
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Dent. Med. , 19 March 2025
Sec. Periodontics
Volume 6 - 2025 | https://doi.org/10.3389/fdmed.2025.1512252
This article is part of the Research Topic Diagnostic and Treatment Strategies for Periodontal Disease View all 5 articles
 Priyanka Padalkar1,†
Priyanka Padalkar1,† Sunaina Shetty Yadadi2,†
Sunaina Shetty Yadadi2,† Gopinath Vivekanandan3
Gopinath Vivekanandan3 Shishir Ram Shetty4
Shishir Ram Shetty4 Mangesh Andhare1
Mangesh Andhare1 Aditi Pashine5
Aditi Pashine5 Vineet Vinay6
Vineet Vinay6 Vijay Desai7,8
Vijay Desai7,8 Raghavendra M. Shetty7,8,9*
Raghavendra M. Shetty7,8,9*
Background: The diagnosis of periodontitis is primarily through clinical and radiographic assessments. However, it is difficult for clinicians to detect incipient periodontitis during the routine clinical assessment. Identifying people at risk for periodontitis and tracking disease development need a dependable biomarker. Currently, no biomarkers meet all the criteria required for an ideal diagnostic test. Therefore, the clinical utility of salivary periostin as a potential screening tool for periodontitis warrants further investigation, particularly through large samples across diverse populations. The present study aimed to investigate salivary periostin levels as a biomarker in individuals with periodontitis and healthy controls.
Methods: Forty-five patients with generalized periodontitis stage III grade A/B and an equivalent number of periodontally healthy controls were evaluated for plaque index (PI), gingival index (GI), pocket probing depth (PPD), and clinical attachment level (CAL). Unstimulated salivary samples from all subjects were taken, and periostin levels were quantified using an ELISA kit.
Results: The average salivary periostin levels were 4.63 in the healthy group and 1.24 in the periodontitis group (P < 0.05). The Spearman coefficient indicated a negative correlation between periostin levels and the gingival index (r = −0.761), plaque index (r = −0.780; P < 0.05), probing pocket depth (PPD) (r = −0.713; P < 0.05) and clinical attachment level (CAL) (r = −0.713; P < 0.05). Linear regression analysis validated the indirect correlation between salivary periostin levels and clinical indicators (Adjusted R square = 0.947).
Conclusions: Salivary periostin levels are associated with periodontal disease. Salivary periostin levels indirectly influence as a non-invasive biomarker of periodontitis. The biomarker periostin is effective for evaluating both healthy and diseased periodontium.
“Prospective healthcare” is an innovative methodology that evaluates an individual's health condition and vulnerability to the potential onset of clinical issues (1–3). The efficient implementation of resources to prevent diseases is crucial for cost-effective healthcare strategies, particularly in middle and low-income nations. Reliable and economical healthcare services can be achieved by advancing diagnostic methods to identify and classify at-risk patients (4). Recent research on molecular biomarkers like genomic biomarkers, transcriptomic biomarkers, proteomic biomarkers and metabolic biomarkers aims to identify quantifiable indicators of physiological, pathological, pharmacological, or genetic processes, which can be utilized to predict diagnosis and prognosis.
Human saliva, being readily accessible, possesses a composite nature and dynamic content, rendering it a viable medium for biomarker detection. It comprises proteins, nucleic acids, lipids, and metabolites that may be associated with oral and systemic diseases (5). Moreover, utilizing salivary fluids for disease activity recognition presents the significant benefits of being cost-effective, quick, and non-invasive. The combined application of modern technologies such as proteomics, metabolomics, lipidomics, and microbiomics has resulted in the development of a novel diagnostic approach termed “salivaomics” (5–9).
Periodontal diseases are significant among oral illnesses due to their elevated prevalence rates. Moreover, these diseases serve as a shared risk factor for various systemic diseases or conditions, including cardiovascular disease, pre-term low birth weight, Type 2 Diabetes Mellitus, multiple sclerosis, lupus erythematosus, oral cancer, polycystic ovarian syndrome, and negative outcomes in COVID-19 patients (10–16). Currently, clinical characteristics serve as conventional methods for identifying and diagnosing periodontitis.
The mechanisms linking periodontitis to other systemic diseases align with clinical findings that associate periodontitis with bacteremia, chronic low-grade inflammation, increased myelopoietic activity, and the effectiveness of local periodontal treatments in reducing systemic inflammation and improving disease activity markers (17–19).
On the other hand, systemic conditions like type 2 diabetes (T2DM) may increase the risk of developing periodontitis by heightening the inflammatory load on the periodontium or altering the periodontal microbiome (17, 20, 21).
The variability of clinical measures in evaluating the onset of risk factors for periodontitis underscores the urgent necessity for dependable disease biomarkers. The development of periodontitis biomarkers is a paramount priority that could transform the prevention and treatment of this condition.
Periostin is a distinctive proteomic biomarker that not only aids in diagnosing disease but also indicates severity, monitors treatment response, and forecasts prognosis in conditions such as cardiac repair, bronchial asthma, and type 2 diabetes mellitus (22–25). “Periostin” is a novel biomarker that plays a key role in processes such as collagen synthesis, cell migration, wound healing, and the regulation of periodontal pathogenesis (25–33). Nevertheless, experimental animal studies provided most of the information regarding salivary periostin levels and periodontitis circumstances compared to human studies (28–33), prompting more human research for external validity. Though there are sufficient studies on the periostin level in gingival crevicular fluid (GCF), the research on the periostin level in saliva is scanty. Hence, the present study aimed to assess salivary periostin levels as a non-invasive biomarker and to correlate with the clinical parameters among healthy and periodontitis patients.
An Analytical Cross-Sectional study was designed, and ethical approval was secured from the Institutional Ethical Committee of the University of Health Sciences Nashik (SYN/PERIO/ADC/BEED/2462/2018). This research adhered to the “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) criteria (34). Figure 1 depicts the flowchart of the investigation.
Participants attending the outpatient Department of Periodontology at Aditya Dental College in Beed, Maharashtra, India, enrolled in this analytical cross-sectional study. The primary author elucidated the technique to all participants. Upon obtaining written informed consent from the volunteered participants, they signed up for the study.
The control group (Group I) comprised healthy patients exhibiting bleeding on probing of less than 10% and probing pocket depth (PPD) of less than 4 mm. The case group (Group II) comprised periodontitis patients aged 30 years and older, possessing at least 2 non-adjacent teeth with probing pocket depth (PPD) ≥ 5 mm, interdental clinical attachment loss (CAL) ≥ 5 mm, positive bleeding on probing, and radiographic evidence of alveolar bone loss classified as generalized periodontitis Stage III Grade B. The participants were categorized as having generalized periodontitis stage III grade A/B or as healthy individuals following a comprehensive intraoral examination per the latest classification of periodontal and peri-implant diseases 2017 (35–37). Individuals who habitually used tobacco in any form, pregnant women, those with a history of systemic disease, individuals who have used antibiotics within the past six months, and those who have received any sort of periodontal treatment during the last two years were excluded from the study.
To ascertain the sample size for our study, we utilized the standard normal deviates for Type I error (α) and Type II error (β), denoted as Zα and Zβ, respectively. In our analysis, Zα is established at 1.9600 and Zβ at 0.8416. We computed the coefficient C, defined as 0.5 * ln[(1 + r)/(1-r)], where r denotes the correlation coefficient (0.461) (23). We determined the overall sample size (N) by the formula N = [(Zα+Zβ)/C]2 + 3. This necessitates a sample size of 35 persons. We included 45 people in each group. This calculation validates that our investigation possesses adequate statistical power to identify the stated effects at a 95% confidence range and 80% power.
This study recruited ninety individuals, comprising an equal number (n = 45) of subjects with periodontitis and healthy controls. Participants' gingival status and periodontal condition were evaluated using established procedures based on Plaque Index scores, Pocket Probing Depth scores (PPD), Gingival Index scores, and Clinical Attachment Level (CAL). Intra-examiner reliability was assessed using Kappa statistics, yielding a value of 0.75. The four regions were documented for the Plaque Index and Gingival Index (38, 39). The distance from the coronal free gingival border to the base of the pocket for each tooth was assessed using the Williams periodontal probe (Hu Friedy, Chicago, IL, USA). The deepest pocket recorded was deemed a PPD score for the specific subject (40). The Clinical Attachment Level (CAL) was measured from the cementoenamel junction to the base of the gingival sulcus/periodontal pocket using a periodontal probe at all six designated sites for probing depth (41). Only the primary author evaluated all patients' PPD and CAL scores to ensure consistency in proprioception assessment. The radiographic examination was conducted to corroborate the clinical diagnosis of widespread periodontitis stage III grade A/B in Group II and to assess the health status of patients in Group I.
Participants were instructed to abstain from consuming food or beverages for a minimum of 2 h before the collection of salivary samples. Unstimulated saliva samples were obtained from all the participants (42, 43). Samples were obtained between 9 a.m. and 12 p.m. to mitigate diurnal oscillations coinciding with the periods, individuals attended the outpatient department. Each volunteer gargled with water approximately 10 min before the initiation of salivary collection. Subsequently, participants were seated on the dental chair, and their saliva was collected passively into a labeled sterile container by instructing them to expectorate until the volume reached 5 ml. All samples underwent centrifugation for 5 min to eliminate turbidity and cellular debris. The samples were preserved at −80°C in Eppendorf tubes until subsequent processing. Following thawing, the periostin concentration in preserved samples was quantified utilizing an ELISA kit and administered following the manufacturer's guidelines (RD191016100; BioVendor Laboratory Medicine, Brno, Czech Republic). The ELISA plate reader (BioTeK Instruments Inc., Winooski, VT, USA) was employed to quantify periostin levels by correlating them with absorbance at a wavelength of 450 nm. The periostin level was expressed as nanograms per milliliter (ng/ml) with a 1–50 ng/ml detection range of the ELISA kit.
The Statistical Package for the Social Sciences (SPSS) software version 21 (SPSS Inc., Chicago, IL, USA) was utilized to analyze the descriptive and inferential statistics of the results. Due to the Kolmogorov–Smirnov test indicating non-normality, the Mann–Whitney U-test was employed to evaluate GI, PI, PPD, CAL, and periostin levels between healthy individuals and patients with periodontitis. A P-value less than 0.05 was deemed statistically significant. The Spearman correlation test evaluated the association between salivary periostin and GI, PI, PPD, and CAL. Additionally, a regression analysis was carried out to assess the impact of independent variables on salivary periostin levels. Receiver-operating characteristic curve (ROC) was carried out to assess the diagnostic accuracy and the area under the curve (AUC) was generated.
Ninety volunteers participated in the current investigation. Group I included 45 individuals, 30 males and 15 females, whereas Group II also comprised 45 individuals, 27 males and 18 females. No significant difference was seen in the distribution of research participants considering gender and age group (Table 1).
All subjects exhibited the presence of periostin in their salivary samples. The average salivary periostin levels in Group I and Group II were 4.63 and 1.24 ng/ml, respectively, indicating a substantial disparity between the groups in our study. The evaluated clinical indicators demonstrated a statistically significant difference between Group I and Group II in gingival index scores, plaque index scores, pocket probing depth, clinical attachment loss, and salivary periostin levels (Table 2; P < 0.001).

Table 2. Intergroup comparison of gingival index, plaque index, pocket probing depth, clinical attachment level and salivary periostin levels.
The Spearman coefficient indicated a negative correlation between periostin levels and the gingival index (r = −0.761; P < 0.001), plaque index (r = −0.780; P < 0.001), probing pocket depth (PPD) (r = −0.713; P < 0.001), and clinical attachment level (CAL) (r = −0.713; P < 0.001) (Table 3). Linear regression analysis further elucidated the robustness of the indirect correlation between salivary periostin levels and the clinical measures employed in the study (Table 4).
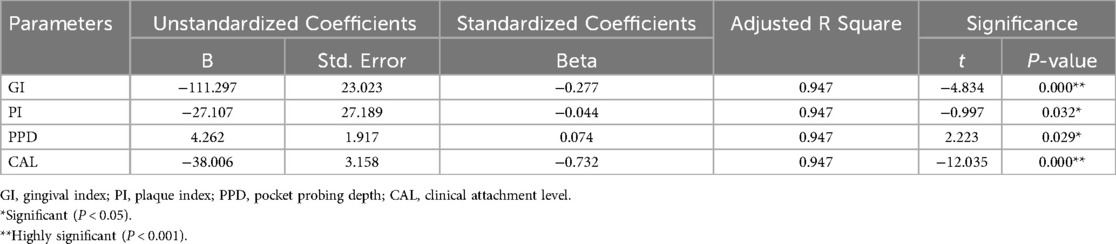
Table 4. Multivariate regression analysis of salivary periostin levels with gingival Index, plaque index, pocket probing depth, clinical attachment level scores.
ROC analysis revealed the area under the curve (AUC) value of 0.952, which indicated excellent diagnostic ability. AUC values range from 0.5 (no discrimination) to 1.0 (perfect discrimination). An AUC of 0.952 suggested that the test variable (salivary periostin level) can distinguish between the positive and negative groups. The P-value was less than 0.001, which was highly significant. This suggested that the observed AUC significantly differs from the null hypothesis value (AUC = 0.5), indicating strong evidence that the test variable is predictive (Figure 2).
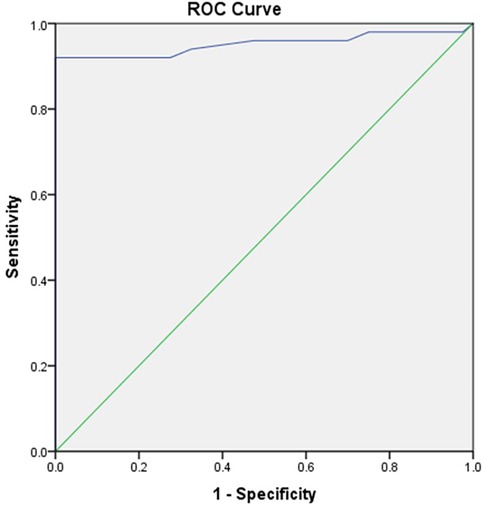
Figure 2. Receiver operating characteristics curve (ROC) depicting area under the curve (AUC) for salivary periostin level (ng/ml).
The periodontium, a highly specialized tissue, supports, protects, and enables proprioception within the oral cavity. This tissue consists of various cells vital for maintaining the integrity of the teeth' structures (43–47). Periodontal disease, which encompasses a broad spectrum of clinical manifestations, can range from mild symptoms, such as gingival bleeding, to more severe forms that result in attachment loss, bone degradation, tooth mobility, and eventual tooth loss. The progression of this disease can have profound consequences on oral health and overall well-being. Epidemiological data suggest that periodontal disease affects approximately 20%–50% of the global population, positioning it as one of the most prevalent oral health issues worldwide. It is ranked as the ninth most common illness-related impairment, impacting millions of individuals across different regions and populations (48, 49).
Periodontal disease arises from intricate interactions between the host immune response and subgingival biofilms. While everyone reacts to this immunomodulation differently, the disease can generally be averted in most cases (50). Current clinical diagnostic measures can only assess prior episodes of tissue destruction, specifically the threshold shift in periodontal cells, which may indicate 2–3 mm of tissue destruction before clinicians can detect a current disease site. Advanced diagnostic tools, such as subtraction radiography, are accessible for diagnosing periodontal disease. Nonetheless, dental care providers' routine application, cost-effectiveness, and patient acceptance results in their underutilization (4).
The pathogenesis of periodontal disease involves a complex interplay between the host's immune response and subgingival biofilms, which harbor a variety of pathogenic microorganisms. While the immune response to these microorganisms varies among individuals, periodontal disease is largely preventable if proper preventive measures are taken (50). However, current diagnostic tools primarily focus on detecting tissue damage after it has already occurred, typically when there is already a significant loss of periodontal tissue, such as a 2–3 mm shift in attachment levels, which may not be identified until substantial destruction has taken place. While useful, conventional diagnostic methods, such as probing and radiographic assessments, are often limited by their inability to detect early disease stages and their reliance on visible or measurable tissue destruction. Although advanced diagnostic techniques, such as subtraction radiography, show promise for detecting periodontal disease in its early stages, their high cost and limited routine application by dental care providers often lead to underutilization that highlights a significant gap in clinical practice and a need for alternative, cost-effective methods for early detection. Therefore, a viable new diagnostic approach that transitions from a “Disease-Centric” model of periodontal care to a “Patient-Centric” model, which evaluates individual patient risk factors, will significantly interest healthcare professionals (50).
A shift towards a more patient-centric approach in periodontal care, where individual risk factors and molecular markers are considered, offers great potential for improving diagnosis and treatment. This personalized approach could provide more targeted, effective interventions and better align with individuals' diverse responses to periodontal disease. Recent advances in “omics” technologies have introduced the possibility of utilizing molecular markers, such as those found in saliva, to predict an individual's predisposition to periodontal disease. These biomarkers are particularly advantageous as they are non-invasive, easily accessible, and can provide real-time insights into an individual's periodontal health.
Castagnola et al. (51) indicated that holistic technologies incorporating omics sciences in saliva could, shortly, accurately predict the complex individuality of each individual by leveraging their distinct traits such as fingerprints and the molecular mechanisms associated with health and disease, and vice versa (51). Periostin has emerged as a promising biomarker in periodontal disease among the various molecular markers. Periostin is a secreted extracellular matrix protein predominantly expressed in periodontal ligaments, where it plays a crucial role in tissue integrity, bone remodeling, and tooth development (52–58). This protein has been identified in several biological fluids, including saliva, where its presence and concentration could potentially indicate periodontal health. The present study involved 90 volunteers divided equally into two groups, Group I and Group II. There were no significant differences in gender or age distribution, which ensured that the results were not biased by these demographic factors. All participants had detectable levels of periostin in their salivary samples, confirming the presence of this biomarker across both groups.
A notable finding of this study is the substantial difference in average salivary periostin levels between the two groups, with Group I exhibiting significantly higher levels (4.63) compared to Group II (1.24). This suggests a potential link between periostin levels, and the clinical condition being investigated, as indicated by the substantial difference in clinical measures between the two groups. Specifically, Group II showed significantly worse clinical indicators, including higher gingival index scores, plaque index scores, probing pocket depth (PPD), and clinical attachment loss (CAL), all of which are consistent with poorer periodontal health.
The correlation analysis further strengthens this relationship, revealing significant negative correlations between salivary periostin levels and clinical measures such as the gingival index (r = −0.761), plaque index (r = −0.780), probing pocket depth (r = −0.713), and clinical attachment level (r = −0.713), all with P-values less than 0.001. These findings suggest that as periostin levels decrease, the clinical indicators of periodontal disease worsen, which aligns with the study of Du and Li (43) indicating that periostin, a matrix protein involved in tissue remodeling, may play a role in the progression of periodontal disease (43).
Linear regression analysis further supported the strength and consistency of this negative correlation, reinforcing the potential utility of salivary periostin levels as an indirect marker of periodontal health. This is particularly significant as it offers a non-invasive method for monitoring periodontal disease progression, which could benefit clinical settings.
The receiver operating characteristic (ROC) analysis also demonstrated an excellent diagnostic ability for salivary periostin, with an area under the curve (AUC) value of 0.952. This high AUC indicates that salivary periostin levels can effectively differentiate between individuals with differing clinical presentations of periodontal health and disease. The AUC value, significantly higher than the null hypothesis value of 0.5, supports the strong predictive value of salivary periostin levels for periodontal disease.
Our investigation found no statistically significant variations in mean age between the control and experimental groups, unlike the findings of Jamesha et al. (50), since the age of Group II in the experimental group was higher than that of the control group (50). Substantial data indicates increased plaque build-up and elevated gingival index scores in people with periodontitis (23, 33, 50, 59, 60) corroborating with the present study.
The results of our investigation indicated that periostin expression in saliva was markedly decreased in patients with periodontitis. In accordance with the current study, research conducted by Aral et al. (33) that measured periostin levels in saliva and gingival crevicular fluid (GCF) in periodontitis patients revealed lower values (33). Similarly, Esfahrood et al. (61) observed findings consistent with the current study in their focused research on salivary periostin and chronic periodontitis (34). The only difference was that the latest 2017 classification of periodontal and peri-implant diseases was used to diagnose periodontitis in the present study. These findings may be attributed to periodontal inflammation, which could impair fibroblast development from totipotent cells, perhaps resulting in less periostin expression.
The findings of our study also indicated that periostin levels in saliva are significantly lower in individuals with periodontitis. This result aligns with studies by Aral et al. (33) and Esfahrood et al. (61), who found decreased periostin levels in the saliva and gingival crevicular fluid (GCF) of periodontitis patients (33, 34). These findings suggest that periodontal inflammation, which impairs the function of fibroblasts in the periodontal ligament, may lead to reduced periostin expression. Chronic inflammation in the periodontium can hinder the expression of periostin, thus contributing to the observed decrease in its levels in saliva and GCF. Research on periostin levels in the gingival crevicular fluid (GCF) of patients with chronic periodontitis indicated reduced periostin levels in these individuals (23, 50, 59, 62–64).
In the recent systematic review and meta-analysis by Abdolalian et al. (65), it was reported that the mean concentration of periostin in GCF is significantly lower in individuals with chronic periodontitis compared to those with gingivitis and healthy individuals (65). These results indicate that periostin may serve as a potential diagnostic marker for chronic periodontitis. Our findings are consistent with these results, although, in our study, we evaluated salivary periostin levels rather than GCF periostin.
In the present study, we found a clear indirect correlation between the presence of periodontitis and alterations in salivary periostin levels, further solidifying periostin's potential as a reliable marker for periodontal tissue inflammation. This non-invasive biomarker could offer several advantages over traditional diagnostic methods, including ease of collection, cost-effectiveness, and detecting early signs of disease before more significant tissue damage occurs. While another study by Aral et al. (33) has also corroborated the effectiveness of salivary periostin as a periodontal biomarker, our findings contribute to the growing body of evidence supporting its use as a practical tool for assessing periodontal conditions (33). Given the emerging importance of molecular biomarkers in modern periodontal diagnostics, salivary periostin holds promise for enhancing the early detection, management, and monitoring of periodontal disease, ultimately improving patient outcomes and progressing toward patient-centered care.
The results of the present study provide strong evidence supporting the potential of salivary periostin as a reliable biomarker for assessing periodontal health. The significant negative correlations with clinical measures of periodontal disease and the excellent diagnostic accuracy shown in the ROC analysis suggest that salivary periostin could be an important tool in the early detection and monitoring of periodontal disease. Further studies are warranted to explore its use in clinical practice, including its potential for early intervention and personalized treatment strategies in periodontal care.
The current investigation employed both subjective and objective approaches to verify the disease state. To reduce observer bias, a single examiner documented all the clinical parameters. Although the sample size is limited, our study offers significant insights into interpreting the population parameter of the dependent variable examined. Salivary periostin holds significant promise for the early detection and management of periodontitis. Through non-invasive tests, it can identify early signs of the disease even before clinical symptoms manifest. Salivary periostin biomarkers could be integrated into diagnostic devices that facilitate chair-side investigations in dental clinics during routine screenings. Once detected, these biomarkers can assist in monitoring disease progression, evaluating treatment effectiveness, and preventing relapses. Additionally, combining these biomarkers with clinical parameters can enhance the precision of periodontitis management, leading to more personalized and effective care for patients.
The present study did not evaluate the relationship between periostin levels and the severity of periodontal disease, which is a limitation. Future research that includes all stages of periodontitis as defined by the latest classification will provide a more complete understanding.Additional multicentric research worldwide is necessary to validate external applicability. The evaluation of the whole biochemical expression of this complex salivary protein by several diagnostic techniques, such as ELISA, quantitative real-time PCR, and mRNA analysis, is necessary. It is necessary to design more clinical trials to evaluate salivary periostin levels to validate this novel biomarker in identifying disease status and measuring its efficacy as a diagnostic tool post-treatment. Periodontitis is a chronic immunomodulatory disease that is influenced by several hormonal and systemic factors. Therefore, exploring fresh perspectives about periostin salivary levels in different periodontal disorders is advisable. Further studies need to be designed to evaluate the impact of systemic conditions/diseases on periostin levels that could confound the diagnosis of periodontitis and could benefit targeted screening of high-risk groups with systemic diseases and periodontitis.
Salivary periostin levels are associated with the periodontal disease. The periostin level was higher in healthy individuals when compared to the individuals with periodontal diseases included in the study. The study revealed significant negative correlations between salivary periostin levels and clinical measures such as the gingival index, plaque index, probing pocket depth, and clinical attachment level. Salivary periostin levels indirectly influence as a non-invasive biomarker of periodontitis. The biomarker periostin is effective for evaluating both healthy and diseased periodontal tissue.
The present study contributes new insights into the role of salivary periostin as a biomarker for periodontitis, which is evaluated within the framework of the latest classification system of the “2017 World Workshop classification of periodontal and peri-implant diseases and conditions” and marks a significant step forward in periodontal research. The non-invasive approach validates the relevance of periostin as a biomarker and supports the growing trend toward using biomarkers for the early detection and management of periodontal diseases. As more studies explore the relationship between salivary periostin and periodontal health, this biomarker will likely play an increasingly central role in clinical practice, enhancing the ability to monitor disease progression and response to treatment.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethical approval was obtained from the Institutional Ethical Committee of the University of Health Sciences Nashik (SYN/PERIO/ADC/BEED/2462/2018). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
PP: Conceptualization, Data curation, Formal analysis, Investigation, Resources, Writing – review & editing. SSY: Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. GV: Data curation, Formal analysis, Supervision, Validation, Writing – review & editing. SRS: Data curation, Resources, Validation, Writing – review & editing. MA: Data curation, Resources, Validation, Writing – review & editing. AP: Formal analysis, Resources, Writing – review & editing. VV: Formal analysis, Resources, Validation, Writing – original draft, Writing – review & editing. VD: Formal analysis, Resources, Visualization, Writing – review & editing. RS: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. The Deanship of Research and Graduate Studies (DRG), Ajman University, Ajman, United Arab Emirates supported the publication fees partially.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdmed.2025.1512252/full#supplementary-material
1. Taba M, Kinney J, Sampath Kumar A, Giannobile WV. Diagnostic biomarkers for oral and periodontal diseases. Dent Clin North Am. (2005) 49(3):551–71. doi: 10.1016/j.cden.2005.03.009
2. Snyderman R, Yoediono Z. Prospective care: a personalized, preventative approach to medicine. Pharmacogenomics. (2005) 7(1):5–9. doi: 10.2217/14622416.7.1.5
3. Tabak LA. Point-of-care diagnostics enter the mouth. Ann N Y Acad Sci. (2007) 1098(1 ):7–14. doi: 10.1196/annals.1384.043
4. Giannobile WV, Beikler T, Kinney JS, Ramseier CA, Morelli T, Wong DT. Saliva as a diagnostic tool for periodontal disease: current state and future directions. Periodontol 2000. (2009) 50(1):52–64. doi: 10.1111/j.1600-0757.2008.00288.x
5. Buzalaf MAR, Carvalho Ortiz AD, Carvalho TS, Fideles SOM, Araújo TT, Moraes SM, et al. Saliva as a diagnostic tool for dental caries, periodontal disease, and cancer: is there a need for more biomarkers? Expert Rev Mol Diagn. (2020) 20(5):543–55. doi: 10.1080/14737159.2020.1743686
6. Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. (2001) 69(3):89–95. doi: 10.1067/mcp.2001.113989
7. Ahsan H. Biomolecules and biomarkers in oral cavity: bioassays and immunopathology. J Immunoassay Immunochem. (2018) 40(1):52–69. doi: 10.1080/15321819.2018.1550423
8. Siqueira WL, Dawes C. The salivary proteome: challenges and perspectives. Proteomics Clin Appl. (2011) 5(11–12):575–9. doi: 10.1002/prca.201100046
9. Ai J, Smith B, Wong DT. Saliva ontology: an ontology-based framework for a salivaomics knowledge base. BMC Bioinformatics. (2010) 11(1):1–8. doi: 10.1186/1471-2105-11-302
10. Humphrey LL, Fu R, Buckley DI, Freeman M, Helfand M. Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis. J Gen Intern Med. (2008) 23(12):2079–86. doi: 10.1007/s11606-008-0787-6
11. Rezaei M, Bayani M, Tasorian B, Mahdian S. The comparison of visfatin levels of gingival crevicular fluid in systemic lupus erythematosus and chronic periodontitis patients with healthy subjects. Clin Rheumatol. (2019) 38(11):3139–43. doi: 10.1007/s10067-019-04708-w
12. Sheu J-J, Lin H-C. Association between multiple sclerosis and chronic periodontitis: a population-based pilot study. Eur J Neurol. (2013) 20(7):1053–9. doi: 10.1111/ene.12103
13. Krüger M, Hansen T, Kasaj A, Moergel M. The correlation between chronic periodontitis and oral cancer. Case Rep Dent. (2013) 2013:1–8. doi: 10.1155/2013/262410
14. Tanguturi S, Nagarakanti S. Polycystic ovary syndrome and periodontal disease: underlying links—a review. Indian J Endocrinol Metab. (2018) 22(2):267. doi: 10.4103/ijem.ijem_577_17
15. Llambés F. Relationship between diabetes and periodontal infection. World J Diabetes. (2015) 6(7):927. doi: 10.4239/wjd.v6.i7.927
16. Al-Maweri SA, Alhajj MN, Halboub E, Tamimi F, Salleh NM, Saleh Al-Ak’hali M, et al. The impact of periodontal disease on the clinical outcomes of COVID-19: a systematic review and meta-analysis. BMC Oral Health. (2023) 23(1):1–15. doi: 10.1186/s12903-023-03378-0
17. Genco RJ, Sanz M. Clinical and public health implications of periodontal and systemic diseases: an overview. Periodontol 2000. (2020) 83(1):7–13. doi: 10.1111/prd.12344
18. Teles R, Wang C-Y. Mechanisms involved in the association between periodontal diseases and cardiovascular disease. Oral Dis. (2011) 17(5):450–61. doi: 10.1111/j.1601-0825.2010.01784.x
19. Bajaj JS, Matin P, White MB, Fagan A, Deeb JG, Acharya C, et al. Periodontal therapy favorably modulates the oral-gut-hepatic axis in cirrhosis. Am J Physiol Gastrointest Liver Physiol. (2018) 315(5):G824–37. doi: 10.1152/ajpgi.00230.2018
20. Xiao E, Mattos M, Vieira GHA, Chen S, Corrêa JD, Wu Y, et al. Diabetes enhances IL-17 expression and alters the oral microbiome to increase its pathogenicity. Cell Host Microbe. (2017) 22(1):120–128.e4. doi: 10.1016/j.chom.2017.06.014
21. Teles F, Wang Y, Hajishengallis G, Hasturk H, Marchesan JT. Impact of systemic factors in shaping the periodontal microbiome. Periodontol 2000. (2021) 85(1):126–60. doi: 10.1111/prd.12356
22. Reshmaa R, Sudarsan S, Arunmozhi U, Kadhiresan R, Jenifer Cynthia RA. Periostin—a forward step in periodontal biomarkers—a narrative review. J Indian Dent Assoc Madras. (2021) 8(3):14–9. doi: 10.37841/jidam_2021_v8_i3_04
23. Sophia K, Suresh S, Sudhakar U, Abdul Cader S, Vardhini VM, Arunachalam LT, et al. Comparative evaluation of serum and gingival crevicular fluid periostin levels in periodontal health and disease: a biochemical study. Cureus. (2020) 12(3):e7218. doi: 10.7759/cureus.7218
24. Sasaki H, Yu C-Y, Dai M, Tam C, Loda M, Auclair D, et al. Elevated serum periostin levels in patients with bone metastases from breast but not lung cancer. Breast Cancer Res Treat. (2003) 77(3):245–52. doi: 10.1023/a:1021899904332
25. Khurshid Z, Mali M, Adanir N, Zafar MS, Khan RS, Latif M. Periostin: immunomodulatory effects on oral diseases. Eur J Dent. (2020) 14(3):462–6. doi: 10.1055/s-0040-1714037
26. Cobo T, Obaya A, Cal S, Solares L, Cabo R, Vega JA, et al. Immunohistochemical localization of periostin in human gingiva. Eur J Histochem. (2015) 59(3):2548. doi: 10.4081/ejh.2015.2548
27. Romanos GE, Asnani KP, Hingorani D, Deshmukh VL. Periostin: role in formation and maintenance of dental tissues. J Cell Physiol. (2013) 229(1):1–5. doi: 10.1002/jcp.24407
28. Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, et al. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor β. J Bone Miner Res. (1999) 14(7):1239–49. doi: 10.1359/jbmr.1999.14.7.1239
29. Kruzynska-Frejtag A, Wang J, Maeda M, Rogers R, Krug E, Hoffman S, et al. Periostin is expressed within the developing teeth at the sites of epithelial–mesenchymal interaction. Dev Dyn. (2004) 229(4):857–68. doi: 10.1002/dvdy.10453
30. Suzuki H, Amizuka N, Kii I, Kawano Y, Nozawa-Inoue K, Kudo A, et al. Immunohistochemical localization of periostin in tooth and its surrounding tissues in mouse mandibles during development. Anat Rec A Discov Mol Cell Evol Biol. (2004) 281A(2 ):1264–75. doi: 10.1002/ar.a.20080
31. Wilde J, Yokozeki M, Terai K, Kudo A, Moriyama K. The divergent expression of periostin mRNA in the periodontal ligament during experimental tooth movement. Cell Tissue Res. (2003) 312(3):345–51. doi: 10.1007/s00441-002-0664-2
32. Afanador E, Yokozeki M, Oba Y, Kitase Y, Takahashi T, Kudo A, et al. Messenger RNA expression of periostin and twist transiently decrease by occlusal hypofunction in mouse periodontal ligament. Arch Oral Biol. (2005) 50(12):1023–31. doi: 10.1016/j.archoralbio.2005.04.002
33. Aral CA, Köseoğlu S, Sağlam M, Pekbağrıyanık T, Savran L. Gingival crevicular fluid and salivary periostin levels in non-smoker subjects with chronic and aggressive periodontitis. Inflammation. (2016) 39(3):986–93. doi: 10.1007/s10753-016-0328-0
34. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. (2008) 61(4):344–9. doi: 10.1016/j.jclinepi.2007.11.008
35. Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, et al. A new classification scheme for periodontal and peri-implant diseases and conditions—introduction and key changes from the 1999 classification. J Periodontol. (2018) 89:S1. doi: 10.1002/jper.18-0157
36. Teles R, Benecha HK, Preisser JS, Moss K, Starr JR, Corby P, et al. Modelling changes in clinical attachment loss to classify periodontal disease progression. J Clin Periodontol. (2016) 43(5):426–34. doi: 10.1111/jcpe.12539
37. Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol. (2018) 89:S1. doi: 10.1002/jper.17-0721
38. Silness J, Löe H. Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. (1964) 22(1):121–35. doi: 10.3109/00016356408993968
39. Löe H, Silness J. Periodontal disease in pregnancy I. prevalence and severity. Acta Odontol Scand. (1963) 21(6):533–51. doi: 10.3109/00016356309011240
40. Hill EG, Slate EH, Wiegand RE, Grossi SG, Salinas CF. Study design for calibration of clinical examiners measuring periodontal parameters. J Periodontol. (2006) 77(7):1129–41. doi: 10.1902/jop.2006.050395
41. Pihlstrom BL. Measurement of attachment level in clinical trials: probing methods. J Periodontol. (1992) 63(12S):1072–7. doi: 10.1902/jop.1992.63.12s.1072
42. Navazesh M, Christensen CM. A comparison of whole mouth resting and stimulated salivary measurement procedures. J Dent Res. (1982) 61(10):1158–62. doi: 10.1177/00220345820610100901
43. Du J, Li M. Functions of periostin in dental tissues and its role in periodontal Tissues’ regeneration. Cell Mol Life Sci. (2017) 74(23):4279–86. doi: 10.1007/s00018-017-2645-3
44. Benatti BB, Silvério KG, Casati MZ, Sallum EA, Nociti FH. Physiological features of periodontal regeneration and approaches for periodontal tissue engineering utilizing periodontal ligament cells. J Biosci Bioeng. (2007) 103(1):1–6. doi: 10.1263/jbb.103.1
45. Beertsen W, McCulloch CAG, Sodek J. The periodontal ligament: a unique, multifunctional connective tissue. Periodontol 2000. (1997) 13(1):20–40. doi: 10.1111/j.1600-0757.1997.tb00094.x
46. Seo B-M, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. (2004) 364(9429):149–55. doi: 10.1016/s0140-6736(04)16627-0
47. Moussad EE-DA, Brigstock DR. Connective tissue growth factor: what’s in a name? Mol Genet Metab. (2000) 71(1–2):276–92. doi: 10.1006/mgme.2000.3059
48. Nazir M, Al-Ansari A, Al-Khalifa K, Alhareky M, Gaffar B, Almas K. Global prevalence of periodontal disease and lack of its surveillance. Scientific World J. (2020) 2020:1–8. doi: 10.1155/2020/2146160
49. Batchelor P. Is periodontal disease a public health problem? Br Dent J. (2014) 217(8):405–9. doi: 10.1038/sj.bdj.2014.912
50. Jamesha F, Maradi A, Chithresan K, Janakiram S, Maddur P, Rangaraju R. Comparison of gingival crevicular fluid periostin levels in healthy, chronic periodontitis, and aggressive periodontitis. J Indian Soc Periodontol. (2018) 22(6):480. doi: 10.4103/jisp.jisp_266_18
51. Castagnola M, Scarano E, Passali GC, Messana I, Cabras T, Iavarone F, et al. Salivary biomarkers and proteomics: future diagnostic and clinical utilities. Acta Otorhinolaryngol Ital. (2017) 37(2):94–101. doi: 10.14639/0392-100x-1598
52. Merle B, Garnero P. The multiple facets of periostin in bone metabolism. Osteoporos Int. (2012) 23(4):1199–212. doi: 10.1007/s00198-011-1892-7
53. Yamada S, Tauchi T, Awata T, Maeda K, Kajikawa T, Yanagita M, et al. Characterization of a novel periodontal ligament-specific periostin isoform. J Dent Res. (2014) 93(9):891–7. doi: 10.1177/0022034514543015
54. Kudo A. Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell. Cell Mol Life Sci. (2011) 68(19):3201–7. doi: 10.1007/s00018-011-0784-5
55. Tanabe H, Takayama I, Nishiyama T, Shimazaki M, Kii I, Li M, et al. Periostin associates with Notch1 precursor to maintain Notch1 expression under a stress condition in mouse cells. PLoS One. (2010) 5(8):e12234. doi: 10.1371/journal.pone.0012234
56. Maruhashi T, Kii I, Saito M, Kudo A. Interaction between periostin and BMP-1 promotes proteolytic activation of lysyl oxidase. J Biol Chem. (2010) 285(17):13294–303. doi: 10.1074/jbc.m109.088864
57. Lindsay G, Matei D, Fishman DA. Periostin secreted by epithelial ovarian carcinoma is a ligand for α(V)β(3) and α(V)β(5) integrins and promotes cell motility. Cancer Res. (2002) 62(18):5358–64.12235007
58. Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, et al. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem. (2007) 101(3):695–711. doi: 10.1002/jcb.21224
59. Balli U, Keles ZP, Avci B, Guler S, Cetinkaya BO, Keles GC. Assessment of periostin levels in serum and gingival crevicular fluid of patients with periodontal disease. J Periodontal Res. (2014) 50(6):707–13. doi: 10.1111/jre.12254
60. Bhardwaj S, Prabhuji MLV, Karthikeyan BV. Effect of non-surgical periodontal therapy on plasma homocysteine levels in Indian population with chronic periodontitis: a pilot study. J Clin Periodontol. (2015) 42(3):221–7. doi: 10.1111/jcpe.12374
61. Esfahrood ZR, Vardian ST, Yadegari Z, Adhim M, Saravi NSV. Periostin levels in saliva of patients with chronic periodontitis. J Indian Soc Periodontol. (2018) 22(1):25–7. doi: 10.4103/jisp.jisp_239_17
62. Padial-Molina M, Volk SL, Rodriguez JC, Marchesan JT, Galindo-Moreno P, Rios HF. Tumor necrosis factor-α and Porphyromonas Gingivalis lipopolysaccharides decrease periostin in human periodontal ligament fibroblasts. J Periodontol. (2013) 84(5):694–703. doi: 10.1902/jop.2012.120078.
63. Kumaresan D, Balasundaram A, Naik VK, Appukuttan DP. Gingival crevicular fluid periostin levels in chronic periodontitis patients following nonsurgical periodontal treatment with low-level laser therapy. Eur J Dent. (2016) 10(4):546–50. doi: 10.4103/1305-7456.195179
64. Hartenbach FARR, Velasquez É, Nogueira FCS, Domont GB, Ferreira E, Colombo APV. Proteomic analysis of whole saliva in chronic periodontitis. J Proteomics. (2020) 213:103602. doi: 10.1016/j.jprot.2019.103602
Keywords: biomarker, periodontitis, periostin, saliva, clinical attachment loss, salivary periostin
Citation: Padalkar P, Yadadi SS, Vivekanandan G, Shetty SR, Andhare M, Pashine A, Vinay V, Desai V and Shetty RM (2025) Salivary periostin levels as a non-invasive biomarker and their clinical correlates among healthy and periodontitis patients—a cross-sectional analytical study. Front. Dent. Med. 6:1512252. doi: 10.3389/fdmed.2025.1512252
Received: 16 October 2024; Accepted: 19 February 2025;
Published: 19 March 2025.
Edited by:
R. M. Baiju, Kerala University of Health Sciences, IndiaReviewed by:
Tonnie Mulli, University of Nairobi, KenyaCopyright: © 2025 Padalkar, Yadadi, Vivekanandan, Shetty, Andhare, Pashine, Vinay, Desai and Shetty. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raghavendra M. Shetty, cmFnaGF2ZW5kcmE3N0B5YWhvby5jb20=; ci5zaGV0dHlAYWptYW4uYWMuYWU=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.