- 1Department of Oral Health Science, Division of Orofacial Pain, College of Dentistry, University of Kentucky, Lexington, KY, United States
- 2College of Dental Medicine - Illinois, Midwestern University, Downers Grove, IL, United States
- 3Periodontics Division, College of Dentistry, University of Kentucky, Lexington, KY, United States
- 4Chief, Periodontics Division, College of Dentistry, University of Kentucky, Lexington, KY, United States
Background: Patients who complain of mucosal burning sensations (i.e., glossodynia and gastro-esophageal reflux disease, GERD) often take multiple medications. However, the relationship between xerogenic medication intake and salivary flow in these patients has not been thoroughly examined.
Methods: A retrospective study of 192 consecutive patients diagnosed with glossodynia (ICD-10-CM) at a regional center over a six-year period was performed. Data from electronic health records were extracted and relationships between medication intake, unstimulated whole salivary flow rate (UWSFR), xerostomia, and GERD were determined by chi-square, t-test, and correlation analysis.
Results: Of 134 records that met inclusion criteria, 87.1% of patients reported daily intake of one or more xerogenic medications. Two or more xerogenic medications were taken significantly more often by patients with glossodynia reporting GERD than those with glossodynia without GERD (p = .02). UWSFR was negatively correlated with number of medications [r(103) = −.277, p = .005] and xerogenic medications [r(103) = −.195, p = .049]. The lowest UWSFR was observed with use of trazodone and cyclobenzaprine.
Conclusions: Daily xerogenic medication intake, hyposalivation, and xerostomia were commonly present and potentially interrelated in patients who suffer from glossodynia and/or GERD.
Practical implications: Clinicians should be aware of the consequences of prescribing multiple and certain xerogenic medications in reducing UWSFR, especially in patients physiologically at risk of hyposalivation such as those suffering from GERD and/or glossodynia.
1. Introduction
Saliva is a complex fluid comprised of major and minor salivary gland secretions important for intraoral hard and soft tissues (1), neutralizing acids in the oral cavity (2), and decreasing the time that acid is in contact with esophageal mucosa (3). A healthy individual produces on average 500 ml–1.5 L of saliva daily, about 0.35 ml/min (4).
Alterations in saliva, quality or quantity, can result from several etiological factors, including systemic conditions (5), such as obesity (6), diabetes (7), autoimmune disease (Sjogren's syndrome) (8), syndromes (Down syndrome) (9), neurodegenerative diseases (Alzheimer's disease) (10), infections [hepatitis (11), HIV-infection (12)]; iatrogenic cause, such as hemodialysis (13), radioactive iodine therapy (14), radio- or chemotherapy (15, 16); environmental factors, like dietary change, tobacco, alcohol or recreational drug use (17); physiological changes (18), including aging (19, 20); or medication intake (21–24). Medications, alone or in combination, are routinely prescribed to treat systemic or local conditions. However, many medications induce dry mouth and low salivary flow (25), which can contribute to mucosal atrophy and fissuring (26), dental caries (27), and fungal infections (28).
Reduced salivary volume may also contribute to glossodynia. Among studies that investigated this association, on average 64% of patients with glossodynia have xerostomia (range 23%–100%) (29–38) and 56% have hyposalivation (range 33%–100%) (29, 31–34, 37–44). Despite the established association between glossodynia and diminished salivary flow, to the best of our knowledge their relationship with xerogenic medications has not been thoroughly examined.
A comorbidity frequently seen among patients with glossodynia is gastro-esophageal reflux disease (GERD), a condition known to produce burning sensation. This can be initiated or maintained from a reduced salivary volume, which may itself contribute to symptoms felt in anatomical structures distal to the mouth (45). In the available literature, eleven studies measured unstimulated salivary flow (UWS) in patients with GERD, and four of these conducted on a total of 351 subjects confirmed a significant reduction in UWS, compared to 297 healthy controls (46–49). Moreover, patients with GERD tend to be managed with further medication intake, that can contribute to decreased salivary volume. Hence, the vicious cycle between xerogenic medications, salivary flow, and GERD may be important. However, few studies have investigated the presence of GERD in patients diagnosed with glossodynia with respect to xerogenic medications (50–55).
Therefore, the objectives of this study were to investigate the patients diagnosed with glossodynia (1) the prevalence of xerogenic medication; (2) the association between salivary flow volume and medication use; (3) which medications were associated with hyposalivation; and (4) the presence and association of xerostomia and hyposalivation with or without GERD. The null hypothesis was that patients with glossodynia are unlikely to take xerogenic medications and have concurrent GERD symptomatology.
2. Methods
A retrospective study was conducted on data collected from consecutive patients with a complaint of burning mouth sensation, seen at the Orofacial Pain Center (University of Kentucky, Lexington, United States) between January 2014 and April 2020. Due to the retrospective design of the study, a sample size calculation was not performed. However, a post-hoc power analysis on a sample size of 103 revealed a power of 81%.
Patient's electronic health records were reviewed for eligibility, according to the following inclusion criteria: (1) diagnosis of glossodynia (International Classification of Disease ICD-10-CM, Diagnosis Code-14.6); and (2) oral burning pain rated greater than 0, on a 0–10 numerical rating scale (NRS, 0 = “no pain” and 10 = “worst possible pain”). Patients were categorized as presenting with GERD symptomatology when they had either a physician diagnosis or self-reported GERD on intake forms. Exclusion criteria included patients with complaints of oral burning due to known condition (anemia, oral lesion, diabetes mellitus, Sjögren's syndrome, vitamin deficiency, or use of angiotensin converting enzyme inhibitors). Although GERD has been classified among underlying medical conditions associated with oral burning symptoms, for the current investigation, participants with a complaint of GERD were included in the study population. The study was approved by the Institutional Review Board of the Office of Research Integrity at the University of Kentucky (Lexington KY, United States; IRB #74332).
2.1. Procedure
All patients underwent a clinical evaluation for their oral burning complaint by the same Diplomate of the American Board of Oral Medicine (C.M.). A thorough medical history was collected with specific questions that investigated chief complaint, systemic medical conditions, medications, parafunctional habits, alcohol consumption, smoking status, daily physical activity, and laboratory results.
2.1.1. Oral burning sensation
The chief complaint was investigated by ascertaining the pain location, quality, frequency, and duration, the presence of taste disturbances, and triggering, aggravating, and relieving factors. Pain intensity was collected on a 0–10 NRS, with 10 being the worst possible pain.
2.1.2. Medications
The number and types of prescribed medication were recorded, with a focus on those known to be xerogenic (21, 22, 56).
2.1.3. Xerostomia
Xerostomia, defined as a subjective complaint of dry mouth (57–59), was assessed by self-report or with the Short-Form Xerostomia Inventory questionnaire (SXI) (60). The following statements on the SXI: “My mouth feels dry”; “My mouth feels dry when eating a meal”; “I have difficulties in eating dry food”; “I have difficulties swallowing certain foods”; and “My lips feel dry” were scored as “Never” 1; “Occasionally” 2; and “Often” 3. A cut-off of 10 or higher was used as clinical definition of “xerostomia” (personal communication with WM Thomson) (60).
2.1.4. Hyposalivation
Unstimulated whole salivary flow rate (UWSFR) was measured by instructing the patient to allow saliva to collect in the mouth and spit into a cup every 20 s for 5 min, while sitting upright and undisturbed in a comfortable position. Samples were collected between 1 pm and 4 pm, with participants refraining from eating or drinking one hour before the procedure (61). Hyposalivation was defined as UWSFR < 0.2 ml/min, which represents the lowest 10th percentile as determined in our clinic (data not shown) and many previous studies (62, 63).
UWSFR of all patients was measured by the same Diplomate of the American Board of Oral Medicine (C.M.) to increase the reproducibility and standardization of the procedure.
2.2. Statistical analysis
Data normality was checked with a Shapiro-Wilk test. Variables were expressed as means and standard deviations. Hyposalivation, GERD symptomatology, and xerostomia were coded as dichotomous variables (1 = yes, 0 = no). Medications and salivary flow rate were coded as continuous variables. Descriptive statistics were completed for patients with data on salivary flow rate and xerostomia. Bivariate correlation analysis was performed to test the association between UWSFR and medication (number of medications, number and types of xerogenic medications), and between UWSFR and GERD. According to GERD symptomatology, the total population was divided in two groups (patients presenting with GERD symptoms = 1; patients not presenting with GERD symptoms = 0). Chi-square and McNemar tests were used when appropriate to compare the two groups in terms of gender, number, and type of medication. Independent t-test was used to compare the two groups in terms of age, medical conditions, pain intensity, xerostomia, and UWSFR. Statistical significance was set at α = 0.05. Data were analyzed with SPSS (IBM SPSS Statistics Macintosh, Version 27.000, IBM Corp, Armonk, NY).
3. Results
Of 192 records identified, 58 were excluded due to missing data or a diagnosis different from glossodynia (IDC-10 14.6). A total of 134 patients diagnosed with glossodynia (mean age of 63.43 ± 11.84, 80.6% females) were included in the analysis (Table 1). The mean pain intensity was 6.30 ± 2.39, and 38.1% reported a taste disturbance. On average, patients had 5.17 ± 3.28 systemic medical conditions, with hypertension being the most common (44.8%), followed by GERD (44.0%) and depression (22.4%). Salivary flow was measured in 104 patients (77.6%). Their mean UWSFR was 0.24 ± 0.30 ml/min, and 68 (65.4%) had hyposalivation. Xerostomia was determined by questionnaire to be present in 76 patients out of 98 (77.6%), and the SXI yielded a mean score of 12.84 ± 5.90. Seventy-eight percent of xerogenic patients had hyposalivation [χ2(104) = 15.11, p = .001]. Of those, 38 (50.7%) reported GERD symptoms.
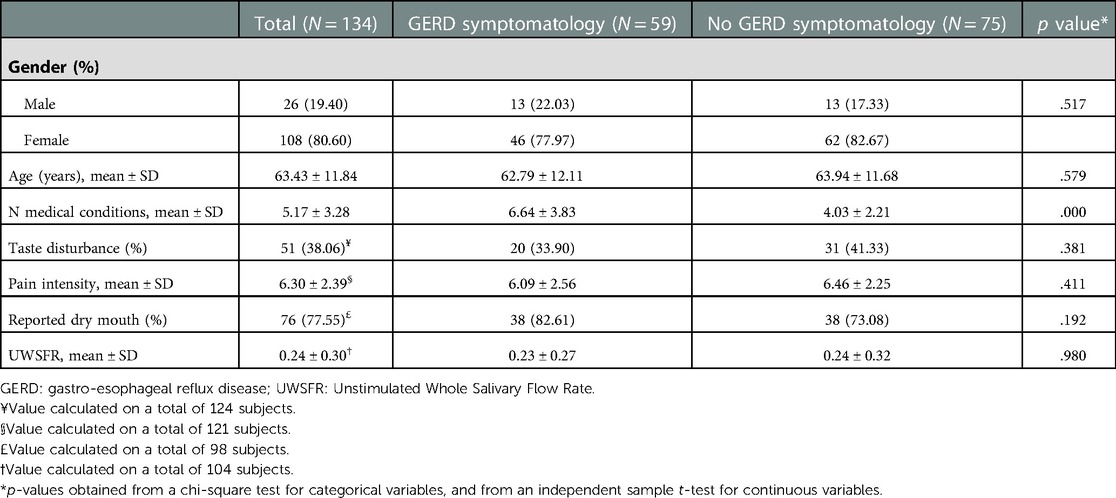
Table 1. Characteristics of the study population and differences between participants with and without GERD symptomatology.
3.1. Medication and salivary flow rate
The mean number of daily prescribed medications for the 134 patients diagnosed with glossodynia was 5.5 ± 3.49. A significant negative correlation was found between the number of medications and UWSFR [r(103) = −.277, p = .005, Figure 1A]. The mean number of daily xerogenic medications of the total sample size was 3.11 ± 2.22. Overall, 72.7% reported taking 2 or more xerogenic medications; 14.4% reported taking 1 xerogenic medication, and 12.9% of the total participants did not report any daily xerogenic medication intake. A negative correlation was found between the number of xerogenic medications and UWSFR [r(103) = −.195, p = .049, Figure 1B]. The most common xerogenic medications were selective serotonin reuptake inhibitors (SSRIs, 31.3%) and non steroid anti-inflammatory drugs (NSAIDS, 13.4%) (Table 2). Daily use of omeprazole was ascertained in 27.6% of the patients. Daily cyclobenzaprine [r(104) = −.201, p = .041] and ibuprofen use [r(104) = −.206, p = .036] were negatively associated with UWSFR. The medication profiles between patients reporting GERD and those not reporting GERD were similar (all p's > 0.05, Table 2). Not surprisingly, daily intake of omeprazole was significantly higher in those with GERD symptoms (39.0% vs. 18.7%, p = .009). Also, two or more xerogenic medications were used significantly more often by patients with glossodynia reporting GERD than those who took fewer than two xerogenic meds [χ2(132) = 7.85, p = .020].
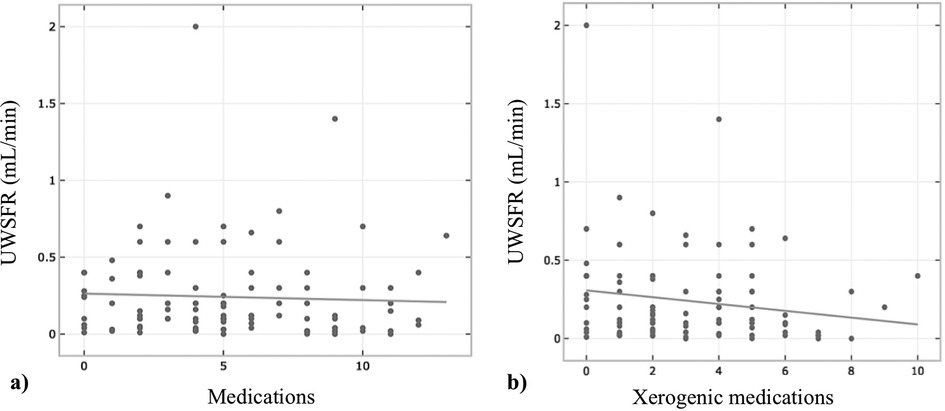
Figure 1. Relationship between number of medications (A) and xerogenic medications (B) and unstimulated whole salivary flow rate (UWSFR) (N = 104).
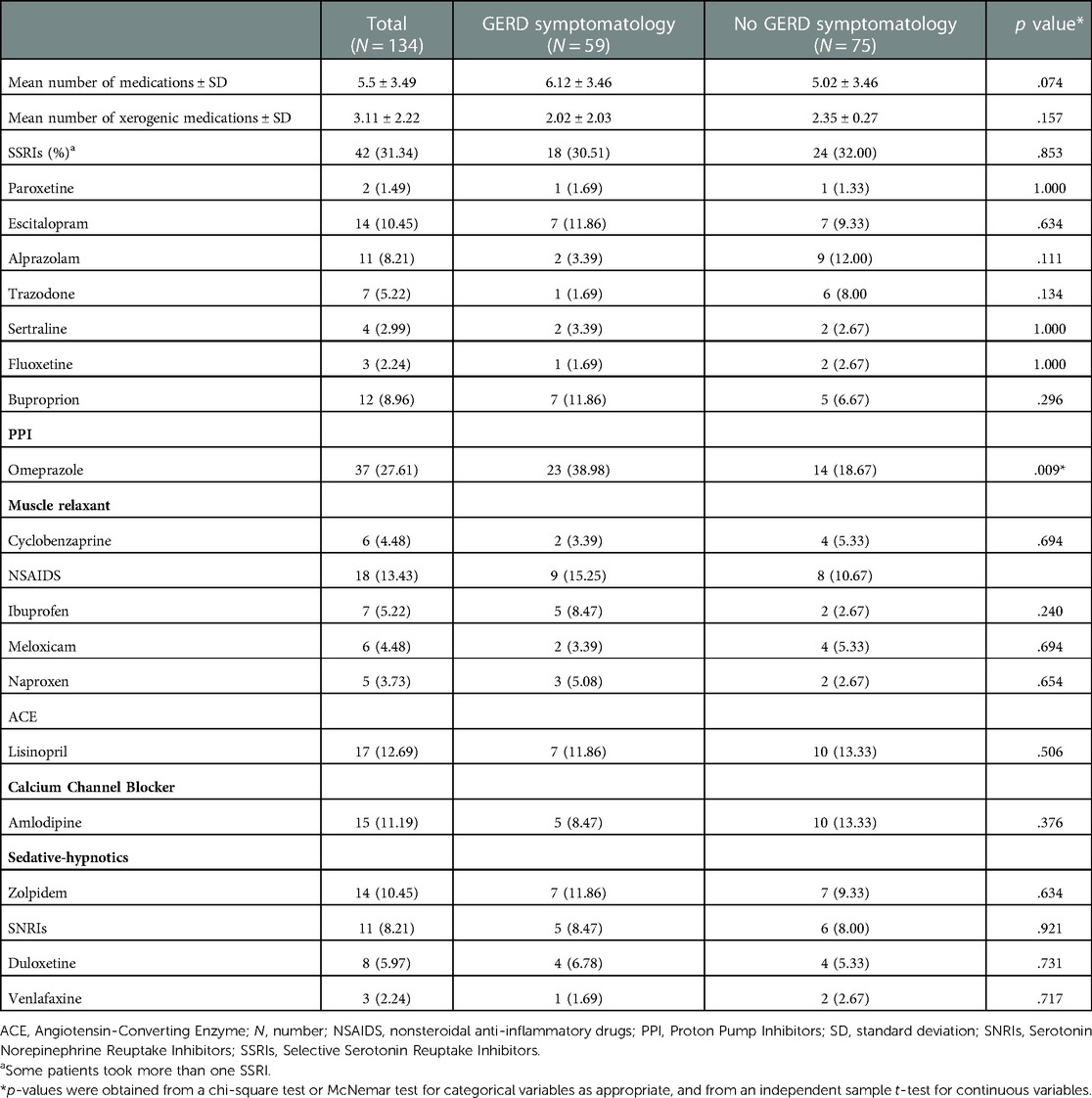
Table 2. Number and type of medication intake in the study population and differences between participants with and without GERD symptomatology.
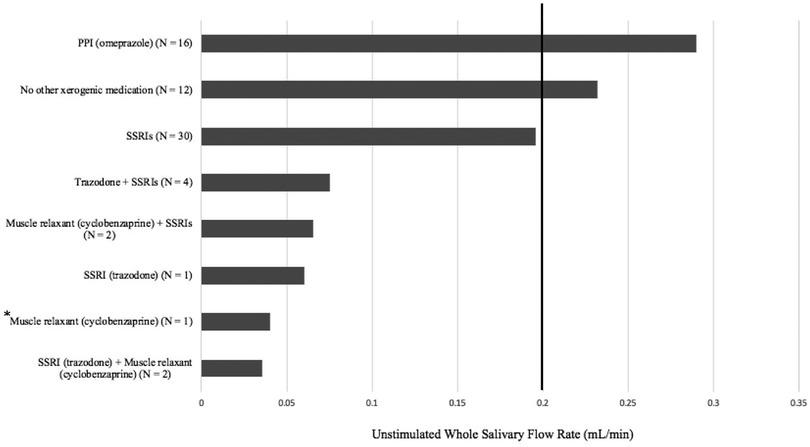
Figure 2 displays the overall mean UWSFR of patients who used xerogenic medications daily. Those with daily intake of trazodone and cyclobenzaprine alone or in combination with another drug had the lowest UWSFR (0.03 ± 0.02 ml/min); those using omeprazole did not present with hyposalivation (0.29 ± 0.19 ml/min).
3.2. UWSFR, xerostomia, and GERD
Fifty-nine of those with glossodynia (44.0%) reported GERD. Of those, 61.4% had hyposalivation. A significant difference was not found in UWSFR between patients who reported GERD symptoms and those who did not (Table 2). A difference in xerostomia was not found between participants with and without GERD [χ2(134) = 2.540, p = .194].
4. Discussion
This study is one of the first to explore the interaction between medication intake and salivary flow in patients with glossodynia, with or without GERD symptomatology. The findings indicate that the majority of patients complaining of intraoral burning took xerogenic medications. A positive association was found between the number of xerogenic medications and low UWSFR in those with glossodynia. A high prevalence of GERD symptomatology (44%) was observed in patients with oral burning sensations and daily xerogenic medication intake. Despite this frequency, the presence of GERD and omeprazole use by themselves were not related to salivary flow rate.
Xerostomia was identified in 78% of patients diagnosed with glossodynia in this study, which is higher than the prevalence of xerostomia found in population-based studies (ranges from 0.9% to 64.8%) (64–67). Several factors may explain the greater prevalence of xerostomia in our patient population. In our clinic, the intake forms ask specifically about xerostomia whereas other studies may not. Also, xerostomia is common with aging and with use of xerogenic medications. Many of our patients took xerogenic medications, and many other studies did not explore the relationship with xerogenic medications (29, 31, 35, 40, 68). Finally, xerostomia is a subjective complaint based on self-reported data that may not be reliable; however, our study attempted to make the condition more objective by the administration of, and use of data from, the SXI form.
Our study demonstrated that 78% of those reporting xerostomia had hyposalivation. The present study utilized a cut-off of 0.2 ml/min to define hyposalivation, because patients below this threshold are in the lowest 10th percentile and often have symptoms or clinical findings consistent with the presence of hyposalivation-associated disorders. This is in contrast to the literature, where a cut-off of 0.1 ml/min is a commonly used definition for hyposalivation, as originally indicated by Ericsson and Hardwick (69). In the current study, hyposalivation was observed in 65% of patients diagnosed with glossodynia, which may be attributed to the age of our patient population and the extensive list of xerogenic medications used in this cohort. Alternatively, this finding may point to the potentially intrinsic common root between hyposalivation and glossodynia. In this regard, the results of the present study corroborate the negative association between number of medications and UWSFR (39, 70–77). Specifically, the lowest UWSFRs were observed in patients with glossodynia who took a daily combination of xerogenic medications, as shown in Figure 2. These observations are consistent with studies that reported difference between monotherapy and polytherapy (75, 78). However, these findings highlight a difficulty with the management of glossodynia; that is, how do clinicians who treat these patients eliminate hyposalivation when xerogenic medications are needed for management of their comorbidities? Novel study designs may be needed to address this concern to better understand confounders vs. root causes of glossodynia.
The findings from this study help support the concept that polypharmacy and hyposalivation underlie burning sensations that occur in the mouth and upper gastro-intestinal tract (53, 79–82). In this regard, 40% of those with hyposalivation also reported GERD symptomatology, although the difference was not statistically significant. Contradictory results are found in the literature, with studies supporting a relationship between hyposalivation and GERD (83–87) and studies that fail to demonstrate a correlation (53, 80, 81, 84). This may be due to the different (1) criteria used to diagnose GERD [endoscopy (53, 81, 84) vs. questionnaires and self-reported reflux symptoms (83, 47)]; and (2) assessment methods of salivary flow [different duration (80, 81, 83), frequency (53, 80, 81), and time (53, 81, 83) of spitting method (53, 80, 81, 83, 84, 85), vs. Saxon method (48, 49), salivary scintigraphy (46), or modified cotton roll method (47)].
This study has limitations. First, the retrospective design did not permit control of confounding factors, such as medical condition, dosage of the medication taken, or allow retrieval of all the data. Hence, missing items were imputed using multiple imputation as appropriate. Future studies with more rigorous methodology, which include the comparison with a healthy control group, are needed to control for factors that may influence the results. Similarly, the association observed between UWSFR and medication intake does not prove causation. Second, medication intake was self-reported and not investigated in terms of daily frequency, dosage, duration, and compliance. These factors have been shown to influence salivary flow rate (71, 86, 87). The sample included mostly elderly females, due to age and gender predilection of glossodynia. Therefore, these findings might not be generalizable to males or larger populations. Moreover, hormonal disturbances may influence the high prevalence of hyposalivation and xerostomia, which was not taken into consideration in the present investigation. As 18.7% of patient without GERD reported to take omeprazole, it is possible that they may have been misclassified. Therefore, we reanalyzed the data by classifying these patients within the GERD group, and these analyses did not alter the results (data not shown). Similarly, the presence of other comorbid systemic conditions was not included in the analysis as a confounding factor. Last, although an interesting trend was seen between UWSFR and type of medication, some of the findings are based on small sample size (e.g., medication use) and should be interpreted with caution until larger studies are performed.
5. Conclusions
These findings suggest that polypharmacy medication intake, hyposalivation, and xerostomia are commonly seen among patients with glossodynia and/or suffering from GERD. Because of these associations and the possible effect of burning sensation secondary to medication-induced hyposalivation, clinicians should be cautious in prescribing xerogenic medications and consider ways to deprescribe xerogenic medications in patients who display concurrent hyposalivation and mucosal burning sensations to help evaluate alleviation of symptoms.
Data availability statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by by the Institutional Review Board (IRB, #74332) of the Office of Research Integrity at the University of Kentucky. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization: CM; data curation: LS, WE; formal analysis: LS; methodology: CM; supervision: CM; roles/writing: LS, CM; writing – review and editing: LS, CM. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mese H, Matsuo R. Salivary secretion, taste and hypo salivation. J Oral Rehabil. (2007) 34:711–23. doi: 10.1111/j.1365-2842.2007.01794.x17824883
2. Helm JF, Dodds WJ, Hogan WJ, Soergel KH, Egide MS, Wood CM. Acid neutralizing capacity of human Saliva. Gastroenterology. (1982) 83:69–74. doi: 10.1016/S0016-5085(82)80286-27075945
3. Helm JF, Dodds WJ, Pelc LR, Palmer DW, Hogan WJ, Teeter BC. Effect of esophageal emptying and Saliva on clearance of acid from the esophagus. New Eng J Med. (1984) 310:284–8. doi: 10.1056/NEJM1984020231005036690951
4. Humphrey SP, Williamson RT. A review of Saliva: normal composition, flow, and function. J Prosthet Dent. (2001) 85:162–9. doi: 10.1067/mpr.2001.11377811208206
5. Saleh J, Zancanaro Figueiredo MA, Cherubini K, Gonçalves Salum F. Salivary hypofunction: an update on aetiology, diagnosis and therapeutics. Arch Oral Biol. (2015) 60:242–55. doi: 10.1016/j.archoralbio.2014.10.00425463902
6. de Andrade PAM, Giovani PA, Araujo DS, de Souza AJ, Pedroni-Pereira A, Kantovitz KR, et al. Shifts in the bacterial community of Saliva give insights on the relationship between obesity and oral Microbiota in adolescents. Arch Microbiol. (2020) 202:1085–95. doi: 10.1007/s00203-020-01817-y32034425
7. Khovidhunkit SO, Suwantuntula T, Thaweboon S, Mitrirattanakul S, Chomkhakhai U, Khovidhunkit W. Xerostomia, hyposalivation, and oral microbiota in type 2 diabetic patients: a preliminary study. J Med Assoc Thai. (2009) 92(9):1220–8. PMID: 19772183.19772183
8. Jensen SB, Vissink A. Salivary gland dysfunction and Xerostomia in sjögren's syndrome. Oral Maxillofac Surg Clin North Am. (2014) 26:35–53. doi: 10.1016/j.coms.2013.09.00324287192
9. Kawai M, Ito N, Ayuse T. Changes in surface tension of Saliva in down syndrome. Eur Rev Med Pharmacol Sci. (2018) 22:6469–74. doi: 10.26355/eurrev_201810_1606030338816
10. Zalewska A, Klimiuk A, Zięba S, Wnorowska O, Rusak M, Waszkiewicz N, et al. Salivary gland dysfunction and salivary redox imbalance in patients with Alzheimer's disease. Sci Rep. (2021) 11:23904. doi: 10.1038/s41598-021-03456-934903846
11. Maldonado JO, Beach ME, Wang Y, Perez P, Yin H, Pelayo E, et al. HCV Infection alters salivary gland histology and Saliva composition. J Dent Res. (2022) 101:534–41. doi: 10.1177/0022034521104939535045743
12. Li Y, Saxena D, Chen Z, Liu G, Abrams WR, Phelan JA, et al. HIV Infection and microbial diversity in Saliva. J Clin Microbiol. (2014) 52:1400–11. doi: 10.1128/JCM.02954-1324523469
13. Yu IC, Liu CY, Fang JT. Effects of hemodialysis treatment on Saliva flow rate and Saliva composition during in-center maintenance dialysis: a cross-sectional study. Ren Fail. (2021) 43:71–8. doi: 10.1080/0886022X.2020.185776933327832
14. Singer MC, Marchal F, Angelos P, Bernet V, Boucai L, Buchholzer S, et al. Salivary and lacrimal dysfunction after radioactive iodine for differentiated thyroid cancer: american head and neck society endocrine surgery section and salivary gland section joint multidisciplinary clinical consensus statement of otolaryngology, ophthalmology, nuclear medicine and endocrinology. Head Neck. (2020) 42:3446–59. doi: 10.1002/hed.2641732812307
15. Arrifin A, Heidari E, Burke M, Fenlon MR, Banerjee A. The effect of radiotherapy for treatment of head and neck cancer on oral Flora and Saliva. Oral Health Prev Dent. (2018) 16:425–9. doi: 10.3290/j.ohpd.a4136430460355
16. Acauan MD, Zancanaro Figueiredo MA, Cherubini K, Gomes AP, Salum FG. Radiotherapy-Induced salivary dysfunction: structural changes, pathogenetic mechanisms and therapies. Arch Oral Biol. (2015) 60:1802–10. doi: 10.1016/j.archoralbio.2015.09.01426454716
17. Rad M, Kakoie S, Niliye Brojeni F, Pourdamghan N. Effect of long-term smoking on whole-mouth salivary flow rate and oral health. J Dent Res Dent Clin Dent Prospects. (2010) 4:110–4. doi: 10.5681/joddd.2010.02823346336
18. Gueiros LA, Soares MSM, Leão JC. Impact of ageing and drug consumption on oral health. Gerodontology. (2009) 26:297–301. doi: 10.1111/j.1741-2358.2009.00284.x19392837
19. Xu F, Laguna L, Sarkar A. Aging-Related changes in quantity and quality of Saliva: where do we stand in our understanding? J Texture Stud. (2019) 50:27–35. doi: 10.1111/jtxs.1235630091142
20. Maciejczyk M, Nesterowicz M, Szulimowska J, Zalewska A. Oxidation, glycation, and carbamylation of salivary biomolecules in healthy children, adults, and the elderly: can Saliva be used in the assessment of aging? J Inflamm Res. (2022) 15:2051–73. doi: 10.2147/JIR.S35602935378954
21. Arany S, Kopycka-Kedzierawski DT, Caprio TV, Watson GE. Anticholinergic medication-related dry mouth and impacts on the salivary glands. Oral Surg Oral Med Oral Pathol Oral Radiol. (2021) 132:662–70. doi: 10.1016/j.oooo.2021.08.01534593340
22. Wolff A, Joshi RK, Ekstrom J, Aframian D, Pedersen AML, Proctor G, et al. A guide to medications inducing salivary gland dysfunction, Xerostomia, and subjective sialorrhea: a systematic review sponsored by the world workshop on oral medicine Vi. Drugs RD. (2017) 17:1–28. doi: 10.1007/s40268-016-0153-9
23. Aliko A, Wolff A, Dawes C, Aframian D, Proctor G, Ekström J, et al. World workshop on oral medicine vi: clinical implications of medication-induced salivary gland dysfunction. Oral Surg Oral Med Oral Pathol Oral Radiol. (2015) 120:185–206. doi: 10.1016/j.ooo.2014.10.02725861957
24. Miranda-Rius J, Brunet-Llobet L, Lahor-Soler E, Farré M. Salivary secretory disorders, inducing drugs, and clinical management. Int J Med Sci. (2015) 12:811–24. doi: 10.7150/ijms.1291226516310
25. Screebny LM, Valdini A. Xerostomia. A negnected symptom. Arch Intern Med. (1987) 147:1333–7. doi: 10.1001/archinte.147.7.13333300589
26. Guggenheimer J, Moore PA. Xerostomia: etiology, recognition and treatment. J Am Dent Assoc. (2003) 134:61–9. doi: 10.14219/jada.archive.2003.001812555958
27. Locker D. Subjective reports of oral dryness in an older adult population. Comm Dent Oral Epidem. (1993) 21:165–8. doi: 10.1111/j.1600-0528.1993.tb00744.x
28. Rossie K, Guggenheimer J. Oral candidiasis: clinical manifestations, diagnosis and treatment. Pract Periodontics Aesthet Dent. (1997) 9:635–42. PMID: 9573835.9573835
29. Lee YC, Hong IK, Na SY, Eun YG. Evaluation of salivary function in patients with burning mouth syndrome. Oral Dis. (2015) 21:308–13. doi: 10.1111/odi.1227024962264
30. Pajukoski H, Meurman JH, Halonen P, Sulkava R. Prevalence of subjective dry mouth and burning mouth in hospitalized elderly patients and outpatients in relation to Saliva, medication, and systemic diseases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2001) 92:641–9. doi: 10.1067/moe.2001.11847811740482
31. Nagler RM, Hershkovich O. Sialochemical and gustatory analysis in patients with oral sensory complaints. J Pain. (2004) 5:56–63. doi: 10.1016/j.jpain.2003.09.00214975379
32. Pekiner FN, Gümrü B, Demirel GY, Ozbayrak S. Burning mouth syndrome and Saliva: detection of salivary trace elements and cytokines. J Oral Pathol Med. (2009) 38:269–75. doi: 10.1111/j.1600-0714.2008.00734.x19141055
33. Soares MS, Chimenos-Küstner E, Subirá-Pifarrè C, Rodríguez de Rivera-Campillo ME, López-López J. Association of burning mouth syndrome with Xerostomia and medicines. Med Oral Patol Oral Cir Bucal. (2005) 10:301–8. PMID: 16056186.16056186
34. Suh KI, Lee JY, Chung JW, Kim YK, Kho HS. Relationship between salivary flow rate and clinical symptoms and behaviours in patients with dry mouth. J Oral Rehabil. (2007) 34:739–44. doi: 10.1111/j.1365-2842.2006.01712.x17824886
35. Toida M, Nanya Y, Takeda-Kawaguchi T, Baba S, Iida K, Kato K, et al. Oral complaints and stimulated salivary flow rate in 1188 adults. J Oral Pathol Med. (2010) 39:407–19. doi: 10.1111/j.1600-0714.2009.00852.x20202092
36. Glazar I, Urek MM, Brumini G, Pezelj-Ribaric S. Oral sensorial complaints, salivary flow rate and mucosal lesions in the institutionalized elderly. J Oral Rehabil. (2010) 37:93–9. doi: 10.1111/j.1365-2842.2009.02027.x19968768
37. Acharya S, Hägglin C, Jontell M, Wenneberg B, Ekström J, Carlén A. Saliva on the oral Mucosa and whole Saliva in women diagnosed with burning mouth syndrome. Oral Dis. (2018) 24:1468–76. doi: 10.1111/odi.1291829917294
38. Hershkovich O, Nagler RM. Biochemical analysis of Saliva and taste acuity evaluation in patients with burning mouth syndrome, Xerostomia and/or gustatory disturbances. Arch Oral Biol. (2004) 49:515–22. doi: 10.1016/j.archoralbio.2004.01.01215126133
39. Poon R, Su N, Ching V, Darling M, Grushka M. Reduction in unstimulated salivary flow rate in burning mouth syndrome. Br Dent J. (2014) 217:E14. doi: 10.1038/sj.bdj.2014.88425303607
40. Spadari F, Venesia P, Azzi L, Veronesi G, Costantino D, Croveri F, et al. Low basal salivary flow and burning mouth syndrome: new evidence in this enigmatic pathology. J Oral Pathol Med. (2015) 44:229–33. doi: 10.1111/jop.1224025155153
41. Rouleau TS, Shychuk AJ, Kayastha J, Lockhart PB, Nussbaum ML, Brennan MT. A retrospective, cohort study of the prevalence and risk factors of oral burning in patients with dry mouth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2011) 111:720–5. doi: 10.1016/j.tripleo.2011.01.04221497523
42. Ko JY, Kim MJ, Lee SG, Kho HS. Outcome predictors affecting the efficacy of clonazepam therapy for the management of burning mouth syndrome (bms). Arch Gerontol Geriatr. (2012) 55:755–61. doi: 10.1016/j.archger.2011.10.00122040716
43. de Souza FT, Amaral TM, dos Santos TP, Abdo EN, Aguiar MC, Teixeira AL, et al. Burning mouth syndrome: a therapeutic approach involving mechanical salivary stimulation. Headache. (2012) 52:1026–34. doi: 10.1111/j.1526-4610.2011.02037.x22084903
44. Dawes C. Summary of: reduction in unstimulated salivary flow rate in burning mouth syndrome. Br Dent J. (2014) 217:364–5. doi: 10.1038/sj.bdj.2014.88525303588
45. Iorgolescu G. Saliva between normal and pathological. Important factors in determining systemic and oral health. J Med Life. (2009) 2:303–7. PMID: 20112475.20112475
46. Kao CH HY, ChangLai SP, Liao KK. Evidence for decreased salivary function in patients with reflux esophagitis. Digestion. (1999) 60:191–5. doi: 10.1007/s00455-005-0016-y10343131
47. Koshiyama S, Tanimura K, Ito K, Funayama S, Hira D, Komase Y, et al. Gastroesophageal reflux-like symptoms are associated with hyposalivation and oropharyngeal problems in patients with asthma. Resp Invest. (2021) 59:114–9. doi: 10.1016/j.resinv.2020.06.004
48. Watanabe M, Nakatani E, Yoshikawa H, Kanno T, Nariai Y, Yoshino A, et al. Oral soft tissue disorders are associated with gastroesophageal reflux disease: retrospective study. BMC Gastroenterol. (2017) 17:92. doi: 10.1186/s12876-017-0650-528784097
49. Yoshikawa H, Furuta K, Ueno M, Egawa M, Yoshino A, Kondo S, et al. Oral symptoms including dental erosion in gastroesophageal reflux disease are associated with decreased salivary flow volume and swallowing function. J Gastroenterol. (2012) 47:412–20. doi: 10.1007/s00535-011-0515-622200941
50. Lechien JE, Hans S, De Marrez LG, Deuqanter D, Rodriguez A, Muls V, et al. Prevalence and features of laryngopharyngeal reflux in patients with primary burning mouth syndrome. Laryngoscope. (2021) 131:1–7. doi: 10.1002/lary.2960432302421
51. Hakeem A, Fitzpatrick SG, Bhattacharyya I, Islam MN, Cohen DM. Clinical characterization and treatment outcome of patients with burning mouth syndrome. Gen Dent. (2018) 66:41–7. PMID: 29714699.29714699
52. Sánchez-Blanco I, Rodriguez-Téllez M, Corcuera-Flores JR, González-Blanco C, Torres-Lagares D, Serrera-Figallo MÁ, et al. Effectiveness of salivary stimulation using Xylitol-malic acid tablets as coadjuvant treatment in patients with gastro-oesophageal reflux disease: early findings. Med Oral Patol Oral Cir Bucal. (2020) 25:e818–26. doi: 10.4317/medoral.23887
53. Di Fede O, Di Liberto C, Occhipinti G, Vigneri S, Lo Russo L, Fedele S, et al. Oral manifestations in patients with gastro-oesophageal reflux disease: a single-center case-control study. J Oral Pathol Med. (2008) 37:336–40. doi: 10.1111/j.1600-0714.2008.00646.x18284539
54. Campisi G, Lo Russo L, Di Liberto C, Di Nicola F, Butera D, Vigneri S, et al. Saliva variations in gastro-oesophageal reflux disease. J Dent. (2008) 36:268–71. doi: 10.1016/j.jdent.2008.01.00318313197
55. Correa MC, Lerco MM, Henry MA. Study in oral cavity alterations in patients with gastroesophageal reflux disease. Arq Gastroenterol. (2008) 45:132–6. doi: 10.1590/S0004-2803200800020000818622467
56. Ristevska I, Armata RS, D’Ambrosio C, Furtado M, Anand L, Katzman MA. Xerostomia: understanding the diagnosis and the treatment of dry mouth. J Fam Med Dise Prev. (2015) 1:2.
57. Gil-Montoya JA, Silvestre FJ, Barrios R, Silvestre-Rangi J. Treatment of Xerostomia and hyposalivation in the elderly: a systematic review. Med Oral Patol Oral Cir Bucal. (2016) 21:e355–66. doi: 10.4317/medoral.2096927031061
58. Lopez-Pintor RM, Casanas E, González-Serrano J, Serrano J, Ramirez L, de Arriba L, et al. Xerosotmia, hyposalivation, and salivary flow in diabetes patients. J Diabetes Res. (2016) 2016:4372852. doi: 10.1155/2016/437285227478847
59. Thakkar JP, Lane C. Hyposalivation and Xerostomia and burning mouth syndrome. Medical management. Oral Maxillofac Surg Clin N Am. (2022) 34:135–46. doi: 10.1016/j.coms.2021.08.002
60. Thomson WM, van der Putten GJ, de Baat C, Ikebe K, Matsuda K, Enoki K, et al. Shortening the Xerostomia inventory. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2011) 112:322–27. doi: 10.1016/j.tripleo.2011.03.02421684773
61. Navazesh M, Christensen CM. A comparison of whole mouth resting and stimulated salivary measurement procedures. J Dent Res. (1982) 61:1158–62. doi: 10.1177/002203458206101009016956596
62. Anderson DJ, Hector MP. Periodontal mechanoreceptors and parotid secretion in animals and man. J Dent Res. (1987) 66:518–23. doi: 10.1177/002203458706600222013476568
63. Dawes C. Physiological factors affecting salivary flow rate, oral sugar clearance, and the sensation of dry mouth in man. J Dent Res. (1987) 66:648–53. doi: 10.1177/00220345870660S1073476629
64. Orellana MF, Lagravére MO, Boychuk DGJ, Major PW, Flores-Mir C. Prevalence of Xerostomia in population-based samples: a systematic review. Publ Health Dent. (2006) 66:152–8. doi: 10.1111/j.1752-7325.2006.tb02572.x
65. Diep MT, Jensen JL, Skudutyte-Rysstad R, Young A, Sødal ATT, Petrovski BÉ, et al. Xerostomia and hyposalivation among a 65-yr-old population living in Oslo, Norway. Eur J Oral Sci. (2021) 129:e12757. doi: 10.1111/eos.1275733501713
66. Wiener CR, Wu B, Crout R, Wiener M, Plassman B, Kao E, et al. Hyposalivation and Xerostomia in dentate older adults. J Am Dent Assoc. (2010) 141:279–84. doi: 10.14219/jada.archive.2010.016120194383
67. Jamieson LM, Thomson WM. Xerostomia: its prevalence and associations in the adult Australian population. Aust Dent J. (2020) 65(Suppl 1):S67–70. doi: 10.1111/adj.1276732583587
68. Imura H, Shimada M, Yamazaki Y, Sugimoto K. Characteristic changes of Saliva and taste in burning mouth syndrome patients. J Oral Pathol Med. (2016) 45(3):231–6. doi: 10.1111/jop.1235026293497
69. Ericsson Y, Hardwick L. Individual diagnosis, prognosis and counselling for caries prevention. Caries Res. (1978) 12:94–102. doi: 10.1159/000260369277298
70. Bardow A, Nyvad B, Nauntofte B. Relationships between medication intake, complaints of dry mouth, salivary flow rate and composition, and the rate of tooth demineralization in situ. Arch Oral Biol. (2001) 46:413–23. doi: 10.1016/s003-9969(01)00003-611286806
71. Navazesh M, Brightman VJ, Pogoda JM. Relationship of medical Status, medications, and salivary flow rates in adults of different ages. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (1996) 81:172–6. doi: 10.1016/s1079-2104(96)80410-08665310
72. Alwaheidi HAA, O’Toole S, Bernabé E. The interrelationship between Xerogenic medication use, subjective oral dryness and tooth wear. J Dent. (2021) 104:103542. doi: 10.1016/j.jdent.2020.10354233276080
73. Leal SC, Bittar J, Portugal A, Falcao DP, Faber J, Zanotta P. Medication in elderly people: its influence on salivary pattern, signs and symptoms of dry mouth. Gerodontology. (2010) 27:129–33. doi: 10.1111/j.1741-2358.2009.00293.x20337727
74. Krunic J, Stojanovic N, Ivkovic N, Stojic D. Salivary flow rate and decayed, missing, and filled teeth (dmft) in female patients with schizophrenia on chlorpromazine therapy. J Dent Sci. (2013) 8:418–24. doi: 10.1016/j.jds.2013.05.004
75. Narhi TO, Meurman JH, Ainamo A, Nevalainen JM, Schmidt-Kaunisaho KG, Siukosaari P, et al. Association between salivary flow rate and the use of systemic medication among 76-, 81-, and 86-year-old inhabitants in Helsinki, Finland. J Dent Res. (1992) 71:1875. doi: 10.1177/002203459207101204011452886
76. Wu AJ, Ship JA. A characterization of Major salivary gland flow rates in the presenceof medications and systemic diseases. Oral Surg Oral Med Oral Pathol. (1993) 76:301–6. doi: 10.1016/0030-4220(93)90258-68378045
77. Meurman JH, Rantonen P. Salivary flow rate, buffering capacity, and yeast counts in 187 consecutive adult patients from kuopio, Finland. Scand J Dent Res. (1994) 102:229–34. doi: 10.1111/j.1600-0722.1994.tb01185.x8091123
78. Djukic L, Roganovic J, Brajovic MD, Bokonjic D, Stojic D. The effects of antihypertensives and type 2 diabetes on salivary flow and total antioxidant capacity. Oral Dis. (2015) 21:619–25. doi: 10.1111/odi.1232525689395
79. Korsten MA, Rosman AS, Fishbein S, Shlein RD, Goldberg HE. Chronic Xerostomia increases esophageal acid exposure and is associated with esophageal injury. Am J Med. (1991) 90:701–6. doi: 10.1016/0002-9343(91)90665-K2042685
80. Sonnenberg A, Steinkamp U, Weise A, Berges W, Wienbeck M, Rohner HG, et al. Salivary secretion in reflux esophagitis. Gastroenterol. (1982) 83:889–95. doi: 10.1016/S0016-5085(82)80021-8
81. Helm JF, Dodds W, Hogan WJ. Salivary response to esophageal acid in normal subjects and patients with reflux esophagitis. Gastroenterol. (1987) 93:1393–7. doi: 10.1016/0016-5085(87)90270-8
82. Namiot Z, Rourk RM, Piascik R, Hetzel DP, Sarosiek J, McCallum RW. Interrelationship between esophageal challenge with mechanical and chemical stimuli and salivary protective mechanisms. Am J Gastroenterol. (1994) 89:581–7 PMID: 8147362.8147362
83. Costa HO, Neto OM, Eckley CA. Is there a relationship between the ph and volume of Saliva and esophageal ph-metry results? Dysphagia. (2005) 20:175–81. doi: 10.1007/s00455-005-0016-y16362506
84. Sarosiek J, Scheurich CJ, Marcinkiewicz M, McCallum RW. Enhancement of salivary esophagoprotection: rationale for a physiological approach to gastroesophageal reflux disease. Gastroenter. (1996) 110:675–81. doi: 10.1053/gast.1996.v110.pm8608875
85. Navazesh M, Christensen CM, Brightman C. Clinical criteria for the diagnosis of salivary gland hypofunction. J Dent Res. (1992) 71:1363–9. doi: 10.1177/002203459207100703011629451
86. Hunter KD, Wilson WS. The effects of antidepressant drugs on salivary flow and content of sodium and potassium ions in human parotid Saliva. Archs Oral Biol. (1995) 40:983–89. doi: 10.1016/0003-9969(95)00079-5
Keywords: adverse effects, burning mouth syndrome, gastroesophageal reflux disease, glossodynia, xerostomia
Citation: Sangalli L, Eldomiaty W and Miller CS (2023) Xerogenic medications may contribute to decreased unstimulated salivary flow in patients with oral burning and/or gastro-esophageal reflux disease. Front. Dent. Med 4:1047235. doi: 10.3389/fdmed.2023.1047235
Received: 17 September 2022; Accepted: 31 January 2023;
Published: 23 February 2023.
Edited by:
Man-Kyo Chung, University of Maryland, United StatesReviewed by:
Mayank Hans, ESIC Medical College, IndiaYeon-Hee Lee, Kyung Hee University Dental Hospital, Republic of Korea
Stefania Brazzoli, Jefferson University Hospitals, United States
© 2023 Sangalli, Eldomiaty and Miller. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linda Sangalli, bHNhbmdhQG1pZHdlc3Rlcm4uZWR1
Specialty Section: This article was submitted to Systems Integration, a section of the journal Frontiers in Dental Medicine
 Linda Sangalli
Linda Sangalli Walied Eldomiaty3
Walied Eldomiaty3