- 1Department of Oral Medicine, Infection, and Immunity, Division of Periodontology, Harvard School of Dental Medicine, Boston, MA, United States
- 2Department of Anatomy, Histology, Embryology, Pathology Anatomy and Pathology Histology, Faculty of Dental Medicine and Health Osijek, J.J. Strossmayer University of Osijek, Osijek, Croatia
- 3Department of Oral Biology, The Goldschleger School of Dental Medicine, Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
- 4Department of Oral Surgery, Faculty of Dentistry, University of Szeged, Szeged, Hungary
This narrative review aims to discuss the incorporation of novel medical concepts and tools into dental practice, with the goal of improving early diagnosis and exploring new personalized treatment options for oral pathologies, such as caries and periodontitis. Preventative dental approaches concentrate on the timely detection of oral infections and the integration of biomarker analysis to recognize pathogenic changes at early stage of disease. Likewise, periodic monitoring after the treatment is relevant to ensure the balance in the oral biofilms and prevent relapse. Additionally, more attention has shifted towards the contributing factors to disease development, such as essential nutrients. Sufficient levels of vitamin C, vitamin D and zinc pre- and post-operatively are employed to boost immune function and reduce the risk of postoperative infections. Omega-3 fatty acids, melatonin, and antioxidants like vitamin E, which have anti-inflammatory properties, are utilized to help minimize excessive inflammation and promote faster recovery. The data presented in this manuscript emphasize the crucial integration of innovative healthcare concepts and tools into dental practices. By adopting a more holistic view of the patient, clinicians can tailor treatments to each individual's predispositions, lifestyle, and oral health conditions. This review also highlights the potential of salivary biomarkers and point-of-care technologies in enhancing early diagnostic accuracy and personalizing treatment. Bridging the gap between oral and systemic health is the most effective approach to improving patient quality of life. These findings underscore the importance of continued interdisciplinary collaboration in dentistry.
Introduction
As the medical and dental fields evolve towards a new paradigm focused on prevention and minimally invasive procedures, both clinicians and patients increasingly advocate for care strategies that are both individualized and preventative. The adoption of telemedicine for continuous monitoring and non-invasive preventive interventions is already supporting this shift. Moreover, as the associations between system diseases and oral health gain more relevance, dental practice gains greater relevance. Furthermore, the recognition of the link between genetic predisposition, lifestyle choices and environmental or dietary factors has been a major aspect of scientific research, addressing the needs of patients based on their epigenetics, biomarkers and socio-economic characteristics with a potential to minimize diagnostic errors and improve treatment outcomes (1).
Oral health-related quality of life (OHRQoL) is an integral part of overall health and well-being. It is a multidimensional construct, including subjective evaluations of the individual's oral health, functional and emotional well-being, expectations and satisfaction with care, and self- perception. Recognized by the World Health Organization (WHO) as a crucial component of the Global Oral Health Program since 2003, the assessment of OHRQoL and extensive research in this area facilitate greater patient involvement in medical treatment, the integration of new approaches, and the monitoring of external factors influencing treatment outcomes (2).
Over the years, the dental approaches have shifted towards preventive measures and timely detection of oral infections. Yet, caries and periodontitis are still the major opponents of the maintenance of oral health (3, 4). These conditions result from dysbiosis in microbial biofilms and host factors, with dietary habits and hygiene playing as main drivers in disease outbreak and progression, as illustrated in Figure 1. Differential analysis between oral pathologies aids in implementing preventative measures and improvement of dental treatment (3, 5).
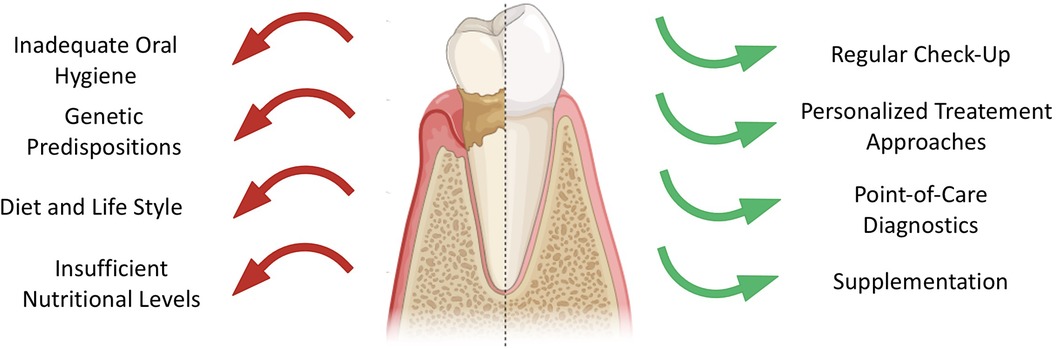
Figure 1. Overview of factors contributing to decreased oral health (left, red arrows) and factors enhancing dental health (right, green arrows).
This narrative review aims to discuss the incorporation of novel medical concepts and tools into dental practice, with the goal of improving early diagnosis and exploring new personalized treatment options for oral pathologies, such as caries and periodontitis.
Salivary screening for biomarkers
Understanding the field of dysbiosis and host- microbe interactions requires expanding our understanding beyond specific pathogens. The role of beneficial bacterial species and how they interact with each other is crucial (6). Host biomarkers also play a very important role on oral ecology, such as receptors for microbiome metabolites, lipid mediators of inflammation, chemokines, cytokines, enzymes, and other proteins. Exploring protein biomarkers not only provides insights into the pathogenic processes but also establishes the groundwork for precision medicine (6).
As previously mentioned, precision medicine demands a comprehensive system approach to integrate multi-level data in the search for accurate and reliable biomarkers of disease activity and novel therapeutic targets, with the aim to improve the management of chronic conditions across all medical domains (6). Biomarkers, measurable substances, serve as valuable data resources for indicating physiological health, screening pathological processes, and assessing responses to therapeutic intervention (6).
Numerous studies were carried out to analyze the relationships between saliva composition and oral diseases including periodontitis (7–10), carious lesions (11), and oral cancer (12, 13). These studies have shown that any shift in oral microbial biofilm and subsequent inflammatory response are reflected in saliva. As a result, saliva has been proposed as a potential medium for disease detection (7), even during early pathogenic changes prior irreversible tissue damage (14). Furthermore, saliva analysis has shown promises in monitoring disease progression (15) and evaluating responses to treatment strategies (15). Utilizing saliva as a diagnostic medium exerts several advantages; its collection is simple and painless, enabling for large quantities to be obtained without posing complications for the patients. Additionally, saliva demonstrates high durability (16).
Within the context of periodontitis, a nuanced understanding reveals three distinct phases: inflammation, connective tissue degradation, and bone resorption. Throughout each phase, the identification of specific biomarkers serves as a diagnostic compass, providing a comprehensive understanding of the patient's current stage of pathologic breakdown (17). The traditional diagnostic methods solely often fail to accurately reflect current disease activity and to identify high risk individuals (18). Hence, the meticulous monitoring of biomarkers stands as a pivotal element, emphasizing the significance of preliminary testing to inform the selection of supplementation dosage and duration. A recent systematic review highlights several biomarkers, including macrophage inflammatory protein-1 alpha (MIP-1α), IL-1β, IL-6, and MMP-8 (matrix metalloproteainase-8), as promising indicators for diagnosing periodontitis (19). Integrating clinical measures with assessments of pathogen and cytokines levels can yield a diagnostic sensitivity of up to 74% for predicting periodontitis progression (20). Future studies should focus on determining the optimal combination of biomarkers that would allow for the early diagnosis of periodontitis, as well as those that could be used to monitor treatment or serve as endpoints for treatments.
Salivary biomarkers can be an effective tool also in detecting caries in earlier stages and assessing a patient's caries risk. These biomarkers include nitrite, nitrate, immunoglobulin A, mucin, histatin and proline- rich proteins. A recent systematic review has demonstrated that nitrite levels in saliva serve as a reliable biomarker for dental caries in children (21). However, further research in this area is necessary. Additionally, the application of Artificial Intelligence (AI) could enhance the capability of salivary biomarkers to accurately diagnose and manage several oral diseases, including caries and periodontitis (22).
Oral-systemic axis
Over the past decades, there has been a growing recognition of the bidirectional connection between oral health, particularly periodontal health, and overall systemic health. This has led to a heightened awareness among physicians regarding the significance of prioritizing oral health alongside systemic health, given that oral cavity serves as a significant reservoir of microorganisms (23). Special high-risk groups emphasize the interplay between oral and systemic health. The suggested mechanisms that facilitate the connection between oral and systemic health involve predisposing and precipitating factors. These encompass genetic elements like gene polymorphisms, environmental influences such as stress and habits like smoking, and dietary choices featuring high-fat or heavily processed foods. Furthermore, medications, microbial dysbiosis, bacteremia, and changes in the host immune response contribute to mediating this relationship (24).
Specifically, periodontitis and periodontal pathogens have been associated with diabetes mellitus, metabolic syndrome, obesity, eating disorders, liver disease, cardiovascular disease, Alzheimer disease, rheumatoid arthritis, adverse pregnancy outcomes, and cancer (24–26).
Diabetes mellitus
Diabetes mellitus is a well- established risk factor for periodontitis (8), with some experts even considering it as “the sixth complication of diabetes mellitus” (27). Additionally, active periodontitis has been shown to adversely impair glycemic control, resulting in hyperglycemia that produces advanced glycation end products (AGE). In turn, AGEs stimulate the overproduction of pro- inflammatory cytokines such as IL-6, IL-1 and Tumor Necrosis Factor alpha (TNF-α). As a consequence, the presence of AGEs in tissues impairs wound healing by disrupting collagen turnover and promoting an excessive neutrophil response to periodontal bacteria. Maintaining glycemic levels within the normal ranges is crucial for preventing periodontal complications (28). For example, studies conducted by Preshaw et al. and Steigmann et al. revealed a marked rise in the risk of periodontal-related issues with higher levels of capillary blood glucose (29, 30). Additionally, periodontal therapy has been shown to have a positive effect in reducing insulin resistance leading to improvement in glycemic control (28).
Coronary heart disease
Porphyromonas gingivalis induces vascular permeability by degrading endothelial adhesion molecules via gingipain-dependent mechanisms. This activity can elevate platelet aggregation, enhance leukocyte adhesion, and increase secretion of pro-inflammatory cytokines, all contributing to the development and progression of atherosclerosis, the primary cause of cardiovascular disease. Immunolocalization studies have identified P. gingivalis in 42% of infectious agents present in atherosclerotic plaques. These microorganisms, located within unstable plaque regions, pose risks such as plaque ulceration, thrombosis, and vascular cell apoptosis (31). Periodontitis results in elevated systemic levels of C- reactive protein, IL-1β, IL-6, IL-8, and TNF-α (32). These systemic markers of inflammation are also considered as predictors of current and future cardiovascular events and disease (30). Untreated periodontitis is linked to elevated plasma levels of LDL, cholesterol and triglycerides, accompanied by a decrease in HDL concentrations (28).
Obesity
Cardiovascular disease symptoms and diabetes mellitus are part of the metabolic syndrome cluster, alongside obesity. Obesity results in an imbalance between increased inflammatory stimuli, reduced anti-inflammatory mechanisms, and persistent low-grade inflammation, triggering periodontitis and further obesity status. Obese patients are 50%–80% more likely to develop of periodontitis compared to non-obese individuals (33, 34).
Alzheimer disease
P. gingivalis has also gained attention for its implication in neurodegenerative disorders, as it can migrate from the oral cavity into the bloodstream into and then reach distal sites, such as the brain. This microbial translocation can lead to local inflammation and buildup of the hallmark signs of Alzheimer disease, including beta-amyloid deposits, tau fragmentation and tangles, and the breakdown of host protective molecules, like apolipoproteins, leading to neuron toxicity (24). Consistent with this, P.gingivalis’ DNA and gingipains have been detected in brains of individuals with Alzheimer's disease, as well as in cerebrospinal fluid (35).
IL-1 gene polymorphism
Individuals with severe periodontal genotypes may exhibit susceptibility alleles more frequently. IL-1, a potent pro-inflammatory cytokine, plays a crucial role in the pathogenesis of numerous chronic human conditions, including cardiovascular, metabolic, and autoimmune diseases (36). In the context of periodontitis, the IL-1 genotype has been shown to regulate local inflammatory processes (9, 37) and conversely, individuals with stage III and IV periodontitis were found to have a higher likelihood of cardiovascular disease compared to those with stage I (adjusted odds ratio 3.59) (38).
Rheumatoid arthritis
Individuals with moderate to severe periodontitis are at a heightened risk of developing rheumatoid arthritis. There's a suggestion that periodontal disease could be a contributing factor in both initiating and sustaining the autoimmune inflammatory response characteristic of rheumatoid arthritis (39). Reviews of studies have shown that erythrocyte sedimentation rate and C- reactive protein levels may be raised in rheumatoid arthritis patients with periodontitis. Furthermore, serum antibodies targeting periodontal pathogenic bacteria, like Porphyromonas gingivalis have been found in the synovium. P. gingivalis’ presence may also worsen rheumatoid arthritis by triggering the production of anticitrullinated protein antibodies (40).
Nutritional support
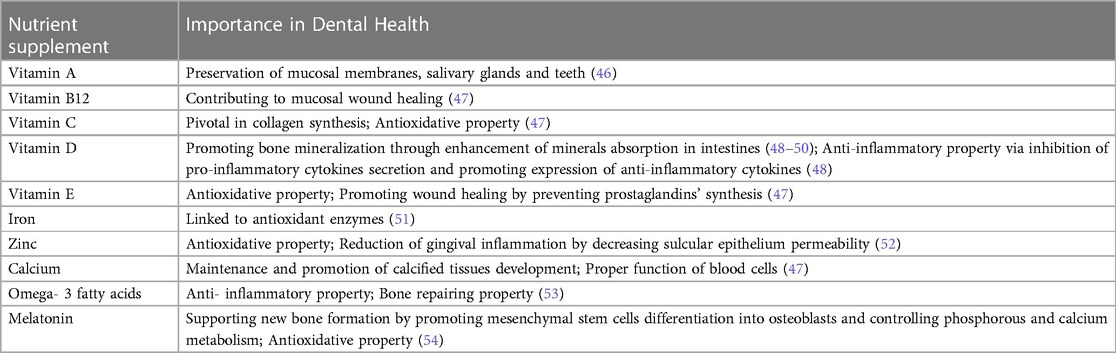
The importance of nutrition and its role in contributing to periodontal disease has been already described (41–43). While a balanced diet is crucial for maintaining periodontal health particularly, it is also essential for optimal tissue repair and immune function before and after periodontal procedures. Supplementation of specific nutrients that could be lacking may be necessary. Therefore, emphasis is placed on nutritional supplements for ensuring optimal regenerative treatment (44, 45). The supplemented nutrients and their role in oral health are summarized in Table 1.
Vitamins
Vitamin A, a fat-soluble vitamin, is crucial for the preservation of the mucosal membranes, salivary glands, and teeth (46). The vitamin B complex family has an importance in cell metabolism, repair, and proliferation. Deficiency in vitamin B can lead to various diseases and symptoms; oral manifestations can be seen as angular cheilitis, glossitis and gingival bleeding. Zong et al.'s recent study revealed a reverse correlation between the severity of periodontitis and serum vitamin B12 levels. Increased levels of vitamin B12 were associated with a decrease in the clinical parameters of periodontitis since vitamin B12 is known to contribute towards mucosal wound healing and facilitating bone health, both of which are necessary for recovery in cases of periodontitis (47).
Vitamin C, also known as ascorbic acid, is a pivotal element for collagen synthesis and preventing oxidative stress. Its deficiency results in Scurvy disease, which has periodontal hallmarks as well, notably bleeding, inflamed and painful gums. Given its beneficial impact on periodontal health, several in vitro studies suggest applying vitamin C in coatings or gels to promote osseointegration of dental implants and improve post- surgical healing. Additionally, for smokers, the application of ascorbic acid can be utilized to reduce the typical periodontal tissue breakdown in these patients (47).
Vitamin D also contributes to oral health preservation by promoting bone mineralization through enhanced absorption of calcium, magnesium, zinc and phosphate in the intestine. Deficiencies in vitamin D have been associated with chronic diseases such as periodontitis and diabetes and with tooth decay (48–50), Extensive studies have explored the anti- inflammatory properties of vitamin D inflammation; vitamin D inhibits the production of pro-inflammatory cytokines like IL-6 and IL-8, which are involved in acute inflammation, while promoting the expression of the anti-inflammatory cytokine IL-10. Furthermore, studies have revealed lower levels of vitamin D levels in patients with periodontitis compared to healthy patients, and its supplementation has been shown to improve periodontal parameters (48). These findings highlight the regulatory role of vitamin D in both the innate and adaptive immune system and its crucial impact on inflammation in the body, particularly in dental aspects (55–57).
Vitamin E plays a crucial role in oral health maintenance by inhibiting the reaction of free radicals, thereby aiding in membrane stabilization. Beyond its antioxidant property, vitamin E has demonstrated positive effects on periodontal health by intruding on the prostaglandins’ synthesis, which is helpful in reducing inflammation level and improving wound healing (47).
Minerals
In a recent study conducted by Chakraborty et al., it was revealed that iron-deficiency anemia is linked to a decline in antioxidant enzymes, leading to increased oxidative stress and a worsening of periodontal diseases (51).
Zinc is reported to decrease the sulcular epithelial permeability by inhibiting the leukocyte activity, thus decreasing gingival inflammation via decreasing gingival fluids. Furthermore, it was noticed that zinc acts to counteract reactive oxygen species and bacterial toxins, promoting healthy periodontium (52).
Calcium is indispensable for the maintenance and development of calcified tissues like bones and teeth. Additionally, it plays a crucial role in the proper functioning of blood cells. A deficiency in dietary calcium can also affect periodontal health. Co-supplementation of calcium and vitamin D is frequently employed and has been shown to have a beneficial impact on the outcomes of periodontal therapy. Research has demonstrated that the local administration of calcium, in the form of hydroxyapatite, improves the osseointegration of dental implants (47).
The protective effects of fluoride against tooth decay have long been recognized. Fluoride works by strengthening enamel and cementum through the formation of fluoroapatite, while also inhibiting bacterial growth and adhesion, effectively preventing caries. Consequently, various forms of topical fluoride, such as mouth washes, toothpaste, gels, foams, and varnishes, are commonly used as preventive measures against dental caries. Furthermore, recognizing its benefits, fluoride has been integrated into different restorative materials like glass ionomers. Systemic fluoride can be administered through water, milk, or capsules, with supplementation ranging from 0.25 to 1 mg per day depending on the existing fluoride concentration in drinking water (47).
Omega-3-fatty acids and melatonin
Omega-3 fatty acids and melatonin are recognized for their anti-inflammatory and bone repairing properties. By inflammation control, omega-3 fatty acids indirectly reduce the excessive breakdown of connective tissue and bone, while also enhancing the blood flow to affected areas. Also, omega-3 fatty acids have an impact on prostaglandin production, thereby altering bone metabolisms (53). On the other hand, melatonin is a hormone which regulates various factors such as calcitonin, growth factors and corticosterone, all of which are involved in bone metabolisms. Moreover, melatonin promotes mesenchymal stem cells differentiation into osteoblasts and can regulate phosphorus and calcium metabolism, thus supporting new bone formation and repair. Melatonin's antioxidant properties enable it to neutralize oxidative stress in the oral cavity, which has been linked to periodontal disease (54). Therefore, supplementation with omega-3 fatty acids and melatonin has been reported to enhance outcomes in regenerative dentistry, plaque score improvements, reduction of gingival inflammation and enhancement of periodontal healing (53, 54).
Point-of-care technologies
Patient involvement
Generally, personalized medicine not only concentrates on the recognition of associations between systemic diseases and contributing factors but also aims to tailor treatments based on an individual's unique characteristics and preferences. Therefore, by involving patients in decision- making discussions regarding medical approaches, clinicians acknowledge the importance of autonomy to make decisions about their healthcare and highlight the effects on their well- being. Furthermore, patients often rely on information from different sources when being confronted with unfamiliar problems. By providing support during the decision- making processes, clinical staff have the opportunity to be transparent about the complexity of the medical situations and inform the patients about genetic testing, biomarkers, treatment options, and potential risks or benefits. This allows the engagement in discussions resulting in a clear understanding of potential treatment options and the implementation of lifestyle modifications and required habit changes. Throughout this process, clinicians are able to address associated uncertainties and promote trust in patient-centered care, which is more likely to result in the adhesion to the treatment plan and, thus, improved patient satisfaction (58, 59).
Periodical monitoring
Regular testing of biomarkers may be beneficial for patients who are at risk for deficiencies or pathogenicity. Constant monitoring allows risk stratification of patients into low to high categories, considering both modifiable and nonmodifiable risk factors, as well as immune-metabolic indicated by biomarkers. The oral phenotype, determined by genetic, environmental, and microbial factors, is reflected by tissue integrity and immune functions that control the pathogenicity of the oral biofilms. Integrating gene biomarkers with conventional risk factors in a personalized medicine approach enables population stratification, aiding in resource allocation for preventive dentistry (60).
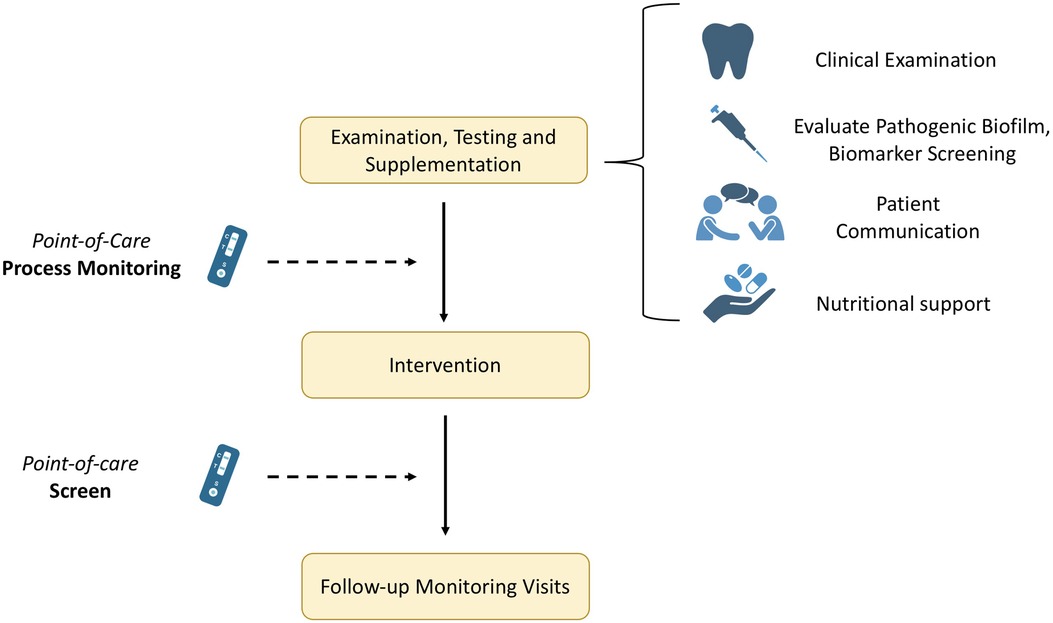
Apart from the rapid diagnosis, novel approaches allow for the implementation of various tests over an extended period to monitor progress or assess healing parameters, thereby preventing complications or relapse (Figure 2). When supplementation is needed, the periodic monitoring of biomarker levels is crucial to follow any fluctuation in serum concentrations. Thus, these novel testing methodologies offer a more advanced approach via the point-of-care testing and the optimization of the whole process. Therefore, mapping the levels before and after e.g., a surgical intervention is easier and with minimized outlay. Likewise, the importance of regular preventative visits is highlighted in literature. Giannobile et al. investigated insurance claims for 16 years for 5,117 adults retrospectively for tooth extraction events and reported significantly reduced tooth loss rates associated with increased preventive visits (60). Hence, emphasizing the importance of patient awareness, enhanced regular check-ups, as well as integration of marker screening and nutritional monitoring in patients’ treatment approaches to support improved clinical outcomes.
Point-of-care testing and triaging
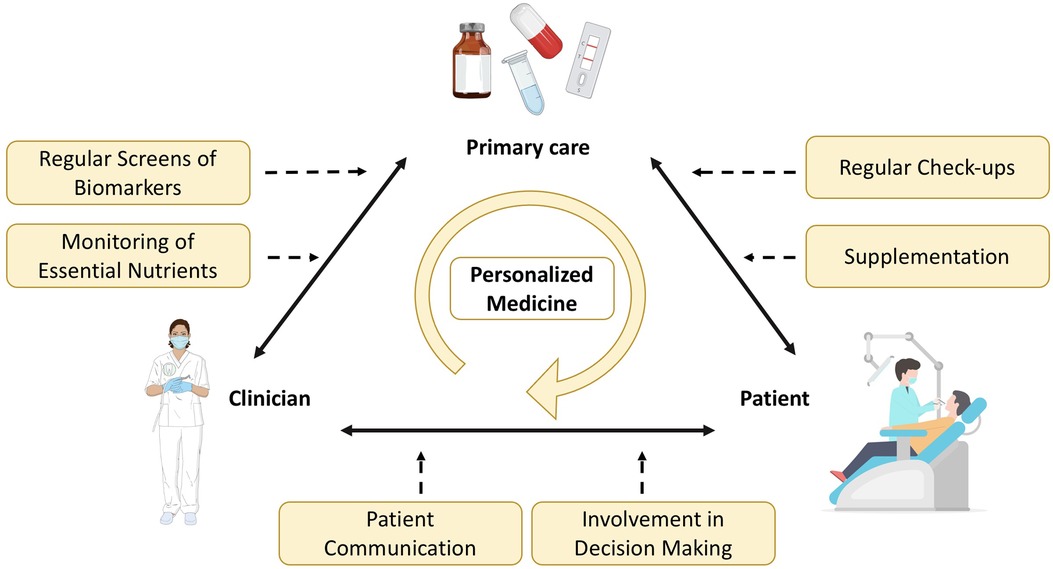
The development of new point-of-care technologies has revolutionized the way clinicians can test marker levels directly in their offices, eliminating the need for external laboratories involvement. This advancement not only allows for rapid diagnostics but also facilitates the formulation of personalized treatment plan (Figure 3). Furthermore, reducing chair time in dental offices improves efficiency and productivity for staff while potentially enhancing the overall patient's dental experience, as patients commonly associate dental procedures with discomfort. By minimizing chair time, patients are spared the inconvenience of scheduling additional appointments at external laboratories and then returning to the dental office for further consultations. Additionally, shorter chair time may contribute to more efficient scheduling and better outcomes. Instead, it can guide and triage the patient to any follow up or consultation in medical practice that are necessary post-screening. This interdisciplinary workflow, starting in the dental office, promotes pre-appointment preparation and excludes any additional testing reducing likelihood of missed cancer screening or skipped appointments for patients with limited time available. Ultimately, reducing chair time encourages patients to prioritize their oral health and ensures that they receive the necessary care promptly.
Integrated primary care
Oral health is increasingly recognized as an integral part of general health, prompting the integration of dental care into primary healthcare services (Figure 3). This shift has put the spotlight on various risk factors such as lifestyle, systemic conditions, diet and smoking, which contribute to oral diseases. Integration strategies not only advocate to raise awareness about oral health but also seek to enhance individual's willingness to prioritize it. The integration of oral health into primary care has been implemented to reduce the burden of oral diseases within the communities and to promote health education and empowerment. For example, the Longwood Harvard Dental Center (HDC) has launched an innovative project known as the Nurse Practitioner-Dentist (NPD) model for Primary Care in a dental practice environment. Recognizing the pivotal role nurse practitioners play in providing comprehensive healthcare services to patients, such as physical examinations, annual wellness check- ups, and counseling on nutrition and tobacco cessation. The program's goal is to optimize the value of each patient encounter by improving outcomes and delivering more holistic care through the seamless integration of these services into the dental visit. Other strategies concentrate on building interdisciplinary networks, training non-dental care providers and implementing e-health technologies (61). The ultimate goal is to integrate oral health into overall health, acknowledging the relationship between oral and systemic well-being.
Conclusion
This review emphasizes the crucial integration of innovative healthcare concepts and tools into dental practices. By adopting a more holistic view of the patient, clinicians can tailor treatments to each individual's predispositions, lifestyle, and oral health conditions. The data also highlights the potential of salivary biomarkers and point-of-care technologies in enhancing early diagnostic accuracy and personalizing treatment. Bridging the gap between oral and systemic health is the most effective approach to improving patient quality of life. These findings underscore the importance of continued interdisciplinary collaboration in dentistry.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
LS: Conceptualization, Formal Analysis, Supervision, Writing – original draft, Writing – review & editing. ŽK: Data curation, Investigation, Visualization, Writing – original draft, Writing – review & editing. JK: Data curation, Investigation, Writing – original draft, Writing – review & editing. KN: Writing – review & editing, Methodology. MF: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Reddy MS, Shetty SR, Vannala V. Embracing personalized medicine in dentistry. J Pharm Bioallied Sci. (2019) 11:S92–6. doi: 10.4103/JPBS.JPBS_297_18
2. Sischo L, Broder HL. Oral health-related quality of life: what, why, how, and future implications. J Dent Res. (2011) 90:1264–70. doi: 10.1177/0022034511399918
3. Sanz M, Beighton D, Curtis MA, Cury JA, Dige I, Dommisch H, et al. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J Clin Periodontol. (2017) 44(Suppl 18):S5–S11.28266109
4. Marsh PD, Zaura E. Dental biofilm: ecological interactions in health and disease. J Clin Periodontol. (2017) 44(Suppl 18):S12–S22. doi: 10.1111/jcpe.12679
5. Larsen T, Fiehn N-E. Dental biofilm infections—an update. APMIS. (2017) 125:376–84. doi: 10.1111/apm.12688
6. Steigmann L, Maekawa S, Sima C, Travan S, Wang C-W, Giannobile WV. Biosensor and lab-on-a-chip biomarker-identifying technologies for oral and periodontal diseases. Front Pharmacol. (2020) 11:588480. doi: 10.3389/fphar.2020.588480
7. Giannobile WV. Salivary diagnostics for periodontal diseases. J Am Dent Assoc. (2012) 143:6S–11. doi: 10.14219/jada.archive.2012.0341
8. Kinney J, Morelli T, Braun T, Ramseier C, Herr A, Sugai J. Erratum. J Dent Res. (2011) 90:1037–1037. doi: 10.1177/0022034511399908
9. Kornman KS, Crane A, Wang HY, di Giovine FS, Newman MG, Pirk FW, et al. The interleukin-1 genotype as a severity factor in adult periodontal disease. J Clin Periodontol. (1997) 24:72–7. doi: 10.1111/j.1600-051X.1997.tb01187.x
10. Salminen A, Gursoy UK, Paju S, Hyvärinen K, Mäntylä P, Buhlin K, et al. Salivary biomarkers of bacterial burden, inflammatory response, and tissue destruction in periodontitis. J Clin Periodontol. (2014) 41:442–50. doi: 10.1111/jcpe.12234
11. Teng F, Yang F, Huang S, Bo C, Xu ZZ, Amir A, et al. Prediction of early childhood caries via spatial-temporal variations of oral Microbiota. Cell Host Microbe. (2015) 18:296–306. doi: 10.1016/j.chom.2015.08.005
12. Wang Y, Springer S, Mulvey CL, Silliman N, Schaefer J, Sausen M, et al. Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Sci Transl Med. (2015) 7:293ra104. doi: 10.1126/scitranslmed.aaa8507
13. Zhang C-Z, Cheng X-Q, Li J-Y, Zhang P, Yi P, Xu X, et al. Saliva in the diagnosis of diseases. Int J Oral Sci. (2016) 8:133–7. doi: 10.1038/ijos.2016.38
14. Steigmann L, Maekawa S, Kauffmann F, Reiss J, Cornett A, Sugai J, et al. Changes in salivary biomarkers associated with periodontitis and diabetic neuropathy in individuals with type 1 diabetes. Sci Rep. (2022) 12:11284. doi: 10.1038/s41598-022-15430-0
15. Paqué PN, Herz C, Jenzer JS, Wiedemeier DB, Attin T, Bostanci N, et al. Microbial analysis of saliva to identify oral diseases using a point-of-care compatible qPCR assay. J Clin Med Res. (2020) 9(9):2945: doi: 10.3390/jcm9092945
16. Chojnowska S, Baran T, Wilińska I, Sienicka P, Cabaj-Wiater I, Knaś M. Human saliva as a diagnostic material. Adv Med Sci. (2018) 63:185–91. doi: 10.1016/j.advms.2017.11.002
17. Korte DL, Kinney J. Personalized medicine: an update of salivary biomarkers for periodontal diseases. Periodontol 2000. (2016) 70:26–37. doi: 10.1111/prd.12103
18. Taylor JJ. Protein biomarkers of periodontitis in saliva. ISRN Inflamm. (2014) 2014:593151. doi: 10.1155/2014/593151
19. Kc S, Wang XZ, Gallagher JE. Diagnostic sensitivity and specificity of host-derived salivary biomarkers in periodontal disease amongst adults: systematic review. J Clin Periodontol. (2020) 47:289–308. doi: 10.1111/jcpe.13218
20. Kinney JS, Morelli T, Oh M, Braun TM, Ramseier CA, Sugai JV, et al. Crevicular fluid biomarkers and periodontal disease progression. J Clin Periodontol. (2014) 41:113–20. doi: 10.1111/jcpe.12194
21. Díaz-Fabregat B, Ramírez-Carmona W, Cannon ML, Monteiro DR, Pessan JP, Antoniali C. Are salivary NO2- / NO2- and NO3- levels biomarkers for dental caries in children? Systematic review and meta-analysis. Nitric Oxide. (2024) 144:11–9. doi: 10.1016/j.niox.2024.01.001
22. Adeoye J, Su Y-X. Artificial intelligence in salivary biomarker discovery and validation for oral diseases. Oral Dis. (2024) 30:23–37. doi: 10.1111/odi.14641
23. Rautemaa R, Lauhio A, Cullinan MP, Seymour GJ. Oral infections and systemic disease–an emerging problem in medicine. Clin Microbiol Infect. (2007) 13:1041–7. doi: 10.1111/j.1469-0691.2007.01802.x
24. Kapila YL. Oral health’s inextricable connection to systemic health: special populations bring to bear multimodal relationships and factors connecting periodontal disease to systemic diseases and conditions. Periodontol 2000. (2021) 87:11–6. doi: 10.1111/prd.12398
25. Ma KS, Hasturk H, Carreras I, Dedeoglu A, Veeravalli JJ, Huang JY, et al. Dementia and the risk of periodontitis: a population-based cohort study. J Dent Res. (2022) 101:270–7. doi: 10.1177/00220345211037220
26. Zepeda-Rivera M, Minot SS, Bouzek H, Wu H, Blanco-Míguez A, Manghi P, et al. A distinct Fusobacterium nucleatum clade dominates the colorectal cancer niche. Nature. (2024) 628:424–32. doi: 10.1038/s41586-024-07182-w
27. Nibali L, Gkranias N, Mainas G, Di Pino A. Periodontitis and implant complications in diabetes. Periodontol 2000. (2022) 90:88–105. doi: 10.1111/prd.12451
28. Mainas G, Ide M, Rizzo M, Magan-Fernandez A, Mesa F, Nibali L. Managing the systemic impact of periodontitis. Medicina (B Aires). (2022) 58(5):621. doi: 10.3390/medicina58050621
29. Steigmann L, Gunaratnam S, Giannobile WV, Van Til M, Daignault-Newton S, Herman WH, et al. Poor glycemic control increases dental risk in a Sri Lankan population. Healthcare (Basel). (2024) 12(3):358. doi: 10.3390/healthcare12030358
30. Preshaw PM, de Silva N, McCracken GI, Fernando DJS, Dalton CF, Steen ND, et al. Compromised periodontal status in an urban Sri Lankan population with type 2 diabetes. J Clin Periodontol. (2010) 37:165–71. doi: 10.1111/j.1600-051X.2009.01519.x
31. Chiu B. Multiple infections in carotid atherosclerotic plaques. Am Heart J. (1999) 138:S534–6. doi: 10.1016/S0002-8703(99)70294-2
32. Isola G, Santonocito S, Lupi SM, Polizzi A, Sclafani R, Patini R, et al. Periodontal health and disease in the context of systemic diseases. Mediators Inflamm. (2023) 2023:9720947. doi: 10.1155/2023/9720947
33. Pamuk F, Kantarci A. Inflammation as a link between periodontal disease and obesity. Periodontol 2000. (2022) 90:186–96. doi: 10.1111/prd.12457
34. Chaffee BW, Weston SJ. Association between chronic periodontal disease and obesity: a systematic review and meta-analysis. J Periodontol (2010) 81(12):1708–24. doi: 10.1902/jop.2010.100321
35. Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, et al. Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. (2019) 5:eaau3333. doi: 10.1126/sciadv.aau3333
36. Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. (2010) 10:89–102. doi: 10.1038/nri2691
37. Offenbacher S, Jiao Y, Kim SJ, Marchesan J, Moss KL, Jing L, et al. GWAS For interleukin-1β levels in gingival crevicular fluid identifies IL37 variants in periodontal inflammation. Nat Commun. (2018) 9:3686. doi: 10.1038/s41467-018-05940-9
38. Sumayin Ngamdu K, Mallawaarachchi I, Dunipace EA, Chuang L-H, Jafri SH, Shah NR, et al. Association between periodontal disease and cardiovascular disease (from the NHANES). Am J Cardiol. (2022) 178:163–8. doi: 10.1016/j.amjcard.2022.05.028
39. Arigbede AO, Babatope BO, Bamidele MK. Periodontitis and systemic diseases: a literature review. J Indian Soc Periodontol. (2012) 16:487–91. doi: 10.4103/0972-124X.106878
40. de Molon RS, Rossa C Jr, Thurlings RM, Cirelli JA, Koenders MI. Linkage of periodontitis and rheumatoid arthritis: current evidence and potential biological interactions. Int J Mol Sci. (2019) 20(18):4541. doi: 10.3390/ijms20184541
41. Niu Y, Wang H, Xu X, Xiao J. The pivotal role of oral microbiota dysbiosis and microbiota-host interactions in diseases. Frontiers Media SA. (2023):198.
42. Niu Y, Xu X, Xiao J. The pivotal role of oral microbiota dysbiosis and microbiota-host interactions in diseases—volume II. Frontiers Media SA. (2023):140.
43. Dawson DR 3rd, Branch-Mays G, Gonzalez OA, Ebersole JL. Dietary modulation of the inflammatory cascade. Periodontol 2000. (2014) 64:161–97. doi: 10.1111/j.1600-0757.2012.00458.x
44. Leaf A, Weber PC. A new era for science in nutrition. Am J Clin Nutr. (1987) 45:1048–53. doi: 10.1093/ajcn/45.5.1048
45. Purvis RJ, Barrie WJ, MacKay GS, Wilkinson EM, Cockburn F, Belton NR. Enamel hypoplasia of the teeth associated with neonatal tetany: a manifestation of maternal vitamin-D deficiency. Lancet. (1973) 2:811–4. doi: 10.1016/S0140-6736(73)90857-X
46. Shodhan Shetty A, Shenoy R, Dasson Bajaj P, Rao A, Ks A, Pai M, et al. Role of nutritional supplements on oral health in adults—a systematic review. F1000Res. (2023) 12:492. doi: 10.12688/f1000research.134299.1
47. Najeeb S, Zafar MS, Khurshid Z, Zohaib S, Almas K. The role of nutrition in periodontal health: an update. Nutrients. (2016) 8(9):530. doi: 10.3390/nu8090530
48. Liang F, Zhou Y, Zhang Z, Zhang Z, Shen J. Association of vitamin D in individuals with periodontitis: an updated systematic review and meta-analysis. BMC Oral Health. (2023) 23:387. doi: 10.1186/s12903-023-03120-w
49. Wang H, Chen W, Li D, Yin X, Zhang X, Olsen N, et al. Vitamin D and chronic diseases. Aging Dis. (2017) 8:346–53. doi: 10.14336/AD.2016.1021
50. Schroth RJ, Levi JA, Sellers EA, Friel J, Kliewer E, Moffatt MEK. Vitamin D status of children with severe early childhood caries: a case-control study. BMC Pediatr. (2013) 13:174. doi: 10.1186/1471-2431-13-174
51. Chakraborty S, Tewari S, Sharma RK, Narula SC, Ghalaut PS, Ghalaut V. Impact of iron deficiency anemia on chronic periodontitis and superoxide dismutase activity: a cross-sectional study. J Periodontal Implant Sci. (2014) 44:57–64. doi: 10.5051/jpis.2014.44.2.57
52. Kaur K, Sculley D, Wallace J, Turner A, Ferraris C, Veysey M, et al. Micronutrients and bioactive compounds in oral inflammatory diseases. Journal of Nutrition & Intermediary Metabolism. (2019) 18:100105. doi: 10.1016/j.jnim.2019.100105
53. Azuma MM, Gomes-Filho JE, Ervolino E, Pipa CB, Cardoso CdB, Andrada AC, et al. Omega 3 fatty acids reduce bone resorption while promoting bone generation in rat apical periodontitis. J Endod. (2017) 43:970–6. doi: 10.1016/j.joen.2017.01.006
54. Škrlec I. The influence of dental implants on the circadian clock and the role of melatonin in the oral cavity. Exploratory Research and Hypothesis in Medicine. (2023) 8:143–9. doi: 10.14218/ERHM.2022.00052
55. Korf H, Decallonne B, Mathieu C. Vitamin D for infections. Curr Opin Endocrinol Diabetes Obes. (2014) 21:431–6. doi: 10.1097/MED.0000000000000108
56. Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. (2010) 10:482–96. doi: 10.1016/j.coph.2010.04.001
57. White JH. Vitamin D metabolism and signaling in the immune system. Rev Endocr Metab Disord. (2012) 13:21–9. doi: 10.1007/s11154-011-9195-z
58. Szabó RM, Buzás N, Braunitzer G, Shedlin MG, Antal MÁ. Factors influencing patient satisfaction and loyalty as perceived by dentists and their patients. Dent J. (2023) 11(9):203. doi: 10.3390/dj11090203
59. Chapple H, Shah S, Caress A-L, Kay EJ. Exploring dental patients’ preferred roles in treatment decision-making—a novel approach. Br Dent J. (2003) 194:321–7. discussion 317. doi: 10.1038/sj.bdj.4809946
60. Giannobile WV, Braun TM, Caplis AK, Doucette-Stamm L, Duff GW, Kornman KS. Patient stratification for preventive care in dentistry. J Dent Res. (2013) 92:694–701. doi: 10.1177/0022034513492336
Keywords: precision medicine, salivary testing, biomarkers, primary care, point-of-care, periodontal diseases
Citation: Steigmann L, Kačarević ŽP, Khoury J, Nagy K and Feres M (2024) Integration of precision medicine into the dental care setting. Front. Dent. Med 5:1398897. doi: 10.3389/fdmed.2024.1398897
Received: 11 March 2024; Accepted: 9 July 2024;
Published: 21 August 2024.
Edited by:
Siddhartha Varma, Krishna Institute of Medical Sciences, IndiaReviewed by:
Maria Contaldo, University of Campania L. Vanvitelli, ItalyJôice Dias Corrêa, Pontifical Catholic University of Minas Gerais, Brazil
Copyright: © 2024 Steigmann, Kačarević, Khoury, Nagy and Feres. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Larissa Steigmann, bGFyaXNzYV9zdGVpZ21hbm5AaHNkbS5oYXJ2YXJkLmVkdQ==
 Larissa Steigmann
Larissa Steigmann Željka Perić Kačarević2
Željka Perić Kačarević2