- 1Division of Oral Diagnostics and Rehabilitation, Department of Dental Medicine, Karolinska Institutet, Huddinge, Sweden
- 2Scandinavian Center for Orofacial Neurosciences (SCON), Huddinge, Sweden
- 3Section for Orofacial Pain and Jaw Function, Department of Dentistry and Oral Health, Aarhus University, Aarhus, Denmark
Introduction: Masticatory function is often impaired in patients with painful temporomandibular disorders (TMD); therefore, more detailed studies on comminution and mixing ability are warranted in well-defined TMD patients with chronic myalgia. Moreover, there is a need to explore the correlation between any changes in perceived pain or fatigue in such patients and the masticatory function.
Materials and methods: Self-assessments using questionnaires regarding pain, oral health, jaw function, masticatory ability, fear of movement, and psychosocial signs were answered by all the participants. A series of chewing tasks involving viscoelastic food and two-colored gum were performed. Optical imaging and analysis were conducted. Bite force as well as characteristics of pain and fatigue were assessed.
Results: In patients, the fragmented soft candy particles were less in number and had larger median of area and minimum Feret's diameter after standardized chewing compared to healthy individuals (P = 0.02). Surprisingly, the two-colored Hue-Check gum was less mixed by the healthy controls since they displayed a greater variance of the hue (P = 0.04). There were significant differences between the patients and the healthy controls in the self-assessed masticatory ability, mainly regarding pain-related variables.
Conclusions: Objectively, TMD patients with chronic myalgia exhibited an impaired masticatory performance with less efficiency in comminuting soft viscoelastic food compared to the pain-free healthy control group. There was an agreement between the patients' self-assessed masticatory ability and the efficiency of their masticatory function.
Introduction
Temporomandibular disorders (TMD) are a collective term embracing chronic pain conditions in the orofacial region, thus affecting the masticatory muscles or the temporomandibular joint and their associated structures (1). Patients with temporomandibular disorders often complain about pain, usually chronic jaw muscle pain (2), and jaw dysfunction, for instance, restricted jaw mobility and chewing difficulties (3). Previous studies indicated that chewing pattern and duration differ in patients with TMD, including painful jaw muscles and TMJ, from controls (4, 5). These studies showed that difficulties in vertical mouth opening and chewing were significantly more prevalent in patients with TMD. Those patients displayed a unilateral chewing pattern, longer duration of chewing, and higher number of chewing strokes during observational monitoring. Further, experimental pain in the masseter muscle induced a decreased electromyographic (EMG) activity of the jaw-closing muscles in their agonist phase during painful mastication (6).
Mastication is the first step of the process of digestion in which food is prepared for swallowing. Chewing is a complex sensory-motor activity that is under the necessary neuromuscular control, leading to a reduction of the food particle size in order to allow further breakdown by salivary enzymes (7). Chewing performance can be described in terms of kinetics and EMG activity (8). The masticatory muscles move the mandible dynamically in a rhythmic pattern in order to bring the teeth into intermittent contact by occluding and opening and generate the force needed to comminute the food (9, 10). Previous studies have shown enhanced muscle activity in healthy pain-free jaw-closing muscles with increased food hardness due to sensory feedback from periodontal receptors and spindle afferents of the jaw-closing muscles (11–15). Also, the prediction of food properties based on information obtained during previous chewing cycles plays an important role in regulating jaw muscle activation during the jaw-closing phase, especially as the mechanical properties of the food are changing during natural mastication (16, 17). Age, gender, jaw muscle size, dental state, salivary flow rate, bolus size, chewing rate, bite force, jaw movements, and TMDs are among the factors that are believed to affect the masticatory performance in humans (18–25).
Objective assessment of masticatory performance or efficiency needs to include variables of comminution and mixing functions (20, 26–30). Several studies have assessed jaw kinematics and masticatory performance objectively in specific patient groups, such as older adults, denture wearers, and patients with dental implants (31–39). Both the objective masticatory efficiency and self-reported masticatory ability aspects are of importance in evaluating the masticatory performance, especially considering the weak and even lack of correlation between them (24, 40).
To the best of our knowledge, there are no studies that investigate either the objective masticatory efficiency of patients with chronic jaw myalgia or the subjective experiences of the ability or difficulty of mastication in these patients. The main aim of the study was to investigate masticatory performance in patients with chronic jaw myalgia using comminution and mixing tests. Furthermore, to explore the correlation between any changes in the perceived pain or fatigue and the masticatory function. We hypothesized that TMD pain patients would show signs of impaired chewing function reflected in larger food particles during the comminution test and larger variance of hue values in the mixing ability test compared to the control group. Also, increased pain and fatigue reports in patients would correlate negatively with efficient chewing. A second aim was to explore these patients' self-assessed masticatory ability.
Materials and methods
This case–control study was conducted at the Department of Dental Medicine, Karolinska Institutet, Huddinge, Sweden. The study was performed in accordance with the Declaration of Helsinki and approved by the Regional Ethical Review Board in Stockholm (DNR: 2014/1394-3).
Participants
Twenty patients with myalgia according to the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) (41) and 20 healthy and pain-free controls were enrolled in this study. The sample size calculation indicated that 17 patients with myalgia and 17 controls were needed to show a mean (SD) difference of 30% between groups, with a power of 80% and a significance level of P < 0.05 (5, 42). The participants were recruited at the University Dental Clinic and the Department of Dental Medicine, Karolinska Institutet, Huddinge, Sweden. Before inclusion, all participants were given both verbal and written information about this study, and an informed written consent was obtained.
The inclusion criteria for the TMD pain patient group were: local myalgia/myofascial pain with referred pain in masseter and/or temporal muscles according to the DC/TMD by Schiffman et al. (41); pain duration of at least 3 months; and current pain with a minimum score of 4 according to numeric rating scale (NRS 0–10). For the healthy individuals, the inclusion criteria were: good general health. Additional inclusion criteria for all participation in the study were: age over 18 years; natural teeth within positions 13–16 and 23–26 with normal relation to antagonistic teeth; and at least 2 premolar/molar occlusal contacts per side in intercuspal position, including single fixed tooth-supported prostheses/crowns or single root-canal-treated teeth. Individuals with missing teeth within positions 13–16 and 23–26 due to orthodontic extractions or aplasia, but no edentulous areas, were included.
The exclusion criteria for the healthy individuals were: a diagnosis of myalgia or myofascial pain according to DC/TMD; and additional palpatory tenderness of the masseter, temporalis muscles, or over the temporomandibular joint (TMJ). Additional exclusion criteria for all participation in the study were: a diagnosis of arthralgia, degenerative joint disease, painful jaw clicking or popping/locking according to DC/TMD; toothache, neuropathic pain in the trigeminal nerve region or referred pain from other regions to the jaw muscles; clinically visible dental pathology or mobility, malocclusions, edentulous areas, single fixed implant prostheses, tooth-supported or implant-supported bridges within positions 13–16 and 23–26 or dentures; tooth wear grade 3 = exposure of pulp or secondary dentine according to the simplified scoring criteria for tooth wear index I by López-Frías et al. (43); General pain conditions or systemic inflammatory diseases (for example, fibromyalgia, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis) or neurological disease (for example, myasthenia gravis, craniomandibular dystonia); whiplash-associated disorder; use of any medication that might influence the response of pain i.e., analgesics, anti-inflammatory or muscle relaxant during 24 h preceding the experiment, use of cannabinoids, or any medication that might influence the neurological function; allergy to any of the substances or food used in the experiment; pregnancy or lactation; and cognitive or physical disability that prevent participation.
Experimental protocol
Prior to the inclusion, all participants answered questionnaires regarding their psychosocial distress and pain characteristics, including pain drawings according to Axis II of DC/TMD, as well as self-assessments regarding their chewing abilities according to the questionnaires mentioned below. The participants were also clinically examined according to Axis I of DC/TMD. The participants were then asked to perform a series of chewing tasks involving six candies and one pair of two-colored gum. Recordings of chewing durations during each chewing task were registered. Pain intensity and self-reported fatigue/exertion were assessed at baseline, after the first two soft candies (S1 + S2), after the two hard candies (H1 + H2), after the chewing gum, and after the last two soft candies (S3 + S4). Maximal voluntary bite force (MVBF) was assessed at baseline and at the end of the experiment. The experimental protocol and sequence of assessments are explained in detail and illustrated in Figure 1. All clinical examinations and assessments, including giving instructions to the participants and monitoring the number of chewing strokes and scans, were performed by one examiner (SAS).
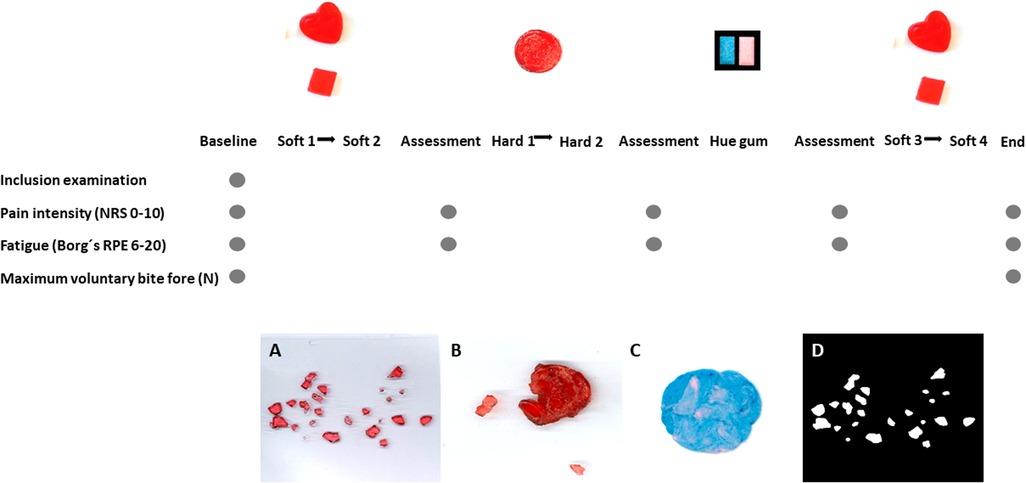
Figure 1. Experimental protocol and illustration samples—the experimental protocol and sequence of assessments are illustrated. Recordings of chewing duration during each chewing task S1 (natural chewing with soft candy), S2 (standardized chewing with soft candy), H1 (natural chewing with hard candy), H2 (standardized chewing with hard candy), Hue-Check gum, S3 (natural chewing with soft candy) and S4 (standardized chewing with soft candy) were registered. Pain intensity and self-reported fatigue/exertion were assessed at baseline and after each chewing sequence, S1 + S2, H1 + H2, Hue-Check gum and S3 + S4. Maximal voluntary bite force was assessed at baseline and at the end of the experiment. The figure presents samples to illustrate images of the fragmented soft candy (A), hard candy after chewing (B) and Hue-Check gum after chewing (C) as well as imaging in Image J (D).
Questionnaires
Self-assessments using questionnaires regarding pain, oral health, jaw function, masticatory ability, fear of movement, and psychosocial signs were answered by all the participants. The questionnaires included were: DC/TMD Axis II (41), graded chronic pain scale (GCPS-7) (44), oral behavior checklist (OBC-21) (45), oral health impact profile (OHIP-14) (46), jaw functional limitation scale (JFLS-20) (47, 48), quality of masticatory function (QMF-28) (49), Tampa scale for kinesiophobia (Tampa-TMD-18) (50), widespread pain index (WPI) and somatic severity scale (SSS) (51), diagnostic criteria for irritable bowel syndrome (Rome IV) (52), pain catastrophizing scale (PCS-13) (53), the patient health questionnaire for depression (PHQ-9) (54), generalized anxiety disorder scale (GAD-7) (55), the patient health questionnaire for physical symptoms (PHQ-15) (56), perceived stress scale (PSS-10) (57) and insomnia severity index (ISI-7) (58).
Test food
Both edible laboratory-fabricated test foods (14, 15, 31, 59, 60) and commercially available foods (36, 42, 61) were used in studying the comminuting function. In the current study, viscoelastic candies were used. Big red heart-shaped jelly candy (Stora Gelé Hjärtan, Konfektyrfabriken Aroma AB) served as soft elastic candy. Red circular-shaped sugar-coated sour gummy gelatin candy (Haribo Syrlingar, Haribo Lakrits AB) served as hard plastic candy. Each one of the heart-shaped soft candy was manually cut and formed into cubes with standardized dimensions of 20 × 20 × 5 mm, all by the same examiner (SAS). The circular-shaped hard candies had standardized dimensions of 20 × 5 mm. For studying the mixing performance, the standardized two-colored Hue-Check gum was used (30, 62), which is a valid and reliable method (26, 63–66) that had been used in several previous studies (67–69). Images of the test food and gum are shown in Figure 1.
Chewing tasks
The participants chewed four pieces of soft candies (S1, S2, S3 and S4) and two pieces of hard candies (H1 and H2). The participants were instructed to chew S1, H1, and S3 as naturally as possible until swallowing onset and then to spit-out instead of swallowing. S2, H2, and S4 would be chewed in a standardized manner using the habitual/preferred side with twenty chewing strokes/chewing cycles. Soft candies S3 and S4 were used to explore possible correlations between the perceived changes in pain or fatigue and the chewing performance. All the candy, together with the saliva, should be spited-out in plastic mugs for later spreading on transparent sheets. The participants were also instructed according to the manual of Schimmel (62) to chew the two-colored Hue-Check gum twenty times as normally as possible and were allowed to change the chewing side during this task. The participants were instructed to rinse their mouth with water after spitting out each candy and gum.
Assessment of bite force
The maximal voluntary bite force (MVBF) in Newton (N) was assessed at baseline and at the end of the experiment. A custom-made bite force transducer (1,000 N, 41.0 × 12.0 × 5.0 mm, length × width × height, Aalborg University, Aalborg, Denmark) was used to assess the MVBF. The bite force transducer was covered with 1 mm rubber in order to avoid any cross-contamination and reduce the risk of tooth fracture and inserted between the first molars either on the right or left side, depending on each participant's habitual or preferred masticatory side. The assessment of MVBF was repeated three times at baseline and at the end of the experiment. The mean of the three assessments was later included in the statistical analyses.
Assessment of pain characteristics and self-reported fatigue/exertion
At baseline, the participants were asked to mark the maximum pain spread on a lateral chart of the face for both the right and left sides separately as well as intra-orally. Pain intensity was assessed at baseline, after S1 + S2, after H1 + H2, and after S3 + S4 using a numeric rating scale (NRS) (0–10), where the end-points were 0 = no pain and 10 = worst imaginable pain (70).
Self-reported fatigue/exertion was assessed at baseline, after S1 + S2, after H1 + H2, and after S3 + S4 using Borg's Rating of Perceived Exertion Scale (Borǵs RPE) (6–20), where 6 is extremely easy effort and 20 is maximum effort (71).
Imaging, Pre-processing, and data analyses
Sieving using silicone-based test food, such as Optosil Comfort, has been used as gold standard method regarding comminution of food (24, 72–75). Since sieving requires a lot of time and special equipment, alternative methods were identified and tested. Studies showed that optical scanning or imaging is a comparable method to sieving and is more time-effective (28, 29, 76–79). Studying the mixing performance using two-colored gum requires optical scanning and image processing as well.
At the end of the chewing experiment, the fragmented candies were immediately manually separated and spread on a transparent sheet with standardized dimensions of 100 mm × 100 mm and left to dry until the next day for later scanning within 24 h. The chewed gum was immediately flattened in a transparent plastic bag with standardized dimensions of 70 mm × 70 mm × 1 mm for scanning within 24 h. The candies were weighed in grams before chewing as well as the transparent sheet that would be used for each candy. The fragmented candies were weighed once again the next day after drying from the saliva they were soaked in. A digital gram scale with a precision of ±0.01 g (Fino Balance Mini; Fino GmbH, Bad Bocklet, Germany) was used throughout the whole experiment.
The scanning apparatus Ricoh eduPrint Scanner was used for the scanning of the pain drawings, the samples of the fragmented candies, and flattened chewing gum. The two-dimentional imaging of the candy particles and the chewing gum was standardized with the following settings: Color → Original type-full color, Resolution → 300 dpi, Scanning-format → Cropped scanning in mm 210 × 297/5 × 5/100 × 100 for the candy samples and 210 × 297/5 × 5/70 × 70 for the chewing gum, and Reproduction ratio → 100%. All of the samples (candy and chewing gum) were scanned with a white paper serving as background.
The candy images were pre-processed by standardized settings in Adobe Photoshop CC software (version 19.1.3, Adobe Systems Incorporated, San Jose, CA, United States) in order to remove any shadows and then analyzed by Fiji Image J (Image Processing and Analysis in Java; National Institutes of Health, United States). The settings used in Fiji Image J were also standardized as follows: Scale → width: 1185, height: 1185, Type → 8-bite, Size → width: 1185, height: 1185, depth: 1, Background → black and white: dark background, Set measurements → area, area fraction and Analyze particles → size (inch): 0-infinity, circularity: 0–1, show: outlines, display results. The results were then converted from inches to millimeters. The results are presented in the number of particles into which the original candies were crushed, the minimum Feret's diameter of the particles since they have an irregular shape and area of the particles in millimeters (mm).
The View Gum©, which is the Hue-Check Gum® own validated analyzing software (freeware), was used for analyzing the variance of hue (VOH) according to the instructions in the attached manual (30, 62, 80).
The Adobe Photoshop CC software (version 19.1.3, Adobe Systems Incorporated, San Jose, CA, United States) was used to count the pixels within the marked total pain area from the scanned drawings in arbitrary units (au). Those pain drawings provided visual illustration and quantitatively described the pattern and location of pain as well as referred pain (81).
Statistical analyses
The statistical analyses were performed using SigmaPlot software version 14.0 (Systat Software Inc., San Jose, CA, United States) and SPSS 27.0 (IBM Corp, Armonk, NY, United States). Normality of the data was tested with the Shapiro-Wilk test. Parametric and non-parametric tests were used to analyze data depending on the outcome of the normality test. The majority of the variables showed a non-normal distribution and a skewness to the right, except for the mouth opening capacity, the variance of the hue, and the chewing duration of the two-colored gum. For MVBF, values were normalized and presented as the percentage change from baseline values. For between-group comparison, unpaired t-test was used for variables on a nominal scale and normal distributed data, while the Mann–Whitney U test was used for variables on an ordinal scale and skewed distributions. For within-group comparisons, paired t-test or Wilcoxon signed rank test were used to test differences between the chewing modes (natural and standardized) and the change in MVBF at the end of the experiment compared to baseline values. The Kruskal–Wallis analysis of variance with Tukey post-hoc test for the associated multiple comparisons was used to test the differences between the chewing tasks (based on type of candy: soft vs. hard; and chewing mode: natural vs. standardized). The Friedman's analysis of variance for repeated measures with Tukey post-hoc test for the associated multiple comparisons was used to test changes in pain intensity and self-reported fatigue/exertion. Spearman correlation test was applied to detect any correlation between objective variables of masticatory performance and self-reported variables, including pain, fatigue, and variables of self-assessed masticatory ability obtained from the questionnaires. Binary logistic regression analyses were carried out to further explore which of the objective and self-reported variables can be considered predictors of masticatory performance. First, condition (healthy or patient) was considered as dependent variable, and the healthy group was set as reference group when analysis was performed to identify objective predictors. Later when exploring predictors among the self-reported variables, the strongest objective predictor identified was used as dependent variable, while the reference cut-off value was generated using the patient group's median value of that identified predictor. Data are reported as frequencies, means, and standard deviation (SD) or median and interquartile range (IQR). Data from the regression analyses are reported in odds ratio (OR) and 95% confidence interval (CI). The significance level was set at P < 0.05.
Results
Participants
The study involved 20 TMD pain patients with median (IQR) age of 26 (10) years as well as 20 healthy, pain-free, age and sex-matched volunteers with median (IQR) age of 25.5 (9.5) years. Both groups were comprised of 16 women and 4 men.
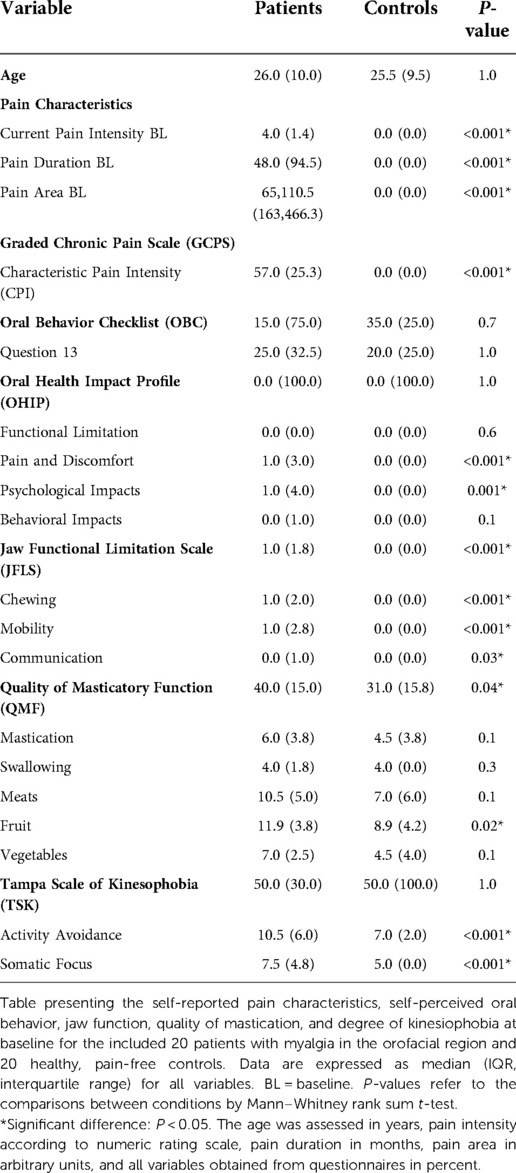
All included patients were diagnosed with myalgia, sub-classified according to DC/TMD into 10% with local myalgia, 35% with myofascial pain and 55% with myofascial pain with referred pain. Further, 95% of the patients were diagnosed with headache associated with TMD. 20% of the patients were diagnosed with disc displacement with reduction. None of the healthy controls had any TMD diagnosis according to DC/TMD. 35% of the patients had low pain intensity and low grade of disability (according to GCPS-7), 60% had high pain intensity and low grade of disability, 5% had moderately limiting high disability, and none of them had severely limiting high disability. Since the controls were all pain-free, the patients showed significantly greater baseline values of pain intensities, pain duration, pain area, reduced pain-free mouth opening capacity as well as fatigue compared to the healthy and pain-free controls (Tables 1, 2) and (Figures 2, 3).
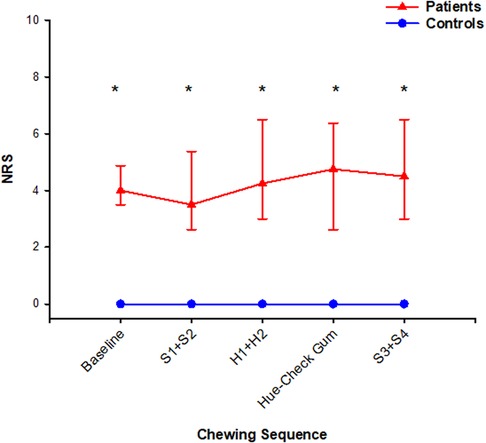
Figure 2. In this figure, median (IQR) of pain intensity scores assessed with numeric rating scale are shown at baseline, after chewing sequences S1 + S2, H1 + H2, Hue-Check gum, S3 + S4 and at the end of the experiment. The patients scored *significantly higher scores than the healthy controls throughout the whole experiment (Mann–Whitney rank sum test; P < 0.001). The pain intensity did not change at any assessment point compared to baseline values in both groups (Friedman ANOVA test/Tukey post-hoc; P > 0.05).
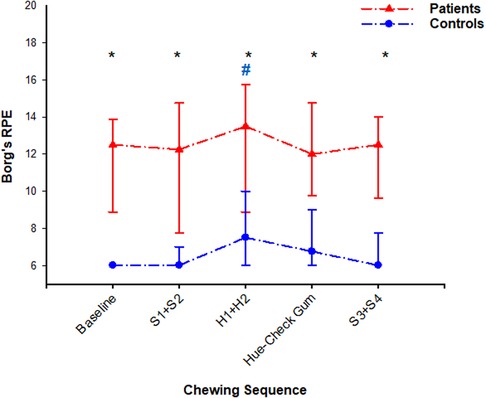
Figure 3. In this figure, median (IQR) of self-reported fatigue scores assessed with Borg's rating of perceived exertion are shown at baseline, after chewing sequences S1 + S2, H1 + H2, Hue-Check gum, S3 + S4 and at the end of the experiment. The patients scored *significantly higher scores than the healthy controls throughout the whole experiment (Mann–Whitney rank sum test; P < 0.001). The fatigue did not change at any assessment point compared to baseline value in the patient group (Friedman ANOVA test/Tukey post-hoc; P > 0.05), but it was #significantly higher after chewing sequence H1 + H2 compared to baseline value in the control group (Friedman ANOVA test/Tukey post-hoc; P = 0.006).
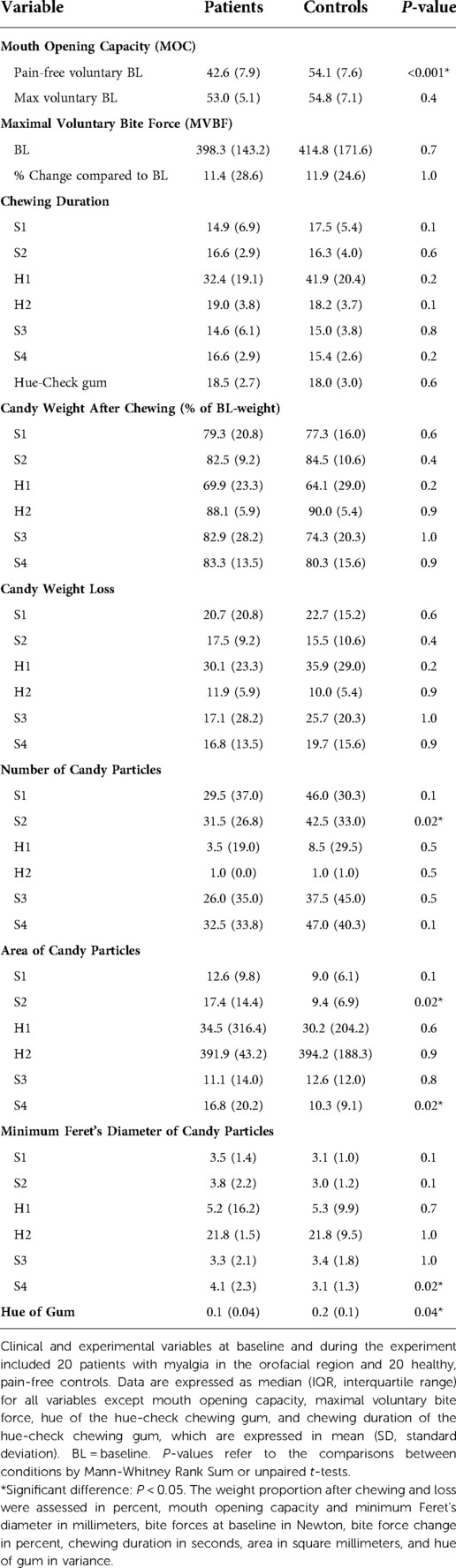
Table 2. Clinical and experimental variables at baseline and during the experiment for 20 patients and 20 controls.
Compared to the controls, the TMD pain patients also showed a greater negative self-perceived impact on their oral health (OHIP-pain and OHIP-psychological impacts), a greater degree of limitations in their jaw functioning (JFLS, QMF, and QMF-fruits), and a greater degree of kinesiophobia (Tampa AA and SF) (P < 0.05; respectively). As shown in Supplementary Table S1, there were no differences between the groups regarding any aspect of psychosocial distress, but the patients with myalgia showed a significantly higher scoring of widespread pain (WPI) and somatic severity (SSS) although no diagnoses of general pain conditions.
Regression analyses revealed that the self-perceived quality of masticatory function (QMF-28) was the strongest self-reported predictor of masticatory performance (OR = 1.02; 95% CI = 1.002–1.04; P = 0.03).
Dentition
The median (IQR) of the total number of the occluding tooth pairs in patients and healthy controls were 12.0 (3.0) and 13.0 (3.8), respectively. Further, the median (IQR) of the post-canine number of the occluding tooth pairs in patients and healthy controls were 8.0 (0.8) and 8.0 (0.0), respectively. There was no difference in the number of neither all occluding tooth pairs nor the post-canine occluding pairs between the condition groups (P = 0.3 and P = 0.2, respectively). All patients exhibited mild to moderate tooth wear; 80% of them had dentition that was generally affected. 85% of the patients displayed mild tooth wear (1st grade), and 15% displayed moderate tooth wear (2nd grade). On the other hand, 85% of the healthy participants exhibited tooth wear, which was only mild. Of those, 53% displayed dentition that was generally affected.
Chewing performance
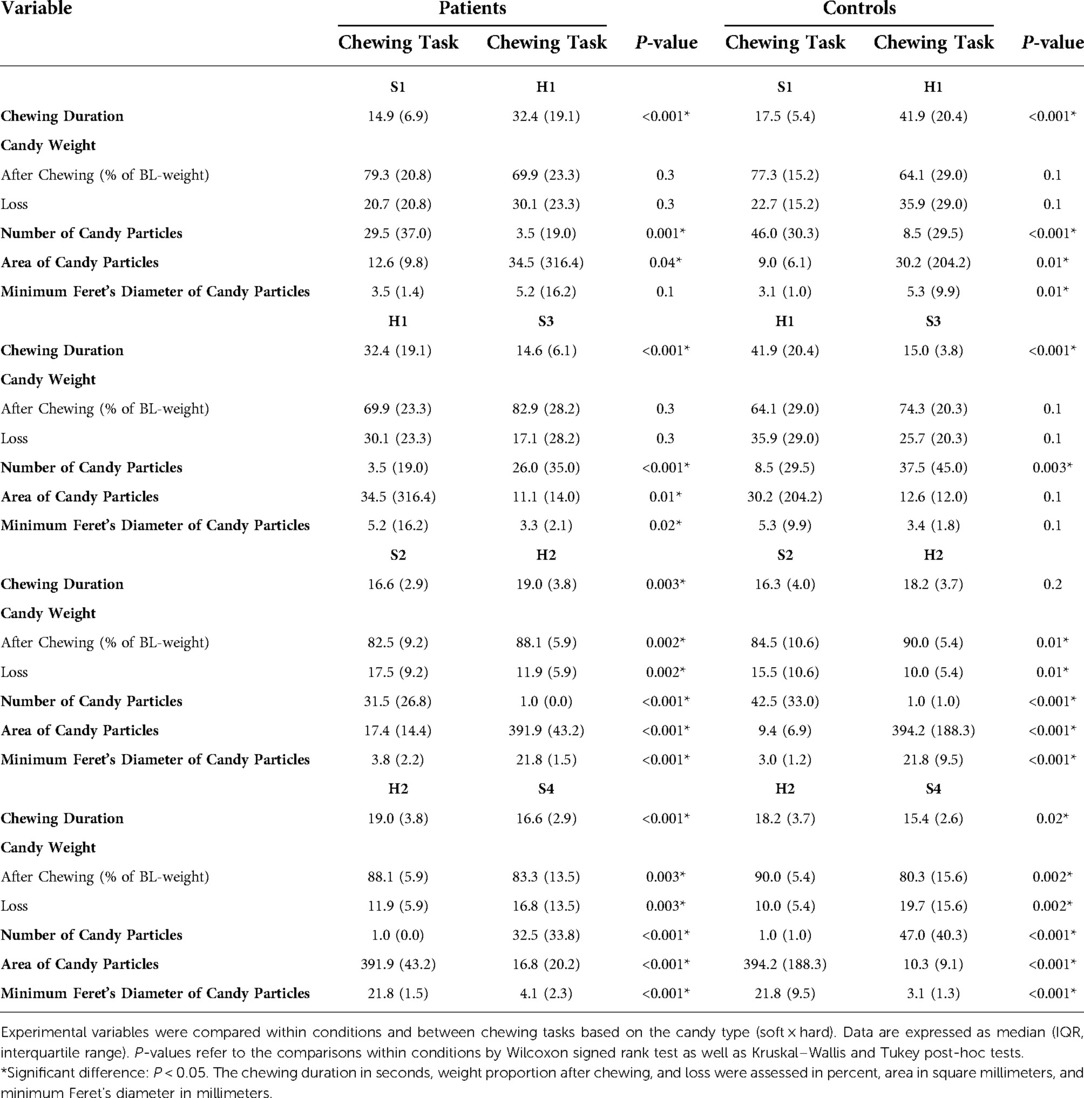
The number of soft candy particles spitted out by TMD patients after standardized chewing (20 chewing cycles) was significantly less, with a significantly larger area and larger minimum Feret's diameter compared to controls. The patients also exhibited a significantly smaller variance of the hue after chewing the two-colored gum than the controls (Table 2).
The different chewing tasks were compared within the groups based on chewing mode (Table 3) and candy type (Table 4). Within the control group, greater weight loss of the soft candy was exhibited in the natural chewing task S1 compared to the standardized task S2 as well as higher number of candy particles was displayed in the natural task S3 compared to standardized task S4 (Table 3).
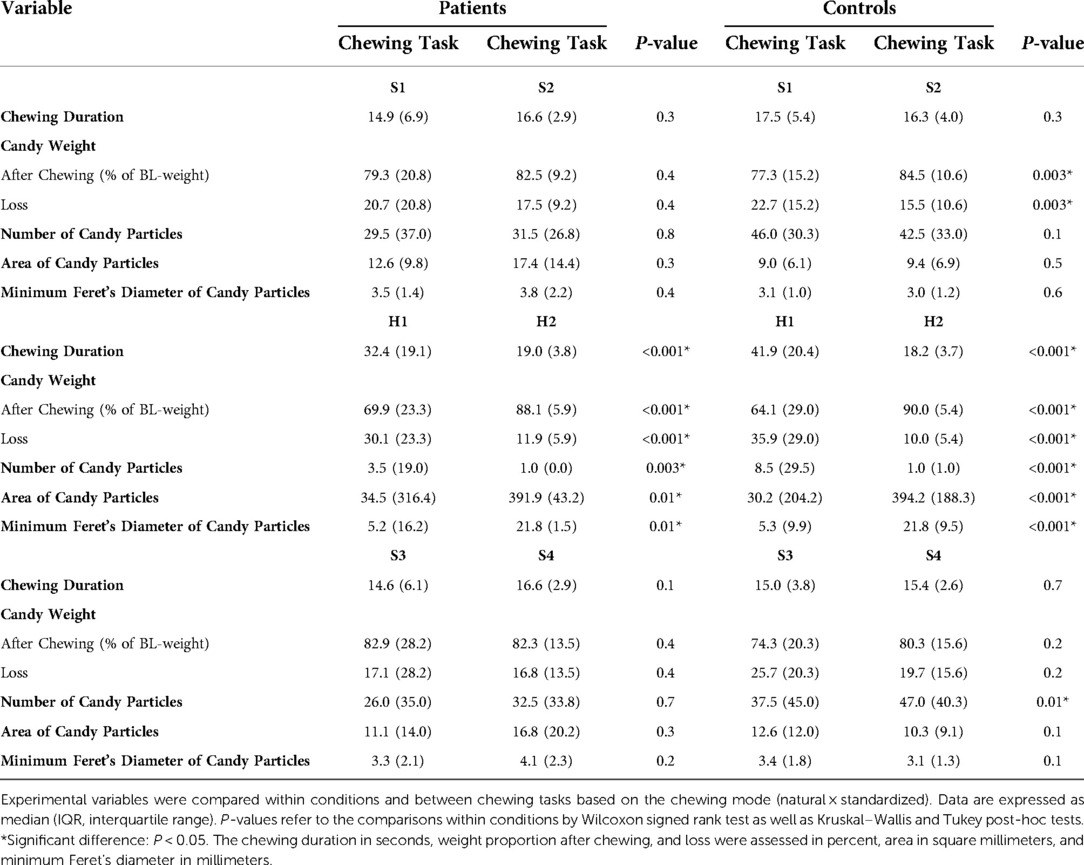
Table 3. Comparisons within conditions and between chewing tasks based on chewing mode (natural × standardized).
Finally, both groups showed a significant increase in the maximal voluntary bite force at the end of the experiment compared to baseline values, with 11.4% for the patients and 11.9% for the controls; P ≤ 0.001 for both groups.
Regression analyses revealed that the number of particles was the strongest objective predictor of masticatory performance (OR = 0.99; 95% CI = 0.98–1.0; P = 0.01).
Chewing pattern
50% of the patients and 45% of the healthy controls declared bilateral (alternate/simultaneous) chewing pattern. 20% of the patients and 5% of the healthy controls declared chronic unilateral chewing pattern and the right side being the dominant chewing side for the majority. On the other hand, 30% of the patients and 50% of the healthy controls declared preferred unilateral chewing pattern with the right side also being the dominant side for the majority. The Hue-Check gum was retrieved from the right side for the majority of the patients but that was not the case for the healthy controls.
Pain and fatigue
Patients scored higher pain intensity than controls at all assessment points of the experiment (Figure 2). Within the patient group, pain intensity after S3 + S4 correlated positively with the number of particles and weight loss in the natural task S3 (Spearman; P = 0.04). Furthermore, pain intensity at baseline correlated positively with the chewing duration of S3 (Spearman; P = 0.04).
Compared to controls, patients scored higher self-reported fatigue at all assessment points of the experiment (Figure 3). Within the healthy group, the self-reported sensation of fatigue was significantly higher after chewing the hard candies H1 + H2 compared to the baseline value (Friedman ANOVA test/Tukey post-hoc; P = 0.006) (Figure 3). In this group, the fatigue after H1 + H2 correlated positively with the fatigue after chewing of the Hue-Check gum (Spearman; P = 0.01) and correlated negatively with the chewing duration of S3 (Spearman; P = 0.01).
The different chewing tasks were compared within the condition groups based on the effect of pain and fatigue changes (Table 5). It is worth mentioning that Figures 2 and 3 show the changes in pain and fatigue, respectively, while Table 5 presents any possible effect of the changes on chewing.
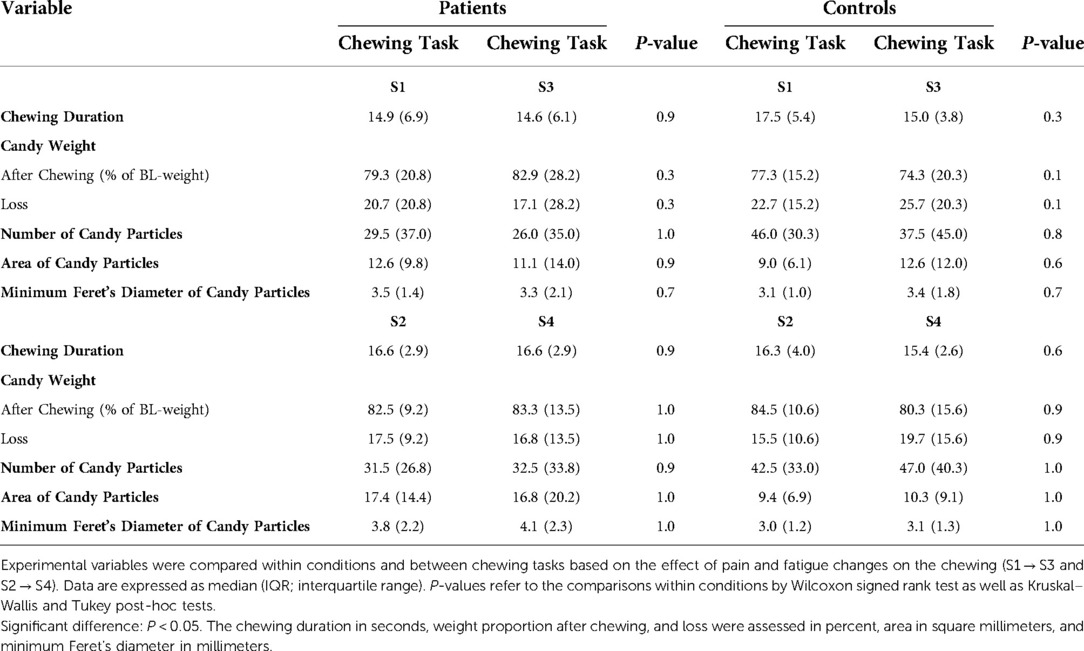
Table 5. Comparisons within conditions and between chewing tasks based on the effect of pain and fatigue changes on chewing (S1 → S3 and S2 → S4).
Discussion
The main finding of this investigation was that the TMD patients with chronic myalgia in the jaw muscles, compared to the healthy controls, exhibited an impaired masticatory performance with less efficiency in food comminution. Such impairment of the masticatory function is also exhibited by other TMD patients with frequent temporomandibular joint clicking and moderate or severe overall symptoms of TMD (82), as well as other patient groups with malocclusions and anomalies (83–85). Efficient chewing performance is indicated by proper food breakdown rather than short chewing duration or low number of chewing cycles (24, 39, 86–89). In patients, the fragmented soft candy particles were less in number and had larger median of area and minimum Feret's diameter after the standardized chewing (20 chewing cycles). The finding is explained by the significant differences in pain (including pain-related variables) and fatigue in patients compared to controls. Further, higher fragmentation of candies in patients correlated positively with more intensified pain. Also, more severe pain facilitated longer chewing duration. That is in agreement with previous results showing that chewing duration correlated positively with TMD severity (5).
Both experimental groups needed, as could be expected, longer chewing duration for the hard candy compared to the soft candy and were able to split the soft candy into higher numbered and smaller particles than the hard candy within the similar chewing modes (S1 and S3 compared to H1; S2 and S4 compared to H2), implying an adaptation to candy hardness (15, 90, 91), and further demonstrating that this adaptability to food hardness did not seem to be negatively affected in TMD pain patients.
Greater weight loss in chewing task S1 than in S2, as well as higher number of particles in S3 compared to S4, were only evident within the healthy control group. Both findings indicate a more efficient crushing of candies in the natural tasks S1 and S3. The controls probably chose to alternate to “the less fatigued” side during the natural tasks S1 and S3, which they were not able to do during the standardized tasks S2 and S4, where they were instructed to chew only on the habitual/preferred side. Alternating sides facilitated more efficient chewing using the less fatigued side and giving the fatigued side an opportunity to recover. The recovery seems to be fast in the healthy controls since the increased fatigue was rather low and the chewing was still efficient. Being able to crush the candy efficiently in S3 after the increased fatigue in H1 + H2 confirms that. A greater weight loss was found to be positively correlated with higher number of particles and negatively correlated with greater particle size (number and size of particles correlate negatively). Swallowing threshold seems to mainly depend on the degree of pulverization (87); smaller particle size facilitates easier swallowing (39, 92), and thereby more unintentional “happened to” swallow incidents occurred, leading to greater weight loss. There is also the risk of inadequate collection of the comminuted particles despite the strict instructions and management (79). That risk increases with more effective fragmentation. Further correlation analyses also showed that during natural chewing (S1 and S3), the chewing duration correlated positively with weight loss. It appears that pain-free masticatory muscles allow longer chewing duration if needed for more efficient comminution of food during natural chewing.
The between-group differences were more evident in the standardized chewing tasks with the soft candy, whereas the within-group differences (specifically the healthy controls) appeared in the natural tasks with the soft candy. The participants were probably more able to adapt their mastication more efficiently during the natural tasks rather than the standardized ones. Standardized tasks are more controlled with a stricter goal orientation. The patients seemed to succeed in their attempt to compensate for any possible pain effect on the masticatory performance during the natural chewing. However, the impairment of the masticatory function in these patients was revealed by standardizing the chewing.
It was an unexpected finding that the two-colored Hue-Check gum was less mixed by the healthy controls since they displayed a greater variance of the hue. There were no differences in the instructions or chewing duration between the condition groups that could explain this finding. Possible explanations could be the significant increased fatigue after the hard candy tasks, the chewing pattern, or the consumption habits of chewing gum. Regarding the fatigue increase and the chewing pattern, the majority of the healthy participants initially indicated that they preferred the right chewing side, but the gum was retrieved from the left side for many instead, implying that they probably chose the less fatigued side since they were allowed to alternate sides during the gum task. The duration for recovery from fatigue between the hard candies and the gum was not as long as (probably not long enough) the more sufficient time between the hard candies and the soft candies (S3 + S4). The controls had to use the less fatigued side which was also the less habitual and less preferred side for longer time during gum chewing. More patients indicated chronic unilateral chewing pattern compared to the controls, which is in line with previous findings (5), implying that they already prefer the less painful masticatory side and had probably been integrating compensating mechanisms for a long time. It was also suggested that the two-colored chewing gum mixing test can be influenced by the chewing-gum consumption habits (93). However, further analyses of question thirteen of the oral behavior checklist (OBC-Q13) showed that there was no difference in the frequency of chewing gum habits between the groups (Table 1).
In the current study, pain in patients seems to affect the chewing strategies, including possible compensating chewing rate and pattern rather than an effect on maximal bite force. However, dynamic changes of the bite force during the masticatory sequences were not assessed in this current study. Higher occlusal bite force was shown to be associated with higher chewing efficiency and smaller particle size (39). Previous studies reported conflicting results, where some did not show differences in the bite force between TMD patients and controls (94, 95) while others did (96–99). Patients may not have a reduced MVBF; they may even develop less muscle fatigue; however, they usually make a slower recovery than healthy individuals (100, 101). This may be an indication of selective activation of motoneurons, depending on the task at hand (9). The number of functional tooth units (102) and morphology, including occlusal contact area and tooth wear (19, 103, 104) might affect the masticatory performance. Those factors can nevertheless be ruled out in the current study since the number of occluding contacts was the same in the two groups. Patients had more tooth wear generally; however, it was shown that there was no effect of tooth wear severity on masticatory performance using comminuting test (105, 106).
There were significant differences between the patients and the healthy controls in the self-assessed masticatory ability, mainly regarding pain-related variables. The patients evaluated the oral health impact profile regarding the impact of pain (OHIP-pain and discomfort) and psychological impacts (OHIP-psychological impacts) with significantly higher scores than the healthy controls (107). It has been shown that there is a moderate correlation between OHIP and JFLS (46). Higher scoring on the jaw functional limitation scale (JFLS) in patients compared to healthy controls (48) indicates a higher impact of the myalgia on the function of the jaw regarding chewing, mobility, and communication. This was in line with the patients' evaluation of the quality of their masticatory function (QMF) since they scored higher when answering questions referring to difficulties encountered with different types of food (specifically fruits) and habitual adaptations that needed to be made (49). Regarding QMF fruits, patients probably eat apples with the necessity of cutting them since the pain-free mouth opening capacity in patients was significantly reduced compared to the controls. Furthermore, the patients showed higher scores both regarding the Tampa scale activity avoidance and somatic focus, indicating a greater fear of jaw movement due to fear of pain increase compared to the controls. This was also in line with the scores exhibited by the patients with TMD in the original study of TSK-TMD (50).
The patients’ self-evaluation of their masticatory ability was in line with the results from the objectively assessed masticatory function, including reduced pain-free mouth opening capacity and impaired efficiency of food breakdown. Although the comminution test and the mixing ability test are believed to correlate positively (108), the finding of a greater variance of the hue in controls might emerge only due to the experimental set-up and the arranged sequence in which the gum was placed or because the Hue-Check gum was not a suitable method for comparing individuals with natural dentitions and normal occlusions (26, 69).
Study limitations
The patient group in this study included four patients (20%) with non-painful disc displacement with reduction which can be considered a limitation. However, previous studies although showing affected masticatory efficiency and altered recruitment of the jaw muscles in patients with disc displacement, did neither report if the disc displacements were painful or not, nor if the studies included only clickings or if poppings and lockings were accepted (109, 110). It is therefore unclear if the disc displacement itself was the reason behind the results in those previous studies. Pain upon clickings and poppings/lockings could affect the results; therefore, they were excluded from the current study. Another limitation of this study is that it only used viscoelastic food. Future studies need to test other types of food with different mechanical and rheological properties or different sizes of the test bolus. There are yet no normative values for particle size regarding the chosen candy to compare with (39). However, the masticatory function in the patients could be considered impaired since the particle size was significantly larger, even though they tended to show an adaptive behavior. Adaptive mastication is defined as the achievement of the same degree of pulverization as normal mastication using compensatory mechanisms pre-swallowing (88). Further, assessing comminution of food involves risks of unintentional swallowing and inadequate collection of the comminuted particles, eventually leading to measurement errors. However, that same risk may occur to a similar extension regardless of whether sieving or optical scanning and imaging are used. Furthermore, the static bite force was only assessed at baseline and at the end of the experiment, omitting information about the dynamic change of the bite force during masticatory sequences. Masticatory studies using dynamic bite force monitoring, EMG, and electrognathographic monitoring are warranted in order to assess other variables dynamically, such as chewing pattern and muscle activity.
Conclusions
Patients with chronic myalgia in the masticatory muscles exhibited an impaired masticatory performance with less efficiency in comminuting soft viscoelastic food compared to the pain-free healthy control group. Higher food fragmentation was positively correlated with more severe pain. There was an agreement between the patients' self-assessed masticatory ability and the efficiency of their masticatory function.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Regional Ethical Review Board in Stockholm. The patients/participants provided their written informed consent to participate in this study.
Author contributions
SAS designed the experimental protocol, performed the research, analyzed the data and images, wrote the manuscript, and made the tables and figures. NCH and AG participated in designing the protocol, data analysis, and manuscript editing. AK participated in data analysis and manuscript editing. PS participated in the planning of the study and editing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The study is financed by Stockholm County Council and Karolinska Institutet (SOF; Styrgruppen för Odontologisk Forskning).
Acknowledgments
We are grateful to Professor Malin Ernberg for her precious input.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdmed.2022.963425/full#supplementary-material.
Abbreviations
au, arbitrary units; Borǵs RPE, Borg's rating of perceived exertion scale; CI, confidence interval; DC/TMD, the Diagnostic Criteria for Temporomandibular Disorders; EMG, electromyographic activity; GAD, generalized anxiety disorder scale; GCPS, graded chronic pain scale; IQR, interquartile range; ISI, insomnia severity index; JFLS, jaw functional limitation scale; MVBF, maximal voluntary bite force; NRS, numeric rating scale; OBC, oral behavior checklist; OHIP, oral health impact profile; OR, odds ratio; PCS, pain catastrophizing scale; PHQ-15, the patient health questionnaire for physical symptoms; PHQ-9, the patient health questionnaire for depression; PSS, perceived stress scale; QMF, quality of masticatory function; SD, standard deviation; SSS, somatic severity scale; TMD, temporomandibular disorders; TMJ, temporomandibular joint; VOH, variance of hue; WPI, widespread pain index.
References
1. Okeson JP, de Kanter RJ. Temporomandibular disorders in the medical practice. J Fam Pract. (1996) 43(4):347–56. https://hdl.handle.net/2066/23081 8874369
2. Schiffman EL, Fricton JR, Haley DP, Shapiro BL. The prevalence and treatment needs of subjects with temporomandibular disorders. J Am Dent Assoc. (1990) 120(3):295–303. doi: 10.14219/jada.archive.1990.0059
3. Sato S, Goto S, Takanezawa H, Kawamura H, Motegi K. Electromyographic and kinesiographic study in patients with nonreducing disk displacement of the temporomandibular joint. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (1996) 81(5):516–21. doi: 10.1016/S1079-2104(96)80039-4
4. Felício CM, Mazzetto MO. Perri angote dos santos C. Masticatory behavior in individuals with temporomandibular disorders. Minerva Stomatol. (2002) 51(4):111–20.
5. Felício CM, Melchior MO, Silva MA, Celeghini RM. Masticatory performance in adults related to temporomandibular disorder and dental occlusion. Pro Fono. (2007) 19(2):151–8. doi: 10.1590/S0104-56872007000200003
6. Svensson P, Houe L, Arendt-Nielsen L. Bilateral experimental muscle pain changes electromyographic activity of human jaw-closing muscles during mastication. Exp Brain Res. (1997) 116(1):182–5. doi: 10.1007/PL00005738
7. van der Bilt A, Engelen L, Pereira LJ, van der Glas HW, Abbink JH. Oral physiology and mastication. Physiol Behav. (2006) 89(1):22–7. doi: 10.1016/j.physbeh.2006.01.025
8. Wilding RJ, Shaikh M. Muscle activity and jaw movements as predictors of chewing performance. J Orofac Pain. (1997) 11(1):24–36.10332308
9. Blanksma NG, van Eijden TM. Electromyographic heterogeneity in the human temporalis and masseter muscles during static biting, open/close excursions, and chewing. J Dent Res. (1995) 74(6):1318–27. doi: 10.1177/00220345950740061201
10. Lund JP. Mastication and its control by the brain stem. Crit Rev Oral Biol Med. (1991) 2(1):33–64. doi: 10.1177/10454411910020010401
11. Lavigne G, Kim JS, Valiquette C, Lund JP. Evidence that periodontal pressoreceptors provide positive feedback to jaw closing muscles during mastication. J Neurophysiol. (1987) 58(2):342–58. doi: 10.1152/jn.1987.58.2.342
12. Morimoto T, Inoue T, Masuda Y, Nagashima T. Sensory components facilitating jaw-closing muscle activities in the rabbit. Exp Brain Res. (1989) 76(2):424–40. doi: 10.1007/BF00247900
13. Liu ZJ, Masuda Y, Inoue T, Fuchihata H, Sumida A, Takada K, et al. Coordination of cortically induced rhythmic jaw and tongue movements in the rabbit. J Neurophysiol. (1993) 69(2):569–84. doi: 10.1152/jn.1993.69.2.569
14. Foster KD, Woda A, Peyron MA. Effect of texture of plastic and elastic model foods on the parameters of mastication. J Neurophysiol. (2006) 95(6):3469–79. doi: 10.1152/jn.01003.2005
15. Peyron MA, Lassauzay C, Woda A. Effects of increased hardness on jaw movement and muscle activity during chewing of visco-elastic model foods. Exp Brain Res. (2002) 142(1):41–51. doi: 10.1007/s00221-001-0916-5
16. Grigoriadis A, Johansson RS, Trulsson M. Temporal profile and amplitude of human masseter muscle activity is adapted to food properties during individual chewing cycles. J Oral Rehabil. (2014) 41(5):367–73. doi: 10.1111/joor.12155
17. Ross CF, Dharia R, Herring SW, Hylander WL, Liu ZJ, Rafferty KL, et al. Modulation of mandibular loading and bite force in mammals during mastication. J Exp Biol. (2007) 210(Pt 6):1046–63. doi: 10.1242/jeb.02733
18. Lujan-Climent M, Martinez-Gomis J, Palau S, Ayuso-Montero R, Salsench J, Peraire M. Influence of static and dynamic occlusal characteristics and muscle force on masticatory performance in dentate adults. Eur J Oral Sci. (2008) 116(3):229–36. doi: 10.1111/j.1600-0722.2008.00530.x
19. Lepley CR, Throckmorton GS, Ceen RF, Buschang PH. Relative contributions of occlusion, maximum bite force, and chewing cycle kinematics to masticatory performance. Am J Orthod Dentofacial Orthop. (2011) 139(5):606–13. doi: 10.1016/j.ajodo.2009.07.025
20. Manly RS. Factors affecting masticatory performance and efficiency among young adults. J Dent Res. (1951) 30(6):874–82. doi: 10.1177/00220345510300062001
21. Buschang PH, Throckmorton GS, Travers KH, Johnson G. The effects of bolus size and chewing rate on masticatory performance with artificial test foods. J Oral Rehabil. (1997) 24(7):522–6. doi: 10.1046/j.1365-2842.1997.00524.x
22. Park S, Shin WS. Differences in eating behaviors and masticatory performances by gender and obesity status. Physiol Behav. (2015) 138:69–74. doi: 10.1016/j.physbeh.2014.10.001
23. Shiau YY, Peng CC, Hsu CW. Evaluation of biting performance with standardized test-foods. J Oral Rehabil. (1999) 26(5):447–52. doi: 10.1046/j.1365-2842.1999.00411.x
24. van der Bilt A. Assessment of mastication with implications for oral rehabilitation: a review. J Oral Rehabil. (2011) 38(10):754–80. doi: 10.1111/j.1365-2842.2010.02197.x
25. Homsi G, Kumar A, Almotairy N, Wester E, Trulsson M, Grigoriadis A. Assessment of masticatory function in older individuals with bimaxillary implant-supported fixed prostheses or with a natural dentition: a case-control study. J Prosthet Dent. (2021). doi: 10.1016/j.prosdent.2021.08.023
26. van der Bilt A, Mojet J, Tekamp FA, Abbink JH. Comparing masticatory performance and mixing ability. J Oral Rehabil. (2010) 37(2):79–84. doi: 10.1111/j.1365-2842.2009.02040.x
27. van der Bilt A, Fontijn-Tekamp FA. Comparison of single and multiple sieve methods for the determination of masticatory performance. Arch Oral Biol. (2004) 49(3):193–8. doi: 10.1016/j.archoralbio.2003.08.007
28. Mahmood WA, Watson CJ, Ogden AR, Hawkins RV. Use of image analysis in determining masticatory efficiency in patients presenting for immediate dentures. Int J Prosthodont. (1992) 5(4):359–66.1520458
29. Mowlana F, Heath MR, Auger D. Automated optical scanning for rapid sizing of chewed food particles in masticatory tests. J Oral Rehabil. (1995) 22(2):153–8. doi: 10.1111/j.1365-2842.1995.tb00249.x
30. Schimmel M, Christou P, Herrmann F, Müller F. A two-colour chewing gum test for masticatory efficiency: development of different assessment methods. J Oral Rehabil. (2007) 34(9):671–8. doi: 10.1111/j.1365-2842.2007.01773.x
31. Grigoriadis A, Johansson RS, Trulsson M. Adaptability of mastication in people with implant-supported bridges. J Clin Periodontol. (2011) 38(4):395–404. doi: 10.1111/j.1600-051X.2010.01697.x
32. Kohyama K, Mioche L, Bourdiol P. Influence of age and dental status on chewing behaviour studied by EMG recordings during consumption of various food samples. Gerodontology. (2003) 20(1):15–23. doi: 10.1111/j.1741-2358.2003.00015.x
33. Mioche L, Bourdiol P, Peyron MA. Influence of age on mastication: effects on eating behaviour. Nutr Res Rev. (2004) 17(1):43–54. doi: 10.1079/NRR200375
34. Weijenberg RA, Lobbezoo F, Knol DL, Tomassen J, Scherder EJ. Increased masticatory activity and quality of life in elderly persons with dementia–a longitudinal matched cluster randomized single-blind multicenter intervention study. BMC Neurol. (2013) 13:26. doi: 10.1186/1471-2377-13-26
35. Grigoriadis J, Trulsson M, Svensson KG. Motor behavior during the first chewing cycle in subjects with fixed tooth- or implant-supported prostheses. Clin Oral Implants Res. (2016) 27(4):473–80. doi: 10.1111/clr.12559
36. Kapur KK, Soman SD. Masticatory performance and efficiency in denture wearers. J Prosthet Dent. (1964) 95(6):407–11. doi: 10.1016/j.prosdent.2006.03.012
37. Rissin L, House JE, Manly RS, Kapur KK. Clinical comparison of masticatory performance and electromyographic activity of patients with complete dentures, overdentures, and natural teeth. J Prosthet Dent. (1978) 39(5):508–11. doi: 10.1016/S0022-3913(78)80181-4
38. Eberhard L, Schneider S, Eiffler C, Kappel S, Giannakopoulos NN. Particle size distributions determined by optical scanning and by sieving in the assessment of masticatory performance of complete denture wearers. Clin Oral Investig. (2015) 19(2):429–36. doi: 10.1007/s00784-014-1266-6
39. Witter DJ, Woda A, Bronkhorst EM, Creugers NH. Clinical interpretation of a masticatory normative indicator analysis of masticatory function in subjects with different occlusal and prosthodontic status. J Dent. (2013) 41(5):443–8. doi: 10.1016/j.jdent.2013.02.004
40. Pedroni-Pereira A, Marquezin MCS, Araujo DS, Pereira LJ, Bommarito S, Castelo PM. Lack of agreement between objective and subjective measures in the evaluation of masticatory function: a preliminary study. Physiol Behav. (2018) 184:220–5. doi: 10.1016/j.physbeh.2017.12.001
41. Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the international RDC/TMD consortium network* and orofacial pain special interest group†. J Oral Facial Pain Headache. (2014) 28(1):6–27. doi: 10.11607/jop.1151
42. Shimada A, Baad-Hansen L, Svensson P. Effect of experimental jaw muscle pain on dynamic bite force during mastication. Arch Oral Biol. (2015) 60(2):256–66. doi: 10.1016/j.archoralbio.2014.11.001
43. López-Frías FJ, Castellanos-Cosano L, Martín-González J, Llamas-Carreras JM, Segura-Egea JJ. Clinical measurement of tooth wear: tooth wear indices. J Clin Exp Dent. (2012) 4(1):e48–53. doi: 10.4317/jced.50592
44. Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. (1992) 50(2):133–49. doi: 10.1016/0304-3959(92)90154-4
45. Markiewicz MR, Ohrbach R, McCall WD. Oral behaviors checklist: reliability of performance in targeted waking-state behaviors. J Orofac Pain. (2006) 20(4):306–16.17190029
46. Larsson P, List T, Lundström I, Marcusson A, Ohrbach R. Reliability and validity of a Swedish version of the oral health impact profile (OHIP-S). Acta Odontol Scand. (2004) 62(3):147–52. doi: 10.1080/00016350410001496
47. Ohrbach R, Larsson P, List T. The jaw functional limitation scale: development, reliability, and validity of 8-item and 20-item versions. J Orofac Pain. (2008) 22(3):219–30.18780535
48. Oghli I, List T, John MT, Häggman-Henrikson B, Larsson P. Prevalence and normative values for jaw functional limitations in the general population in Sweden. Oral Dis. (2019) 25(2):580–7. doi: 10.1111/odi.13004
49. Muller K, Morais J, Feine J. Nutritional and anthropometric analysis of edentulous patients wearing implant overdentures or conventional dentures. Braz Dent J. (2008) 19(2):145–50. doi: 10.1590/S0103-64402008000200011
50. Visscher CM, Ohrbach R, van Wijk AJ, Wilkosz M, Naeije M. The Tampa scale for kinesiophobia for temporomandibular disorders (TSK-TMD). Pain. (2010) 150(3):492–500. doi: 10.1016/j.pain.2010.06.002
51. Häuser W, Jung E, Erbslöh-Möller B, Gesmann M, Kühn-Becker H, Petermann F, et al. Validation of the fibromyalgia survey questionnaire within a cross-sectional survey. PLoS One. (2012) 7(5):e37504. doi: 10.1371/journal.pone.0037504
52. Palsson OS, Whitehead WE, van Tilburg MA, Chang L, Chey W, Crowell MD, et al. Rome IV diagnostic questionnaires and tables for investigators and clinicians. Gastroenterology. (2016) 150(6):1481–91. doi: 10.1053/j.gastro.2016.02.014
53. Kemani MK, Grimby-Ekman A, Lundgren J, Sullivan M, Lundberg M. Factor structure and internal consistency of a Swedish version of the pain catastrophizing scale. Acta Anaesthesiol Scand. (2019) 63(2):259–66. doi: 10.1111/aas.13246
54. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
55. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. (2006) 166(10):1092–7. doi: 10.1001/archinte.166.10.1092
56. Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. (2002) 64(2):258–66. doi: 10.1097/00006842-200203000-00008
57. Nordin M, Nordin S. Psychometric evaluation and normative data of the Swedish version of the 10-item perceived stress scale. Scand J Psychol. (2013) 54(6):502–7. doi: 10.1111/sjop.12071
58. Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity Index as an outcome measure for insomnia research. Sleep Med. (2001) 2(4):297–307. doi: 10.1016/S1389-9457(00)00065-4
59. Lassauzay C, Peyron MA, Albuisson E, Dransfield E, Woda A. Variability of the masticatory process during chewing of elastic model foods. Eur J Oral Sci. (2000) 108(6):484–92. doi: 10.1034/j.1600-0722.2000.00866.x
60. Nokubi T, Yoshimuta Y, Nokubi F, Yasui S, Kusunoki C, Ono T, et al. Validity and reliability of a visual scoring method for masticatory ability using test gummy jelly. Gerodontology. (2013) 30(1):76–82. doi: 10.1111/j.1741-2358.2012.00647.x
61. Al-Ali F, Heath MR, Wright PS. Simplified method of estimating masticatory performance. J Oral Rehabil. (1999) 26(8):678–83. doi: 10.1046/j.1365-2842.1999.00429.x
62. Schimmel M, Christou P, Miyazaki H, Halazonetis D, Herrmann FR, Müller F. A novel colourimetric technique to assess chewing function using two-coloured specimens: validation and application. J Dent. (2015) 43(8):955–64. doi: 10.1016/j.jdent.2015.06.003
63. Liedberg B, Owall B. Masticatory ability in experimentally induced xerostomia. Dysphagia. (1991) 6(4):211–3. doi: 10.1007/BF02493529
64. Liedberg B, Owall B. Oral bolus kneading and shaping measured with chewing gum. Dysphagia. (1995) 10(2):101–6. doi: 10.1007/BF00440079
65. Prinz JF. Quantitative evaluation of the effect of bolus size and number of chewing strokes on the intra-oral mixing of a two-colour chewing gum. J Oral Rehabil. (1999) 26(3):243–7. doi: 10.1046/j.1365-2842.1999.00362.x
66. Tarkowska A, Katzer L, Ahlers MO. Assessment of masticatory performance by means of a color-changeable chewing gum. J Prosthodont Res. (2017) 61(1):9–19. doi: 10.1016/j.jpor.2016.04.004
67. Kaya MS, Güçlü B, Schimmel M, Akyüz S. Two-colour chewing gum mixing ability test for evaluating masticatory performance in children with mixed dentition: validity and reliability study. J Oral Rehabil. (2017) 44(11):827–34. doi: 10.1111/joor.12548
68. Silva LC, Nogueira TE, Rios LF, Schimmel M, Leles CR. Reliability of a two-colour chewing gum test to assess masticatory performance in complete denture wearers. J Oral Rehabil. (2018) 45(4):301–7. doi: 10.1111/joor.12609
69. Speksnijder CM, Abbink JH, van der Glas HW, Janssen NG, van der Bilt A. Mixing ability test compared with a comminution test in persons with Normal and compromised masticatory performance. Eur J Oral Sci. (2009) 117(5):580–6. doi: 10.1111/j.1600-0722.2009.00675.x
70. Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Anderson JA. Studies with pain rating scales. Ann Rheum Dis. (1978) 37(4):378–81. doi: 10.1136/ard.37.4.378
72. Edlund J, Lamm CJ. Masticatory efficiency. J Oral Rehabil. (1980) 7(2):123–30. doi: 10.1111/j.1365-2842.1980.tb00428.x
73. Sánchez-Ayala A, Vilanova LS, Costa MA, Farias-Neto A. Reproducibility of a silicone-based test food to masticatory performance evaluation by different sieve methods. Braz Oral Res. (2014) 28:1–8. doi: 10.1590/1807-3107BOR-2014.vol28.0004
74. Sánchez-Ayala A, Farias-Neto A, Vilanova LS, Costa MA, Paiva AC, Carreiro AF, et al. Reproducibility, reliability, and validity of fuchsin-based beads for the evaluation of masticatory performance. J Prosthodont. (2016) 25(6):446–52. doi: 10.1111/jopr.12348
75. Elgestad Stjernfeldt P, Sjögren P, Wårdh I, Boström AM. Systematic review of measurement properties of methods for objectively assessing masticatory performance. Clin Exp Dent Res. (2019) 5(1):76–104. doi: 10.1002/cre2.154
76. van der Bilt A, van der Glas HW, Mowlana F, Heath MR. A comparison between sieving and optical scanning for the determination of particle size distributions obtained by mastication in man. Arch Oral Biol. (1993) 38(2):159–62. doi: 10.1016/0003-9969(93)90201-V
77. van der Bilt A, Abbink JH, Mowlana F, Heath MR. A comparison between data analysis methods concerning particle size distributions obtained by mastication in man. Arch Oral Biol. (1993) 38(2):163–7. doi: 10.1016/0003-9969(93)90202-W
78. Eberhard L, Schindler HJ, Hellmann D, Schmitter M, Rammelsberg P, Giannakopoulos NN. Comparison of particle-size distributions determined by optical scanning and by sieving in the assessment of masticatory performance. J Oral Rehabil. (2012) 39(5):338–48. doi: 10.1111/j.1365-2842.2011.02275.x
79. Salazar S, Hori K, Uehara F, Okawa J, Shibata A, Higashimori M, et al. Masticatory performance analysis using photographic image of gummy jelly. J Prosthodont Res. (2020) 64(1):48–54. doi: 10.1016/j.jpor.2019.04.010
80. Halazonetis DJ, Schimmel M, Antonarakis GS, Christou P. Novel software for quantitative evaluation and graphical representation of masticatory efficiency. J Oral Rehabil. (2013) 40(5):329–35. doi: 10.1111/joor.12043
81. Wright EF. Referred craniofacial pain patterns in patients with temporomandibular disorder. J Am Dent Assoc. (2000) 131(9):1307–15. doi: 10.14219/jada.archive.2000.0384
82. Henrikson T, Ekberg EC, Nilner M. Masticatory efficiency and ability in relation to occlusion and mandibular dysfunction in girls. Int J Prosthodont. (1998) 11(2):125–32.9709601
83. English JD, Buschang PH, Throckmorton GS. Does malocclusion affect masticatory performance? Angle Orthod. (2002) 72(1):21–7. doi: 10.1043/0003-3219(2002)0722.0.CO;2
84. Feldman RS, Kapur KK, Alman JE, Chauncey HH. Aging and mastication: changes in performance and in the swallowing threshold with natural dentition. J Am Geriatr Soc. (1980) 28(3):97–103. doi: 10.1111/j.1532-5415.1980.tb00240.x
85. Ngom PI, Diagne F, Aïdara-Tamba AW, Sene A. Relationship between orthodontic anomalies and masticatory function in adults. Am J Orthod Dentofacial Orthop. (2007) 131(2):216–22. doi: 10.1016/j.ajodo.2005.03.027
86. Berretin-Felix G, Genaro KF, Trindade IE, Trindade Júnior AS. Masticatory function in temporomandibular dysfunction patients: electromyographic evaluation. J Appl Oral Sci. (2005) 13(4):360–5. doi: 10.1590/S1678-77572005000400009
87. Horio T, Kawamura Y. Effects of texture of food on chewing patterns in the human subject. J Oral Rehabil. (1989) 16(2):177–83. doi: 10.1111/j.1365-2842.1989.tb01331.x
88. Woda A, Hennequin M, Peyron MA. Mastication in humans: finding a rationale. J Oral Rehabil. (2011) 38(10):781–4. doi: 10.1111/j.1365-2842.2011.02235.x
89. Helkimo E, Carlsson GE, Helkimo M. Chewing efficiency and state of dentition. A methodologic study. Acta Odontol Scand. (1978) 36(1):33–41. doi: 10.3109/00016357809026364
90. Almotairy N, Kumar A, Grigoriadis A. Effect of food hardness on chewing behavior in children. Clin Oral Investig. (2020) 25:1203–16. doi: 10.1007/s00784-020-03425-y 32613432
91. Grigoriadis A, Kumar A, Åberg MK, Trulsson M. Effect of sudden deprivation of sensory inputs from periodontium on mastication. Front Neurosci. (2019) 13:1316. doi: 10.3389/fnins.2019.01316
92. Lucas PW, Luke DA. Is food particle size a criterion for the initiation of swallowing? J Oral Rehabil. (1986) 13(2):127–36. doi: 10.1111/j.1365-2842.1986.tb00645.x
93. Vaccaro G, Peláez JI, Gil-Montoya JA. The influence of habitual consumption of chewing gums in the outcome of masticatory performance tests using two-coloured chewing gums. Sci Rep. (2019) 9(1):6543. doi: 10.1038/s41598-019-42918-z
94. Pereira-Cenci T, Pereira LJ, Cenci MS, Bonachela WC. Del bel cury AA. Maximal bite force and its association with temporomandibular disorders. Braz Dent J. (2007) 18(1):65–8. doi: 10.1590/S0103-64402007000100014
95. Hotta PT, Hotta TH, Bataglion C, Pavão RF, Siéssere S, Regalo SC. Bite force in temporomandibular dysfunction (TMD) and healthy complete denture wearers. Braz Dent J. (2008) 19(4):354–7. doi: 10.1590/S0103-64402008000400012
96. Kogawa EM, Calderon PS, Lauris JR, Araujo CR, Conti PC. Evaluation of maximal bite force in temporomandibular disorders patients. J Oral Rehabil. (2006) 33(8):559–65. doi: 10.1111/j.1365-2842.2006.01619.x
97. Testa M, Geri T, Pitance L, Lentz P, Gizzi L, Erlenwein J, et al. Alterations in jaw clenching force control in people with myogenic temporomandibular disorders. J Electromyogr Kinesiol. (2018) 43:111–7. doi: 10.1016/j.jelekin.2018.07.007
98. Ahlberg JP, Kovero OA, Hurmerinta KA, Zepa I, Nissinen MJ, Könönen MH. Maximal bite force and its association with signs and symptoms of TMD, occlusion, and body mass index in a cohort of young adults. Cranio. (2003) 21(4):248–52. doi: 10.1080/08869634.2003.11746258
99. Pizolato RA, Gavião MB, Berretin-Felix G, Sampaio AC, Trindade Junior AS. Maximal bite force in young adults with temporomandibular disorders and bruxism. Braz Oral Res. (2007) 21(3):278–83. doi: 10.1590/S1806-83242007000300015
100. Hagberg C, Agerberg G, Hagberg M. Discomfort and bite force in painful masseter muscles after intramuscular injections of local anesthetic and saline solution. J Prosthet Dent. (1986) 56(3):354–8. doi: 10.1016/0022-3913(86)90019-3
101. Lyons MF, Baxendale RH. Masseter muscle relaxation rate in volunteers with a myogenous craniomandibular disorder. J Oral Rehabil. (1995) 22(5):355–64. doi: 10.1111/j.1365-2842.1995.tb00785.x
102. Hatch JP, Shinkai RS, Sakai S, Rugh JD, Paunovich ED. Determinants of masticatory performance in dentate adults. Arch Oral Biol. (2001) 46(7):641–8. doi: 10.1016/S0003-9969(01)00023-1
103. Yoshimura M, Fueki K, Garrett N, Ohyama T. Influence of food platform width of mandibular removable partial denture on food mixing ability. J Oral Rehabil. (2006) 33(5):335–40. doi: 10.1111/j.1365-2842.2005.01570.x
104. Luke DA, Lucas PW. Chewing efficiency in relation to occlusal and other variations in the natural human dentition. Br Dent J. (1985) 159(12):401–3. doi: 10.1038/sj.bdj.4805742
105. Sterenborg BAMM, Kalaykova SI, Loomans BAC, Huysmans MDNJ. Impact of tooth wear on masticatory performance. J Dent. (2018) 76:98–101. doi: 10.1016/j.jdent.2018.06.016
106. Sterenborg BAMM, Kalaykova SI, Loomans BAC, Huysmans MDNJ. Corrigendum to “impact of tooth wear on masticatory performance” [J. Dent. 76 (2018) 98-101]. J Dent. (2019) 82:101. doi: 10.1016/j.jdent.2019.01.003
107. Larsson P, John MT, Hakeberg M, Nilner K, List T. General population norms of the Swedish short forms of oral health impact profile. J Oral Rehabil. (2014) 41(4):275–81. doi: 10.1111/joor.12137
108. Sato S, Fueki K, Sato H, Sueda S, Shiozaki T, Kato M, et al. Validity and reliability of a newly developed method for evaluating masticatory function using discriminant analysis. J Oral Rehabil. (2003) 30(2):146–51. doi: 10.1046/j.1365-2842.2003.01050.x
109. Di Giacomo P, Ferrato G, Serritella E, Polimeni A, Di Paolo C. Muscular pattern in patients with temporomandibular joint disc displacement with reduction: an electromyographical assessment. Clin Ter. (2020) 171(5):e414–20. doi: 10.7417/CT.2020.2251
Keywords: chewing, mastication, masticatory efficiency, masticatory ability, myalgia, myofascial pain, TMD
Citation: Al Sayegh S, Christidis N, Kumar A, Svensson P and Grigoriadis A (2022) Masticatory performance in patients with jaw muscle pain: A case–control study. Front. Dent. Med 3:963425. doi: 10.3389/fdmed.2022.963425
Received: 8 June 2022; Accepted: 18 October 2022;
Published: 17 November 2022.
Edited by:
Vincenzo Grassia, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Michał Ginszt, Medical University of Lublin, PolandRossana Patricia Rotolo, University of Campania Luigi Vanvitelli, Italy
© 2022 Al Sayegh, Christidis, Kumar, Svensson and Grigoriadis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samaa Al Sayegh, c2FtYWEuYWwuc2F5ZWdoQGtpLnNl
Specialty Section: This article was submitted to Reconstructive Dentistry, a section of the journal Frontiers in Dental Medicine
 Samaa Al Sayegh
Samaa Al Sayegh Nikolaos Christidis
Nikolaos Christidis Abhishek Kumar
Abhishek Kumar Peter Svensson
Peter Svensson Anastasios Grigoriadis
Anastasios Grigoriadis