
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Digit. Health , 17 August 2022
Sec. Health Technology Implementation
Volume 4 - 2022 | https://doi.org/10.3389/fdgth.2022.909519
 Tim Adams1
Tim Adams1 Sophie Wagner1
Sophie Wagner1 Melanie Baldinger2
Melanie Baldinger2 Incinur Zellhuber1
Incinur Zellhuber1 Michael Weber1
Michael Weber1 Daniel Nass3
Daniel Nass3 Rainer Surges3*
Rainer Surges3*
Background: Recent research has shown that photoplethysmography (PPG) based wearable sensors offer a promising potential for chronic disease monitoring. The aim of the present study was to assess the performance of an in-ear wearable PPG sensor in acquiring valid and reliable heart rate measurements in a clinical setting, with epileptic patients.
Methods: Patients undergoing video-electroencephalography (EEG) monitoring with concomitant one-lead electrocardiographic (ECG) recordings were equipped with an in-ear sensor developed by cosinuss°.
Results: In total, 2,048 h of recording from 97 patients with simultaneous ECG and in-ear heart rate data were included in the analysis. The comparison of the quality-filtered in-ear heart rate data with the reference ECG resulted in a bias of 0.78 bpm with a standard deviation of ±2.54 bpm; Pearson’s Correlation Coefficient PCC = 0.83; Intraclass Correlation Coefficient ICC = 0.81 and mean absolute percentage error MAPE = 2.57.
Conclusion: These data confirm that the in-ear wearable PPG sensor provides accurate heart rate measurements in comparison with ECG under realistic clinical conditions, especially with a signal quality indicator. Further research is required to investigate whether this technology is helpful in identifying seizure-related cardiovascular changes.
Current advancements in optical wearable sensors have made them an attractive tool in disease monitoring, in which patients’ physiological and behavioral metrics are captured in a continuous, unobtrusive way (1). A notable percentage of the optical sensing devices use the PPG, a non-invasive technology, measuring optical variations based on blood volume changes (2). Heart rate is one of the key neurophysiological metrics when monitoring patients’ condition. In PPG measurements a certain proportion of the emitted light is absorbed through tissue. Since tissue absorption depends on the local blood volume, it is possible to infer the heart rate from the ratio of absorbed to reflected light, which is measured by a photodiode.
In a medical setting, heart rate is typically measured by an ECG, involving electrodes on the skin. This method is uncomfortable for the patient, not suitable for continuous, long-term monitoring, especially when patients move. PPG-based heart rate monitors can be a promising alternative to ECG. The comparison of PPG and ECG in heart rate monitoring has already been discussed in a number of studies. An example of raw signals from a PPG sensor and an ECG is provided in Supplementary Materials, Figure S1. Some studies comparing the two methods found differences, other groups reported an overall strong agreement (for an overview see (3)). Nevertheless, these studies had diverse experimental settings, measurement locations and/or methods of analysis. Results on the influence of the sensor location are sparse (see for example (4)). Ear-worn PPG devices have been discussed in several studies as an alternative to ECG (5–7). When the sensor was placed on the earlobe, moderate to good accordance between PPG and ECG was shown (8–10). Overall, previous studies have shown that the PPG is a promising technology regarding its use in ear-worn wearables. However, most of the studies mentioned evaluated non-commercial, prototype ear-worn devices (11, 12).
Recent research has shown that alterations in heart rate can be used as a biomarker in epilepsy monitoring (13–16). Epilepsy is a common neurological disease affecting about 0.6% of the world’s population (17). It is characterized by recurrent seizures with highly variable symptoms, ranging from mild pure body sensations to severe symptoms such as loss of body movements control and consciousness (18). A significant proportion of seizures are not recalled by patients for various reasons, hampering the accurate documentation of disease activity by seizure diaries held by patients (19). Therefore novel treatment options allowing real-time seizure monitoring in daily life can benefit patients tremendously by reducing unpredictability and improving their quality of life (20, 21). In this context, epilepsy monitoring is one of the potential medical applications of in-ear wearable PPG sensors.
Within the scope of the EPItect project (22), we hypothesize that PPG-based sensors placed inside the ear canal are well protected from movement-related artifacts and have a potential to accurately capture all necessary biosignals that are required to detect a wide range of epileptic seizures. As a fundamental step towards this goal, here we aimed to evaluate the performance of an in-ear PPG-based wearable sensor in acquiring accurate and reliable heart rate with respect to an ECG. This CE-marked device, cosinuss° One (developed by Cosinuss GmbH, Munich, Germany), is capable of detecting heart rate by using PPG signals, head movements by measuring acceleration in three dimensions and core body temperature via contact temperature sensor.
Our results demonstrate that the cosinuss° One with PPG-technology can derive heart rate with high accuracy in a real-life clinical setting, in an epileptic patient population. Our findings also indicate the necessity of a signal qualifier for identifying medically useful data points. To our knowledge, this study is the first to conduct a detailed validation of a commercial in-ear heart rate monitor using PPG in a clinical setting.
Patients (18 years) with refractory epilepsy, who underwent video-EEG monitoring in the Department of Epileptology in University of Bonn Medical Center, for non-invasive presurgical evaluation, syndrome diagnosis, or monitoring of seizure control, were included in the study. Informed consent was obtained from all subjects involved in the study. Subjects participated in long-term VEEG-monitoring for 1–13 days depending on the individual diagnosis and the amount of seizures recorded. During that time, videos of the subjects were recorded as well as the EEG, pulse oximetry, and one-lead ECG. Additionally, the patients wore the cosinuss° One in-ear sensor, which recorded their heart rate, PPG-signals, core body temperature and the 3D-acceleration data. Every six hours the study nurses changed the in-ear sensor to a fully charged device. The study was approved by the local medical ethics committee (Ethikkommission der Medizinischen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn, No. 355/16) and conducted in accordance with the Declaration of Helsinki.
To record the PPG signals, we used the in-ear sensor cosinuss° One (Cosinuss GmbH, Munich, Germany), which is shown in Figure 1. It is a mobile wearable sensor that continuously measures the core body temperature, heart rate and acceleration. In addition, it records the intervals between heart beats (known as RR intervals), fluctuations of which is correlated with heart rate variability. The cosinuss° One incorporates a PPG sensor element (combining a light-emitting diode (LED) and a photodiode), a resistance temperature sensor and a 3D-accelerometer. For heart rate measurements the so-called circummission method uses green light, which is emitted into the ear canal by a LED (23). The photodiode measures the proportion of the light reflected. The heart rate is automatically calculated by the sensor device and can directly be derived from the data output.
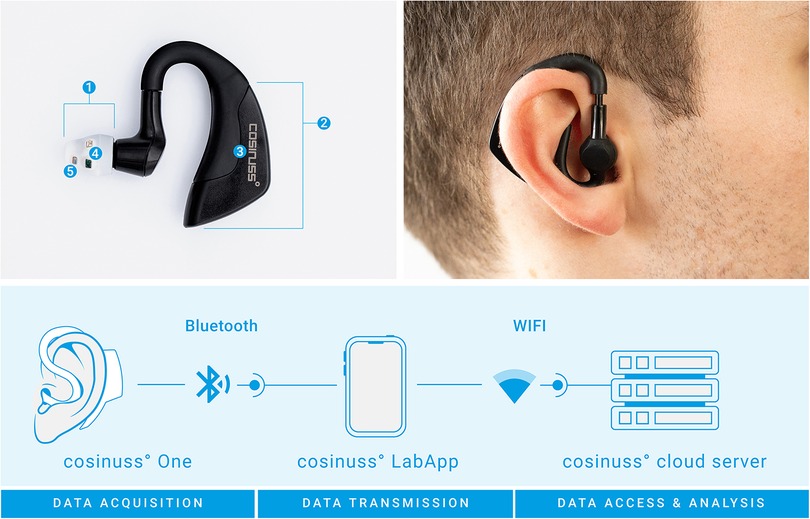
Figure 1. Concept of the cosinuss° One in-ear sensor used as a wearable to detect heart rate in the study. Top left image: (1) sensor head, (2) processing unit, (3) accelerometer, (4) temperature sensor, (5) PPG sensor elements. Bottom image: Acquired data are sent to the cosinuss° LabApp smartphone application via Bluetooth, which are then automatically uploaded to the cloud via a Wi-Fi connection.
The sensor has a size of (H) 45 (W) 38 (D) 18 mm and a weight of 6.5 g. Close contact of the sensor with the tissue inside the ear canal is crucial for a precise measurement. For this reason, the LED, photodiode and temperature sensor are placed on a soft silicone shield that fits firmly into the ear canal. The sensor head is available in three sizes (S, M, L) to adapt to different ear canal sizes of children, adults and elderly. The processing unit is behind the ear. Battery life of the sensor is at least 7 h according to the manufacturer (24). The cosinuss° One is certified with the consumer CE-mark.
The one-lead ECG was recorded at lead I with a sampling rate of 256 Hz, using the Micromed system (Micromed S.p.A., Treviso, Italy). The data collected from the in-ear sensor was recorded in real-time with a sampling rate of 50 Hz for the PPG and acceleration data, and approximately 1 Hz for the vital parameters (heart rate with RR intervals, core body temperature). The data were continuously transferred from the sensor to an Android smartphone using Bluetooth Low Energy (Bluetooth 4.2), without compression. From the Android device the data was automatically sent to the cosinuss° cloud server via a Wi-Fi connection for further analysis and archiving of the data using the cosinuss° LabApp (see Figure 1). All personal identifying information and medical health data on the cosinuss° cloud server are pseudonymized. Only authorized persons have access to the data.
Data was analyzed using Python 3. Heart rate data from the in-ear sensor is directly derived from the cosinuss° cloud server. Data from the ECG recording was exported and uploaded to the same cloud server by the clinical staff. For the ECG data the R-wave peaks are detected using the well-approved Pan-Tompkins-algorithm (25). From the resulting RR-intervals, the heart rate values in units of beats per minute (bpm) are obtained.
The in-ear sensor provides a signal quality indicator (QI, ranging from 0–100 (a.u.), with 100 being the most reliable). It is calculated at each heart rate data point and indicates its reliability by measuring the PPG signal dominance in relation to the perturbations. Before the following steps in data processing, this quality measure is smoothed and then used to remove data points that do not exceed a specified quality threshold. With the choice of this threshold, a trade-off between improvement of statistical values and loss of data is made.
To determine the threshold QI value, a sensitivity analysis was performed. It was assessed how data loss and mean absolute percentage error (MAPE) changes with an increasing quality threshold. MAPE is computed as follows:
where is the reference value and is the value as measured by the in-ear device. How exactly these simultaneous data points and were extracted is explained in more detail in 2.4.2. The measure of data loss is obtained by comparing the absolute number of available raw data points before and after filtering for quality. The results can be seen in Figure 2. The higher the threshold, the more data will be removed by the quality filter, while the results of the statistical analysis performed on the remaining data improve. Using the smallest threshold, where the QI needs to be larger than 0, is already an improvement to performing no quality filtering at all. It is mainly because the QI is set to 0 for each data point where the QI’s -value and the corresponding heart rate data point’s -value differ by more than 2.5 s and when the heart rate is not within the range of 40 to 148 bpm. For thresholds 20 or larger, there is a near-linear relationship between the threshold of the quality indicator and percentage of data points that are removed based on this threshold, ranging from 11% data loss at a threshold of 20 to 53% data loss at a threshold of 80. For thresholds >80, the slope is slightly steeper, reaching 100% data loss at a threshold of 100. As expected, the MAPE drops with increasing QI threshold. Between thresholds of 40 and 80, the slope of decline is the steepest. Using a quality threshold of 0, the MAPE is at 4.14, and from a threshold of 70 upwards, the MAPE is below 2.6.
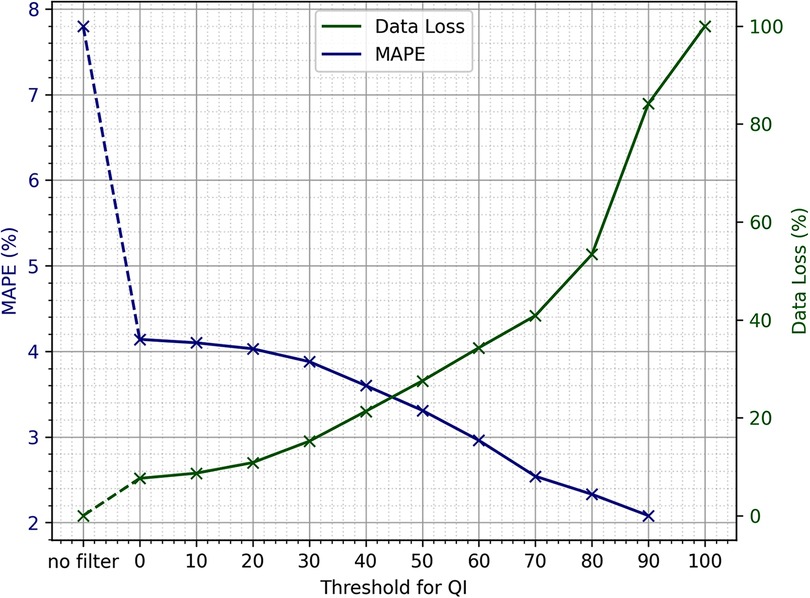
Figure 2. Sensitivity analysis of the quality indicator threshold, provided by the in-ear sensor to be used for quality filtering, in relation to mean absolute percentage error (MAPE) and data loss.
The sensitivity analysis shows that the choice of the exact threshold can be made at liberty, as the trade-off between data loss and MAPE is nearly linear if using thresholds of 80 and lower. If the primary goal of an evaluation is to include as much of the original data as possible, the smallest threshold would suffice to obtain reasonable heart rate values. Due to the large dataset available, it was decided that losing a larger portion of data in order to optimize for quality would still leave sufficient amounts of data for the analysis. As the data loss incline gets steeper upwards of a QI threshold of 80, and the MAPE is well below 3 upwards of 70, it was decided to use 70 as the QI threshold.
Only heart rate data points that exceeded the quality threshold were kept, removing about 40% of the data of the in-ear sensor. The main reason for this large percentage is the fact that the quality threshold discards long periods of measurement if the patient is not assigned a well-fitting sensor size or if the device is not worn properly in the ear canal. The reference ECG signal also underwent data processing in order to detect and remove unreliable data points due to motion artifacts and poor placement of the electrodes. A value was considered an outlier, and was discarded, if it deviated by more than 8 bpm from the median of itself and the 14 data points that follow it. If, within this area of 15 data points, more than three values were considered outliers, all 15 points were discarded. This step reduced the ECG data by 23%.
Figure 3 shows an example recording of heart rate over three hours. As a relatively high quality indicator predicts, the in-ear sensor coincides well with the ECG data. An enlarged segment of this example can be seen in Figure 3, bottom plot. In contrast, a recording that exhibits critically low quality values for a certain period of time is shown in Figure 4. The quality indicator (shown in green), while stable at first, drops below the critical threshold of 70 (indicated by the dashed line) after one hour, most likely because the sensor tip has lost its firm contact with the ear canal tissue. During this time, the in-ear sensor’s data points are dismissed due to their unreliability. Towards the end of the measurement, the quality rises above the threshold again, as the sensor may have gotten readjusted into the ear.
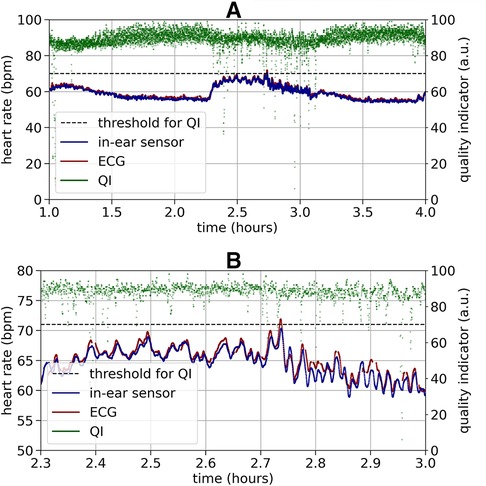
Figure 3. Top plot: Example of a high quality heart rate recording (bpm) over three hours by the ECG (red) and the in-ear sensor (blue). The signal quality indicator of the in-ear sensor (green) is above the quality threshold of 70 (indicated by the dashed line) at nearly all times of the recording. The heart rate data of the in-ear sensor and of the ECG overlap in a way that the red line of the ECG is completely hidden by the blue line of the in-ear sensor. Bottom plot: For better visibility this figure shows a short clipping of the recording above. During a period of relatively high variance in the heart rate, compared to the rest of the measurement, the heart rate values obtained by the ECG (red) and the in-ear sensor (blue) still show high agreement.
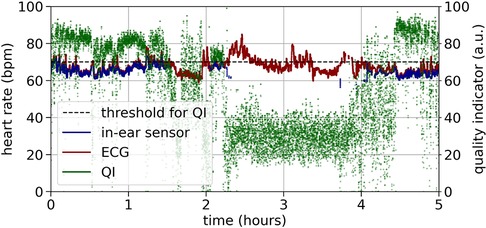
Figure 4. Example of a critical quality heart rate recording (bpm) over five hours by the ECG (red) and the in-ear sensor (blue). The signal quality indicator of the in-ear sensor (green) returns values below the quality threshold of 70 (indicated by the dashed line) for certain periods of time.
After data cleansing, the heart rate data of the ECG and the in-ear sensor were matched based on their respective time stamps, keeping only simultaneous data points of sufficient quality where signals from both sources were available.
For further analysis, the data points were combined into time windows of two seconds. The average value of each time bin as provided by the in-ear sensor is compared to the respective average value of the ECG recording, testing both precision and reliability. Correlation between recordings was measured using Pearson’s correlation coefficient (PCC). Since high correlation does not necessarily guarantee high agreement, the Intraclass Correlation Coefficient—ICC (3,1) as defined by Shrout and Fleiss (26)—is considered to be a more suitable measure to quantify an instrument’s accuracy (27). Besides the ICC, MAPE was computed to assess accuracy. To test precision, Bland-Altman plots were employed, which plot the difference between two samples over their mean value (28). Thus, it shows the accordance of the two measurements, while also visualizing whether a measurement error is systematic, i.e. correlated with the value it is measuring. The mean value over all deviations within a recording session leads to an absolute bias of the two measurement methods and the lower (LLOA) and upper (ULOA) limits of agreement can be derived using the standard deviation (SD): and , respectively. For the following evaluation, the parameters deduced from the Bland-Altman plots (LLOA, ULOA, mean, standard deviation) were taken from a conjoined Bland-Altman plot of all data points of each person. PCC, R-squared, ICC, and MAPE were calculated for each measurement individually and then averaged per person.
A total of 174 subjects (age: years; gender: male 46%, female 54%) participated in this study. At the beginning of the study a prototype in-ear sensor with a red and infrared LED was used. The reason for that was to be able to derive more physiological signs (such as the blood oxygen saturation) from the PPG signal. However, during the recordings with the first 65 patients, we observed substantial motion artifacts, which led to unreliable heart rate measurements. Since the PPG signals from green LED are more robust in dealing with motion artifacts, after the first 65 patients, we decided to switch to the cosinuss° One in-ear sensor using a green LED, to acquire more reliable heart rate measurements. Only data recorded with the cosinuss° One was taken into account for further analysis. During the initial trial a few recordings of the in-ear sensor were missed due to technical issues. Furthermore, not all ECG data was stored permanently for the first half of the study, where only the first 12–24 h recording per patient was stored. This results in a total of 97 subjects and 2048.46 h of recording including ictal as well as interictal activity.
After the quality filtering and smoothing of the simultaneous ECG and PPG data -as described in Section D, the heart rate data from the in-ear sensor and the ECG amount to 1396.77 h of recording. Using recordings from 97 different patients, this leads to an average of 14.4 h of data per patient.
To analyze the effectiveness of the quality indicator on the in-ear heart rate measurement, we used Bland-Altman plots (28) and visualized the deviations of heart rate data from the in-ear sensor and the ECG, as shown in Figure 5. Data shown in Figure 5, subplot A), comprises a recording time of approximately 63 h. All data points originating from the in-ear sensor were used without filtering. There are some significant deviations of the in-ear sensor’s heart rate measurements to the ones of the ECG. Most deviations occur around a heart rate of 60–70 bpm, because the majority of this patient’s heartbeats were in this range. Said deviations are mostly positive, meaning that the ECG generally returned higher values than the in-ear sensor. The mean bias and standard deviation lead to a lower and upper limit of agreement of bpm and bpm for this patient. Figure 5, subplot B), shows the same patient’s data; however, the in-ear sensor’s quality indicator was taken into consideration, removing all points that were below the threshold of 70, leaving 46 h of data. As a result, the upper limit of agreement improves to bpm and the lower limit rises to bpm.
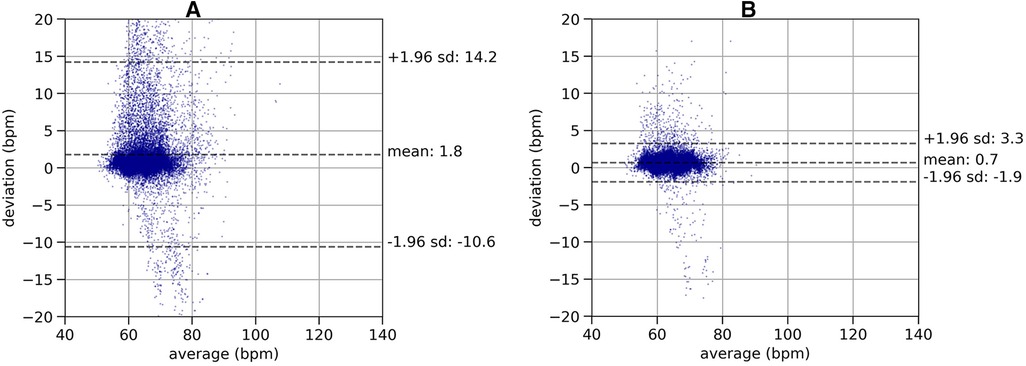
Figure 5. Plot (A): Bland-Altman plot of a recording of heart rate without quality filtering, comparing the in-ear sensor’s heart rate measurement with the ECG over a recording time of 63 h. The plot shows the deviations of the heart rate values (-axis) relative to the mean of the two methods (-axis). The dashed lines indicate the mean value of the deviations (bias) and the lower (LLOA) and upper (ULOA) limits of agreement as calculated as . This leads to a bias of 1.8 bpm and limits of agreement of bpm and bpm. Plot (B): Bland-Altman plot of a recording of heart rate with quality filtering, comparing the in-ear sensor’s heart rate measurement with the ECG over a recording time of 46 h. The same data set as in left plot is shown, removing the data points below the quality threshold. The lower (LLOA) and upper (ULOA) limits of agreement as calculated as . Removing low quality data points leads to a bias of 0.7 bpm and limits of agreement of bpm and bpm.
After quality-filtering of all in-ear heart rate measurements, the comparison of the in-ear sensor and the ECG leads to a bias of 0.78 bpm and a standard deviation of 2.54 bpm. Table I visualizes the effectiveness of the quality filter by comparing statistical results using all data points to statistical results keeping only points of sufficient quality. The overall limits of agreement amounted to bpm and bpm; the average ; while the average PCC scored slightly higher, at 0.84 over all recordings; the average MAPE was 2.43%. Removing unreliable points significantly improves all statistical parameters. As an example, Figure 6 shows a box plot of the ICC value over all measurements.
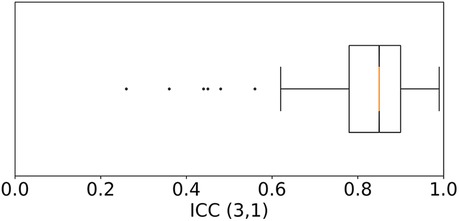
Figure 6. Box plot of the Intraclass Correlation Coefficient—ICC (3,1) as defined by Shrout and Fleiss (26)—comparing the quality filtered in-ear heart rate data to the heart rate from ECG over all 97 patients. Each recording was weighted by its duration. The median is at 0.85. 50% of the data points lie within the range of 0.78–0.9. Six outliers that score lower than 0.62 were identified.
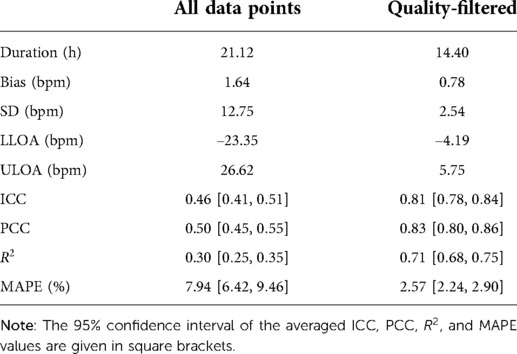
Table I. The resulting statistical values averaged over all 97 patients. Values are average duration, bias, standard deviation (SD), lower limit of agreement (LLOA), upper limit of agreement (ULOA), Intraclass Correlation Coefficient (ICC), Pearson’s Correlation Coefficient (PCC), the coefficient of determination, R2, in this case the squared Pearson’s correlation coefficient, and the mean absolute percentage error (MAPE).
The present study examined the accuracy of the cosinuss° One, an in-ear wearable sensor utilizing PPG-technology, for measuring heart rate in a real-life clinical setting, in comparison with ECG. The results prove that the in-ear PPG sensor can provide a valid heart rate measurement if further improved by filtering algorithms. Though achieving moderate results using all data points, applying the quality indicator of the in-ear sensor improved all statistical parameters by removing unreliable data points. If less data loss is desired, the quality threshold can be decreased without disproportionally increasing the MAPE, as both parameters have a near linear relationship to the threshold.
PCC and ICC were calculated to test the correlation of the in-ear sensor with the reference ECG device. The fact that the PCC (0.83) scored slightly higher than the ICC (0.81) is not surprising, as the in-ear sensor cosinuss° One shows a more or less constant bias of 0.8 bpm, and the PCC measure returns high correlation for linear relationships between recordings. In medicine, there is no consensus whether the ICC is a sufficient measure of quality for a heart rate measurement device. According to Rosner’s definition (29), the value obtained by the cosinuss° One in the present study signifies an excellent agreement, while according to Koo et al. (27), it signifies a good agreement. Fokkema et al. (30) suggest to interpret ICC values as excellent, good, moderate, and low agreement thresholds, if they are 0.90; 0.75–0.90; 0.60–0.75; and 0.60, respectively. This interpretation of ICC values indicates a good agreement between the cosinuss° One and the ECG recordings in this case.
Testing the accuracy of the in-ear sensor using MAPE resulted in 2.57%. There is no standard cut-off value for acceptable MAPEs, however Nelson et al. (31) consider a MAPE 10% as an indicator of inaccuracy, whereas Fokkema et al. (30) suggest a threshold of 5% for a value to be considered accurate. Etiwy et al. (32) tested the accuracy of four PPG-based wrist-worn heart rate measurement devices as well as the Polar H7 chest strap, comparing all to standard ECG limb leads. The devices were tested on 80 patients in cardiac rehabilitation while they were at rest, on a treadmill and on a stationary cycle. The Polar strap achieved the best results in all disciplines, reaching a MAPE of during the resting period. The other wearable devices reached MAPEs between (Apple watch on the stationary cycle) and (Garmin device on the stationary cycle). Therefore, applying the previous interpretations of the MAPE in similar contexts, it can be concluded that the MAPE achieved by the in-ear sensor cosinuss° One in here () is comparably low and can be considered as accurate.
Bland-Altman analysis revealed that the cosinuss° One had a bias of 0.78 bpm and 95% of differences fall within 5.75 and 4.19 bpm of the ECG (ULOA - LLOA 9.94 bpm). As the differences were calculated by subtracting the in-ear sensor’s value from the one of ECG’s and the mean bias is positive, we conclude that the in-ear sensor tends to slightly underestimate heart rate values. This result is in line with the findings of Passler et al. (6). In this previous work by Passler et al., the cosinuss° One in-ear sensor was compared and validated against an ECG. However, this validation was done on 20 healthy subjects during graded cycling, under laboratory conditions. Results of the in-ear sensor showed a bias of 0.4 bpm and 95% of all differences within 4.38 and 5.17 bpm of the ECG (ULOA - LLOA 9.55 bpm) during resting conditions with a heart rate 90‘bpm. Furthermore the study demonstrated an excellent agreement (; ) between the cosinuss° One and ECG recordings during resting conditions (mean heart rate standard deviation of bpm as measured by the cosinuss° One) and a good agreement () at maximum heart rate (mean heart rate standard deviation of bpm as measured by the cosinuss° One). There is no indication that the quality indicator of the in-ear sensor was taken into account. Therefore, we argue that the quality filtering algorithm of the cosinuss° One significantly improves the accuracy of the HR measurements, especially when applied in realistic clinical settings where a certain contingent of application errors must be assumed.
There are however some limitations to the performed study that could be addressed in future research. First, the study focused on the heart rate recordings of stationary patients, who were at resting-state. This leads to recordings of mostly moderate heart rate values and small changes. Therefore the accuracy of the in-ear sensor during periods of activity needs to be assessed in further work. Not yet understood is the more or less constant bias of 0.8 bpm on average below the ECG heart rate. Second, it must be noted that the current analysis did not take into account whether the patients also had cardiac diseases, which would affect the reproducibility of the results.
The third limitation of this study is that, while all patients experienced epileptic seizures during the recording periods, it was not assessed to what extent the seizure-related data was retained after quality filtering. We expect that the measurement quality deteriorates during seizures where muscles cramp and the heart rate increases. However, the validation of cosinuss° One against ECG is still a necessary prerequisite for future research on seizure detection. Taking into account the fact that the heart rate provided by the in-ear sensor proved to be reliable, a sudden deterioration in the quality may thus be used as a factor in seizure detection. While the loss of sensor-to-skin contact might be the main reason for decreased signal quality of the sensor, another one is seizure occurrence. If information is lost during seizures purely due to the event itself, the sensor will regain contact immediately afterwards, at which point the heart rate will be at a higher level and then gradually return to normal during the next minutes. This pattern, an example of which is shown in Supplementary Figure S2, could be detected and used for further analysis.
Furthermore, there are examples where the sensor’s signal quality stayed reasonably high during a seizure, as in Supplementary Figure S2, bottom plot, allowing for accurate heart rate measurements during the entire process.
The next step is to investigate the feasibility of using the heart rate, acceleration and PPG signals from the in-ear sensor to detect epileptic seizures. In a first attempt, Henze et al. (33) evaluated a multimodal approach by combining the 3D acceleration and heart rate data from the in-ear sensor developed by cosinuss°. They trained a selection of classification models to be used on the EPItect project dataset to detect tonic-clonic seizures. The labels originated from the project’s video-EEG recordings, which was annotated by medical professionals, to mark the on- and offset of seizures. This previous study found that using the combination of heart rate and acceleration data, in comparison to acceleration data only, indeed correlated with a higher number of tonic-clonic seizures. Bruno et al. (34) concluded in their systematic review on preictal heart rate changes that heart rate measurements could be valuable in identifying seizures prior to their apparent onset, although additional research is necessary to clearly mark down those patients that might benefit from heart rate monitoring. Furthermore, El Atrache et al. (35) identified changes in the PPG signal using wearable wristbands in the periictal periods of patients with focal impaired awareness seizures (FIAS) and concluded that FIAS detection using PPG data is feasible.
In the light of recent findings, we speculate that seizure detection will likely comprise a combination of features derived from multiple biosignals during, before and after the event. To make the in-ear optical sensor suitable for usage in epilepsy practice, future research should focus on the feasibility of using the heart rate, acceleration and PPG signals of the in-ear sensor to detect epileptic seizures and the impact of movement on the accuracy of the PPG-signal.
This validation study showed that the in-ear sensor cosinuss° One with PPG-technology can determine heart rate with high accuracy compared to single-lead ECG. The signal quality indicator of the sensor provides robust filtering of the unreliable PPG data, significantly increasing the overall accuracy of the heart rate measurements, which is critical in everyday clinical practice. This is the first paper to describe a detailed validation of heart rate derived from a commercial in-ear PPG sensor in a realistic clinical setting.
In the grand scheme of clinical epilepsy practice, our main question remains as whether in-ear PPG-based measurements can be used for epileptic seizure detection. Since epileptic patients may ultimately benefit from continuous, real-time monitoring and seizure detection using the in-ear sensor, establishing its accuracy in this specific setting was a crucial first step.
Subsequent studies should be conducted to explore whether the various biosignals measured by the wearable device might be useful to detect a broad spectrum of epileptic seizures.
The raw data supporting the conclusions of this article will be made available by the authors upon qualified requests.
The studies involving human participants were reviewed and approved by Ethikkommission der Medizinischen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn (No. 355/16). The patients/participants provided their written informed consent to participate in this study.
Conceptualization & project supervision, RS; Software & data curation, TA; Formal analysis & interpretation, TA and SW; Investigation, RDN; Data visualization, SW; Draft of the manuscript, TA, SW and MB; Critical review & editing, IZ, MW, RDN and RS All authors have read and agreed to the published version of the manuscript.
This research was funded by Federal Ministry of Education and Research of Germany in the framework of EPItect (project number 16SV7482).
The device used in this study is a commercial product of Cosinuss GmbH, which is (or was) the employer of TA, SW, MB, IZ and MW. The reviewer [OH] declared a shared affiliation with the author(s) [MB] to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdgth.2022.909519/full#supplementary-material.
1. Guo Y, Liu X, Peng S, Jiang X, Xu K, Chen C, et al. A review of wearable and unobtrusive sensing technologies for chronic disease management. Comput Biol Med (2021) 129:104163. doi: 10.1016/j.compbiomed.2020.104163
2. Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas (2007) 28:R1. doi: 10.1088/0967-3334/28/3/R01
3. Schäfer A, Vagedes J. How accurate is pulse rate variability as an estimate of heart rate variability?: a review on studies comparing photoplethysmographic technology with an electrocardiogram. Int J Cardiol (2013) 166:15–29. doi: 10.1016/j.ijcard.2012.03.119
4. Tamura T. Current progress of photoplethysmography and SPO 2 for health monitoring. Biomed Eng Lett (2019) 9:21–36. doi: 10.1007/s13534-019-00097-w
5. Vogel S, Hulsbusch M, Starke D, Leonhardt S. In-ear heart rate monitoring using a micro-optic reflective sensor. In 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. Lyon, France: IEEE (2007). p. 1375–8.
6. Passler S, Müller N, Senner V. In-ear pulse rate measurement: a valid alternative to heart rate derived from electrocardiography? Sensors (Switzerland) (2019) 19:3641. doi: 10.3390/s19173641
7. Tigges T, Büchler T, Pielmuş A, Klum M, Feldheiser A, Hunsicker O, et al. Assessment of in-ear photoplethysmography as a surrogate for electrocardiography in heart rate variability analysis. In: World Congress on Medical Physics and Biomedical Engineering 2018. Prague, The Czech Republic: Springer (2019). p. 293–7.
8. Weinschenk SW, Beise RD, Lorenz J. Heart rate variability (HRV) in deep breathing tests and 5-min short-term recordings: agreement of ear photoplethysmography with ecg measurements, in 343 subjects. Eur J Appl Physiol (2016) 116:1527–35. doi: 10.1007/s00421-016-3401-3
9. Nakajima K, Tamura T, Miike H. Monitoring of heart and respiratory rates by photoplethysmography using a digital filtering technique. Med Eng Phys (1996) 18:365–72. doi: 10.1016/1350-4533(95)00066-6
10. Poh M-Z, Swenson NC, Picard RW. Motion-tolerant magnetic earring sensor and wireless earpiece for wearable photoplethysmography. IEEE Trans Inf Technol Biomed (2010) 14:786–94. doi: 10.1109/TITB.2010.2042607
11. Hülsbusch M, Blazek V, Herzog M, Vogel S, Wartzek T, Starke D, et al. Development of a miniaturized in-ear pulse oximeter for long term monitoring of risk patients. In: Dössel O, Schlegel WC, editors. World Congress on Medical Physics and Biomedical Engineering; 2009 Sep 7–12; Munich, Germany. Berlin, Heidelberg: Springer Berlin Heidelberg (2010). p. 779–81.
12. Venema B, Blanik N, Blazek V, Schiefer J, Leonhardt S. A feasibility study evaluating innovative in-ear pulse oximetry for unobtrusive cardiovascular homecare monitoring during sleep. In: 2013 IEEE Point-of-Care Healthcare Technologies (PHT). Bangalore, India: IEEE (2013). p. 124–7. doi: 10.1109/PHT.2013.6461300
13. Surges R, Scott CA, Walker MC. Enhanced QT shortening and persistent tachycardia after generalized seizures. Neurology (2010) 74:421–6. doi: 10.1212/WNL.0b013e3181ccc706
14. Jansen K, Lagae L. Cardiac changes in epilepsy. Seizure (2010) 19:455–60. doi: 10.1016/j.seizure.2010.07.008
15. De Cooman T, Varon C, Hunyadi B, Van Paesschen W, Lagae L, Van Huffel S. Online automated seizure detection in temporal lobe epilepsy patients using single-lead ecg. Int J. Neural Syst (2017) 27:1750022. doi: 10.1142/S0129065717500228
16. Kim T, Nguyen P, Pham N, Bui N, Truong H, Ha S, et al. Epileptic seizure detection and experimental treatment: a review. Front Neurol (2020) 11:701. doi: 10.3389/fneur.2020.00701
17. Picot M-C, Baldy-Moulinier M, Daurès J-P, Dujols P, Crespel A. The prevalence of epilepsy and pharmacoresistant epilepsy in adults: a population-based study in a western european country. Epilepsia (2008) 49:1230–8. doi: 10.1111/epi.2008.49.issue-7
18. Thijs RD, Surges R, O’Brien TJ, Sander JW. Epilepsy in adults. Lancet (2019) 393:689–701. doi: 10.1016/S0140-6736(18)32596-0
19. Hoppe C, Poepel A, Elger CE. Epilepsy: accuracy of patient seizure counts. Arch Neurol (2007) 64:1595–9. doi: 10.1001/archneur.64.11.1595
20. Stirling RE, Cook MJ, Grayden DB, Karoly PJ. Seizure forecasting and cyclic control of seizures. Epilepsia (2021) 62(Suppl 1):S2–S14. doi: 10.1111/epi.16541
21. Proix T, Truccolo W, Leguia MG, Tcheng TK, King-Stephens D, Rao VR, et al. Forecasting seizure risk in adults with focal epilepsy: a development and validation study. Lancet Neurol (2021) 20:127–35. doi: 10.1016/S1474-4422(20)30396-3
22. EPItect (Project Number: 16SV7482). Sensorische Anfallsdetektion (2019). Available from: https://www.epitect.de/ (Accessed March 17, 2022).
23. Kreuzer J. Alltagstaugliche sensorik: kontinuierliches monitoring von körperkerntemperatur und sauerstoffsättigung [dissertation]. Munich: Technical University of Munich (2009).
24. Cosinuss GmbH. cosinuss° One technical data sheet. Munich: Cosinuss GmbH (2019). Available from: https://support.cosinuss.com/wp-content/uploads/2020/06/COS˙2019-02-12˙Datenblatt˙EN˙V1.4.pdf
25. Pan J, Tompkins WJ. A real-time QRS detection algorithm. IEEE Trans Biomed Eng (1985) 32(3):230–6. doi: 10.1109/TBME.1985.325532
26. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull (1979) 86:420. doi: 10.1037/0033-2909.86.2.420
27. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med (2016) 15:155–63. doi: 10.1016/j.jcm.2016.02.012
28. Bland JM, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet (1986) 327:307–10. doi: 10.1016/S0140-6736(86)90837-8
30. Fokkema T, Kooiman TJ, Krijnen WP, Van der Schans CP, De Groot M. Reliability and validity of ten consumer activity trackers depend on walking speed. Med Sci Sports Exerc (2017) 49:793–800. doi: 10.1249/MSS.0000000000001146
31. Nelson MB, Kaminsky LA, Dickin DC, Montoye AH. Validity of consumer-based physical activity monitors for specific activity types. Med Sci Sports Exerc (2016) 48:1619–28. doi: 10.1249/MSS.0000000000000933
32. Etiwy M, Akhrass Z, Gillinov L, Alashi A, Wang R, Blackburn G, et al. Accuracy of wearable heart rate monitors in cardiac rehabilitation. Cardiovasc Diagn Ther (2019) 9:262. doi: 10.21037/cdt
33. Henze J, Houta S, Surges R, Kreuzer J, Bisgin P. Multimodal detection of tonic–clonic seizures based on 3D acceleration and heart rate data from an in-ear sensor. In: Del Bimbo A, Cucchiara R, Sclaroff S, Farinella GM, Mei T, Bertini M, Escalante HJ, Vezzani R, editors. Pattern Recognition. ICPR International Workshops and Challenges. Cham: Springer International Publishing (2021). p. 490–502.
34. Bruno E, Biondi A, Richardson MP, RADAR-CNS Consortium. Pre-ictal heart rate changes: a systematic review and meta-analysis. Seizure (2018) 55:48–56. doi: 10.1016/j.seizure.2018.01.003
Keywords: validation, heart rate, photoplethysmography, in-ear, wearable sensor, continuous monitoring, epilepsy
Citation: Adams T, Wagner S, Baldinger M, Zellhuber I, Weber M, Nass D and Surges R (2022) Accurate detection of heart rate using in-ear photoplethysmography in a clinical setting. Front. Digit. Health 4:909519. doi: 10.3389/fdgth.2022.909519
Received: 31 March 2022; Accepted: 3 August 2022;
Published: 17 August 2022.
Edited by:
Mandal Subhamoy Indian Institute of Technology Kharagpur, IndiaReviewed by:
Basak Kausik JIS Institute of Advanced Studies and Research, India© 2022 Adams, Wagner, Baldinger, Zellhuber, Weber, Nass and Surges. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rainer Surges cmFpbmVyLnN1cmdlc0B1a2Jvbm4uZGU=
Specialty Section: This article was submitted to Health Technology Implementation, a section of the journal Frontiers in Digital Health
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.