible for all organizations. One solution would be to explore whether health information exchanges could be leveraged for collaborative monitoring and updating where local resources are insufficient. Broader research and policy discussions are needed as we think creatively about such multi-level, coordinated efforts to ensure the benefits of predictive tools are available and practical for health care organizations serving all communities.
While some metrics appear more robust to dataset shift, performance drift has been documented in measures of discrimination, calibration, and clinical utility (7, 8, 10, 16, 38). Monitoring metrics relevant to an AI-enabled tool’s use case is critical to understanding whether changes in performance warrant updating or whether updating may have little impact on model use and outcomes. For example, the number needed to screen was identified by the VSAIL team as the target metric for monitoring and stabilizing model performance as this impacts the cost-benefit analysis of clinics adopting the tool (39). For models deployed in diverse clinical contexts or across multiple tools, tracking a matrix of performance measures would provide insights supporting a variety of user perspectives (12, 40). Monitoring recommendations should thus include components that are agnostic to the performance metrics under consideration (e.g., selection of measurement), as well as components regarding metric selection.
Monitoring performance alone is insufficient; model managers need to be able to determine when observed deterioration in performance warrants intervention. Drift detection methods surveil temporal performance to alert users to statistically significant changes (41, 42) and have been applied to monitoring clinical prediction models. (34, 38) Methods vary in their ability to handle multiple forms and speeds of performance drift, as well as in their applicability to clinical contexts where calibration is of interest (43). Best practice recommendations will need to provide a decision framework for selecting between drift detection approaches, including considerations of whether detection algorithms are model-independent; can handle data streams of individual or batched observations, and are flexible in their ability to monitor prediction errors using a variety of metrics.
We note small differences in performance may be detected by the statistical tests underlying drift detection algorithms. However, statistically significant differences in performance may not directly translate into clinically meaningful differences. In such cases, users may question the value of updating or pausing a model in response to detections of small statistically significant, but not clinically important performance drift. The magnitude of acceptable inaccuracy and performance variability likely varies by use case. For example, performance drift is most likely to impact clinical utility when the calibration of predictions near clinically relevant decision thresholds or near classification cut-points deteriorates. Understanding whether, when, and how performance drift affects the clinical utility of predictions for decision-making is key to detecting meaningful changes in monitored models. Defining and measuring clinically acceptable performance and defining clinically relevant decision boundaries remains an open area of research. Subsequent research and guidance will need to address tailoring drift detection algorithms to place more import on clinically important changes in model performance.
In addition to performance metrics, the inputs of AI models could be monitored. This may involve evaluating data streams for changes in predictor distributions and associations (17), as well as establishing teams to actively evaluate external influences in clinical guidelines, software systems, data standards, and health care policies (6). Tracking external influences would allow teams to recognize structural changes that could render a model unreliable and plan customized updating approaches. Changes in data stream features, however, may not necessitate updating unless and until they affect the model accuracy in clinically meaningful ways. Best practices will need to address integrating insights from performance monitoring and evaluations of factors impacting model inputs to promote stable performance while efficiently and conservatively updating models. Additional research could investigate strategies for monitoring these non-performance aspects of AI models and policies for disseminating information across health systems when new practices are anticipated to impact widely adopted models.
When updating is initiated by pre-established schedules or detected performance drift, model managers must choose between a range of updating methods – from recalibration to retraining to model revision. As not all methods will be feasible, permissible, or successful in all situations, research and recommendations are needed to guide updating practice.
Although retraining with a cohort of recent observations may be established practice, this approach fails to build on the knowledge encoded in existing models, can be susceptible to overfitting, and may not improve performance above that achieved through recalibration (11, 44–47). For health systems with smaller populations, concerns regarding performance instability when retraining complex models may be more pronounced. Several methods have been developed to compare updating approaches on a particular cohort and recommend the approach that most improves accuracy (17, 45, 46). These methods, however, test for statistically rather than clinically significant differences across potential updates and do not consider whether the recommended update sufficiently restores performance. As methods for establishing clinically relevant decision thresholds mature, testing procedures for selecting updating methods could be implemented with weighted scoring rules to emphasize accuracy in critical regions. Future research should consider expanding options for optimizing decisions using varied performance metrics; increasing test efficiency, particularly for computationally intensive models; methods for evaluating whether updating provides clinically meaningful improvement; and recommendations for cases in which available updating methods do not restore models to acceptable levels of accuracy.
Model updating with current recalibration, retraining, and model revision methods has been developed, evaluated, and applied primarily in research databases. In production systems, interactions between users and AI-enabled decision support tools will, if successful, alter treatment decisions and improve patient outcomes. As a result, the observed data in production systems will be biased and updates using these biased data may reduce future model utility by updating away useful signals (48). These feedback loops created by successful clinical AI tools pose new challenges to updating practice that requires additional methodological research to better characterize the problem; to distinguish between dataset shift and performance changes due to model interventions; and to develop novel algorithms and updating approaches that are robust to confounding by intervention.
The clinical AI lifecycle is incomplete without components to monitor and stabilize accuracy in evolving clinical environments. Despite the diverse landscape of AI-enabled tools, common challenges to model maintenance impact new and existing implementations regardless of clinical domain and underlying modeling algorithms. Methods development for model monitoring and updating is accelerating, yet open questions for the design of maintenance programs, those described here and more, require additional research and scientific consensus to devise best practices. Establishing best practices is critical to designing AI-enabled tools that deliver reliable predictions, promote adoption, and realize the promise of AI to improve patient care.
The datasets presented in this article are not readily available because VUMC patient data used in this study are not publicly available. Requests to access the datasets should be directed to Sharon Davis,c2hhcm9uLmUuZGF2aXMuMUB2dW1jLm9yZw==.
The studies involving human participants were reviewed and approved by Vanderbilt University Medical Center's Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
SED and MEM developed the initial themes of this manuscript. SED and CGW conducted data management and analysis. SED drafted the manuscript. All authors contributed to the article and approved the submitted version.
CW and the Vanderbilt Suicide Attempt and Ideation Likelihood model (VSAIL) were supported by funding from the National Institutes of Health (R01 MH121455; R01 MH120122; R01 MH116269), the Military Suicide Research Consortium (W81XWH-10-2-0181), and Vanderbilt University Medical Center’s Evelyn Selby Stead Fund for Innovation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Amarasingham R, Patzer RE, Huesch M, Nguyen NQ, Xie B. Implementing electronic health care predictive analytics: considerations and challenges. Health Aff. (2014) 33(7):1148–54. doi: 10.1377/hlthaff.2014.0352
2. Smith J. Setting the agenda: an informatics-led policy framework for adaptive CDS. J Am Med Inform Assoc. (2020) 27(12):1831–3. doi: 10.1093/jamia/ocaa239
3. Matheny ME, Thadaney Israni S, Ahmed M, Whicher D. Artificial intelligence in health care: the hope, the hype, the promise, the peril. Washington, DC: National Academy of Medicine (2019).
4. Jenkins DA, Martin GP, Sperrin M, Riley RD, Debray TPA, Collins GS, et al. Continual updating and monitoring of clinical prediction models: time for dynamic prediction systems? Diagn Progn Res. (2021) 5(1):1. doi: 10.1186/s41512-020-00090-3
5. Petersen C, Smith J, Freimuth RR, Goodman KW, Jackson GP, Kannry J, et al. Recommendations for the safe, effective use of adaptive CDS in the US healthcare system: an AMIA position paper. J Am Med Inform Assoc. (2021) 28(4):677–84. doi: 10.1093/jamia/ocaa319
6. Finlayson SG, Subbaswamy A, Singh K, Bowers J, Kupke A, Zittrain J, et al. The clinician and dataset shift in artificial intelligence. N Engl J Med. (2021) 385(3):283–6. doi: 10.1056/NEJMc2104626
7. Hickey GL, Grant SW, Murphy GJ, Bhabra M, Pagano D, McAllister K, et al. Dynamic trends in cardiac surgery: why the logistic EuroSCORE is no longer suitable for contemporary cardiac surgery and implications for future risk models. Eur J Cardiothorac Surg. (2013) 43(6):1146–52. doi: 10.1093/ejcts/ezs584
8. Davis SE, Lasko TA, Chen G, Siew ED, Matheny ME. Calibration drift in regression and machine learning models for acute kidney injury. J Am Med Inform Assoc. (2017) 24(6):1052–61. doi: 10.1093/jamia/ocx030
9. Minne L, Eslami S, De Keizer N, De Jonge E, De Rooij SE, Abu-Hanna A. Effect of changes over time in the performance of a customized SAPS-II model on the quality of care assessment. Intensive Care Med. (2012) 38(1):40–6. doi: 10.1007/s00134-011-2390-2
10. Wong A, Cao J, Lyons PG, Dutta S, Major VJ, Otles E, et al. Quantification of sepsis model alerts in 24 US hospitals before and during the COVID-19 pandemic. JAMA Netw Open. (2021) 4(11):e2135286. doi: 10.1001/jamanetworkopen.2021.35286
11. Davis SE, Greevy RA, Lasko TA, Walsh CG, Matheny ME. Comparison of prediction model performance updating protocols: using a data-driven testing procedure to guide updating. Proceedings of the AMIA Annual Symposium Bethesda, MD: American Medical Informatics Association (2019). pp. 1002–10.
12. Jiang X, Osl M, Kim J, Ohno-Machado L. Calibrating predictive model estimates to support personalized medicine. J Am Med Inform Assoc. (2012) 19(2):263–74. doi: 10.1136/amiajnl-2011-000291
13. Van Calster B, Vickers AJ. Calibration of risk prediction models: impact on decision-analytic performance. Med Decis Making. (2015) 35(2):162–9. doi: 10.1177/0272989X14547233
14. Quinonero-Candela J, Sugiyama M, Schwaighofer A, Lawrence N. Dataset shift in machine learning. Cambridge, MA: The MIT Press (2009).
15. Luijken K, Wynants L, van Smeden M, Van Calster B, Steyerberg EW, Groenwold RHH, et al. Changing predictor measurement procedures affected the performance of prediction models in clinical examples. J Clin Epidemiol. (2020) 119:7–18. doi: 10.1016/j.jclinepi.2019.11.001
16. Davis SE, Lasko TA, Chen G, Matheny ME. Calibration drift among regression and machine learning models for hospital mortality. Proceedings of the AMIA Annual Symposium Bethesda, MD: American Medical Informatics Association (2017). pp. 625–34.
17. Guo LL, Pfohl SR, Fries J, Posada J, Fleming SL, Aftandilian C, et al. Systematic review of approaches to preserve machine learning performance in the presence of temporal dataset shift in clinical medicine. Appl Clin Inform. (2021) 12(4):808–15. doi: 10.1055/s-0041-1735184
18. Luo W, Phung D, Tran T, Gupta S, Rana S, Karmakar C, et al. Guidelines for developing and reporting machine learning predictive models in biomedical research: a multidisciplinary view. J Med Internet Res. (2016) 18(12):e323. doi: 10.2196/jmir.5870
19. Jenkins DA, Sperrin M, Martin GP, Peek N. Dynamic models to predict health outcomes: current status and methodological challenges. Diagn Prognostic Res. (2018) 2:23.
20. Morse KE, Brown C, Fleming S, Todd I, Powell A, Russell A, et al. Monitoring approaches for a pediatric chronic kidney disease machine learning model. Appl Clin Inform. (2022) 13(2):431–8. doi: 10.1055/s-0042-1746168
21. Walsh CG, Johnson KB, Ripperger M, Sperry S, Harris J, Clark N, et al. Prospective validation of an electronic health record-based, real-time suicide risk model. JAMA Netw Open. (2021) 4(3):e211428. doi: 10.1001/jamanetworkopen.2021.1428
22. Otles E, Oh J, Li B, Bochinski M, Joo H, Ortwine J, et al. Mind the performance gap: examining dataset shift during prospective validation. Proceedings of the 6th Machine Learning for Healthcare Conference Proceedings of Machine Learning Research (2021). pp. 506–34.
23. van Walraven C, Wong J, Forster AJ. LACE+ index: extension of a validated index to predict early death or urgent readmission after hospital discharge using administrative data. Open Med. (2012) 6(3):e80–90.23696773
24. Walsh C, Ribeiro J, Franklin J. Predicting risk of suicide attempts over time through machine learning. Clin Psychol Sci. (2017) 5(3):457–69. doi: 10.1177/2167702617691560
25. Van Calster B, Nieboer D, Vergouwe Y, De Cock B, Pencina MJ, Steyerberg EW. A calibration hierarchy for risk models was defined: from utopia to empirical data. J Clin Epidemiol. (2016) 74:167–76. doi: 10.1016/j.jclinepi.2015.12.005
26. Lu JH, Callahan A, Patel BS, Morse KE, Dash D, Pfeffer MA, et al. Assessment of adherence to reporting guidelines by commonly used clinical prediction models from a single vendor: a systematic review. JAMA Netw Open. (2022) 5(8):e2227779. doi: 10.1001/jamanetworkopen.2022.27779
27. Gianfrancesco MA, Tamang S, Yazdany J, Schmajuk G. Potential biases in machine learning algorithms using electronic health record data. JAMA Intern Med. (2018) 178(11):1544–7. doi: 10.1001/jamainternmed.2018.3763
28. Paulus JK, Kent DM. Predictably unequal: understanding and addressing concerns that algorithmic clinical prediction may increase health disparities. NPJ Digit Med. (2020) 3:99. doi: 10.1038/s41746-020-0304-9
29. Selbst A, Boyd D, Friedler S, Venkatasubramanian S, Vertesi J., Fairness and abstraction in sociotechnical systems. ACM Conference of Fairness, Accountability, and Transparency New York, NY: Association for Computing Machinery (2019). pp. 59–68.
30. Pfohl SR, Foryciarz A, Shah NH. An empirical characterization of fair machine learning for clinical risk prediction. J Biomed Inform. (2021) 113:103621. doi: 10.1016/j.jbi.2020.103621
31. Beutel A, Chen J, Doshi T, Qian H, Woodruff A, Luu C, et al. Putting fairness principles into practice: challenges, metrics, and improvements. 2019 AAAI/ACM Conference on AI, Ethics, and Society New York, NY: Association for Computing Machinery (2019). pp. 453–9.
32. ACS NSQIP Surgical Risk Calculator. Available at: https://riskcalculator.facs.org/RiskCalculator/index.jsp. (accessed April 30, 2022).
33. Jung K, Kashyap S, Avati A, Harman S, Shaw H, Li R, et al. A framework for making predictive models useful in practice. J Am Med Inform Assoc. (2021) 26(6)1149–58.
34. Davis SE, Greevy RA Jr., Lasko TA, Walsh CG, Matheny ME. Detection of calibration drift in clinical prediction models to inform model updating. J Biomed Inform. (2020) 112:103611. doi: 10.1016/j.jbi.2020.103611
35. Riley RD, Debray TPA, Collins GS, Archer L, Ensor J, van Smeden M, et al. Minimum sample size for external validation of a clinical prediction model with a binary outcome. Stat Med. (2021) 40(19):4230–51. doi: 10.1002/sim.9025
36. Riley RD, Collins GS, Ensor J, Archer L, Booth S, Mozumder SI, et al. Minimum sample size calculations for external validation of a clinical prediction model with a time-to-event outcome. Stat Med. (2022) 41(7):1280–95. doi: 10.1002/sim.9275
37. Steyerberg EW, Borsboom GJ, van Houwelingen HC, Eijkemans MJ, Habbema JD. Validation and updating of predictive logistic regression models: a study on sample size and shrinkage. Stat Med. (2004) 23(16):2567–86. doi: 10.1002/sim.1844
38. Minne L, Eslami S, de Keizer N, de Jonge E, de Rooij SE, Abu-Hanna A. Statistical process control for monitoring standardized mortality ratios of a classification tree model. Methods Inf Med. (2012) 51(4):353–8. doi: 10.3414/ME11-02-0044
39. Ross EL, Zuromski KL, Reis BY, Nock MK, Kessler RC, Smoller JW. Accuracy requirements for cost-effective suicide risk prediction among primary care patients in the US. JAMA Psychiatry. (2021) 78(6):642–50. doi: 10.1001/jamapsychiatry.2021.0089
40. Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. (2010) 21(1):128–38. doi: 10.1097/EDE.0b013e3181c30fb2
41. Gama J, Žliobaitė I, Bifet A, Pechenizkiy M, Bouchachia A. A survey on concept drift adaptation. ACM Comput Surv (CSUR). (2014) 46(4):44. doi: 10.1145/2523813
42. Bifet A, Gavalda R. Learning from time-changing data with adaptive windowing. Proceedings of the 2007 SIAM International Conference on Data Mining. Philadephia, PA: Society for Industrial and Applied Mathematics (2007). pp. 443–8.
43. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. BMJ Qual Saf. (2003) 12(6):458–64. doi: 10.1136/qhc.12.6.458
44. Toll DB, Janssen KJ, Vergouwe Y, Moons KG. Validation, updating and impact of clinical prediction rules: a review. J Clin Epidemiol. (2008) 61(11):1085–94. doi: 10.1016/j.jclinepi.2008.04.008
45. Vergouwe Y, Nieboer D, Oostenbrink R, Debray TP, Murray GD, Kattan MW, et al. A closed testing procedure to select an appropriate method for updating prediction models. Stat Med. (2017) 36(28):4529–39. doi: 10.1002/sim.7179
46. Davis SE, Greevy RA, Fonnesbeck C, Lasko TA, Walsh CG, Matheny ME. A nonparametric updating method to correct clinical prediction model drift. J Am Med Inform Assoc. (2019) 26(12):1448–57. doi: 10.1093/jamia/ocz127
47. Su TL, Jaki T, Hickey GL, Buchan I, Sperrin M. A review of statistical updating methods for clinical prediction models. Stat Methods Med Res. (2018) 27(1):185–97. doi: 10.1177/0962280215626466
Keywords: dataset shift, model updating, machine learning, risk model surveillance, artificial intelligence
Citation: Davis SE, Walsh CG and Matheny ME (2022) Open questions and research gaps for monitoring and updating AI-enabled tools in clinical settings. Front. Digit. Health 4:958284. doi: 10.3389/fdgth.2022.958284
Received: 31 May 2022; Accepted: 11 August 2022;
Published: 2 September 2022.
Edited by:
Mark Sendak, Duke Institute for Health Innovation, United StatesReviewed by:
Jonathan Lu, Stanford University School of Medicine, United States© 2022 Davis, Walsh and Matheny. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sharon E. Davis c2hhcm9uLmUuZGF2aXMuMUB2dW1jLm9yZw==
Specialty Section: This article was submitted to Health Informatics, a section of the journal Frontiers in Digital Health
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.