- Philip Morris International Research and Development, Philip Morris Products, Neuchâtel, Switzerland
Objectives: We conducted a 6-month randomized clinical study to evaluate the impact of exposure to the aerosol of the Tobacco Heating System (THS), a smoke-free alternative to cigarettes, on changes in periodontal parameters after scaling and root planing (SRP) for periodontitis in subjects who were either continuing to smoke cigarettes or had switched to THS.
Material and methods: Smokers with generalized periodontitis were randomized to continue smoking cigarettes or switch to THS use. They underwent SRP for up to 8 weeks, with dental assessments conducted at baseline and at 3 and 6 months after the first treatment.
Results: After SRP treatment, all groups showed improvements in the mean full-mouth probing depth (PD), full-mouth clinical attachment level (CAL), gingival inflammation score, plaque control record (PCR), and bleeding on probing (BoP). There were no statistically significant intergroup differences. However, as compared to smokers, THS users showed a trend toward more favorable outcomes in BoP, PCR, and PD improvement at sites with higher initial PD (≥7 mm).
Conclusions: Our results indicate that SRP improves the course of periodontitis similarly in cigarette smokers and THS users. The beneficial effects of this treatment might mask the favorable changes that may occur upon modifying one of the several periodontitis risk factors, such as cigarette smoking.
Clinical trial registration: ClinicalTrials.gov, identifer: NCT03364751.
Introduction
Periodontitis is the sixth most common disease in the world (1). It is an inflammatory disease affecting the gums, connective tissue, and the alveolar bone surrounding and supporting the teeth (2). It has a complex etiology, in which specific pathogenic bacterial strains trigger the inflammatory host response leading to tissue destruction (3, 4).
Smoking has been shown to increase the risk of developing periodontal diseases (5–9). The magnitude of the relative risk estimates for periodontal disease associated with smoking varies from 1.4 to 5.0 in different studies (8). The standard of care treatment for periodontitis, scaling and root planing (SRP), is reported to be less effective in smokers than in non-smokers. Notably, three meta-analyses showed the negative impact of smoking on periodontal responses to SRP treatment, with smokers having significantly less pocket depth (PD) reduction and clinical attachment level (CAL) gain than non-smokers (10–12). In addition, the microbiome of smokers differs from that of never smokers; the former microbiome is rich in pathogenic bacteria, but poor in commensal and aerobic bacteria, partly explaining why smokers are more prone to developing periodontal diseases (13). In patients with periodontitis who quit smoking for 12 months, the subgingival microbiome has been observed to change toward a healthier composition (14, 15).
The role of nicotine in the development and severity of periodontitis remains unclear (16, 17). It is generally described as having effects on gingival blood flow, cytokine production, and functions of immune cells, such as neutrophils (18).
Smoking cessation is the best way to decrease the risk of developing smoking-related diseases. However, for smokers who would otherwise continue smoking cigarettes, switching to heated tobacco products may provide beneficial effects on smoking-related disease risk due to the reduced levels of toxicants produced by the heating, rather than burning, of tobacco. Philip Morris International (PMI) has developed and marketed the Tobacco Heating System (THS) in more than 60 countries under the IQOSTM brand name. Pre-clinical data on gingival (19) and buccal epithelial (20) human organotypic cultures exposed to THS aerosol showed minor histopathological alterations and minimal cytotoxicity compared with exposure to cigarette smoke, along with a significantly reduced inflammatory response. A recent systematic review of electronic cigarettes (e-cigarettes) did not demonstrate any effect of vaping as compared to non-smoking on periodontitis (21). The suggested absence of an effect on the disease by vaping should ideally be compared to the effects of current smoking or former smoking on the disease. Additionally, the time since switching to e-cigarettes may be a critical variable that should be taken into consideration.
Considering the available pre-clinical data for THS and the published data on the effect of smoking cessation on SRP treatment outcomes, we sought to evaluate whether switching from cigarette to THS resulted in more favorable SRP treatment outcomes in patients with chronic generalized periodontitis as compared to continued cigarette smoking.
Material and methods
Study design and setting
This was a randomized, controlled, two-arm, parallel-group, multicenter study that compared the treatment outcomes of patients with chronic generalized periodontitis who switched from cigarettes to THS and those of patients who continued to smoke cigarettes for 6 months. This open-label study was conducted in 26 dental clinics in Japan, located mainly in Tokyo and the Kyushu region. Prior to the initiation of any study procedures, the protocol was approved at each site by the relevant institutional review board. All participants gave informed consent prior to enrollment. The study was registered and the results were disclosed in the U.S. Clinical Trials Registry with the ClinicalTrials.gov identifier NCT03364751. The description of the study protocol was published previously (22). The study was completed on December 15, 2018.
Patients were randomized via an interactive web response system (IWRS) into one of the two study arms, the THS arm and the cigarette arm, in a 1:1 ratio, using stratified randomization based on daily cigarette consumption over the month (30 days) prior to the first visit (V1; 10–19 cigarettes/day vs. >19 cigarettes/day) and disease severity (<5-mm PD vs. ≥5-mm PD) in smokers with chronic generalized periodontitis. In each arm, a quota was applied to ensure that patients with PD ≥5 mm represented at least 50% of the randomized patients.
Subjects visited the clinics at the time of screening/enrolment/baseline visit (V1), at randomization, corresponding to the first SRP treatment visit (V2), and at 3 months (V3) and 6 months (V4) post-randomization. They also attended as many unscheduled visits as deemed necessary by the investigators for subsequent SRP treatment. All SRP treatment had to be completed within 8 weeks after V2.
Study population
Male and female Japanese adult smokers, aged ≥30 years, diagnosed with chronic generalized periodontitis as per Japanese guidelines (i.e., more than 30% of diseased teeth with a PD ≥4 mm, considering only teeth that do not need to be extracted or whose mobility grade is <3) (23) were eligible for this study if they had smoked at least 10 cigarettes per day on average (no brand restrictions) for at least 5 consecutive years. Current smoking status was verified with a urinary cotinine test (cotinine ≥200 ng/mL). Furthermore, subjects had at least 15 natural teeth, excluding the teeth that needed to be extracted or whose mobility grade was ≥3. They were excluded from the study if they had received root planing therapy within the 6 months prior to V1, surgical periodontal therapy within 3 years prior to V1, or if they had been treated within the 3 months prior to V1 with systemic antibiotics, or with topical antibiotics applied in the mouth.
Subjects who had any clinically relevant diseases (for example, diabetes), had fewer than 15 natural teeth, wore orthodontic appliances, or had received an SRP treatment within 6 months or periodontal surgery within 3 years prior to the start of the study were excluded. Additionally, subjects were excluded if they had received antibiotics within 3 months prior to the first visit or if they were treated chronically with steroid or non-steroidal anti-inflammatory drugs.
Quotas were used to ensure adequate representation of subjects' disease severity (<5 vs. ≥5 mm PD, with PD ≥5 mm representing at least 50% of the study population) and cigarette consumption prior to the beginning of the study (10–19 cigarettes/day vs. >19 cigarettes/day).
The sample size was calculated to have at least 80% power to detect an effect difference of 0.25 mm based on Rosa et al. (24) and Preshaw et al. (25) on the PD change from baseline between subjects who had switched to THS and subjects who continued smoking after 6 months, assuming 25% non-adherence or drop-out.
Product adherence and product-use categories
The number of cigarettes, HeatSticksTM, and other nicotine/tobacco-containing products used, as self-reported by the subjects on paper-based questionnaires, was used to monitor product use and evaluate adherence to product allocation.
Based on the number of self-reported cigarettes, HeatSticks, and other nicotine/tobacco-containing products used, subjects were categorized into product-use categories, independently of their randomization arm, either as THS users (adherent subjects to THS use: ≥30 HeatSticks per month, but <30 cigarettes per month), cigarette users (adherent subjects to cigarette smoking: ≥30 cigarettes per month, but <30 HeatSticks per month), dual users (≥30 HeatSticks per month and ≥30 cigarettes per month), or “other” (for those who used other nicotine-containing products, such as e-cigarettes). We chose a threshold of 30, based on the definition of a daily smoker and on the fact that epidemiological studies have shown that even one cigarette per day increases the risk of developing a tobacco-related disease (26, 27).
Study assessments
As described previously (22), assessments at 3 and 6 months included the following: mean reduction in PD and CAL at sites with an initial PD ≥4 mm after mechanical periodontal therapy, change in mean full-mouth PD and CAL, mean PD and CAL changes at sites with an initial PD of <5, 5–6, 6–7 mm, and >7 mm, change in the number of sites with PD <5, 5–6, >6–7, and >7 mm, changes in gingival index (GI) score, tooth mobility (grade), PCR, and BoP scores. Examiners attended a calibration session before the initiation of the study (22). Furthermore, the number of self-reported products used was recorded every month. Biomarkers of exposure (BoExp) to nicotine, that is, N-[methylnitrosamino]-1-[3-pyridyl]-1-butanol (NNK), and acrylonitrile, that is, nicotine equivalents (NEQ), total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (total NNAL), and cyanoethyl mercapturic acid (2CyEMA; also named CEMA in former publications), were measured in spot urine as indicators of overall exposure to cigarette smoking in patients throughout the study. We recorded adverse effects (AE) and severe AEs (SAE) throughout the study. Gingival crevicular fluid was sampled to assess levels of inflammatory mediators, before and 3 months after SRP treatment, using multiplex immunoassays and parallel reaction monitoring (28). Subgingival plaque samples were collected to analyze bacterial composition at baseline and at 6 months after the SRP treatment.
As reported previously (22), subjects were treated using SRP as per the standard of care recommended by the Japanese Society of Periodontology (23). The first SRP treatment was performed on visit 2, and then the number of visits for these treatments was left up to the investigator to decide, but all treatments had to be completed within 8 weeks. Only SRP was part of the care provided, unless otherwise decided by the investigator. Blinding of the examiners to the randomization arm of their patients was attempted by asking the patients to wash their hands before the examination (to remove the smell of tobacco smoke), and the examiners all wore the same type of mask that neutralized odors (22).
Data set analysis and statistical methods
The primary objective of the study was to demonstrate a PD reduction in subjects switching to THS as compared to continuing smoking. This was evaluated through a mixed model for repeated measurements in subjects who had an initial PD ≥4 mm according to subjects' product-use categories, as further detailed in the publication by Pouly et al. (22). This “As Exposed Set” consisted of all randomized subjects with at least one post-randomization product-use experience, who had at least one valid non-safety assessment after randomization and had no major protocol deviation that could influence the evaluability of the study data. For the evaluation of the study's primary objective, we calculated estimates of PD difference for cigarette users against the THS user category and cigarette users against the dual user category, testing for multiple covariates that could have contributed to the study outcomes using a likelihood ratio approach. The final statistical model to determine changes in PD included the PD change from baseline, as the dependent variable, adjusted for daily cigarette consumption at baseline; disease severity at baseline; full mouth PD at baseline; time of visit; product-use category; interaction between the visit and product-use category; average daily cigarette use at each visit; number of target tooth/teeth extracted; number of non-measured target tooth/teeth extracted; and baseline tooth mobility.
One of the secondary objectives of the study was a comparison of the change in PD at 3 months and CAL at 3 and 6 months in all product-use categories with the changes in the cigarette smoking category. The same statistical approach as for the primary objective was followed.
Analysis of other objectives, including the changes in levels of BoExp and inflammatory mediators in the GCF, was descriptive only. Total NNAL, 2CyEMA, and NEQ levels (concentration data adjusted for creatinine) were analyzed on a logarithmic scale. Geometric means and related confidence intervals were calculated.
The safety endpoints were summarized using the Safety Set consisting of all enrolled subjects with at least one valid safety assessment by actual exposure (product-use categories).
All statistical tests were one sided and conducted at the 2.5% level, with two-sided 95% confidence intervals (CI). All other results are presented in a descriptive manner with 95% CIs.
Microbiome sampling and analysis
Subgingival plaque samples were collected from the mesio-buccal surface of the four selected target teeth (with an initial PD ≥4 mm), using a sterile Gracey curette, pooled, and aliquoted into OMNIgene•ORAL OMR-110 tubes from DNA Genotek (DNA Genotek, Ottawa, Canada). Microbiome DNA was isolated using ZymoBIOMICS DNA microprep kits (Zymo Research, Irvine, CA, USA). The amount of extracted DNA was quantified with a Thermo Fisher Scientific Qubit 2.0 (Thermo Fisher Scientific, Waltham, MA, USA) using Qubit dsDNA HS and BR Assay Kits.
We manually prepared DNA sequencing libraries with the Nugen Celero™ DNA-Seq Library Preparation Kit (NuGEN Technologies, San Carlos, CA, USA). Library molarity was measured using an Agilent Fragment Analyzer (Agilent Technologies, Santa Clara, CA, USA) with the DNF-473 NGS Fragment Kit (1–6,000 bp). Microbiome DNA sequencing libraries were normalized to 1 nM and pooled equimolarly for sequencing on an Illumina HiSeq4000 Sequencer (Illumina, San Diego, CA, USA) using an Illumina HiSeq 3000/4000 PE Cluster Kit and an Illumina HiSeq 3000/4000 SBS Kit (300 cycles). We used Illumina's bcl2fastq2 Conversion Software v2.20 for demultiplexing sequencing data.
Taxonomic profiles were created for each sample using the Biobakery Metagenomics Analysis pipeline, specifically the output of the MetaPhlan2 tool (Harvard T.H. Chan School of Public Health, Boston, MA, USA) (29). Samples with fewer than the minimum read number (10M paired-end reads) were not considered for statistical analysis. The individual profiles were merged into a single table for downstream statistical analysis. Comparisons were performed on the relative abundance at the species level. The effects of the SRP treatment in each arm were determined using the paired-sample Wilcoxon test on the subset of subjects for which samples were available at both visits (V1 [baseline] and V4 [6 months]). We performed statistical calculations in R using the “Wilcox” function with the “exact” argument set to “FALSE.” To account for multiple testing, we used a False Discovery Rate (FDR) correction with the Benjamini–Hochberg method (p.adjust function in R, with the method set to “BH,” with all other parameters set to default).
Results
Recruitment
The first subject was recruited in November 2017, and the last subject completed the study in December 2018.
Participant flow
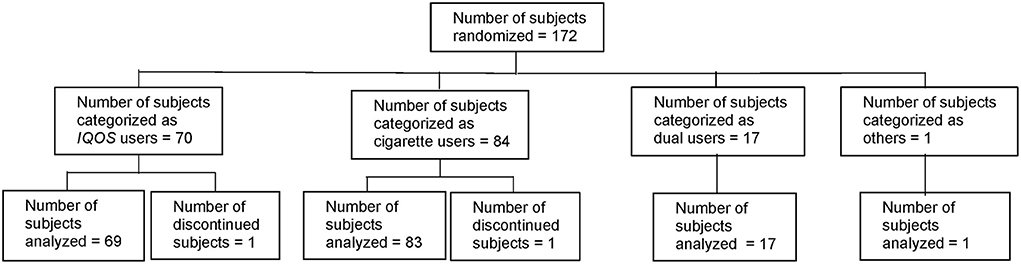
Of the 185 subjects screened, six were considered to be screening failures (five because of unmet eligibility criteria and one because of withdrawal by the subject). Among the 179 enrolled subjects, seven were excluded prior to randomization (three because of unmet eligibility criteria after V1, two because of informed consent withdrawal, and two because the required number of patients had been reached before V2). Thus, 172 subjects were randomized to either the THS arm or the cigarette arm (87 to the THS arm and 85 to the cigarette arm) (Figure 1).
The subjects started to use their assigned product after randomization. Of the 172 randomized subjects, 170 subjects completed the study (86 in the THS arm and 84 in the cigarette arm); thus, 98.8% of all randomized subjects completed the study. One subject in the THS arm (classified in the THS user category) was discontinued due to withdrawal of informed consent 140 days after randomization (between V3 and V4). Another subject in the cigarette arm (classified in the cigarette user category) was lost to follow-up 193 days after randomization (did not complete V4).
Data sets analyzed
The As Exposed Set consisted of 172 subjects (70 THS users, 84 cigarette users, 17 dual users, and one in the “other” category).
The Safety Set (N = 179) comprised 70 THS users, 91 cigarette users, 17 dual users, and one “other” subject. The Safety Set included seven subjects who were enrolled but not randomized; these subjects were all categorized as cigarette users.
For simplification purposes, assessment results for the single “other” subject are not presented in this manuscript, but the results are available on ClinicalTrials.gov.
Baseline data
A summary of demographic and baseline characteristics is shown for subjects who were part of the analysis in the As Exposed Set in Table 1, presented according to product-use categories.
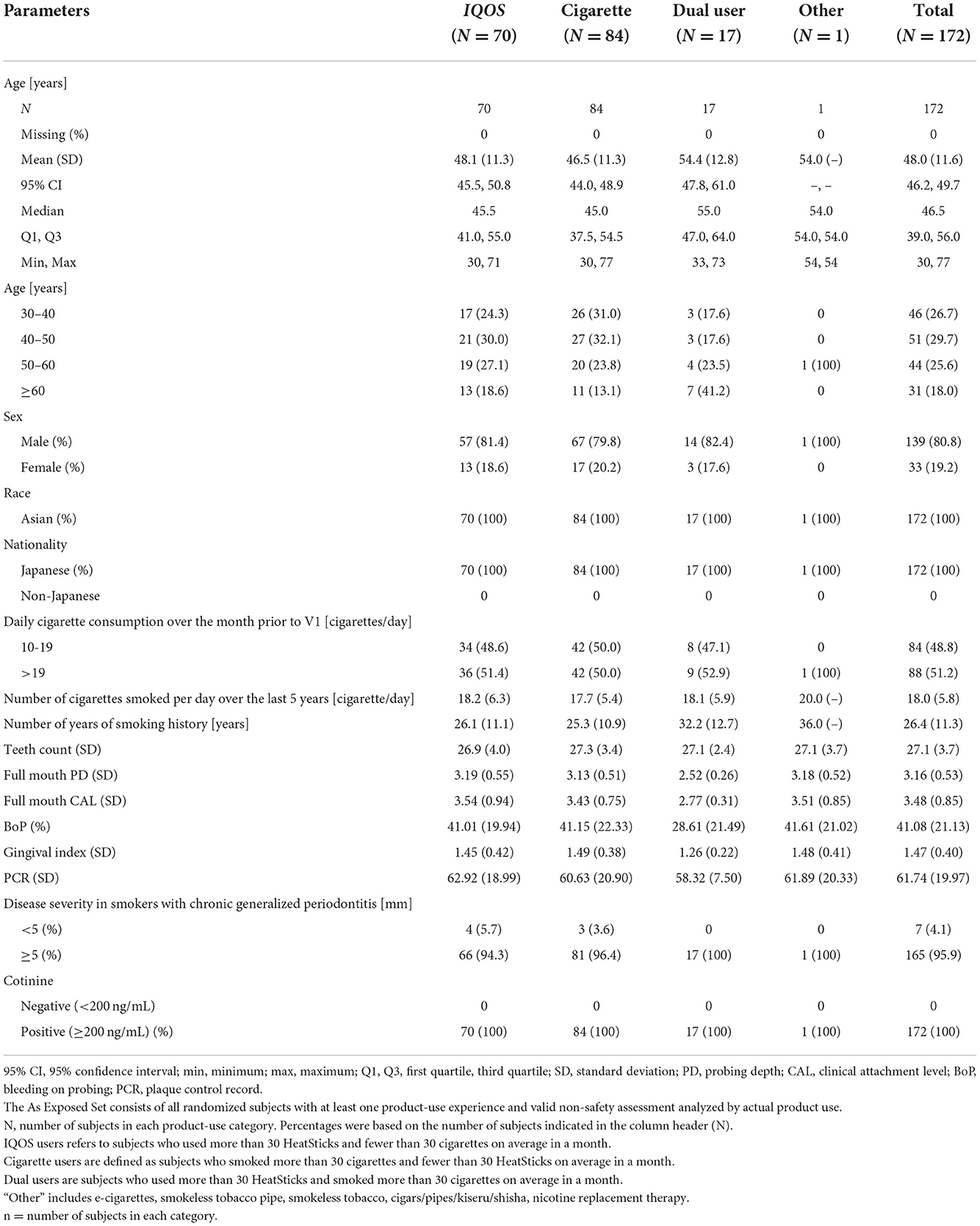
Table 1. Summary of participants' demographic characteristics by product-use category in the As Exposed Set.
The demographic and baseline characteristics, excluding age, were balanced across categories. All subjects were Japanese, and the ratio of males to females was about 4:1 in all groups. The median age was higher for the dual users than for the THS and cigarette user groups, as was the percentage of subjects in the age group ≥60 years. Approximately half of the subjects in each product-use category smoked more than 19 cigarettes/day over the month prior to V1, as per eligibility criteria.
A large majority of subjects in each product-use category had initial PD values of ≥5 mm, based on the most severely diseased tooth, as expected from the study design. The dual users appear to have had less severe disease, as indicated by the lower full-mouth PD and CAL values, and lower BoP, GI, and PCR values. All groups had a comparable number of teeth at baseline.
Mean PD and CAL changes
As detailed previously, the primary objective of the study was to demonstrate the effect of switching to IQOS use, compared to continued cigarette smoking, on the response of PD to mechanical periodontal therapy after 6 months. Secondary objectives included response of PD after 3 months and CAL after 3 and 6 months.
The mean PD and CAL values at all sites with initial PD ≥4 mm and the change from baseline for the As Exposed Set are presented in Figure 2, with summarized descriptive statistics at each visit presented according to the product-use category in Supplementary Table 1.
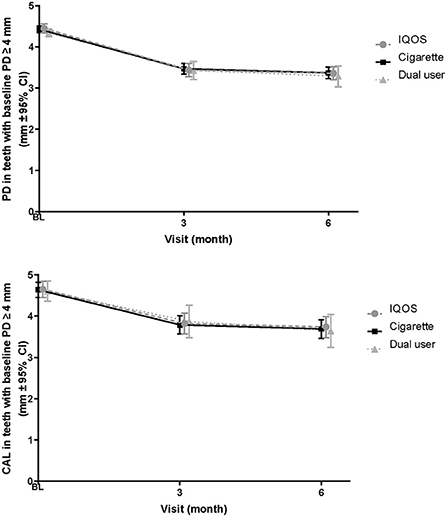
Figure 2. Changes in PD and CAL in sites with initial probing depth ≥4 mm—As Exposed Set. BL, baseline; PD, probing depth; CAL, Clinical attachment level; CI, confidence interval.
The mean PD in THS users decreased from 4.45 mm (baseline) to 3.44 mm (3 months, corresponding to a −1.01 mm change) and 3.36 mm (6 months); this corresponded to an overall change of −1.09 mm. Similarly, the values in cigarette users and dual users decreased from 4.43 and 4.34 mm (baseline) to 3.47 and 3.43 mm (3 months), corresponding to changes of −0.96 and −0.91 mm, respectively; at 6 months, the values further decreased to 3.37 and 3.29 mm, corresponding to an overall change of −1.06 mm in each group.
The mean full-mouth CAL values in THS users decreased from 3.48 mm (baseline) to 3.10 mm (3 months), corresponding to a −0.38 mm change, which was sustained at 6 months. Similarly, the values in cigarette users and dual users decreased from 3.52 and 3.34 mm (baseline) to 3.13 and 3.15 mm (3 months), corresponding to changes of −0.40 and −0.20 mm, respectively; at 6 months, the values further decreased to 3.09 and 2.97 mm, corresponding to overall changes of −0.44 and −0.37 mm in cigarette users and dual users, respectively.
Although the PD values decreased at 6 months from baseline within each product-use category, no statistically significant differences in the change in Least Squares (LS) means from baseline were found between cigarette and THS users (0.068 [95% CI, −0.060 to 0.196] p-value = 0.297) or between cigarette and dual users (−0.064 [95% CI, −0.273 to 0.146]; p-value = 0.550) (Supplementary Table 2). Similar to the analysis of the primary objective for PD at 6 months, the PD values decreased from baseline at 3 months in all product-use categories; however, no statistically significant differences in the change in LS means from baseline were found between cigarette and THS users or between cigarette and dual users at 3 months.
The CAL values also decreased from baseline at 3 and 6 months in all product-use categories. However, no statistically significant differences in the change in LS means from baseline were found between cigarette and THS users (0.092 [95% CI, −0.064 to 0.247]; p-value = 0.246) or between cigarette and dual users (−0.105 [95% CI, −0.359 to 0.150]; p-value = 0.417) at 6 months (Supplementary Table 2). Similarly, no statistically significant differences in the change in LS means from baseline were found between cigarette and THS users or between cigarette and dual users at 3 months.
Mean PD and CAL changes in sites with different initial PD levels
Mean PD value and the change from baseline, according to sites with different initial PD levels (<4, 4 to <5, 5 to <6, 6 to <7, and ≥7 mm), at each visit are summarized according to the product-use category for the As Exposed Set, using descriptive statistics (Figure 3A).
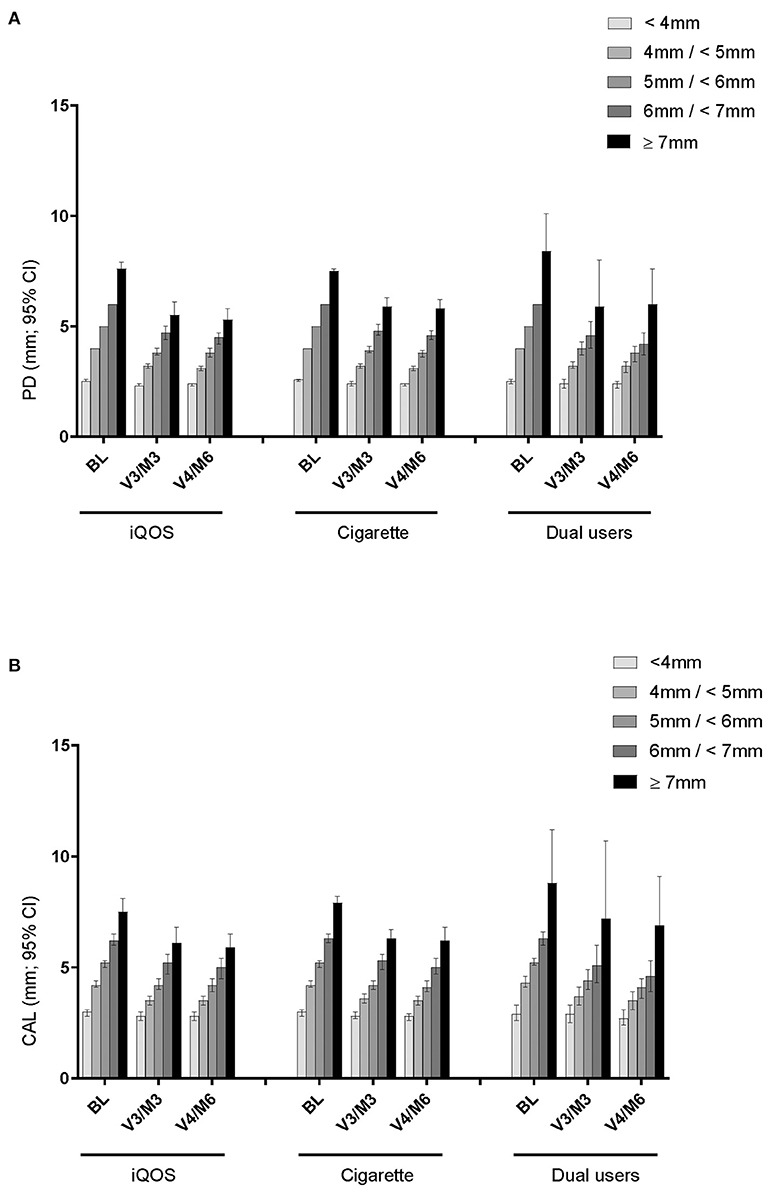
Figure 3. Mean PD and CAL change in sites with different initial PD level. (A) PD of sites based on initial PD (mm) level. (B) CAL of sites based on initial PD (mm) level.
After SRP treatment, the mean PD values had decreased by 3 and 6 months at all sites categorized by the initial PD level, except for the very shallow pockets (<4 mm). The magnitude of decrease appeared to be somewhat larger in sites with higher initial PD and in THS users than in cigarette users at both 3 and 6 months.
Comparably, after SRP treatment, full-mouth CAL values decreased at 3 and 6 months in pockets that were >4 mm PD at baseline (Figure 3B). The profiles in each initial PD group were similar across the product-use categories and did not differ between product-use categories throughout the study. Unlike PD, however, CAL changes did not show a trend of greater change in sites with higher initial PD and in THS users.
Other periodontal parameters
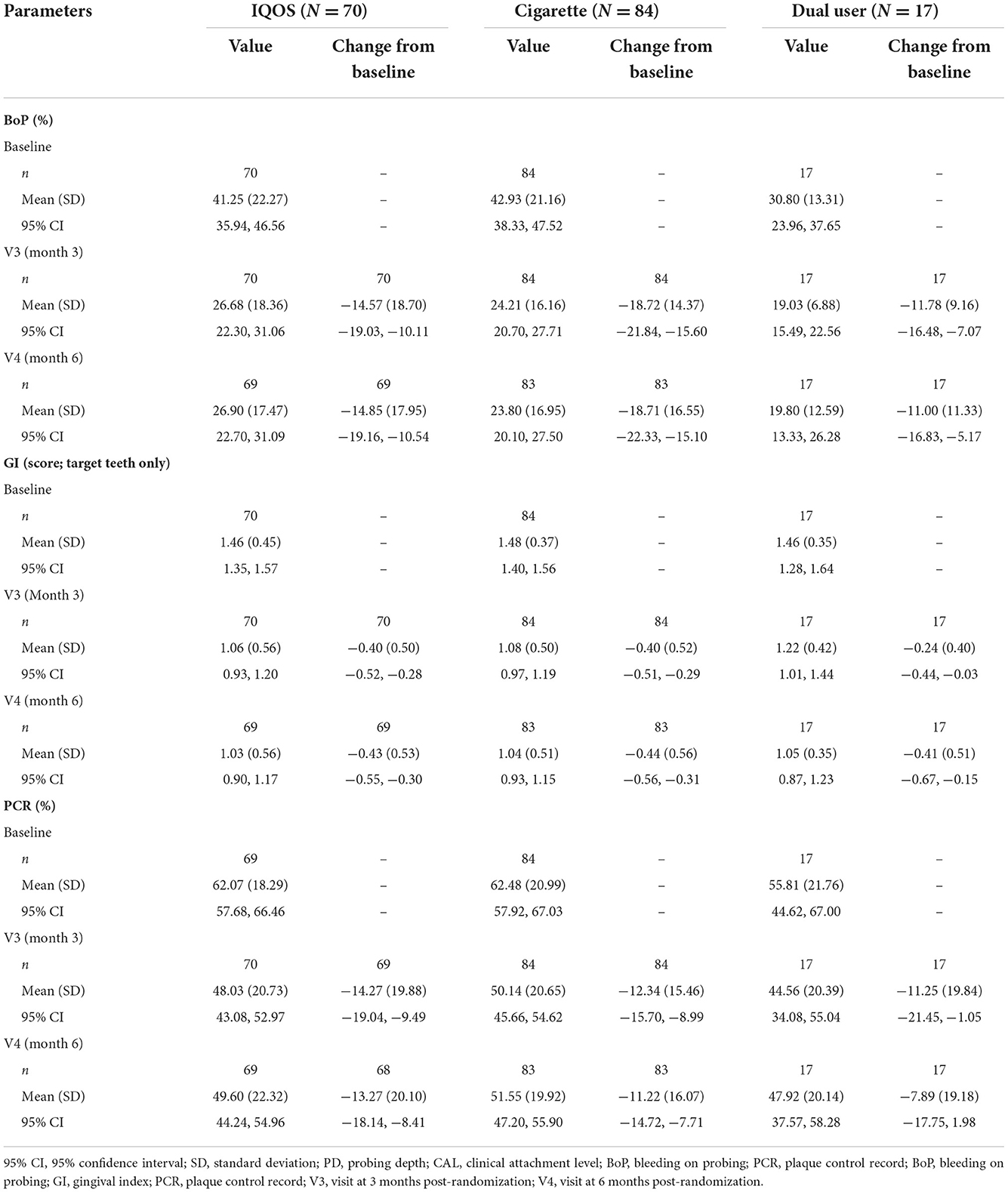
Descriptive statistics for the other periodontal parameters are presented in Table 2.
The profiles of BoP scores were generally similar among the product-use categories, with most of the decreases observable at 3 months and being sustained at 6 months. The magnitude of decrease was generally similar among the product-use categories, except for a slightly greater decrease seen in cigarette users than in THS or dual users at 3 and 6 months.
The GI was scored from six surfaces of each of the target teeth. The GI scores were similar among the product-use categories, with comparable decreases observed at 3 and 6 months. The magnitude of decrease was similar among the product-use categories.
The profiles of mean PCR were generally similar among the product-use categories, with comparable decreases observed at 3 and 6 months. The magnitude of decrease was similar among the product-use categories, with a slightly greater decrease at 3 and 6 months in THS users than in cigarette users or dual users.
At baseline, a higher grade of tooth mobility was observed in subjects with higher initial PD levels in THS and cigarette users. In dual users, all subjects were categorized into the initial PD level of <4 mm. After SRP treatment, almost no changes were found in tooth mobility at V3 and V4, irrespective of the initial PD levels, and the profiles were almost the same in all product-use categories.
Product use and exposure to smoke constituents
Exposure to nicotine, as assessed by urinary NEQ adjusted to creatine values from spot urine collection during the visits, remained largely unchanged throughout the study, in all product-use categories, as shown in Supplementary Figure 1 and Supplementary Table 3. No significant changes were found at 3 or 6 months. On the other hand, urinary 2-CyEMA and total NNAL values adjusted to creatinine values were decreased significantly in THS users as compared to cigarette users, by 83.9 and 87.0% at 3 and 6 months, respectively, for 2-CyEMA, and by 66.3% and 68.5%, respectively, for total NNAL. These values remained relatively stable in cigarette smokers and did not change as markedly in dual users (decreases of 7.7% to 35.7% for 2CyEMA and of 2.4% to 17% for total NNAL at 3 and 6 months, respectively).
Inflammatory mediators in the gingival crevicular fluid
Changes in inflammatory mediators in the GCF are summarized in Figure 4 and Supplementary Table 4, with levels for THS and dual users presented as ratios relative to the levels in cigarette users. For most of the 46 assessed mediators, the geometric mean ratio of the levels in THS users to those in cigarette users at 3 months increased slightly (<1.5-fold), while 12 mediators showed a statistically significant increase in their levels in THS users as compared to cigarette users. Among them, C-reactive protein (CRP) had the highest geometric mean ratio (2.853 [95% CI, 1.521 to 5.351]), followed by matrix metalloproteinase 8 (MMP-8) (1.418 [95% CI, 1.067 to 1.886]), [C-X-C motif] ligand 1 CXCL1 (1.403 [95% CI, 1.022 to 1.926]), and tumor necrosis factor α (TNFα) [(1.400 [95% CI, 1.036 to 1.890]).
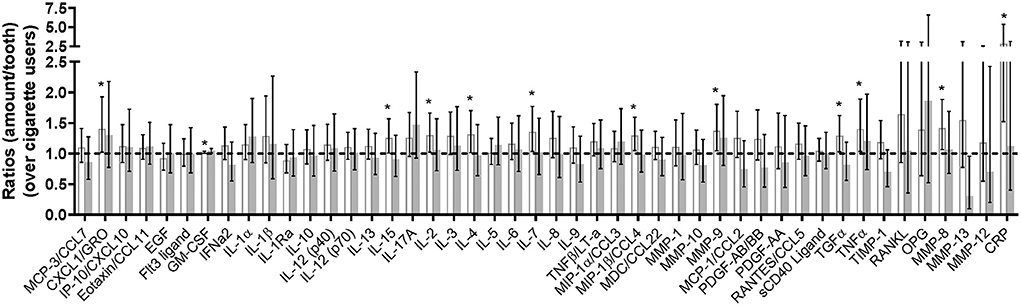
Figure 4. Changes in multiplex assay- and parallel reaction monitoring-analyzed inflammatory mediators (As Exposed Set). Bars represent the ratio of geometric means between THS users, dual users, and cigarette smokers (95% CI); white bars, IQOS users; gray bars, dual users; dotted line, levels for cigarette smokers. MCP-3/CCL7, chemokine (C-C motif) ligand 7 [pg/tooth]; CXCL1/GRO, chemokine (C-X-C motif) ligand 1 [pg/tooth]; IP-10/CXCL10, chemokine (C-X-C motif) ligand 10 [pg/tooth]; Eotaxin/CCL11; eotaxin-1 [pg/tooth]; EGF, epidermal growth factor [pg/tooth]; Flt3 ligand, fms-related tyrosine kinase 3 ligand [pg/tooth]; GM-CSF, granulocyte-macrophage colony-stimulating factor [pg/tooth]; IFNα2, interferon alpha-2 [pg/tooth]; IL-1α, interleukin-1 alpha [pg/tooth]; IL-1β, interleukin-1 beta [pg/tooth]; IL-1Ra, interleukin-1 receptor antagonist [pg/tooth]; IL-10, interleukin-10 [pg/tooth]; IL-12 (p40), interleukin-12 [pg/tooth]; IL-12 (p70), interleukin-12 beta [pg/tooth]; IL-13, interleukin-13 [pg/tooth]; IL-15, interleukin-15 [pg/tooth]; IL-17A/CTLA8, interleukin-17 [pg/tooth]; IL-2, interleukin-2 [pg/tooth]; IL-3, interleukin-3 [pg/tooth]; IL-4, interleukin-4 [pg/tooth]; IL-5, interleukin-5 [pg/tooth]; IL-6, interleukin-6 [pg/tooth]; IL-7, interleukin-7 [pg/tooth]; IL-8/CXCL8, interleukin-8 [pg/tooth]; IL-9, interleukin-9 [pg/tooth]; TNFβ/LT-α, lymphotoxin-alpha [pg/tooth]; MIP-1α/CCL3, macrophage inflammatory protein-1 alpha; MIP-1β/CCL4, macrophage inflammatory protein-1 beta [pg/tooth]; MDC/CCL22, macrophage-derived chemokine [pg/tooth]; MMP-1, matrix metalloproteinase-1 [pg/tooth]; MMP-10, matrix metalloproteinase-10 [pg/tooth]; MMP-9, matrix metalloproteinase-9 [pg/tooth]; MCP-1/CCL2, monocyte chemotactic protein-1 [pg/tooth]; PDGF-AB/BB, platelet derived growth factor isoform AB/BB [pg/tooth]; PDGF-AA, platelet derived growth factor isoform AA [pg/tooth]; RANTES/CCL5, regulated upon activation normal T-cell expressed and secreted [pg/tooth]; sCD40L, soluble CD40 ligand [pg/tooth]; TGFα, transforming growth factor alpha [pg/tooth]; TNFα, tumor necrosis factor [pg/tooth]; TIMP-1, tissue inhibitor of metalloproteinase-1 [pg/tooth]; RANKL, receptor activator nuclear kappa B ligand [pg/tooth]; OPG, osteoprotegerin [pg/tooth]; MMP-8, matrix metalloproteinase-8 [pg/tooth]; MMP-13, matrix metalloproteinase-13 [pg/tooth]; MMP-12, matrix metalloproteinase-12 [pg/tooth]; CRP, C-reactive protein [pg/tooth]. The * symbol indicates the value of p < 0.05 for ratio between cigarette smokers and THS users or dual users.
On the other hand, for the geometric mean ratio of the inflammatory mediator levels in dual users to those in cigarette users at 3 months, no apparent overall trends were found, despite the statistically significant increase reported for granulocyte-macrophage colony-stimulating factor (GM-CSF) and the statistically significant decrease for matrix metalloproteinase 13. (MMP-13).
Microbiome composition
Supplementary Table 5 shows the microbe species that were significantly differentially abundant between baseline and 3 months in the overall pooled subjects, as well as in each product-use category. Overall, SRP treatment resulted in lower abundance of several bacterial species in all product-use categories at 3 months from baseline. The levels of bacteria that form part of the red complex, an aggregate of three oral bacteria (Tannerella forsythia, Porphyromonas gingivalis, and Treponema denticola) usually responsible for severe clinical manifestation of periodontal disease (30), were significantly reduced at 3 months from baseline in all product-use categories, including cigarette users.
Considering each product-use category, cigarette users had a slightly greater decrease in the abundance of some bacterial species than THS users at 3 months from baseline, with statistically significant differences in three classified bacterial species, Olsenella uli, Pseudoramibacter alactolyticus, and Porphyromonas gingivalis. When comparing the relative abundance of bacterial species in THS users vs. cigarette users at 3 months, no significant changes were observed (data not shown).
Safety
Two SAEs were reported in cigarette users, while no SAEs were reported in other categories (THS users; dual users; “other” users). There were no severe AEs and no AEs that led to subject discontinuation from the study. Only one AE related to the investigational product (IP: THS or cigarette) was reported in dual users, and there were no AEs related to IPs in other product-use categories.
There was no significant difference in the incidence of AEs related to study procedures between IQOS users (5 of 70 subjects, 7.1%) and cigarette users (4 of 91 subjects, 4.4%). The incidence of AEs related to study procedures in dual users (2 of 17 subjects, 11.8%) was higher than those in IQOS users and cigarette users, although this category included a smaller number of subjects.
Eighty-nine subjects used THS at least once in this study, and 42 device events were reported in 30 subjects (33.7%). None of these events led to an AE. The most frequently reported device events were “Holder heater broken” (23 events) and “Holder does not charge when inserted into the mobile unit” (10 events).
Discussion
This study was designed to demonstrate the effect of switching to THS use as compared to continued cigarette smoking on the response of PD to mechanical periodontal therapy, by evaluating the mean PD reduction at all sites with initial PD ≥4 mm at 6 months after SRP treatment. The definition of chronic generalized periodontitis was based on the guidelines established by the Japanese Society of Periodontology (23). i.e., before the publication of the new periodontitis classification scheme (31). We observed a PD reduction of approximately 1 mm from baseline at 6 months, regardless of whether a subject switched to THS or continued smoking cigarettes, with no statistically significant differences between cigarette and THS users or between cigarette and dual users. The differences between cigarette and THS users were smaller than the effect size of 0.25 (0.5) mm, which was estimated from the published data on smokers and non-smokers, from which the sample size was calculated (24, 25). Similarly, comparable CAL reductions from baseline were also observed at 3 and 6 months, regardless of whether a subject switched to THS or continued smoking cigarettes.
Previous reports showed that PD changes in smokers at 6 months after SRP treatment were within the same ranges as those found in the present study, varying from −0.64 to −1.13 mm (32–36). However, analyses of the results differed between studies in terms of statistical approaches, for example, whether adjustments were made for baseline values or other covariates. The same studies showed differences in PD in non-smokers and smokers ranging from −0.58 to −1.98 mm, with PD changes generally greater for non-smokers. Published studies that also considered former smokers or quitters showed larger changes in PD after SRP treatment in former smokers than in smokers (36, 37). Two longitudinal studies on smoking cessation showed PD differences at 6 months after SRP treatment of −0.86 mm and −1.25 mm (24), and −0.91 mm and −1.23 mm (25) in smokers and quitters, respectively, with a smaller PD change in smokers. All these studies indicated a detrimental effect of smoking on periodontal improvement after SRP treatment, except for the reports of Pucher et al. (34), Ryder et al. (36), and Kanmaz et al. (38), which showed no or minimal differences between smokers and quitters. Even though the benefit of smoking cessation on SRP treatment effects has been reported in several publications, the data were not always consistent, the differences were very small, and they were mostly not statistically different. Additionally, the importance of considering differences in baseline values was highlighted by Preus et al. (39), who found differences between baseline-adjusted and unadjusted analyses of the effect of smoking on the change in periodontal parameters after SRP. Their unadjusted analysis results indicated that this treatment had a larger effect in smokers than in former or never smokers, whereas the results of the baseline-adjusted analysis indicated no such differences. Several publications on the effects of SRP in smokers vs. non-smokers may thus have been biased by not taking into account covariates, such as baseline values.
Changes in PD are often considered less clinically relevant than changes in CAL, which is measured from a fixed reference point and is thus a more valid metric and stable indicator of improvement in periodontal health than PD (40). PD has, however, often been used as the primary endpoint in periodontal studies assessing the effects of smoking or smoking cessation on SRP treatment (10).
In line with the results on PD and CAL, improvements were observed following SRP treatment in all product-use categories for almost all other periodontal parameters assessed in this study, excluding tooth mobility.
The magnitude of the descriptive changes in PCR from baseline were comparable among all product-use categories, with a slightly greater change in THS users than in cigarette users, despite the limitation that scaling maintenance treatment was performed at each visit, thereby limiting the possibility of observing differences over such short periods.
The mean change in BoP was slightly greater in cigarette users than in THS users, while the changes in GI were comparable among the product-use categories. In general, published data showed no significant difference in bleeding between smokers and non-smokers at 6 months after SRP treatment (24, 25, 35, 36). However, smoking has clearly been shown to suppress the bleeding response, without SRP treatment, while, within weeks of biochemically validated cessation of cigarette smoking, BoP increases (41–43). The lower rate of BoP in cigarette smokers than in THS users after SRP treatment in this study is therefore likely to be an indicator of improvement in the oral health status of THS users, similar to that observed upon smoking cessation, but this requires further confirmation. The timeframe of this increased bleeding after smoking cessation seems to be relatively short, that is, weeks to a few months, and might have been missed in studies with visits after 3 months or more. Thus, only the tail-end of this phenomenon would have been caught in the present study.
The differences between THS and cigarette users in terms of responses by disease severity (such as higher initial PD level) remain unclear from the results of this study: full mouth PD at 3 months seemed to be slightly more improved in THS users with a baseline disease severity <5 mm PD, involving only seven subjects, while PD gain was increased in THS users for sites with an initial PD ≥7 mm. Taken together, these results suggest that the SRP treatment by itself was inducing most of the changes, which may have masked a potential beneficial effect of switching to THS.
In this study, self-reported consumption of tobacco or nicotine-containing products was used to evaluate overall product use, which has limitations given that there is a possibility of recall bias. This is supported by some values of 2CyEMA and total NNAL that were clearly higher than the median value in some subjects from the THS user group. Based on self-reported product use, most subjects used their assigned product almost exclusively, except for 17 subjects who used about 50% cigarettes and 50% HeatSticks. No significant differences were found in nicotine exposure among cigarette, dual, and THS users during the 6 months. Exposure to NNK and acrylonitrile were reduced by approximately 70% or more in THS users, which was as expected, as THS use has been shown to decrease exposure to toxic smoke constituents (44–47). This suggests that most subjects were able to switch to THS and obtain adequate levels of nicotine and a satisfactory product experience when they replaced their cigarettes with THS.
Exploratory analyses suggest that the recovery of all periodontal parameters after SRP treatment across all product-use categories was associated with a slightly higher inflammatory response in THS users than in cigarette users and dual users, as indicated by a small increase in many of the mediator levels measured in the GCF. In THS users, the levels of most mediators measured in the GCF increased slightly (around 1.5-fold) as compared to baseline, while they were unchanged or slightly decreased in cigarette users and dual users. It has been reported that smokers have reduced clinical signs of inflammation and GCF volumes and flow rate as compared to non-smokers (42). In addition, the concentrations of inflammatory markers detected in the GCF of smokers with severe chronic periodontitis were decreased as compared to non-smokers (48). Such data might be explained by the immunosuppressive effects and decreased angiogenesis, which have been observed with cigarette smoking (43). Quitting smoking, on the other hand, has been suggested to restore the microcirculation in the gingiva, so that BoP appears after a few weeks of cessation and restores GCF secretion (41, 42). The slight restoration in the inflammatory response observed in this study in the THS users is thus coherent with what has been described for periodontitis patients who quit smoking.
Although the overall oral microbiome composition remained unchanged, SRP was successful in reducing the abundance of several pathogenic bacterial species at 6 months in the pooled group. Bacterial species, such as O. uli, P. alactolyticus, and P. gingivalis, associated with periodontal diseases and conditions (49–51), were significantly decreased at 6 months, although the differences between cigarette users and THS users did not reach statistical significance. It is possible that the beneficial effect of the SRP therapy may have masked the potential favorable effects of switching to THS, as proposed for the periodontal endpoints.
The strengths of this study include the number of subjects, which was one of the largest samples ever tested in a longitudinal study on periodontitis and SRP. Furthermore, the statistical analysis was adjusted with many co-variates, including baseline values. Moreover, the variability of the periodontal measurements can be considered to be low, considering that 26 different investigators were involved in the study. Measurement of PD or CAL is highly variable, and prone to error, as described by Leroy et al. (52). For this reason, we implemented a calibration session for the investigators at the beginning of the study.
The study has some limitations, such as not having a smoking-abstinence arm. Because THS delivers nicotine while reducing the levels of other smoke toxicants significantly, the extent to which nicotine might have had an effect cannot be excluded without having a smoking-abstinence arm as a reference. The reason for not including such an arm was largely based on operational and recruitment aspects, as well as the need for biochemical verification of the true abstinence status of the subjects (53), as many subjects would have failed to remain abstinent (54). Since the initiation of the study, a number of clinical studies on the oral effects of e-cigarettes, which deliver reduced levels of harmful constituents while still delivering nicotine, have been published, but most of them indicate that more research is needed, including larger studies, as summarized in a recent review (55) and in a recent meta-analysis (21). Another limitation of the study may be its duration. Even though most published data on SRP have focused on improvements after 3–6 months, more time may have been needed to observe an improvement when switching to THS. Finally, several investigators noted some potential Hawthorne effects in their subjects, with smokers generally taking better care of their oral hygiene than they would usually have done outside of this clinical setting.
The present findings showed that smokers, dual users, and THS users all responded well to non-surgical periodontal treatment during the 6-month follow-up. The study found that when THS users and cigarette smokers received the same SRP treatment for periodontitis, there was no difference in the treatment outcome for either group of subjects. Further studies would be required to better understand the long-term potential benefit of THS on oral health.
Data availability statement
The data sets generated and/or analyzed the current study are available from the corresponding author upon reasonable request.
Ethics statement
The studies involving human participants were reviewed and approved by SONE Clinic Institutional Review Board 3-32-8 Shinjuku, Shinjuku-ku, Tokyo JAPAN. The patients/participants provided their written informed consent to participate in this study.
Author contributions
SP, WN, and CH designed the study. SP, WN, CH, and PH wrote the manuscript with support from FZ, JB, GL, and NB. All authors discussed the results and contributed to the final manuscript.
Funding
Philip Morris International is the sole source of funding and sponsor of this study.
Acknowledgments
The authors acknowledge the bioanalysis performed by the genomics (Dariusz Peric, Remi Dulize, and David Bornand) and proteomics team (Thomas Schneider, Celine Merg, Maica Corciulo, Yvan Eb-Levadoux, and Edina Kishazi) and sample management team (Sam Ansari and Edouard Dargaud) under the supervision of Nikolai V. Ivanov at PMI R&D.
Conflict of interest
All authors were employed by Philip Morris Products S.A. (PMP) or worked for PMP under contractual agreements. The authors declare that Philip Morris International is the sole source of funding and sponsor of this study. The authors were solely involved in the study design, collection, analysis, interpretation of data, the writing of this article and the decision to submit it for publication.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdmed.2022.915079/full#supplementary-material
References
1. Frencken JE, Sharma P, Stenhouse L, Green D, Laverty D, Dietrich T, et al. Global epidemiology of dental caries and severe periodontitis–a comprehensive review. J Clin Periodontol. (2017) 44:S94–S105. doi: 10.1111/jcpe.12677
2. Hernandez M, Dutzan N, García-Sesnich J, Abusleme L, Dezerega A, Silva N, et al. Host-pathogen interactions in progressive chronic periodontitis. J Dent Res. (2011) 90:1164–70. doi: 10.1177/0022034511401405
3. Van Dyke T, Serhan C. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res. (2003) 82:82–90. doi: 10.1177/154405910308200202
4. Griffen AL, Beall CJ, Campbell JH, Firestone ND, Kumar PS, Yang ZK, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. (2012) 6:1176–85. doi: 10.1038/ismej.2011.191
5. Kaldahl WB, Johnson GK, Patil KD, Kalkwarf KL. Levels of cigarette consumption and response to periodontal therapy. J Periodontol. (1996) 67:675–81. doi: 10.1902/jop.1996.67.7.675
6. Torrungruang K, Nisapakultorn K, Sutdhibhisal S, Tamsailom S, Rojanasomsith K, Vanichjakvong O, et al. The effect of cigarette smoking on the severity of periodontal disease among older Thai adults. J Periodontol. (2005) 76:566–72. doi: 10.1902/jop.2005.76.4.566
7. Ojima M, Hanioka T, Tanaka K, Inoshita E, Aoyama H. Relationship between smoking status and periodontal conditions: findings from national databases in Japan. J Periodontal Res. (2006) 41:573–9. doi: 10.1111/j.1600-0765.2006.00915.x
8. Warnakulasuriya S, Dietrich T, Bornstein MM, Casals Peidro E, Preshaw PM, Walter C, et al. Oral health risks of tobacco use and effects of cessation. Int Dent J. (2010) 60:7–30. doi: 10.1922/IDJ_2532Warnakulasuriya24
9. Johannsen A, Susin C, Gustafsson A. Smoking and inflammation: evidence for a synergistic role in chronic disease. Periodontology. (2014) 64:111–26. doi: 10.1111/j.1600-0757.2012.00456.x
10. Labriola A, Needleman I, Moles DR. Systematic review of the effect of smoking on nonsurgical periodontal therapy. Periodontology (2005) 37:124–137. doi: 10.1111/j.1600-0757.2004.03793.x
11. Chambrone L, Preshaw PM, Rosa EF, Heasman PA, Romito GA, Pannuti CM, et al. Effects of smoking cessation on the outcomes of non-surgical periodontal therapy: a systematic review and individual patient data meta-analysis. J Clin Periodontol. (2013) 40:607–15. doi: 10.1111/jcpe.12106
12. Chang J, Meng HW, Lalla E, Lee CT. The impact of smoking on non-surgical periodontal therapy: a systematic review and meta-analysis. J Clinic periodontol. (2020) 20:13384. doi: 10.1111/jcpe.13384
13. Mason MR, Preshaw PM, Nagaraja HN, Dabdoub SM, Rahman A, Kumar PS, et al. The subgingival microbiome of clinically healthy current and never smokers. ISME J. (2015) 9:268–72. doi: 10.1038/ismej.2014.114
14. Fullmer SC, Preshaw PM, Heasman PA, Kumar PS. Smoking cessation alters subgingival microbial recolonization. J Dent Res. (2009) 88:524–8. doi: 10.1177/0022034509338676
15. Delima SL, McBride RK, Preshaw PM, Heasman PA, Kumar PS. Response of subgingival bacteria to smoking cessation. J Clin Microbiol. (2010) 48:2344–9. doi: 10.1128/JCM.01821-09
16. Ramoa CP, Eissenberg T, Sahingur SE. Increasing popularity of waterpipe tobacco smoking and electronic cigarette use: implications for oral. Healthcare. (2017) 52:813–23. doi: 10.1111/jre.12458
17. Holliday RS, Campbell J, Preshaw PM. Effect of nicotine on human gingival, periodontal ligament and oral epithelial cells. a systematic review of the literature. J Dentistr. (2019) 19:30. doi: 10.1016/j.jdent.2019.05.030
18. Malhotra R, Kapoor A, Grover V, Kaushal S. Nicotine and periodontal tissues. J Indian Soc Periodontol. (2010) 14:72–9. doi: 10.4103/0972-124X.65442
19. Zanetti F, Titz B, Sewer A, Lo Sasso G, Scotti E, Schlage WK, et al. Comparative systems toxicology analysis of cigarette smoke and aerosol from a candidate modified risk tobacco product in organotypic human gingival epithelial cultures: a 3-day repeated exposure study. Food Chemic Toxicol. (2017) 101:15–35. doi: 10.1016/j.fct.2016.12.027
20. Zanetti F, Sewer A, Mathis C, Iskandar AR, Kostadinova R, Schlage WK, et al. Systems toxicology assessment of the biological impact of a candidate modified risk tobacco product on human organotypic oral epithelial cultures. Chem Res Toxicol. (2016) 29:1252–69. doi: 10.1021/acs.chemrestox.6b00174
21. Figueredo CA, Abdelhay N, Figueredo CM, Catunda R, Gibson MP. The impact of vaping on periodontitis: a systematic review. Clin Exp Dent Res. (2020) 20:360. doi: 10.1002/cre2.360
22. Pouly S, Ng WT, Benzimra M, Soulan A, Blanc N, Zanetti F, et al. Effect of switching to the Tobacco Heating System vs. continued cigarette smoking on chronic generalized periodontitis treatment outcome: protocol for a randomized controlled multicenter study. JMIR Res Protoc. (2021) 10:e15350. doi: 10.2196/15350
23. Japanese Society of Periodontology. Japanese Society of Periodontology Clinical Practice Guideline for the Periodontal Treatment. (2015). Available online at: http://www.perio.jp/publication/upload_file/guideline_perio_plan2015.pdf (accessed July 14, 2017).
24. Rosa EF, Corraini P, de Carvalho VF, Inoue G, Gomes EF, Lotufo JP, et al. A prospective 12-month study of the effect of smoking cessation on periodontal clinical parameters. J Clin Periodontol. (2011) 38:562–71. doi: 10.1111/j.1600-051X.2011.01723.x
25. Preshaw PM, Heasman L, Stacey F, Steen N, McCracken GI, Heasman PA, et al. The effect of quitting smoking on chronic periodontitis. J Clin Periodontol. (2005) 32:869–79. doi: 10.1111/j.1600-051X.2005.00779.x
26. Bjartveit K, Tverdal A. Health consequences of smoking 1-4 cigarettes per day. Tob Control. (2005) 14:315–20. doi: 10.1136/tc.2005.011932
27. Hackshaw A, Morris JK, Boniface S, Tang JL, Milenkovic D. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. Bmj. (2018) 360:j5855. doi: 10.1136/bmj.j5855
28. Rauniyar N. Parallel reaction monitoring: a targeted experiment performed using high resolution and high mass accuracy mass apectrometry. Int J Mol Sci. (2015) 16:28566–81. doi: 10.3390/ijms161226120
29. Truong DT, Franzosa EA, Tickle TL, Scholz M, Weingart G, Pasolli E, et al. MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat Methods. (2015) 12:902–3. doi: 10.1038/nmeth.3589
30. Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL. Microbial complexes in subgingival plaque. J Clinic Periodontol. (1998) 25:134–44. doi: 10.1111/j.1600-051X.1998.tb02419.x
31. Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol. (2018) 89(Suppl 1):S173–82. doi: 10.1002/jper.17-0721
32. Preber H, Bergstrom J. The effect of non-surgical treatment on periodontal pockets in smokers and non-smokers. J Clin Periodontol. (1986) 13:319–23. doi: 10.1111/j.1600-051X.1986.tb02229.x
33. Preber H, Linder L, Bergstrom J. Periodontal healing and periopathogenic microflora in smokers and non-smokers. J Clin Periodontol. (1995) 22:946–52. doi: 10.1111/j.1600-051X.1995.tb01800.x
34. Pucher JJ, Shibley O, Dentino AR, Ciancio SG. Results of limited initial periodontal therapy in smokers and non-smokers. J Periodontol. (1997) 68:851–6. doi: 10.1902/jop.1997.68.9.851
35. Palmer RM, Matthews JP, Wilson RF. Non-surgical periodontal treatment with and without adjunctive metronidazole in smokers and non-smokers. J Clin Periodontol. (1999) 26:158–63. doi: 10.1034/j.1600-051X.1999.260305.x
36. Ryder MI, Pons B, Adams D, Beiswanger B, Blanco V, Bogle G, et al. Effects of smoking on local delivery of controlled-release doxycycline as compared to scaling and root planing. J Clin Periodontol. (1999) 26:683–91. doi: 10.1034/j.1600-051X.1999.261008.x
37. Grossi SG, Zambon J, Machtei EE, Schifferle R, Andreana S, Genco RJ, et al. Effects of smoking and smoking cessation on healing after mechanical periodontal therapy. J Am Dent Assoc. (1997) 128:599–607. doi: 10.14219/jada.archive.1997.0259
38. Kanmaz B, Lappin DF, Nile CJ, Buduneli N. Effects of smoking on non-surgical periodontal therapy in patients with periodontitis stage III or IV, and grade C. J Periodontol. (2019) 19:141. doi: 10.1002/JPER.19-0141
39. Preus HR, Sandvik L, Gjermo P, Baelum V. Baseline adjustment and change revisited: effect of smoking on change in periodontal status following periodontal therapy. Eur J Oral Sci. (2014) 122:89–99. doi: 10.1111/eos.12111
40. Smiley CJ, Tracy SL, Abt E, Michalowicz BS, John MT, Gunsolley J, et al. Evidence-based clinical practice guideline on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J Am Dent Assoc. (2015) 146:525–35. doi: 10.1016/j.adaj.2015.01.026
41. Nair P, Sutherland G, Palmer RM, Wilson RF, Scott DA. Gingival bleeding on probing increases after quitting smoking. J Clin Periodontol. (2003) 30:435–7. doi: 10.1034/j.1600-051X.2003.20039.x
42. Morozumi T, Kubota T, Sato T, Okuda K, Yoshie H. Smoking cessation increases gingival blood flow and gingival crevicular fluid. J Clin Periodontol. (2004) 31:267–72. doi: 10.1111/j.1600-051X.2004.00476.x
43. Buduneli N, Scott DA. Tobacco-induced suppression of the vascular response to dental plaque. Mol Oral Microbiol. (2018) 33:271–82. doi: 10.1111/omi.12228
44. Lüdicke F, Picavet P, Baker G, Haziza C, Poux V, Lama N, et al. Effects of switching to the Menthol Tobacco Heating System 2.2, smoking abstinence, or continued cigarette smoking on clinically relevant risk markers: a randomized, controlled, open-label, multicenter study in sequential confinement and ambulatory settings (Part 2). Nicotine Tob Res. (2018) 20:173–182. doi: 10.1093/ntr/ntx028
45. Haziza C, de La Bourdonnaye G, Donelli A, Poux V, Skiada D, Weitkunat R, et al. Reduction in exposure to selected Harmful and Potentially Harmful Constituents approaching those observed upon smoking abstinence in smokers switching to the menthol Tobacco Heating System 2.2 for three months (part 1). Nicotine Tob Res. (2019) 22:539–548. doi: 10.1093/ntr/ntz013
46. Haziza C, de La Bourdonnaye G, Merlet S, Benzimra M, Ancerewicz J, Donelli A, et al. Assessment of the reduction in levels of exposure to harmful and potentially harmful constituents in Japanese subjects using a novel tobacco heating system compared with conventional cigarettes and smoking abstinence: a randomized controlled study in confinement. Regulat Toxicol Pharmacol. (2016) 81:489–99. doi: 10.1016/j.yrtph.2016.09.014
47. Haziza C, de La Bourdonnaye G, Skiada D, Ancerewicz J, Baker G, Picavet P, et al. Evaluation of the tobacco heating system 2.2. part 8,5.-day randomized reduced exposure clinical study in Poland. Regul Toxicol Pharmacol. (2016) 2:S139-50. doi: 10.1016/j.yrtph.2016.11.003
48. Tymkiw KD, Thunell DH, Johnson GK, Joly S, Burnell KK, Cavanaugh JE, et al. Influence of smoking on gingival crevicular fluid cytokines in severe chronic periodontitis. J Clin Periodontol. (2011) 38:219–28. doi: 10.1111/j.1600-051X.2010.01684.x
49. Goker M, Held B, Lucas S, Nolan M, Yasawong M, Glavina Del Rio T, et al. Complete genome sequence of Olsenella uli type strain (VPI D76D-27C). Stand Genomic Sci. (2010) 3:76–84. doi: 10.4056/sigs.1082860
50. Rafiei M, Kiani F, Sayehmiri F, Sayehmiri K, Sheikhi A, Zamanian Azodi M, et al. Study of Porphyromonas gingivalis in periodontal diseases: A systematic review and meta-analysis. Med J Islam Repub Iran. (2017) 31:62. doi: 10.14196/mjiri.31.62
51. Siqueira JF. Rocas IN, Alves FR, Silva MG. Bacteria in the apical root canal of teeth with primary apical periodontitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2009) 107:721–6. doi: 10.1016/j.tripleo.2009.01.042
52. Leroy R, Eaton KA, Savage A. Methodological issues in epidemiological studies of periodontitis—how can it be improved? BMC Oral Health. (2010) 10:8. doi: 10.1186/1472-6831-10-8
53. Benowitz NL, Bernert JT, Foulds J, Hecht SS, Jacob P, Jarvis MJ, et al. Biochemical verification of tobacco use and abstinence: 2019 update. Nicotine Tob Res. (2020) 22:1086–97. doi: 10.1093/ntr/ntz132
54. Scheuermann TS, Richter KP, Rigotti NA, Cummins SE, Harrington KF, Sherman SE, et al. Accuracy of self-reported smoking abstinence in clinical trials of hospital-initiated smoking interventions. Addiction. (2017) 112:2227–36. doi: 10.1111/add.13913
Keywords: smoking, heated tobacco product, periodontitis, oral health, root planing, scaling
Citation: Pouly S, Ng WT, Blanc N, Hession P, Zanetti F, Battey JND, de La Bourdonnaye G, Heremans A and Haziza C (2022) Effect of switching from cigarette smoking to the use of the tobacco heating system on periodontitis treatment outcome: Periodontal parameter results from a multicenter Japanese study. Front. Dent. Med. 3:915079. doi: 10.3389/fdmed.2022.915079
Received: 07 April 2022; Accepted: 28 June 2022;
Published: 22 July 2022.
Edited by:
Gaetano Isola, University of Catania, ItalyReviewed by:
Egle Ramanauskaite, Lithuanian University of Health Sciences, LithuaniaCássia Araujo, São Paulo State University, Brazil
Fouzia Tarannum, Mathrusri Ramabai Ambedkar Dental College and Hospital, India
Copyright © 2022 Pouly, Ng, Blanc, Hession, Zanetti, Battey, de La Bourdonnaye, Heremans and Haziza. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sandrine Pouly, c2FuZHJpbmUucG91bHlAcG1pLmNvbQ==
 Sandrine Pouly
Sandrine Pouly Wee Teck Ng
Wee Teck Ng Paul Hession
Paul Hession Filippo Zanetti
Filippo Zanetti James N. D. Battey
James N. D. Battey Guillaume de La Bourdonnaye
Guillaume de La Bourdonnaye Christelle Haziza
Christelle Haziza