- 1Restorative Dentistry Unit, the School of Clinical Dentistry, University of Sheffield, Sheffield, United Kingdom
- 2Healthy Lifespan Institute, University of Sheffield, Sheffield, United Kingdom
- 3Oxford Uehiro Centre for Practical Ethics, University of Oxford, Oxford, United Kingdom
- 4Department of Civil Law Disciplines, V.N. Karazin Kharkiv National University, Kharkiv, Ukraine
- 5Bristol Dental Hospital, University Hospitals Bristol and Weston NHS Foundation Trust, Bristol, United Kingdom
- 6Centre for Craniofacial and Regenerative Biology, FoDOCS, King’s College London, London, United Kingdom
- 7Institute of Animal Physiology and Genetics, Brno, Czech Republic
Periodontal disease (PD), a widespread non-communicable disease, affects over 90% of the global population with no known cure. Current management strategies focus on the stabilisation of disease progression, which is successfully achieved to a limited extent. Yet the never-ending battle between bacteria and the gingiva involves a complex interplay between genetic, microbial and environmental factors, demanding innovative approaches to improve the prevention and stabilisation of this disease. Glucose is the body's source of energy and research has shown that dysregulation of the glucose metabolism impacts PD establishment and progression, as well as the development of systemic non-communicable diseases. Metformin, a drug known for its efficacy in diabetes treatment via controlling glucose metabolism, also demonstrated cardioprotective effects, increased longevity, and anti-inflammatory properties. Metformin has been used in gel format in clinical trials for non-surgical treatment of PD, however, its systemic use in normoglycemic individuals with PD is less explored. A recent study presented compelling evidence of metformin's preventive potential, impacting PD and markers of systemic health involved in metabolic health linked to improvement of lifespan. Therefore, this review discusses the aspects of ageing as a concept in the periodontium and the potential benefits of modulating glucose metabolism through metformin to prevent PD, indirectly preventing systemic conditions involved in multi-morbidity, addressing a critical gap in current management. It also examines the choice between implementation of behaviour change and/or medication as a strategy to add to current oral hygiene strategies. Finally, it discusses the ethical implications of prescribing systemic medication in dentistry.
Graphical Abstract The never-ending battle between gingiva and biofilm. Characters of the illustration: Yellow fighter, Bac; Red fighter, Gingiva; Toothbrush, Coach Toothy; Bone with cap, Coach Bony; Round Tablet with Lightening sign, Coach metformin; Orange circle in the audience, Glucose; Purple triangle in the audience, Insulin; Yellow round crusty characters in the audience, Bacteria.
Introduction
Periodontal disease (PD), in any form, affects over 90% of the global population (1). Unfortunately, PD has no cure known to date, and the only treatment available is to stabilize it to avoid progression. Given that PD develops as a result of gene-environmental interaction, a cure will only be available when capacity-altering, biologically-based interventions are safe and compatible with the demands of distributive justice (2, 3). In light of PD's epidemiology and complex pathogenesis, seeking to implement curative strategies may not be currently the best way to tackle the global burden of the disease. Therefore, it becomes clear that there is a need to enhance prevention and stabilization of PD via the development of novel and accessible strategies to complement current methodologies.
PD is a non-communicable disease (NCD) with direct links to systemic health, including some life-threatening diseases, such as cardiovascular diseases, diabetes, and obesity (4). Commonly, these conditions develop during one's lifespan, and the risk of these conditions tends to increase with age, influenced by a combination of genetic, environmental, and lifestyle factors (5). A significant proportion of the population develops PD at a younger age (30 years old) when compared to other systemic NCDs (6, 7). Given the similarities between the host's metabolic-mediated inflammatory response driving PD progression and other NCDs, early systemic management of PD may reduce the impact of these systemic diseases on individuals, improving public health.
With the increasing life expectancy and a growing proportion of elderly individuals in the general population, there is a pressing need to comprehend the reasons behind the escalating susceptibility to chronic morbidity, disability, and frailty associated with ageing, making it a key public health concern. Given the high prevalence of PD and its association with ageing, further understanding on how ageing affects the oral tissues and microbiome is essential to evolving dentistry beyond the current standard of practice, where management of disease does not account for ageing.
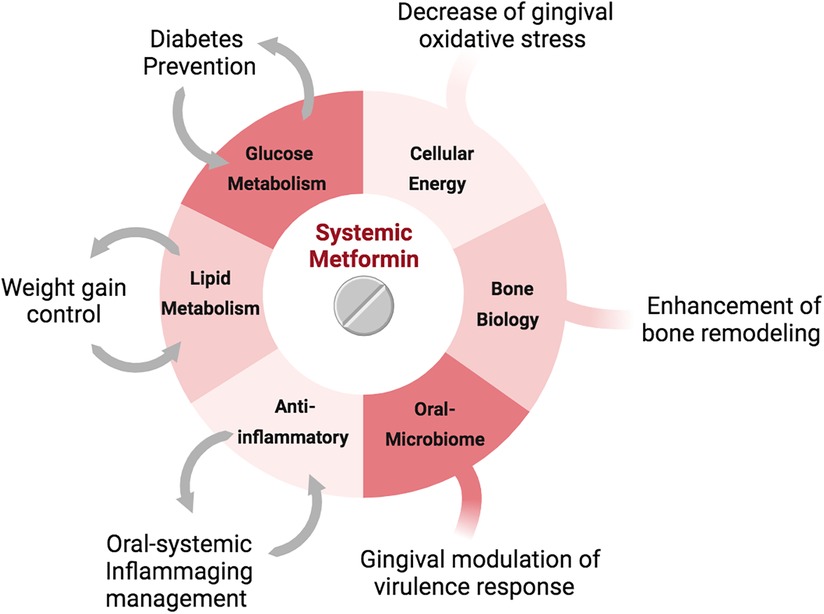
Most recently, glucose metabolism modulation has been indicated as a key enhancer of longevity by decreasing NCD incidence (8–10), and in PD literature, it is widely known that dysregulation of glucose metabolism accelerates PD progression. Biofilm build-up and glycaemic peaks are both transient physiological factors of human physiology, and current PD management strategies do not tackle glycemia nor have an impact on overall systemic NCD prevention. Therefore, this article investigates the potential of implementation of glucose metabolism modulation in PD management as a form of oral-systemic preventive strategy. Although many pharmacological glucose modulatory drugs exist in the market, in this article we offer a rationale for the implementation of metformin use in the clinics, as this is a cost-effective and globally available medication used as a first-line treatment for type 2 diabetes. Additionally, we further discuss the global health impact of dentists prescribing medication to treat oral disease and improve general health.
Ageing in the periodontium
The aging process within the periodontium, encompassing the gingiva, cementum, periodontal ligament, and alveolar bone, involves several interconnected biological phenomena. Collagen changes, primarily associated with alterations in the composition, density, and structure of collagen fibres, emerge as a common theme affecting both the gingiva and the periodontal ligament (11, 12). This crucial structural component undergoes modifications with age, impacting the overall health and resilience of these tissues. Diminished vascularity and blood supply constitute another shared factor, influencing the gingiva and periodontal ligament. This reduction in blood flow can compromise oxygen and nutrient delivery, affecting tissue health and repair (13, 14). Additionally, both tissues are subject to the influence of chronic inflammation and immune changes associated with aging. This inflammaging phenomenon dysregulates the immune response and can contribute to increased susceptibility to periodontal diseases and impaired tissue healing in both the gingiva and periodontal ligament (15). These shared factors impact the structural support and regenerative potential of these tissues, underscoring the interconnected nature of the aging process within the periodontium.
Nevertheless, tissue-specific factors also contribute to the aging of individual components of this structure. For cementum, mineralization changes and reduced cellular activity emerge as key factors affecting its hardness and resilience. Moreover, accumulation of microdamage, influenced by mechanical stresses and normal masticatory forces, further compromises the structural integrity of the cementum and periodontal ligament (16, 17). Finally, ageing influences the alveolar bone due to reduced bone turnover [20], changes in mineralization (18), altered cellular activity (19), hormonal shifts (20), and responsiveness to mechanical loading (21), all of which lead to a complex interplay of factors affecting bone density and quality. Most specifically, and in need of further investigation, hormonal fluctuations, especially in postmenopausal women, have been linked to changes in periodontal tissues that are more favourable to periodontal disease breakdown (22).
Plaque and glycemia during the lifespan: secrete double agents?
The presence of the oral microbiome is natural in every human being; therefore, the formation of plaque-biofilm is a natural biological process. Humans without oral hygiene intervention naturally accumulate plaque-biofilm around teeth, leading to PD (23); therefore, plaque levels need to be decreased to below the threshold of natural accumulation to prevent PD disease and development.
Similarly, blood sugar levels naturally fluctuate all day long, spiking when food is consumed. Continuous glucose build-up in the bloodstream can, over time, cause damage to human health even in normoglycemic humans; therefore, maintaining blood glucose at stable levels improves metabolic health preventing issues such as insulin resistance, obesity, and Type 2 diabetes (24); all of which have a direct impact on PD progression (4).
The catalyst for transitioning from stable chronic gingivitis to destructive periodontitis is still uncertain, yet it is understood that PD initiates due to biofilm accumulation, and inflammation is the mediator of the disease progression (25). Given the constant bacterial challenge from natural biofilm accumulation, the periodontal cells are subject to persistent oxidative stress and DNA damage (26, 27). Energy is necessary for constant DNA repair, precise cell turnover, and inflammatory regulation of the periodontal tissues. Glycolysis is the cellular metabolic pathway responsible for the breakdown of glucose to generate energy. Recent research suggests that glycolysis intermediates play a role in supporting DNA repair processes (28), however, little is known about this in the periodontal tissues. Yet, it is possible to infer that cellular glycolysis is important for periodontal stability as the impairment of a fine-tuned glycolytic cascade, due to insulin resistance or lack thereof, leads to increased risk of periodontitis breakdown (29). Further, continuous fluctuation of high peaks of blood glucose levels throughout the lifespan has been found to influence the development of conditions that share inflammatory risk factors with PD (30).
Although the biological interaction between glucose metabolism and plaque is not yet fully connected and elucidated, further investigations of these two natural daily fluctuant biological processes may unveil key information about the pathogenesis of PD. Moreover, systemic glucose modulatory strategies could be the missing link to enhancing PD prevention and simultaneously mitigating against other NCDs over one's lifespan.
Current state of metformin use
Metformin is a cost-effective, globally available, and renowned medication used as a first-line treatment for type 2 diabetes (31). It operates by enhancing insulin sensitivity in the body's cells, thereby facilitating improved glucose utilization. Its appeal arises from its proven ability to regulate blood glucose levels without causing undue hypoglycemia, a common concern with some other antidiabetic medications.
Epidemiological studies have drawn the attention of researchers to therapeutic benefits of metformin beyond glycemic control. Metformin has been associated with a range of cardioprotective effects, reduced incidence of cancer and mortality, and increased longevity (31, 32). Adjoining these processes is the ability of Metformin to suppress inflammation, modulate the gastrointestinal microbiota, enhance osteoblast differentiation, repress osteoclast activity, and regulate stem cell aging, all of which indicate potential to highly impact PD management.
Aside from a wide range of in vitro research investigating the effects of metformin on the periodontium, several clinical studies exist looking at local administration of metformin gel when treating periodontitis (non-surgically), including non-diabetic patients (Table 1). Despite these positive findings, there was still no recommendation for topical metformin gel use in the management of stages I-III periodontitis by the European Federation of Periodontology and American Academy of Periodontology guidelines (44), due to it being an off-label use of the medication without a published formulation of the gel.
Repurposing systemic metformin as an oral-systemic preventive medicine
PD management is in need of a holistic approach to address its immediate symptoms and underlying systemic risk factors as a preventive measure, prior to their establishment. Little is known about the systemic use of metformin on the periodontium of normoglycemic humans. However, systemic metformin has been shown in vivo to improve bone levels in a periodontal treatment model (45), and in a clinical trial to improve the gingival crevicular fluid inflammatory surrogates, simultaneously preventing weight gain in class 1 obese pregnant women (46). These research pieces indicate that systemic metformin use has potential to impact both oral and systemic management of complex NCD in normoglycemic humans.
Further in a recent study, Neves et al. (47) investigated the efficacy of metformin in preventing and managing PD and its underlying associated risk factors, in healthy, non-obese, normoglycemic animals and humans (Figure 1). The in vivo results showed that systemic metformin use prior to inoculation of Porphyromonas gingivalis (P. gingivalis), halted P. gingivalis-associated periodontal bone loss, modulating the composition of the oral microbiome. Further, since metformin is considered a longevity drug, the study also investigated the role of metformin in preventing age-related periodontal bone loss, finding that long-term systemic metformin use reduced 54% of age-related periodontal bone loss. Using single-cell RNA sequencing analysis, Neves et al. (47) revealed that systemic use of metformin modulated the cellular energy metabolism related to glycolysis and mitochondrial function, as well as differentiation capacity of the gingiva. This resulted in enhanced preventive capacity against periodontal disease establishment, affecting the host-microbiome axis, even in the presence of dysbiosis.
Apart from the effect on the periodontium, the study also investigated how preventive systemic metformin impacted systemic associated risk factors, such as blood glucose levels and weight gain. The animals managed with metformin maintained significantly lower blood glucose levels compared to controls in all in vivo models. Furthermore, it demonstrated that PD induction increased systemic blood glucose levels, a feature of the disease that could potentially further explain the destructive synergy between PD and Diabetes. With regards to body weight, the in vivo results corroborate with the human weight gain prevention study, as animals on long-term systemic metformin were four times lighter than those on water.
Translationally, Neves et al. (47) ran the first randomized control trial using systemic metformin as an adjunct to periodontal disease treatment in normoglycemic and non-obese humans, as a first step to investigate the potential use of metformin in PD management. Using 850 mg of metformin once daily for 10 days, the study revealed comparable adverse effects to placebo and notable impacts on clinical periodontal parameters (overall additional PPD reduction of 0.3 mm and 1.09 mm in pockets ≥7 mm), similar to those of antibiotic therapies (48). Furthermore, systemic metformin treatment modulated systemic inflammation (IL-6 −0.9 pg/ml, hsCRP −2.15 mg/L), and systemic glucose metabolism, significantly enhancing insulin sensitivity compared to placebo. These findings suggest a potential for metformin to modulate co-morbidity risk factors and reduce age-related chronic conditions, laying a foundation for innovative approaches in PD management with metformin supplementation and this grant application.
The study also found that systemic metformin treatment modulated systemic inflammation and glucose metabolism, independently of any dietarian intake. Metformin administered as an adjunct to periodontal treatment stabilised fasting glucose and hsCRP, and improved insulin sensitivity, which together are markers associated with better metabolic health resulting in a lower likelihood of age-related chronic conditions.
Medication or behaviour change?
Innovation in PD management demands a critical decision between implementing behavioural changes and medications, significantly impacting public health strategy. Patients striving to control their condition require a comprehensive approach, merging individual agency, preventive measures, and a holistic long-term well-being strategy.
Embracing lifestyle modifications, such as adopting a balanced diet, engaging in regular physical activity, and managing stress, can yield a myriad of benefits that extend beyond glucose control. Unlike medications, behaviour change empowers individuals to actively participate in their health journey, fostering long-term habits that promote overall well-being. Medications, on the other hand, offer a targeted and often swift approach to managing risk factors and mitigating the progression of various conditions. The benefit of medications in NCD prevention lies in their ability to address specific biochemical pathways or physiological processes, providing a reliable and measurable approach to managing risk factors.
Currently, oral hygiene (OH) stands as the sole strategy preventing PD establishment. However, its efficacy hinges on patient adherence, proficient OH techniques, and manual dexterity. OH proves limited for PD cases related to systemic diseases, genetic predisposition, or alterations in the oral environment due to detrimental habits.
Clinically, when considering adding to current PD prevention management strategies, dentists can emphasize the essential role of lifestyle changes in NCD prevention and PD impact. Moreover, our class can and should work in collaboration with Nutritionists despite the lack of consensus on the optimal PD management diet. However, dentistry's exclusive reliance on behavioural change, coupled with additional dietary-focused policies targeted at PD prevention to complement OH may overwhelm and discourage patients, especially those from less fortunate backgrounds focused on sustenance rather than dieting. It may also not have an additive effect to those who have no health services provided in their vicinity due to lack of health care workforce. Given dentistry's lack of a medication arsenal for PD's inflammatory biochemistry in secondary prevention, providing chemoprophylactic agents to control risk factors complements ongoing behavioural change efforts, preventing PD onset or progression and targeting NCD risk factors, ultimately impacting lifespan positively. This integrated approach addresses multifaceted challenges and access disparities, fortifying comprehensive PD management.
Where is the line between dentistry and medicine?
An important consideration when strategizing systemic medication for oral diseases is whether dentistry is crossing the ethical boundary of its scope of practice. The boundary between medicine and dentistry lies in the direction of activity and the object of treatment, diagnosis and prevention. Medicine focuses on diseases of the entire human body, while dentistry focuses on the oral cavity and face, which have unique symptoms and management strategies. However, when an oral disease treatment has the scope to prevent systemic diseases, this boundary becomes blurry. While specific medical ethics may not directly apply to dental practice, fundamental values such as ethics, loyalty, and fiduciary relations should align across both professions (49). Here we try to shed light on the discussion about the autonomy within dentistry for prescription of systemic medication.
Medical practitioners can find signs of oral diseases but are not responsible for treating dental problems and so, should not attempt to manage a condition requiring dental skills unless they have appropriate training (50). Similarly, dentists can find signs of more severe disease when treating the oral cavity but also should not be responsible for treating the condition, requiring referring the patient to an appropriate medical practitioner. However, PD is highly common (more common than cardiovascular diseases), detrimental to wellbeing, affects quality of life and is directly linked to systemic health, including some life-threatening diseases (51). Therefore, when considering the impact of systemic interventions to prevent and manage PD and indirectly preventing systemic diseases, it could be argued that not intervening is a sign of neglect to overall health.
Crossing the boundary of oral-systemic health intervention is not a novelty in dentistry, dentists already prescribe systemic medications for various conditions related to oral health or systemic health concerns with oral manifestations. Yet, systemic medication prescription also comes with risk of misconduct, an example of this, is the misuse of antibiotics by dentists (52). Therefore, for systemic interventions to be rolled out for clinical use, tight collaboration with practitioners of medicine is essential for a safe practice. However, the current lack of autonomy to prescribe alternative medications for management of PD disease could hinder the progress and effectiveness of oral-systemic health intervention. With the necessary safeguards and collaboration with medical professionals, autonomy in prescribing medications to enhance oral health interventions would empower dentists to contribute significantly to the prevention and management of systemic diseases at the global level.
Future directions for metformin repurposing
The integration of systemic metformin prescription for management and treatment of PD still needs further investigations and discussion for a concentrated widespread repurposing. Larger trials need to be done and investigations of the dose response for PD management need to be ascertained, as metformin prescription dosage is currently based on stabilising dysregulated systemic glucose metabolisms, not multi-morbidity prevention in normoglycemic patients. Further optimisation of the length of prescription is still to be ascertained so that the treatment can positively impact oral-systemic disease development and progression. Given that signs of multimorbidity prevention were seen when using metformin for PD management, the surrogate markers of these conditions, such as systemic glucose levels, lipid levels, and inflammatory trends on normoglycemic patients with PD need to be further investigated. Finally, investigations on patients with geriatric conditions associated with physical impairment affecting oral hygiene activities are needed as they could benefit from the indirect impact of metformin use for PD management. Together, these steps could lead to integration of PD disease management with metformin as a means to manage age-related morbidities prior to their development over a lifespan, impacting the global incidence of PD-associated risk factors, such as cardiovascular disease, obesity, diabetes, and cognitive decline.
Conclusion
Here, we contextualize PD within the broader framework of non-communicable diseases (NCDs), highlighting its significant impact on systemic health and its association with ageing, underscoring the interconnectedness of PD with systemic health conditions and ageing-related changes in oral tissues and the microbiome. Further, we offered a comprehensive exploration of PD management and prevention strategies, emphasizing the potential of systemic glucose metabolism modulation, particularly through the use of metformin, as a promising avenue for oral-systemic preventive medicine. Finally, we addressed pertinent ethical considerations regarding the role of dentistry in systemic medication prescription, advocating for a collaborative approach between dental and medical professionals to ensure safe and effective oral-systemic health interventions, with implications for improving global public health outcomes.
Author contributions
VN: Conceptualization, Data curation, Supervision, Writing – original draft, Writing – review & editing. VS: Conceptualization, Writing – original draft, Writing – review & editing. JD: Data curation, Writing – original draft, Writing – review & editing. PS: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
I would like to thank the artist who created the graphical abstract out of a script I wrote.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Peres MA, Macpherson LMD, Weyant RJ, Daly B, Venturelli R, Mathur MR, et al. Oral diseases: a global public health challenge. Lancet. (2019) 394(10194):249–60. doi: 10.1016/S0140-6736(19)31146-8
2. Savulescu J. Genetic interventions and the ethics of enhancement of human beings. In: Steinbock B, editor. The Oxford Handbooks of Bioethics. Oxford: Oxford University Press (2009). p. 516–35.
3. Neves V, Pugh J, Savulescu J. Beyond oral hygiene, are capacity-altering, biologically based interventions within the moral domain of dentistry? Br Dent J. (2021) 231:277–80. doi: 10.1038/s41415-021-3335-y
4. Ramseier CA, Woelber JP, Kitzmann J, Detzen L, Carra MC, Bouchard P. Impact of risk factor control interventions for smoking cessation and promotion of healthy lifestyles in patients with periodontitis: a systematic review. J Clin Periodontol. (2020) 47(Suppl 22):90–106. doi: 10.1111/jcpe.13240
5. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. (2006) 3(11):e442. doi: 10.1371/journal.pmed.0030442
6. Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. (2005) 366(9499):1809–20. doi: 10.1016/S0140-6736(05)67728-8
7. Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. (2012) 91(10):914–20. doi: 10.1177/0022034512457373
8. Hammel MC, Stein R, Kratzsch J, Vogel M, Eckert AJ, Triatin RD, et al. Fasting indices of glucose-insulin-metabolism across life span and prediction of glycemic deterioration in children with obesity from new diagnostic cut-offs. Lancet Reg Health Eur. (2023) 30:100652. doi: 10.1016/j.lanepe.2023.100652
9. Ishii M. What is good for the heart is good for brain glucose metabolism. Lancet Healthy Longev. (2023) 4(9):e448–9. doi: 10.1016/S2666-7568(23)00167-8
10. Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, et al. Interventions to slow aging in humans: are we ready? Aging Cell. (2015) 14(4):497–510. doi: 10.1111/acel.12338
11. Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontol 2000. (2002) 28(1):12–55. doi: 10.1034/j.1600-0757.2002.280102.x
12. Bosshardt DD, Lang NP. The junctional epithelium: from health to disease. J Dent Res. (2005) 84(1):9–20. doi: 10.1177/154405910508400102
13. Nanci A, Bosshardt DD. Structure of periodontal tissues in health and disease. Periodontol 2000. (2006) 40(1):11–28. doi: 10.1111/j.1600-0757.2005.00141.x
14. Jin K. A microcirculatory theory of aging. Aging Dis. (2019) 10(3):676–83. doi: 10.14336/AD.2019.0315
15. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. (2014) 69(Suppl 1):S4–9. doi: 10.1093/gerona/glu057
16. Lim WH, Liu B, Mah SJ, Chen S, Helms JA. The molecular and cellular effects of ageing on the periodontal ligament. J Clin Periodontol. (2014) 41(10):935–42. doi: 10.1111/jcpe.12277
17. Menicanin D, Hynes K, Han J, Gronthos S, Bartold PM. Cementum and periodontal ligament regeneration. In: Bertassoni L, Coelho P, editors. Engineering Mineralized and Load Bearing Tissues. Advances in Experimental Medicine and Biology. Vol. 881. Cham: Springer (2015). doi: 10.1007/978-3-319-22345-2_12
18. Ono R, Katsumata A, Fujikawa Y, Takahira E, Yamamoto T, Kanamura N. Sex differences and age-related changes in the mandibular alveolar bone mineral density using a computer-aided measurement system for intraoral radiography. Sci Rep. (2024). 14:7386. doi: 10.1038/s41598-024-57805-5
19. Marie PJ. Cellular regulation of bone remodeling. Calcif Tissue Int. (1985) 37(4):401–5. doi: 10.1007/BF02553710
20. Khosla S. Update on estrogens and the skeleton. J Clin Endocrinol Metab. (2010) 95(8):3569–77. doi: 10.1210/jc.2010-0856
21. Turner CH. Three rules for bone adaptation to mechanical stimuli. Bone. (1998) 23(5):399–407. doi: 10.1016/S8756-3282(98)00118-5
22. Lee DJ, Wu L, Shimono M, Piao Z, Green DW, Lee JM, et al. Differential mechanism of periodontitis progression in postmenopause. Front Physiol. (2018) 9:1098. doi: 10.3389/fphys.2018.01098
23. Löe H, Anerud A, Boysen H, Smith M. The natural history of periodontal disease in man. The rate of periodontal destruction before 40 years of age. J Periodontol. (1978) 49(12):607–20. doi: 10.1902/jop.1978.49.12.607
24. Blonde L, Umpierrez GE, Reddy SS, McGill JB, Berga SL, Bush M, et al. American association of clinical endocrinology clinical practice guideline: developing a diabetes mellitus comprehensive care plan-2022 update. Endocr Pract. (2022) 28(10):923–1049. doi: 10.1016/j.eprac.2022.08.002
25. Curtis MA, Diaz PI, Van Dyke TE. The role of the microbiota in periodontal disease. Periodontol 2000. (2020) 83(1):14–25. doi: 10.1111/prd.12296
26. Zamora-Perez AL, Zúñiga-González GM, Gómez-Meda BC, Lazalde-Ramos BP, Ortiz-García YM, Morales-Velazquez G, et al. Periodontal disease and nuclear and oxidative DNA damage. Insights into various aspects of oral health. InTech. (2017). doi: 10.5772/intechopen.68446
27. Kumar J, Teoh SL, Das S, Mahakknaukrauh P. Oxidative stress in oral diseases: understanding its relation with other systemic diseases. Front Physiol. (2017) 8:693. doi: 10.3389/fphys.2017.00693
28. Bhatt AN, Chauhan A, Khanna S, Rai Y, Singh S, Soni R, et al. Transient elevation of glycolysis confers radio-resistance by facilitating DNA repair in cells. BMC Cancer. (2015) 15:335. doi: 10.1186/s12885-015-1368-9
29. Chapple IL, Genco R. Diabetes and periodontal diseases: consensus report of the joint EFP/AAP workshop on periodontitis and systemic diseases. J Periodontol. (2013) 84(4 Suppl):S106–12. doi: 10.1902/jop.2013.1340011
30. Flynn MC, Kraakman MJ, Tikellis C, Lee MKS, Hanssen NMJ, Kammoun HL, et al. Transient intermittent hyperglycemia accelerates atherosclerosis by promoting myelopoiesis. Circ Res. (2020) 127(7):877–92. doi: 10.1161/CIRCRESAHA.120.316653
31. Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. (2014) 20(6):953–66. doi: 10.1016/j.cmet.2014.09.018
32. Zheng J, Xu M, Yang Q, Hu C, Walker V, Lu J, et al. Efficacy of metformin targets on cardiometabolic health in the general population and non-diabetic individuals: a Mendelian randomization study. EBioMedicine. (2023) 96:104803. doi: 10.1016/j.ebiom.2023.104803
33. Arslaan M, Karim N, Fatima M. Clinical efficacy of 1% metformin and alendronate intra-pocket gel in moderate localized chronic periodontitis. Rawal Med J. (2022) 47(1):112–5.
34. Bashir S, Gopalakrishnan D, Martande S. Locally delivered 1.2% simvastatin gel and 1% metformin gel in chronic periodontitis patients. Saudi J Oral Dent Res. (2022) 7(8):182–91. doi: 10.36348/sjodr.2022.v07i08.001
35. Shah K, Parikh H, Duseja S. Comparative evaluation of metformin gel and chlorhexidine gel as adjunct to scaling and root planning in the treatment of chronic periodontitis: a clinical study. Int J Dentistry Res. (2022) 7(3):63–7. doi: 10.31254/dentistry.2022.7305
36. Mirza M, Karim N, Baksh Khadri W, Asghar S. Clinical efficacy of 1% metformin gel and systemic doxycycline in chronic periodontitis: a randomised clinical trial. J Liaquat Uni Med Health Sci. (2021) 20(3):204–8. doi: 10.22442/jlumhs.2021.00844
37. Pundir AJ, Gupta S, Pundir S, Bhatnagar S, Agrawal N, Sultana N. Evaluation of efficacy of subgingival delivered 1% metformin gel as an adjunct to scaling and root planing in the treatment of chronic periodontitis. Int J Med Sci Curr Res. (2021) 4(1):225–33.
38. Maatouq AG, Elshinnawi UM, Raafat IM, Mahsoub N. Therapeutic efficacy of locally delivered metformin gel versus clindamycin gel in chronic periodontal diseases. Mansoura J Dentistry. (2019) 6(24):10–4. doi: 10.21608/mjd.2019.199352
39. Mushtaq I, Shukla P, Malhotra G, Dahiya V, Kataria P, Joshi CS. Comparative evaluation of 1% metformin gel as an adjunct to scaling and root planing in the treatment of chronic periodontitis with scaling and root planing alone: a randomised controlled clinical trial. Int J Oral Care Res. (2018) 6(2):79–88.
40. Hasan F, Najam R, Simjee SU. Metformin- a non-conventional drug for the local treatment of periodontal diseases: a randomized clinical control study. Med Forum. (2017) 28(6):162–6.
41. Kasseem AA, Elsayed Issa DA, Kotry GS, Faird RM. Thiolated alginate-based multiple layer mucoadhesive films of metformin for intra-pocket local delivery: in vitro characterization and clinical assessment. Drug Dev Ind Pharm. (2017) 43(1):120–31. doi: 10.1080/03639045.2016.1224895
42. Santo Grace U, Sankari M. Effect of locally administered 1% metformin gel in the treatment of chronic periodontitis. J Pharm Sci Res. (2017) 9(9):1463–5.
43. Sreedhar A, Sikkander S, Pai J, Walvekar A, Baramappa R, Varkey A. Clinical and biochemical evaluation of efficacy of 1% metformin gel as an adjunct to SRP in chronic periodontitis- a split mouth placebo controlled study. Int J Sci Res. (2017) 6(12):78–82.
44. Sanz M, Herrera D, Kebschull M, Chapple I, Jepsen S, Beglundh T, et al. Treatment of stage I-III periodontitis-the EFP S3 level clinical practice guideline. J Clin Periodontol. (2020) 47 Suppl 22(Suppl 22):4–60. doi: 10.1111/jcpe.13290. Erratum in: J Clin Periodontol. 2021 Jan;48(1):163.32383274
45. Araújo AA, Pereira ASBF, Medeiros CACX, Brito GAC, Leitão RFC, Araújo LS, et al. Effects of metformin on inflammation, oxidative stress, and bone loss in a rat model of periodontitis. PLoS One. (2017) 12(8):e0183506. doi: 10.1371/journal.pone.0183506
46. Zúñiga Curz CA, Calzada Mendoza CC, Miranda Mondragón ID, Bustamante Bacame A, Portilla Robertson J, Ocharán Hernández E. Efecto del manejo de la obesidad clase I con metformina sobre actividad de metaloproteinasas en pacientes con periodontitis crónica [effect of the management of class I obesity with metformin on metalloproteinase activity in patients with chronic periodontitis]. Nutr Hosp. (2019) 36(5):1095–100. Spanish. doi: 10.20960/nh.02602
47. Neves VCM, Satie Okajima L, Elbahtety E, Joseph S, Daly J, Menon A, et al. Repurposing metformin for periodontal disease management as a form of oral-systemic preventive medicine. J Transl Med. (2023) 21(1):655. doi: 10.1186/s12967-023-04456-1
48. Teughels W, Feres M, Oud V, Martín C, Matesanz P, Herrera D. Adjunctive effect of systemic antimicrobials in periodontitis therapy: a systematic review and meta-analysis. J Clin Periodontol. (2020) 47(Suppl 22):257–81. doi: 10.1111/jcpe.13264
49. Freukel DA, Lurie Y. Responsibility and liability in health care: some differences between dentistry and medicine. Med Law. (2002) 21(3):605–15. PMID: 12437206.
50. British Medical Association. Patients Presenting With Dental Problems. The British Medical Association (2019). Available online at: https://www.bma.org.uk/advice-and-support/gp-practices/gp-service-provision/patients-presenting-with-dental-problems (Accessed November 01, 2023).
51. The Economist. Time to Take Gum Disease Seriously: the Societal and Economic Impact of Periodontitis. Available online at: https://impact.economist.com/perspectives/healthcare/time-take-gum-disease-seriously-societal-and-economic-impact-periodontitis (Accessed November 01, 2024).
Keywords: periodontal disease, metformin, ageing, preventive medicine, glycaemic control, multimorbidity prevention
Citation: Neves VCM, Savchenko V, Daly J and Sharpe P (2024) Periodontal ageing and its management via pharmacological glucose modulation. Front. Dent. Med 5: 1415960. doi: 10.3389/fdmed.2024.1415960
Received: 11 April 2024; Accepted: 12 June 2024;
Published: 1 July 2024.
Edited by:
Yuqi Guo, New York University, United StatesReviewed by:
Xinyuan Liu, New York University, United StatesZi Wang, New York University, United States
© 2024 Neves, Savchenko, Daly and Sharpe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vitor C. M. Neves, di5uZXZlc0BzaGVmZmllbGQuYWMudWs=
 Vitor C. M. Neves
Vitor C. M. Neves Viktor Savchenko
Viktor Savchenko James Daly
James Daly Paul Sharpe
Paul Sharpe