- School of Dentistry, Section of Periodontics, University of California, Los Angeles, Los Angeles, CA, United States
Systems biology is a promising scientific discipline that allows an integrated investigation of host factors, microbial composition, biomarkers, immune response and inflammatory mediators in many conditions such as chronic diseases, cancer, neurological disorders, and periodontitis. This concept utilizes genetic decoding, bioinformatic, flux-balance analysis in a comprehensive approach. The aim of this review is to better understand the current literature on systems biology and identify a clear applicability of it to periodontitis. We will mostly focus on the association between this condition and topics such as genomics, transcriptomics, proteomics, metabolomics, as well as contextualize delivery systems for periodontitis treatment, biomarker detection in oral fluids and associated systemic conditions.
Introduction
Systems biology is a promising scientific discipline that aims at understanding the biological organisms and the multilevel interconnections between their different cell constituents (1, 2). It utilizes different quantitative, experimental and computational methods to decode genetic information, protein activities and signaling pathways in the cells, tissues, and organisms (3–5).
An important applicability of this field is the tracking of molecular changes that occur in pathological events (6) such as periodontitis. Periodontitis is a chronic multifactorial inflammatory disease associated with dysbiotic plaque biofilms and characterized by progressive destruction of the tooth-supporting apparatus (7).
In order understand the pathogenesis of periodontitis, studies have reported isolated biological regulatory mechanisms instead of utilizing integrated systems (8–12). Biological systems study models allow for the integrated investigation of local host factors, microbial composition, in periodontal tissue as well as systemic immune response (13). It better replicates biology of the biofilm-gingival interface in specific patients and provides insight into their clinical management (6, 14).
Unfortunately, this approach is in its infancy, and there is limited data available, especially as it relates to periodontitis. In this review, we will mostly focus on systems biology as an emerging approach to periodontal studies, with the potential of bringing accurate diagnosis and treatment closer to a translational reality, as well as creating a scientific basis for future studies to elucidate and enrich this thematic.
Key Principles of Systems Biology
Systems biology is a new discipline that studies the molecular diversity of living systems, by identifying principles and patterns and integrating them through complex models of regulatory mechanisms (15). It involves computational analysis, mathematics, and physical concepts (1, 2). Furthermore, it focuses on complex interactions within biological systems, using a holistic approach to conducting modern research (14).
Despite being a new field and integrating biological systems in its entirety, systems biology can also be analyzed from the perspective of traditional scientific methodology including test-based hypothesis (3). When we think about the differences between healthy conditions and illnesses, it is certainly expected that there are different protein expressions in these seconds due to the disturbances suffered at the genetic level. This is what systems biology is based on when addressing inflammatory processes and diseases in general (16). The investigation encompasses genomics, transcriptomics, proteomics, metabolomics with the goal of data integration. In this review we will discuss each one in detail with the objective of intertwining all of them.
The investigation of candidate molecular biomarkers for the diagnosis and prognosis of inflammatory diseases through bioinformatics analysis of gene expression datasets is an example of systems biology application (11). Specifically, regarding oral diseases, in the last decade many studies have been using systems biology in their searches, although this knowledge field is not completely explicit (17–21). Furthermore, recent advances in sequencing technologies allow researchers to profile disease-associated microbial communities, quantify microbial metabolic activities and host transcriptional responses, and correlate to oral diseases such as periodontitis (22–25). The potential limitations in systems biology refer precisely to the scarce literature and the diversity of imprecise definitions. Classic terms such as “a new type of biology” or “the successor to molecular biology” cannot fully explain this new discipline that aims to integrate biology and technology from different points of view (1, 3). These limitations could be overcome by an extensive and continuous discussion and, even more, by a formal meeting between biologists and scientists from different areas with the aim of standardizing concepts, classifications and objectives of this complex knowledge.
Systems Biology Concepts in Periodontitis
Systemic Decoding of Periodontal Inflammation
According to the current model of periodontitis pathogenesis, the complex interactions between plaque bacteria, host genetic factors and acquired environmental stressors must be considered (25, 26). Although bacteria present in the plaque biofilm initiate inflammation, intrinsic host factors and environmental stressors modulate the magnitude, duration, and extent of an inflammatory response (27, 28). Understanding the molecular mechanisms underlying the pathogenesis as well as the development of efficient therapeutics is even more important since periodontitis is linked to other metabolic and/or systemic diseases including diabetes, cardiovascular diseases, and rheumatoid arthritis (23, 29–31). Currently, many studies have evaluated genomics, proteomics and metabolomics in an isolated and non-integrated manner, therefore, combining all these approaches in a systematic way will allow us to better understand and treat the disease.
Genomics in Periodontitis
The identification of the causal variant(s) that increase the susceptibility/resistance to periodontitis can be done by Genome-wide Associated Studies (GWAS). In fact, GWAS has provided insight into novel loci and biological processes plausibly implicated in this complex biofilm-dependent disease (31–38) through human and animal studies (17, 39, 40). Other studies have also discussed the current knowledge on genomics considering inflammatory cytokines and polymorphisms related to periodontitis (35, 36, 41, 42).
The meta-analysis by Munz et al. (43), that included 16 studies comprising 5,095 cases and 9,908 controls, identified novel risk loci of periodontitis. The same group performed a GWAS meta-analysis on coronary artery disease and periodontitis. The study revealed that the molecular pathway shared by these two conditions involves a novel risk locus (VAMP8) (44).
The use of GWAS to understand periodontitis has suggested that there are many potential contributors to periodontitis, reinforced that some genes should be further investigated and validated the importance of many genes that we already know are relevant to periodontitis. Moving forward the integration of data obtained from GWAS in patients and in animal models with the microbiome and proteome will allow a broader view in the mechanisms underlying periodontitis and may serve as a foundation for a more personalized treatment approach (17, 40, 45).
Transcriptomics in Periodontitis
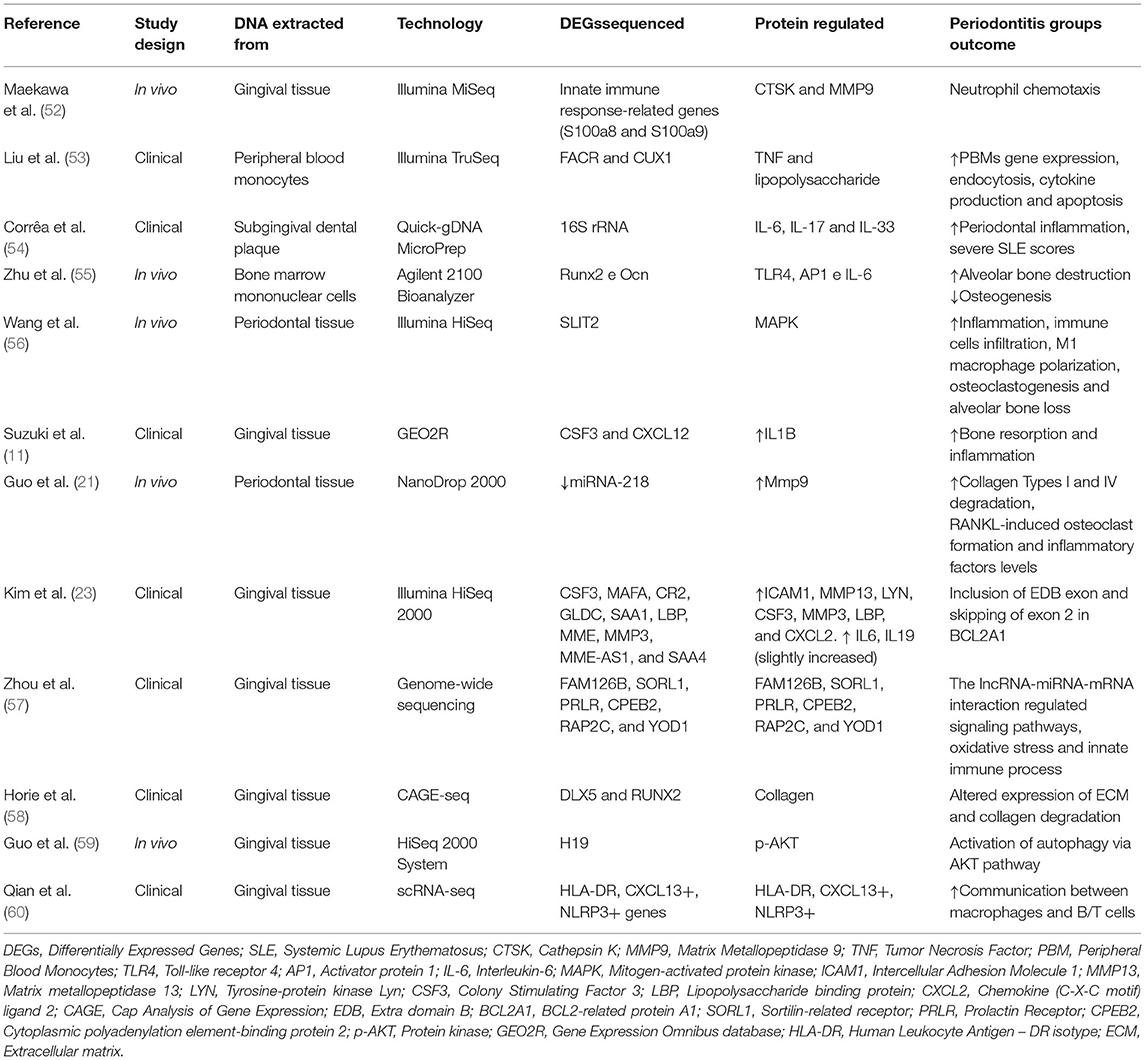
Transcriptomics refers to the complete set of gene transcripts or RNA transcribed in a given cell type, tissue or organism for a specific physiological or pathological condition (46). It studies and interprets the key functional output of the genome, comparing cells or tissues under defined conditions or disease states to identify changes in gene expression (47–49). Transcriptomics can efficiently narrow down candidate genes associated with multifactorial diseases, assist in the investigation of underlying mechanisms of diseases and the identification of biomarkers for diagnosis and prognosis (6, 11, 50, 51). Studies evaluating the transcriptome of periodontitis have been performed human and in animal models (Table 1).
When evaluating the transcriptome, clinical studies have observed increased periodontal inflammation due to increased oxidative stress, innate immune response regulation (57), and collagen degradation (18, 23, 58, 60). For instance, Suzuki et al. (11) investigated a candidate molecular biomarker for diagnosis and prognosis of periodontitis through bioinformatic analysis of pooled microarray gene expression datasets in Gene Expression Omnibus (GEO). The study observed that IL-1β is one of the upstream regulators of CSF3 and CXCL12, both up-regulated and related to the inflammation process and bone loss in periodontitis (Table 1) (11).
A clinical study performed RNA sequencing (RNA-seq) of peripheral blood monocytes (PBMs) in periodontitis cases and identified 380 genes transcribed from differentially expressed isoforms (DEx) and suggested a more functionally active monocyte transcriptome in periodontitis patients compared to healthy individuals. Furthermore, several of the genes identified as associated with periodontitis are known for interacting with invading microorganisms (53).
In murine models of periodontitis, transcriptomics have associated: (a) proteins present in bone marrow cells with increased alveolar bone destruction and decreased osteogenesis (55); (b) MAPK with increased inflammation, M1 macrophage polarization, osteoclastogenesis (56) and (c) Mmp9 with collagen type I and IV degradation, besides RANKL-induced osteoclast formation, both in the DNA extracted from periodontal tissue (21).
For instance, in trying to identify the relevant differentially expressed genes and clarify the mechanism underlying alveolar bone loss using ligature-induced periodontitis in mice, Maekawa et al. (52) performed an enrichment analysis of gene ontology terms. This study revealed that neutrophil chemotaxis and inflammatory responses were significantly enriched in the gingival tissues around teeth where periodontitis was initiated through a ligature model (52).
A study aiming to obtain insights into periodontitis etiology combined GWAS and transcriptomic data from mouse and humans. This study was able to further identify two sets of periodontitis (chronic and aggressive) (61).
More recently, the literature has shown advanced technologies to perform single-cell RNA sequencing (scRNA-seq) in terms of detection of periodontitis and its specific changes. While Takada et al. (62) identified that the Mohawk homeobox transcription factor (Mkx) expressed in the periodontal ligament of Wistar rats was involved in ankylosis and periodontitis, Chen et al. (63) investigated the osteoimmunological microenvironment in the periodontitis development by scRNA-seq and identified more than 50 thousand cells isolated from healthy patients, individuals with severe chronic periodontitis, and others with severe chronic periodontitis. Among other findings, enrichment of TNFRSF21+ fibroblasts with high expression of CXCL13 was detected in patients with periodontitis compared to healthy subjects.
In addition to these authors, Agrafioti et al. (64) also used single-cell RNA-sequencing and decoded the role of macrophages in periodontitis and type 2 diabetes. They observed high expression of a subunit of NF-κB called RELA in gingival macrophages of patients with periodontitis with diabetes.
Transcriptomics has advanced our field and is necessary to help understand periodontitis pathogenesis and to facilitate the development of more precise biomarkers for disease prevention, diagnosis and prognosis. The current task is to combine and utilize the resulting data sets to benefit patient care (65). Additional related studies and their applied technology can be seen in Table 1.
Proteomics in Periodontitis
Proteomics is the study of the entire set of proteins expressed by a cell, tissue or a organism at a particular state/time (66–68). It supplements the other “omics” technologies such as genomic and transcriptomics (69). In recent years, several studies have discussed the use of proteomics in periodontitis (65, 70–73).
Many animal studies have performed proteomics analysis of periodontitis (74–78). For instance, it has identified interleukin 4 (IL-4) as having protective role in bone destruction due to its anti-osteoclastogenic action (77).
Human studies evaluating periodontitis through proteomics have identified many proteins as being different in health and diseases (79–81). For instance, Bostanci el al. evaluating gingival crevicular fluid identified that one hundred and nineteen proteins are different between health patients and disease subjects (82). Mertens et al. evaluating salivary samples identified hemopexin, plasminogen and α-fibrinogen in the saliva of patients with periodontitis compared with healthy subjects (81). A recent case-control study analyzed the salivary proteome of individuals with chronic periodontitis and observed that there is an increase in acidic proline-rich phosphoprotein, submaxillary gland androgen-regulated protein, histatin, fatty acid binding protein, thioredoxin and cystatin in patients with periodontitis (79).
The growing use of proteomic techniques has allowed our field to better understand the pathogenesis and biomarkers identification for diagnosis and prognosis.
Metabolomic Approaches to Periodontitis
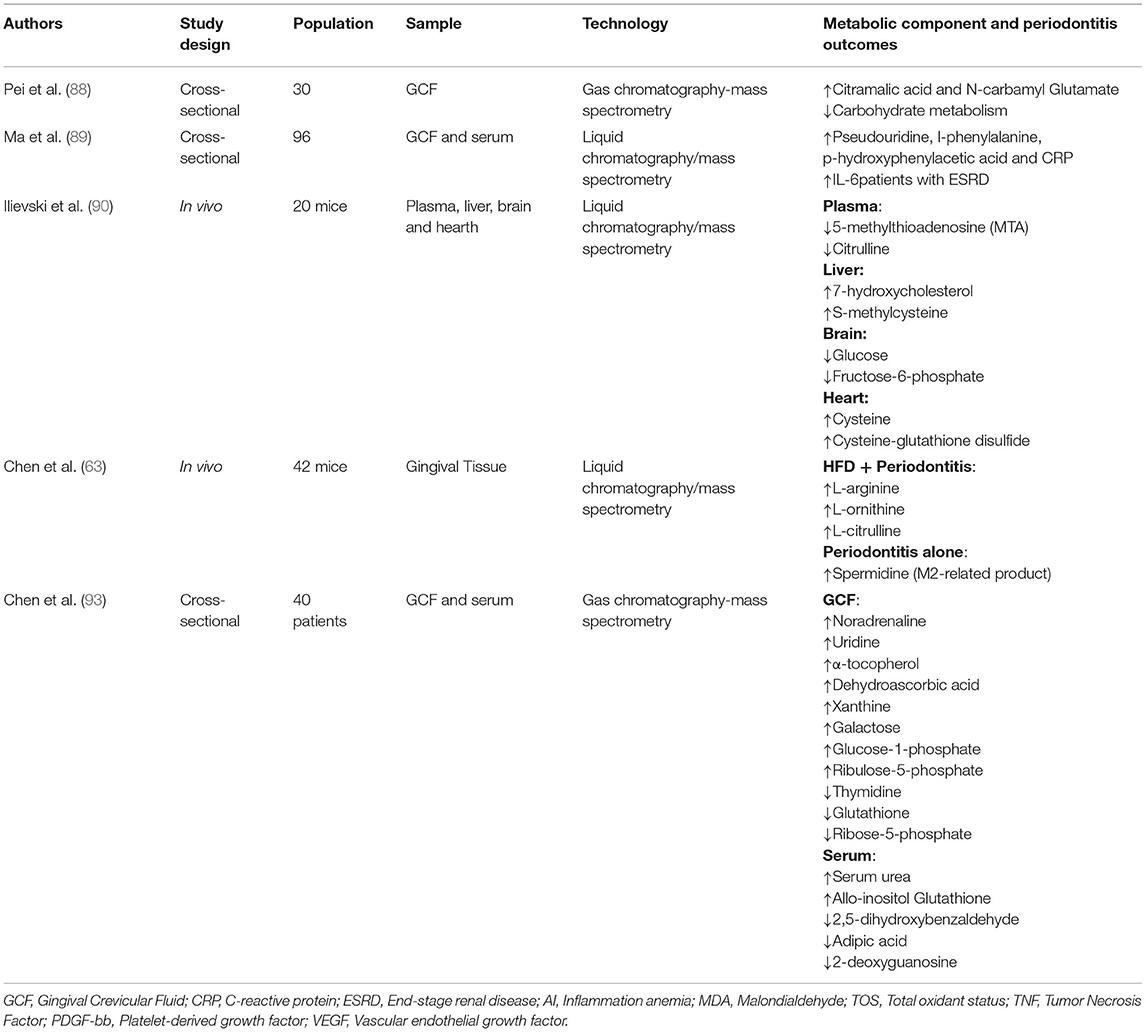
Periodontal inflammation triggers biological reactions that can consequently be expressed in metabolic changes (80, 83). The most common way of analyzing a metabolic network is using flux-balance analysis, which involves calculating the flow of metabolites through the network in steady state (84–86). In recent years, the metabolic networks of whole organisms have become available and, for instance, the metabolism of intestinal microbial communities has been reconstructed (87). In oral biology, despite extensive studies published on the variation in the oral microbiota and metabolic profiles of patients with periodontitis, information is still lacking regarding the correlation between host-bacterial interactions and biochemical metabolism (88).
Thus far, there have been several longitudinal, cross-sectional and animal studies evaluating the relationship between periodontitis and metabolic components behavior (63, 88–90). When periodontal tissue is in an inflammatory state, inflammatory factors such as cytokines, bacterial antigens, various cells, metabolites, and other degradation products are released in the gingival crevicular fluid (GCF) (89, 91, 92). By analyzing the oral microbiome and oral metabolome in periodontitis, in addition to identifying biomarker molecules, a recent cross-sectional study detected functional changes in basic metabolism including vitamins, energy and cofactor (88).
Another cross-sectional study analyzed metabolic profiles in individuals with aggressive periodontitis and identified, among other differences, an increase in dehydroascorbic acid and a decrease in thymidine in gingival crevicular fluid when compared to healthy individuals (93).
Marchesan et al. (94) performed an analysis of microbiome microarray data and metabolite data from saliva and assessed the relationships among plaque microbial composition, salivary bacterial metabolites and periodontitis phenotypes in a well-established stent-induced biofilm overgrowth clinical model. Several newly identified putative periodontal pathogenic species in the Synergizes and Treponema phyla were significantly associated with periodontitis parameters (94). The differential detection of biomarker metabolites in the GCF, in addition to being an excellent diagnostic tool, can also be an important strategy in the treatment of periodontitis (88).
In addition to human studies, in vivo studies have also applied methodologies based on metabolomic approaches. For example, an interesting animal study determined the metabolic effects of periodontitis beyond the mouth such as decreased plasma lysolipid metabolites, increased liver bile acid synthesis, decreased brain glucose and increased cysteine and changes in redox homeostasis in the heart (90).
Applying a similar method, another animal study validated the association between high fat diet (HFD) and periodontitis in arginine metabolism (related to M1/M2 macrophage phenotypes) and observed that HFD was able to increase L-arginine levels. Periodontitis alone showed enhanced spermidine, a product related to M2 macrophages (63). Additional related studies can be seen in Table 2.
Optimizing Drug Delivery Systems for Periodontitis Treatment
Classic conventional therapy for periodontitis treatment, including mechanical plaque removal, is sometimes unable to eliminate bacterial debris in hard-to-reach areas. The literature is rich in studies that defend and highlight the role of complementary therapies whose proposal is precisely to assist in this bacterial control or immune-regulation, including chemical drugs, probiotics, photodynamic therapy, and systemic antibiotics (95–99).
In terms of technological innovations and broader approaches focused on systems biology, advanced drug delivery strategies using biodegradable nanocarriers have been proposed to avoid problems such as toxicity and antibiotic resistance in periodontitis treatment (100). Among them, a recent study developed computer simulations using an in situ localized nanogel drug delivery system for periodontitis and noted that the formulated nanoemulgel exhibited a remarkable release of 92.4% of quercetin at the end of 6 h, as compared to that of pure quercetin-loaded gel (<3% release) (101). The versatility of distinct nanocarriers allowing for improvement of their loading and releasing capabilities could be used for microbiological control, periodontal regeneration, and/or immunomodulation (100).
Systems biology approaches optimizing drug delivery have been described by several authors will most likely emerge as alternatives to the classical treatment of periodontitis (102–107).
Utilization of Oral Fluids for the Detection of Biomarkers and Microbiome
Oral fluids may offer the basis for patient-specific diagnostic tests for periodontitis because it can be easily collected and contains local and systemic-derived biomarkers. Among them, there is the GCF which consists of an inflammatory exudate originating from the gingival plexus of blood vessels in the gingival corium, subjacent to the epithelium lining of the dentogingival space (91). In clinical health, the periodontium secretes an inflammatory infiltrate within the crevicular sulcus (19, 45, 108, 109). In pathological situations, such as periodontitis, as the disease progresses, the GCF volume increases and inflammatory mediators including cytokines, arachidonic acid metabolites and enzymes are upregulated (110).
An ever-expanding pool of GCF proteins associated with periodontal health or disease has been cataloged over the years, particularly with the recent implementation of proteomic technologies which provide a broad qualitative and quantitative insight of the proteins present in gingival crevicular fluid (82).
In a recent study, Kim et al. performed proteomic analysis of GCF and identified galectin-10 as a biomarker for periodontitis (111). Batschkus et al., in turn, aiming to identify and quantify proteins obtained from CGF of patients with periodontitis, observed three hundred and seventeen different proteins trrough by SDS-PAGE and high-resolution mass spectrometry (108).
A similar study, published by Marinho et al. compared the relative abundance of 104 proteins in the GCF from individuals with type 2 diabetes mellitus with and without periodontitis. In patients with diabetes there was an increase in titin, neutrophil elastase and myeloperoxidase while cathelicidin antimicrobial peptide decreased in periodontitis cases and annexin decreased in healthy patients (112).
The use of salivary biomarkers has been heavily employed in recent years, including in omics approaches because it can be easily collected and it has low cost (113). For instance, specific host- or bacteria-derived biomarkers detected in saliva could indicate the presence or progression/remission of periodontitis (70, 79, 114, 115). Other recent studies made discoveries in the proteomics field and saliva diagnosis, including multiplexing platforms development (116) and rapid-test-kit with Shotgun proteomics (73).
Systemic Conditions, Periodontitis and Systems Biology
Systemic diseases with increased inflammation are frequently linked to increased risk of periodontitis (117, 118). Some of the systemic conditions linked to periodontitis described below are diabetes mellitus, cardiovascular diseases, metabolic syndrome and systemic lupus erythematosus.
Diabetes mellitus, a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both (119), shares a bidirectional relationship with periodontitis (120). Because of that, there have been many studies that addressed such conditions simultaneously under different clinical and biological perspectives and with different study designs (121–125). As an example, sequencing 16S rDNA in subgingival dental plaque from patients with type 2 diabetes allowed the authors to quantify 110 metabolites and 415 lipids, but the most important findings were that the cross-omics correlation analysis revealed a novel microbial metabolic pathway and significant associations of host-derived proteins with periodontitis (126).
Other systemic conditions associated with periodontitis are cardiovascular diseases, whose incidence, especially coronary artery disease and atherosclerosis, have been increasing alarmingly (127–129). Atherosclerosis and periodontitis utilize common inflammatory signaling pathways and in addition, bacteria associated with periodontitis have been identified in atherosclerotic plaque specimens (92, 130–132). A review by Mirnejad et al. focused on the most important bacterial species involved in cardiovascular diseases and periodontitis presented recent findings about the proteomic evaluation of virulence factors of these bacteria (132). Another review by Pietiäinen et al. (133) summarized possible molecular mediators between the dysbiotic oral microbiota and atherosclerotic processes, and, among others, included a study that performed Pyrosequencing of bacterial 16S rRNA genes in oral swabs which did not reveal significant differences between patients with atherosclerosis and controls (133, 134).
System biology studies have also been able to link metabolic syndrome, a group of conditions defined by the presence of obesity, dyslipidemia, hypertension, and deglycation leading to an increased risk of diabetes and cardiovascular disease (118) to periodontitis (135, 136). An association with systemic lupus erythematosus (SLE) and periodontal disease has also been observed by sequencing the V4 region of the 16S gene (54).
Studies focusing on how periodontal inflammation may affect systemic conditions are necessary, not only to investigate the mechanisms underlying this inflammatory process, but also to establish therapeutic strategies (137). Applying the concepts of system biology in these investigations is as important as the need to clarify this new discipline and integrate it with classical disciplines such as periodontal medicine and systemic diseases.
Some possible future directions for systems biology in periodontitis may include computer-aided design software development and finite element analysis for inflammatory assessment, bone loss, and bone regeneration, as well as innovative computed radiology and ultrasound for tissue regeneration analysis.
In the clinical field of implantology there is a remarkable applicability of new technologies, including computed radiology and resonance frequency analysis (RFA) of osseointegration around implants. An example of this is the Osstell® device, which consists of a small piezoelectric transducer in which the RFA is automatically converted into an index that informs the Implant Stability Coefficient via software (138).
Conclusions
This review analyzed different studies in periodontology that have applied partial systems biology concepts in their thematic and methodological approaches. Even though, it is an emerging area, it is strongly intertwined with what has been studied for years in terms of periodontitis diagnosis and treatment. Going from traditional biological approaches to a systemic investigation model, adding OMICS technology, has already taken important steps. Although translating the OMICS data into clinical approaches is challenging, the oral microbiome plays a key role for different systemic or oral conditions such as periodontitis. Therefore, profiling subgingival bacterial communities by omics methods is a great tool for early detection of periodontitis biomarkers. It is suggested that new studies need to be developed not only to enrich this topic, but also to better investigate periodontitis and other oral conditions.
Author Contributions
DS and SM conducted bibliographic research and manuscript writing. FP contributed to the writing and guided the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) for supporting this manuscript.
References
1. Boogerd FC, Bruggeman FJ, Hofmeyr JHS, Westerhoff HV. Systems Biology: Philosophical Foundations. Elsevier (2007). p. 342.
2. Ahn AC, Tewari M, Poon CS, Phillips RS. The limits of reductionism in medicine: could systems biology offer an alternative? PLoS Med. (2006) 3:e208. doi: 10.1371/journal.pmed.0030208
4. Koide T, Pang WL, Baliga NS. The role of predictive modelling in rationally re-engineering biological systems. Nat Rev Microbiol. (2009) 7:297–305. doi: 10.1038/nrmicro2107
5. Tavassoly I, Goldfarb J, Iyengar R. Systems biology primer: the basic methods and approaches. Essays Biochem. (2018) 62:487–500. doi: 10.1042/EBC20180003
6. Zeidán-Chuliá F, Gürsoy M, Neves de Oliveira BH, Özdemir V, Könönen E, et al. A systems biology approach to reveal putative host-derived biomarkers of periodontitis by network topology characterization of MMP-REDOX/NO and apoptosis integrated pathways. Front Cell Infect Microbiol. (2016) 5:102. doi: 10.3389/fcimb.2015.00102
7. Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol. (2018) 89:S173–82. doi: 10.1002/JPER.17-0721
8. Alvarez C, Suliman S, Almarhoumi R, Vega ME, Rojas C, Monasterio G, et al. Regulatory T cell phenotype and anti-osteoclastogenic function in experimental periodontitis. Sci Rep. (2020) 10:19018. doi: 10.1038/s41598-020-76038-w
9. Qi W, Yang X, Ye N, Li S, Han Q, Huang J et al. TLR4 gene in the regulation of periodontitis and its molecular mechanism. Exp Ther Med. (2019) 18:1961–6. doi: 10.3892/etm.2019.7809
10. Shi T, Jin Y, Miao Y, Wang Y, Zhou Y, Lin X. IL-10 secreting B cells regulate periodontal immune response during periodontitis. Odontology. (2020) 108:350–7. doi: 10.1007/s10266-019-00470-2
11. Suzuki A, Horie T, Numabe Y. Investigation of molecular biomarker candidates for diagnosis and prognosis of chronic periodontitis by bioinformatics analysis of pooled microarray gene expression datasets in Gene Expression Omnibus (GEO). BMC Oral Health. (2019) 19:52. doi: 10.1186/s12903-019-0738-0
12. Xu S, Jiang C, Liu H, Zhang H, Liao H, Wang X, et al. Integrin-α9 and its corresponding ligands play regulatory roles in chronic periodontitis. Inflammation. (2020) 43:1488–97. doi: 10.1007/s10753-020-01226-9
13. Kornman KS. Mapping the pathogenesis of periodontitis: a new look. J Periodontol. (2008) 79:1560–8. doi: 10.1902/jop.2008.080213
14. Adeola HA, Papagerakis S, Papagerakis P. Systems biology approaches and precision oral health: a circadian clock perspective. Front Physiol. (2019) 10:399. doi: 10.3389/fphys.2019.00399
15. Kim JT, Eils R. Systems biology and artificial life: towards predictive modeling of biological systems. Artif Life. (2008) 14:1–2. doi: 10.1162/artl.2008.14.1.1
16. Hood L, Heath JR, Phelps ME, Lin B. Systems biology and new technologies enable predictive and preventative medicine. Science. (2004) 306:640–3. doi: 10.1126/science.1104635
17. Hiyari S, Green E, Pan C, Lari S, Davar M, Davis R, et al. Genomewide association study identifies Cxcl family members as partial mediators of LPS-induced periodontitis. J Bone Miner Res. (2018) 33:1450–63. doi: 10.1002/jbmr.3440
18. Ahn SH, Chun S, Park C, Lee JH, Lee SW, Lee TH. Transcriptome profiling analysis of senescent gingival fibroblasts in response to Fusobacterium nucleatum infection. PLoS ONE. (2017) 12:e0188755. doi: 10.1371/journal.pone.0188755
19. Carneiro LG, Venuleo C, Oppenheim FG, Salih E. Proteome data set of human gingival crevicular fluid from healthy periodontium sites by multidimensional protein separation and mass spectrometry. J Periodontal Res. (2012) 47:248–62. doi: 10.1111/j.1600-0765.2011.01429.x
20. Coêlho MC, Queiroz IC, Viana Filho JMC, Aquino SG, Persuhn DC, Oliveira NFP. miR-9-1 gene methylation and DNMT3B (rs2424913) polymorphism may contribute to periodontitis. J Appl Oral Sci. (2020) 28:e20190583. doi: 10.1590/1678-7757-2019-0583
21. Guo J, Zeng X, Miao J, Liu C, Wei F, Liu D, et al. MiRNA-218 regulates osteoclast differentiation and inflammation response in periodontitis rats through Mmp9. Cell Microbiol. (2019) 21:e12979. doi: 10.1111/cmi.12979
22. Kebschull M, Demmer RT, Grün B, Guarnieri P, Pavlidis P, Papapanou PN. Gingival tissue transcriptomes identify distinct periodontitis phenotypes. J Dent Res. (2014) 93:459–68. doi: 10.1177/0022034514527288
23. Kim YG, Kim M, Kang JH, Kim HJ, Park JW, Lee JM, et al. Transcriptome sequencing of gingival biopsies from chronic periodontitis patients reveals novel gene expression and splicing patterns. Hum Genomics. (2016) 10:28. doi: 10.1186/s40246-016-0084-0
24. Sawle AD, Kebschull M, Demmer RT, Papapanou PN. Identification of master regulator genes in human periodontitis. J Dent Res. (2016) 95:1010–7. doi: 10.1177/0022034516653588
25. Zhang S, Yu N, Arce RM. Periodontal inflammation: Integrating genes and dysbiosis. Periodontol. (2020) 82:129–142. doi: 10.1111/prd.12267
26. Golub LM, Lee HM. Periodontal therapeutics: Current host-modulation agents and future directions. Periodontol. (2020) 82:186–204. doi: 10.1111/prd.12315
27. Löe H, Anerud A, Boysen H, Morrison E. Natural history of periodontal disease in man. Rapid, moderate and no loss of attachment in Sri Lankan laborers 14 to 46 years of age. J Clin Periodontol. (1986) 13:431–45. doi: 10.1111/j.1600-051X.1986.tb01487.x
28. Löe H, Anerud A, Boysen H, Smith M. The natural history of periodontal disease in man. The rate of periodontal destruction before 40 years of age. J Periodontol. (1978) 49:607–20. doi: 10.1902/jop.1978.49.12.607
29. Bullon P, Morillo JM, Ramirez-Tortosa MC, Quiles JL, Newman HN, Battino M. Metabolic syndrome and periodontitis: is oxidative stress a common link? J Dent Res. (2009) 88:503–18. doi: 10.1177/0022034509337479
30. Linden GJ, Lyons A, Scannapieco FA. Periodontal systemic associations: review of the evidence. J Periodontol. (2013) 84:S8–S19. doi: 10.1902/jop.2013.1340010
31. Bae SC, Lee YH. Causal association between periodontitis and risk of rheumatoid arthritis and systemic lupus erythematosus: a Mendelian randomization. Z Rheumatol. (2020) 79:929–36. doi: 10.1007/s00393-019-00742-w
32. Cirelli T, Nepomuceno R, Orrico SRP, Rossa C Jr, Cirelli JA, North KE, et al. Validation in a Brazilian population of gene markers of periodontitis previously investigated by GWAS and bioinformatic studies. J Periodontol. (2021) 92:689–703. doi: 10.1002/JPER.20-0126
33. Czesnikiewicz-Guzik M, Osmenda G, Siedlinski M, Nosalski R, Pelka P, Nowakowski D, et al. Causal association between periodontitis and hypertension: evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur Heart J. (2019) 40:3459–70. doi: 10.1093/eurheartj/ehz646
34. Sun YQ, Richmond RC, Chen Y, Mai XM. Mixed evidence for the relationship between periodontitis and Alzheimer's disease: a bidirectional Mendelian randomization study. PLoS ONE. (2020) 15:e0228206. doi: 10.1371/journal.pone.0228206
35. Offenbacher S, Jiao Y, Kim SJ, Marchesan J, Moss KL, Jing L, et al. GWAS for Interleukin-1β levels in gingival crevicular fluid identifies IL37 variants in periodontal inflammation. Nat Commun. (2018) 9:3686. doi: 10.1038/s41467-018-05940-9
36. Offenbacher S, Divaris K, Barros SP, Moss KL, Marchesan JT, Morelli T, et al. Genome-wide association study of biologically informed periodontal complex traits offers novel insights into the genetic basis of periodontal disease. Hum Mol Genet. (2016) 25:2113–29. doi: 10.1093/hmg/ddw069
37. Divaris K, Monda KL, North KE, Olshan AF, Reynolds LM, Hsueh WC, et al. Exploring the genetic basis of chronic periodontitis: a genome-wide association study. Hum Mol Genet. (2013) 22:2312–24. doi: 10.1093/hmg/ddt065
38. Agler CS, Shungin D, Ferreira Zandoná AG, Schmadeke P, Basta PV, Luo J, et al. Protocols, methods, and tools for genome-wide association studies (GWAS) of dental traits. Methods Mol Biol. (2019) 1922:493–509. doi: 10.1007/978-1-4939-9012-2_38
39. Nashef A, Qabaja R, Salaymeh Y, Botzman M, Munz M, Dommisch H, et al. Integration of murine and human studies for mapping periodontitis susceptibility. J Dent Res. (2018) 97:537–46. doi: 10.1177/0022034517744189
40. Shusterman A, Munz M, Richter G, Jepsen S, Lieb W, Krone B, et al. The PF4/PPBP/CXCL5 gene cluster is associated with periodontitis. J Dent Res. (2017) 96:945–52. doi: 10.1177/0022034517706311
41. Yu N, Zhang J, Phillips ST, Offenbacher S, Zhang S. Impaired function of epithelial plakophilin-2 is associated with periodontal disease. J Periodontal Res. (2021) 56:1046–57. doi: 10.1111/jre.12918
42. Marchesan JT, Jiao Y, Moss K, Divaris K, Seaman W, Webster-Cyriaque J, et al. Common polymorphisms in IFI16 and AIM2 genes are associated with periodontal disease. J Periodontol. (2017) 88:663–72. doi: 10.1902/jop.2017.160553
43. Munz M, Richter GM, Loos BG, Jepsen S, Divaris K, Offenbacher S, et al. Meta-analysis of genome-wide association studies of aggressive and chronic periodontitis identifies two novel risk loci. Eur J Hum Genet. (2019) 27:102–13. doi: 10.1038/s41431-018-0265-5
44. Munz M, Richter GM, Loos BG, Jepsen S, Divaris K, Offenbacher S, et al. Genome-wide association meta-analysis of coronary artery disease and periodontitis reveals a novel shared risk locus. Sci Rep. (2018) 8:13678. doi: 10.1038/s41598-018-31980-8
45. Rody WJ Jr, Holliday LS, McHugh KP, Wallet SM, Spicer V, Krokhin O. Mass spectrometry analysis of gingival crevicular fluid in the presence of external root resorption. Am J Orthod Dentofacial Orthop. (2014) 145:787–98. doi: 10.1016/j.ajodo.2014.03.013
46. Piétu G, Alibert O, Guichard V, Lamy B, Bois F, Leroy E, et al. Novel gene transcripts preferentially expressed in human muscles revealed by quantitative hybridization of a high density cDNA array. Genome Res. (1996) 6:492–503. doi: 10.1101/gr.6.6.492
47. Chambers DC, Carew AM, Lukowski SW, Powell JE. Transcriptomics and single-cell RNA-sequencing. Respirology. (2019) 24:29–36. doi: 10.1111/resp.13412
48. Kan M, Shumyatcher M, Himes BE. Using omics approaches to understand pulmonary diseases. Respir Res. (2017) 18:149. doi: 10.1186/s12931-017-0631-9
49. Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. (2009) 10:57–63. doi: 10.1038/nrg2484
50. Kebschull M, Hülsmann C, Hoffmann P, Papapanou PN. Genome-wide analysis of periodontal and peri-implant cells and tissues. Methods Mol Biol. (2017) 1537:307–26. doi: 10.1007/978-1-4939-6685-1_18
51. Chen M, Cai W, Zhao S, Shi L, Chen Y, Li X, et al. Oxidative stress-related biomarkers in saliva and gingival crevicular fluid associated with chronic periodontitis: a systematic review and meta-analysis. J Clin Periodontol. (2019) 46:608–22. doi: 10.1111/jcpe.13112
52. Maekawa S, Onizuka S, Katagiri S, Hatasa M, Ohsugi Y, Sasaki N, et al. RNA sequencing for ligature induced periodontitis in mice revealed important role of S100A8 and S100A9 for periodontal destruction. Sci Rep. (2019) 9:14663. doi: 10.1038/s41598-019-50959-7
53. Liu YZ, Maney P, Puri J, Zhou Y, Baddoo M, Strong M, et al. RNA-sequencing study of peripheral blood monocytes in chronic periodontitis. Gene. (2016) 581:152–60. doi: 10.1016/j.gene.2016.01.036
54. Corrêa JD, Calderaro DC, Ferreira GA, Mendonça SM, Fernandes GR, Xiao E, et al. Subgingival microbiota dysbiosis in systemic lupus erythematosus: association with periodontal status. Microbiome. (2017) 5:34. doi: 10.1186/s40168-017-0252-z
55. Zhu LF Li L, Wang XQ, Pan L, Mei YM, Fu YW, Xu Y. M1 macrophages regulate TLR4/AP1 via paracrine to promote alveolar bone destruction in periodontitis. Oral Dis. (2019) 25:1972–82. doi: 10.1111/odi.13167
56. Wang L, Zheng J, Pathak JL, Chen Y, Liang D, Yang L, et al. SLIT2 Overexpression in periodontitis intensifies inflammation and alveolar bone loss, possibly via the activation of MAPK pathway. Front Cell Dev Biol. (2020) 8:593. doi: 10.3389/fcell.2020.00593
57. Zhou H, Chen D, Xie G, Li J, Tang J, Tang L. LncRNA-mediated ceRNA network was identified as a crucial determinant of differential effects in periodontitis and periimplantitis by high-throughput sequencing. Clin Implant Dent Relat Res. (2020) 22:424–50. doi: 10.1111/cid.12911
58. Horie M, Yamaguchi Y, Saito A, Nagase T, Lizio M, Itoh M, et al. Transcriptome analysis of periodontitis-associated fibroblasts by CAGE sequencing identified DLX5 and RUNX2 long variant as novel regulators involved in periodontitis. Sci Rep. (2016) 6:33666. doi: 10.1038/srep33666
59. Guo R, Huang Y, Liu H, Zheng Y, Jia L, Li W. Long non-coding RNA H19 participates in periodontal inflammation via activation of autophagy. J Inflamm Res. (2020) 13:635–46. doi: 10.2147/JIR.S276619
60. Qian SJ, Huang QR, Chen RY, Mo JJ, Zhou LY, Zhao Y, et al. Single-Cell RNA sequencing identifies new inflammation-promoting cell subsets in asian patients with chronic periodontitis. Front Immunol. (2021) 12:711337. doi: 10.3389/fimmu.2021.711337
61. Nashef A, Matthias M, Weiss E, Loos BG, Jepsen S, van der Velde N, et al. Translation of mouse model to human gives insights into periodontitis etiology. Sci Rep. (2020) 10:4892. doi: 10.1038/s41598-020-61819-0
62. Takada K, Chiba T, Miyazaki T, Yagasaki L, Nakamichi R, Iwata T, et al. Single cell rna sequencing reveals critical functions of mkx in periodontal ligament homeostasis. Front Cell Dev Biol. (2022) 10:795441. doi: 10.3389/fcell.2022.795441
63. Chen ZY, Xu TT, Liang ZJ, Zhao L, Xiong XQ, Xie KK, et al. Untargeted and targeted gingival metabolome in rodents reveal metabolic links between high-fat diet-induced obesity and periodontitis. J Clin Periodontol. (2021) 48:1137–48. doi: 10.1111/jcpe.13486
64. Agrafioti P, Morin-Baxter J, Tanagala KKK, Dubey S, Sims P, Lalla E, et al. Decoding the role of macrophages in periodontitis and type 2 diabetes using single-cell RNA-sequencing. FASEB J. (2022) 36:e22136. doi: 10.1096/fj.202101198R
65. Rizal MI, Soeroso Y, Sulijaya B, Assiddiq BF, Bachtiar EW, Bachtiar BM. Proteomics approach for biomarkers and diagnosis of periodontitis: systematic review. Heliyon. (2020) 6:e04022. doi: 10.1016/j.heliyon.2020.e04022
66. Gupta A, Govila V, Saini A. Proteomics - the research frontier in periodontics. J Oral Biol Craniofac Res. (2015) 5:46–52. doi: 10.1016/j.jobcr.2015.01.001
67. Häkkinen L, Uitto VJ, Larjava H. Cell biology of gingival wound healing. Periodontol. (2000) 24:127–52. doi: 10.1034/j.1600-0757.2000.2240107.x
68. Aslam B, Basit M, Nisar MA, Khurshid M, Rasool MH. Proteomics: technologies and their applications. J Chromatogr Sci. (2017) 55:182–96. doi: 10.1093/chromsci/bmw167
69. Wilkins MR, Sanchez JC, Gooley AA, Appel RD, Humphery-Smith I, Hochstrasser DF, et al. Progress with proteome projects: why all proteins expressed by a genome should be identified and how to do it. Biotechnol Genet Eng Rev. (1996) 13:19–50. doi: 10.1080/02648725.1996.10647923
70. Bostanci N, Bao K, Greenwood D, Silbereisen A, Belibasakis GN. Periodontal disease: From the lenses of light microscopy to the specs of proteomics and next-generation sequencing. Adv Clin Chem. (2019) 93:263–90. doi: 10.1016/bs.acc.2019.07.006
71. Guzman YA, Sakellari D, Arsenakis M, Floudas CA. Proteomics for the discovery of biomarkers and diagnosis of periodontitis: a critical review. Expert Rev Proteomics. (2014) 11:31–41. doi: 10.1586/14789450.2014.864953
72. Rong X, Xiang L, Li Y, Yang H, Chen W, Li L, et al. Chronic periodontitis and Alzheimer disease: a putative link of serum proteins identification by 2D-DIGE proteomics. Front Aging Neurosci. (2020) 12:248. doi: 10.3389/fnagi.2020.00248
73. Shin MS, Kim YG, Shin YJ, Ko BJ, Kim S, Kim HD. Deep sequencing salivary proteins for periodontitis using proteomics. Clin Oral Investig. (2019) 23:3571–80. doi: 10.1007/s00784-018-2779-1
74. Nagata M, Iwasaki K, Akazawa K, et al. Conditioned medium from periodontal ligament stem cells enhances periodontal regeneration. Tissue Eng Part A. (2017) 23:367–77. doi: 10.1089/ten.tea.2016.0274
75. Bao K, Li X, Kajikawa T, Toshiharu A, Selevsek N, Grossmann J, et al. Pressure cycling technology assisted mass spectrometric quantification of gingival tissue reveals proteome dynamics during the initiation and progression of inflammatory periodontal disease. Proteomics. (2020) 20:e1900253. doi: 10.1002/pmic.201900253
76. Lin P, Niimi H, Ohsugi Y, Tsuchiya Y, Shimohira T, Komatsu K, et al. Application of ligature-induced periodontitis in mice to explore the molecular mechanism of periodontal disease. Int J Mol Sci. (2021) 22:8900. doi: 10.3390/ijms22168900
77. Freire MS, Oliveira NG, Lima SMF, Porto WF, Martins DCM, Silva ON, et al. IL-4 absence triggers distinct pathways in apical periodontitis development. J Proteomics. (2021) 233:104080. doi: 10.1016/j.jprot.2020.104080
78. Sun L, Girnary M, Wang L, Jiao Y, Zeng E, Mercer K, et al. IL-10 dampens an IL-17-mediated periodontitis-associated inflammatory network. J Immunol. (2020) 204:2177–91. doi: 10.4049/jimmunol.1900532
79. Hartenbach FARR, Velasquez É, Nogueira FCS, Domont GB, Ferreira E, Colombo APV. Proteomic analysis of whole saliva in chronic periodontitis. J Proteomics. (2020) 213:103602. doi: 10.1016/j.jprot.2019.103602
80. Jurdziński KT, Potempa J, Grabiec AM. Epigenetic regulation of inflammation in periodontitis: cellular mechanisms and therapeutic potential. Clin Epigenetics. (2020) 12:186. doi: 10.1186/s13148-020-00982-7
81. Mertens B, Orti V, Vialaret J, Gibert P, Relaño-Ginés A, Lehmann S, et al. Assessing a multiplex-targeted proteomics approach for the clinical diagnosis of periodontitis using saliva samples. Bioanalysis. (2018) 10:35–45. doi: 10.4155/bio-2017-0218
82. Bostanci N, Belibasakis GN. Gingival crevicular fluid and its immune mediators in the proteomic era. Periodontol. (2018) 76:68–84. doi: 10.1111/prd.12154
83. Kurgan S, Kantarci A. Molecular basis for immunohistochemical and inflammatory changes during progression of gingivitis to periodontitis. Periodontol. (2018) 76:51–67. doi: 10.1111/prd.12146
84. Anand S, Mukherjee K, Padmanabhan P. An insight to flux-balance analysis for biochemical networks. Biotechnol Genet Eng Rev. (2020) 36:32–55. doi: 10.1080/02648725.2020.1847440
85. Orth JD, Thiele I, Palsson BØ. What is flux balance analysis? Nat Biotechnol. (2010) 28:245–8. doi: 10.1038/nbt.1614
86. Thomas JP, Modos D, Korcsmaros T, Brooks-Warburton J. Network biology approaches to achieve precision medicine in inflammatory bowel disease. Front Genet. (2021) 12:760501. doi: 10.3389/fgene.2021.760501
87. Magnúsdóttir S, Heinken A, Kutt L, Ravcheev DA, Bauer E, Noronha A, et al. Generation of genome-scale metabolic reconstructions for 773 members of the human gut microbiota. Nat Biotechnol. (2017) 35:81–9. doi: 10.1038/nbt.3703
88. Pei J, Li F, Xie Y, Liu J, Yu T, Feng X. Microbial and metabolomic analysis of gingival crevicular fluid in general chronic periodontitis patients: lessons for a predictive, preventive, and personalized medical approach. EPMA J. (2020) 11:197–215. doi: 10.1007/s13167-020-00202-5
89. Ma X, Wang Y, Wu H, Li F, Feng X, Xie Y, et al. Periodontal health related-inflammatory and metabolic profiles of patients with end-stage renal disease: potential strategy for predictive, preventive, and personalized medicine. EPMA J. (2021) 12:117–28. doi: 10.1007/s13167-021-00239-0
90. Ilievski V, Kinchen JM, Prabhu R, Rim F, Leoni L, Unterman TG, et al. Experimental periodontitis results in prediabetes and metabolic alterations in brain, liver and heart: global untargeted metabolomic analyses. J Oral Biol. (2016) 3. doi: 10.13188/2377-987X.1000020
91. Barros SP, Williams R, Offenbacher S, Morelli T. Gingival crevicular fluid as a source of biomarkers for periodontitis. Periodontol. (2016) 70:53–64. doi: 10.1111/prd.12107
92. Atarbashi-Moghadam F, Havaei SR, Havaei SA, Hosseini NS, Behdadmehr G, Atarbashi-Moghadam S. Periopathogens in atherosclerotic plaques of patients with both cardiovascular disease and chronic periodontitis. ARYA Atheroscler. (2018) 14:53–7. doi: 10.22122/arya.v14i2.1504
93. Chen HW, Zhou W, Liao Y, Hu SC, Chen TL, Song ZC. Analysis of metabolic profiles of generalized aggressive periodontitis. J Periodontal Res. (2018) 53:894–901. doi: 10.1111/jre.12579
94. Marchesan JT, Morelli T, Moss K, Barros SP, Ward M, Jenkins W, et al. Association of synergistetes and cyclodipeptides with periodontitis. J Dent Res. (2015) 94:1425–31. doi: 10.1177/0022034515594779
95. Katsikanis F, Strakas D, Vouros I. The application of antimicrobial photodynamic therapy (aPDT, 670 nm) and diode laser (940 nm) as adjunctive approach in the conventional cause-related treatment of chronic periodontal disease: a randomized controlled split-mouth clinical trial. Clin Oral Investig. (2020) 24:1821–7. doi: 10.1007/s00784-019-03045-1
96. Martin-Cabezas R, Davideau JL, Tenenbaum H, Huck O. Clinical efficacy of probiotics as an adjunctive therapy to non-surgical periodontal treatment of chronic periodontitis: a systematic review and meta-analysis. J Clin Periodontol. (2016) 43:520–30. doi: 10.1111/jcpe.12545
97. Pretzl B, Sälzer S, Ehmke B, Schlagenhauf U, Dannewitz B, Dommisch H, et al. Administration of systemic antibiotics during non-surgical periodontal therapy-a consensus report. Clin Oral Investig. (2019) 23:3073–85. doi: 10.1007/s00784-018-2727-0
98. Ren C, McGrath C, Jin L, Zhang C, Yang Y. The effectiveness of low-level laser therapy as an adjunct to non-surgical periodontal treatment: a meta-analysis. J Periodontal Res. (2017) 52:8–20. doi: 10.1111/jre.12361
99. Zhao H, Hu J, Zhao L. Adjunctive subgingival application of Chlorhexidine gel in nonsurgical periodontal treatment for chronic periodontitis: a systematic review and meta-analysis. BMC Oral Health. (2020) 20:34. doi: 10.1186/s12903-020-1021-0
100. Cafferata EA, Alvarez C, Diaz KT, Maureira M, Monasterio G, González FE, et al. Multifunctional nanocarriers for the treatment of periodontitis: Immunomodulatory, antimicrobial, and regenerative strategies. Oral Dis. (2019) 25:1866–78. doi: 10.1111/odi.13023
101. Aithal GC, Nayak UY, Mehta C, Narayan R, Gopalkrishna P, Pandiyan S, et al. Localized in situ nanoemulgel drug delivery system of quercetin for periodontitis: development and computational simulations. Molecules. (2018) 23:1363. doi: 10.3390/molecules23061363
102. Lecio G, Ribeiro FV, Pimentel SP, Reis AA, da Silva RVC, Nociti-Jr F, et al. Novel 20% doxycycline-loaded PLGA nanospheres as adjunctive therapy in chronic periodontitis in type-2 diabetics: randomized clinical, immune and microbiological trial. Clin Oral Investig. (2020) 24:1269–79. doi: 10.1007/s00784-019-03005-9
103. Pérez-Pacheco CG, Fernandes NAR, Primo FL, Tedesco AC, Bellile E, Retamal-Valdes B, et al. Local application of curcumin-loaded nanoparticles as an adjunct to scaling and root planing in periodontitis: Randomized, placebo-controlled, double-blind split-mouth clinical trial. Clin Oral Investig. (2021) 25:3217–27. doi: 10.1007/s00784-020-03652-3
104. Baranov N, Popa M, Atanase LI, Ichim DL. Polysaccharide-based drug delivery systems for the treatment of periodontitis. Molecules. (2021) 26:2735. doi: 10.3390/molecules26092735
105. Trame MN, Biliouris K, Lesko LJ, Mettetal JT. Systems pharmacology to predict drug safety in drug development. Eur J Pharm Sci. (2016) 94:93–5. doi: 10.1016/j.ejps.2016.05.027
106. Kloft C, Trame MN, Ritter CA. Systems pharmacology in drug development and therapeutic use - A forthcoming paradigm shift. Eur J Pharm Sci. (2016) 94:1–3. doi: 10.1016/j.ejps.2016.07.014
107. Abhyankar V, Bland P, Fernandes G. The role of systems biologic approach in cell signaling and drug development responses-a mini review. Med Sci. (2018) 6:43. doi: 10.3390/medsci6020043
108. Batschkus S, Cingoez G, Urlaub H, Miosge N, Kirschneck C, Meyer-Marcotty P, et al. New albumin-depletion strategy improves proteomic research of gingival crevicular fluid from periodontitis patients. Clin Oral Investig. (2018) 22:1375–84. doi: 10.1007/s00784-017-2213-0
109. Ngo LH, Darby IB, Veith PD, Locke AG, Reynolds EC. Mass spectrometric analysis of gingival crevicular fluid biomarkers can predict periodontal disease progression. J Periodontal Res. (2013) 48:331–41. doi: 10.1111/jre.12012
110. Moriya Y, Obama T, Aiuchi T, Sugiyama T, Endo Y, Koide Y, et al. Quantitative proteomic analysis of gingival crevicular fluids from deciduous and permanent teeth. J Clin Periodontol. (2017) 44:353–62. doi: 10.1111/jcpe.12696
111. Kim JS, Cho IH, Kim KH, Hwang YS. Identification of galectin-10 as a biomarker for periodontitis based on proteomic analysis of gingival crevicular fluid. Mol Med Rep. (2021) 23:123. doi: 10.3892/mmr.2020.11762
112. Marinho MC, Pacheco ABF, Costa GCV, Ortiz ND, Zajdenverg L, Sansone C. Quantitative gingival crevicular fluid proteome in type 2 diabetes mellitus and chronic periodontitis. Oral Dis. (2019) 25:588–95. doi: 10.1111/odi.12996
113. Gürsoy UK, Pussinen PJ, Salomaa V, Syrjäläinen S, Könönen E. Cumulative use of salivary markers with an adaptive design improves detection of periodontal disease over fixed biomarker thresholds. Acta Odontol Scand. (2018) 76:493–6. doi: 10.1080/00016357.2018.1441436
114. Cuevas-Córdoba B, Santiago-García J. Saliva: a fluid of study for OMICS. OMICS. (2014) 18:87–97. doi: 10.1089/omi.2013.0064
115. Meleti M, Quartieri E, Antonelli R, Pezzi ME, Ghezzi B, Viani MV, et al. Metabolic profiles of whole, parotid and submandibular/sublingual saliva. Metabolites. (2020) 10:318. doi: 10.3390/metabo10080318
116. Kaczor-Urbanowicz KE, Martín Carreras-Presas C, Kaczor T, Tu M, Wei F, Garcia-Godoy F, et al. Emerging technologies for salivaomics in cancer detection. J Cell Mol Med. (2017) 21:640–7. doi: 10.1111/jcmm.13007
117. Graves DT, Corrêa JD, Silva TA. The oral microbiota is modified by systemic diseases. J Dent Res. (2019) 98:148–56. doi: 10.1177/0022034518805739
118. Jepsen S, Suvan J, Deschner J. The association of periodontal diseases with metabolic syndrome and obesity. Periodontol. (2020) 83:125–53. doi: 10.1111/prd.12326
119. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. (2018) 138:271–81. doi: 10.1016/j.diabres.2018.02.023
120. Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, et al. Scientific evidence on the links between periodontal diseases and diabetes: consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol. (2018) 45:138–49. doi: 10.1111/jcpe.12808
121. Baeza M, Morales A, Cisterna C, Cavalla F, Jara G, Isamitt Y, et al. Effect of periodontal treatment in patients with periodontitis and diabetes: systematic review and meta-analysis. J Appl Oral Sci. (2020) 28:e20190248. doi: 10.1590/1678-7757-2019-0248
122. Joshipura KJ, Muñoz-Torres FJ, Dye BA, Leroux BG, Ramírez-Vick M, Pérez CM. Longitudinal association between periodontitis and development of diabetes. Diabetes Res Clin Pract. (2018) 141:284–93. doi: 10.1016/j.diabres.2018.04.028
123. Mauri-Obradors E, Merlos A, Estrugo-Devesa A, Jané-Salas E, López-López J, Viñas M. Benefits of non-surgical periodontal treatment in patients with type 2 diabetes mellitus and chronic periodontitis: a randomized controlled trial. J Clin Periodontol. (2018) 45:345–53. doi: 10.1111/jcpe.12858
124. Wu CZ, Yuan YH, Liu HH Li SS, Zhang BW, Chen W, et al. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health. (2020) 20:204. doi: 10.1186/s12903-020-01180-w
125. Zheng J, Chen S, Albiero ML, Vieira GHA, Wang J, Feng JQ, et al. Diabetes activates periodontal ligament fibroblasts via NF-κB in vivo. J Dent Res. (2018) 97:580–8. doi: 10.1177/0022034518755697
126. Overmyer KA, Rhoads TW, Merrill AE, Ye Z, Westphall MS, Acharya A, et al. Proteomics, lipidomics, metabolomics, and 16s dna sequencing of dental plaque from patients with diabetes and periodontal disease. Mol Cell Proteomics. (2021) 20:100126. doi: 10.1016/j.mcpro.2021.100126
127. Naderi S, Merchant AT. The association between periodontitis and cardiovascular disease: an update. Curr Atheroscler Rep. (2020) 22:52. doi: 10.1007/s11883-020-00878-0
128. Priyamvara A, Dey AK, Bandyopadhyay D, Katikineni V, Zaghlol R, Basyal B, et al. Periodontal inflammation and the risk of cardiovascular disease. Curr Atheroscler Rep. (2020) 22:28. doi: 10.1007/s11883-020-00848-6
129. Sanz M, Marco Del Castillo A, Jepsen S, Gonzalez-Juanatey JR, D'Aiuto F, Bouchard P, et al. Periodontitis and cardiovascular diseases: consensus report. J Clin Periodontol. (2020) 47:268–88. doi: 10.1111/jcpe.13189
130. Gode S, Sarp TZ, Saribas S, Ergin S, Kasnak G, Dinc HO, et al. The prevalence of periodontal pathogenic bacteria in atherosclerotic cardiovascular disease. Clin Lab. (2020) 66. doi: 10.7754/Clin.Lab.2020.191146
131. Hamilton JA, Hasturk H, Kantarci A, Serhan CN, Van Dyke T. Atherosclerosis, periodontal disease, and treatment with resolvins. Curr Atheroscler Rep. (2017) 19:57. doi: 10.1007/s11883-017-0696-4
132. Mirnejad R, Razeghian-Jahromi I, Sepehrimanesh M, Zibaeenezhad MJ, Lopez-Jornet P. A proteomic analysis of the virulence factors of three common bacterial species involved in periodontitis and consequent possible atherosclerosis: a narrative review. Curr Protein Pept Sci. (2018) 19:1124–30. doi: 10.2174/1389203719666180625111449
133. Pietiäinen M, Liljestrand JM, Kopra E, Pussinen PJ. Mediators between oral dysbiosis and cardiovascular diseases. Eur J Oral Sci. (2018) 126:26–36. doi: 10.1111/eos.12423
134. Fåk F, Tremaroli V, Bergström G, Bäckhed F. Oral microbiota in patients with atherosclerosis. Atherosclerosis. (2015) 243:573–8. doi: 10.1016/j.atherosclerosis.2015.10.097
135. Lamster IB, Pagan M. Periodontal disease and the metabolic syndrome. Int Dent J. (2017) 67:67–77. doi: 10.1111/idj.12264
136. Moon KH. Screening of genetic factor in the interaction between periodontitis and metabolic traits using candidate gene association study (CGAS). Biochem Genet. (2019) 57:466–74. doi: 10.1007/s10528-018-9899-9
137. Beck JD, Papapanou PN, Philips KH, Offenbacher S. Periodontal medicine: 100 years of progress. J Dent Res. (2019) 98:1053–62. doi: 10.1177/0022034519846113
Keywords: systems biology, periodontitis, genetic decoding, genome, transcriptome, proteome, metabolome, meta-transcriptome
Citation: Silva DNdA, Monajemzadeh S and Pirih FQ (2022) Systems Biology in Periodontitis. Front. Dent. Med. 3:853133. doi: 10.3389/fdmed.2022.853133
Received: 12 January 2022; Accepted: 17 March 2022;
Published: 25 April 2022.
Edited by:
Francisco Nociti, Universidade Estadual de Campinas, BrazilReviewed by:
Oleh Andrukhov, University Dental Clinic Vienna, AustriaSasanka Chukkapalli, Texas A & M University College Station, United States
Copyright © 2022 Silva, Monajemzadeh and Pirih. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Flavia Queiroz Pirih, ZnBpcmloQGRlbnRpc3RyeS51Y2xhLmVkdQ==
 Davi Neto de Araújo Silva
Davi Neto de Araújo Silva Sepehr Monajemzadeh
Sepehr Monajemzadeh Flavia Queiroz Pirih
Flavia Queiroz Pirih