- 1Department of Prosthodontics, Faculty of Medicine Carl Gustav Carus, TU Dresden, Dresden, Germany
- 2Department of Pharmacognosy and Natural Products Chemistry, Faculty of Pharmacy, National and Kapodistrian University of Athens, Athens, Greece
- 3Department of Operative Dentistry and Periodontology, Medical Center, Faculty of Medicine, University of Freiburg, Freiburg, Germany
- 4Institute of Medical Microbiology and Hygiene, Faculty of Medicine, University of Freiburg, Freiburg, Germany
- 5Institute for Medical Biometry and Statistics, Faculty of Medicine and Medical Center, University of Freiburg, Freiburg, Germany
- 6Clinic of Conservative and Preventive Dentistry, Center of Dental Medicine University of Zurich, Zurich, Switzerland
Introduction: A range of disinfectant mouthwashes are available for oral hygiene. The gold standard is Chlorhexidine digluconate (CHX), which, like other available products, cannot be used without side effects in the long term. However, in recent years, therapy with herbal products, often considered antiquated, has regained considerable interest. Therefore, the search for plant compounds as an alternative to existing oral disinfectants is meaningful.
Methods: In this study, eleven Mediterranean plant extracts were tested for their antimicrobial effect in vitro. Methanol extracts of the following plants were produced by the pharmaceutical faculty of the University of Athens: Mentha aquatica, Mentha longifolia, Sideritis euboea, Sideritis syriaca, Stachys spinosa, Satureja parnassica, Satureja thymbra, Lavandula stoechas, Achillea taygetea, Phlomis cretica, and Vaccinium myrtillus. The extracts were dissolved for microdilution experiments at concentrations ranging from 10 to 0.019 mg/ml. The oral pathogens tested were Streptococcus mutans, Streptococcus oralis, Streptococcus sobrinus, Prevotella intermedia, Fusobacterium nucleatum, Parvimonas micra, Porphyromonas gingivalis, and Candida albicans. Enterococcus faecalis, Staphylococcus aureus, and Escherichia coli were used as references.
Results: All extracts, except the methanol extract of V. myrtillus, showed an antibacterial effect at concentrations ranging from 10 to 0.15 mg/ml. None of the extracts exhibited a significant antifungal effect. In general, the anaerobic pathogens could be inhibited and killed at lower concentrations compared to the aerobic pathogens. S. oralis also showed good susceptibility to the extracts. Additionally, the extracts' ability to inhibit biofilm formation by S. mutans was tested. L. stoechas at a concentration of 0.3 mg/ml showed a moderate inhibitory effect. The extracts of L. stoechas, S. thymbra, S. parnassica, and the methanol extract of V. myrtillus were effective at concentrations up to 1.25 mg/ml. P. cretica was able to inhibit and kill S. mutans at a concentration of 0.6 mg/ml, but its effectiveness in biofilm inhibition significantly decreased at 2.5 mg/ml.
Discussion: The study's hypothesis that all extracts would exhibit an antimicrobial effect was thus confirmed.
Introduction
A quarter of all medical prescriptions consist of drugs based on substances derived from plants or synthetic analogues derived from plants (1). Particularly in developing countries, plant-based drugs form the foundation of healthcare. Traditional medicine encompasses over 20,000 plant species, all of which hold potential as sources for new medicines (2). The diversity of structural compounds in plants is immense, offering numerous chemical structures that could potentially be effective (3). Effective natural plant products may include secondary plant compounds, organic compounds, phytochemical components, or bioactive compounds. Prominent plant secondary compounds comprise alkaloids, terpenes, flavonoids, and phenols (4). Maintaining proper oral hygiene is crucial for preventing oral diseases and involves regular removal and prevention of biofilm formation. Alongside mechanical removal using toothbrushes and oral hygiene aids, mouthwashes are utilized to support daily oral hygiene practices (5).
The Mediterranean region is home to a rich diversity of flora, many of which have long been used in traditional medicine for their therapeutic properties. These plants are not only vital to the region's ecosystem but also play a crucial role in local economies, as they are sources of food, medicinal products, and essential oils.
Chlorhexidine digluconate (CHX) serves as the gold standard for disinfecting mouthwashes. A 0.2% CHX solution is commonly employed to prevent biofilm formation in the oral cavity. CHX, being cationic, interacts with the negatively charged bacterial surface, disrupting the cell membrane and leading to bacterial death (6). However, prolonged use of CHX can result in undesired side effects such as extrinsic discoloration of teeth, changes in mucosal membranes, temporary taste disturbances, and increased calculus formation (5). CHX, due to its non-selective toxicity towards bacterial cells, can also cause harm to bone or mucosal cells (7, 8). Therefore, there is a need to explore alternatives to CHX.
Natural antimicrobials, especially those derived from plant extracts, play a crucial role in reducing plaque accumulation by targeting the bacteria involved in biofilm formation. These natural extracts possess antimicrobial and anti-inflammatory properties, showing great promise in managing peri-implantitis by addressing plaque buildup and biofilm development, two key factors in the onset and progression of the disease. Compounds found in these extracts, such as flavonoids, polyphenols, and alkaloids, exhibit strong antimicrobial properties that can prevent bacterial adhesion and disrupt biofilm formation on teeth and gums (9, 10). For example, green tea polyphenols, particularly epigallocatechin gallate (EGCG), have been found to effectively inhibit bacterial growth and reduce plaque formation (11). Similarly, extracts from neem and licorice demonstrate antimicrobial effects that help limit plaque accumulation by interfering with bacterial cell adhesion and their metabolic processes (12, 13). By minimizing plaque buildup, these natural antimicrobials contribute to improved oral health and decrease the risk of developing periodontal disease. Incorporating these plant-based solutions into oral hygiene practices may provide a complementary approach to conventional dental care.
The effects of the extracts on Gram-positive and facultative anaerobic microorganisms, such as Streptococcus mutans, Streptococcus sobrinus, and Streptococcus oralis, were investigated. Additionally, the impact on Gram-negative and anaerobic pathogens including Porphyromonas gingivalis, Prevotella intermedia, and Fusobacterium nucleatum, as well as the Gram-positive anaerobic bacterium Parvimonas micra and the fungus Candida albicans, were assessed. Reference microorganisms consisted of the Gram-positive facultative anaerobic pathogens Staphylococcus aureus and Enterococcus faecalis, along with the Gram-negative facultative anaerobe Escherichia coli. In previous studies, medical devices in contact with S. mutans increased the risk of infection. A variety of treatment options have been applied to treat such infections (14–16). The use of herbal drugs may impact this interaction by reducing microbial load and promoting alternative therapies (17). The antibacterial properties of Mentha spp., Sideritis spp., Lavandula spp., Satureja spp., Phlomis spp., and Stachys spp. against diverse Gram-positive and Gram-negative microorganisms have been highlighted in various studies (18–22). However, their impact on a variety of oral microorganisms has yet to be elucidated.
The 11 Mediterranean plants were selected based on their traditional medicinal uses, availability, and previous evidence of antimicrobial properties. These plants are known to contain bioactive compounds, such as essential oils and phenolic compounds, which have demonstrated potential antimicrobial and biofilm-inhibitory effects. A wide concentration range (from 10 to 0.15 mg/ml) was tested to determine both antimicrobial efficacy (Minimum Inhibitory Concentrations, or MICs) and biofilm-inhibitory effects. This approach aligns with previous studies and ensures that both high and low activity thresholds are included for reliable in vitro testing.
The growing global dependence on plant-based compounds for medical applications highlights the importance of exploring their potential in managing oral health. With a quarter of all medical prescriptions based on plant-derived substances and over 20,000 plant species employed in traditional medicine, the structural diversity of bioactive compounds in plants presents significant opportunities for discovering new therapeutic agents (23–25). This is particularly relevant for tackling issues like plaque accumulation and biofilm formation, which are central to oral diseases such as peri-implantitis and periodontitis. Given the limitations of commonly used antimicrobial agents like chlorhexidine digluconate (CHX), which can cause side effects such as tooth discoloration, changes in mucosal tissues, and potential cytotoxicity, there is an increasing demand for natural alternatives that are both safer and equally effective (26, 27). Plant extracts, rich in secondary metabolites such as flavonoids, terpenes, and phenolic compounds, have shown antimicrobial and biofilm-inhibitory effects against a variety of microorganisms (28). These natural compounds can disrupt bacterial adhesion, inhibit biofilm formation, and reduce microbial load, making them promising candidates for oral hygiene applications (29).
This study aims to evaluate the antimicrobial and biofilm-inhibitory potential of selected Mediterranean plant extracts, focusing on their activity against a range of Gram-positive and Gram-negative oral microorganisms, including Streptococcus mutans, Porphyromonas gingivalis, and Candida albicans. By investigating the efficacy of these extracts at various concentrations, the study seeks to identify plant-based alternatives to conventional antimicrobials that could be integrated into oral care strategies, ultimately contributing to improved management of conditions related to oral biofilm. The objective of this study was to assess the antimicrobial potential of the provided plant extracts in order to identify extracts suitable for further in vivo investigations. The plants examined in this study are indigenous to the Mediterranean region. They include Mentha longifolia (mint), Mentha aquatica (mint), Lavandula stoechas (lavender), Sideritis syriaca (ironwort, mountain tea), Sideritis Euboea (ironwort, mountain tea), Satureja parnassica (savory), Satureja thymbra (savory), Phlomis cretica (Cretan Jerusalem Sage), and Stachys spinosa (hedgenettle), belonging to the Lamiaceae family. Achillea taygetea (yarrow) belongs to the Asteraceae family, and Vaccinium myrtillus (bilberries, blueberries) belongs to the Ericaceae family. This study was conducted with the hypothesis that all extracts, particularly at higher concentrations, would exhibit antimicrobial effects.
Materials and methods
Plant extracts
Plant materials from eleven distinct plant species were gathered from different locations within the Greek periphery. The selected plant species included: Mentha longifolia L., Lavandula stoechas L., Sideritis syriaca L., Mentha aquatica L., Satureja thymbra L., Satureja parnassica Heldr. & Sart. ex Boiss., Phlomis cretica C. Presl, Sideritis euboea Heldr., Stachys spinosa L., Achillea taygetea Boiss. & Heldr., and Vaccinium myrtillus L. In the case of Vaccinium myrtillus, the focus was on collecting the fruits, while for the remaining plants, the aerial components were collected.
Extraction process
The collected plant specimens underwent thorough grinding (Allenwest-Eac ltd, Brighton and Hove, United Kingdom) to achieve finely homogeneous powders, which were then subjected to ultrasound-assisted extraction (UAE) (30, 31). This process utilized an Elma S 100H (Elmasonic, Elma Schmidbauer GmbH, Singen, Germany) instrument, with a solvent mixture of MeOH/Water 80:20, for an extraction duration of 15 min at room temperature. The ratio of plant material to solvent was maintained at 1/10 (w/v). To ensure comprehensive extraction, the procedure was repeated twice for each sample. Following extraction, the solvents were meticulously evaporated under reduced pressure utilizing a Buchi Rotavapor R-200 rotary evaporator, maintaining a temperature of 40°C, until dryness was achieved.
High performance thin layer chromatography (HPTLC) analysis
To generate the fingerprinting profiles of the diverse extracts, a Camag HPTLC instrument setup was employed (32, 33). Solutions of the extracts were formulated by dissolving 10 mg of each extract in 1 ml of hydroalcoholic. For the application of plant extract samples onto Thin layer chromatography (TLC) plates measuring 20 cm × 10 cm (silica gel 60, F254, Merck), the Automatic TLC Sampler (ATS4, CAMAG, Muttenz, Switzerland) was utilized, controlled by the VisionCats 2.3 software platform (CAMAG, Muttenz, Switzerland). The TLC Sampler was configured according to standard parameters: 6 tracks with 8 mm bands, an 8 mm distance from the lower edge, 20 mm from both left and right edges, and a spacing of 10.4 mm between individual tracks. The applied volume for each sample was 8 μl. The ensuing development of plates was performed within an automatic development chamber (ADC2), adhering to established guidelines: 20 min of chamber saturation with filter paper, 10 min of plate conditioning at 33% relative humidity (using MgCl2), and a subsequent 5-min plate drying period. The mobile phase employed was dichloromethane/methanol/water (70:30:4; v/v/v) for polar extracts, while ethyl acetate, methanol/water/formic acid (50:10:7:1; v/v/v/v) containing highly polar substances was chosen for other extracts. Imaging at both 254 and 366 nm was captured using a Visualizer 2 Documentation System (CAMAG, Muttenz, Switzerland).
Bacterial and fungal strains
Seven bacterial strains of the oral microflora and the yeast Candida albicans DSM 1386 (German Collection of Microorganisms and Cell Cultures) were tested. Reference microorganisms were Enterococcus faecalis ATCC 29212 and Escherichia coli ATCC 25923 found in the intestinal tract and Staphylococcus aureus ATCC 25923, a colonizer of the skin surface. The reference organisms were used to compare the oral inhibitory effect with the general antimicrobial activity. Streptococcus sobrinus DSM 20381, Streptococcus mutans DSM 20523, Streptococcus oralis ATCC 35037, Enterococcus faecalis ATCC 29212 and Staphylococcus aureus ATCC 25923 are facultative anaerobic Gram-positive bacteria. Escherichia coli ATCC 25922 is also facultative anaerobic but Gram-negative. The tested bacteria Porphyromonas gingivalis W 381, Prevotella intermedia ATCC 25611, Fusobacterium nucleatum ATCC 25586 and Parvimonas micra ATCC 23195 are obligate anaerobe pathogens. The bacterial strain E. faecalis T9 was isolated in the Department of Dental Conservation and Periodontology of the University Hospital Freiburg. After thawing of the pathogens, subcultures were created. The facultative anaerobic pathogens were cultivated on Columbia-blood agar plates (CBA) plates. E. coli, S. aureus, E. faecalis and C. albicans for 18 h at 37°C and humid heat, the streptococci for 24 h at 37°C humid heat and 5%–10% CO2. The subcultures of the obligate anaerobic bacteria were cultured on yeast-cystein blood Agar (HCB) plates under exclusion of oxygen for 48 h at 37°C anaerobic incubation chambers (Anaerocult® IS, Merck Chemicals GmbH, Darmstadt, Germany). Colonies of facultatively anaerobic bacteria and C. albicans were mixed in 0.9% NaCl up to a McFarland turbidity of 0.5 and 1 for C. albicans. The McFarland turbidity was tested by DensiCheck (BioMèrieux SA, Marcy-l'Étoile Frankreich). The obligate anaerobic bacteria were adjusted in Wilkens-Chalgren Anaerobe Broth (WCB, IMMH Freiburg) in a McFarland turbidity of 0,5. A comparable number of colony forming units (CFU) should be available per well of the microtiter plate. 5 × 105 CFU of the facultative anaerobic bacteria, 5 × 106 CFU of the obligate anaerobic bacteria and 5 × 104 CFU of the fungus.
Determination and evaluation of the minimum inhibitory concentration (MIC)
In shafts 2–12 of the microtiter plates, the corresponding nutrient medium was first presented. BBL™ Mueller Hinton II Broth (MHB; Becton Dickinson GmbH, Heidelberg, Deutschland) as culture medium of the facultatively anaerobic germs and C. albicans, sterile WCB for the obligate anaerobic germs. The plant extracts were dissolved in Dimethylsulfoxide (DMSO) and 1:10 in the respective Culture medium diluted. Thus, the initial concentration for all extracts was 10 mg/ml. The experiments were performed in duplicate. The 96 well micotiter plate was divided up as follows. In rows A-H; column 2–12, 100 µl culture medium were added according to the germ to be tested. In column 1 200 µl of the respective diluted extract were pipetted at the initial concentration of 10 mg/ml. Using a multichannel pipette, 100 µl of the first column were removed and mixed with the bouillon in the second column. 100 µl were taken from this column, halving the extract concentration. This procedure was used up to a dilution of 0.0019 mg/ml. In order to exclude antimicrobial effects of DMSO, DMSO dilution series were tested in a double test. The initial concentration was 20% DMSO and diluted to 0.0004% using the same procedure. As a positive control, 0.1% CHX was tested on each plate in duplicate and diluted down to 0.0002%. Column 11 of each plate contained WCB or MHB and was inoculated as growth control. Column 12 contained only WCB and MHB as blank values for optical comparison. The inoculation of each well up to the column blank value was carried out with 5 µl germ suspension. Only one germ was inoculated per plate (31, 34). Subsequently, the plates with the facultative anaerobic germs and C. albicans were incubated at 37°C and 5%–10% CO2 atmospheric pressure for 24 h. The anaerobically inoculated plates were also incubated at 37°C for 48 h under anaerobic conditions. Inocula were prepared from one of the growth controls to check the achievement of the desired CFU. In parallel to the inoculum control, smears from the last dilution series were fractionated and incubated in the CO2 oven. If aerobic growth became visible the next day, the experiment was discarded.
The turbidity in the wells was assessed under a magnifying lamp. The MIC was defined as the lowest concentration of each active substance at which a visible inhibition of bacterial growth was induced. This means the MIC was visually determined at the concentration at which no turbidity was visible or no growth was visible according to the growth control comparison. If the MIC values in the duplicate extract were different, the higher concentration was evaluated as MIC. If there was more than one concentration level difference, the experiment was repeated. The possibly inhibitory effect of the solvent DMSO was also considered. An extract concentration of 10 mg/ml contains 10% DMSO. Thus, an extract effect could only be considered if the MIC for DMSO was greater than 10% in the same experimental approach. The growth was divided into three strengths, which were recorded in the laboratory protocol with +, ++, +++. The decisive factor is the last well without visible growth. This determines the MIC. The reported values represent the mean values, and the experiments were conducted in duplicate.
Determination and evaluation of the minimum bactericidal concentration (MBC)
To determine the minimum bactericidal concentration, 10 µl were spread out from each well on a quarter of the culture medium to the dilution at which bacterial growth was clearly visible. In the case of facultatively anaerobic bacteria and C. albicans, incubation was performed on CBA plates at 37°C and 5%–10% CO2 atmospheric pressure for 24–48 h. The anaerobes were incubated under anaerobic conditions on HCB culture media at 37°C for 4–5 days.
The MBC was defined as a drop in growth of 99.9%. As a guideline, ten colony-forming units per 10 µl smear were allowed. The CFU was determined visually (31). The reported values represent the mean values, and the experiments were conducted in duplicate.
Determination and evaluation of biofilm formation
The experiments to test the inhibition of biofilm formation were developed after the submission of a paper described in 2014 (35). To test the inhibition of biofilm formation, the clinical isolate R 15-8 of S. mutans was used as a biofilm forming, facultative anaerobic bacterium. S. mutans R15-8 was isolated from an infected root canal of a tooth that had undergone dental treatment in the Department of Operative Dentistry and Periodontology, University of Freiburg, Germany. The biofilm-forming bacterium E. faecalis was used as a control germ. Subcultures were created and incubated at 37°C and 5%–10% CO2 atmospheric pressure for 24 h. The next day, the isolates were cultivated in tryptic soy broth (TSB) overnight with the addition of sucrose (Merck KGaA, Darmstadt, Deutschland). The TSB culture medium used includes 2.5 g/L glucose, which facilitates the formation of glucan as a biofilm matrix by S. mutans. The CFU of each overnight culture were determined on CBA. The live bacterial count was in a range of 108 CFU/ml. Each well of a 96 well tissue-culture plates (Greiner bio-one, Frickenhausen, Germany) was filled with 180 µl fresh tryptic soy broth (TSB). One extract per plate was tested in a quadruple experiment, again DMSO and CHX dilutions were used as controls. Dilutions from 10 to 0.019 mg/ml were tested. Analogous to the MIC determination CHX from 0.1% to 0.0002% and DMSO from 20% to 0.004%. Afterwards, 20 µl of the overnight culture were pipetted before incubation of the microtiter plates for 24 h at 37°C and 5%–10% CO2 atmospheric pressure. After incubation, the liquid was discarded. The plates were washed three times with 200 µl phosphate buffered salt solution (PBS; Life Technologies Inc., Carlsbad, CA, USA) and air dried for 10 min. With 0.1% crystal violet (Carl Roth GmbH + Co KG, Karlsruhe, Deutschland) staining for 10 min was performed. After washing three times with 200 µl distilled water and drying the plates for 10 min at 60°C, the dye was dissolved with 50 µl 99.9% alcohol each. The optical density was determined at 595 nm with a microtiter plate photometer (Tecan Group AG, Männedorf, Switzerland).
Three categories were formed on the basis of thresholds. The first threshold value was formed by adding three times the value of the standard deviation of the negative control to the actual measured value of the negative control. The second threshold was defined as three times the value of the first threshold. Values below the first threshold value were used to inhibit biofilm formation. Values higher than the first threshold value, but lower as the second, were considered moderate biofilm formation. Values above the second threshold were considered as strong biofilm formation. This approach was utilized to eliminate false positive results for biofilm formation.
Statistical analysis
For analysis of the biofilm plate assay, T-tests were applied between the logarithmic adsorption values (basis 10) of the extracts and the two control groups, respectively, with a Bonferroni-correction due to multiple testing. For graphical presentation of the results scatter plots were used. All computations were done with STATA (Version 17.0, College Station, TX, USA).
Results
All extracts, except the hydroalcoholic extract of V. myrtillus, showed an antibacterial effect at concentrations ranging from 10 to 0.15 mg/ml. The most significant and consistent results were generally obtained with anaerobic bacteria. However, inhibition of the yeast was challenging or minimal. Overall, The extracts of L. stoechas, S. thymbra, S. parnassica, and the hydroalcoholic extract of V. myrtillus yielded an antibiofilm effect at concentrations up to 1.25 mg/ml.
HPTLC analysis
To obtain the chemical profile of the plant extracts, a rapid and accurate analytical method was developed using HPTLC (High-Performance Thin Layer Chromatography). Visualization of the plates at 254 and 366 nm revealed that the extracts possessed a diverse chemical content, and major active compound categories were detected. The analysis primarily indicated the presence of phenolic compounds. Rosmarinic acid was identified as the main compound in Mentha, Lavandula, Satureja, Phlomis, and Stachys. Phenylethanol glycosides, such as acteoside, and flavonoid glucosides of hypolaetin, methylhypolaetin, isoscutellarein, and methylisoscutellarein, were found to be the main compounds in Sideritis species. A. taygetea extracts were rich in flavonoids, specifically derivatives of apigenin and luteolin. V. myrtillus extracts were abundant in anthocyanins, particularly cyanidin-3-glucoside and delphinidin-3-glucoside.
Sideritis euboea and Satureja thymbra
Sideritis euboea has demonstrated effectiveness against all anaerobic bacteria, as well as against S. aureus and S. oralis, exhibiting inhibitory and bactericidal effects at dilutions of 1.25 mg/ml, as indicated in Table 1. The most notable results were observed against P. gingivalis and P. micra, with minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values of 0.3 mg/ml each.
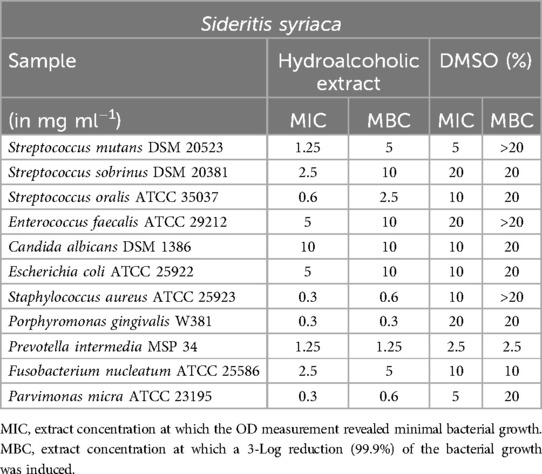
Sideritis syriaca (Table 2) demonstrated effective inhibition against S. mutans, with a minimum inhibitory concentration (MIC) of 1.25 mg/ml. Additionally, S. aureus, P. gingivalis, and P. micra displayed MIC values of 0.6 mg/ml or below, and except for S. oralis, minimum bactericidal concentration (MBC) values within the same range. However, for P. intermedia, clear results could not be obtained as the MIC/MBC values of the extract and DMSO were too close to each other. The same was observed for E. coli and C. albicans.
Mentha longifolia and Mentha aquatica
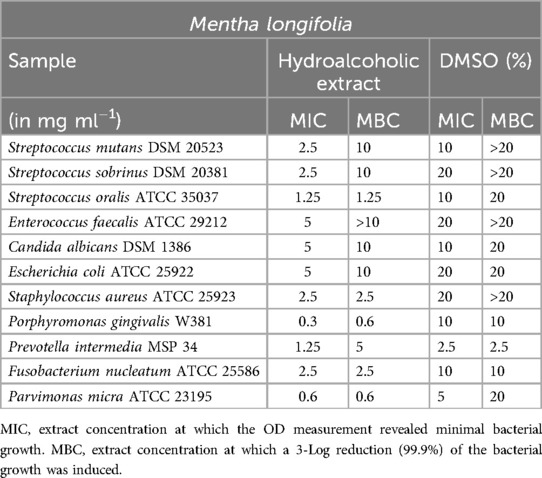
For Mentha longifolia (Table 3), inhibitory effects were observed at a concentration of 2.5 mg/ml against S. mutans, S. sobrinus, and S. aureus. Notably, P. gingivalis showed a minimum inhibitory concentration (MIC) of 0.3 mg/ml and a minimum bactericidal concentration (MBC) of 0.6 mg/ml, while P. micra exhibited an MIC of 0.6 mg/ml and an MBC of 0.6 mg/ml, indicating susceptibility to lower concentrations as is typical for anaerobic bacteria. However, for P. intermedia, the MIC and MBC values were indistinguishable from those of the DMSO control.
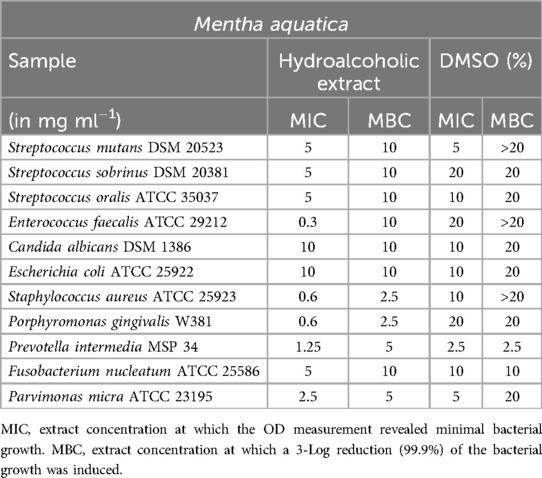
Mentha aquatica (Table 4) exhibited notable results in relation to E. faecalis. The extract demonstrated inhibition of the bacteria at a concentration of 0.3 mg/ml, although the minimum bactericidal concentration (MBC) was found to be at a concentration of 10 mg/ml. However, no significant results were obtained against the tested Streptococcus species. The values for P. intermedia, F. nucleatum, and P. micra were also similar to those of the DMSO controls, making them inconclusive. As for C. albicans and E. coli, the observed effects can be attributed to the DMSO rather than the extract, suggesting no direct impact of the extract on these microorganisms.
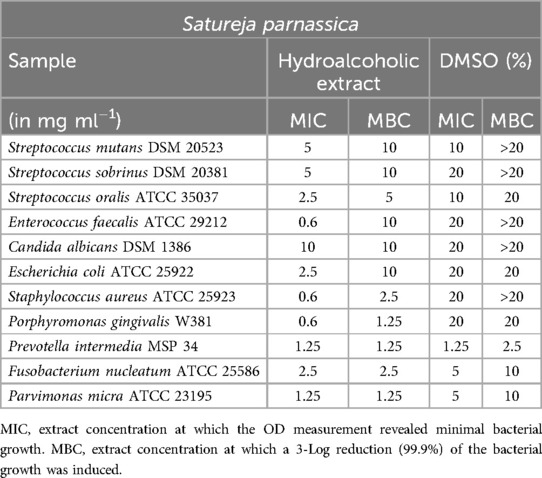
Satureja thymbra and Satureja parnassica
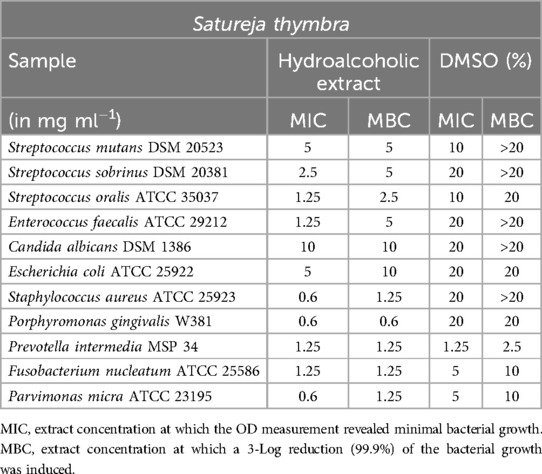
Satureja thymbra (Table 5) yielded favorable results in inhibiting anaerobic bacteria and S. oralis. However, it is worth noting that the possibility of a DMSO effect cannot be completely ruled out when testing against F. nucleatum. E. faecalis also demonstrated sensitivity to the extract, with a minimum inhibitory concentration (MIC) of 1.25 mg/ml.
Satureja parnassica showed a limited impact on S. mutans, as indicated in Table 6. However, for S. oralis, a minimum inhibitory concentration (MIC) of 2.5 mg/ml and a minimum bactericidal concentration (MBC) of 5 mg/ml were determined. Similar to Mentha aquatica, S. parnassica exhibited a low MIC (0.6 mg/ml) against E. faecalis, although the MBC was significantly higher at 10 mg/ml. S. aureus, P. gingivalis, and P. micra displayed sensitivity to the extract's effects.
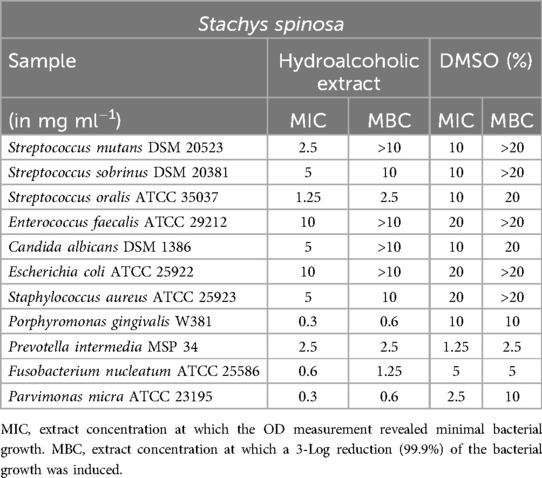
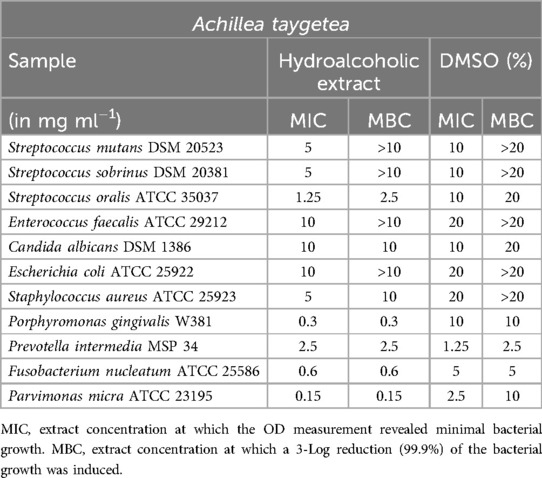
Stachys spinosa and Achillea taygetea
Table 7 provides a summary of the results for the Stachys spinosa extract. Similar to the hydroalcoholic extract of Satureja thymbra, excellent values were observed between 0.3 and 1.25 mg/ml for P. gingivalis, F. nucleatum, and P. micra. However, when testing against P. intermedia, the possibility of a DMSO effect could not be excluded. A significant effect against S. oralis was evident. However, weak minimum inhibitory concentration (MIC) values were obtained for the other tested microorganisms, and these values could not be confirmed by the minimum bactericidal concentration (MBC).
The extract of Achillea taygetea exhibited similar effects, as shown in Table 8. Good minimum inhibitory concentration (MIC) values ranging from 1.25 mg/ml and minimum bactericidal concentration (MBC) values below 2.5 mg/ml were achieved against S. oralis, P. gingivalis, and F. nucleatum. For P. micra, both MIC and MBC were even observed at a concentration of 0.15 mg/ml, indicating strong inhibitory and bactericidal effects. However, no clear extract effect could be demonstrated against S. mutans, S. sobrinus, E. faecalis, E. coli, and P. intermedia. Furthermore, the extract did not exhibit significant inhibition against C. albicans.
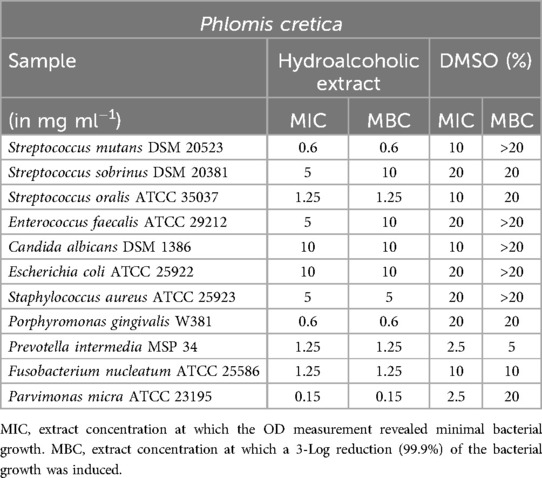
Phlomis cretica
Phlomis cretica (Table 9) demonstrated reliable activity against the anaerobic bacteria P. gingivalis, F. nucleatum, and P. micra, as well as the facultative anaerobic bacterium S. oralis. Notably, the extract exhibited excellent MIC and MBC values of 0.6 mg/ml for S. mutans, underscoring its effectiveness against this particular microorganism.
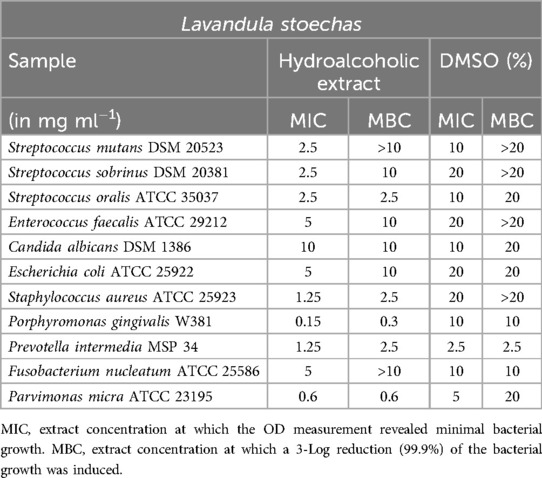
Lavandula stoechas
The testing of Lavandula stoechas (Table 10) revealed minimum inhibitory concentration (MIC) concentrations of 2.5 mg/ml for S. mutans, S. sobrinus, and S. oralis. However, the minimum bactericidal concentration (MBC) for S. mutans and S. sobrinus was relatively high at 10 mg/ml compared to the MIC value. P. gingivalis could be inhibited at a very low concentration of 0.15 mg/ml, and at twice that concentration, it was effectively killed. S. aureus (MIC = 1.25 mg/ml; MBC = 2.5 mg/ml) and P. micra (MIC and MBC = 0.6 mg/ml) demonstrated the expected sensitivity to the extract.
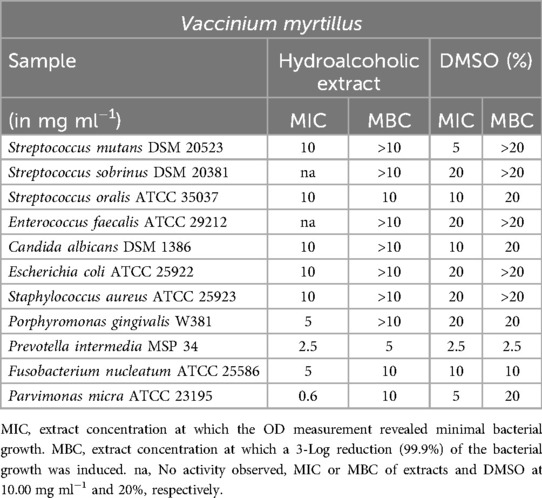
Vaccinium myrtillus
The hydroalcoholic extract of Vaccinium myrtillus (Table 11) exhibited minimal notable effects. The measured inhibitory concentration of 0.6 mg/ml for P. micra is likely not significant, as it aligns closely with the effects of DMSO, particularly considering the MBC of 10 mg/ml and the MBC of 20 mg/ml.
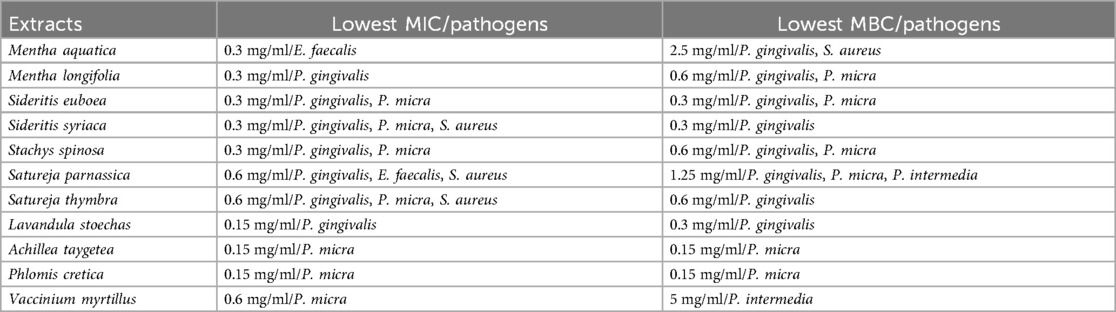
Key findings are summarized in Table 12.
Results of the biofilm plate assay
The extracted values were compared to the controls of DMSO and CHX, and the significance was evaluated (Figure 1). The significance level of less than 1% or less than 5% is depicted in the diagrams. At a concentration of 10 mg/ml, all extracts, except S. thymbra, exhibited high biofilm inhibition (p ≤ 0.024). DMSO already showed scattered but moderate inhibition at high concentrations. At a lower concentration of 5 mg/ml, A. taygetea extract demonstrated significantly increased values of biofilm formation (p = 1). P. cretica fell into the category of mild biofilm inhibitors at this concentration (p = 1). Extracts of M. longifolia (p = 0.007), L. stoechas (p = 0.001), S. thymbra (p = 0.001), S. parnassica (p = 0.001), and V. myrtillus (p = 0.001) exhibited high levels of biofilm inhibition at 2.50 mg/ml.
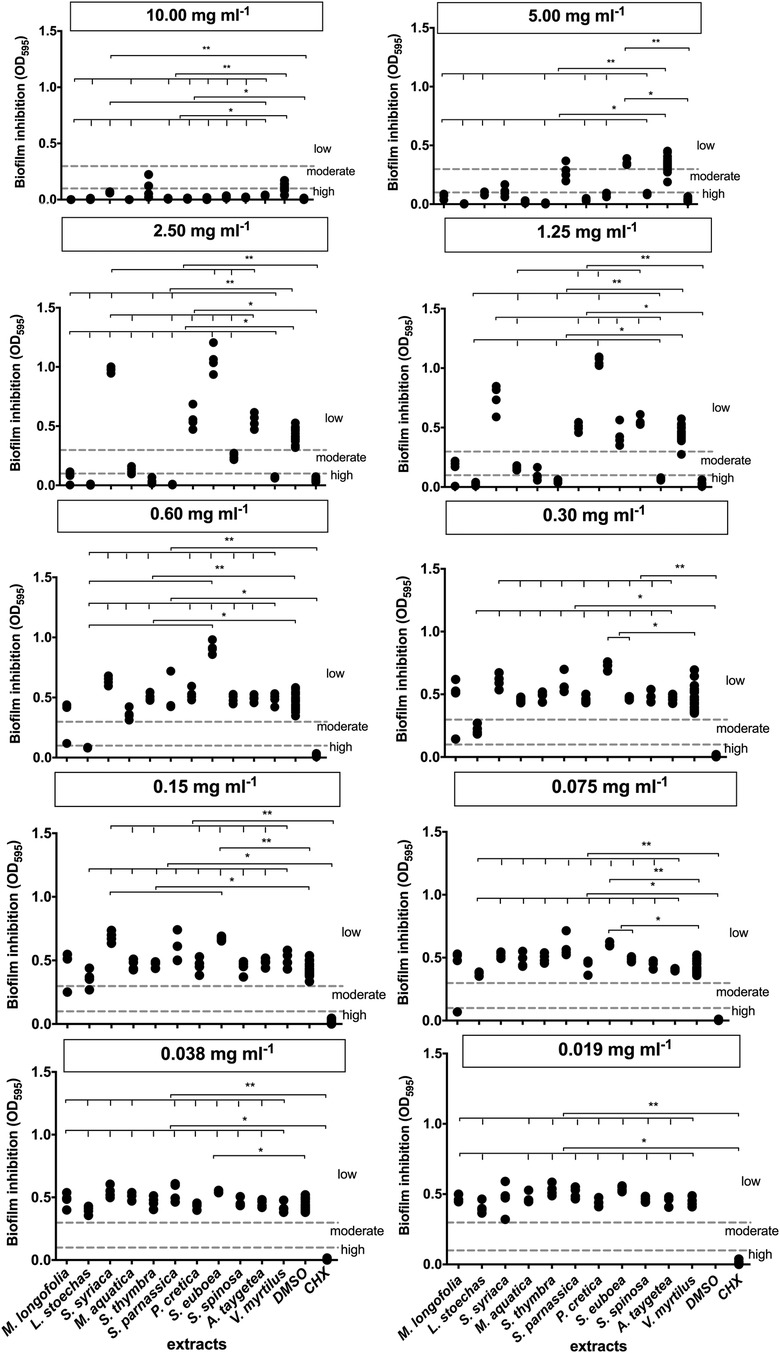
Figure 1. Results of the biofilm inhibition test. **Significance less then 1%, *Significance less then 5%.
Furthermore, extracts of L. stoechas (p = 0.001), S. parnassica (p = 0.0001), and V. myrtillus (p = 0.0001) showed activity at 1.25 mg/ml. The optical density (OD) of S. euboea (p = 0.0001) was notably above the second threshold value. Only L. stoechas (p = 0.001) achieved biofilm formation inhibition at 0.60 mg/ml. M. longifolia (p = 1) exhibited a measurement in the moderate range, which should be considered as an outlier. From a concentration of 0.015 mg/ml, L. stoechas (p = 1) reached the threshold for mild biofilm inhibition but lost effectiveness at 0.075 mg/ml. M. longifolia again exhibited an outlier without evaluation.
None of the tested extracts produced moderate biofilm inhibition at a concentration of 0.038 mg/ml. Consequently, at an even lower concentration of 0.019 mg/ml, very weak or no inhibition was observed.
Discussion
The selected plants are commonly found in the Mediterranean region. M. longifolia, L. stoechas, S. syriaca, M. aquatica, S. thymbra, S. parnassica, P. cretica, S. euboea, and S. spinosa belong to the Lamiaceae family, while A. taygetea belongs to the Asteraceae family, and V. myrtillus belongs to the Ericaceae family. Testing a greater variety of oral bacteria would yield more comprehensive information regarding the antimicrobial activity of the extracts. However, in our manuscript, we included both Gram-negative and Gram-positive bacteria because the cell envelope is crucial for the mechanisms of all antimicrobial compounds in general. Additionally, we also included the most common oral fungus, Candida albicans.
The tested oral streptococcal strains, frequently isolated from the supragingival oral biofilm, have been associated with dental caries according to the ecological plaque hypothesis (36). Conversely, the tested anaerobic oral bacteria (F. nucleatum, P. gingivalis, P. intermedia, and Parvimonas micra) have been frequently isolated from the subgingival oral biofilm, which is associated with periimplantitis and periodontitis (37–39). Candida albicans, frequently isolated from the supragingival oral biofilm, has been associated with early childhood caries (40, 41). Enterococcus faecalis has been described as one of the major infectious bacteria in endodontic infections (42).
An analysis of tea derived from S. syriaca demonstrated potent antimicrobial activity against S. aureus, indicating strong inhibitory effects. Additionally, the tea exhibited moderate inhibition of E. coli and E. faecalis, suggesting a moderate impact on these bacterial strains (43). The significant effect of S. syriaca on S. aureus was further supported by this experiment, with a minimum inhibitory concentration (MIC) of 0.3 mg/ml and a minimum bactericidal concentration (MBC) of 0.6 mg/ml. In a previous study conducted in 2001, S. syriaca was also attributed with high antimicrobial activity. This effect was mainly attributed to the presence of carvacrol, which was found in substantial amounts in the essential oil of S. syriaca (44). Among other things, carvacrol is utilized in other experiments as a rationale for its antimicrobial effect against microorganisms (45).
In a previous study, both M. aquatica and M. longifolia demonstrated a significant antimicrobial effect, which is consistent with the findings of the current work (46). Furthermore, the antimicrobial activity of a hydroalcoholic extract of M. longifolia against S. aureus and E. coli was reported at concentrations of 1, 3, and 5 mg/ml (47). These values align closely with the minimum inhibitory concentrations (MIC) determined in this study, which were 5 mg/ml for E. coli and 2.5 mg/ml for S. aureus.
The essential oils of the two Satureja species have undergone multiple testing in various studies. In a study conducted in 2006, essential oils were extracted from S. parnassica and S. thymbra, and their compositions were differentiated based on the time of harvest. Carvacrol and thymol were identified as the primary isomers in each oil produced. Carvacrol was found to be the predominant component during the flowering period, while thymol became more abundant shortly before and after flowering. Additionally, the levels of precursor compounds of these substances increased over time. The oils obtained from the flowering plants exhibited the lowest minimum inhibitory concentration (MIC) values. Furthermore, the study confirmed the inherently stronger effect of these oils on Gram-positive pathogens compared to Gram-negative ones, as mentioned in the above cited research (48).
S. spinosa exhibited a notable effect on Gram-negative anaerobic bacteria in the experiments. It also achieved moderate biofilm inhibition at a concentration of 2.5 mg/ml. There are currently no other antimicrobial investigations of these plant substances available for comparison with the obtained results. In a chemical analysis of the above-ground parts of the plant as a hydroalcoholic extract, stachyspinosides, belonging to the flavonoid group, as well as three secondary plant substances in the form of iridoids, were detected (49).
A. taygetea demonstrated an effect on the Gram-positive pathogen S. oralis (MIC = 1.25 mg/ml; MBC = 2.5 mg/ml), but not on the other streptococci or E. faecalis. Additionally, no significant effect was observed against the tested fungus. However, the Gram-negative anaerobes and P. micra exhibited greater sensitivity to the extract.
Chemical analysis of the extract of A. taygetea revealed the presence of α- and β-pinene, camphene, 1,8-cineole, camphor, and α-terpineol in significant proportions compared to other Achillea species (50). The demonstrated antimicrobial effect of A. taygetea is attributed to 1,8-cineole and camphor based on individual tests of these components (50, 51).
There is currently no available literature on the antimicrobial activity of P. cretica specifically, but other Phlomis species have been studied. For example, the essential oil of Phlomis lantana was found to contain significant amounts of α-pinene, limonene, and trans-caryophyllene. This oil was tested for its minimal inhibitory concentration against Gram-negative and Gram-positive bacteria, as well as fungi including C. albicans. It exhibited a stronger effect against Gram-negative pathogens like P. aeruginosa and E. faecalis compared to Gram-positive ones. Further testing of the three mentioned chemical components for their minimal inhibitory concentration against microorganisms revealed that the activity of the oil was likely attributed to α-pinene, while limonene was found to be completely inactive (52). In the present study, the most notable effect of P. cretica was observed on the Gram-positive bacterium P. micra. Other tested Gram-negative bacteria also showed sensitivity, except for E. coli, which required a higher concentration (MIC and MBC at 10 mg/ml). Additionally, the Gram-positive streptococci S. mutans and S. oralis were significantly affected, while the inhibition of biofilm formation by P. cretica was only observed at higher concentrations.
In this study, L. stoechas demonstrated moderate biofilm inhibition at low concentrations of 0.30 mg/ml. However, in another study by Gursoy et al., an essential oil of L. stoechas was not included in the biofilm experiments due to its high minimum inhibitory concentration (MIC) values (53). Chemical analysis conducted in the study by Gursoy et al. identified camphor, fenchone, and 1,8-cineole (also known as eucalyptol) as the main components of the essential oil (53–55). In the literature, fenchone, one of the main components, has been evaluated as weakly antimicrobial, which may explain the limited antimicrobial behavior of L. stoechas essential oil observed in Dadalioglu's study. However, in the experiments described here, L. stoechas extract exhibited an effect on the tested anaerobic bacteria as well as on S. aureus and S. oralis.
V. myrtillus (blueberry) has been extensively studied for its antioxidant and hypoglycemic effects in existing research. A qualitative analysis of blueberry stems, leaves, and fruits has shown that all components of the plant are suitable sources of phenolic compounds (56). In this study, the hydroalcoholic extract of blueberry exhibited an effect on P. micra with a minimum inhibitory concentration (MIC) of 0.6 mg/ml, but this effect was diminished by a minimum bactericidal concentration (MBC) of 10 mg/ml. Regarding the inhibition of biofilm formation, the extract demonstrated a significant inhibition at concentrations up to 1.25 mg/ml, but at lower concentrations, it was just above the threshold for low inhibitors. This observation is consistent with a review that described the inhibition of biofilm formation by blueberry against S. mutans as moderate when compared to its related fruit, cranberry (57). Recent studies have examined Vaccinium myrtillus (blueberry) for its antioxidant, hypoglycemic, and antimicrobial properties (58, 59). The hydroalcoholic extract showed an inhibitory effect on P. micra, with a minimum inhibitory concentration (MIC) of 0.6 mg/ml, but the bactericidal activity required a much higher concentration of 10 mg/ml, indicating a weak effect. The extract also inhibited biofilm formation at concentrations up to 1.25 mg/ml, though lower concentrations had marginal effects, consistent with previous findings on Streptococcus mutans. Overall, while V. myrtillus has antimicrobial activity, its effectiveness in biofilm inhibition and bactericidal action is less pronounced compared to related species like Vaccinium macrocarpon (cranberry), warranting further research into its active compounds and potential synergies (60–62).
Herbal products have been extensively studied for their antimicrobial properties due to their diverse bioactive compounds. Previous research has demonstrated the antimicrobial effects of Satureja thymbra and Satureja parnassica, with essential oils rich in carvacrol and thymol showing strong inhibitory activity against both Gram-positive and Gram-negative bacteria, particularly Staphylococcus aureus and Escherichia coli (63). Similarly, Mentha longifolia and Mentha aquatica have exhibited significant antimicrobial activity in multiple studies, with hydroalcoholic extracts displaying low minimum inhibitory concentration (MIC) values against S. aureus and E. coli (30, 64). This activity is attributed to their high concentrations of rosmarinic acid and menthol. Vaccinium myrtillus (blueberry) has also been investigated for its phenolic compounds, which effectively inhibit biofilm formation by Streptococcus mutans and related oral pathogens, exhibiting comparable activity to that of cranberry (65). Additionally, L. stoechas, which contains camphor, fenchone, and 1,8-cineole, has shown moderate antimicrobial effects, particularly against anaerobic bacteria and fungi, although previous studies reported variability in biofilm inhibition depending on the extraction methods used (66, 67). Other Mediterranean plants, such as Phlomis lantana and Sideritis syriaca, have demonstrated antimicrobial properties against both oral and systemic pathogens (68, 69). These effects are primarily attributed to their diterpenoids, flavonoids, and phenolic compounds. The antimicrobial and antibiofilm properties of these plants align with the ecological plaque hypothesis, highlighting their potential role in managing oral diseases caused by pathogenic biofilms. Despite these promising findings, more comprehensive studies are required to confirm their efficacy, particularly in vivo, and to evaluate their safety and potential for incorporation into oral care products.
This study offers valuable insights, but it has several limitations. It was conducted in vitro, which does not fully replicate the complexities of the oral environment, such as the presence of saliva and host-microbe interactions. Furthermore, only a limited range of oral microorganisms were tested, omitting many that are relevant to biofilm complexity. There may also be challenges in achieving effective concentrations of the plant extracts in clinical applications. The exact mechanisms of action, as well as any potential synergistic or antagonistic effects of the compounds within the extracts, remain unclear. Additionally, the study did not evaluate cytotoxicity on human tissues nor did it include comparisons with other oral hygiene products. Future research should address these gaps through in vivo studies, broader microbial testing, and safety evaluations. Potential biases in extraction methods could arise from solvent effects, such as the use of DMSO, which may affect microbial growth at higher concentrations. To ensure repeatability, MIC/MBC and biofilm assays were performed in duplicate with standardized protocols, including appropriate controls and statistical analysis, to minimize variability and confirm consistent results.
All tested extracts exhibited antimicrobial effects. However, none of the extracts were effective at concentrations as low as 0.15 mg/ml, and most of them showed effectiveness at concentrations ranging from 2.5 to 5 mg/ml. This can present challenges in terms of procurement, production, and dosage forms. Consequently, it seems logical to attribute the observed effects to specific ingredients or the interaction of multiple ingredients. However, comprehensive analytical methods beyond HPTLC analysis are needed to elucidate and understand the precise mechanisms of action. It is recommended that further studies investigate the hypothesis attributing the antimicrobial and antibiofilm effects to specific pure substances within the tested extracts. Additional tests on the toxicity of the plant extracts introduced in our study are also required. In the in vitro experiments conducted in our study, chlorhexidine digluconate (CHX) served as a positive control. To further evaluate the effects of mouthwashes containing CHX compared to the tested plant extracts, future studies should be conducted in vivo (70, 71).
The antimicrobial properties of Mediterranean plant extracts present promising applications in oral healthcare products like mouthwashes and toothpaste. These extracts can inhibit oral pathogens, particularly anaerobic bacteria and biofilm formation, making them potential natural alternatives to traditional disinfectants like CHX. However, there are challenges in translating these findings into clinical practice, including the need for standardization of extract concentrations and ensuring the stability of active compounds, which can degrade over time. Future research should investigate the synergistic effects of different plant extracts or their combinations with established antimicrobials to enhance efficacy and broaden their spectrum of activity, while also addressing issues of formulation stability. Additionally, vitamin C contributes to reducing biofilm formation by supporting immune function and preventing bacterial adhesion. A deficiency in vitamin C can impair immune responses, promoting the development of biofilms (72). The roughness of materials also affects biofilm formation, as rough surfaces facilitate bacterial attachment, while smooth surfaces offer greater resistance (73). Therefore, combining adequate levels of vitamin C with smooth dental materials could improve the effectiveness of plant extract-based oral products.
In conclusion, all the tested extracts demonstrated a significant antimicrobial effect against anaerobic oral microorganisms. This finding indicates their potential use as mouth disinfectants in future clinical studies. Notably, the extracts from L. stoechas, S. thymbra, S. parnassica, and the hydroalcoholic extract of V. myrtillus were able to inhibit biofilm formation at concentrations of up to 1.25 mg/ml. This promising result suggests these extracts could be strong candidates for further clinical research. Additionally, future studies could explore the safety and efficacy of incorporating these extracts into long-term oral hygiene practices and their potential use in treating periodontal diseases.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
NB: Investigation, Writing – original draft. AA: Formal analysis, Methodology, Writing – review & editing. AA-A: Project administration, Supervision, Writing – review & editing. EH: Funding acquisition, Resources, Supervision, Writing – review & editing. AS: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. AW: Data curation, Formal analysis, Investigation, Software, Writing – review & editing. KV: Data curation, Formal analysis, Writing – review & editing. LK: Conceptualization, Supervision, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. There was no additional external funding received for this study. Bettina Spitzmüller is acknowledged for her technical assistance during the biofilm plate assay.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Generative AI was used in the creation of this manuscript. During the preparation of this work the authors used ChatGPT in order to improve the language and readability of the manuscript. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdmed.2025.1535753/full#supplementary-material
Supplementary Figure S1 | HPTLC analysis of the hydroalcoholic extracts at 254 nm (panel A) and 366 nm (panel B).
Abbreviations
CHX, chlorhexidine; HPTLC, high performance thin layer chromotography; CBA, Columbia blood agar plates; HCB, yeast-cysteine blood agar plates; CFU, colony forming units; MIC, minimal inhibitory concentration; MBC, minimal bactericidal concentration; MHB, BBL™ Mueller Hinton II Broth-Cation-Adjusted; WCB, Wilkens chalgren Bouillon; DMSO, dimethylsulfoxide; TSB, tryptic soy broth; PBS, phosphate buffered saline; OD, optical density.
References
1. Hotwani K, Baliga S, Sharma K. Phytodentistry: use of medicinal plants. J Complement Integr Med (2014) 11(4):233–51. doi: 10.1515/jcim-2013-0015
2. Lahlou M, El Mahi M, Hamamouchi J. Evaluation of antifungal and mollusuicidial activities of Moroccan Zizyphus lotus (L.) Desf. Ann Pharm Fr. (2002) 60(6):410–4.12514508
3. Gyawali R, Ibrahim SA. Natural products as antimicrobial agents. Food Control. (2014) 46:412–29. doi: 10.1016/j.foodcont.2014.05.047
4. Kenny CR, Furey A, Lucey B. A post-antibiotic era looms: can plant natural product research fill the void? Br J Biomed Sci (2015) 72(4):191–200. doi: 10.1080/09674845.2015.11665752
5. Takenaka S, Ohsumi T, Noiri Y. Evidence-based strategy for dental biofilms: current evidence of mouthwashes on dental biofilm and gingivitis. Jpn Dent Sci Rev. (2019) 55(1):33–40. doi: 10.1016/j.jdsr.2018.07.001
6. Shen Y, Zhao J, de la Fuente-Nunez C, Wang Z, Hancock RE, Roberts CR, et al. Experimental and theoretical investigation of multispecies oral biofilm resistance to chlorhexidine treatment. Sci Rep. (2016) 6:27537. doi: 10.1038/srep27537
7. Chang YC, Huang FM, Tai KW, Chou MY. The effect of sodium hypochlorite and chlorhexidine on cultured human periodontal ligament cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2001) 92(4):446–50. doi: 10.1067/moe.2001.116812
8. Proksch S, Strobel SL, Vach K, Abouassi T, Tomakidi P, Ratka-Kruger P, et al. Melatonin as a candidate therapeutic drug for protecting bone cells from chlorhexidine-induced damage. J Periodontol. (2014) 85(12):e379–89. doi: 10.1902/jop.2014.140279
9. Suphangul S, Pujarern P, Rokaya D, Kanchanasobhana C, Rungsiyakull P, Chaijareenont P. Comparison of plaque accumulation between Titanium and PEEK healing abutments. J Funct Biomater. (2024) 15(11):334. doi: 10.3390/jfb15110334
10. Kathayat G, Selvido DI, Skallevold HE, Rokaya MB, Bhattarai BP, Marya A, et al. Natural oral care and herbal products for oral diseases and oral hygiene maintenance. In: Chauhan DN, Singh PR, Chauhan NS, Shah K, editors. Pharmacological Studies in Natural Oral Care. Hoboken, NJ: Wiley (2023). p. 47–59.
11. Aragão MGB, He X, Aires CP, Corona SAM. Epigallocatechin gallate reduces the virulence of cariogenic Streptococcus mutans biofilm by affecting the synthesis of biofilm matrix components. Arch Oral Biol. (2024) 164:105990. doi: 10.1016/j.archoralbio.2024.105990
12. AlDehlawi H, Jazzar A. The power of licorice (Radix glycyrrhizae) to improve oral health: a comprehensive review of its pharmacological properties and clinical implications. Healthcare. (2023) 11(21):2887. doi: 10.3390/healthcare11212887
13. Jabeen B, Mirani ZA, Lone MA, Nirkhiwale A, Farooqui WA, Aslam K, et al. Comparison of chlorhexidine gluconate, sodium hypochlorite, neem extract, and microwave radiation for disinfection of type IV dental stone. Eur J Dent. (2024). doi: 10.1055/s-0044-1788631
14. Chai M, An M, Zhang X. Construction of a TiO(2)/MoSe(2)/CHI coating on dental implants for combating Streptococcus mutans infection. Mater Sci Eng C Mater Biol Appl. (2021) 129:112416. doi: 10.1016/j.msec.2021.112416
15. Kaypetch R, Anuwongnukroh N, Dechkunakorn S, Wichai W, Tua-Ngam P, Tantivitayakul P, et al. Novel vinegar solution for denture-cleansing agent. J Oral Sci. (2023) 65(2):117–20. doi: 10.2334/josnusd.22-0385
16. Benine-Warlet J, Brenes-Alvarado A, Steiner-Oliveira C. Potassium iodide enhances inactivation of Streptococcus mutans biofilm in antimicrobial photodynamic therapy with red laser. Photodiagnosis Photodyn Ther. (2022) 37:102622. doi: 10.1016/j.pdpdt.2021.102622
17. Mathai K, Anand S, Aravind A, Dinatius P, Krishnan AV, Mathai M. Antimicrobial effect of ginger, garlic, honey, and lemon extracts on Streptococcus mutans. J Contemp Dent Pract. (2017) 18(11):1004–8. doi: 10.5005/jp-journals-10024-2165
18. Bouymajane A, Filali FR, El Majdoub YO, Ouadik M, Abdelilah R, Cavò E, et al. Phenolic compounds, antioxidant and antibacterial activities of extracts from aerial parts of Thymus zygis subsp. gracilis, Mentha suaveolens and Sideritis incana from Morocco. Chem Biodivers. (2022) 19(3):e202101018. doi: 10.1002/cbdv.202101018
19. Hendel N, Sarri M, Sarri D, Seghiour S, Napoli E, Selloum M, et al. Phytochemical analysis, antibacterial and antifungal effect of Lavandula dentata L. essential oil and methanol extract. Nat Prod Res. (2023) 38(20):1–10. doi: 10.1080/14786419.2023.2252973
20. Fierascu I, Dinu-Pirvu CE, Fierascu RC, Velescu BS, Anuta V, Ortan A, et al. Phytochemical profile and biological activities of Satureja hortensis L.: a review of the last decade. Molecules (2018) 23(10):2458. doi: 10.3390/molecules23102458
21. Alyamani AA, Albukhaty S, Aloufi S, AlMalki FA, Al-Karagoly H, Sulaiman GM. Green fabrication of zinc oxide nanoparticles using Phlomis leaf extract: characterization and in vitro evaluation of cytotoxicity and antibacterial properties. Molecules (2021) 26(20):6140. doi: 10.3390/molecules26206140
22. Ferhat M, Erol E, Beladjila KA, Çetintaş Y, Duru ME, Öztürk M, et al. Antioxidant, anticholinesterase and antibacterial activities of Stachys guyoniana and Mentha aquatica. Pharm Biol. (2017) 55(1):324–9. doi: 10.1080/13880209.2016.1238488
23. Milutinovici RA, Chioran D, Buzatu R, Macasoi I, Razvan S, Chioibas R, et al. Vegetal compounds as sources of prophylactic and therapeutic agents in dentistry. Plants. (2021) 10(10):2148. doi: 10.3390/plants10102148
24. Zhang Z, Yu Y, Zhu G, Zeng L, Xu S, Cheng H, et al. The emerging role of plant-derived exosomes-like nanoparticles in immune regulation and periodontitis treatment. Front Immunol. (2022) 13:896745. doi: 10.3389/fimmu.2022.896745
25. Li W, Xiang Z, Yu W, Huang X, Jiang Q, Abumansour A, et al. Natural compounds and mesenchymal stem cells: implications for inflammatory-impaired tissue regeneration. Stem Cell Res Ther. (2024) 15(1):34. doi: 10.1186/s13287-024-03641-3
26. Gürgan CA, Zaim E, Bakirsoy I, Soykan E. Short-term side effects of 0.2% alcohol-free chlorhexidine mouthrinse used as an adjunct to non-surgical periodontal treatment: a double-blind clinical study. J Periodontol. (2006) 77(3):370–84. doi: 10.1902/jop.2006.050141
27. Bartsch S, Kohnert E, Kreutz C, Woelber JP, Anderson A, Burkhardt AS, et al. Chlorhexidine digluconate mouthwash alters the oral microbial composition and affects the prevalence of antimicrobial resistance genes. Front Microbiol. (2024) 15:1429692. doi: 10.3389/fmicb.2024.1429692
28. Milia EP, Sardellitti L, Eick S. Antimicrobial efficiency of Pistacia lentiscus L. derivates against oral biofilm-associated diseases: a narrative review. Microorganisms (2023) 11(6):1378. doi: 10.3390/microorganisms11061378
29. Narwal E, Choudhary J, Kumar M, Amarowicz R, Kumar S, Radha , et al. Botanicals as promising antimicrobial agents for enhancing oral health: a comprehensive review. Crit Rev Microbiol. (2024) 51(1):1–24. doi: 10.1080/1040841X.2024.2321489
30. Hickl J, Argyropoulou A, Al-Ahmad A, Hellwig E, Skaltsounis AL, Wittmer A, et al. Unleashing nature’s defense: potent antimicrobial power of plant extracts against oral pathogens and Streptococcus mutans biofilms. Front Oral Health. (2024) 5:1469174. doi: 10.3389/froh.2024.1469174
31. Hickl J, Argyropoulou A, Sakavitsi ME, Halabalaki M, Al-Ahmad A, Hellwig E, et al. Mediterranean herb extracts inhibit microbial growth of representative oral microorganisms and biofilm formation of Streptococcus mutans. PLoS One. (2018) 13(12):e0207574. doi: 10.1371/journal.pone.0207574
32. Sklirou AD, Angelopoulou MT, Argyropoulou A, Chaita E, Boka VI, Cheimonidi C, et al. Phytochemical study and in vitro screening focusing on the anti-aging features of various plants of the Greek flora. Antioxidants. (2021) 10(8):1206. doi: 10.3390/antiox10081206
33. Mikropoulou EV, Petrakis EA, Argyropoulou A, Mitakou S, Halabalaki M, Skaltsounis LA. Quantification of bioactive lignans in sesame seeds using HPTLC densitometry: comparative evaluation by HPLC-PDA. Food Chem (2019) 288:1–7. doi: 10.1016/j.foodchem.2019.02.109
34. Al-Ahmad A, Laird D, Zou P, Tomakidi P, Steinberg T, Lienkamp K. Nature-inspired antimicrobial polymers–assessment of their potential for biomedical applications. PLoS One. (2013) 8(9):e73812. doi: 10.1371/journal.pone.0073812
35. Al-Ahmad A, Ameen H, Pelz K, Karygianni L, Wittmer A, Anderson AC, et al. Antibiotic resistance and capacity for biofilm formation of different bacteria isolated from endodontic infections associated with root-filled teeth. J Endod. (2014) 40(2):223–30. doi: 10.1016/j.joen.2013.07.023
36. Anderson AC, Al-Ahmad A, Schlueter N, Frese C, Hellwig E, Binder N. Influence of the long-term use of oral hygiene products containing stannous ions on the salivary microbiome—a randomized controlled trial. Sci Rep. (2020) 10(1):9546. doi: 10.1038/s41598-020-66412-z
37. Al-Ahmad A, Muzafferiy F, Anderson AC, Wölber JP, Ratka-Krüger P, Fretwurst T, et al. Shift of microbial composition of peri-implantitis-associated oral biofilm as revealed by 16S rRNA gene cloning. J Med Microbiol. (2018) 67(3):332–40. doi: 10.1099/jmm.0.000682
38. Abusleme L, Dupuy AK, Dutzan N, Silva N, Burleson JA, Strausbaugh LD, et al. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J (2013) 7(5):1016–25. doi: 10.1038/ismej.2012.174
39. Curtis MA, Diaz PI, Van Dyke TE. The role of the microbiota in periodontal disease. Periodontol 2000. (2020) 83(1):14–25. doi: 10.1111/prd.12296
40. Rapala-Kozik M, Surowiec M, Juszczak M, Wronowska E, Kulig K, Bednarek A, et al. Living together: the role of Candida albicans in the formation of polymicrobial biofilms in the oral cavity. Yeast. (2023) 40(8):303–17. doi: 10.1002/yea.3855
41. Al-Ahmad A, Auschill TM, Dakhel R, Wittmer A, Pelz K, Heumann C, et al. Prevalence of Candida albicans and Candida dubliniensis in caries-free and caries-active children in relation to the oral microbiota-a clinical study. Clin Oral Investig. (2016) 20(8):1963–71. doi: 10.1007/s00784-015-1696-9
42. Anderson AC, Jonas D, Huber I, Karygianni L, Wölber J, Hellwig E, et al. Enterococcus faecalis from food, clinical specimens, and oral sites: prevalence of virulence factors in association with biofilm formation. Front Microbiol. (2015) 6:1534. doi: 10.3389/fmicb.2015.01534
43. Goulas V, Exarchou V, Kanetis L, Gerothanassis IP. Evaluation of the phytochemical content, antioxidant activity and antimicrobial properties of mountain tea (Sideritis syriaca) decoction. J Funct Foods. (2014) 6:248–58. doi: 10.1016/j.jff.2013.10.014
44. Aligiannis N, Kalpoutzakis E, Chinou IB, Mitakou S, Gikas E, Tsarbopoulos A. Composition and antimicrobial activity of the essential oils of five taxa of Sideritis from Greece. J Agric Food Chem. (2001) 49(2):811–5. doi: 10.1021/jf001018w
45. Vazquez-Sanchez D, Galvao JA, Mazine MR, Gloria EM, Oetterer M. Control of Staphylococcus aureus biofilms by the application of single and combined treatments based in plant essential oils. Int J Food Microbiol. (2018) 286:128–38. doi: 10.1016/j.ijfoodmicro.2018.08.007
46. Mimica-Dukić N, Bozin B, Soković M, Mihajlović B, Matavulj M. Antimicrobial and antioxidant activities of three Mentha species essential oils. Planta Med. (2003) 69(5):413–9. doi: 10.1055/s-2003-39704
47. Khan RA, Khan F, Ahmed M, Shah AS, Khan NA, Khan MR, et al. Phytotoxic and antibacterial assays of crude methanolic extract of Mentha longifolia (Linn.). Afr J Pharm Pharmacol. (2011) 5(12):1530–3. doi: 10.5897/AJPP11.374
48. Chorianopoulos N, Evergetis E, Mallouchos A, Kalpoutzakis E, Nychas G-J, Haroutounian SA. Characterization of the essential oil volatiles of Satureja thymbra and Satureja parnassica: influence of harvesting time and antimicrobial activity. J Agric Food Chem (2006) 54(8):3139–45. doi: 10.1021/jf053183n
49. Kotsos M, Aligiannis N, Mitaku S, Skaltsounis AL, Charvala C. Chemistry of plants from Crete: stachyspinoside, a new flavonoid glycoside and iridoids from Stachys spinosa. Nat Prod Lett (2001) 15(6):377–86. doi: 10.1080/10575630108041307
50. Magiatis P, Skaltsounis AL, Chinou I, Haroutounian SA. Chemical composition and in vitro antimicrobial activity of the essential oils of three Greek Achillea species. Z Naturforsch C. (2002) 57(3-4):287–90. doi: 10.1515/znc-2002-3-415
51. Kyriakopoulou I, Magiatis P, Skaltsounis AL, Aligiannis N, Harvala C. Samioside, a new phenylethanoid glycoside with free-radical scavenging and antimicrobial activities from Phlomis samia. J Nat Prod. (2001) 64(8):1095–7. 10.1021/np010128+11520237
52. Couladis M, Tanimanidis A, Tzakou O, Chinou IB, Harvala C. Essential oil of Phlomis lanata growing in Greece: chemical composition and antimicrobial activity. Planta Med (2000) 66(7):670–2. doi: 10.1055/s-2000-8631
53. Gursoy UK, Gursoy M, Gursoy OV, Cakmakci L, Kononen E, Uitto VJ. Anti-biofilm properties of Satureja hortensis L. essential oil against periodontal pathogens. Anaerobe (2009) 15(4):164–7. doi: 10.1016/j.anaerobe.2009.02.004
54. Dadalioglu I, Evrendilek GA. Chemical compositions and antibacterial effects of essential oils of Turkish oregano (Origanum minutiflorum), bay laurel (Laurus nobilis), Spanish lavender (Lavandula stoechas L.), and fennel (Foeniculum vulgare) on common foodborne pathogens. J Agric Food Chem (2004) 52(26):8255–60. doi: 10.1021/jf049033e
55. Dursun G, Ozgursoy OB, Kemal O, Coruh I. One-year follow-up results of combined use of CO2 laser and cold instrumentation for Reinke’s edema surgery in professional voice users. Eur Arch Otorhinolaryngol. (2007) 264(9):1027–32. doi: 10.1007/s00405-007-0309-x
56. Bujor OC, Le Bourvellec C, Volf I, Popa VI, Dufour C. Seasonal variations of the phenolic constituents in bilberry (Vaccinium myrtillus L.) leaves, stems and fruits, and their antioxidant activity. Food Chem (2016) 213:58–68. doi: 10.1016/j.foodchem.2016.06.042
57. Puupponen-Pimia R, Nohynek L, Alakomi HL, Oksman-Caldentey KM. Bioactive berry compounds-novel tools against human pathogens. Appl Microbiol Biotechnol. (2005) 67(1):8–18. doi: 10.1007/s00253-004-1817-x
58. Khalifa HO, Kamimoto M, Shimamoto T, Shimamoto T. Antimicrobial effects of blueberry, raspberry, and strawberry aqueous extracts and their effects on virulence gene expression in Vibrio cholerae. Phytother Res. (2015) 29(11):1791–7. doi: 10.1002/ptr.5436
59. Wood E, Hein S, Mesnage R, Fernandes F, Abhayaratne N, Xu Y, et al. Wild blueberry (poly)phenols can improve vascular function and cognitive performance in healthy older individuals: a double-blind randomized controlled trial. Am J Clin Nutr. (2023) 117(6):1306–19. doi: 10.1016/j.ajcnut.2023.03.017
60. Kim D, Hwang G, Liu Y, Wang Y, Singh AP, Vorsa N, et al. Cranberry flavonoids modulate cariogenic properties of mixed-Species biofilm through exopolysaccharides-matrix disruption. PLoS One. (2015) 10(12):e0145844. doi: 10.1371/journal.pone.0145844
61. Philip N, Bandara H, Leishman SJ, Walsh LJ. Inhibitory effects of fruit berry extracts on Streptococcus mutans biofilms. Eur J Oral Sci. (2019) 127(2):122–9. doi: 10.1111/eos.12602
62. Sundararajan A, Rane HS, Ramaraj T, Sena J, Howell AB, Bernardo SM, et al. Cranberry-derived proanthocyanidins induce a differential transcriptomic response within Candida albicans urinary biofilms. PLoS One. (2018) 13(8):e0201969. doi: 10.1371/journal.pone.0201969
63. Hagh LG, Arefian A, Farajzade A, Dibazar S, Samiea N. The antibacterial activity of “Satureja hortensis” extract and essential oil against oral bacteria. Dent Res J. (2019) 16(3):153–9. doi: 10.4103/1735-3327.255741
64. Tourabi M, El Ghouizi A, Nouioura G, Faiz K, Elfatemi H, El-Yagoubi K, et al. Phenolic profile, acute and subacute oral toxicity of the aqueous extract from Moroccan Mentha longifolia L. aerial part in Swiss Albino mice model. J Ethnopharmacol. (2024) 319(Pt 2):117293. doi: 10.1016/j.jep.2023.117293
65. Satoh Y, Ishihara K. Investigation of the antimicrobial activity of bilberry (Vaccinium myrtillus L.) extract against periodontopathic bacteria. J Oral Biosci. (2020) 62(2):169–74. doi: 10.1016/j.job.2020.01.009
66. Aabouch F, Annemer S, Satrani B, Ettaleb I, Kara M, Ghanmi M, et al. Assessing the optimal antibacterial action of Lavandula stoechas L., Thymus zygis L., and Eucalyptus camaldulensis Dehnh essential oils. Life. (2024) 14(11):1424. doi: 10.3390/life14111424
67. Angioni A, Barra A, Coroneo V, Dessi S, Cabras P. Chemical composition, seasonal variability, and antifungal activity of Lavandula stoechas L. ssp. stoechas essential oils from stem/leaves and flowers. J Agric Food Chem. (2006) 54(12):4364–70. doi: 10.1021/jf0603329
68. Farooq A, Ali S, Ullah H, Khan A, Nawaz F, Farooq S, et al. GC-MS assisted determination of 26 compounds in Phlomis Stewartii extract exhibiting antioxidant, antifungal, and antibacterial properties. Chem Biodivers. (2024) 21(12):e202401068. doi: 10.1002/cbdv.202401068
69. Öztürk Sarıkaya SB, Zehiroğlu C. Endemic mountain tea (Sideritis dichotoma huter): antioxidant and antibacterial activities, mineral content by ICP-MS and phytochemical content by LCHR-MS. Chem Biodivers. (2023) 20(12):e202301453. doi: 10.1002/cbdv.202301453
70. Günther M, Karygianni L, Argyropoulou A, Anderson AC, Hellwig E, Skaltsounis AL, et al. The antimicrobial effect of Rosmarinus officinalis extracts on oral initial adhesion ex vivo. Clin Oral Investig. (2022) 26(6):4369–80. doi: 10.1007/s00784-022-04400-5
71. Kurz H, Karygianni L, Argyropoulou A, Hellwig E, Skaltsounis AL, Wittmer A, et al. Antimicrobial effects of Inula viscosa extract on the in situ initial oral biofilm. Nutrients (2021) 13(11):4029. doi: 10.3390/nu13114029
72. Rosa A, Pujia AM, Arcuri C. The protective role antioxidant of vitamin C in the prevention of oral disease: a scoping review of current literature. Eur J Dent. (2024) 18(4):965–70. doi: 10.1055/s-0044-1786845
Keywords: Mediterranean herb extracts, oral mouthwashes, antimicrobial activity, biofilm inhibition, Streptococcus mutans
Citation: Bartels N, Argyropoulou A, Al-Ahmad A, Hellwig E, Skaltsounis AL, Wittmer A, Vach K and Karygianni L (2025) Antibiofilm potential of plant extracts: inhibiting oral microorganisms and Streptococcus mutans. Front. Dent. Med. 6:1535753. doi: 10.3389/fdmed.2025.1535753
Received: 27 November 2024; Accepted: 24 March 2025;
Published: 4 April 2025.
Edited by:
Analú Barros De Oliveira, São Paulo State University, BrazilReviewed by:
Dinesh Rokaya, Ajman University, United Arab EmiratesAlessio Rosa, University of Rome Tor Vergata, Italy
Copyright: © 2025 Bartels, Argyropoulou, Al-Ahmad, Hellwig, Skaltsounis, Wittmer, Vach and Karygianni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lamprini Karygianni, bGFtcHJpbmkua2FyeWdpYW5uaUB6em0udXpoLmNo
 Nomi Bartels1
Nomi Bartels1 Aikaterini Argyropoulou
Aikaterini Argyropoulou Ali Al-Ahmad
Ali Al-Ahmad Alexios Leandros Skaltsounis
Alexios Leandros Skaltsounis Annette Wittmer
Annette Wittmer Lamprini Karygianni
Lamprini Karygianni