- 1University of Utah College of Pharmacy, Salt Lake City, UT, United States
- 2Department of Medicinal Chemistry, L.S. Skaggs College of Pharmacy, University of Utah, Salt Lake City, UT, United States
- 3Department of Pharmacotherapy, L.S. Skaggs College of Pharmacy, University of Utah, Salt Lake City, UT, United States
Digital therapeutics (DTx, mobile medical apps, software as a medical device) are rapidly emerging as clinically effective treatments for diverse chronic diseases. For example, the Food and Drug Administration (FDA) has recently authorized a prescription virtual reality (VR) app for treatment of moderate to severe low back pain. The FDA has also approved an adjunct digital therapy in conjunction with buprenorphine for opioid use disorder, further illustrating opportunities to integrate digital therapeutics with pharmacotherapies. There are ongoing needs to disseminate knowledge about advances in digital interventions among health care professionals, policymakers, and the public at large. This mini-review summarizes accumulating clinical evidence of digital interventions delivered via virtual reality and mobile apps to improve opioid-based analgesia. We identified relevant randomized controlled trials (RCTs) using Embase and PubMed databases which reported pain scores with a validated pain scale (e.g., visual analog scales, graphic rating scale, numeric rating scale) and use of a digital intervention in conjunction with opiates. Among identified RCTs, the majority of studies reported improved pain scores in the digital intervention group, as compared to “treatment as usual” group. Our work suggests that VR and mobile apps can be used as adjunct digital therapies for pain management. We discuss these findings in the context of how digital health technologies can transform patient-centered pharmacy care.
Introduction
Pain management is a complex, multifaceted challenge that has become a major public health crisis, with an estimated 126.1 million US adults suffering from pain (1). In 2016, over 60 million patients filled or refilled one or more prescriptions for opioid analgesics (1). Although opioid-based analgesia is frequently used to treat both acute and chronic pain, health care professionals (physicians, physician assistants, pharmacists, and nurses) have limited knowledge on opioid analgesic therapies (2). In addition to inadequate pain relief, the use of opioids for pain management is challenged by significant adverse effects including physical dependence, tolerance, sedation, dizziness, constipation, nausea, vomiting, and respiratory depression (3). Trends in opioid prescription and the associated mortality continue to be problematic not only in the US, but also in other countries (4, 5). Multimodal approaches for pain management such as combination therapy with both nonpharmacological means in addition to traditional pharmacological therapeutics can be effective in achieving optimal control of pain (6, 7). Many aspects of pain management and the opioid epidemic may effectively be addressed by shifting clinical practice to using more non-pharmacological and non-invasive treatments (8), including “digital analgesics” interventions (9–13) and mobile apps to support opioid tapering (14, 15).
Digital health technologies encompass diverse software-based tools which can improve health and therapy outcomes for many chronic diseases. Digital therapeutics (DTx), also known as mobile medical applications, are software-based interventions intended to treat specific medical conditions (16–18). To provide evidence-based therapies, DTx receive marketing authorization (software as a medical device, or SaMD) from regulatory agencies. In the US, the FDA has approved and cleared several digital therapeutics for the treatment of diabetes (type 1 and 2), ADHD, asthma, COPD, chronic low back pain, chronic insomnia, substance use disorder and opioid use disorder. It is also noteworthy that two non-profit organizations, namely The Digital Medicine Society (www.dimesociety.org/) and The Digital Therapeutics Alliance (dtxalliance.org/) are dedicated to advance and promote this rapidly evolving branch of digital health.
Pioneering work on the SnowWorld virtual reality (VR) video game for burn patients illustrates early efforts to bring digital interventions for pain to clinical practice (19–22). There has been an increasing number of clinical studies on VR and mobile apps to improve pain management and relief (6, 9–11, 23–39). For example, a 12-week RCT of a multidisciplinary back pain mobile app (Kaia) showed significant reduction of pain intensity in patients with non-specific low back pain (40). In 2020, the FDA granted a breakthrough medical device designation to a VR app, RelieVRx (previously named EaseVRx), for treatment of intractable low back pain and treatment-resistant fibromyalgia. In 2021, RelieVRx received the FDA authorization for marketing a prescription virtual reality app for treatment of moderate to severe low back pain (41). These advances in digital interventions highlight opportunities for their use as adjunct therapies in combination with diverse analgesic drugs.
To the best of our knowledge, there are currently no review studies focused on effects of digital interventions on opioid-based pain management. Given the increasing number of clinical studies on VR and mobile apps for pain, there is a need for systematic reviews and meta-analyses (SR/MA) of the impact of DTx on different types of pain in combination with analgesic medications. The objectives of this mini-review are: (1) to summarize findings from currently published RCTs focused on adjunct digital interventions (VR and mobile apps) for opioid-based pain management, and (2) to encourage future SR/MA studies on adjunct digital interventions for pain in combination with specific analgesic drugs, including opioids, NSAIDs and others. We further discuss our findings in the context of how digital therapeutics can impact patient-centered pharmacy care.
Adjunct Digital Interventions For Opioid-Based Analgesia
In order to identify adjunct digital interventions for pain management in conjunction with opioid-based analgesia, EMBASE and PubMed databases were searched for relevant RCTs, systematic reviews and meta-analyses. Database search with keywords “pain,” “acute pain,” “chronic pain,” “cancer pain,” “burn pain,” “postoperative pain,” “pain management,” “virtual reality,” “VR,” “web-based,” “phone app,” “mobile app,” “opioid” and “digital therapeutics” identified nine RCTs which met the following inclusion criteria: (1) reported digital interventions were compared to pharmacological interventions alone, (2) reported pain scores with a validated pain scale (e.g., visual analog scales, graphic rating scale, numeric rating scale), and (3) reported use of concomitant opioids. Studies that did not explicitly report use of opioids or use of a validated pain scale were excluded. In addition to searching the databases, we also examined RCTs evaluated in recent systematic reviews and meta-analyses on digital interventions for pain for those clinical studies that matched inclusion/exclusion criteria mentioned above (30, 32–34).
As summarized in Table 1, our search yielded nine RCTs which met inclusion and exclusion criteria. A majority of RCTs examined effects of digital interventions in burn pain patients (a total of n = 227), whereas two studies were focused on cancer pain. Regarding types of digital interventions, a vast majority of studies used VR apps. Eight studies demonstrated significant reduction in one or more pain outcomes (19, 42–45, 47–49), whereas one RCT reported no significant changes in pain intensity, as compared to the control groups. Based on the RCTs listed in Table 1, these findings suggest that adjunct digital interventions can improve pain scores or reduce medication use in opioid-based analgesia.
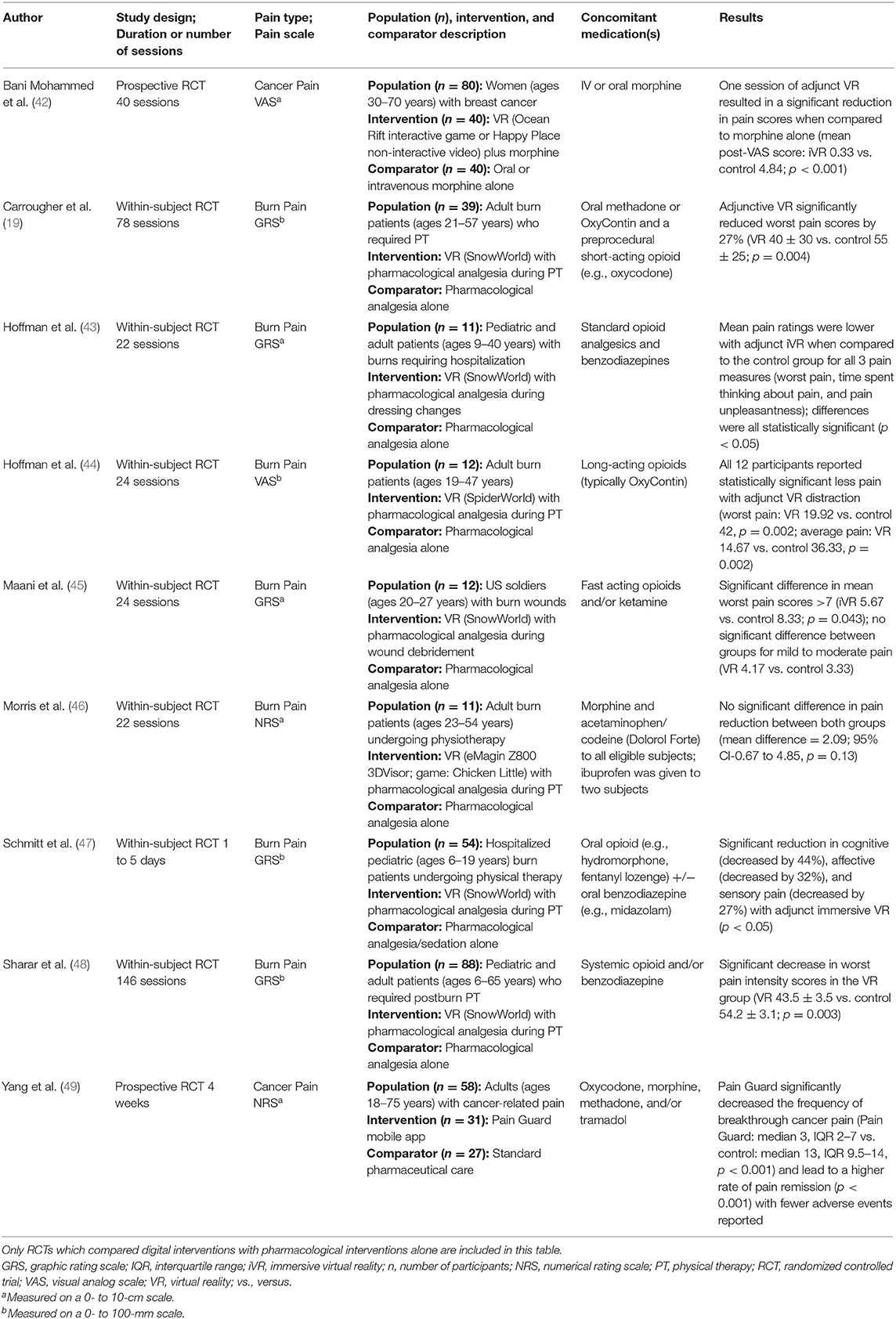
Table 1. Summary of randomized controlled trials of digital interventions in patients with acute or chronic pain.
Two additional RCTs investigated digital interventions in pain patients taking opioid analgesics, but they did not meet all three inclusion criteria (the comparator was not pharmacological treatment alone) (13, 50). In one RCT examining digital intervention in breast cancer surgery patients, the treatment group showed significant reduction of time (by 5 days) toward cessation of opioid medications, as compared to the control group (digital health education) (13). In another RCTs, VR app intervention (as compared to standard iPad use) did not change postoperative pain scores nor opioid consumption in pediatric patients (50). As discussed below, with more ongoing RCTs studying digital interventions and opioid-based analgesia in pain patients, our results justify near-future SR/MA study to evaluate clinical efficacy of adjunct VR and mobile apps in conjunction with analgesics to improve pain management.
Discussion
There are ongoing needs to mitigate the opioid crisis in the United States (51, 52). To increase awareness about potential benefits of digital interventions for pain management, this mini-review project focused on whether virtual reality and mobile apps can improve opioid-based analgesia. Our findings suggest that VR applications can offer clinical-grade interventions for opioid-sparing pain management, and are in accord with conclusions from a recent systematic-review and meta-analysis that “Virtual reality is an effective pain reduction measurement added to analgesics for burn patients undergoing dressing change or physical therapy.” (32). The FDA authorization to market RelieVRx as a prescription virtual reality pain treatment further emphasizes opportunities to combine digital interventions with analgesics (41). It is noteworthy that clinical evidence for digital interventions in pain management is still limited and needs additional multi-center RCTs to validate their clinical efficacy and effectiveness in patients with various pain conditions (30–34, 53).
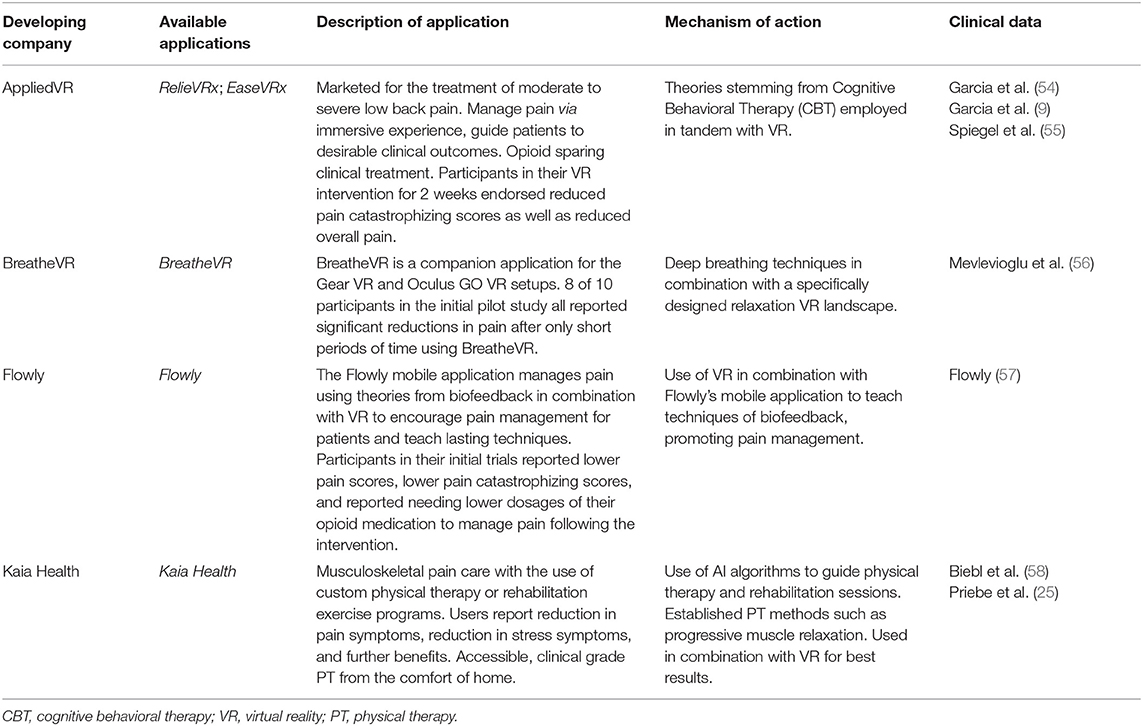
As shown in Table 2, there are several VR and mobile applications currently available for patients and health care providers as tools for improving pain management. RelieVRx has received the FDA authorization (through de novo regulatory pathway) to be marketed as a prescription virtual reality pain treatment for adult patients with chronic low back pain (41). Kaia Health is a mobile app intended for adults with acute or chronic, non-specific musculoskeletal pain, which received class II medical device status in Europe, while is marketed in the US under the FDA enforcement discretion. Flowly VR and biofeedback app is presented as “opioid-sparing pain management device” (www.flowly.world/), but to the best of our knowledge, Flowly has not received the FDA authorization as a medical device, as of writing this mini-review. While digital health technologies are rapidly evolving and expanding, we believe that this article will encourage health care professionals to explore opportunities to integrate digital interventions with pharmacotherapies for improved pain management.
Bringing digital interventions for pain to clinical practice is challenged by complexity of workflow in pain management (59). Mobile apps have been recognized as opportunities to improve pharmacy practice (60–63). Pharmacists often work in interdisciplinary care teams and make recommendations to both providers and patients about pharmacologic and non-pharmacologic interventions, including pain management (64–66). Given an important role of pharmacists in opioid stewardship and prevention of future opioid crisis (67, 68), we hypothesize here that pharmacists recommendations to integrate digital therapeutics with opioid-based analgesia will improve outcomes of opioid tapering programs (69–73). Recently, the Academy of Managed Care Pharmacy convened a forum that brought digital therapeutic innovators, payers, pharmacy benefit managers, and other key stakeholders to discuss the role of digital interventions as therapeutic options (74). While implementation of digital health technologies within health care systems is both inevitable and challenging (75–77), it will be important for payers to consider their health care coverage, especially as more evidence emerges with the potential opportunity of lowering overall health care costs and increasing clinical outcomes. An initial cost-effectiveness analysis of the reimbursement rate for digital therapeutics for low back pain suggests economic benefits for health care in Germany (78).
Integration of digital interventions with drug-based therapies is illustrated by the FDA approval of a prescription adjunct digital therapeutic, namely reSET-O® PDT, in conjunction with buprenorphine for opioid use disorder (OUD). This adjunct digital intervention was shown to improve therapy and health care outcomes, including cost-effectiveness (79–84). From the perspective of long-term therapy outcomes for chronic diseases, patients could benefit from research and development of both adjunct digital therapeutics and drug+digital combination therapies (using drug-device combination product regulatory pathway, where drug is combined with a mobile app approved as SaMD) (18, 85–89). Although drug+digital combination therapies offer a full integration of pharmacotherapy and non-pharmacological intervention, to the best of our knowledge there are no currently known such drug-device combination products. Other future prospects for improved patient-centered pain management may include integration of drug-based analgesia with patient education delivered via digital health technologies (29, 90, 91), and integration of digital health technologies with self-care and therapeutic home environment (92).
A limitation of this mini-review is a lack of systematic review methodology and meta-analysis, thus precluding to draw evidence-based conclusions on effectiveness of digital interventions for opioid-based analgesia. Given that clinical studies on digital interventions for reduction of opioid use in pain management is a very active area of research (e.g., from ClinicalTrials.gov: NCT04139564, NCT04010266, NCT03851042, NCT04273919, NCT04416555 and others), it is prudent to wait for more published results from all relevant RCTs. Another limitation of this project is a focus on opioid-based treatments, rather than on opioids and non-steroidal anti-inflammatory drugs (NSAIDs). This is due to a limited number of clinical studies which report use of specific pain medications when evaluating VR or mobile apps in pain management. We hope that despite these limitations, this mini-review will raise awareness on how digital interventions can improve patient-centered pharmacy care for pain and for other medical conditions.
Given complex and unmet needs to address the opioid crisis (52, 93), this review supports several actionable recommendations to be considered. Educating health care professionals, patients and policymakers about the FDA-approved VR and mobile apps for pain should be led by both patient advocacy groups (e.g., The American Chronic Pain Association and the US Pain Foundation) and professional organizations (e.g., The American College of Physicians and The American Academy of Neurologists). Integrated healthcare systems and hospitals can create VR simulation centers for patient education about their diagnosis and treatment options including digital interventions (94, 95). Educating pharmacists, nurses and physician assistants about digital health technologies will accelerate clinical workflow redesign to incorporate their “internal champions” roles in decision making for pain management (64–66, 77, 96). For opioid prescription and tapering for chronic pain, revisions and updates to the CDC guidelines and payer pharmacy coverage should include the use of digital therapeutics for pain relief and management (97). Lastly, increasing social media campaigns (98, 99), and direct-to-consumer advertising of VR and mobile apps for pain will expand public awareness about digital therapeutics, and will also impact prescribing practices in the future (100, 101).
Conclusion
Our mini-review suggests that both VR and mobile apps can be used as adjunct digital therapies in conjunction with opioid-based analgesics for pain management. Such interventions, which are applicable to hospital, hospital at home and stay-at-home care, can improve patient-centered pharmacy care and opioid tapering outcomes. Rapidly evolving digital health technologies create opportunities to integrate pharmacotherapies with non-pharmacological treatments for pain, while regulatory approval of commercially available digital interventions as DTx for pain management is critical for reimbursement and health care implementation.
Author Contributions
HG and GB: conceptualization, literature search and review, and manuscript writing. ZB: literature search and review and manuscript writing. LT: literature review and manuscript writing. All authors contributed to the article and approved the submitted version.
Funding
GB acknowledges a research support by the ALSAM Foundation Grant.
Conflict of Interest
GB is a founder and owner of OMNI Self-care, LLC, a health promotion company creating digital content for disease self-management and is a co-inventor on two issued US patents 9,569,562 and 9,747,423 “Disease Therapy Game Technology” and patent-pending application “Multimodal Platform for Treating Epilepsy”. These patents are related to digital health technologies, and are owned by the University of Utah. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hagemeier NE. Introduction to the opioid epidemic: the economic burden on the healthcare system and impact on quality of life. Am J Manag Care. (2018) 24:S200–S6.
2. Williamson C, Martin BJ, Argoff C, Gharibo C, McCarberg B, Atkinson T, et al. Pain management and opioid therapy: persistent knowledge gaps among primary care providers. J Pain Res. (2021) 14:3223–34. doi: 10.2147/JPR.S316637
3. Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. (2008) 11(2 Suppl):S105–20. doi: 10.36076/ppj.2008/11/S105
4. Kurdi A. Opioids and gabapentinoids utilisation and their related-mortality trends in the United Kingdom primary care setting, 2010–2019: a cross-national, population-based comparison study. Front. Pharmacol. (2021) 12:732345. doi: 10.3389/fphar.2021.732345
5. Bedson J, Chen Y, Hayward RA, Ashworth J, Walters K, Dunn KM, et al. Trends in long-term opioid prescribing in primary care patients with musculoskeletal conditions: an observational database study. Pain. (2016) 157:1525–31. doi: 10.1097/j.pain.0000000000000557
6. Shebib R, Bailey JF, Smittenaar P, Perez DA, Mecklenburg G, Hunter S. Randomized controlled trial of a 12-week digital care program in improving low back pain. NPJ Digit Med. (2019) 2:1. doi: 10.1038/s41746-018-0076-7
7. Amorim AB, Pappas E, Simic M, Ferreira ML, Jennings M, Tiedemann A, et al. Integrating Mobile-health, health coaching, and physical activity to reduce the burden of chronic low back pain trial (IMPACT): a pilot randomised controlled trial. BMC Musculoskelet Disord. (2019) 20:71. doi: 10.1186/s12891-019-2454-y
8. Qaseem A, Wilt TJ, McLean RM, Forciea MA, Clinical Clinical Guidelines Committee of the American College of P. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the american college of physicians. Ann Intern Med. (2017) 166:514–530. doi: 10.7326/M16-2367
9. Garcia LM, Birckhead BJ, Krishnamurthy P, Sackman J, Mackey IG, Louis RG, et al. An 8-week self-administered at-home behavioral skills-based virtual reality program for chronic low back pain: double-blind, randomized, placebo-controlled trial conducted during COVID-19. J Med Internet Res. (2021) 23:e26292. doi: 10.2196/26292
10. Won AS, Bailey J, Bailenson J, Tataru C, Yoon IA, Golianu B. Immersive virtual reality for pediatric pain. Children. (2017) 4:52. doi: 10.3390/children4070052
11. Irvine AB, Russell H, Manocchia M, Mino DE, Cox Glassen T, Morgan R, et al. Mobile-Web app to self-manage low back pain: randomized controlled trial. J Med Internet Res. (2015) 17:e1. doi: 10.2196/jmir.3130
12. Pronk Y, Peters M, Sheombar A, Brinkman JM. Effectiveness of a mobile eHealth app in guiding patients in pain control and opiate use after total knee replacement: randomized controlled trial. JMIR Mhealth Uhealth. (2020) 8:e16415. doi: 10.2196/16415
13. Darnall BD, Ziadni MS, Krishnamurthy P, Flood P, Heathcote LC, Mackey IG, et al. “My surgical success”: effect of a digital behavioral pain medicine intervention on time to opioid cessation after breast cancer surgery-a pilot randomized controlled clinical trial. Pain Med. (2019) 20:2228–37. doi: 10.1093/pm/pnz094
14. Magee M, Gholamrezaei A, McNeilage AG, Dwyer L, Sim A, Ferreira M, et al. Evaluating acceptability and feasibility of a mobile health intervention to improve self-efficacy in prescription opioid tapering in patients with chronic pain: protocol for a pilot randomised, single-blind, controlled trial. BMJ Open. (2022) 12:e057174. doi: 10.1136/bmjopen-2021-057174
15. Magee MR, McNeilage AG, Avery N, Glare P, Ashton-James CE. mHealth interventions to support prescription opioid tapering in patients with chronic pain: qualitative study of patients' perspectives. JMIR Form Res. (2021) 5:e25969. doi: 10.2196/25969
16. Patel NA, Butte AJ. Characteristics and challenges of the clinical pipeline of digital therapeutics. NPJ Digit Med. (2020) 3:159. doi: 10.1038/s41746-020-00370-8
17. Shuren J, Patel B, Gottlieb S. FDA Regulation of mobile medical apps. JAMA. (2018) 320:337–8. doi: 10.1001/jama.2018.8832
18. Sverdlov O, van Dam J, Hannesdottir K, Thornton-Wells T. Digital therapeutics: an integral component of digital innovation in drug development. Clin Pharmacol Ther. (2018) 104:72–80. doi: 10.1002/cpt.1036
19. Carrougher GJ, Hoffman HG, Nakamura D, Lezotte D, Soltani M, Leahy L, et al. The effect of virtual reality on pain and range of motion in adults with burn injuries. J Burn Care Res. (2009) 30:785–91. doi: 10.1097/BCR.0b013e3181b485d3
20. Hoffman HG. Virtual-reality therapy. Sci Am. (2004) 291:58–65. doi: 10.1038/scientificamerican0804-58
21. Hoffman HG, Seibel EJ, Richards TL, Furness TA, Patterson DR, Sharar SR. Virtual reality helmet display quality influences the magnitude of virtual reality analgesia. J Pain. (2006) 7:843–50. doi: 10.1016/j.jpain.2006.04.006
22. Hoffman HG, Chambers GT, Meyer WJ 3rd, Arceneaux LL, Russell WJ, Seibel EJ, et al. Virtual reality as an adjunctive non-pharmacologic analgesic for acute burn pain during medical procedures. Ann Behav Med. (2011) 41:183–91. doi: 10.1007/s12160-010-9248-7
23. Bailey JF, Agarwal V, Zheng P, Smuck M, Fredericson M, Kennedy DJ, et al. Digital care for chronic musculoskeletal pain: 10,000 participant longitudinal cohort study. J Med Internet Res. (2020) 22:e18250. doi: 10.2196/18250
24. Thurnheer SE, Gravestock I, Pichierri G, Steurer J, Burgstaller JM. Benefits of mobile apps in pain management: systematic review. JMIR Mhealth Uhealth. (2018) 6:e11231. doi: 10.2196/11231
25. Priebe JA, Haas KK, Moreno Sanchez LF, Schoefmann K, Utpadel-Fischler DA, Stockert P, et al. Digital treatment of back pain versus standard of care: the cluster-randomized controlled trial, rise-uP. J Pain Res. (2020) 13:1823–38. doi: 10.2147/JPR.S260761
26. Chi B, Chau B, Yeo E, Ta P. Virtual reality for spinal cord injury-associated neuropathic pain: Systematic review. Ann Phys Rehabil Med. (2018) 62:49–57. doi: 10.1016/j.rehab.2018.09.006
27. Tashjian VC, Mosadeghi S, Howard AR, Lopez M, Dupuy T, Reid M, et al. Virtual reality for management of pain in hospitalized patients: results of a controlled trial. JMIR Ment Health. (2017) 4:e9. doi: 10.2196/mental.7387
28. Darnall BD, Krishnamurthy P, Tsuei J, Minor JD. Self-administered skills-based virtual reality intervention for chronic pain: randomized controlled pilot study. JMIR Form Res. (2020) 4:e17293. doi: 10.2196/17293
29. Garcia LM, Birckhead BJ, Krishnamurthy P, Mackey I, Sackman J, Salmasi V, et al. Three-month follow-up results of a double-blind, randomized placebo-controlled trial of 8-week self-administered at-home behavioral skills-based virtual reality (VR) for chronic low back pain. J Pain. (2021) 23:822–840. doi: 10.1016/j.jpain.2021.12.002
30. Huang Q, Lin J, Han R, Peng C, Huang A. Using virtual reality exposure therapy in pain management: a systematic review and meta-analysis of randomized controlled trials. Value Health. (2022) 25:288–301. doi: 10.1016/j.jval.2021.04.1285
31. Lewkowicz D, Slosarek T, Wernicke S, Winne A, Wohlbrandt AM, Bottinger E. Digital therapeutic care and decision support interventions for people with low back pain: systematic review. JMIR Rehabil Assist Technol. (2021) 8:e26612. doi: 10.2196/26612
32. Luo H, Cao C, Zhong J, Chen J, Cen Y. Adjunctive virtual reality for procedural pain management of burn patients during dressing change or physical therapy: a systematic review and meta-analysis of randomized controlled trials. Wound Repair Regen. (2019) 27:90–101. doi: 10.1111/wrr.1
33. Chuan A, Zhou JJ, Hou RM, Stevens CJ, Bogdanovych A. Virtual reality for acute and chronic pain management in adult patients: a narrative review. Anaesthesia. (2021) 76:695–704. doi: 10.1111/anae.15202
34. Zheng C, Chen X, Weng L, Guo L, Xu H, Lin M, et al. Benefits of Mobile Apps for Cancer Pain Management: Systematic Review. JMIR Mhealth Uhealth. (2020) 8:e17055. doi: 10.2196/17055
35. O'Connor S, Mayne A, Hood B. Virtual reality-based mindfulness for chronic pain management: a scoping review. Pain Manag Nurs. (2022). doi: 10.1016/j.pmn.2022.03.013
36. Grassini S. Virtual reality assisted non-pharmacological treatments in chronic pain management: a systematic review and quantitative meta-analysis. Int J Environ Res Public Health. (2022) 19. doi: 10.3390/ijerph19074071
37. Găină MA, Szalontay AS, Ştefănescu G, Bălan GG, Ghiciuc CM, Bolo? A, et al. State-of-the-art review on immersive virtual reality interventions for colonoscopy-induced anxiety and pain. J Clin Med. (2022) 11:1670. doi: 10.3390/jcm11061670
38. He ZH, Yang HM, Dela Rosa RD, De Ala MB. The effects of virtual reality technology on reducing pain in wound care: a meta-analysis and systematic review. Int Wound J. (2022). doi: 10.1111/iwj.13785 [Epub ahead of print].
39. Nagpal AS, Raghunandan A, Tata F, Kibler D, McGeary D. Virtual reality in the management of chronic low back pain: a scoping review. Front Pain Res. (2022) 3:856935. doi: 10.3389/fpain.2022.856935
40. Toelle TR, Utpadel-Fischler DA, Haas KK, Priebe JA. App-based multidisciplinary back pain treatment versus combined physiotherapy plus online education: a randomized controlled trial. Npj Digit Med. (2019) 2:34. doi: 10.1038/s41746-019-0109-x
41. Rubin R. Virtual reality device is authorized to relieve back pain. JAMA. (2021) 326:2354. doi: 10.1001/jama.2021.22223
42. Bani Mohammad E, Ahmad M. Virtual reality as a distraction technique for pain and anxiety among patients with breast cancer: a randomized control trial. Palliative and Supportive Care. (2019) 17:29–34. doi: 10.1017/S1478951518000639
43. Hoffman HG, Patterson DR, Seibel E, Soltani M, Leahy L, Sharar SR. Virtual reality pain control during burn wound debridement in the hydrotank. Clin J Pain. (2008) 24:299–304. doi: 10.1097/AJP.0b013e318164d2cc
44. Hoffman HG, Patterson DR, Carrougher GJ. Use of virtual reality for adjunctive treatment of adult burn pain during physical therapy: a controlled study. Clin J Pain. (2000) 16:244–50. doi: 10.1097/00002508-200009000-00010
45. Maani CV, Hoffman HG, Morrow M, Maiers A, Gaylord K, McGhee LL, et al. Virtual reality pain control during burn wound debridement of combat-related burn injuries using robot-like arm mounted VR goggles. Journal of Trauma: Injury, Infection and Critical Care. (2011) 71:S125–S30. doi: 10.1097/TA.0b013e31822192e2
46. Morris LD, Louw QA, Crous LC. Feasibility and potential effect of a low-cost virtual reality system on reducing pain and anxiety in adult burn injury patients during physiotherapy in a developing country. Burns. (2010) 36:659–64. doi: 10.1016/j.burns.2009.09.005
47. Schmitt YS, Hoffman HG, Blough DK, Patterson DR, Jensen MP, Soltani M, et al. A randomized, controlled trial of immersive virtual reality analgesia, during physical therapy for pediatric burns. Burns. (2011) 37:61–8. doi: 10.1016/j.burns.2010.07.007
48. Sharar SR, Carrougher GJ, Nakamura D, Hoffman HG, Blough DK, Patterson DR. Factors influencing the efficacy of virtual reality distraction analgesia during postburn physical therapy: preliminary results from 3 ongoing studies. Arch Phys Med Rehabil. (2007) 88:S43–S9. doi: 10.1016/j.apmr.2007.09.004
49. Yang J, Weng L, Chen Z, Cai H, Lin X, Hu Z, et al. Development and testing of a mobile app for pain management among cancer patients discharged from hospital treatment: randomized controlled trial. JMIR mHealth and uHealth. (2019) 7:e12542. doi: 10.2196/12542
50. Specht BJ, Buse CR, Phelps JR, Phillips MR, Chiavacci SD, Harrell LE, et al. Virtual reality after surgery—a method to decrease pain after surgery in pediatric patients. Am Surg.
51. Stoicea N, Costa A, Periel L, Uribe A, Weaver T, Bergese SD. Current perspectives on the opioid crisis in the US healthcare system: a comprehensive literature review. Medicine. (2019) 98:e15425. doi: 10.1097/MD.0000000000015425
52. Humphreys K, Shover CL, Andrews CM, Bohnert ASB, Brandeau ML, Caulkins JP, et al. Responding to the opioid crisis in North America and beyond: recommendations of the Stanford-Lancet Commission. Lancet. (2022) 399:555–604. doi: 10.1016/S0140-6736(21)02252-2
53. Pfeifer AC, Uddin R, Schröder-Pfeifer P, Holl F, Swoboda W, Schiltenwolf M. Mobile application-based interventions for chronic pain patients: a systematic review and meta-analysis of effectiveness. J Clin Med. (2020) 9:3557. doi: 10.3390/jcm9113557
54. Garcia LM, Darnall BD, Krishnamurthy P, Mackey IG, Sackman J, Louis RG, et al. Self-administered behavioral skills-based at-home virtual reality therapy for chronic low back pain: protocol for a randomized controlled trial. JMIR Res Protoc. (2021) 10:e25291. doi: 10.2196/25291
55. Spiegel B, Fuller G, Lopez M, Dupuy T, Noah B, Howard A, et al. Virtual reality for management of pain in hospitalized patients: a randomized comparative effectiveness trial. PLoS ONE. (2019) 14:e0219115. doi: 10.1371/journal.pone.0219115
56. Mevlevioglu D, Murphy D, Tabirca S. Visual respiratory feedback in virtual reality exposure therapy: a pilot study. ACM International Conference on Interactive Media Experiences. Virtual Event, USA: Association for Computing Machinery (2021). p. 1–6. doi: 10.1145/3452918.3458799
58. Biebl JT, Rykala M, Strobel M, Kaur Bollinger P, Ulm B, Kraft E, et al. App-based feedback for rehabilitation exercise correction in patients with knee or hip osteoarthritis: prospective cohort study. J Med Internet Res. (2021) 23:e26658. doi: 10.2196/26658
59. Sarkar U, Lee JE, Nguyen KH, Lisker S, Lyles CR. Barriers and facilitators to the implementation of virtual reality as a pain management modality in academic, community, and safety-net settings: qualitative analysis. J Med Internet Res. (2021) 23:e26623. doi: 10.2196/26623
60. Aungst TD. Integrating mHealth and mobile technology education into the pharmacy curriculum. Am J Pharm Educ. (2014) 78:19. doi: 10.5688/ajpe78119
61. Aungst TD. Medical applications for pharmacists using mobile devices. Ann Pharmacother. (2013) 47:1088–95. doi: 10.1345/aph.1S035
62. Aungst TD, Miranda AC, Serag-Bolos ES. How mobile devices are changing pharmacy practice. Am J Health Syst Pharm. (2015) 72:494–500. doi: 10.2146/ajhp140139
63. AMCP partnership forum: digital therapeutics-what are they and where do they fit in pharmacy and medical benefits? J Manag Care Spec Pharm. (2020) 26:674–81. doi: 10.18553/jmcp.2020.19418
64. Boren LL, Locke AM, Friedman AS, Blackmore CC, Woolf R. Team-based medicine: incorporating a clinical pharmacist into pain and opioid practice management. PM&R. (2019) 11:1170–7. doi: 10.1002/pmrj.12127
65. Kang I, Urick B, Vohra R, Ives TJ. Physician-pharmacist collaboration on chronic non-cancer pain management during the opioid crisis: a qualitative interview study. Res Social Adm Pharm. (2019) 15:1027–31. doi: 10.1016/j.sapharm.2019.04.052
66. Giannitrapani KF, Glassman PA, Vang D, McKelvey JC, Thomas Day R, Dobscha SK, et al. Expanding the role of clinical pharmacists on interdisciplinary primary care teams for chronic pain and opioid management. BMC Fam Pract. (2018) 19:107. doi: 10.1186/s12875-018-0783-9
67. Salwan A, Hagemeier NE, Tudiver F, Dowling-McClay K, Foster KN, Arnold J, et al. Community pharmacist engagement in opioid use disorder prevention and treatment behaviors: a descriptive analysis. J Am Pharm Assoc. (2003) 60:e173–e8. doi: 10.1016/j.japh.2020.06.008
68. Chisholm-Burns MA, Spivey CA, Sherwin E, Wheeler J, Hohmeier K. The opioid crisis: Origins, trends, policies, and the roles of pharmacists. Am J Health Syst Pharm. (2019) 76:424–35. doi: 10.1093/ajhp/zxy089
69. Firemark AJ, Schneider JL, Kuntz JL, Papajorgji-Taylor D, Dickerson JF, Thorsness LA, et al. “We need to taper.” Interviews with clinicians and pharmacists about use of a pharmacy-led opioid tapering program. Pain Med. (2021) 22:1213–22. doi: 10.1093/pm/pnaa442
70. Kuntz JL, Schneider JL, Firemark AJ, Dickerson JF, Papajorgji-Taylor D, Reese KR, et al. A pharmacist-led program to taper opioid use at kaiser permanente northwest: rationale, design, and evaluation. Perm J. (2020) 24:19.216. doi: 10.7812/TPP/19.216
71. Page J, Traver R, Patel S, Saliba C. Implementation of a proactive pilot health plan-driven opioid tapering program to decrease chronic opioid use for conditions of the back and spine in a medicaid population. J Manag Care Spec Pharm. (2018) 24:191–6. doi: 10.18553/jmcp.2018.24.3.191
72. Hundley L, Spradley S, Donelenko S. Assessment of outcomes following high-dose opioid tapering in a Veterans Healthcare System. J Opioid Manag. (2018) 14:89–101. doi: 10.5055/jom.2018.0436
73. Darnall BD, Fields HL. Clinical and neuroscience evidence supports the critical importance of patient expectations and agency in opioid tapering. Pain. (2022) 163:824–6. doi: 10.1097/j.pain.0000000000002443
74. AMCP Partnership Forum Develops Steps to Strengthen Evaluation of Digital Therapeutics: Academy of Managed Care Pharmacy (2021).
75. Kubo A, Kurtovich E, McGinnis M, Aghaee S, Altschuler A, Quesenberry C Jr, et al. A randomized controlled trial of mhealth mindfulness intervention for cancer patients and informal cancer caregivers: a feasibility study within an integrated health care delivery system. Integr Cancer Ther. (2019) 18:1534735419850634. doi: 10.1177/1534735419850634
76. Avalos LA, Aghaee S, Kurtovich E, Quesenberry C Jr, Nkemere L, McGinnis MK, et al. A mobile health mindfulness intervention for women with moderate to moderately severe postpartum depressive symptoms: feasibility study. JMIR Ment Health. (2020) 7:e17405. doi: 10.2196/17405
77. Marwaha JS, Landman AB, Brat GA, Dunn T, Gordon WJ. Deploying digital health tools within large, complex health systems: key considerations for adoption and implementation. Npj Digit Med. (2022) 5:13. doi: 10.1038/s41746-022-00557-1
78. Lewkowicz D, Wohlbrandt AM, Bottinger E. Digital therapeutic care apps with decision-support interventions for people with low back pain in germany: cost-effectiveness analysis. JMIR Mhealth Uhealth. (2022) 10:e35042. doi: 10.2196/35042
79. Velez FF, Colman S, Kauffman L, Ruetsch C, Anastassopoulos K. Real-world reduction in healthcare resource utilization following treatment of opioid use disorder with reSET-O, a novel prescription digital therapeutic. Expert Rev Pharmacoecon Outcomes Res. (2021) 21:69–76. doi: 10.1080/14737167.2021.1840357
80. Maricich YA, Xiong X, Gerwien R, Kuo A, Velez F, Imbert B, et al. Real-world evidence for a prescription digital therapeutic to treat opioid use disorder. Curr Med Res Opin. (2021) 37:175–83. doi: 10.1080/03007995.2020.1846023
81. Maricich YA, Gerwien R, Kuo A, Malone DC, Velez FF. Real-world use and clinical outcomes after 24 weeks of treatment with a prescription digital therapeutic for opioid use disorder. Hosp Pract. (1995) 2021:1–8. doi: 10.1080/21548331.2021.1974243
82. Velez FF, Huang D, Mody L, Malone DC. Five-year budget impact of a prescription digital therapeutic for patients with opioid use disorder. Expert Rev Pharmacoecon Outcomes Res. (2022) 1–9. doi: 10.1080/14737167.2022.2016396
83. Velez FF, Malone DC. Cost-Effectiveness analysis of a prescription digital therapeutic for the treatment of opioid use disorder. J Mark Access Health Policy. (2021) 9:1966187. doi: 10.1080/20016689.2021.1966187
84. Velez FF, Luderer HF, Gerwien R, Parcher B, Mezzio D, Malone DC. Evaluation of the cost-utility of a prescription digital therapeutic for the treatment of opioid use disorder. Postgrad Med. (2021) 133:421–7. doi: 10.1080/00325481.2021.1884471
85. Bulaj G. Combining non-pharmacological treatments with pharmacotherapies for neurological disorders: a unique interface of the brain, drug-device, and intellectual property. Front Neurol. (2014) 5:126. doi: 10.3389/fneur.2014.00126
86. Metcalf CS, Huntsman M, Garcia G, Kochanski AK, Chikinda M, Watanabe E, et al. Music-enhanced analgesia and antiseizure activities in animal models of pain and epilepsy: toward preclinical studies supporting development of digital therapeutics and their combinations with pharmaceutical drugs. Front Neurol. (2019) 10:277. doi: 10.3389/fneur.2019.00277
87. Raijada D, Wac K, Greisen E, Rantanen J, Genina N. Integration of personalized drug delivery systems into digital health. Adv Drug Deliv Rev. (2021) 176:113857. doi: 10.1016/j.addr.2021.113857
88. Bulaj G, Clark J, Ebrahimi M, Bald E. From precision metapharmacology to patient empowerment: delivery of self-care practices for epilepsy, pain, depression and cancer using digital health technologies. Front Pharmacol. (2021) 12:612602. doi: 10.3389/fphar.2021.612602
89. Afra P, Bruggers CS, Sweney M, Fagatele L, Alavi F, Greenwald M, et al. Mobile software as a medical device (SaMD) for the treatment of epilepsy: development of digital therapeutics comprising behavioral and music-based interventions for neurological disorders. Front Hum Neurosci. (2018) 12:171. doi: 10.3389/fnhum.2018.00171
90. Darnall BD, Roy A, Chen AL, Ziadni MS, Keane RT, You DS, et al. Comparison of a single-session pain management skills intervention with a single-session health education intervention and 8 sessions of cognitive behavioral therapy in adults with chronic low back pain: a randomized clinical trial. JAMA Netw Open. (2021) 4:e2113401. doi: 10.1001/jamanetworkopen.2021.13401
91. Ziadni MS, Gonzalez-Castro L, Anderson S, Krishnamurthy P, Darnall BD. Efficacy of a single-session “empowered relief” zoom-delivered group intervention for chronic pain: randomized controlled trial conducted during the COVID-19 pandemic. J Med Internet Res. (2021) 23:e29672. doi: 10.2196/29672
92. Huntsman DD, Bulaj G. Healthy dwelling: design of biophilic interior environments fostering self-care practices for people living with migraines, chronic pain, and depression. Int J Environ Res Public Health. (2022) 19:2248. doi: 10.3390/ijerph19042248
93. Volkow ND, Blanco C. The changing opioid crisis: development, challenges and opportunities. Mol Psychiatry. (2021) 26:218–33. doi: 10.1038/s41380-020-0661-4
94. Bekelis K, Calnan D, Simmons N, MacKenzie TA, Kakoulides G. Effect of an immersive preoperative virtual reality experience on patient reported outcomes: a randomized controlled trial. Ann Surg. (2017) 265:1068–73. doi: 10.1097/SLA.0000000000002094
95. Chen G, Zhao Y, Xie F, Shi W, Yang Y, Yang A, et al. Educating outpatients for bowel preparation before colonoscopy using conventional methods vs virtual reality videos plus conventional methods: a randomized clinical trial. JAMA Netw Open. (2021) 4:e2135576. doi: 10.1001/jamanetworkopen.2021.35576
96. Lagisetty P, Smith A, Antoku D, Winter S, Smith M, Jannausch M, et al. A physician-pharmacist collaborative care model to prevent opioid misuse. Am J Health Syst Pharm. (2020) 77:771–80. doi: 10.1093/ajhp/zxaa060
97. Togun AT, Karaca-Mandic P, Wurtz R, Jeffery MM, Beebe T. Association of 3 CDC opioid prescription guidelines for chronic pain and 2 payer pharmacy coverage changes on opioid initiation practices. J Manag Care Spec Pharm. (2021) 27:1352–64. doi: 10.18553/jmcp.2021.27.10.1352
98. Allen HG, Stanton TR, Di Pietro F, Moseley GL. Social media release increases dissemination of original articles in the clinical pain sciences. PLoS ONE. (2013) 8:e68914. doi: 10.1371/journal.pone.0068914
99. Suman A, Armijo-Olivo S, Deshpande S, Marietta-Vasquez J, Dennett L, Miciak M, et al. A systematic review of the effectiveness of mass media campaigns for the management of low back pain. Disabil Rehabil. (2021) 43:3523–51. doi: 10.1080/09638288.2020.1743777
100. Beilfuss S, Linde S. Pharmaceutical opioid marketing and physician prescribing behavior. Health Econ. (2021) 30:3159–85. doi: 10.1002/hec.4424
Keywords: pharmacotherapy, analgesics, mHealth, smartphone apps, therapeutic video games, serious video games, opioid epidemic, health care
Citation: Giravi HY, Biskupiak Z, Tyler LS and Bulaj G (2022) Adjunct Digital Interventions Improve Opioid-Based Pain Management: Impact of Virtual Reality and Mobile Applications on Patient-Centered Pharmacy Care. Front. Digit. Health 4:884047. doi: 10.3389/fdgth.2022.884047
Received: 25 February 2022; Accepted: 16 May 2022;
Published: 13 June 2022.
Edited by:
Tim Campellone, University of California, Berkeley, United StatesReviewed by:
Shabbir Syed Abdul, Taipei Medical University, TaiwanCopyright © 2022 Giravi, Biskupiak, Tyler and Bulaj. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hayam Y. Giravi, aGF5YW0uZ2lyYXZpQHBoYXJtLnV0YWguZWR1; Grzegorz Bulaj, YnVsYWpAcGhhcm0udXRhaC5lZHU=
 Hayam Y. Giravi1*
Hayam Y. Giravi1* Grzegorz Bulaj
Grzegorz Bulaj