
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Dent. Med., 06 December 2022
Sec. Reconstructive Dentistry
Volume 3 - 2022 | https://doi.org/10.3389/fdmed.2022.973738
This article is part of the Research TopicGlobal Excellence in Dental Medicine: EuropeView all 4 articles
Medication-related osteonecrosis of the jaws (MRONJ) is a relatively rare but serious adverse drug reaction in patients under bone-targeting or antiangiogenic medication for malignant or osteometabolic diseases. The pathogenesis of MRONJ is multifactorial with the inhibition of osteoclasts and angiogenesis considered to play a key role in an individually susceptible environment, thus its prevalence is highly differentiated according to each study. Even though MRONJ has been reported since 2003 and the literature is expanding rapidly about possible risk factors, prevention and treatment options, the successful management with no recurrence is still under controversy. The conservative non-surgical (optimal oral hygiene, systemic antibiotic therapy) and surgical procedures (debridement, sequestrectomy or bone resection) are considered the treatment of choice especially at the early stages. Adjuvant therapies have been proposed to further stimulate bone and tissue healing such as teriparatide, bone morphogenic proteins, platelet concentrates, hyperbaric oxygen, ozone therapy, photodynamic therapy and lasers with promising results. The need to develop minimally invasive treatment protocols using novel technologies in particular for those patients with severe medical histories has been highlighted in the literature. The clinical protocol that has been developed in our dental department, which is effectively contributing to MRONJ management and combines Photobiomodulation therapy (PBMT) with platelet-rich fibrin (A-PRF), will be presented in this article.
Osteonecrosis of the Jaw (ONJ) is a relatively rare but serious adverse drug reaction that can occur in patients suffering from malignant or osteometabolic diseases and treated with various agents used to prevent bone complications. It has been first reported by Robert Marx (2003) in patients receiving Bisphosphonates (BPs) such as pamidronate or zoledronic acid due to their inhibition of the osteoclastic activity leading to the suppression of bone turnover. Thus, the term bisphosphonate-related osteonecrosis of the jaws (BRONJ) has been used for more than a decade and has been specifically associated with the intravenous (IV) use of BPs for tumor-associated bone conditions.
In addition to BPs, ONJ has been associated with various anticancer therapy agents including classic chemotherapy combinations, anti-resorptive agents (Denosumab), angiogenesis inhibitors, tyrosine kinase inhibitors (TKIs), inhibitors of mammalian target of rapamycin and immunotherapeutic agents (1). Thus, the term has been modified to Medication-Related Osteonecrosis of the Jaw according to the American Association of Oral and Maxillofacial Surgeons (AAOMS 2014) (2).
MRONJ is defined as an adverse drug reaction resulting in bone exposure or bone that can be probed through an intraoral or extraoral fistula(e) in the maxillofacial region which has persisted longer than 8 weeks. Moreover, the patient should be treated, currently or previously, with antiresorptive or antiangiogenic agents and should have no history of radiation therapy or obvious metastatic disease to the jaws (2).
Pathogenesis of MRONJ is still unclear but is likely to be multifactorial and the inhibition of osteoclasts and angiogenesis are thought to play a key role. MRONJ is usually triggered by local traumas, mostly affecting the mandible, such as tooth extractions, minor dentoalveolar surgery and ill-fitting dentures. Genetic or individual susceptibility is strongly involved in pathogenesis since MRONJ does not occur in all patients (1, 3, 4).
Among the most frequent symptoms are delayed wound healing, tissue infection and swelling, numbness, paresthesia, pain or altered sensation. In many cases patients may be asymptomatic (5, 6).
The diagnosis of MRONJ is usually performed radiographically (e.g., panoramic x-rays, CBCT) with the presence of osteolysis, thickening of lamina dura, widening of periodontal space, sequestra or fractures. Radiologic imaging is assumed to be a fundamental tool for diagnostic completion, defining the extent of the disease at the skeletal level, staging and the correct therapeutic planning. Where clinically exposed necrotic bone cannot be seen, or in cases of doubtful diagnosis, additional investigations such as PET scans and MRI may further help in identifying early areas of bone involvement (7).
According to the AAOMS position paper (2014), MRONJ is divided into five stages: At risk stage, there is no apparent necrotic bone or any kind of clinical complaints in patients receiving antiresorptive or antiangiogenic medication. Stage 0, even though there is no clinical evidence of necrotic bone, nonspecific symptoms or clinical and radiographic findings are present. Stage I is defined by the exposed and necrotic bone or a fistula through which the bone can be probed in patients who have no evidence of infection and are asymptomatic. In Stage II, in addition to the exposed and necrotic bone or the fistula there is also evidence of infection, with the patients usually being symptomatic. Lastly, Stage III requires the presence of exposed and necrotic bone or a fistula through which the bone can be probed, evidence of infection and one of the following clinical signs: exposed necrotic bone which extends beyond the region of the alveolar bone (i.e., maxillary sinus, inferior border and ramus in the mandible), extraoral fistula, pathological fracture, oroantral or oronasal communication, or osteolysis which extends to the inferior border of the sinus or mandibular floor (2, 8, 9).
Treatment strategies range from conservative local wound care to aggressive resective surgery of all necrotic bone. Conservative strategies include systemic antibiotics, oral antibacterial rinses, and debridement of loose necrotic bone that no longer has soft tissue coverage. Recent literature demonstrates that disease prevention with dental exams and treatment before initiating antiresorptive/antiangiogenic therapy is the most effective method to decrease ONJ incidence. In the conservative management of patients with active ONJ, the treatment goal is focused on preventing disease progression using antibiotics and chlorhexidine mouth rinses. More invasive treatment strategies, such as for patients in stage II and III, may include local curettage and debridement, flap advancement and resective surgery (10). Critical information for the best treatment outcome between conservative and surgical approach, through randomised control studies, is still lacking (11, 12). Recently, different therapeutic modalities complementary to surgical intervention have been proposed. These modalities include autologous platelet concentrates, bone morphogenetic proteins, hyperbaric oxygen, ozone therapy, teriparatide, mesenchymal stem cells, pentoxifylline, lasers, photodynamic therapy and fluorescence-guided bone surgery. All these are considered adjunctive therapies (8, 13–15).
Autologous platelet concentrates have been proposed as part of a surgical intervention and they are widely used in modern dentistry for their regenerative properties and the amelioration of bone healing. Plasma-rich fibrin (PRF), initially introduced by Choukroun (2001), is a healing biomaterial slowly remodeled similar to a blood clot and has a great potential for bone and soft tissue regeneration without inflammatory reactions. It can be used alone or in combination with bone grafts, promoting hemostasis, proliferation, osteoblast differentiation, bone growth and maturation (16).
Photobiomodulation therapy (PBMT), previously termed low-level laser therapy (LLLT), is the local application of a monochromatic light energy in the red and near infra-red wavelengths to provide tissue repair, pain alleviation, neural stimulation, modulation of inflammatory reaction and oedema reduction (17). PBM produced by lasers or LED emitting into the so-called “therapeutic window” (between approximately 600–1200 nm), with doses that follow the rules of “biphasic-dose response” has been shown to enhance cell proliferation of fibroblasts, keratinocytes, endothelial cells and lymphocytes. The effects of laser irradiation expand the organic matrix of bone and increase the mitotic index of osteoblasts, stimulating their proliferation and differentiation. This increases the number of osteoblastic cells, enhances their activity and promotes bone formation. Due to the beneficial biomodulative effects on hard and soft tissue healing that PBMT offers, it has been considered as a promising adjunctive treatment method for MRONJ (13, 18–20).
The clinical success during MRONJ therapy is achieved through the healing improvement of the MRONJ site and its transition from a higher to a lower MRONJ stage, or the complete healing without after-effects and no recurrence during the follow-up period of at least 12 months (2). This is not always feasible, especially for those patients with a severe medical history who are not eligible for extensive surgery. Thus, the increasing need for better clinical results has led to various research groups to develop minimally invasive treatment protocols using novel technologies and combine different therapeutic modalities. This will have positive effects on patients’ quality of life (13, 14, 21–24).
The clinical protocol that has been developed in our dental department, which is effectively contributing to MRONJ management and combines PBMT with platelet-rich fibrin (A-PRF), will be presented.
A 60-year-old Caucasian female presented at the dental department of Alexandra Hospital in Athens with concerns about a non-healing oral lesion in the right mandibular premolar area. During anamnesis, the patient reported the use of alendronate (70 mg once a week for >4years) against her mild osteoporosis. While being on drug holiday for more than 3 months, the right mandibular second premolar (№ 45) and the left mandibular wisdom tooth (№ 38) had been extracted at the same session. The premolar, endodontically treated, had a root fracture while the wisdom tooth suffered from repeated periodontal abscesses.
The clinical and radiographic evaluation revealed a properly healed area of the left mandibular wisdom and a non-healing wound with progressive bone loss for more than 6 months of the premolar, regardless of the extensive use of various antibiotics and topical antiseptics. Since the patient's medical history was negative for radiation therapy or obvious metastatic disease to the jaws, the extended use of alendronate hypothesised the possibility of an MRONJ (Stage II) case. Due to the inadequate conservative approach, the patient was informed and agreed to proceed with an alternative protocol that will effectively remove the necrotic bone, promoting bone and tissue healing with the use of A-PRF membranes and Photobiomodulation therapy (Figure 1).
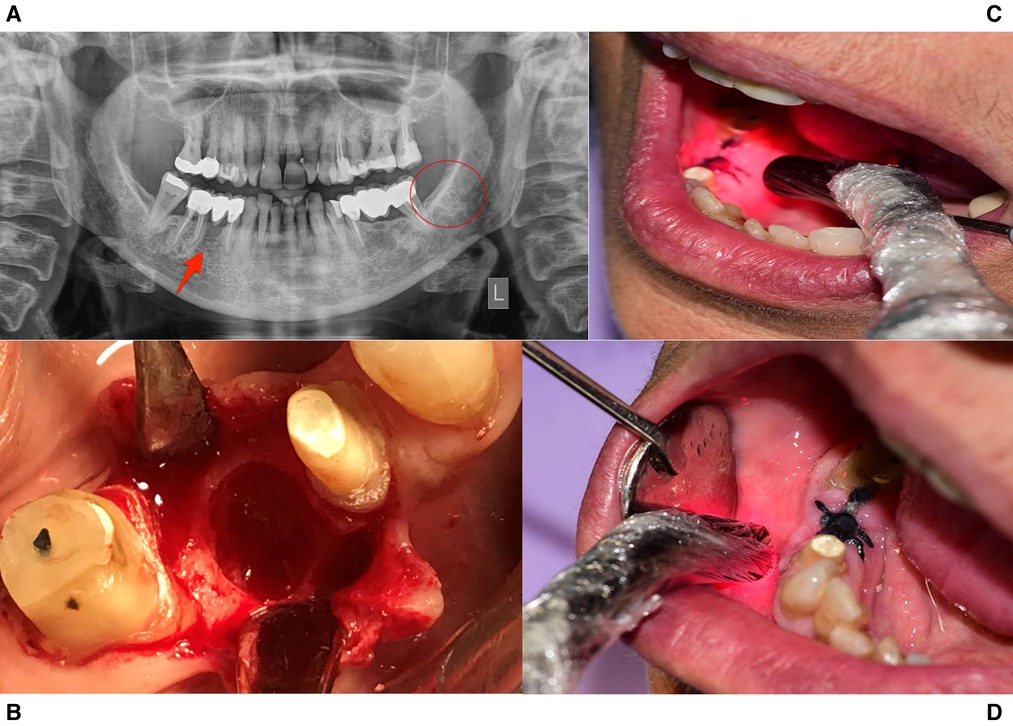
Figure 1. Treatment protocol for case 1. (A) Panoramic Radiographic Image. A non-healing wound present in tooth site #45, while the lower left 3rd molar area had healed properly. (B) The non-healing site during operation. (C,D) Intraoral PBMT protocol with 660 nm and 810 nm probes.
All surgical procedures were performed under local anesthesia (Articaine Hydrochloride 4% with Epinephrine 1:200000). Mucoperiosteal flaps were elevated around the osteonecrotic area and all inflammatory tissues were thoroughly removed. Piezosurgery (Piezotome, Solo, Satelec, France) was applied, firmly moving over the necrotic bone while an integrated saline solution was cooling the area to maintain low temperature at the surgical site. A-PRF membranes were obtained according to Choukroun's protocol. The patient's blood was collected in 10 ml tubes without anticoagulant and centrifugation was immediately performed at 1300 rpm for 14 min (PRF Duo Quattro, France). The slow polymerisation generated fibrin network membranes, similar to natural clotting, which were placed into the socket covering the whole area followed by tension-free suturing (silk 4/0).
Oral systemic antibiotics were prescribed (amoxicillin/clavulanic acid 675 mg, 2 times daily and metronidazole 500 mg, 3 times daily for 10 days), and daily rinses with chlorhexidine (0.12% twice daily for 15 days) were requested.
PBM started immediately after surgery. A device with two laser emitting probes at 810 nm (GaAlAs) and 660 nm (InGaAlP) (THOR Photomedicine Ltd) was used over the wound top and edges. The radiation parameters for the 810 nm probe were: Average Power 200 mW, Beam Area 0.09 cm2, Irradiance 1.97 W/cm2, Time per Point 30 s, Energy 6 Joules, Fluence 59 J/cm2 and Total Energy 2 × 6 = 12 Joules, while for the 660 nm probe were: Average Power of 75 mW, Beam area 2.54 cm2, Irradiance 0.026 W/cm2, Time per Point 30 s, Energy 2.25 Joules, Fluence 0.75 J/cm2 and Total Energy 3 × 2.25 = 6.75 Joules. The total delivered dose per session was 12 + 6.75 = 18.75 Joules (25). PBMT was repeated 3 times a week for three weeks.
The Histopathologic examination concluded in “inflammatory cell infiltration with basophilic bacterial colonies and irregular peripheral bone resorption with prominent reversal lines.” The findings were compatible with MRONJ due to alendronate use for more than 3 years.
During the 6 months follow-up period the patient has been asymptomatic with no clinical and radiographic signs of ONJ recurrence while the residual bone and alveolar mucosa have healed properly (Figure 2).
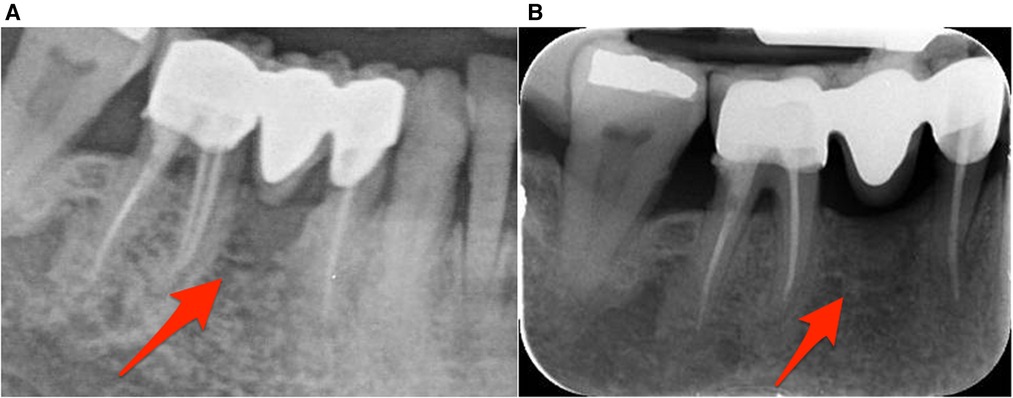
Figure 2. Radiographic image of the lower right premolar-molar region. (A) Radiographic image during patient's initial visit. (B) Radiographic image taken 6 months later revealing bone remodeling.
A 55-year-old Caucasian male was referred to the dental department from the Hematology Oncology department of our hospital with pain, swelling, purulent secretion and exposed bone in the mandible.
His medical anamnesis included a diagnosis of Acute Lymphoblastic Leukemia—Ph + (2010). He had been treated with the GMALL protocol (dexamethasone, vincristine, idarubicin, peg-Asparaginase, cyclophosphamide, mercaptopurine) + tyrosine- kinase inhibitor Imatinib (400 mg per day) followed by the 2nd stage -consolidation- of treatment (vincristine, methotrexate, etoposide, cytabine, dexamethasone, aracytin) + Imatinib (400 mg per day) with complete molecular remission of the disease. After 3.5 years of treatment with no signs of recurrence, the patient continued the maintenance treatment only with Imatinib (400 mg per day) for 4 years.
Eight months ago, the patient underwent an extraction in a private office of tooth #37.
A non-healing wound at the lower left mandible was present during the clinical examination while a radiolucent area surrounded by opacity at the extraction site was revealed in the panoramic x-ray. The clinical and radiographic evaluation, in accordance with the negative history for local bone metastases or radiation therapy or even the use of bone-targeting agents, hypothesised the possibility of a MRONJ (Stage II) case due to Imatinib (Figure 3).
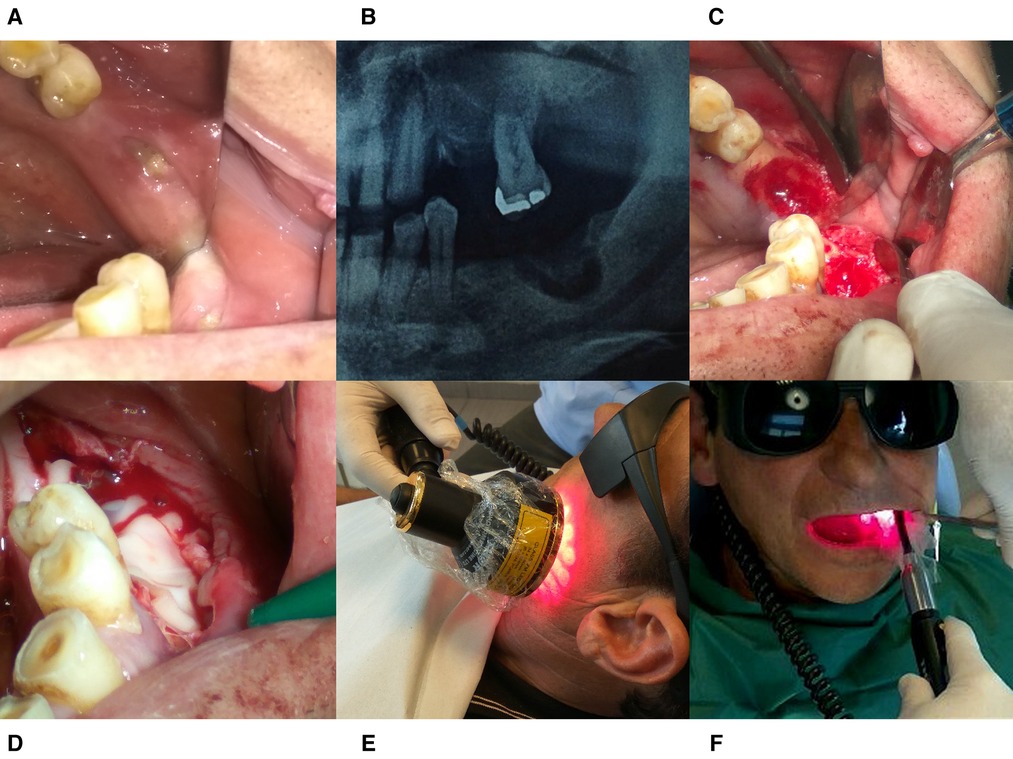
Figure 3. Treatment protocol for case 2. (A) Intraoral image taken at the patient’s initial visit. (B) Radiographic Image before operation. (C) The surgical area after debridement with piezotome. (D) PRF- membrane placement into the socket. (E) Extraoral PBM therapy with 69 LED Cluster 660/850 nm. (F) Intraoral PBM therapy with Laser 660 and 810 nm probes.
The immediate discontinuation of Imatinib had been decided and a conservative approach was followed using oral systemic antibiotic treatment: amoxicillin (1 g, 3 times daily for 10 days) followed by clindamycin (300 mg, 3 times daily for 7 days) and antiseptic mouthwashes (chlorhexidine 0.20%, 2 times daily for 30 days).
The follow-up examination after 6 weeks showed no clinical signs of improvement, thus after informing the patient and in agreement with the hematology oncology department a surgical debridement of the area with the use of piezotome was performed in combination with the placement of A-PRF membrane into the socket as described in the first case. Immediately after tension-free suturing with silk (4/0), PBMT was applied for 3 consecutive days.
For the PBMT the same device was used (THOR Photomedicine, Ltd). Except for the intraoral laser probes (810/660 nm) and settings as previously described, additionally a 69 Cluster LED (34 × 660 nm + 35 × 850 nm) probe was used extra-orally with radiation parameters: Power = 1390 mW, Frequency 2.5 Hz, PdAvg = 44,6 mW/cm², Time per Point 60 s and Total Energy of 84 Joules. The Total Delivered Dose per session was 12 + 6.75 + 84 = 102.75 Joules.
Oral systemic antibiotics (amoxicillin/clavulanic acid 675 mg and metronidazole 500 mg, 3 times daily for 10 days) and daily rinses with chlorhexidine 0.12%, twice daily for 15 days were prescribed.
The Histopathologic Examination revealed “necrotic bone trabeculae with inflammatory infiltration and basophilic bacterial colonies”, findings that confirmed the primary hypothesis of MRONJ (stage II) related to Imatinib.
The patient has been asymptomatic in the follow-up period with no clinical or radiographic signs of ONJ recurrence, and is therefore on maintenance second-line imatinib.
The radiographic evaluation of the area, 3 and 6 months later, verified the reverse tendency of the osteolytic process to a new bone remodeling of the area (Figure 4).
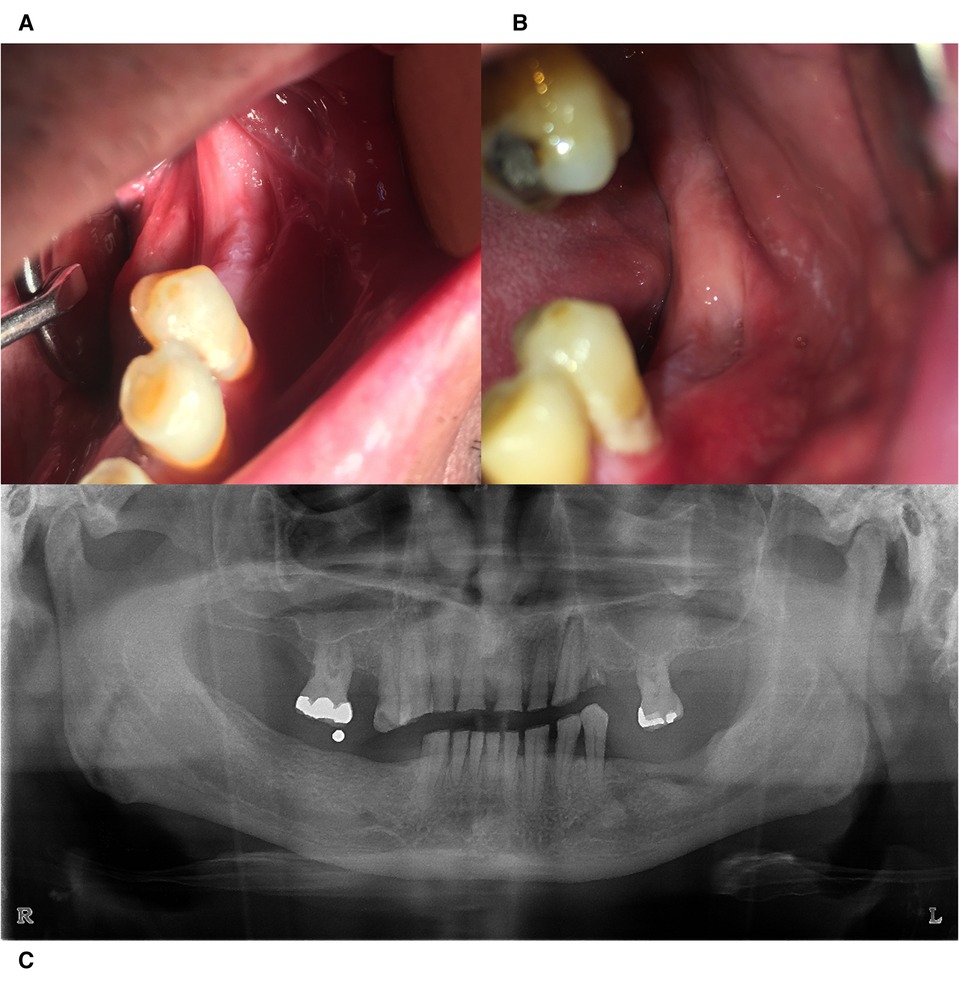
Figure 4. Clinical evaluation 3 months (A) and 6 months (B) postoperatively. (C) Radiographic image taken 6 months postoperatively with signs of bone remodeling.
The management of MRONJ is still a challenge for oral and maxillofacial surgeons, particularly related to the presence of anti-resorptive, antiangiogenic or other targeted therapies. According to the AAOMS position paper (2014), a recommended treatment should limit the infection, relieve pain and control necrosis progression, which may not always be achieved with the antibiotic prescription and the surgical intervention (2). Successful surgical therapy for MRONJ was defined by Ristow and Pautke as the complete removal of all necrotic bone, the total repair and recoating of the overlying mucosa and not experiencing any symptoms in a period of at least 4 months (26). In order to achieve better clinical outcomes, the additional use of adjuvant methods such as PRF and PBMT due to their favourable tissue repair properties was proposed.
The concept of stage-specific treatment has gained attention among clinicians who are choosing different approaches to apply for each particular stage of the disease (27). Non-invasive treatment protocols are preferred for patients at risk or at stage 0, with preventive measures, good oral hygiene, 0.12% chlorhexidine mouthwashes and routine dental follow-up (1, 14). Advanced stages of established MRONJ require antibiotic therapy in combination with surgical intervention (debridement/resection). This is the reason why in many cases, especially with medically compromised patients, the additional use of various adjunctive therapies was proposed and resulted in beneficial outcomes (3, 13, 14, 24, 27–29).
In the cases presented above, both in MRONJ stage II, one patient had been under BPs therapy (alendronate) for more than 4 years while the second one had used a tyrosine-kinase inhibitor (Imatinib) which is rarely described in the literature that can cause ONJ (30). Both patients received an approach that combines antibiotic therapy with the surgical debridement of the affected area using piezosurgery. Additionally, A-PRF membranes were inserted into the viable bone tissue followed by PBMT immediately after suturing.
It is apparent that the exposed bone favours microbial contamination which lead to increased tissue inflammation and necrosis. The complete removal of necrotic bone with the smoothening of the sharp bone edges and infection control are inevitably necessary. Piezosurgery devices, due to their cavitation effect, directly killing bacteria, with low thermal trauma and controlled bony cuts maintaining the continuity of the vital bone, have proven their effectiveness in MRONJ surgical treatments (14, 23, 24, 27, 31).
Since the ability of oral tissues to repair properly is compromised by the negative effect of antiresorptive/antiangiogenic medication in various cell lines, the healing support of both hard and soft tissues is the main objective for a successful outcome (10, 20, 32).
Autologous Platelet Concentrates (Platelet Rich Plasma, Plasma Rich in Growth Factors, Leukocyte- and Platelet-Rich Fibrin) have been used in oral surgery as they have shown promising results in hard and soft tissue regeneration (33, 34). Literature proposed Platelet-Rich Fibrin (PRF) as an adjuvant therapy for MRONJ as it promotes the migration and proliferation of both fibroblasts and osteoblasts, enhancing the healing process (24, 35). A-PRF is a variation of the original L-PRF using much lower centrifugation speed, a slightly larger centrifugation time and glass tubes. A-PRF membranes promote the differentiation of macrophages and release high quantities of growth factors (PDGF, VEFG, TGF-β) activating a cascade of events inducing the revascularisation of injured tissues (24, 36). There are several reports in the literature assessing that the use of PRF seems to be a good alternative for the prevention of MRONJ promoting a high-rate success of surgical interventions and improving wound healing (14, 27, 34).
Photobiomodulation therapy has gained interest as a promising therapeutic modality for MRONJ management due to its ability to reduce drug-associated cytotoxicity and promote inherent healing capacity of multiple oral cell lines including keratinocytes, fibroblasts and osteoblasts (32, 37). PBM stimulates proliferation and differentiation of osteoblasts in vitro and in vivo and also increases the activity of alkaline phosphatase and osteocalcin expression, leading to increased bone formation (18, 38) Moreover, PBM is a non-invasive method with biomodulative properties that increase collagen synthesis, accelerate the resolution of inflammatory processes, promote neoangiogenesis, and fasten the tissue repair process (21, 29). Intraorally and extraorally administered PBMT is believed to be more effective for the resolution of inflammation and postoperative oedema, promoting lymphatic relaxation via vasodilation, reducing patients' morbidity (39, 40). Clinical studies have indicated positive outcomes of Photobiomodulation as monotherapy on post-extraction healing process, while others have shown higher therapeutic success rate when PBMT was combined with conventional or surgical interventions (13, 41–43).
Recently the association of microbial growth control with systemic antibiotics and local irrigation, the favorable biomechanical effect on necrotic bone removal with Piezosurgery, the osteoinductive properties of PRF along with the biomodulative properties of PBMT as a multidisciplinary approach has given promising results in MRONJ management (14, 21, 24, 29). Such an approach has been regularly followed in our dental department with patients referred from the Hematology/Oncology department, suffering from MRONJ. Most of the cases treated were successful, with no recurrence in the 12-month follow-up period. Our findings contribute to previous literature by offering an alternative approach especially for those cases that cannot postpone the bone targeting or antiangiogenic medication. It is apparent that more prospectively controlled studies with larger sample sizes are still needed to verify the beneficial effects of such complementary protocols.
All patients treated either with bone-targeting agents or anti-angiogenic drugs are at risk of developing MRONJ. The incidence of ONJ is estimated to increase due to the urgent need for new biological therapies.
In the present case report, a combined protocol aimed at controlling microbial infection with systemic antibiotics and local mouth rinses, debriding minimally the necrotic bone with the use of piezotome and the additional promotive effect on wound healing from osteoinductive (PRF) and biomodulative (PBM) factors, was shown to be a beneficial alternative for the management of MRONJ. In order to minimise patient risk, patients with a severe medical history who are not eligible for extensive surgery are probably the most suitable candidates.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
KV: conceptualization and supervision. KV and TFV: contributed to the literature review. TFV: writing- original draft preparation. TFV and KV: review and editing of the manuscript. KV and ST: contributed to the surgical treatment. TFV and EG: contributed to PBM treatment. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Nicolatou-Galitis O, Schiødt M, Mendes RA, Ripamonti C, Hope S, Drudge-Coates L, et al. Medication-related osteonecrosis of the jaw: definition and best practice for prevention, diagnosis, and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol. (2019) 127(2):117–35. doi: 10.1016/j.oooo.2018.09.008
2. Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrota B, et al. American Association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw- 2014 update. J Oral Maxillofac Surg. (2014) 72:1938–56. doi: 10.1016/j.joms.2014.04.031
3. Otto S, Pautke C, Van den Wyngaert T, Niepel D, Schiødt M. Medication-related osteonecrosis of the jaw: prevention, diagnosis and management in patients with cancer and bone metastasis. Cancer Treat Rev. (2018) 69:177–87. doi: 10.1016/j.ctrv.2018.06.007
4. Longo F, Guida A, Aversa C, Pavone E, Di Costanzo G, Ramaglia L, et al. Platelet rich plasma in the treatment of bisphosphonate-related osteonecrosis of the jaw: personal experience and review of the literature. Int J Dent. (2014) 2014:7. doi: 10.1155/2014/298945
5. Patel S, Choyee S, Uyanne J, Nguyen A, Lee P, Sedghizadeh P, et al. Non-exposed bisphosphonate-related osteonecrosis of the jaw: a critical assessment of current definition, staging, and treatment guidelines. Oral Dis. (2012) 18(7):625–32. doi: 10.1111/j.1601-0825.2012.01911.x
6. Bedogni A, Fusco V, Agrillo A, Campisi G. Learning from experience. Proposal of a refined definition and staging system for bisphosphonate-related osteonecrosis of the jaw (BRONJ). Oral Dis. (2012) 18(6):621. doi: 10.1111/j.1601-0825.2012.01903.x
7. Campisi G, Mauceri R, Bertoldo F, Bettini G, Biasotto M, Colella G, et al. Medication-Related osteonecrosis of jaws (MRONJ) prevention and diagnosis: Italian consensus update 2020. Int J Environ Res Public Health. (2020) 17(16):5998. doi: 10.3390/ijerph17165998
8. Govaerts D, Piccart F, Ockerman A, Coropciuc R, Politis C, Jacobs R. Adjuvant therapies for MRONJ: a systematic review. Bone. (2020) 141:115676. doi: 10.1016/j.bone.2020.115676
9. Ruggiero SL, Dodson TB, Aghaloo T, Carlson ER, Ward BB, Kademani D. American association of oral and maxillofacial Surgeons's position paper on medication- related osteonecrosis of the jaws—2022 update. J Oral Maxillofac Surg. (2022) 80(5):920–43. doi: 10.1016/j.joms.2022.02.008
10. Aghaloo T, Hazboun R, Tetradis S. Pathophysiology of osteonecrosis of the jaws. Oral Maxillofac Surg Clin. (2015) 27(4):489–96. doi: 10.1016/j.coms.2015.06.001
11. Vescovi P, Merigo E, Meleti M, Manfredi M, Fornaini C, Nammour S, et al. Conservative surgical management of stage I bisphosphonate-related osteonecrosis of the jaw. Int J Dent. (2014) 2014:107690. doi: 10.1155/2014/107690
12. Tenore G, Zimbalatti A, Rocchetti F, Graniero F, Gaglioti D, Mohsen A, et al. Management of medication-related osteonecrosis of the jaw (MRONJ) using leukocyte-and platelet-rich fibrin (L-PRF) and photobiomodulation: a retrospective study. J Clin Med. (2020) 9(11):3505. doi: 10.3390/jcm9113505
13. Del Vecchio A, Tenore G, Pergolini D, Rocchetti F, Palaia G, Romeo U. The role of the laser photobiomodulation (PBM) in the management of patients at risk or affected by MRONJ. Oral. (2022) 2(1):7–15. doi: 10.3390/oral2010002
14. Nica DF, Riviș M, Roi CI, Todea CD, Duma V-F, Sinescu C. Complementarity of photo-biomodulation, surgical treatment, and antibiotherapy for medication-related osteonecrosis of the jaws (MRONJ). Medicina. (2021) 57(2):145. doi: 10.3390/medicina57020145
15. Goker F, Grecchi E, Grecchi F, Francetti L, Del Fabbro M. Treatment of medication-related osteonecrosis of the jaw (MRONJ). A systematic review. Eur Rev Med Pharmacol Sci. (2021) 25:2662–73. doi: 10.26355/eurrev_202103_25430
16. Kobayashi E, Flückiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, et al. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig. (2016) 20(9):2353–60. doi: 10.1007/s00784-016-1719-1
17. Chung H, Dai T, Sharma SK, Huang Y-Y, Carroll JD, Hamblin MR. The nuts and bolts of low-level Laser (light) therapy. Ann Biomed Eng. (2012) 40(2):516–33. doi: 10.1007/s10439-011-0454-7
18. Weber JBB, Pinheiro ALB, de Oliveira MG, Oliveira FAM, Ramalho LMP. Laser therapy improves healing of bone defects submitted to autologus bone graft. Photomed Laser Surg. (2006) 24(1):38–44. doi: 10.1089/pho.2006.24.38
19. Medrado AP, Soares AP, Santos ET, Reis SRA, Andrade ZA. Influence of laser photobiomodulation upon connective tissue remodeling during wound healing. J. Photochem. Photobiol. B, Biol. (2008) 92(3):144–52.
20. El-Hayes KA, Zaky AA, Ibrahim ZA, Allam GFA, Allam MF. Usage of low level Laser biostimulation and platelet rich fibrin in bone healing: experimental study. Dent Med Probl. (2016) 53(3):338–44. doi: 10.17219/dmp/62973
21. Martins MAT, Martins MD, Lascala CA, Curi MM, Migliorati CA, Tenis CA, et al. Association of laser phototherapy with PRP improves healing of bisphosphonate-related osteonecrosis of the jaws in cancer patients: a preliminary study. Oral Oncol. (2012) 48(1):79–84. doi: 10.1016/j.oraloncology.2011.08.010
22. Merigo E, Rocca J-P, Pinheiro AL, Fornaini C. Photobiomodulation therapy in oral medicine: a guide for the practitioner with focus on new possible protocols. Photobiomodul Photomed Laser Surg. (2019) 37(11):669–80. doi: 10.1089/photob.2019.4624
23. Kagami H, Inoue M, Kobayashi A, Taguchi A, Li X, Yoshizawa M. Issues with the surgical treatment of antiresorptive agent-related osteonecrosis of the jaws. Oral Dis. (2018) 24(1-2):52–6. doi: 10.1111/odi.12783
24. Merigo E, Cella L, Oppici A, Arbasi MC, Clini F, Fontana M, et al. Combined approach to treat medication-related osteonecrosis of the jaws. J Lasers Med Sci. (2018) 9(2):92–100. doi: 10.15171/jlms.2018.19
25. Jenkins PA, Carroll JD. How to report low-level Laser therapy (LLLT)/photomedicine dose and beam parameters in clinical and laboratory studies. Photomed Laser Surg. (2011) 29(12):785–7. doi: 10.1089/pho.2011.9895
26. Ristow O, Pautke C. Auto-fluorescence of the bone and its use for delineation of bone necrosis. Int J Oral Maxillofac Surg. (2014) 43(11):1391–3. doi: 10.1016/j.ijom.2014.07.017
27. Şahin O, Akan E, Tatar B, Ekmekcioğlu C, Ünal N, Odabaşı O. Combined approach to treatment of advanced stages of medication-related osteonecrosis of the jaw patients. Braz J Otorhinolaryngol. (2021) 88(4):613–20. doi: 10.1016/j.bjorl.2021.04.004
28. Weber JB, Camilotti RS, Ponte ME. Efficacy of laser therapy in the management of bisphosphonate-related osteonecrosis of the jaw (BRONJ): a systematic review. Lasers Med Sci. (2016) 31(6):1261–72. doi: 10.1007/s10103-016-1929-4
29. Rodríguez-Sánchez MDP, Statkievicz C, de Mello-Neto JM, Toro LF, Farnezzi Bassi AP, Garcia VG, et al. The effectiveness of the low-level Laser, antibiotic and surgical therapy in the treatment of medication-related osteonecrosis of the jaws: a case report. J Lasers Med Sci. (2020) 11(1):98–103. doi: 10.15171/jlms.2020.16
30. Gupta L, Dholam K, Janghel Y, Gurav SV. Osteonecrosis of the jaw associated with imatinib therapy in myeloproliferative neoplasm: a rare case report. Oral Surg Oral Med Oral Pathol Oral Radiol. (2021) 131(5):e157–62. doi: 10.1016/j.oooo.2020.10.005
31. Blus C, Szmukler-Moncler S, Giannelli G, Denotti G, Orrù G. Use of ultrasonic bone surgery (piezosurgery) to surgically treat bisphosphonate-related osteonecrosis of the jaws (BRONJ). A case series report with at least 1 year of follow-up. Open Dent J. (2013) 7:94–101. doi: 10.2174/1874210601307010094
32. Reid IR, Bolland MJ, Grey AB. Is bisphosphonate-associated osteonecrosis of the jaw caused by soft tissue toxicity? Bone. (2007) 41(3):318–20. doi: 10.1016/j.bone.2007.04.196
33. Del Fabbro M, Gallesio G, Mozzati M. Autologous platelet concentrates for bisphosphonate-related osteonecrosis of the jaw treatment and prevention. A systematic review of the literature. Eur J Cancer. (2015) 51(1):62–74. doi: 10.1016/j.ejca.2014.10.015
34. Miranda M, Gianfreda F, Raffone C, Antonacci D, Pistilli V, Bollero P. The role of platelet-rich fibrin (PRF) in the prevention of medication-related osteonecrosis of the jaw (MRONJ). BioMed Res Int. (2021) 2021:4948139. doi: 10.1155/2021/4948139
35. Fortunato L, Bennardo F, Buffone C, Giudice A. Is the application of platelet concentrates effective in the prevention and treatment of medication-related osteonecrosis of the jaw? A systematic review. J Craniomaxillofac Surg. (2020) 48(3):268–85. doi: 10.1016/j.jcms.2020.01.014
36. Pavlovic V, Ciric M, Jovanovic V, Trandafilovic M, Stojanovic P. Platelet-rich fibrin: basics of biological actions and protocol modifications. Open Med. (2021) 16(1):446–54. doi: 10.1515/med-2021-0259
37. Arany PR. Craniofacial would healing with photobiomodulation therapy: new insights and current challenges. J Dent Res. (2016) 95(9):977–84. doi: 10.1177/0022034516648939
38. Ferrante M, Petrini M, Trentini P, Perfetti G, Spoto G. Effect of low-level laser therapy after extraction of impacted lower third molars. Lasers Med Sci. (2013) 28(3):845–9. doi: 10.1007/s10103-012-1174-4
39. Epstein JB, Raber-Durlacher JE, Lill M, Linhares YPL, Chang J, Barasch A, et al. Photobiomodulation therapy in the management of chronic oral graft-versus-host disease. Support Care Cancer. (2017) 25(2):357–64. doi: 10.1007/s00520-016-3401-1
40. Bensadoun R-J. Photobiomodulation or low-level laser therapy in the management of cancer therapy-induced mucositis, dermatitis and lymphedema. Curr Opin Oncol. (2018) 30(4):226–32. doi: 10.1097/CCO.0000000000000452
41. Scoletta M, Arduino PG, Reggio L, Dalmasso P, Mozzati M. Effect of low-level Laser irradiation on bisphosphonate-induced osteonecrosis of the jaws: preliminary results of a prospective study. Photomed Laser Surg. (2010) 28(2):179–84. doi: 10.1089/pho.2009.2501
42. Vescovi P, Giovannacci I, Merigo E, Meleti M, Manfredi M, Fornaini C, et al. Tooth extractions in high-risk patients under bisphosphonate therapy and previously affected with osteonecrosis of the jaws. J Craniofac Surg. (2015) 26(3):696–9. doi: 10.1097/SCS.0000000000001665
Keywords: MRONJ, photobiomodulation, lasers, platelet concentrates, osteonecrosis, jaw, wound healing
Citation: Valamvanos K, Valamvanos T-F, Toumazou S and Gartzouni E (2022) The combined use of photobiomodulation therapy and platelet-rich fibrin for the management of two MRONJ stage II cases: An alternative approach. Front. Dent. Med 3: 973738. doi: 10.3389/fdmed.2022.973738
Received: 20 June 2022; Accepted: 17 November 2022;
Published: 6 December 2022.
Edited by:
Nasim Chiniforush, Tehran University of Medical Sciences, IranReviewed by:
Maria Contaldo, University of Campania L. Vanvitelli, Italy© 2022 Valamvanos, Valamvanos, Toumazou and Gartzouni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Konstantinos Valamvanos, ay52YWxhbXZhbm9zQHlhaG9vLmNvbQ==
Specialty Section: This article was submitted to Reconstructive Dentistry, a section of the journal Frontiers in Dental Medicine
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.