- 1School of Dental Sciences, Universiti Sains Malaysia, Kelantan, Malaysia
- 2Department of Prosthetic Dentistry, College of Dentistry, University of Mosul, Mosul, Iraq
- 3Department of Orthodontics, Faculty of Dentistry, Ibn Al-Nafis University, Sana’a, Yemen
- 4Pharmacure Pharmacy, Azhar Private Hospital, Muscat, Sultanate of Oman
- 5Department of Cariology, Odontology School, Sahlgrenska Academy, Gothenburg University, Göteborg, Sweden
- 6Department of Applied Dental Sciences, Faculty of Applied Medical Sciences, Jordan University of Science and Technology, Irbid, Jordan
- 7Dental Research Unit, Center for Global Health Research, Saveetha Medical College and Hospital, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai, India
Introduction: Biodentine is a well-known endodontic material that is applied in various endodontic therapies. Enterococcus faecalis (E. faecalis) is associated with endodontic failure and persistent periapical infection. The purpose of this systematic review was to summarize the available evidence regarding the antibacterial activity of Biodentine against E. faecalis and to compare it to other commercial endodontic materials.
Methods: An electronic search of literature was conducted in PubMed, Scopus, Web of Science, and Google Scholar in addition to a manual search in specialized journals up to May 2024. The eligibility criteria, data extraction, and evaluation of risk of bias were assessed by two independent authors. The risk of bias was evaluated in accordance with Modified CONSORT checklist items for pre-clinical in vitro studies on dental materials.
Results: Out of 343 studies, thirty-seven fulfilled the inclusion criteria and were included in this review. Thirty studies reported a good antibacterial efficacy of Biodentine against E. faecalis. Biodentine was superior to or, at least, as efficacious as MTA, MTA Angelus, GIC, RMGIC, DiaRoot BioAggregate, NeoPutty, iRoot FS, MTA Repair HP, MTA Biorep, Well-Root PT, Activa, NeoMTA 2, Calcimol LC, TotalFill, and IRM. The findings were supported by studies with medium to high risk of bias (low quality).
Conclusions: Considering the limitations of this systematic review, there is accumulating evidence on the antibacterial activity of Biodentine against E. faecalis in context of endodontics. However, randomized clinical trials with well-designed and robust methodologies are required in order to provide information about its clinical behaviour.
1 Introduction
The success of endodontic treatment relies on an accurate diagnosis and a definitive treatment plan (1). Oral bacteria have a significant role in the development and progression of pulpal and periapical diseases, as well as in the failure of endodontic treatment (2). Most inflammatory pulpal and periapical diseases are initially treated with conservative nonsurgical treatments (3). Inadequate cleaning of the root canal and persistent/secondary intraradicular infection attributes to re-infection of the root canal, and leads to endodontic failure (4). Surgical intervention (such as apicoectomy and retrograde filling) becomes necessary to save the tooth when nonsurgical treatments have failed (3).
Certain bacteria are frequently found in infected root-filled teeth (5). E. faecalis is commonly detected in the cases of failed endodontic treatment (5), with a percentage rate of 77% (6). E. faecalis is a facultatively anaerobic Gram-positive coccus (7) that exhibits high resistance to antimicrobial agents and tolerates low-nutrient and highly alkaline environments (7–9). In addition, it has the ability to penetrate dentinal tubules (10) and form biofilms on the root canal walls (11).
Following endodontic treatment of chronic periodontal disease, E. faecalis is often associated with persistent intra-radicular and extra-radicular infections (12), although recent evidence indicates that it is not considered the key pathogen in root canal infection (13). The short-term application of intracanal dressing is often insufficient in eradicating bacteria, as the medicament fails to reach the intended sites (14). Furthermore, the inflammation can be efficiently reduced by antibiotics in acute or chronic apical periodontitis (15); however, a complete relief is often hindered by the presence of unreachable bacteria within the canal system (16).
Surgical intervention is the treatment of choice if conventional endodontic treatment/re-treatment fails to save the tooth. This approach involves the removal of persistent pathogens by debridement of infected periradicular tissue, resection of root-end (apicoectomy), and obturation of retrograde root canal (root-end filling) (17). If there are remaining intracanal bacteria, the tight root-end filling will seal the apical termination of root canal and encases the remaining bacteria (18). Therefore, it is important for the root-end filling materials to have antibacterial properties.
Biodentine is one of the well-known root-end filling materials which has drawn attention in recent years. It was introduced by Septodont in 2009 as a dentin replacement material. The powder consists of tricalcium, dicalcium silicate, calcium carbonate and oxide filler, iron oxide shade, and zirconium oxide (as radiopacifier). The liquid mainly contains calcium chloride in an aqueous solution (as an accelerator) with an admixture of hydrosoluble polymer (as a water reducing agent) (19). The hydration of the calcium silicate components leads to the formation of calcium silicate hydrate and calcium hydroxide, the latter of which directly promotes antimicrobial effects (20). The studies have shown that Biodentine is biocompatible (21), stimulates odontoblast differentiation (22) and reparative dentin formation (23, 24), and has an adequate sealing ability (25). Therefore, Biodentine is considered a suitable material for application in various endodontic therapies including surgical endodontics (19).
Many studies have investigated the antibacterial activity of this material against E. faecalis. However, there is a conflicting overview of the existing findings to determine the effectiveness of Biodentine against E. faecalis. This review aims to systematically summarize the available evidence on the antibacterial activity of Biodentine material against E. faecalis and to compare it to other commercial endodontic materials.
2 Methodology
2.1 Review question
The following PICOS guided the formulation of the research question: P (population): Bacterial cultures of E. faecalis; I (intervention): Biodentine; C (comparators) commercial endodontic materials; O (outcomes) Inhibition or reduction in bacterial growth; S (study design) all relevant in vitro and in vivo studies. Based on the PICOS components, the review question is: Is Biodentine effective in inhibiting the growth of E. faecalis, and how does its effectiveness compare to that of other commercial endodontic materials?
The null hypothesis for this review is: Biodentine exhibits antibacterial activity against E. faecalis that is not significantly different from, or superior to, other commercial endodontic materials.
2.2 Literature search
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) (26).
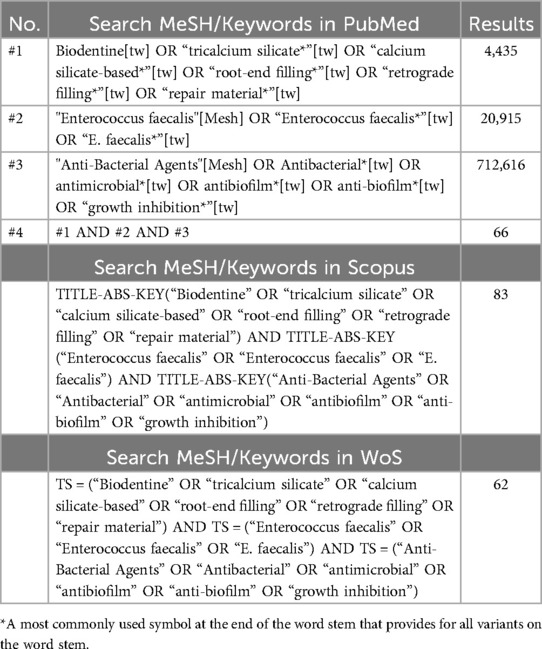
A comprehensive literature search was conducted in March 2024 (and updated in May 2024) in four electronic databases, PubMed, Scopus, Web of Science, and Google Scholar, for the studies published since 2009 (the date of Biodentine introduction) up to May 2024. A combination of search keywords and Medical Subject Headings (MeSH) terms with Boolean operators (AND, OR) was used, as shown in Table 1. In addition, a manual search was conducted in the following dental materials- and endodontics-related journals: International Endodontic Journal, Journal of Endodontics, Australian Endodontic Journal, Endodontology, Dental Materials, Dental Materials Journal, and Oral Surgery, Oral Medicine, Oral Pathology, and Oral Radiology, to find out articles that did not appear in the electronic search of the above database outcome.
2.3 Inclusion criteria
This review included all the studies in the English language, conducted in vivo on both human and animal subjects as well as in vitro on any type of laboratory model.
2.4 Exclusion criteria
Studies were excluded based on the following criteria: studies that evaluated the antibacterial activity of Biodentine against bacterial species other than E. faecalis; studies that investigated the response of E. faecalis to endodontic materials other than Biodentine; studies involving E. faecalis mixed with other bacterial species; studies assessing modified versions of Biodentine; studies that examined the dentin/Biodentine interface; and review studies, case reports, and case series.
2.5 Study selection and data extraction
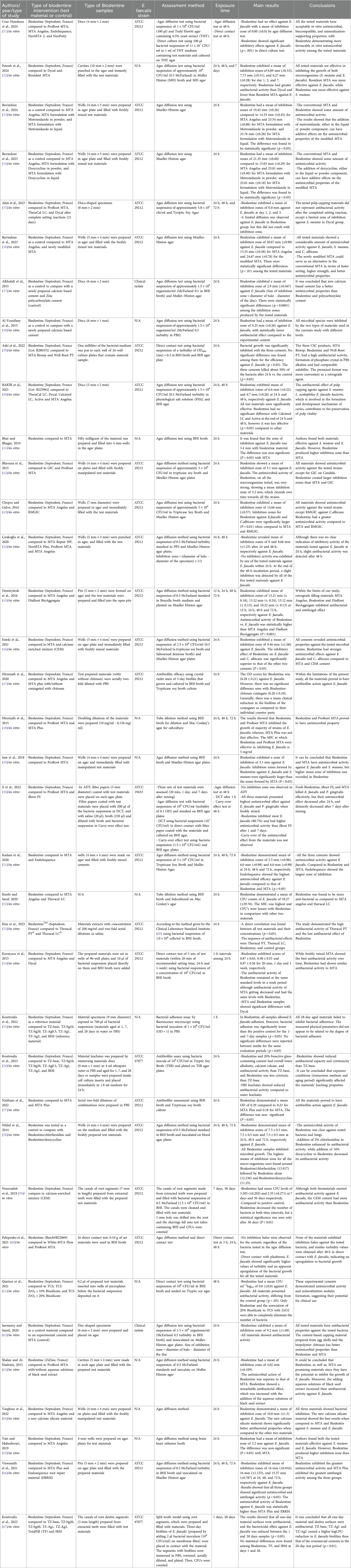
In accordance with PRISMA guidelines, two authors (HS and NS) independently screened the included articles and extracted the necessary information. Initially, the title and abstract of each article were assessed and the appropriate studies were retrieved and then thoroughly and carefully examined for eligibility and inclusion in the review. EndNote X8 (Clarivate Analytics, PA, USA) was used to eliminate all duplicated studies and manage the study citations list. In a case of discrepancy between the authors, a discussion was done with a third author (AT) and came to a decision. After screening the included studies, the following data were extracted: authors, year and type of study, type of Biodentine intervention, type of Biodentine samples, E. faecalis strain, assessment method, exposure time, main results, and conclusion.
A narrative synthesis was performed to address the diversity in study designs, interventions, and outcomes. The studies were categorized based on their methodologies, type of Biodentine intervention, and the results of the antibacterial activity against E. faecalis. The findings were qualitatively summarized, with a focus on identifying shared patterns and notable discoveries across the studies.
2.6 Assessment of risk of bias
The quality and risk of bias of the included studies were assessed independently by two authors (HS and SA) in accordance with Modified CONSORT checklist items for pre-clinical in vitro studies on dental materials [Figgion et al. (27)]. The criteria included eight domains: intervention, outcomes, sample size calculation, specimen randomization, implementation, operator blinded, statistical analysis, and results (outcomes and estimation). During the assessment process, each domain was reported as YES if the corresponding parameter was explicitly described or NO if the parameter was absent or not fully declared. The third author (AT) resolved the discrepancy between the two authors. The overall risk of bias for each study was determined based on the number of “YES” as: 1–3 refers to high bias; 4–6 refers to medium bias; and 7–8 refers to low bias.
3 Results
The search in electronic databases retrieved a total of 343 articles [PubMed = 66, Scopus = 83, Web of Science = 62, Google scholar = 120 (top 120 relevant studies), and hand searching in journals = 12]. By the electronic de-duplication, 148 studies were excluded. Then, an independent and comprehensive reading of the titles and abstracts of the remaining 195 articles were performed and 155 articles which did not meet the inclusion criteria were excluded. After retrieving the articles and thoroughly examined them for inclusion and eligibility, three articles were excluded with reasons. Finally, the remaining 37 studies that fulfilled the inclusion criteria were included in this review study. The excluded full-text articles (n = 3) were due to the following reasons: E. faecalis was mixed with other bacterial species forming dual and multispecies biofilm models (28); A modified Biodentine was used in the antibacterial activity assessment (29); and the absence of a pure Biodentine control (30). The strategy search in the electronic database in this review study has been summarized in Figure 1.
3.1 Risk of bias
As illustrated in Table 2, none of the included studies met all the criteria of risk of bias. Of the 37 studies in this systematic review, only 5 studies (13.51%) had a medium risk of bias, whereas the remaining 32 studies (86.48%) showed a high risk of bias. Most of the studies failed or did not clearly describe the sample size calculation, specimen randomization, implementation, and operator blinded parameters.

Table 2. Risk of bias of the studies in accordance with modified CONSORT checklist [figgion et al. (27)].
3.2 General characteristics and assessment methods
The characteristics and details of the included studies are summarized in Table 3. All the 37 studies included in this review article were in vitro studies (31–67) and no in vivo studies were found. Of these studies, twenty-three studies employed an agar diffusion test (ADT) for assessing the antibacterial activity (32–38, 40–46, 49, 51, 58, 60, 62–66) by measuring the inhibition zone around the test material. Three studies used direct contact test (DCT) to count the colony forming units (CFUs) (39, 61) or optical density (OD) (54). Three studies used antibiofilm assay reading the OD (47, 57) or Log (CFU + 1)/ml (56) of adherent stained biofilm. Two studies used the tube dilution method (48, 52), recording the minimal inhibitory concentration (MIC), and one study used bacterial adhesion assay (55) by a confocal laser scanning microscope to image the viable bacteria. One used the broth dilution method (53) to read the OD. The study by Ji et al. (50) used three methods, ADT, DCT, and carry-over effect test, while Nourzadeh et al. (59), and Koutroulis et al. (67), used the dentine block model to test the antibacterial activity of test materials and CFUs were counted. The study by Cruz Hondares et al. (31), employed two methods, ADT and direct culture test.
3.3 Biodentine intervention in the included studies
Twenty-three studies investigated the antibacterial activity of Biodentine compared to other commercial endodontic materials such as MTA Biorep, Well-Root PT, TheraCal LC, Theracal PT, Dycal, Calcimol LC, Activa, iRoot FS, Endosequence root repair material (ERRM), ProRoot MTA, MTA Angelus, MTA, MTA Repair HP, NeoMTA Plus, NeoMTA 2, NeoPutty, White-MTA Flow, MTA Plus, GIC, RMGIC, Rootdent MTA, DiaRoot BioAggregate and Calcium-enriched mixture (CEM) (31, 32, 35, 39–46, 48–54, 57, 59, 60, 65, 66), whereas fourteen studies investigated the antibacterial activity of Biodentine as a control/reference material compared to experimental materials (33, 34, 36–38, 47, 55, 56, 58, 61–64, 67).
3.4 Effectiveness of biodentine against E. faecalis
Of the thirty-seven studies included in this review article, thirty studies reported a good antibacterial activity of Biodentine against E. faecalis (32–34, 36, 38–43, 45–49, 51–59, 61–66), one study reported a limited antibacterial activity of Biodentine (37), four studies reported a conflicting antibacterial activity, whereas Ji et al. (50) study exhibited no bacterial inhibition for Biodentine using ADT and carry-over effect test while a good antibacterial activity was shown using DCT, Cruz Hondares et al. (31) found no antibacterial activity using ADT, while a significant inhibitory effect was detected using direct culture test, a study by Çırakoğlu et al. (44) revealed no bacterial inhibition at 24 h while a limited antibacterial activity was found at 48 h, and a study by Koutroulis et al. (67) revealed antibacterial activity at 24 h, while no bacterial count reduction was observed at 28 days. Only two studies reported negative antibacterial activity of Biodentine against E. faecalis using both ADT and DCT (60) and ADT (35).
The antibacterial activity of Biodentine at various time intervals has been conducted in thirteen studies (32, 35, 40, 44, 45, 48, 51, 54, 58–60, 66, 67). In three studies, there was no statistically significant difference (p > 0.05) in the antibacterial activity of Biodentine against E. faecalis at different time intervals (40, 48, 66). Other studies reported an increased antibacterial activity (P < 0.01) at 30 days compared to 7 days (59), and at 48 h compared to 24 h (44). However, a reduction was reported in two studies at 72 h compared to 12, 24, and 48 h (P < 0.001) (45), and in 24 h compared to 28 days (p < 0.05) (67). One study (32) reported increased antibacterial activity at 48 h compared to 24 h, followed by a decrease at day 7 (p = .012). The level of Biodentine's antibacterial activity remained nearly in same level across three other studies (51, 54, 58), while two studies reported no antibacterial activity (35, 60).
The antibacterial activity of different time interval-aged Biodentine against E. faecalis has been shown in two studies (55, 56). A study by Koutroulis et al. (55) revealed no significant difference (p > 0.05) in the antibacterial activity of Biodentine aged for 1, 7, and 28 days in water or FBS. While, a study by Koutroulis et al. (56) found that bacterial inhibition in water leachates increased over time when the medium was not refreshed (days 7–28) but decreased when the medium was changed. However, FBS leachates demonstrated lower antibacterial activity compared to water leachates.
3.5 The antibacterial activity of biodentine in comparison to commercial endodontic materials
Eleven studies investigated the antibacterial efficacy of Biodentine compared to MTA against E. faecalis (31, 35, 41, 42, 48–51, 57, 62, 63). The studies reported a significant (p < 0.05) superiority of Biodentine in killing E. faecalis in 3 studies (48, 49, 63), and non-significantly (p > 0.05) in 5 studies (31, 41, 42, 50, 51), which indicates a clinical reduction in bacterial growth. Whereas, Biodentine was inferior in bacterial inhibition compared to MTA in 2 studies, significantly (p < 0.05) in one study (62), and non-significantly (p > 0.05) in one study (57). A study by Akin et al. (35) demonstrated no antibacterial efficacy for Biodentine and MTA after a complete setting reaction.
The comparison of Biodentine and MTA Angelus against E. faecalis appeared in 13 studies. Biodentine exhibited significantly (p < 0.05) greater antibacterial activity in 8 studies (31, 33, 34, 36, 43, 45, 46, 52) and non-significantly (p > 0.05) in 2 studies (64, 65), whereas MTA Angelus exhibited significantly (p < 0.05) greater antibacterial activity in one study (40) and non-significantly (p > 0.05) in 1 study (47). One study (54) reported lower (p > 0.05) antibacterial activity for Biodentine at immediate time point and day 1, and higher (p > 0.05) activity at 1 week compared to MTA Angelus.
Four studies investigated the effectiveness of MTA Plus and Biodentine against E. faecalis (47, 48, 57, 66). Of these studies, two studies (48, 66) reported better antibacterial activity (p < 0.05) for Biodentine compared to MTA Plus. Whereas, two studies (47, 57) showed that MTA Plus was superior to Biodentine with no significant difference (p > 0.05). Two studies evaluated the effectiveness of CEM in comparison to Biodentine against E. faecalis (46, 59). One study (46) reported significantly (p < 0.05) better antibacterial activity for Biodentine, and one study (59) reported that Biodentine was significantly (p < 0.05) inferior compared to CEM. In addition, of the 3 studies investigated ERRM in comparison to Biodentine against E. faecalis (31, 51, 66). Biodentine exhibited significantly (p < 0.05) better antibacterial activity in one study (66), and non-significantly (p > 0.05) in one study (31); whereas, one study (51) reported that ERRM had significantly (p < 0.05) better antibacterial activity than Biodentine.
The antibacterial activity of Biodentine compared to IRM was reported in three studies (55, 56, 67). In two studies (55, 67), Biodentine demonstrated antibacterial activity similar (p > 0.05) to that of IRM. In the other study (56), Biodentine exhibited statistically higher antibacterial activity in leachates prepared over 28 days without a medium change, similar activity to IRM in leachates prepared over 28 days with weekly medium changes, and lower antibacterial activity in leachates prepared in the cell culture insert (0–24 h). One study by Koutroulis et al. (67), compared the antibacterial activity of Biodentine and TotalFill, finding that both exhibited similar (p > 0.05) antibacterial effects at 1 and 28 days.
Four studies evaluated TheraCal LC against E. faecalis and compared to Biodentine (35, 40, 52, 53). One study (52) showed that Biodentine had greater antibacterial activity than TheraCal LC, with no significant difference (p > 0.05). Whereas TheraCal LC had significantly (p < 0.05) better antibacterial activity in one study (40) and non-significantly (p > 0.05) in one study (53). Furthermore, the study by Akin et al. 2024 (35) reported no antibacterial activity for TheraCal LC and Biodentine after the complete setting reaction. Additionally, the antibacterial activity of Biodentine was compared to Dycal in 4 studies (32, 35, 40, 54). Biodentine had significantly (p < 0.05) greater antibacterial activity than Dycal at different time intervals in one study (32). In addition, Biodentine was non-significantly (p > 0.05) superior in inhibiting bacterial growth immediately and 24 h and significantly (p < 0.05) at 1 week in one study (54), whereas, Dycal had significantly (p < 0.05) higher antibacterial activity in one study (40) at 24 h and 48 h. The study by Akin et al. (35) revealed no antibacterial activity for Dycal and Biodentine after a complete setting reaction. Furthermore, the antibacterial efficacy was significantly (p < 0.05) superior in Biodentine compared to GIC (42), RMGIC (43), DiaRoot BioAggregate (45), NeoPutty (31), and iRoot FS (50). Biodentine had greater antibacterial activity with non-significant differences (p > 0.05) compared to MTA Repair HP (44), MTA Biorep (39), Well-Root PT (39), Activa (40), NeoMTA 2 (31), and Calcimol LC (40). Finally, the antibacterial activity was significantly (p < 0.05) inferior in Biodentine compared to Theracal PT (53), Rootdent MTA (32), and Zinc polycarboxylate cement (37).
4 Discussion
Endodontic failure is generally attributed to the lack of proper cleaning of the root canal system and leakage of bacteria into the periradicular tissues. When the infection persists after endodontic treatment/re-treatment, an apicoectomy is indicated and a root-end filling material is placed to prevent re-infection of the root canal. Therefore, the antibacterial activity of endodontic materials is essential for the treatment success in order to prevent or delay infection and extend the lifetime of restorations. Biodentine, a calcium silicate-based material, has gained popularity in recent years due to its various clinical applications, including root-end filling procedures (69, 70). However, there is a shortage of studies and conflicting results regarding the antibacterial activity of Biodentine (71).
E. faecalis is an anaerobic bacterium associated with endodontic failure and persistent periapical infection (72). Although it is not part of the root canal system's microbial flora (73), it is discovered in the oral cavity from contaminated food. It was suggested that this bacterium penetrates the root canal system through several ways, including the lack of coronal seal after root canal treatment, dentinal fractures, carious progression in contiguity with the root canal system during pulp necrosis or inflammation, bloodstream, and through root fracture or lateral canals (74). This species has been reported to exhibit varying degrees of resistance to several antimicrobial agents (75) and intracanal dressings such as calcium hydroxide (76), making its eradication from the root canals challenging. This review focused on the studies that investigated the antibacterial activity of Biodentine against E. faecalis, as it is one of the main bacteria and most commonly involved in studies on persistent periapical infections (13). Although a recently published review article (71) identified the antimicrobial efficacy of Biodentine, it provided brief coverage and limited details regarding its specific efficacy against E. faecalis.
The current review revealed that Biodentine has good antibacterial activity against E. faecalis, with thirty out of 37 included studies reporting positive effectiveness. One study exhibited limited effectiveness, four showed conflicting results, and only two demonstrated negative effectiveness. The outcomes of the included studies appear to have minor discrepancies likely due to their different methodologies such as assessment methods, concentration of the microorganism, and the amount of test materials. The result of ADT is semi-quantitative and depends on the solubility and diffusability of the material within the agar medium (77). Solid endodontic materials may not be diffusible (50). The carry-over effect may also be affected by the insolubility of the material (50). Whereas DCT is a quantitative and reproducible method allowing testing of water-insoluble materials and accurately mimics the contact between the materials and microorganisms (54, 77). It demonstrates the material's bactericidal or bacteriostatic effects regardless of its diffusibility in the medium (78). Therefore, ADT is less sensitive in evaluating the antibacterial activity of calcium silicate-based cement compared to direct culture test (31) and DCT (50), which explains the conflicting outcomes between the ADT and other evaluating methods in the same study.
The antibacterial effect of Biodentine is mainly attributed to its alkalinity and calcium release. The cement hydration process generates a colloidal gel and releases calcium hydroxide, which inhibits bacterial growth. Additionally, as Biodentine sets, its pH rises to 12.5 which prevents bacterial growth and disinfects the surrounding area (46, 79). Moreover, it was reported that Biodentine inhibits microbial adherence, resulting in a strong antibacterial activity (79).
For the persistence of the antibacterial activity of Biodentine at different time intervals, conflicting evidence was reported among the included studies, where 3 out of 13 studies demonstrated statistical similarities over time, 2 demonstrated an increase in antibacterial activity, and 3 demonstrated nearly the same level. One demonstrated an increase followed by a decreased antibacterial activity. While a reduction was reported in 2 studies, and no bacterial inhibition in 2 studies. The antibacterial activity of Biodentine against E. faecalis appears to be influenced by both aging time and the medium used, with conflicting results between studies. One study exhibited no difference in antibacterial activity across different aging periods in either water or FBS, while another study found that bacterial inhibition in water leachates increased over time when the medium was not refreshed but decreased when refreshed. Additionally, FBS leachates showed lower antibacterial activity compared to water.
This review revealed that Biodentine was superior to or, at least, as efficacious as several commercial endodontic materials against E. faecalis such as MTA [in 8 (31, 41, 42, 48–51, 63) out of 11 studies], MTA Angelus [in 11 (31, 33, 34, 36, 43, 45, 46, 52, 54, 64, 65) out of 13 studies], GIC (42), RMGIC (43), DiaRoot BioAggregate (45), NeoPutty (31), iRoot FS (50), MTA Repair HP (44), MTA Biorep (39), Well-Root PT (39), Activa (40), NeoMTA 2 (31), Calcimol LC (40), TotalFill (67), and IRM (55, 56, 67). Whereas, the antibacterial activity of Biodentine was inferior to Theracal PT (53), Rootdent MTA (32), and Zinc polycarboxlate cement (37). Furthermore, it was not feasible to reach a summary on the comparative antibacterial effects of Biodentine in comparison to MTA Plus, CEM, ERRM, TheraCal LC, and Dycal because of the conflicting results among the included studies.
4.1 Limitations and strengths of the study
The current evidence indicates that Biodentine is a superior material in endodontic treatment with potent antibacterial activity against E. faecalis. However, this evidence lacks a sufficient clinical base to support Biodentine for routine use in inhibiting E. faecalis growth. All the included studies and collected data were in vitro, which is unreliable in determining Biodentine's clinical potential. Although in vitro studies with high-quality and well-designed methodology offer helpful solutions for clinical issues, randomized controlled clinical trials reveal the most dependable and robust outcomes (80). Another limitation of this review was the use of various antibacterial assessment methods (such as ADT, DCT, antibiofilm assay, tube dilution method, bacterial adhesion assay, broth dilution method, carry-over effect test, direct culture test, and dentine block model), and heterogeneity in their procedures among the studies [such as bacterial strains, evaluation times, amount of test materials sample, and various measurements (CFUs and OD) used in DCT and antibiofilm assay]. The lack of standardization and evaluation criteria among the included studies caused the cross-study comparison to be hard to execute, and ultimately conducting a meta-analysis was not practical (81). It is important to highlight the necessity of developing standardized methods for the evaluation of antibacterial activity. A further limitation was that some studies examined the antibacterial activity of Biodentine on planktonic bacteria (such as tube dilution method, broth dilution method, direct culture test, and carry-over effect test). This model does not closely resemble in vivo or clinical circumstances because the bacteria are present as complex biofilm communities in the oral cavity (82). Contrastingly to planktonic cells, biofilm structures provide resistance against antimicrobial agents as bacteria are embedded in a hydrated polymeric matrix that serves as a shield to protect bacterial growth (83, 84). The methodological quality evaluation in this review indicated that the findings were supported by studies with medium to high risk of bias (low quality). Lastly, even though the protocol of this study was not registered, the PRISMA guidelines were strictly followed.
This review provides several key strengths that enhance the validity and reliability of the study, including a comprehensive literature search in reputable databases that ensures a broad coverage of relevant studies. The review also employed clear eligibility criteria and rigorously evaluated the risk of bias using the Modified CONSORT checklist items for pre-clinical in vitro studies on dental materials, to ensure the reliability of the findings. In addition, it offers a comparative analysis of Biodentine's antibacterial effect relative to other commercial endodontic materials against E. faecalis, providing a more relevant understanding of Biodentne's relative efficacy in clinical practice. Finally, the main finding of this study, the strong antibacterial activity of Biodentine in inhibiting the growth of E. faecalis, was strongly supported by a substantial number of studies demonstrating favorable effects against E. faecalis. This consistent evidence highlights the Biodentine's significant potential as an endodontic material and supports the acceptance of the null hypothesis of the review.
4.2 Implications of the results for biodentine's clinical practice
The results of this systematic review suggest that Biodentine's strong antibacterial activity against E. faecalis has significant implications for clinical practice. Clinicians can confidently use Biodentine to help prevent bacterial infection, promote healing, and improve treatment outcomes, particularly in cases with persistent or resistant infections. In addition, it may help prevent re-infection and treatment failure in these cases. Its use could also aid in reducing inflammation, leading to faster recovery time and fewer post-treatment complications, such as post-operative infections or flare-ups, thereby enhancing endodontic treatments. Its comparability or superiority to other materials, such as MTA and GIC, supports its potential as a preferred choice in clinical applications, including root-canal sealing and apical surgery. This is further reinforced by its favorable biological and physicochemical properties, as well as its cost-effectiveness. The findings could also guide future clinical trials, inform material selection, and help clinicians optimize infection control in endodontic procedures for better patient care.
Overall, the current review provides clear evidence that Biodentine has a strong efficacy in inhibiting the growth of E. faecalis. In addition, most of the studies supported that the antibacterial activity of Biodentine increases or stays nearly at the same level over time. Furthermore, Biodentine was superior to or, at least, as efficacious as MTA, MTA Angelus, GIC, RMGIC, DiaRoot BioAggregate, NeoPutty, iRoot FS, MTA Repair HP, MTA Biorep, Well-Root PT, Activa, NeoMTA 2, Calcimol LC, TotalFill, and IRM.
5 Conclusion
Considering the limitations of this systematic review, there is accumulating evidence of the antibacterial activity of Biodentine against E. faecalis in the context of endodontics. However, randomized clinical trials with well-designed and robust methodologies are required to provide information about its clinical behaviour. The findings were supported by studies with medium to high risk of bias (low quality). There is a demand for low-risk-of-bias studies to further evaluate the finding's reliability. Furthermore, it is highly suggested to develop standardized methods to evaluate the antibacterial efficacy of endodontic materials in in vitro investigations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
HS: Methodology, Writing – review & editing, Writing – original draft, Conceptualization, Formal Analysis, Investigation, Project administration, Supervision. NS: Writing – original draft, Conceptualization, Formal Analysis, Investigation, Supervision, Writing – review & editing. SA: Writing – original draft, Writing – review & editing. MSA: Data curation, Writing – review & editing. AT: Methodology, Writing – review & editing, Formal Analysis.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
HS, Hasan Subhi; NS, Nashwah Subhi; SA, Salah Alhaidary; MSA, Mahmood S. Azeez; AT, Abedelmalek Tabnjh; ADT, agar diffusion test; CEM, calcium-enriched mixture; CFUs, colony forming units; DCT, direct contact test; E. faecalis, Enterococcus faecalis; ERRM, endosequence root repair material; GIC, glass ionomer cement; MeSH, medical subject headings; MIC, minimal inhibitory concentration; MTA, mineral trioxide aggregate; OD, optical density; PRISMA, preferred reporting items for systematic reviews and meta-analyses; RMGIC, resin-modified glass ionomer cement.
References
1. Yeng T, Messer H, Parashos P. Treatment planning the endodontic case. Aust Dent J. (2007) 52:S32–7. doi: 10.1111/j.1834-7819.2007.tb00523.x
2. Tabassum S, Khan FR. Failure of endodontic treatment: the usual suspects. Eur J Dent. (2016) 10(01):144–7. doi: 10.4103/1305-7456.175682
3. Fernandes M, de Ataide I. Nonsurgical management of periapical lesions. J Conserv Dent. (2010) 13(4):240. doi: 10.4103/0972-0707.73384
4. Siqueira JF Jr. Aetiology of root canal treatment failure: why well-treated teeth can fail. Int Endod J. (2001) 34(1):1–10. doi: 10.1046/j.1365-2591.2001.00396.x
5. Rôças IN, Jung IY, Lee CY, Siqueira JF Jr. Polymerase chain reaction identification of microorganisms in previously root-filled teeth in a south Korean population. J Endod. (2004) 30(7):504–8. doi: 10.1097/00004770-200407000-00011
6. Siqueira JF Jr, Rôças IN. Polymerase chain reaction–based analysis of microorganisms associated with failed endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2004) 97(1):85–94. doi: 10.1016/S1079-2104(03)00353-6
7. Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J Endod. (2006) 32(2):93–8. doi: 10.1016/j.joen.2005.10.049
8. Sedgley C, Lennan S, Clewell D. Prevalence, phenotype and genotype of oral enterococci. Oral Microbiol Immunol. (2004) 19(2):95–101. doi: 10.1111/j.0902-0055.2004.00122.x
9. Portenier I, Haapasalo H, Ørstavik D, Yamauchi M, Haapasalo M. Inactivation of the antibacterial activity of iodine potassium iodide and chlorhexidine digluconate against Enterococcus faecalis by dentin, dentin matrix, type-I collagen, and heat-killed microbial whole cells. J Endod. (2002) 28(9):634–7. doi: 10.1097/00004770-200209000-00002
10. Haapasalo M, Ørstavik D. In vitro infection and of dentinal tubules. J Dent Res. (1987) 66(8):1375–9. doi: 10.1177/00220345870660081801
11. Wang L, Dong M, Zheng JB, Song QY, Yin W, Li Y, et al. Relationship of biofilm formation and gelE gene expression in Enterococcus faecalis recovered from root canals in patients requiring endodontic retreatment. J Endod. (2011) 37(5):631–6. doi: 10.1016/j.joen.2011.02.006
12. Zehnder M, Guggenheim B. The mysterious appearance of enterococci in filled root canals. Int Endod J. (2009) 42(4):277–87. doi: 10.1111/j.1365-2591.2008.01537.x
13. Swimberghe R, Coenye T, De Moor R, Meire M. Biofilm model systems for root canal disinfection: a literature review. Int Endod J. (2019) 52(5):604–28. doi: 10.1111/iej.13050
14. Sjögren U, Figdor D, Spångberg L, Sundqvist G. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int Endod J. (1991) 24(3):119–25. doi: 10.1111/j.1365-2591.1991.tb00117.x
15. Braz-Silva PH, Bergamini ML, Mardegan AP, De Rosa CS, Hasseus B, Jonasson P. Inflammatory profile of chronic apical periodontitis: a literature review. Acta Odontol Scand. (2019) 77(3):173–80. doi: 10.1080/00016357.2018.1521005
16. Dioguardi M, Di Gioia G, Illuzzi G, Arena C, Caponio VCA, Caloro GA, et al. Inspection of the microbiota in endodontic lesions. Dent J. (2019) 7(2):47. doi: 10.3390/dj7020047
17. Verma J, Ahuja V. Apicoectomy—a review. J Dent Panacea. (2021) 3(1):15–9. doi: 10.18231/j.jdp.2021.004
18. Wu MK, Dummer P, Wesselink P. Consequences of and strategies to deal with residual post-treatment root canal infection. Int Endod J. (2006) 39(5):343–56. doi: 10.1111/j.1365-2591.2006.01092.x
19. Malkondu Ö, Kazandağ MK, Kazazoğlu E. A review on biodentine, a contemporary dentine replacement and repair material. BioMed Res Int. (2014) 2014. doi: 10.1155/2014/160951
20. Estrela C, Cintra LTA, Duarte MAH, Rossi-Fedele G, Gavini G, Sousa-Neto MD. Mechanism of action of bioactive endodontic materials. Braz Dent J. (2023) 34(1):1–11. doi: 10.1590/0103-6440202305278
21. Tomás-Catalá CJ, Collado-González M, García-Bernal D, Oñate-Sánchez RE, Forner L, Llena C, et al. Biocompatibility of new pulp-capping materials NeoMTA plus, MTA repair HP, and biodentine on human dental pulp stem cells. J Endod. (2018) 44(1):126–32. doi: 10.1016/j.joen.2017.07.017
22. Jung JY, Woo SM, Lee BN, Koh JT, Nor JE, Hwang YC. Effect of biodentine and bioaggregate on odontoblastic differentiation via mitogen-activated protein kinase pathway in human dental pulp cells. Int Endod J. (2015) 48(2):177–84. doi: 10.1111/iej.12298
23. Nowicka A, Wilk G, Lipski M, Kolecki J, Buczkowska-Radlinska J. Tomographic evaluation of reparative dentin formation after direct pulp capping with ca (OH) 2, MTA, biodentine, and dentin bonding system in human teeth. J Endod. (2015) 41(8):1234–40. doi: 10.1016/j.joen.2015.03.017
24. Nowicka A, Lipski M, Parafiniuk M, Sporniak-Tutak K, Lichota D, Kosierkiewicz A, et al. Response of human dental pulp capped with biodentine and mineral trioxide aggregate. J Endod. (2013) 39(6):743–7. doi: 10.1016/j.joen.2013.01.005
25. Solanki NP, Venkappa KK, Shah NC. Biocompatibility and sealing ability of mineral trioxide aggregate and biodentine as root-end filling material: a systematic review. J Conserv Dent. (2018) 21(1):10. doi: 10.4103/JCD.JCD_45_17
26. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
27. Faggion CM Jr. Guidelines for reporting pre-clinical in vitro studies on dental materials. J Evid Based Dent Pract. (2012) 12(4):182–9. doi: 10.1016/j.jebdp.2012.10.001
28. Jerez-Olate C, Araya N, Alcántara R, Luengo L, Bello-Toledo H, González-Rocha G, et al. In vitro antibacterial activity of endodontic bioceramic materials against dual and multispecies aerobic-anaerobic biofilm models. Aust Endod J. (2022) 48(3):465–72. doi: 10.1111/aej.12587
29. Elsaka SE, Elnaghy AM, Mandorah A, Elshazli AH. Effect of titanium tetrafluoride addition on the physicochemical and antibacterial properties of biodentine as intraorfice barrier. Dent Mater. (2019) 35(1):185–93. doi: 10.1016/j.dental.2018.11.019
30. Patri G, Bansal S, Lath H, Chatterjee I, Majee N, Sinha Y. Comparative evaluation of the antibacterial efficacy of two experimental calcium silicate-based intracanal medicaments: an in vitro study. J Conserv Dent Endod. (2024) 27(4):419–23. doi: 10.4103/JCDE.JCDE_74_24
31. Cruz Hondares T, Hao X, Zhao Y, Lin Y, Napierala D, Jackson JG, et al. Antibacterial, biocompatible, and mineralization inducing properties of calcium silicate-based cements. Int J Paediatr Dent. (2024) 34(6):843–52. doi: 10.1111/ipd.13185
32. Fetouh ME, Noaman KM, Abed Elghany AA. Antimicrobial evaluation of different pulp capping materials. Al-Azhar J Dent Sci. (2024) 27(1):1–9. doi: 10.21608/ajdsm.2022.158675.1369
33. Ravindran V, Jeevanandan G, Veeraraghavan VP, El-Sherbiny M, Bohairi AL, Mardini YR, et al. Comparative evaluation of physical and antimicrobial properties of metronidazole incorporated formulation of mineral trioxide aggregate an in vitro study. J Int Dent Med Res. (2024) 17:127–35.
34. Ravindran V, Jeevanandan G, Veeraraghavan VP, El-Sherbiny M, Alnamly JM, Nawaf SA, et al. Comparative evaluation of physical and antimicrobial properties of doxycycline incorporated formulation of mineral trioxide aggregate-an in vitro study. J Int Dent Med Res. (2023) 16(4):1501–9.
35. Akin D, Ates M, Atalayin Özkaya Ç. Antibacterial activity of different pulp capping materials after completed setting reaction. Ege Üniv Dis Hekim Fak Derg. (2023) 44(2):109–15. doi: 10.5505/eudfd.2023.24392
36. Ravindran V, Jeevanandan G. Comparative evaluation of the physical and antimicrobial properties of mineral trioxide aggregate, biodentine, and a modified fast-setting mineral trioxide aggregate without tricalcium aluminate: an in vitro study. Cureus. (2023) 15(8):e42856. doi: 10.7759/cureus.42856
37. Alkhalidi EF, Alsalman TH, Taqa AA. Antibacterial properties of new calcium based cement prepared from egg shell. Edorium J Dent. (2015) 2:21–8. doi: 10.5348/D01-2015-6-OA-4
38. Al-Yousifany NN, Chakmakchi M, Taqa AA. Preparation and evaluation of antimicrobial activity of novel calcium based dental cement. Int J Enhanced Res Sci Technol Eng. (2015) 4(3):99–105.
39. Ashi T, Mancino D, Hardan L, Bourgi R, Zghal J, Macaluso V, et al. Physicochemical and antibacterial properties of bioactive retrograde filling materials. Bioengineering. (2022) 9(11):624. doi: 10.3390/bioengineering9110624
40. Bakir EP, Bakir Ş, Samican Ü. Comparison of antibacterial effects of pulp capping materials. Selcuk Dent J. (2021) 8(2):553–60. doi: 10.15311/selcukdentj.896007
41. Bhat SA, Bhagat RK. Evaluation of antibacterial efficiency of different pulp capping materials method against E. faecalis and S. mutans: an in vitro study. Int J Appl Dent Sci. (2019) 5(3):13–5.
42. Bhavna V, Chaitanya KP, Gandi P, Patel J, Dola B, Reddy RB. Evaluation of antibacterial and antifungal activity of new calcium-based cement (biodentine) compared to MTA and glass ionomer cement. J Conserv Dent. (2015) 18(1):44–6. doi: 10.4103/0972-0707.148892
43. Chopra MS, Gulve MN. Evaluation of the antibacterial and antifungal activity of three retrograde filling materials: an in vitro study. Int J Contemp Med Res. (2016) 3(8):2286–8.
44. Çırakoğlu S, Baddal B, İslam A. The effectiveness of laser-activated irrigation on the apical microleakage qualities of MTA repair HP and NeoMTA plus in simulated immature teeth: a comparative study. Materials. (2020) 13(15):3287. doi: 10.3390/ma13153287
45. Demiryurek OE, Ozyurek T, Gulhan T, Keskin C. Evaluation of antibacterial and antifungal activity of calcium silicate based retrograde filling materials. Int J Appl Dent Sci. (2016) 2(2):85–8.
46. Esteki P, Jahromi MZ, Tahmourespour A. In vitro antimicrobial activity of mineral trioxide aggregate, biodentine, and calcium-enriched mixture cement against Enterococcus faecalis, Streptococcus mutans, and Candida albicans using the agar diffusion technique. Dent Res J. (2021) 18(3). doi: 10.4103/1735-3327.310032
47. Hiremath G, Singh M, Kulkarni B, Potdar R, Naik B. Antibiofilm efficacy of root-end filling materials against Enterococcus faecalis-an in vitro study. Endodontology. (2020) 32(2):86–90. doi: 10.4103/endo.endo_13_20
48. Hiremath GS, Kulkarni RD, Naik BD. Evaluation of minimal inhibitory concentration of two new materials using tube dilution method: an in vitro study. J Conserv Dent. (2015) 18(2):159–62. doi: 10.4103/0972-0707.153056
49. Jain AS, Gupta AS, Agarwal R. Comparative evaluation of the antibacterial activity of two biocompatible materials ie biodentine and MTA when used as a direct pulp capping agent against Streptococcus mutans and Enterococcus faecalisan in vitro study. Endodontology. (2018) 30(1):66–8. doi: 10.4103/endo.endo_66_17
50. Ji M, Chi Y, Wang Y, Xiong K, Chen X, Zou L. An in vitro evaluation of antimicrobial activity of a fast-setting endodontic material. Sci Rep. (2022) 12(1):16021. doi: 10.1038/s41598-022-20454-7
51. Kadam R, Shashikiran S, Mapara P, Gaonkar N, Gugawad S, Hadakar S, et al. Comparative evaluation of antimicrobial efficacy of mineral trioxide aggregate, biodentine, EndoSequence against bacterial strains of Enterococcus faecalis-an in vitro study. J Evol Med Dent Sci. (2020) 9(28):1988–92. doi: 10.14260/jemds/2020/433
52. Kawle S, Saraf PA. Evaluation of antimicrobial efficacy of three calcium silicatebased materials using tube dilution: an in vitro study. IP Indian J Conserv Endod. (2020) 5(3):109–13. doi: 10.18231/j.ijce.2020.026
53. Kim M, Lee SH, Shin DH. In vitro study of the biological and physical properties of dual-cure resin-modified calcium silicate-based cement. Dent J. (2023) 11(5):120. doi: 10.3390/dj11050120
54. Koruyucu M, Topcuoglu N, Tuna EB, Ozel S, Gencay K, Kulekci G, et al. An assessment of antibacterial activity of three pulp capping materials on Enterococcus faecalis by a direct contact test: an in vitro study. Eur J Dent. (2015) 9(02):240–5. doi: 10.4103/1305-7456.156837
55. Koutroulis A, Valen H, Ørstavik D, Kapralos V, Camilleri J, Sunde PT. Surface characteristics and bacterial adhesion of endodontic cements. Clin Oral Investig. (2022) 26(12):6995–7009. doi: 10.1007/s00784-022-04655-y
56. Koutroulis A, Valen H, Ørstavik D, Kapralos V, Camilleri J, Sunde PT. Effect of exposure conditions on chemical properties of materials for surgical endodontic procedures. Eur J Oral Sci. (2023) 131(4):e12943. doi: 10.1111/eos.12943
57. Naithani N, Nazneen L, Patil P, Patil OA, Deepali KJ, Mathew R. Efficacy of MTA, MTA plus, biodentine on E. faecalis biofilm. J Adv Med Dent Sci Res. (2022) 10(3):1–3. doi: 10.21276/jamdsr
58. Nikhil V, Madan M, Agarwal C, Suri N. Effect of addition of 2% chlorhexidine or 10% doxycycline on antimicrobial activity of biodentine. J Conserv Dent. (2014) 17(3):271–5. doi: 10.4103/0972-0707.131795
59. Nourzadeh M, Amini A, Fakoor F, Asgary S. Antimicrobial activity of calcium-enriched mixture cement and biodentine on Enterococcus faecalis: an in vitro study. Iran Endod J. (2019) 14(1):18–22. doi: 10.22037/iej.v14i1.22745
60. Pelepenko LE, Saavedra F, Antunes TBM, Bombarda GF, Gomes B, Zaia AA, et al. Physicochemical, antimicrobial, and biological properties of white-MTAFlow. Clin Oral Investig. (2021) 25(2):663–72. doi: 10.1007/s00784-020-03543-7
61. Queiroz MB, Torres FFE, Rodrigues EM, Viola KS, Bosso-Martelo R, Chavez-Andrade GM, et al. Development and evaluation of reparative tricalcium silicate-ZrO(2) -biosilicate composites. J Biomed Mater Res B Appl Biomater. (2021) 109(4):468–76. doi: 10.1002/jbm.b.34714
62. Sarmamy HM, Saeed DH. Antibacterial properties of new cement based capping material prepared from egg shell and biopolymer (chitosan). Medico-Legal Update. (2020) 20(1):1265–71. doi: 10.37506/v20/i1/2020/mlu/194476
63. Shalan LA, Al-Hashimi MK. Antibacterial effects of mineral trioxide aggregate and biodentine TM after the addition of different concentrations of black seed aqueous solutions. J Baghdad Coll Dent. (2015) 27(1):48–53. doi: 10.12816/0015264
64. Varghese NS, Jeevanandan G, Rajeshkumar S. Comparative evaluation of antimicrobial properties of a novel cal-cium silicate cement: an in vitro study. J Coast Life Med. (2022) 10(1):97–103.
65. Vats S, Maheshwari P. Comprehensive estimation and evaluation of antimicrobial efficiency of different pulp capping materials: an (in vitro) original study. J Adv Med Dent Sci Res. (2019) 7(5):25–8. doi: 10.21276/jamdsr
66. Viswanath G, Tilakchand M, Naik BD, Kalabhavi AS, Kulkarni RD. Comparative evaluation of antimicrobial and antifungal efficacy of bioactive root-end filling materials: an in vitro study. J Conserv Dent. (2021) 24(2):148–52. doi: 10.4103/JCD.JCD_548_19
67. Koutroulis A, Valen H, Orstavik D, Kapralos V, Camilleri J, Sunde PT. Antibacterial activity of root repair cements in contact with dentin-an ex vivo study. J Funct Biomater. (2023) 14(10):511. doi: 10.3390/jfb14100511
68. Pa W. Clinical and Laboratory Standards Institute: Performance Standards for Antimicrobial Susceptibility Testing: 20th Informational Supplement Document M100-S20. Wayne, PA, USA: Clinical and Laboratory Standards Institute (2016).
69. Khandelwal A, Karthik J, Nadig RR, Jain A. Sealing ability of mineral trioxide aggregate and biodentine as root end filling material, using two different retro preparation techniques-an in vitro study. Int J Contemp Dent Med Rev. (2015) 2015(2015):1–6. doi: 10.15713/ins.ijcdmr.48
70. Dawood AE, Parashos P, Wong RH, Reynolds EC, Manton DJ. Calcium silicate-based cements: composition, properties, and clinical applications. J Investig Clin Dent. (2017) 8(2):e12195. doi: 10.1111/jicd.12195
71. Ruiz-Linares M, de Oliveira Fagundes J, Solana C, Baca P, Ferrer-Luque CM. Current status on antimicrobial activity of a tricalcium silicate cement. J Oral Sci. (2022) 64(2):113–7. doi: 10.2334/josnusd.21-0439
72. Alghamdi F, Shakir M. The influence of Enterococcus faecalis as a dental root canal pathogen on endodontic treatment: a systematic review. Cureus. (2020) 12(3):e7257. doi: 10.7759/cureus.7257
73. Sagsen B, Er O, Esel D, Yagmur G, Altintop Y. In vitro pharmacodynamic activities of root canal sealers on Enterococcus faecalis. J Contemp Dent Pract. (2009) 10(3):35–42. doi: 10.5005/jcdp-10-3-35
74. Gutmann JL, Manjarrés V. Historical and contemporary perspectives on the microbiological aspects of endodontics. Dent J. (2018) 6(4):49. doi: 10.3390/dj6040049
75. Barbosa-Ribeiro M, De-Jesus-Soares A, Zaia AA, Ferraz CC, Almeida JF, Gomes BP. Antimicrobial susceptibility and characterization of virulence genes of Enterococcus faecalis isolates from teeth with failure of the endodontic treatment. J Endod. (2016) 42(7):1022–8. doi: 10.1016/j.joen.2016.03.015
76. Evans M, Davies JK, Sundqvist G, Figdor D. Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide. Int Endod J. (2002) 35(3):221–8. doi: 10.1046/j.1365-2591.2002.00504.x
77. Eldeniz AU, Hadimli HH, Ataoglu H, Ørstavik D. Antibacterial effect of selected root-end filling materials. J Endod. (2006) 32(4):345–9. doi: 10.1016/j.joen.2005.09.009
78. Weiss E, Shalhav M, Fuss Z. Assessment of antibacterial activity of endodontic sealers by a direct contact test. Dent Traumatol. (1996) 12(4):179–84. doi: 10.1111/j.1600-9657.1996.tb00511.x
79. Al-Ahmad A, Haendel M, Altenburger MJ, Karygianni L, Hellwig E, Wrbas KT, et al. Biodentine inhibits the initial microbial adhesion of oral microbiota in vivo. Antibiotics. (2022) 12(1):4. doi: 10.3390/antibiotics12010004
80. AlShwaimi E, Bogari D, Ajaj R, Al-Shahrani S, Almas K, Majeed A. In vitro antimicrobial effectiveness of root canal sealers against Enterococcus faecalis: a systematic review. J Endod. (2016) 42(11):1588–97. doi: 10.1016/j.joen.2016.08.001
81. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (updated March 2011): The Cochrane Library. Chichester, UK: John Wiley & Sons, Ltd (2011).
82. Rocco CJ, Davey ME, Bakaletz LO, Goodman SD. Natural antigenic differences in the functionally equivalent extracellular DNABII proteins of bacterial biofilms provide a means for targeted biofilm therapeutics. Mol Oral Microbiol. (2017) 32(2):118–30. doi: 10.1111/omi.12157
83. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. (1999) 284(5418):1318–22. doi: 10.1126/science.284.5418.1318
Keywords: antibacterial, biodentine, Enterococcus faecalis, endodontic treatment, endodontic failure, systematic review
Citation: Subhi H, Subhi N, Alhaidary S, Azeez MS and Tabnjh AK (2025) Antibacterial activity of biodentine against Enterococcus faecalis: a systematic review. Front. Dent. Med 5:1498353. doi: 10.3389/fdmed.2024.1498353
Received: 18 September 2024; Accepted: 30 December 2024;
Published: 21 January 2025.
Edited by:
Carla Sipert, University of São Paulo, BrazilReviewed by:
Cristiane Cantiga-Silva, São Paulo State University (Unesp), BrazilArbaz Sajjad, Penang International Dental College, Malaysia
Andreas Koutroulis, University of Oslo, Norway
Copyright: © 2025 Subhi, Subhi, Alhaidary, Azeez and Tabnjh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abedelmalek Kalefh Tabnjh, abedelmalek.kalefh.tabnjh@gu.se; Hasan Subhi, hsnsuaz@gmail.com
 Hasan Subhi
Hasan Subhi