- College of Dental Medicine, Western University of Health Sciences, Pomona, CA, United States
The aim of this mini review is to investigate the connection between oral microbiome dysbiosis and systemic diseases. Many systemic conditions can have oral manifestations and cause worsening in oral diseases. For example, uncontrolled type 2 diabetes has been associated with worsening of periodontal disease. Other inflammatory diseases or autoimmune diseases may predispose to oral mucositis, mucosal ulcers, xerostomia, and higher susceptibility to oral infections. This review will outline common systemic diseases such as metabolic, cardiovascular, and immunologic disorders as they relate to oral manifestations and changes in the oral microbiome composition.
Introduction
The oral microbiome is a complex environment comprised of over 1,000 species of bacteria, viruses, fungi, and protozoa (1). While bacteria predominate the microbiome in the oral cavity and will be the primary focus of this discussion, the significance of the viruses, fungi, and protozoa is not to be ignored. Commensal bacteria and other natural inhabitant microorganisms in the oral cavity coexist to maintain health in the oral microbiome. Research is ongoing to explore the link between health of the oral microbiome with systemic health. Specifically, current research is interested in the connection between oral dysbiosis and low-grade inflammatory diseases. Several key conditions characterize dysbiosis, such as loss of diversity in microbial population, loss of the benefits of “healthy” microbes, and expansion of pathogenic microbes (1). Interspecies relationships, categorized as synergistic, signaling, or antagonistic, are disrupted when loss of diversity occurs, contributing to dysbiosis in the oral microbiome. Loss in benefits of a healthy oral microflora can decrease the host immune response and increase susceptibility to external disease, as well as opportunistic infections, which occur as commensal microbes shift to pathogenic microbes in response to the changing ecosystem.
This review discusses the link between oral dysbiosis and common systemic diseases, as there is often a connection between oral health, traditionally viewed as a local ecosystem, and systemic health. Among the systemic diseases that will be discussed are metabolic diseases, which include diabetes mellitus and many cardiovascular diseases. Autoimmune conditions, such as Lupus and Sjogren's, as well as immunosuppression, which can include pregnancy, will also be discussed, as they may contribute to an alteration in host immune response. One example of this established connection of oral diseases and systemic health is the association of periodontal disease in the presence of uncontrolled diabetes as well as during pregnancy. The aim of this review is to explore oral dysbiosis and its connection with systemic diseases, which will serve as foundational knowledge for future research relating to oral health maintenance in systemic disease conditions and for the investigation of novel therapeutics with potential biomarker specificity.
Metabolic diseases
Diabetes
Diabetes is a growing area of concern for the United States and worldwide. Prevalence has tripled worldwide from 2006 to 2017, with over 30 million in the United States alone (2). In preliminary animal studies, diabetes has been shown to increase the pathogenicity of previously commensal bacteria, now termed pathobionts. The increase in pathogenicity of these pathobionts is evidenced by an increase in inflammation, osteoclastogenesis and periodontal bone loss (3). The increase in pathogenicity has been linked to IL-17, a cytokine that activates pro-inflammatory mediators such as IL-6 and RANKL. Inhibition of IL-17 reduces the pathogenicity of the oral microbiome in diabetic animal hosts (4). The connection with IL-17 indicates that host inflammation plays a role in the indirect activation of pathobionts and oral dysbiosis. Oral dysbiosis in patients with diabetes includes an increase in Capnocytophaga, Porphyromonas gingivalis, and Tannerellla forsythia. In uncontrolled diabetes and consequent hyperglycemia, longitudinal studies have shown an increased shift in Proteobacteria and Firmicutes (4). In addition to increasing levels of pathobionts, bacterial diversity is also decreased in the oral microbiome. Table 1 summarizes several of the oral manifestation changes and oral dysbiosis in the presence of several systemic diseases discussed throughout this review.
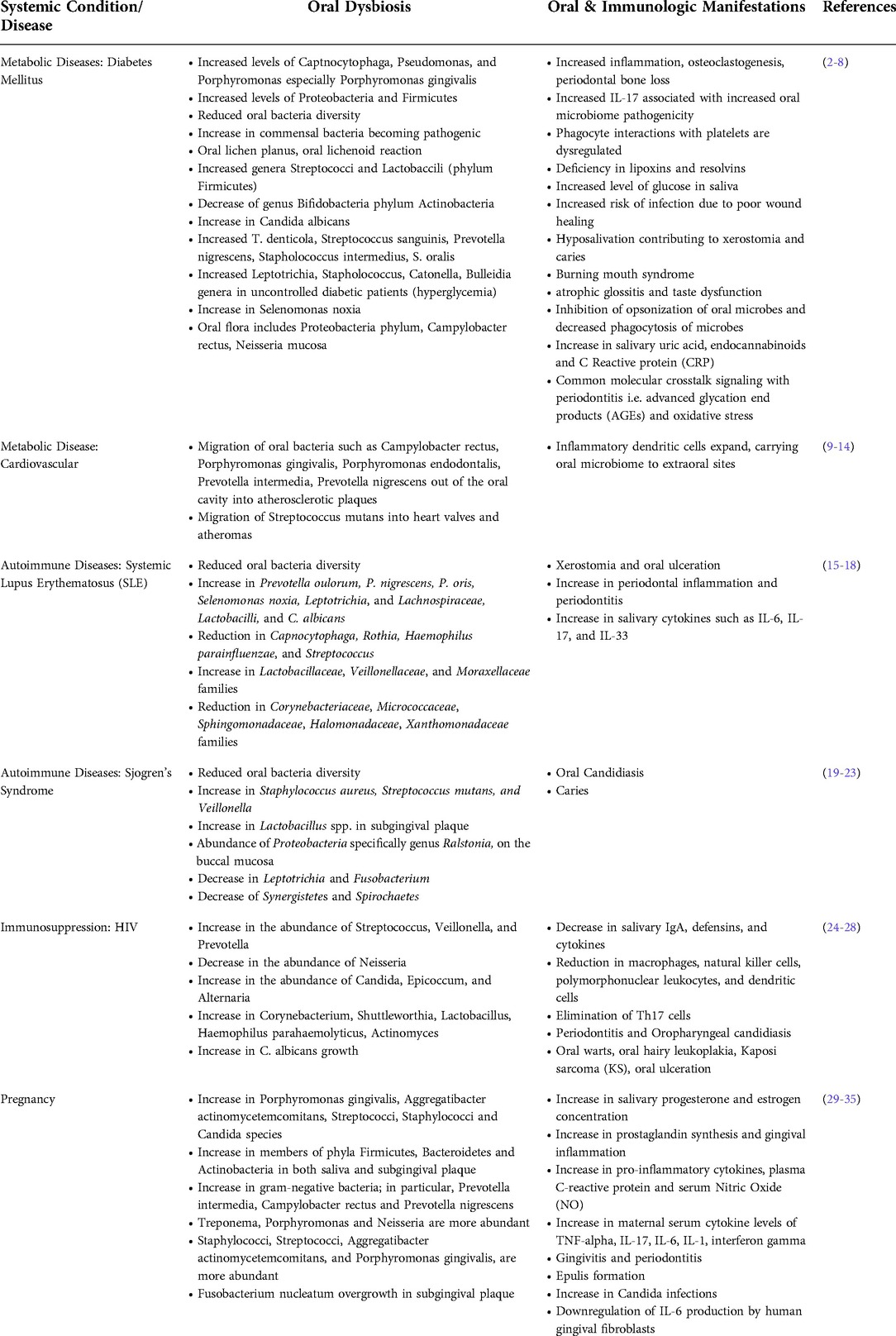
Table 1. Overview of oral dysbiosis and oral manifestation changes associated with common systemic conditions and diseases.
Temporary shifts and upregulation of pathogens alone does not constitute dysbiosis, it is the persistence of pathogens in the oral microbiome. Oral dysbiosis caused by the persistent increase of commensal bacteria is largely associated with chronic inflammation in diabetics as the result of the host immune response. In type 2 diabetes in particular, the phagocyte interactions with platelets are dysregulated, compromising restoration of inflamed tissue back to health (5). Complications of dysregulation from diabetes include increased risk for periodontitis, dental caries, oral infection, salivary dysfunction, taste dysfunction, delayed wound healing, tongue abnormalities, halitosis, and lichen planus (6). The bi-directional relationship between diabetes and periodontitis is most well established. Type 1 diabetics are 44.7% more likely to have periodontal disease and type 2 diabetics are 45%–88% more likely to have periodontal disease (6). Both periodontal disease and diabetes are marked by pro-inflammatory immune activity and share common molecular crosstalk signaling involving advanced glycation end products (AGEs) and oxidative stress (7).
Taste dysfunction in diabetics can contribute to an increase in sugar consumption, leading to other metabolic complications such as obesity. Obesity shares many risk factors with diabetes. Although the exact mechanism has not been established, obese patients have been found to be at increased risk for diabetes and cardiovascular disease (8). Evidence shows the oral microbiome of obese patients has a shift in sialic acid, phosphorous and peroxidase activity. Salivary uric acid, endocannabinoids and C-reaction protein also see an increased shift. Oral dysbiosis in obese patients may also contribute to dental caries and periodontal disease due to a decrease in total salivary flow rate (8).
Cardiovascular disease
Heart disease and stroke are the main causes of death among diabetic patients. The two-way relationship between periodontal disease and diabetes has prompted a closer look at oral dysbiosis and atherosclerotic cardiovascular disease (ASCVD) (9). This umbrella term of ASCVD encompasses coronary artery disease, cerebrovascular disease, or peripheral arterial disease due to atherosclerosis. Atherosclerosis is a chronic inflammatory condition affecting arterial blood vessels. Oral dysbiosis is associated with ASCVD via both indirect and direct mechanisms. We will first examine the direct mechanism via low-level bacteremia in which oral bacteria enter the blood stream and invade the arterial wall. Inflammatory dendritic cells expand outside of the oral cavity, carrying P. gingivalis and other pathobionts to extraoral sites such as arterial walls, contributing to comorbid conditions such as atherosclerosis, hypertension and other cardiovascular diseases (9). A recent meta-analysis confirmed the presence of both pathogenic bacteria such as Porphyromonas gingivalis, and commensal bacteria in atherosclerotic plaque samples. Commensal bacteria specific to atherosclerotic plaque samples included Campylobacter rectus, Porphyromonas endodontalis, Prevotella intermedia, and Prevotella nigrescens (10). Streptococcus mutans has also been found in biopsies from heart valves and atheromas (11). These pathobionts, P. gingivalis in particular, modulate the immune response leading to oral dysbiosis (12). P. gingivalis along with Tannerella forsythia and Treponema denticola make up the “red complex,” which is responsible for severe manifestations of periodontal disease (13). Periodontal disease has been shown to significantly increase the risk of ASCVD. While a causal relationship has not been established between periodontal disease and atherosclerosis, studies have shown periodontal treatment has a beneficial effect on the prevention and control of ASCVD (14).
The indirect mechanism by which oral dysbiosis contributes to ASCVD is through activation of both the innate and adaptive immune response, leading to inflammation. Pathobionts in the oral cavity are activated by chronic inflammation. The role of oral dysbiosis in inducing the inflammatory response is evident by the increase of inflammatory cytokines, C-reactive protein (CRP), white blood cells, fibrinogen, and intercellular adhesion molecules. Activation of the innate and adaptive immune response results in arterial hypertension, Treg dysregulation, macrophage proliferation and migration and plaque erosion and rupture (14). Together, both the direct and indirect mechanisms contribute to ASCVD by increasing plaque formation and triggering stable atherosclerotic lesions to destabilize, increasing the risk of plaque rupture. This can lead to peripheral artery disease (PAD) and life-threatening conditions such as acute coronary syndrome (ACS), and stroke.
Autoimmune diseases
Systemic lupus erythematosus (SLE)
Systemic Lupus Erythematosus (SLE) is an autoimmune disease where the immune system attacks its own tissues. The prevalence of SLE worldwide is 0.02–0.24 (15). Even though SLE exhibits a genetic component, research suggests that the host's microbiome imbalance termed “dysbiosis” might play a role. As a result, many studies have been conducted to understand the connection between gut microbiota and SLE pathogenesis. However, only a few studies have examined the relationship between oral microbiota and SLE.
Some of the oral manifestations of SLE include xerostomia, oral ulcers, hyposalivation, and increased periodontal disease (16). Their increased risk of periodontal disease is due to elevated levels of cytokines such as IL-16, IL-17 and IL-33 correlating with systemic inflammatory pathways (15). Human studies have specifically linked elevated inflammatory cytokine levels to oral microbiome dysbiosis, with elevated populations of Prevotella oulorum, P. nigrescen P. oris, Selenomonas noxia, Leptotrichia, and Lachnospiraceae (17). It has also been observed that bacteria commonly found in periodontal health, such as Capnocytophaga, Rothia, Haemophilus parainfluenzae, and Streptococcus, are depleted in patients with SLE (17). A recent study conducted by Li et al., showed that patients with SLE have an oral microbiota that is imbalanced and reduced in diversity. It was found that SLE patients had an oral microbiome that is enriched in the Lactobacillaceae, Veillonellaceae and Moraxellaceae families but reduced in the Corynebacteriaceae, Micrococcaceae, Sphingomonadaceae, Halomonadaceae, Xanthomonadaceae families (15). Furthermore, the genera Veillonella and Haemophilus were overabundant in SLE patients with oral ulcers indicating their possible role in this oral manifestation (18).
Sjögren's syndrome
Sjögren's syndrome (SS) is a chronic autoimmune disease that targets the exocrine glands and the extra-glandular epithelial tissues. Salivary and lacrimal glands are attacked the most causing hyposalivation and dry eyes, respectively (19). Due to the reduction in saliva production in SS patients, oral mucosal barrier is impaired favoring dysbiosis and colonization with pathogenic microorganisms (20). As a result, the relationship between oral microbiome dysbiosis and SS has been an area of interest in research. It has been hypothesized that SS alters the saliva composition, which consequently causes alterations in the oral microbiome (19). Oral microbiome dysbiosis is evident due to an increase in certain microorganisms such as Capnocytophaga, Dialister, Fusobacterium, Helicobacter, Streptococcus, and Veilonella and a decrease in Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans in SS patients (21).
Moreover, the clinical finding of hyposalivation in SS has been linked to an increased number of the genera Candida and Lactobacillus, specifically C. albicans and S. mutans (22). Therefore, SS patients are more susceptible to dental caries and oral candidiasis infections. Furthermore, some researchers have focused on studying the role of oral microbiome in the pathogenesis of SS. Evidence shows that certain oral bacteria such as P. disiens, and C. ochracea, produce peptides that may induce an immune response by activation of Ro60-reactive T cells (23), eliciting an inflammatory response. In SS patients, oral dysbiosis has been found with an increase of Firmicutes and a decrease in Spirochaetes indicating that oral bacteria have an impact on the host's immune response (23).
Immunosuppression
Human immunodeficiency virus (HIV) is an infection that attacks the host's immune system and is characterized by a major depletion in the CD4+ T cells (24). According to the World Health Organization, approximately 37.7 million people were infected with HIV worldwide by the end of 2020 and about 73% were undergoing antiretroviral (ART) treatment. Both the disease (HIV) and the treatment (ART) have shown to have an impact on the composition and diversity of the oral microbiome. Evidence shows that oral microbiome dysbiosis is present in HIV-infected individuals due to alterations in salivary secretory components, innate and adaptive immune responses, and the physiological function of saliva (24).
Salivary secretory components, such as IgA, defensins, and cytokines are diminished, impairing the oral cavity's local immunity and thus converting commensal microorganisms to pathogenic microorganisms (25). In addition, the function of innate immune cells, such as macrophages, dendritic cells, natural killer cells and polymorphonuclear leukocytes is weakened allowing pathogens, such as bacteria, viruses, and fungi to colonize in the oral mucosa (26). Therefore, HIV-infected patients have a higher load and diversity of pathogenic microorganisms resulting in an increased risk of oral infections. Furthermore, studies have shown that HIV patients might have defects in their adaptive immune response (25). For example, some studies have demonstrated that HIV infection show a decrease in T helper 17 (Th17) cells, which secrete IL-17 leading to the secretion of β-defensins (25). Th17 cells aid in fighting against oral fungal infections and might prevent inflammation of the oral mucosa (24) while β-defensins specifically inhibit C. albicans growth (25). Due to the elimination of Th17 in HIV infected individuals, oral candidiasis infection especially oropharyngeal oral candidiasis (OPC), is one of the most common oral manifestations in those patients.
Several oral diseases occur in patients with HIV and those receiving ART due to dysbiosis in the oral microbiome. Some studies have shown that dysbiosis occurs in the lingual microbiome where pathogenic Veillonella, Prevotella, Megasphaera, and Campylobacter species are enriched, while commensal Streptococcus is depleted (27). Several studies of saliva have reported that Streptococcus mutans, Lactobacillus, Candida, Haemophilus parahaemolyticus, Actinomyces, Neisseria subflava, and Corynebacterium diphtheriae species are more abundant in patients with HIV infection (28). Although ART can decrease the occurrence of oral lesions in HIV patients, it cannot prevent oral microbiome dysbiosis. In fact, recent studies have shown a higher level of pathogenic Neisseria and Haemophilus in patients undergoing ART (28). Ultimately, the imbalance seen between the host's immune response and oral microbiome causes HIV-infected individuals to be more susceptible to oral diseases, such as periodontal disease (24). Other common oral manifestations due to oral microbiome dysbiosis in HIV patients include hairy leukoplakia, Kaposi's sarcoma, oral ulcers, and oral warts.
Pregnancy
As it has been illustrated above, oral microbial dysbiosis is linked to the pathogenesis of systemic diseases. It was also shown that systemic diseases contribute to oral microbial dysbiosis, which in turn causes oral diseases such as periodontitis and dental caries. Microorganisms residing in the oral cavity have a major role in host metabolism, immunity, and overall health. Therefore, it is not surprising that significant changes in the oral microbiota are present during pregnancy in which metabolic and immunological states are altered (29). Several studies have explored the differences in the oral microbiota diversity and composition between pregnant and nonpregnant women. Those studies have revealed that during pregnancy, the microbial diversity remains stable, but the composition of the oral microbiome experiences a pathogenic shift (30). When the bacterial composition of saliva and subgingival plaque (SGP) was studied in pregnant women, it was observed that members of phyla Firmicutes, Bacteroidetes and Actinobacteria were abundant in both saliva and SGP (31). Furthermore, recent studies showed that during the first and second trimester, Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, Streptococci, Staphylococci, and Candida species are increased in pregnant women (31), predisposing these individuals to an increased risk of oral diseases, such as gingivitis and worsening of periodontal diseases.
During pregnancy, the female sex hormones estrogen and progesterone fluctuate, mediating the shift in oral microbiome (32). Recent evidence has revealed that the genera Neisseria, Porphyromonas, and Treponema are overrepresented in pregnant women (33). The changes in the oral microbiome are consistent with the high prevalence of pregnancy gingivitis (29). Prospective studies have reported that the oral microbiome levels of Po. gingivalis, Tr. denticola, Pr. intermedia, Ta. forsythia, Campylobacter rectus, A. actinomycetemcomitans, and Fretibacterium sp. are associated with the formation of dental plaque and gingival inflammation in pregnancy (33). During pregnancy, some common oral manifestations are gingivitis, periodontitis, bone loss, high pocket depths, bleeding upon probing, etc.
Pregnancy oral manifestations, such as gingivitis and periodontitis have been linked to adverse pregnancy outcomes such as pre-eclampsia, low birth weight, miscarriage, and preterm labor (34). A study by Barak et al. concluded that bacteria found in the oral cavity can translocate to the placenta (35). Some studies showed that women who gave birth to preterm babies and were diagnosed with gingivitis during pregnancy had an abundance of Fusobacterium nucleatum bacteria in their placenta (34). This indicates that bacteria found in the subgingival plaque translocated to the placenta (34). In addition, periodontal pathogens such as P. gingivalis increase the risk of preterm birth (33). One possible mechanism behind these adverse pregnancy outcomes is believed to be related to periodontal pathogens causing a maternal immune-inflammatory response (33). Periodontal pathogens, such as P. gingivalis and F. nucleatum, release pro-inflammatory cytokines, specifically, IL-1, IL-6, and IL-8, which consequently lead to preterm birth (32). The same pro-inflammatory cytokines are believed to cause an immune-inflammatory response in the oral cavity that results in bone loss, gingival edema, and bleeding (32).
Conclusion
In this mini review, we discuss the changes in the composition and diversity of the oral microbiome that occur in connection with metabolic diseases, autoimmune diseases, immunosuppression, and pregnancy. Oral microbial dysbiosis observed in systemic diseases reduces microbial diversity and shifts bacterial composition by increasing pathogenic bacteria and decreasing commensal health-associated bacteria. A common consequence of this oral dysbiosis is oral diseases and infections, such as periodontitis, gingivitis, oral ulcers, oral candidiasis, and dental caries. As it has been illustrated, it is the interaction between the pathogens and the host's immune response that results in oral disease. For example, some microbial pathogens upregulate the release of pro-inflammatory cytokines resulting in gingivitis and periodontitis. However, the specific molecular events underlying inflammatory cell signaling events that can alter the immune response may need further investigation. Furthermore, a potential area of future research can investigate new therapeutics with potential biomarker specificity.
Author contributions
FMG and NTD contributed to the literature searches, data collection, analysis, and interpretation, and writing of the manuscript. DS contributed to the design of the review, literature search methodology, writing and revision of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Radaic A, Kapila YL. The oralome and its dysbiosis: new insights into oral microbiome-host interactions. Comput Struct Biotechnol J. (2021) 19:1335–60. doi: 10.1016/j.csbj.2021.02.010
2. Genco RJ, Borgnakke WS. Diabetes as a potential risk for periodontitis: association studies. Periodontol 2000. (2020) 83:40–5. doi: 10.1111/prd.12270
3. Curtis MA, Zenobia C, Darveau RP. The relationship of the oral microbiota to periodontal health and disease. Cell Host Microbe. (2011) 10(4):302–6. doi: 10.1016/j.chom.2011.09.008
4. Xiao E, Mattos M, Vieira GHA, Chen S, Corrêa JD, Wu Y, et al. Diabetes enhances IL-17 expression and alters the oral microbiome to increase its pathogenicity. Cell Host Microbe. (2017) 22:120–128.e4. doi: 10.1016/j.chom.2017.06.014
5. Van dyke TE. Pro-resolving mediators in the regulation of periodontal disease. Mol Aspects Med. (2017) 58:21–36. doi: 10.1016/j.mam.2017.04.006
6. Ahmad R, Haque M. Oral health messiers: diabetes mellitus relevance. Diabetes Metab Syndr Obes. (2021) 14:3001–15. doi: 10.2147/DMSO.S318972
7. A L, Tawfik AN, Islamoglu H, Gobriel HS, Ali N, Ansari P, et al. Periodontitis and diabetes mellitus co-morbidity: a molecular dialogue. J Oral Biosci. (2021) 63:360–9. doi: 10.1016/j.job.2021.10.006
8. Choromańska K, Choromańska B, Dąbrowska E, Bączek W, Myśliwiec P, Dadan J, et al. Saliva of obese patients - is it different? Postepy Hig Med Dosw. (2015) 69:1190–5. doi: 10.5604/17322693.1176778
9. Meghil MM, Cutler CW. Oral microbes and mucosal dendritic cells, “spark and flame” of local and distant inflammatory diseases. Int J Mol Sci. (2020) 21:1–17. doi: 10.3390/ijms21051643
10. Fernandes CP, Oliveira FA, Silva PG, Alves AP, Mota MR, Montenegro RC, et al. Molecular analysis of oral bacteria in dental biofilm and atherosclerotic plaques of patients with vascular disease. Int J Cardiol. (2014) 174:710–2. doi: 10.1016/j.ijcard.2014.04.201
11. Nakano K, Inaba H, Nomura R, Nemoto H, Takeda M, Yoshioka H, et al. Detection of cariogenic Streptococcus mutans in extirpated heart valve and atheromatous plaque specimens. J Clin Microbiol. (2006) 44:3313–7. doi: 10.1128/JCM.00377-06
12. Kriebel K, Hieke C, Müller-hilke B, Nakata M, Kreikemeyer B. Oral biofilms from symbiotic to pathogenic interactions and associated disease -connection of periodontitis and rheumatic arthritis by peptidylarginine deiminase. Front Microbiol. (2018) 9:53. doi: 10.3389/fmicb.2018.00053
13. Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL jr. Microbial complexes in subgingival plaque. J Clin Periodontol. (1998) 25:134–44. doi: 10.1111/j.1600-051X.1998.tb02419.x
14. Czerniuk MR, Surma S, Romańczyk M, Nowak JM, Wojtowicz A, Filipiak KJ. Unexpected relationships: periodontal diseases: atherosclerosis-plaque destabilization? from the teeth to a coronary event. Biology (Basel). (2022) 11:1–20. doi: 10.3390/biology11020272
15. Li BZ, Zhou HY, Guo B, Chen WJ, Tao JH, Cao NW, et al. Dysbiosis of oral microbiota is associated with systemic lupus erythematosus. Arch Oral Biol. (2020) 113:104708. doi: 10.1016/j.archoralbio.2020.104708
16. Jensen JL, Bergem HO, Gilboe IM, Husby G, Axéll T. Oral and ocular sicca symptoms and findings are prevalent in systemic lupus erythematosus. J Oral Pathol Med. (1999) 28:317–22. doi: 10.1111/j.1600-0714.1999.tb02047.x
17. Corrêa JD, Calderaro DC, Ferreira GA, Mendonça SM, Fernandes GR, Xiao E, et al. Subgingival microbiota dysbiosis in systemic lupus erythematosus: association with periodontal status. Microbiome. (2017) 5:34. doi: 10.1186/s40168-017-0252-z
18. Bik EM, Long CD, Armitage GC, Loomer P, Emerson J, Mongodin EF, et al. Bacterial diversity in the oral cavity of 10 healthy individuals. Isme J. (2010) 4:962–74. doi: 10.1038/ismej.2010.30
19. Tsigalou C, Stavropoulou E, Bezirtzoglou E. Current insights in microbiome shifts in Sjogren's syndrome and possible therapeutic interventions. Front Immunol. (2018) 9:1106. doi: 10.3389/fimmu.2018.01106
20. Bellando-randone S, Russo E, Venerito V, Matucci-cerinic M, Iannone F, Tangaro S, et al. Exploring the oral microbiome in rheumatic diseases, state of art and future prospective in personalized medicine with an AI approach. J Pers Med. (2021) 11:4–8. doi: 10.3390/jpm11070625
21. Sharma D, Sandhya P, Vellarikkal SK, Surin AK, Jayarajan R, Verma A, et al. Saliva microbiome in primary Sjögren's Syndrome reveals distinct set of disease-associated microbes. Oral Dis. (2020) 26:295–301. doi: 10.1111/odi.13191
22. Almståh IA, Wikström M, Stenberg I, Jakobsson A, Fagerberg-mohlin B. Oral microbiota associated with hyposalivation of different origins. Oral Microbiol Immunol. (2003) 18:1–8. doi: 10.1034/j.1399-302X.2003.180101.x
23. Siddiqui H, Chen T, Aliko A, Mydel PM, Jonsson R, Olsen I. Microbiological and bioinformatics analysis of primary Sjogren's Syndrome patients with normal salivation. J Oral Microbiol. (2016) 8:31119. doi: 10.3402/jom.v8.31119
24. S L, B S, He QS, Wu H, Zhang T. Alterations in the oral microbiome in HIV infection: causes, effects and potential interventions. Chin Med J (Engl). (2021) 134:2788–98. doi: 10.1097/CM9.0000000000001825
25. Heron SE, Elahi S. HIV infection and compromised mucosal immunity: oral manifestations and systemic inflammation. Front Immunol. (2017) 8:241. doi: 10.3389/fimmu.2017.00241
26. Feller L, Altini M, Khammissa RA, Chandran R, Bouckaert M, Lemmer J. Oral mucosal immunity. Oral Surg Oral Med Oral Pathol Oral Radiol. (2013) 116:576–83. doi: 10.1016/j.oooo.2013.07.013
27. Dang AT, Cotton S, Sankaran-walters S, Li CS, Lee CY, Dandekar S, et al. Evidence of an increased pathogenic footprint in the lingual microbiome of untreated HIV infected patients. BMC Microbiol. (2012) 12:153. doi: 10.1186/1471-2180-12-153
28. Coker MO, Mongodin EF, El-kamary SS, Akhigbe P, Obuekwe O, Omoigberale A, et al. Immune status, and not HIV infection or exposure, drives the development of the oral microbiota. Sci Rep. (2020) 10:10830. doi: 10.1038/s41598-020-67487-4
29. Neuman H, Koren O. The pregnancy microbiome. Nestle Nutr Inst Workshop Ser. (2017) 88:1–9. doi: 10.1159/000455207
30. Balan P, Chong YS, Umashankar S, Swarup S, Loke WM, Lopez V, et al. Keystone species in pregnancy gingivitis: a snapshot of oral microbiome during pregnancy and postpartum period. Front Microbiol. (2018) 9:2360. doi: 10.3389/fmicb.2018.02360
31. Fujiwara N, Tsuruda K, Iwamoto Y, Kato F, Odaki T, Yamane N, et al. Significant increase of oral bacteria in the early pregnancy period in Japanese women. J Investig Clin Dent. (2017) 8:3–7. doi: 10.1111/jicd.12189
32. Massoni RSS, Aranha AMF, Matos FZ, Guedes OA, Borges ÁH, Miotto M, et al. Correlation of periodontal and microbiological evaluations, with serum levels of estradiol and progesterone, during different trimesters of gestation. Sci Rep. (2019) 9:11762. doi: 10.1038/s41598-019-48288-w
33. Lin W, Jiang W, Hu X, Gao L, Ai D, Pan H, et al. Ecological shifts of supragingival microbiota in association with pregnancy. Front Cell Infect Microbiol. (2018) 8:24. doi: 10.3389/fcimb.2018.00024
34. Zakaria ZZ, Al-rumaihi S, Al-absi RS, Farah H, Elamin M, Nader R, et al. Physiological changes and interactions between microbiome and the host during pregnancy. Front Cell Infect Microbiol. (2022) 12:824925. doi: 10.3389/fcimb.2022.824925
Keywords: oral dysbiosis, metabolic disease, cardiovascular disease, immunologic disorders, autoimmune disease, mucositis, xerostomia
Citation: Georges FM, Do NT and Seleem D (2022) Oral dysbiosis and systemic diseases. Front. Dent. Med 3:995423. doi: 10.3389/fdmed.2022.995423
Received: 15 July 2022; Accepted: 31 August 2022;
Published: 22 September 2022.
Edited by:
Thais Manzano Parisotto, Sao Francisco University, BrazilReviewed by:
Joice Dias Correa, Pontifícia Universidade Católica de Minas Gerais, Brazil© 2022 Georges, Do and Seleem. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dalia Seleem, RGFsaWEuc2VsZWVtQHdlc3Rlcm51LmVkdQ==
†These authors contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Systems Integration, a section of the journal Frontiers in Dental Medicine
 F. M. Georges
F. M. Georges N. T. Do
N. T. Do D. Seleem
D. Seleem