- Jiangsu Key Laboratory of Oral Disease, Affiliated Stomatological Hospital, Nanjing Medical University, Nanjing, China
Stem cells derived from human exfoliated deciduous teeth (SHEDs) are considered a promising cell population for cell-based or cell-free therapy and tissue engineering because of their proliferative, multipotency and immunomodulator. Based on recent studies, we find that SHEDs show the superior ability of nerve regeneration in addition to the potential of osteogenesis, odontogenesis owing to their derivation from the neural crest. Besides, much evidence suggests that SHEDs have a paracrine effect and can function as immunomodulatory regents attributing to their capability of secreting cytokines and extracellular vesicles. Here, we review the characteristic of SHEDs, their multipotency to regenerate damaged tissues, specifically concentrating on bones or nerves, following the paracrine activity or immunomodulatory benefits of their potential for clinical application in regenerative medicine.
Introduction
Stem cells isolated from the pulp in exfoliated deciduous teeth (SHEDs) are one of the dental mesenchymal cells derived from cranial neural crest cells (NCCs) (1). It is well established that SHEDs are shown to be a population of highly proliferative, clonogenic cells, compared to other dental stem cells, such as periodontal ligament stem cells (PDLSCs) (2), dental pulp stem cells (DPSCs) (3). They maintain characteristic immunophenotypes in vitro or cryopreserved (4). They express stem cell markers (OCT4, c-Myc and Nanog Etc.) and positively express early mesenchymal stem-cell surface markers, including but not limited to STRO-1, CD146 (MUC18), CD13, CD29, CD44, CD56, CD73, CD90, CD105, CD166 while negatively express hematopoietic markers, such as CD14, CD19, CD24, CD31, CD34 and CD45, CD117, CD133 and CD11b/c, and HLA-DR, which can be used for their identification. However, the positive rate of immune-phenotype may change with passages (5, 6). It also has been shown that SHEDs culture showed a higher proportion of epithelioid cells, while DPSCs showed a higher proportion of spindle-shaped fibroblastoid cells (7). Given that SHEDs are derived from dental pulp tissues of early age groups, higher embryonic markers are expressed in SHEDs, determining their lineage propensity toward a specific destination if no particular intervention is performed (8). Furthermore, SHEDs exhibit specific stemness, such as the capability of multi-differentiation and self-renewal, and can develop into other cell lineages. It has been reported that SHEDs can be induced toward osteoblasts/odontoblasts, neurocytes, chondrocytes, adipocytes, neuro-glial cells, smooth muscle cells (9), vessels (10), epitheliocytes (11), hepatocytes (12), endotheliocytes (11), retinal photoreceptor-like cells (13) and pancreatic β cell-like cells (14) and so on.
More and more pieces of evidence indicate that functional recovery and remodeling in lesions not only rely on their multipotency but also on their protective and anti-inflammatory action by the paracrine mechanism of grafted SHEDs. Immunomodulatory effects of SHEDs are also of good value in cell therapy or cell-free therapy owing to the capacity to interact with the local inflammatory microenvironment. The underlying mechanisms mainly rely on effective cytokines or extracellular vesicles (EVs) paracrine functions. These paracrine activities discovered in recent years encompassed remarkable modulatory effects in various autoimmune and inflammatory diseases. It has been found that SHEDs showed alleviating effects on nervous system diseases, including spinal cord injury (15, 16), Parkinson's disease (6, 17–20), trigeminal neuralgia (21), cerebral ischemia (22), Alzheimer's disease (23), encephalomyelitis (24). Besides, SHEDs also played a vital role in autoimmune diseases, such as rheumatoid arthritis (25), diabetes (26). Other system inflammation like acute kidney injury (27), liver fibrosis/acute liver failure (28–30), osteoarthritic (31), acute respiratory distress syndrome (ARDS) (32) could also benefit from SHEDs for the protective effects underlying immunomodulatory activities.
This article addresses the multipotency and epigenetic types of machinery of SHEDs along with their paracrine activity and immunomodulatory benefits, as evident from the published literature. We aim to review the multi-lineage differentiation of SHEDs, their potential to regenerate damaged tissues, and their potential therapeutic value via immunomodulatory, conducive to understanding their potential for clinical application in regenerative medicine.
Multipotency
SHEDs are noteworthy for their easy accessibility from teeth with painless collection procedures, which provide the powerful potential for regeneration engineering among all dental-derived stem cells. Since being discovered and identified by Miura in 2003, SHEDs have been proved to differentiate into a series of target cells under induction conditions in vitro and used for tissue regeneration by transplantation in vivo (33). SHEDs express characteristic markers under both maintaining medium and conditional medium, and if recruited in lesions, they may differentiate into target cells and promote the directional differentiation of local stem/progenitor cells, achieving regeneration and repair (34). Pulp-dentin complex regeneration is challenging in dental regeneration medicine. The application of dental stem cells like SHEDs extends the tooth longevity in terms of regenerative endodontics and even brings a brighter future of tooth regeneration (35). Cell-based therapy of SHEDs may play a remarkable role in treating nerve injury diseases or neurodegenerative disorders given neurogenesis.
Osteogenesis/Odontogenesis
According to conventional osteo-inducing methods, SHEDs were induced with an osteogenic cocktail of b-glycerophosphate, dexamethasone or retinoic acid (36), and ascorbic acid. Early osteogenic associated genes and proteins [alkaline phosphatase gene (ALP), runt-related transcription factor 2 (RUNX2), collagen type I alpha 1 (COL1A1)] and late osteogenic, odontogenic differentiation marker [osteopontin (OPN), osteocalcin (OCN), osteoprotegerin (OPG), DSPP and DMP-1] upregulated. However, the receptor activator of nuclear factor κB ligand (RANKL) and the OPG/RANKL ratio downregulated during osteogenesis. It is controversial whether SHEDs have a better osteogenic and odontogenic potential than other dental stem cells. For example, Sabbagh's observations demonstrated that DPSCs might have a better osteogenic and odontogenic potential than SHEDs (5). However, in the other two studies, SHEDs exerted significantly higher osteogenic differentiation potential than human dental pulp stem cells (hDPSCs) and bone marrow mesenchymal stem cells (hBMSCs) (37, 38). Gene expression profiles indicated that bone morphogenetic protein (BMP-4) was expressed much higher in SHEDs than in BMMSCs (39). Revealing the regulation mechanism of SHEDs' osteopotential can better guide clinical application. For example, one recent study by Sebastian et al. revealed that the proinflammatory cytokine-IL-17A promoted the proliferative and enhanced mineralization activity of SHEDs (40). Zhai found that human β defensin 4 (HBD4) promoted osteogenic/odontogenic differentiation of SHEDs stimulated by proinflammatory cytokines and considered HBD4 a suitable candidate for vital pulp therapy in future clinic application (41).
Cell sheets derived from stem cells can also give rise to in vitro calcification and in vivo bone repair. Lee et al. have successfully verified that SHED cell sheets could survive and develop into osteogenic tissue to a greater level of maturity after engrafted inside the cleft palate models (42). Biocompatible scaffolds can support mesenchymal stem cells to proliferate and differentiate optimally. Several biocompatible scaffolds up to the present have been found to accelerate bone remodeling. Prahasanti's study showed that SHED-incorporated carbonate apatite scaffold (CAS) enhances bone remodeling through upregulating bone morphogenetic 2 and 7 expressions (BMP2 and BMP-7) and downregulation of matrix metalloproteinase-8 (MMP-8) (43). Enamel Matrix Derivative (EMD) showed the highest cell viability and potential for enhanced mineralization (44). Non-coding RNAs (ncRNAs) have been essential contributors to cell biology. For example, Hsa-miR-1287 was capable of downregulating CD105 expression, which could be used to enhance osteogenesis in SHEDs (45). Mitochondrial biogenesis might be a therapeutic target for improving the osteogenesis of SHEDs. Han's study found mitochondrial dysfunction impaired bone metabolism and osteoporosis, which could be reversed by bezafibrate-treated cells (46).It should be noted that there are few studies on epigenetic regulation, including but not limited to ncRNAs on osteogenic differentiation of SHEDs.
Neurogenesis
Due to the neurogenesis potential of SHEDs, cell-based therapy is one of the promising treatments of neurological disorders, such as spinal cord injury (SCI), Parkinson's disease (PD), Etc. Since 2003, it has been shown that SHEDs express several different neuro-glial cell markers in the growth medium, such as nestin, glial fibrillary acidic protein (GFAP), neuron-specific enolase (NSE), neurofilament medium-chain (NFM), 2',3'-cyclic nucleotide-3'-phosphodiesterase (CNPase), βIII-tubulin, glutamic acid decarboxylase (GAD), and neuron-specific nuclear protein (NeuN), indicating the embryonic neural crest origin of SHEDs. After inductive neural culture in neurobasal media containing B27 supplement, epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF), expression levels of neuronal markers including βIII-tubulin, GAD, and NeuN were increased, which meant that SHEDs differentiated into neurons (47–49). Meanwhile, SHEDs developed multi-cytoplasmic processes immunoreactive to MAP2 and Tau antibodies (1, 50).
SHEDs can also be induced to form neural-like spheres in vitro and further differentiated into specific dopaminergic neurons under adherent conditions for long-term serum-free culture with cytokines including sonic hedgehog, fibroblast growth factor 8, glial cell line-derived neurotrophic factor, and forskolin. Wang's study showed that transplantation of neural-like spheres derived from SHEDs into the striatum of parkinsonian rats significantly improved the behavioral disorders, the number of TH-positive (tyrosine hydroxylase) cells and the protective effect on endogenous dopaminergic neurons, indicating SHED spheres were of potential therapeutic value (6, 51). In 2018, by way of two steps' induction, SHEDs were induced into spiral ganglion neuron-like cells (SGNs) with highly expressed βIII-tubulin, GATA binding protein 3 (GATA3) and tropomyosin receptor kinase B (52).
High proliferation of SHEDs and enhancement of their differentiation into neuron-like cells could bring about desirable therapeutic applications. The latest research showed that Rho-associated kinase inhibitor Y-27632 combined with Noggin potently promote neuronal differentiation and increase the proliferation of SHEDs via activation of the caspase signaling cascades (53).
Many studies suggested that DPSCs have a more tremendous neuronal differentiation potential than SHEDs. However, some observations showed the comparable plasticity of neuronal differentiation (52). Referring to Heng's review, there are different protocols for neural induction of SHEDs in vitro (54). It should be addressed that appropriate neural induction protocols and differentiation stage of SHEDs are required for various therapeutic and non-therapeutic applications.
Other Tissue Regeneration
It has been established that vascular endothelial growth factor (VEGF) can induce endothelial-vasculogenic differentiation of SHEDs, and SHEDs might be a more satisfactory source of perivascular cells for in vivo angiogenesis than other stem cells, such as umbilical vein endothelial cells (55). The latest study by Zhang et al. revealed that p53/p21 acted as an inverse regulator of vasculogenic differentiation through Bmi-1, a significant regulator of stem cell self-renewal (10). In addition to multipotential of craniofacial tissues, SHEDs also had cholangiogenic/hepatocytes- differentiated potential under the stimulation of tumor necrosis factor-alpha (TNF-α) (12, 56) and could differentiate into endothelial cells via inhibition of TGF-β-SMAD2/3 signaling (11). It has also been proved that SHEDs could be induced to insulin-secreting β cell-like cells (14), functional smooth muscle cells (9), peripheral neurocytes (57) and retinal photoreceptor-like cells (13). The various cell types derived from SHEDs are shown in Table 1.
Paracrine Activity and Immunomodulatory
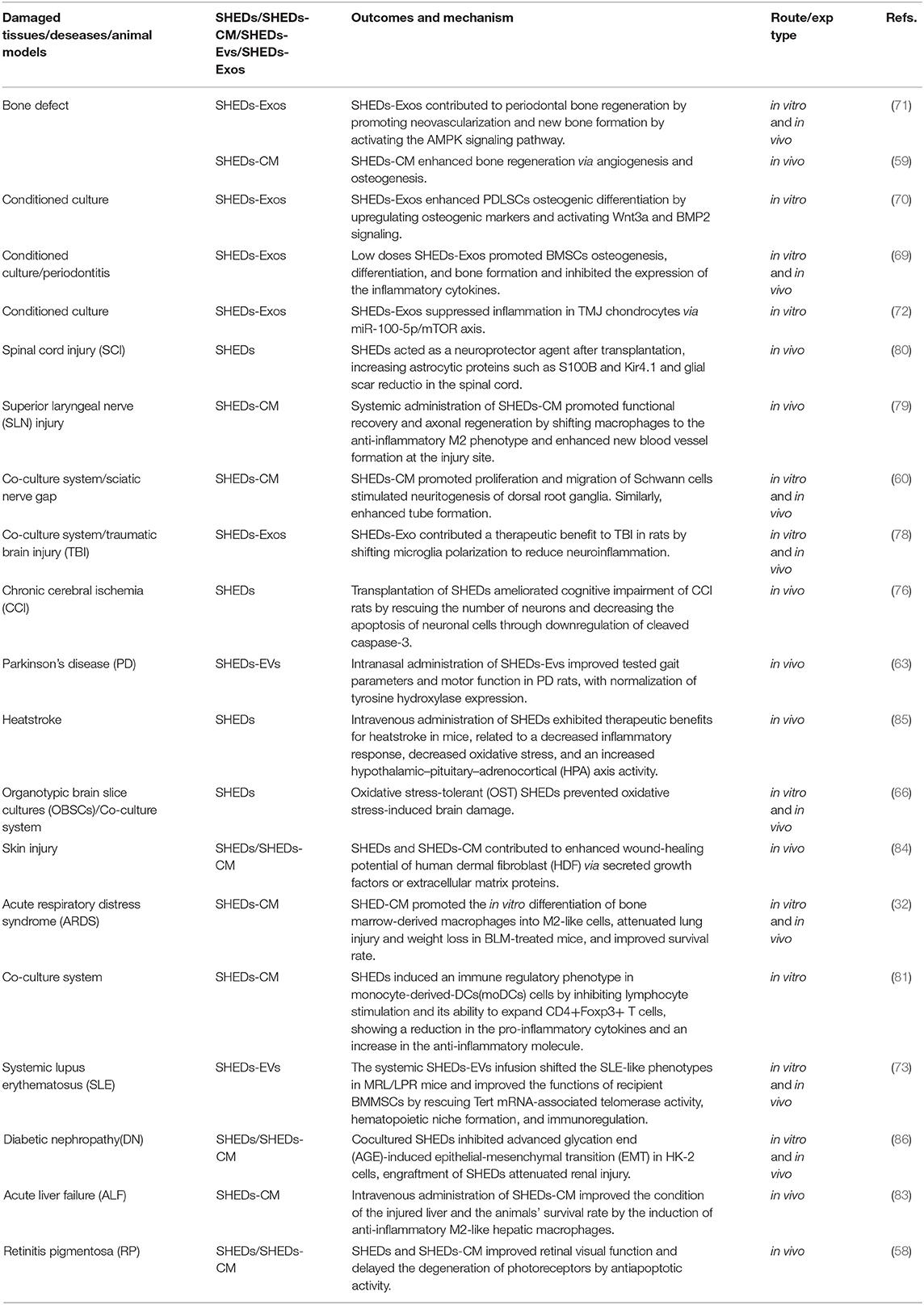
Apart from proliferative and regenerative potential, SHEDs and other oral mesenchymal stems/progenitor cells (MSCs) can interact with the local inflammatory microenvironment through multiple impressive paracrine functions. Many studies have clarified that serum-free conditioned media of SHEDs (SHED-CM) or extracellular vesicles (EVs) had a modulatory function on other cells or tissues, indicating that cell-free based therapy is an effective strategy in regenerative medicine. For example, in a recent study on treating an osteoarthritis (OA) model, the results showed that SHED-CM could increase matrix proteins and suppress MMP-13 expression by downregulation of NF-kB, which gives protective action for chondrocytes (31). Li et al. study showed that SHEDs-CM has therapeutic effects on Retinitis pigmentosa (RP) by antiapoptotic activity (58). In Hiraki et al. study, SHED-CM contributed to more bone regeneration and faster bone maturation in the mouse calvarial bone defect model compared with transplanting SHEDs alone. They found that SHED-CM contains abundant bone metabolism-related markers (OPG, OPN, BMP-2 and BMP-4) and angiogenesis-related markers (M-CSF, MCP-1, ANG, bFGF, VEGF-C and VEGF-A). In addition, neurotrophic family (BDNF, beta-NGF, GDNF and NT-3) angiogenesis-related genes were also included, thus creating a more desirable extracellular microenvironment for peripheral nerve regeneration (59, 60). Yamada et al. also examined high-expressed cytokines secreted from SHEDs, such as growth factors (hepatocyte growth factor (HGF), chemokines (stromal cell-derived factor 1, SDF-1) and matrix metalloproteinases-3 (MMP-3) (61). These cytokines might be closely related to proliferation, differentiation and anti-inflammatory by paracrine effect (62). Fujii, NARBUTE, Chen et al. proved the therapeutic efficacy via intranasal administration of SHED-EVs in a rat model of Parkinson's disease (PD), with significant improvement in behavioral level and histological level (17, 18, 63).
It has been shown that decellularized matrix (DECM) from SHEDs also exerted a profound effect on the adhesion, proliferation and osteogenic differentiation capacity of DPSCs (64), which may be attributed to microenvironment remodeling and recruitment of stem/progenitor cells (65). Xiao et al. treated SHEDs with H2O2 to induce oxidative stress-tolerant SHEDs and co-cultivated them with organotypic brain slice cultures, suggesting that they were significantly superior to regular SHEDs in inhibiting inflammation protecting brain tissues (66).
EVs, known as nanosized membrane structures released by cells, can participate in organ homeostasis by transferring RNA, microRNA, and proteins to modulate the inflammatory environment. For example, BM-MSC-derived exosomes promoted the regeneration/repair of the periodontal ligament and temporomandibular joint and suppressed the inflammatory response by activating AKT, ERK, and AMPK signaling pathways (67). Many studies clarified that SHED-EVs enhanced tissue remodeling and regeneration in an inflammatory microenvironment, such as bones, cartilage, nerves and so on (68). In Wei's study, SHED-derived exosomes (SHED-Exo) promoted BMSCs osteogenesis, reduced apoptosis and inhibited the inflammatory cytokines IL-6 and TNF-α in the periodontitis mouse model (69). Wang's studies revealed that SHED-Exos promoted PDLSCs osteogenic differentiation by activating BMP/Smad signaling and Wnt/β-catenin. The vital molecules-Wnt3a and BMP2 were detected in SHED-Exos and mediated the osteogenic differentiation of PDLSCs (70). In periodontal defect rat models, Wu et al. discovered that SHED-Exos contribute to periodontal bone regeneration by promoting neovascularization and new bone formation through activating the AMPK signaling pathway (71). Luo et al. found that SHED-Exos acted as an anti-inflammatory agent in temporomandibular joint chondrocytes via miR-100-5p/mTOR axis (72). One of the latest researches showed that systemic transplantation of SHEDs-EVs treated systemic lupus erythematosus (SLE)-like disorders in MRL/LPR mice by rescuing Tert mRNA-associated telomerase activity, hematopoietic niche formation, and immunoregulation, but with an impaired effect by using RNA-depleted SHED-EVs (73). The underlying mechanism of these factors and EVs of SHEDs remains further studied.
Several experiments have expounded modulatory functions of SHEDs-CM or SHED-EVs to immunocyte, including microglia/macrophage, astrocyte, dendritic cell (DCs) and T cell (74). It is well known that microglia play a fundamental role in the initiation and support of chronic neuroinflammation (75), such as spinal cord injury, cerebral injury (76), trigeminal neuralgia (21) and so on. Microglia will polarize into M1 and M2 states along with the inflammatory microenvironment. In 2016, 2017, and 2019, it has been shown that both SHEDs-CM and SHED-EVs could act as a potent immunomodulator of human microglial cells. The regulation mechanism was mainly decided by a shift in the microglia/macrophage phenotype from M1 to CD206+ M2, reduced inflammatory cell infiltration and proinflammatory cytokine expression (24, 77, 78). In 2018, Tsuruta et al. demonstrated the therapeutic effect of SHEDs-CM on the injured superior laryngeal nerve. The functional mechanism converted macrophages to the anti-inflammatory M2 phenotype and new blood vessel formation at the injury site (79). Nicola et al. shed light on the fact that SHEDs transplantation contributed to tissue and motor neuron preservation by reducing the early neuronal apoptosis and interfering with the balance between anti-and pro-apoptotic factors. Besides, SHEDs could act as a neuroprotector agent, promoting the tissue plasticity and modulating early astrocyte response and reducing neuronal excitability. However, the paracrine signaling mechanism was unclear (80). In 2018 and 2021, Asadi-Golshan et al. study showed that intraspinal administration of SHED-CM loaded in collagen hydrogel was more advantageous in SCI rats, with remarkable functional recovery (15, 16).
Silva and his colleagues co-cultured DCs with SHEDs, observing that immune phenotype in DCs was regulated, with a decrease in expression of BDCA-1, CD11c, CD40, CD80, CD83 and CD86. In addition, co-culturing peripheral blood lymphocytes with these co-cultured DCs inhibited proliferation of CD4+/CD8+ T cells, reducing the pro-inflammatory cytokines (IL-2, TNF-α and IFN-γ), and increasing the anti-inflammatory molecule IL-10. The results showed that SHEDs directly or indirectly acted as an immune modulator for both DCs and lymphocytes (81). SHEDs exhibited more potent immunomodulatory characteristics via suppressing the proliferation of stimulated T, inhibiting Th17 cell differentiation, and increasing the ratio of regulatory T cells (Tregs) in vivo (74, 82).
Paracrine activity and immunomodulatory effects of SHEDs are not limited to mentioned above. More and more shreds of evidence indicated that application of SHEDs-CM or SHEDs-EVs is positive in quite a lot of systemic diseases such as Alzheimer's disease (23), rheumatoid arthritis (25), acute liver failure (29, 83), liver fibrosis (28, 30), acute kidney injury (27), skin rejuvenation (84), heatstroke (85), diabetic nephropathy (86), Etc. Table 2 shows the therapeutic potential of SHEDs via paracrine activity and immunomodulatory.
Future Prospects
Multipotency of SHEDs holds countless applications in regenerative medicine and tissue engineering, with the most important clinical function in osteogenesis/odontogenesis and neurogenesis, while thoroughly understanding the specific regulatory and regenerative molecular mechanism is waiting for intensive study before their wide application in the clinic, such as in epigenetic regulation of ncRNAs, DNA modification, histone modification and chromatin remodeling. Because of the relative newer stem cell population and conventional wisdom of waste, SHEDs are less studied than DPSCs, which may have more researchable space. The abundance of pulp tissue from exfoliated deciduous teeth does not affect the number of SHEDs obtained. We can harvest a large number of SHEDs in a shorter time due to their high proliferative capacity and stemness maintenance, indicating they are the ideal tool for studying the regeneration of maxillofacial tissue (87). Based on the gene expression profile of DPSCs and SHEDs, higher expression in SHEDs were observed for genes that participate in pathways related to cell proliferation and extracellular matrix (88). On account of its advantages of abundant cell supply with minimal invasion and a higher proliferation capability, SHEDs could be a desirable option as a cell source for potential therapeutic applications. Another viewpoint some researchers lean-to is that SHEDs can promote osteoclastogenesis as a result of physiological root resorption of deciduous teeth in mixed dentition (89), which also inspire us to explore the mechanism of the balance of osteogenesis and osteoclastogenesis.
Besides, developing unified induction protocols for target cells is urgent on the basis of the characteristic of SHEDs. SHEDs-CM or SHEDs-EVs can functionally mirror the parent cell and contribute to the regeneration and repair of the damaged tissues. Many pieces of evidence show the benefits of SHEDs on pathological microglia/macrophage, astrocyte, dendritic cell (DCs) and T cell by regulating proliferation, shifting cell phenotype and modulating cytokine production of immune cells. Hence, they can also be considered as an ideal tool for immunomodulation in clinical applications. Subtype screening or epigenetic modification of SHEDs might have more potential for cell-based or cell-free therapy. Soluble growth factors and exosomes derived from SHEDs requires advancing investigation. It should also be noted that more clinical trials are required before SHEDs' practical and safe application in the clinic. In addition, based on the long-term considerations, the banking of SHEDs and cytokines or exosomes derived from SHEDs might be an ideal therapeutic strategy.
Author Contributions
RG: design and conception, manuscript writing, and final approval of the manuscript. JY: design and conception, manuscript revising, financial support, and final approval of the manuscript. Both authors have read and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. PNAS. (2003) 100:5807–5812. doi: 10.1073/pnas.0937635100
2. Trivanovic D, Jaukovic A, Popovic B, Krstic J, Mojsilovic S, Okic-Djordjevic I, et al. Mesenchymal stem cells of different origin: Comparative evaluation of proliferative capacity, telomere length and pluripotency marker expression. Life Sci. (2015) 141:61–73. doi: 10.1016/j.lfs.2015.09.019
3. Wang H, Zhong Q, Yang T, Qi Y, Fu M, Yang X, et al. Comparative characterization of SHED and DPSCs during extended cultivation in vitro. Mol Med Rep. (2018) 17:6551–9. doi: 10.3892/mmr.2018.8725
4. Lee HS, Jeon M, Kim SO, Kim SH, Lee JH, Ahn SJ, et al. Characteristics of stem cells from human exfoliated deciduous teeth (SHED) from intact cryopreserved deciduous teeth. Cryobiology. (2015) 71:374–83. doi: 10.1016/j.cryobiol.2015.10.146
5. Sabbagh J, Ghassibe-Sabbagh M, Fayyad-Kazan M, Al-Nemer F, Fahed JC, Berberi A, et al. Differences in osteogenic and odontogenic differentiation potential of DPSCs and SHED. J Dent. (2020) 101:103413. doi: 10.1016/j.jdent.2020.103413
6. Xiao Z, Lei T, Liu Y, Yang Y, Bi W, Du H. The potential therapy with dental tissue-derived mesenchymal stem cells in Parkinson's disease. Stem Cell Res Ther. (2021) 12:5. doi: 10.1186/s13287-020-01957-4
7. Shekar R, Ranganathan K. Phenotypic and growth characterization of human mesenchymal stem cells cultured from permanent and deciduous teeth. Indian J Dent Res. (2012) 23:838–9. doi: 10.4103/0970-9290.111281
8. Shi X, Mao J, Liu Y. Pulp stem cells derived from human permanent and deciduous teeth: Biological characteristics and therapeutic applications. Stem Cells Transl Med. (2020) 9:445–64. doi: 10.1002/sctm.19-0398
9. Xu JG, Zhu SY, Heng BC, Dissanayaka WL, Zhang CF. TGF-beta1-induced differentiation of SHED into functional smooth muscle cells. Stem Cell Res Ther. (2017) 8:10. doi: 10.1186/s13287-016-0459-0
10. Zhang Z, Oh M, Sasaki JI, Nor JE. Inverse and reciprocal regulation of p53/p21 and Bmi-1 modulates vasculogenic differentiation of dental pulp stem cells. Cell Death Dis. (2021) 12:644. doi: 10.1038/s41419-021-03925-z
11. Xu JG, Gong T, Wang YY, Zou T, Heng BC, Yang YQ, et al. Inhibition of TGF-beta Signaling in SHED Enhances Endothelial Differentiation. J Dent Res. (2018) 97:218–25. doi: 10.1177/0022034517733741
12. Ohkoshi S, Hirono H, Nakahara T, Ishikawa H. Dental pulp cell bank as a possible future source of individual hepatocytes. World J Hepatol. (2018) 10:702–7. doi: 10.4254/wjh.v10.i10.702
13. Li X, Xie J, Zhai Y, Fang T, Rao N, Hu S, et al. Differentiation of stem cells from human exfoliated deciduous teeth into retinal photoreceptor-like cells and their sustainability in vivo. Stem Cells Int. (2019) 2019:2562981. doi: 10.1155/2019/2562981
14. Kim G, Shin KH, Pae EK. Zinc up-regulates insulin secretion from beta cell-like cells derived from stem cells from human exfoliated deciduous tooth (SHED). Int J Mol Sci. (2016) 17. doi: 10.3390/ijms17122092
15. Asadi-Golshan R, Razban V, Mirzaei E, Rahmanian A, Khajeh S, Mostafavi-Pour Z, et al. Sensory and motor behavior evidences supporting the usefulness of conditioned medium from dental pulp-derived stem cells in spinal cord injury in rats. Asian Spine J. (2018) 12:785–93. doi: 10.31616/asj.2018.12.5.785
16. Asadi-Golshan R, Razban V, Mirzaei E, Rahmanian A, Khajeh S, Mostafavi-Pour Z, et al. Efficacy of dental pulp-derived stem cells conditioned medium loaded in collagen hydrogel in spinal cord injury in rats: Stereological evidence. J Chem Neuroanat. (2021) 116:101978. doi: 10.1016/j.jchemneu.2021.101978
17. Chen YR, Lai PL, Chien Y, Lee PH, Lai YH, Ma HI, et al. Improvement of impaired motor functions by human dental exfoliated deciduous teeth stem cell-derived factors in a rat model of Parkinson's disease. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21113807
18. Fujii H, Matsubara K, Sakai K, Ito M, Ohno K, Ueda M, et al. Dopaminergic differentiation of stem cells from human deciduous teeth and their therapeutic benefits for Parkinsonian rats. Brain Res. (2015) 1613:59–72. doi: 10.1016/j.brainres.2015.04.001
19. Zhang N, Lu X, Wu S, Li X, Duan J, Chen C, et al. Intrastriatal transplantation of stem cells from human exfoliated deciduous teeth reduces motor defects in Parkinsonian rats. Cytotherapy. (2018) 20:670–86. doi: 10.1016/j.jcyt.2018.02.371
20. Jarmalaviciute A, Tunaitis V, Pivoraite U, Venalis A, Pivoriunas A. Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine-induced apoptosis. Cytotherapy. (2015) 17:932–9. doi: 10.1016/j.jcyt.2014.07.013
21. Bai X, Zhang X, Wang C, Liu Y, Liu X, Fan Y, et al. Stem cells from human exfoliated deciduous teeth attenuate trigeminal neuralgia in rats. Stem Cells Int. (2021) 2021:8819884. doi: 10.1155/2021/8819884
22. Inoue T, Sugiyama M, Hattori H, Wakita H, Wakabayashi T, Ueda M. Stem cells from human exfoliated deciduous tooth-derived conditioned medium enhance recovery of focal cerebral ischemia in rats. Tissue Eng Part A. (2013) 19:24–9. doi: 10.1089/ten.tea.2011.0385
23. Mita T, Furukawa-Hibi Y, Takeuchi H, Hattori H, Yamada K, Hibi H, et al. Conditioned medium from the stem cells of human dental pulp improves cognitive function in a mouse model of Alzheimer's disease. Behav Brain Res. (2015) 293:189–97. doi: 10.1016/j.bbr.2015.07.043
24. Shimojima C, Takeuchi H, Jin S, Parajuli B, Hattori H, Suzumura A, et al. Conditioned medium from the stem cells of human exfoliated deciduous teeth ameliorates experimental autoimmune encephalomyelitis. J Immunol. (2016) 196:4164–71. doi: 10.4049/jimmunol.1501457
25. Zhang Q, Li Q, Zhu J, Guo H, Zhai Q, Li B, et al. Comparison of therapeutic effects of different mesenchymal stem cells on rheumatoid arthritis in mice. PeerJ. (2019) 7:e7023. doi: 10.7717/peerj.7023
26. Izumoto-Akita T, Tsunekawa S, Yamamoto A, Uenishi E, Ishikawa K, Ogata H, et al. Secreted factors from dental pulp stem cells improve glucose intolerance in streptozotocin-induced diabetic mice by increasing pancreatic beta-cell function. BMJ Open Diabetes Res Care. (2015) 3:e000128. doi: 10.1136/bmjdrc-2015-000128
27. Hattori Y, Kim H, Tsuboi N, Yamamoto A, Akiyama S, Shi Y, et al. Therapeutic potential of stem cells from human exfoliated deciduous teeth in models of acute kidney injury. PLoS ONE. (2015) 10:e0140121. doi: 10.1371/journal.pone.0140121
28. Hirata M, Ishigami M, Matsushita Y, Ito T, Hattori H, Hibi H, et al. Multifaceted therapeutic benefits of factors derived from dental pulp stem cells for mouse liver fibrosis. Stem Cells Transl Med. (2016) 5:1416–24. doi: 10.5966/sctm.2015-0353
29. Ito T, Ishigami M, Matsushita Y, Hirata M, Matsubara K, Ishikawa T, et al. Secreted ectodomain of SIGLEC-9 and MCP-1 synergistically improve acute liver failure in rats by altering macrophage polarity. Sci Rep. (2017) 7:44043. doi: 10.1038/srep44043
30. Yamaza T, Alatas FS, Yuniartha R, Yamaza H, Fujiyoshi JK, Yanagi Y, et al. in vivo hepatogenic capacity and therapeutic potential of stem cells from human exfoliated deciduous teeth in liver fibrosis in mice. Stem Cell Res Ther. (2015) 6:171. doi: 10.1186/s13287-015-0154-6
31. Muhammad SA, Nordin N, Hussin P, Mehat MZ, Abu Kasim NH, Fakurazi S. Protective effects of stem cells from human exfoliated deciduous teeth derived conditioned medium on osteoarthritic chondrocytes. PLoS ONE. (2020) 15:e0238449. doi: 10.1371/journal.pone.0238449
32. Wakayama H, Hashimoto N, Matsushita Y, Matsubara K, Yamamoto N, Hasegawa Y, et al. Factors secreted from dental pulp stem cells show multifaceted benefits for treating acute lung injury in mice. Cytotherapy. (2015) 17:1119–29. doi: 10.1016/j.jcyt.2015.04.009
33. Seo B, Sonoyama W, Yamaza T, Coppe C, Kikuiri T, Akiyama K, et al. SHED repair critical-size calvarial defects in mice. Oral Dis. (2008) 14:428–434. doi: 10.1111/j.1601-0825.2007.01396.x
34. Morillo CMR, Sloniak MC, Goncalves F, Villar CC. Efficacy of stem cells on bone consolidation of distraction osteogenesis in animal models: a systematic review. Braz Oral Res. (2018) 32:e83. doi: 10.1590/1807-3107bor-2018.vol32.0083
35. Farea M, Husein A, Halim AS, Berahim Z, Nurul AA, Mokhtar KI, et al. Cementoblastic lineage formation in the cross-talk between stem cells of human exfoliated deciduous teeth and epithelial rests of Malassez cells. Clin Oral Investig. (2016) 20:1181–91. doi: 10.1007/s00784-015-1601-6
36. Chadipiralla K, Yochim JM, Bahuleyan B, Huang CY, Garcia-Godoy F, Murray PE, et al. Osteogenic differentiation of stem cells derived from human periodontal ligaments and pulp of human exfoliated deciduous teeth. Cell Tissue Res. (2010) 340:323–33. doi: 10.1007/s00441-010-0953-0
37. Winning L, El Karim IA, Lundy FT A. Comparative Analysis of the Osteogenic Potential of Dental Mesenchymal Stem Cells. Stem Cells Dev. (2019) 28:1050–8. doi: 10.1089/scd.2019.0023
38. Nakajima K, Kunimatsu R, Ando K, Ando T, Hayashi Y, Kihara T, et al. Comparison of the bone regeneration ability between stem cells from human exfoliated deciduous teeth, human dental pulp stem cells and human bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. (2018) 497:876–82. doi: 10.1016/j.bbrc.2018.02.156
39. Hara K, Yamada Y, Nakamura S, Umemura E, Ito K, Ueda M. Potential characteristics of stem cells from human exfoliated deciduous teeth compared with bone marrow-derived mesenchymal stem cells for mineralized tissue-forming cell biology. J Endod. (2011) 37:1647–52. doi: 10.1016/j.joen.2011.08.023
40. Sebastian AA, Kannan TP, Norazmi MN, Nurul AA. Interleukin-17A promotes osteogenic differentiation by increasing OPG/RANKL ratio in stem cells from human exfoliated deciduous teeth (SHED). J Tissue Eng Regen Med. (2018) 12:1856–66. doi: 10.1002/term.2706
41. Zhai Y, Wang Y, Rao N, Li J, Li X, Fang T, et al. Activation and biological properties of human beta defensin 4 in stem cells derived from human exfoliated deciduous teeth. Front Physiol. (2019) 10:1304. doi: 10.3389/fphys.2019.01304
42. Lee JM, Kim HY, Park JS, Lee DJ, Zhang S, Green DW, et al. Developing palatal bone using human mesenchymal stem cell and stem cells from exfoliated deciduous teeth cell sheets. J Tissue Eng Regen Med. (2019) 13:319–27. doi: 10.1002/term.2811
43. Prahasanti C, Nugraha AP, Saskianti T, Suardita K, Riawan W, Ernawati DS. Exfoliated human deciduous tooth stem cells incorporating carbonate apatite scaffold enhance BMP-2, BMP-7 and attenuate MMP-8 expression during initial alveolar bone remodeling in wistar rats (Rattus norvegicus). Clin Cosmet Investig Dent. (2020) 12:79–85. doi: 10.2147/CCIDE.S245678
44. Dahake PT, Panpaliya NP, Kale YJ, Dadpe MV, Kendre SB, Bogar C. Response of stem cells from human exfoliated deciduous teeth (SHED) to three bioinductive materials - an in vitro experimental study. Saudi Dent J. (2020) 32:43–51. doi: 10.1016/j.sdentj.2019.05.005
45. Ishiy FAA, Fanganiello RD, Kobayashi GS, Kague E, Kuriki PS, Passos-Bueno MR. CD105 is regulated by hsa-miR-1287 and its expression is inversely correlated with osteopotential in SHED. Bone. (2018) 106:112–20. doi: 10.1016/j.bone.2017.10.014
46. Taguchi T, Yanagi Y, Yoshimaru K, Zhang XY, Matsuura T, Nakayama K, et al. Regenerative medicine using stem cells from human exfoliated deciduous teeth (SHED): a promising new treatment in pediatric surgery. Surg Today. (2019) 49:316–22. doi: 10.1007/s00595-019-01783-z
47. Hochuli AHD, Senegaglia AC, Selenko AH, Fracaro L, Brofman PRS. Dental Pulp from Human Exfoliated Deciduous Teeth-derived Stromal Cells Demonstrated Neuronal Potential: in vivo and In vitro Studies. Curr Stem Cell Res Ther. (2021) 16:495–506. doi: 10.2174/1574888X16666210215160402
48. Nourbakhsh N, Soleimani M, Taghipour Z, Karbalaie K, Mousavi SB, Talebi A, et al. Induced In vitro differentiation of neural-like cells from human exfoliated deciduous teeth-derived stem cells. Int J Dev Biol. (2011) 55:189–95. doi: 10.1387/ijdb.103090nn
49. AE, Alifarja. S, Nourbakhsh. N, Talebi. A. Messenger RNA expression patterns of neurotrophins during transdifferentiation of stem cells from human-exfoliated deciduous teeth into neural-like cells. Avicenna J Med Biotech. (2014) 6:21–26.
50. Taghipour Z, Karbalaie K, Kiani A, Niapour A, Bahramian H, Nasr-Esfahani MH, et al. Transplantation of undifferentiated and induced human exfoliated deciduous teeth-derived stem cells promote functional recovery of rat spinal cord contusion injury model. Stem Cells Dev. (2012) 21:1794–802. doi: 10.1089/scd.2011.0408
51. Wang J, Wang X, Sun Z, Wang X, Yang H, Shi S, et al. Stem cells from human-exfoliated deciduous teeth can differentiate into dopaminergic neuron-like cells. Stem Cells Dev. (2010) 19:1375–83. doi: 10.1089/scd.2009.0258
52. Gonmanee T, Thonabulsombat C, Vongsavan K, Sritanaudomchai H. Differentiation of stem cells from human deciduous and permanent teeth into spiral ganglion neuron-like cells. Arch Oral Biol. (2018) 88:34–41. doi: 10.1016/j.archoralbio.2018.01.011
53. Yang S, Xin C, Zhang B, Zhang H, Hao Y. Synergistic effects of Rho kinase inhibitor Y-27632 and Noggin overexpression on the proliferation and neuron-like cell differentiation of stem cells derived from human exfoliated deciduous teeth. IUBMB Life. (2020) 72:665–76. doi: 10.1002/iub.2208
54. Heng BC, Lim LW, Wu W, Zhang C. An overview of protocols for the neural induction of dental and oral stem cells in vitro. Tissue Eng Part B Rev. (2016) 22:220–50. doi: 10.1089/ten.teb.2015.0488
55. Kim JH, Kim GH, Kim JW, Pyeon HJ, Lee JC, Lee G, et al. in vivo angiogenic capacity of stem cells from human exfoliated deciduous teeth with human umbilical vein endothelial cells. Mol Cells. (2016) 39:790–6. doi: 10.14348/molcells.2016.0131
56. Yuniartha R, Yamaza T, Sonoda S, Yoshimaru K, Matsuura T, Yamaza H, et al. Cholangiogenic potential of human deciduous pulp stem cell-converted hepatocyte-like cells. Stem Cell Res Ther. (2021) 12:57. doi: 10.1186/s13287-020-02113-8
57. Beigi MH, Ghasemi-Mobarakeh L, Prabhakaran MP, Karbalaie K, Azadeh H, Ramakrishna S, et al. in vivo integration of poly(ε-caprolactone)/gelatin nanofibrous nerve guide seeded with teeth derived stem cells for peripheral nerve regeneration. J Biomed Mater Res A. (2014) 109:572. doi: 10.1002/jbm.a.35119
58. Li XX, Yuan XJ, Zhai Y, Yu S, Jia RX, Yang LP, et al. Treatment with stem cells from human exfoliated deciduous teeth and their derived conditioned medium improves retinal visual function and delays the degeneration of photoreceptors. Stem Cells Dev. (2019) 28:1514–26. doi: 10.1089/scd.2019.0158
59. Hiraki T, Kunimatsu R, Nakajima K, Abe T, Yamada S, Rikitake K, et al. Stem cell-derived conditioned media from human exfoliated deciduous teeth promote bone regeneration. Oral Dis. (2020) 26:381–90. doi: 10.1111/odi.13244
60. Sugimura-Wakayama Y, Katagiri W, Osugi M, Kawai T, Ogata K, Sakaguchi K, et al. Peripheral nerve regeneration by secretomes of stem cells from human exfoliated deciduous teeth. Stem Cells Dev. (2015) 24:2687–99. doi: 10.1089/scd.2015.0104
61. Yamada Y, Nakamura-Yamada S, Umemura-Kubota E, Baba S. Diagnostic cytokines and comparative analysis secreted from exfoliated deciduous teeth, dental pulp, and bone marrow derived mesenchymal stem cells for functional cell-based therapy. Int J Mol Sci. (2019) 20. doi: 10.3390/ijms20235900
62. Mussano F, Genova T, Petrillo S, Roato I, Ferracini R, Munaron L. Osteogenic differentiation modulates the cytokine, chemokine, and growth factor profile of ASCs and SHED. Int J Mol Sci. (2018) 19. doi: 10.3390/ijms19051454
63. Narbute K, Pilipenko V, Pupure J, Dzirkale Z, Jonavice U, Tunaitis V, et al. Intranasal administration of extracellular vesicles derived from human teeth stem cells improves motor symptoms and normalizes tyrosine hydroxylase expression in the substantia nigra and striatum of the 6-hydroxydopamine-treated rats. Stem Cells Transl Med. (2019) 8:490–9. doi: 10.1002/sctm.18-0162
64. Heng BC, Zhu S, Xu J, Yuan C, Gong T, Zhang C. Effects of decellularized matrices derived from periodontal ligament stem cells and SHED on the adhesion, proliferation and osteogenic differentiation of human dental pulp stem cells In vitro. Tissue Cell. (2016) 48:133–43. doi: 10.1016/j.tice.2015.12.004
65. Xu Y, Zhou J, Liu C, Zhang S, Gao F, Guo W, et al. Understanding the role of tissue-specific decellularized spinal cord matrix hydrogel for neural stem/progenitor cell microenvironment reconstruction and spinal cord injury. Biomaterials. (2021) 268:120596. doi: 10.1016/j.biomaterials.2020.120596
66. Xiao L, Saiki C, Okamura H. Oxidative stress-tolerant stem cells from human exfoliated deciduous teeth decrease hydrogen peroxide-induced damage in organotypic brain slice cultures from adult mice. Int J Mol Sci. (2019) 20. doi: 10.3390/ijms20081858
67. Zhou LL, Liu W, Wu YM, Sun WL, Dorfer CE, Fawzy El-Sayed KM. Oral mesenchymal stem/progenitor cells: the immunomodulatory masters. Stem Cells Int. (2020) 2020:1327405. doi: 10.1155/2020/1327405
68. Stanko P, Altanerova U, Jakubechova J, Repiska V, Altaner C. Dental mesenchymal stem/stromal cells and their exosomes. Stem Cells Int. (2018) 2018:8973613. doi: 10.1155/2018/8973613
69. Wei J, Song Y, Du Z, Yu F, Zhang Y, Jiang N, et al. Exosomes derived from human exfoliated deciduous teeth ameliorate adult bone loss in mice through promoting osteogenesis. J Mol Histol. (2020) 51:455–66. doi: 10.1007/s10735-020-09896-3
70. Wang M, Li J, Ye Y, He S, Song J. SHED-derived conditioned exosomes enhance the osteogenic differentiation of PDLSCs via Wnt and BMP signaling In vitro. Differentiation. (2020) 111:1–11. doi: 10.1016/j.diff.2019.10.003
71. Wu J, Chen L, Wang R, Song Z, Shen Z, Zhao Y, et al. Exosomes secreted by stem cells from human exfoliated deciduous teeth promote alveolar bone defect repair through the regulation of angiogenesis and osteogenesis. ACS Biomater Sci Eng. (2019) 5:3561–71. doi: 10.1021/acsbiomaterials.9b00607
72. Luo P, Jiang C, Ji P, Wang M, Xu J. Exosomes of stem cells from human exfoliated deciduous teeth as an anti-inflammatory agent in temporomandibular joint chondrocytes via miR-100-5p/mTOR. Stem Cell Res Ther. (2019) 10:216. doi: 10.1186/s13287-019-1341-7
73. Sonoda S, Murata S, Kato H, Zakaria F, Kyumoto-Nakamura Y, Uehara N, et al. Targeting of deciduous tooth pulp stem cell-derived extracellular vesicles on telomerase-mediated stem cell niche and immune regulation in systemic lupus erythematosus. J Immunol. (2021) 206:3053–63. doi: 10.4049/jimmunol.2001312
74. Shang L, Shao J, Ge S. Immunomodulatory functions of oral mesenchymal stem cells: Novel force for tissue regeneration and disease therapy. J Leukoc Biol. (2021) 110:539–52. doi: 10.1002/JLB.3MR0321-766R
75. Sasaki A. Microglia and brain macrophages: an update. Neuropathology. (2017) 37:452–64. doi: 10.1111/neup.12354
76. Zhu S, Min D, Zeng J, Ju Y, Liu Y, Chen X. Transplantation of stem cells from human exfoliated deciduous teeth decreases cognitive impairment from chronic cerebral ischemia by reducing neuronal apoptosis in rats. Stem Cells Int. (2020) 2020:6393075. doi: 10.1155/2020/6393075
77. Jonavice U, Tunaitis V, Kriauciunaite K, Jarmalaviciute A, Pivoriunas A. Extracellular vesicles can act as a potent immunomodulators of human microglial cells. J Tissue Eng Regen Med. (2019) 13:309–18. doi: 10.1002/term.2810
78. Li Y, Yang YY, Ren JL, Xu F, Chen FM, Li A. Exosomes secreted by stem cells from human exfoliated deciduous teeth contribute to functional recovery after traumatic brain injury by shifting microglia M1/M2 polarization in rats. Stem Cell Res Ther. (2017) 8:198. doi: 10.1186/s13287-017-0648-5
79. Tsuruta T, Sakai K, Watanabe J, Katagiri W, Hibi H. Dental pulp-derived stem cell conditioned medium to regenerate peripheral nerves in a novel animal model of dysphagia. PLoS ONE. (2018) 13:e0208938. doi: 10.1371/journal.pone.0208938
80. Nicola F, Marques MR, Odorcyk F, Petenuzzo L, Aristimunha D, Vizuete A, et al. Stem cells from human exfoliated deciduous teeth modulate early astrocyte response after spinal cord contusion. Mol Neurobiol. (2019) 56:748–60. doi: 10.1007/s12035-018-1127-4
81. Silva Fde S, Ramos RN, de Almeida DC, Bassi EJ, Gonzales RP, Miyagi SP, et al. Mesenchymal stem cells derived from human exfoliated deciduous teeth (SHEDs) induce immune modulatory profile in monocyte-derived dendritic cells. PLoS One. (2014) 9:e98050. doi: 10.1371/journal.pone.0098050
82. Yamaza T, Kentaro A, Chen C, Liu Y, Shi Y, Shi S. Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res Ther. (2010) 1:5. doi: 10.1186/scrt5
83. Matsushita Y, Ishigami M, Matsubara K, Kondo M, Wakayama H, Goto H, et al. Multifaceted therapeutic benefits of factors derived from stem cells from human exfoliated deciduous teeth for acute liver failure in rats. J Tissue Eng Regen Med. (2017) 11:1888–96. doi: 10.1002/term.2086
84. Ueda M, Nishino Y. Cell-based cytokine therapy for skin rejuvenation. J Craniofac Surg. (2010) 21:1861–6. doi: 10.1097/SCS.0b013e3181f43f0a
85. Tseng LS, Chen SH, Lin MT, Lin YC. Transplantation of human dental pulp-derived stem cells protects against heatstroke in mice. Cell Transplant. (2015) 24:921–37. doi: 10.3727/096368914X678580
86. Rao N, Wang X, Xie J, Li J, Zhai Y, Li X, et al. Stem cells from human exfoliated deciduous teeth ameliorate diabetic nephropathy in vivo and in vitro by inhibiting advanced glycation end product-activated epithelial-mesenchymal transition. Stem Cells Int. (2019) 2019:2751475. doi: 10.1155/2019/2751475
87. Abdullah MF, Abdullah SF, Omar NS, Mahmood Z, Fazliah Mohd Noor SN, Kannan TP, et al. Proliferation rate of stem cells derived from human dental pulp and identification of differentially expressed genes. Cell Biol Int. (2014) 38:582–90. doi: 10.1002/cbin.10229
88. Nakamura S, Yamada Y, Katagiri W, Sugito T, Ito K, Ueda M. Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J Endodont. (2009) 35:1536–42. doi: 10.1016/j.joen.2009.07.024
Keywords: stem cells derived from human exfoliated deciduous teeth, multipotency, immunomodulatory, regenerative medicine, paracrine activity
Citation: Guo R and Yu J (2022) Multipotency and Immunomodulatory Benefits of Stem Cells From Human Exfoliated Deciduous Teeth. Front. Dent. Med. 3:805875. doi: 10.3389/fdmed.2022.805875
Received: 31 October 2021; Accepted: 10 January 2022;
Published: 15 February 2022.
Edited by:
Luyuan Jin, Capital Medical University, ChinaReviewed by:
Yan Liu, Peking University Hospital of Stomatology, ChinaTakehito Ouchi, Tokyo Dental College, Japan
Copyright © 2022 Guo and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinhua Yu, eXVqaW5odWFAbmptdS5lZHUuY24=
 Rong Guo
Rong Guo Jinhua Yu
Jinhua Yu