- 1Department of Craniofacial Biology, School of Dental Medicine, University of Colorado, Aurora, CO, United States
- 2Graduate Periodontics Program, School of Dental Medicine, University of Colorado, Aurora, CO, United States
Introduction: Natural color of dentin is affected by many variables, including anatomical variations, age, how much dentin is exposed, or how much enamel is covering the dentin. Chlorhexidine gluconate (CHX) has been observed to cause tooth staining, especially of exposed dentin. Risk factors for CHX staining include the amount of time for CHX utilization amongst others. Interestingly, the temperature of the rinse when used has been identified as a risk factor. However, no evidence of the effect of temperature is available in the literature. The purpose of this study was to determine the effect of temperature on dentin staining due to CHX exposure.
Methods: Two studies were done. The first a pilot study at room temperature to determine the time needed to establish staining solutions, a method to evaluate stain intensity, and establish the time needed to stain dentin samples in vitro. The second study exposed dentin samples on a twice daily basis to a 1 min soak in CHX at different temperatures, followed by a period in an unstimulated saliva mixed with black tea mixture. Temperatures tested were 4, 23, 37 and 50°C. Control samples were exposed to only black tea and saliva (no CHX) and tested at 23°C.
Results: The pilot study found that the combination of CHX and black tea causes dentin staining. From this data the sample size needed for the second experiment was calculated, requiring 12 samples per group. Sixty dentin samples were divided amongst 5 groups. The data from this study showed significant darkening of the dentin samples over 18 days. The 4 and 23°C CHX rinses resulted in significant staining compared to the control samples. The 37 and 50°C CHX rinses did not stain significantly more than the control samples.
Conclusions: Chlorhexidine has the ability to cause tooth staining in the presence of chromogens such as those in black tea. Significant darkening was observed at lower temperatures (4 and 23°C) over 18 days, therefore dental professionals may wish to advise gently warming the CHX rinse toward 37°C prior to use to reduce the risk of staining.
Introduction
Tooth stains result from the accumulation of chromogens which are compounds that have color or darker shades that are either accumulated in the tooth (intrinsic) or on the tooth (extrinsic). Chromogens fall into two categories: large organic compounds that have conjugated double bonds in their chemical structure; and metal containing compounds (1). Extrinsic discoloration is caused by any molecule that can attach to the surface of the tooth and remain for varying periods of time, depending on the type of molecule and the reaction that takes place on its application. Chromogens are present in tobacco, tea, coffee, red wine, spices, vegetables, medicines and dental plaque. Color of the stain depends on the molecules present in the source and can range from yellow to dark brown or black. Additionally, similar chromogens may indirectly cause staining by binding to the dental acquired pellicle. The combination of chromogen with polyvalent metal salts such as iron supplements and cationic antiseptics, often without color such as chlorhexidine gluconate (CHX) will produce the black and brown stains (2–4).
The use of CHX has become widespread for the purpose of treating periodontitis (5). One of the recognized side effects of CHX is brown staining on teeth and tongue (6). The mechanism of CHX staining is still not completely understood, however it is thought to be a non-enzyme browning, or process of condensation and polymerization of carbohydrates, peptides and proteins, and creation of melanoidins, the deterioration of the chlorhexidine molecule that releases parachloranilin, resulting in denaturing of protein and formation of metal sulfides (7). Some of the first studies on staining were done in the seventies (8). In vitro studies by Addy et al. (9) and Prayitno et al. (10) demonstrated that dietary products with tannin-containing substances (chromogens) such as tea, red wine will increase the level of CHX staining. It has been reported that CHX stain is more pronounced on exposed dentin than enamel (11), therefore we chose to use dentin for our studies rather than enamel.
There are many studies that involve chromogens to test the coloring ability of CHX, however there are very few studies that evaluate the temperature of the CHX solution in tooth staining. It is not known if the chosen range in temperature can affect chlorhexidine's propensity to cause staining of the enamel. Therefore, the purpose of this inquiry was to test the hypothesis that at lower temperatures, 0.12% chlorhexidine gluconate oral rinse solution will significantly reduce the extrinsic staining of teeth, and at higher temperature the extrinsic staining will significantly increase.
Materials and Methods
IRB Approvals
The Colorado Multiple Institutional Review Board (COMIRB) has approved the collection of unidentified human saliva (COMIRB 13-2935) and the collection of unidentified human teeth from the clinic waste stream for research purposes.
Human teeth that met the inclusion criteria; non-molar teeth that did not have restorations or preexisting stains, were chosen randomly from the supply of teeth extracted by students and faculty of Colorado University School of Dental Medicine Surgery. Teeth were stored in sterilizing tooth storage solution until the experiments.
Preliminary Study
To determine the best possible plan of approach for this study, our team first performed a small-scale pilot study using dentin because CHX stains on dentin are more pronounced than enamel (11). The pilot study helped to determine the sample size from its power, and further methodology. Six human non-molar teeth that had been stored in a sterilizing tooth storage solution were sectioned in halves. See Figure 1 for the steps for sample preparation. The dentin (interior) surfaces were marked with 3 registration marks to be used to define the areas of interest for each sample. Six halves were assigned to Group A (CHX and saliva) and the other 6 assigned to Group B (CHX and black tea). The groups were exposed to CHX at room temperature for an interval of 2 min a day for 20 days (Group A) or 7 days (Group B).
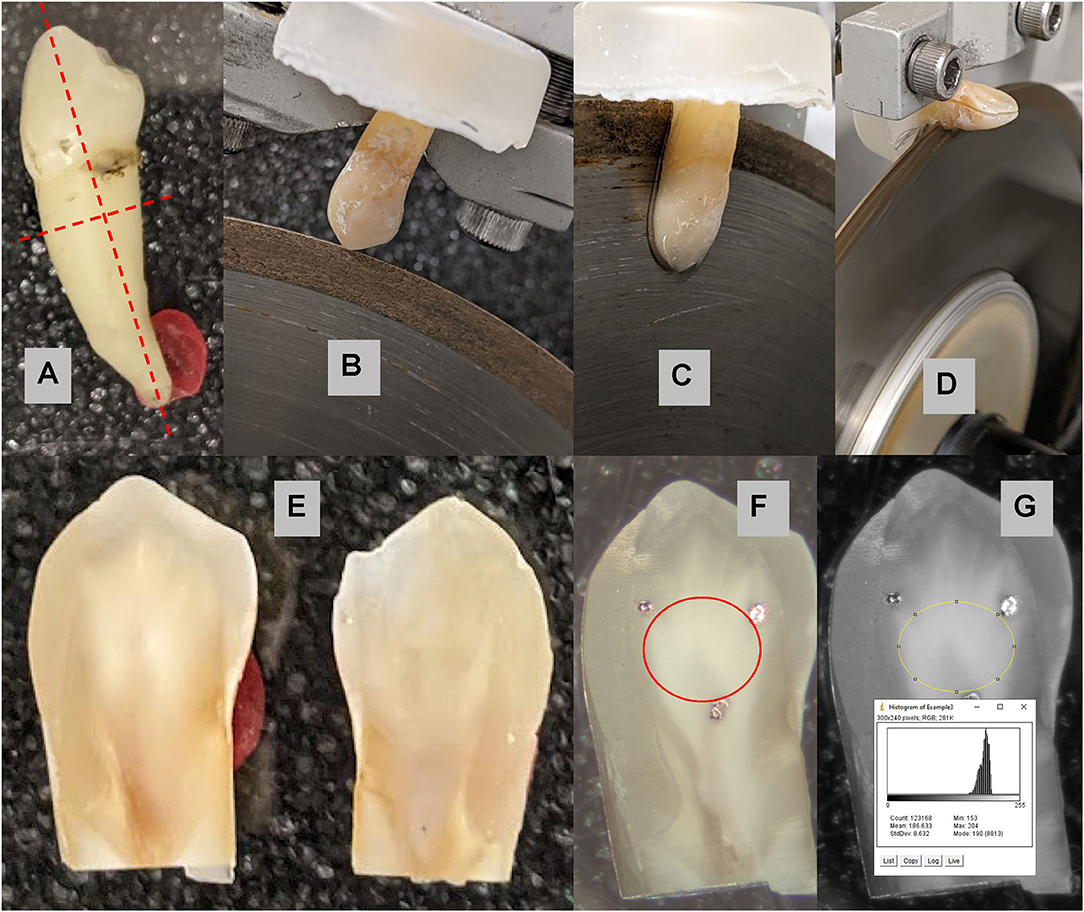
Figure 1. Sample preparation: (A) a non-molar tooth was selected to be bisected and the root removed 2.5–3 mm below the dentin-enamel junction as indicated by the red dashed lines. (B) The tooth is mounted on a slow speed water-cooled diamond saw; and (C) bisected through the crown into the root. (D) The root is removed 2.5–3 mm below the dentin-enamel junction. (E) Two samples from each tooth are (F) marked with three black dots as fiduciary markers indicating the area of interest for the experiments. The area of the dentin inside of the markers marked in red is analyzed for shade change. (G) The average pixel value within the area of interest was 186.6 ± 8.6 on the 256 grayscale range.
The standard tea solution was prepared using one tea sachet of Assam black tea (Two Leaves and a Bud by www.twoleavestea.com) per 100 ml of boiling water which was left to stand for 3 min, decanted and then cooled to room temperature.
Whole unstimulated saliva was collected at the same time each day, centrifuged at 12,000 rpm and using a pipette transferred into a separate container avoiding the heavier solid pellet at the bottom of the test tube. All staining solutions were composed of equal amounts of saliva and tea, including the control.
The teeth were photographed using a microscope (Meiji binocular scope) under a ring light with a built-in digital camera. To ensure proper color standard, black laboratory tape and a white calcium-phosphate (hydroxyapatite) tablet were placed next to each tooth that was photographed. The calibration white and black areas were included in each photograph, see Figure 2 for an example. Exact light setting, exposure (360.584 ms), gain (2.39), gamma (1.55) settings on the camera and magnification in microscope were ensured. Digital images were taken in color and then converted to 8-bit grayscale yielding 256 gray intensities (pixel values) over the full range from black to white for analysis by Image-J software (NIH). The pixel values for the white, and black calibration areas were measured by use of Image-J software (NIH). Figure 1G shows the distribution and average pixel value of the area of interest. These values were converted to percent grayscale where 0 % was the black calibration area and 100 % was the white calibration area. In this way, variations in light or exposure are corrected such that images can be compared over time. The average pixel value for the dentin area of interest was determined and the percent grayscale interpolated from the observed black-white range. The lower the % grayscale the darker the sample. The analysis for all of the teeth was done by AY and repeated by CC. The Houston method was used to determine the coefficient of reliability between the two evaluations which was 96% (12).

Figure 2. An example of a tooth sample with the reference black and white areas needed for assessment of stain.
Main Study
Based on the pilot study, the full study required 60 samples. Thirty human teeth were sectioned in halves to yield 60 samples. The dentin surface was marked with 3 black dots to define the area of interest for each sample for serial comparisons. At that time all teeth were photographed, as described above, and kept in 100% centrifuged whole saliva for 48 h at room temperature to ensure that the teeth remained hydrated.
Samples were divided into five groups of 12 samples: four groups to test tooth exposure to CHX equilibrated at different temperatures and the fifth group served as the control. Sample halves from the same tooth were never together within a group, otherwise the samples were randomly assigned to the groups.
Bottles of CHX from the same lot were equilibrated at 4, 25, 37 or 50°C. Group 1 was assigned to 25°C, Group 2 was at 4°C, Group 3 was at 37°C, Group 4 at 50°C, and Group 5 was at room temperature with exposure to only saliva/tea (no CHX) served as the control.
This experiment consisted of constant exposure of teeth to saliva-tea mixture, however twice per day, generally 8:00 am and 4:00 pm, the teeth were exposed to a 1 min soak in 2 ml of 0.12% CHX at the appropriate temperature for each Group. Tea with newly collected saliva were mixed and each Group was submerged back into it until the next day. On days 2, 4, 8, 12, 15, and 18 each batch was photographed following the methods described above.
Data Collection
Photographs taken using the Meiji binocular scope using the method described for the pilot study. Images were stored on the laboratory hard drive until evaluation. Image-J software (NIH) was used to analyze the gray-scale percentage for comparisons.
Statistical Analysis Methods
For the pilot study an ANOVA was used to determine differences over time. Based on a power analysis from the pilot study data the appropriate sample size was calculated. Statistical analysis of the main data set was performed using ANOVA Two-Factor with Replication and a Student's t-test to determine the statistical significance of the difference between mean values of day 0 and day 18 of the samples.
Results
In the pilot study, the mean and standard deviation were calculated for each group (n = 6) at each day of measurement. Figure 3 shows the results from Groups 1 and 2. For Group 1 (CHX/saliva/no tea) where there were no significant differences over time (p = 0.8308, ANOVA). Figure 4 shows Group 2 (CHX/saliva/tea) where significant staining was observed from day 0 to day 7 (p = 0.0295, t-test). The sample size for the main study was calculated from the observed variation Group B of ± 6 % grayscale and an assumed clinically significant change in shade of the teeth of 7 % grayscale. Twelve samples per each experimental group was calculated to be needed to achieve a power of 80% at an alpha of 0.05.
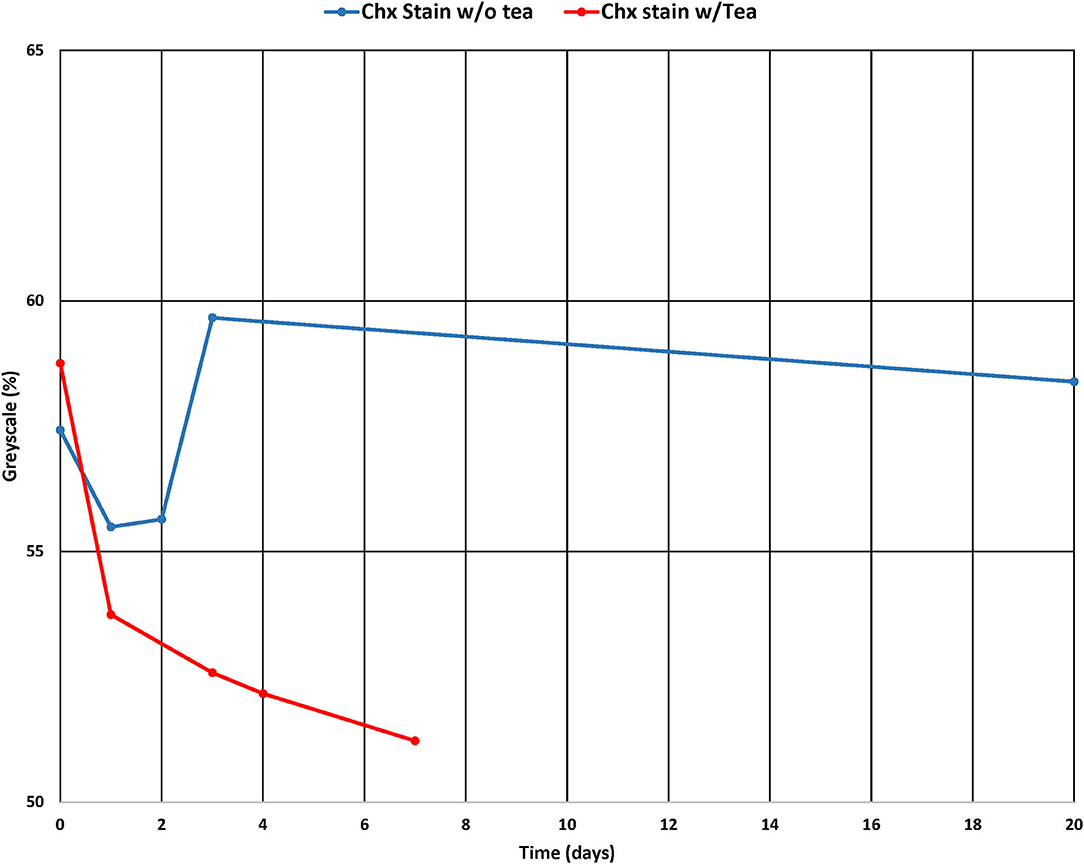
Figure 3. Grayscale % of the control samples and CHX-tea exposed samples over time of the pilot study.
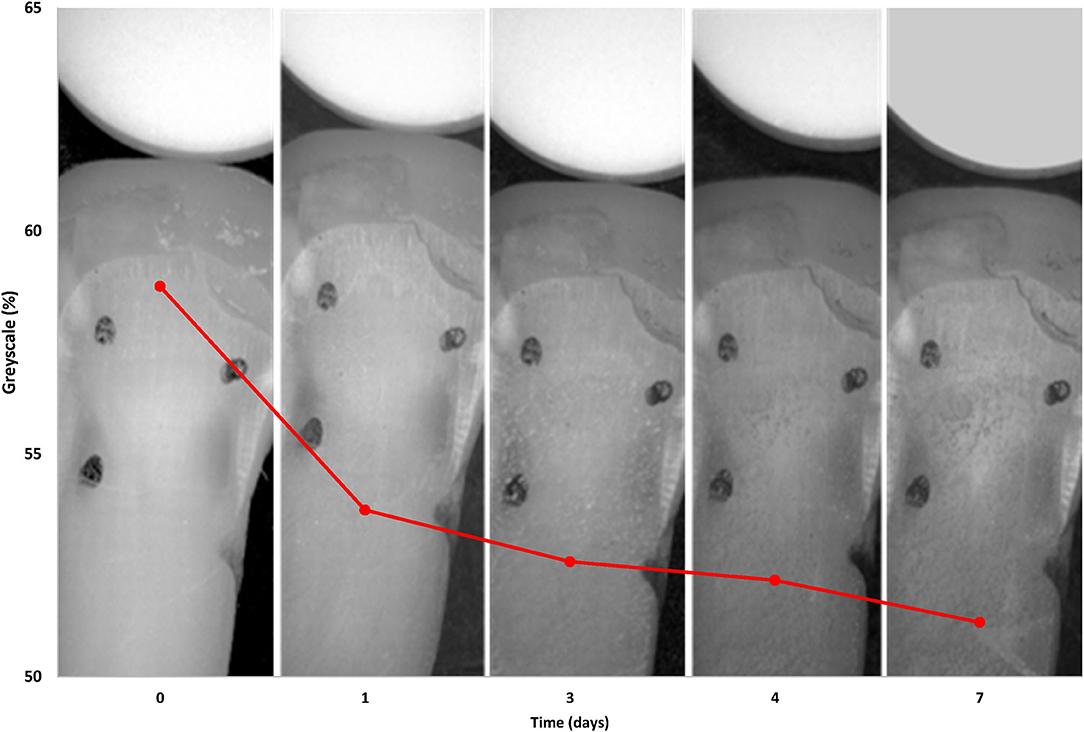
Figure 4. The grayscale % graph of the CHX-tea exposed group overlaid on an image of the dentin tooth samples showing the effect of CHX-tea staining over time.
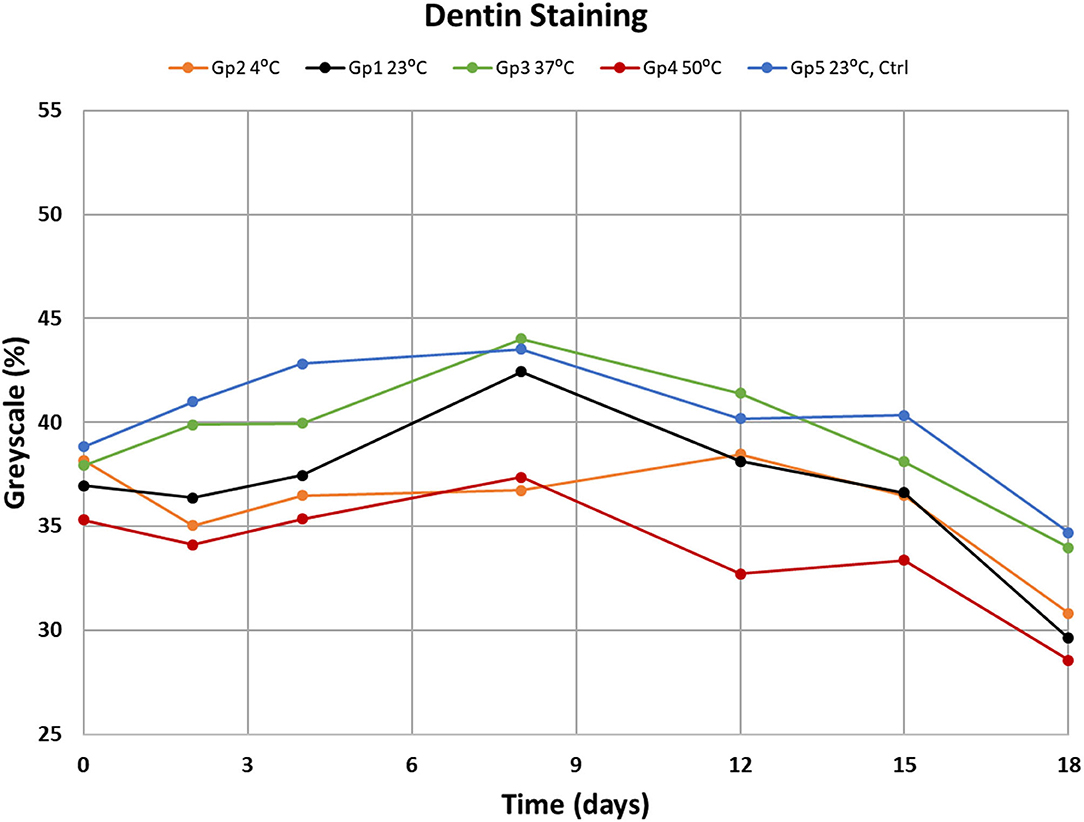
In the main study on the effect of temperature the staining over 18 days led to darker samples (Figure 5). The analysis of variance among groups and days showed that there were statistically significant changes over time (p = 0.0001) and temperature (p = 0.0003). The ANOVA test did not find significant differences due to interactions between Groups and Days. Comparisons between temperature groups showed significant darkening for the groups for 4 and 23°C at p = 0.0285 and p = 0.0447, respectively. With 19.3% darkening of the dentin at 4°C and 19.9% change at 25°C in after 18 days of experimentation. At 37 and 50°C the no statistically significant change in grayscale % was observed with p = 0.2343 and 0.2267, respectively. The control group (23°C without tea exposure) did not change significantly with p = 0.1599. A graphical analysis of the data (Figure 6) showed a polynomial relationship between the starting grayscale value and the 18-day grayscale value with R2 value of 0.7663 (p < 0.001). In almost every case the 18-day grayscale % value is lower (darker) than the initial 0-day value. The shape of the curve indicates that the greatest darkening occurred on the dentin that started with the lightest amount of color and the samples with the darkest color at the start did not change much.
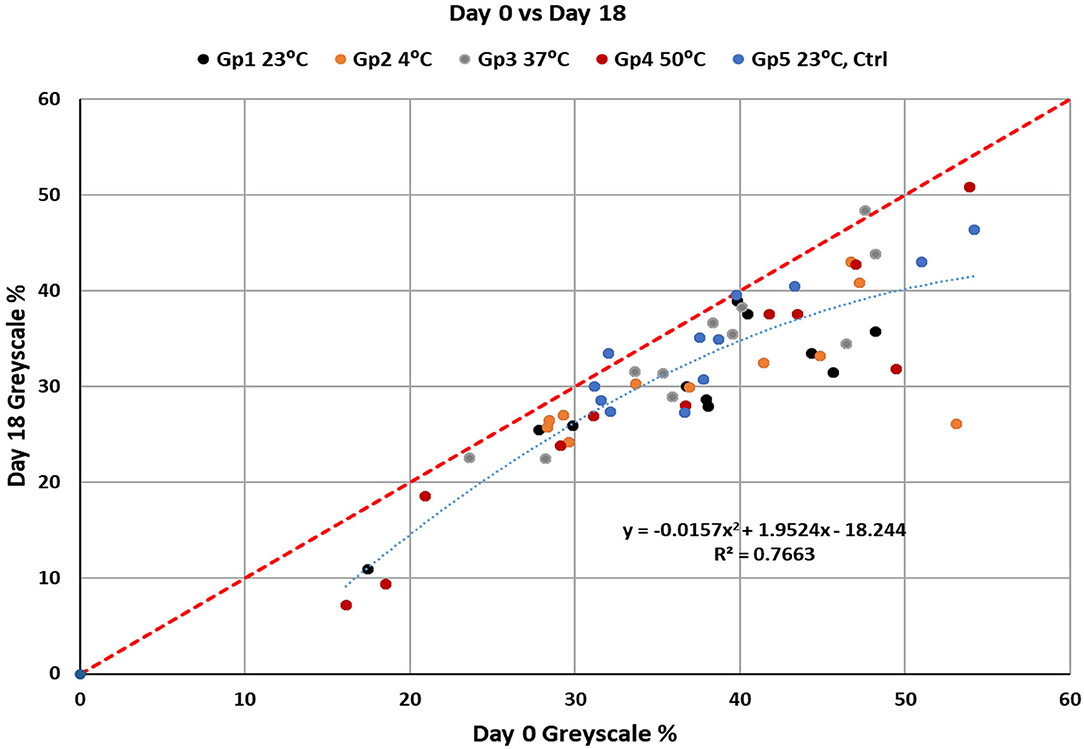
Figure 6. The relationship between change in grayscale % from the value at day 0 to day 18. The polynomial fit of the data shows that lighter dentin samples will take up more change in grayscale % than darker dentin samples over time.
Discussion
Our data are consistent with previous studies on CHX staining where tooth staining progresses with usage duration (13). Our hypothesis that staining increases as the temperature of the CHX increases is rejected. We found that the increment of staining over 18 days was highest at the lower temperatures tested.
The microscopic structure of teeth and specifically the enamel, crystalline structure of which has the remarkable hardness, however it remains permeable to a limited amount of even smaller substances (14). There have been a number of theories attempting to provide the most feasible theory for CHX's ability to stain tooth enamel. One of those pertains to the fact that the chemical denatures the biofilm which results in the deposition of sulfides (4). Another theory is that CHX binds to organic iron released from blood cells and oxidizes the bacterial remains, causing darkening and discoloration of the enamel and dentin (15). Temperature of the CHX rinse has been suggested as a possible factor that leads to staining, however no studies are available to substantiate the suggestion.
Therefore this study was carried out to determine how temperature affects the capacity of CHX to stain dentin. We chose a range of temperatures to test based on natural and environmental factors that pertain to a person's daily life leading to question whether our environment can affect treatments using CHX. The lowest temperature, 4°C, corresponds to refrigerated rinse; room temperature, 23°C, is the likely storage temperature of the rinse; body temperature, 37°C, is the temperature the rinse may reach while in use; and the highest temperature, 50°C, was set at the hottest safe temperature for hot liquid drinks (16). The control sample was tested at room temperature to correspond to the most likely temperature for CHX rinse storage.
It's also important to mention the reasoning behind choosing the method used to collect and process saliva for utilization. To avoid biological rhythmic variation, in the same way it was mentioned in Sheen et al. (17) have pointed out that unstimulated saliva has a tendency to react with CHX to create greater amounts of staining. They postulated that stimulated saliva has less effect on pellicle formation and less mucins that absorb stain (17).
Limitations
Limitations of this study include that a 1 min immersion in the CHX rinse may not have been sufficient to develop the maximum difference in coloration (18) especially when compared to the 3-week immersion in the solution described by Kouadio et al. (19). The natural saliva that was used, although centrifuged to settle the debris, was probably inconsistent from day to day and thus might not be considered to be a standardized solution for immersing the teeth despite controlling the timing of collection. The time span of our experiments, 18-days, may not have been sufficiently long to develop stains that are significantly different between the CHX temperatures tested. Our method of evaluation of shade change is based on the relative grayscale of the photographs and not the spectral color of the teeth. These limitations reduce the generalizability of our results to other laboratories, yet these limitations still allow an evaluation of temperature effects on dentin staining.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
CC helped with the experimental design, data analysis and writing the manuscript. AY performed the experiments, collected the data, and writing the manuscript. KF oversaw the project including experimental design and writing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Funding for the publication fees comes from the University of Colorado Foundation, Carey Laboratory Foundation Account. Funding for this project came from the Department of Periodontics and the Department of Craniofacial Biology.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Carey CM. Tooth whitening: what we now know. J Evid Base Dent Pract. (2014) 14:70–6. doi: 10.1016/j.jebdp.2014.02.006
2. Sulieman M. An overview of bleaching techniques: I. History, chemistry, safety and legal aspects. Dent Update. (2004) 31:608–16. doi: 10.12968/denu.2004.31.10.608
3. Flötra L, Gjermo P, Rölla G, Waerhaug J. Side effects of chlorhexidine mouth washes. Scand J Dent Res. (1971) 79:119–25. doi: 10.1111/j.1600-0722.1971.tb02001.x
4. Watts A, Addy M. Tooth discolouration and staining: a review of the literature. Br Dent J. (2001) 190:309–16. doi: 10.1038/sj.bdj.4800959
5. Lang NP, Brecx MC. Chlorhexidine digluconate- an agent for chemical plaque control and prevention of gingival inflammation. J Perio Res. (1986) 21:74–89. doi: 10.1111/j.1600-0765.1986.tb01517.x
6. Addy M, Moran J, Newcombe R, Warren P. The comparative tea staining potential of phenolic, chlorhexidine and anti-adhesive mouthrinses. J Clin Periodontol. (1995) 22:923–8. doi: 10.1111/j.1600-051X.1995.tb01796.x
7. Bernardi F, Pincelli MR, Carloni S, Gatto MR, Montebugnoli L. Chlorhexidine with an anti discoloration system. A comparative study. Int J Dent Hyg. (2004) 2:122–6. doi: 10.1111/j.1601-5037.2004.00083.x
8. Ellingsen JE, Rolla G, Eriksen HM. Extrinsic dental stain caused by chlorhexidine and other denaturing agents. J Clin Periodontol. (1982) 9:317–22. doi: 10.1111/j.1600-051X.1982.tb02098.x
9. Addy M, Prayitno S, Taylor L, Cadogan S. An in vitro study of the role of dietary factors in the aetiology of tooth staining associated with the use of chlorhexidine. J Periodontal Res. (1979) 14:403–10. doi: 10.1111/j.1600-0765.1979.tb00238.x
10. Prayitno S, Taylor L, Cadogan S, Addy M. An in vivo study of dietary factors in the aetiology of tooth staining associated with the use of chlorhexidine. J Periodontal Res. (1979) 14:411–7. doi: 10.1111/j.1600-0765.1979.tb00239.x
11. Hofer D, Meier A, Sener B, Guggenheim B, Attin T. Schmidlin PR. Biofilm reduction and staining potential of a 005% chlorhexidine rinse containing essential oils. Int J Dent Hyg. (2011) 9:60–7. doi: 10.1111/j.1601-5037.2009.00437.x
12. Houston WJB. The analysis of errors in orthodontic measurements. Am J Orthod. (1983) 83:382–90. doi: 10.1016/0002-9416(83)90322-6
13. Zanatta FB, Antoniazzi RP. Rosing CK. Staining and calculus formation after 012% chlorhexidine rinses in plaque-free and plaque covered surfaces: a randomized trial. J Appl Oral Sci. (2010) 18:515–21. doi: 10.1590/S1678-77572010000500015
14. Paulsen DF. Chapter 15. Digestive tract. In: Histology & Cell Biology: Examination & Board Review, 5e. New York, NY: The McGraw-Hill Companies (2010).
15. Pani SC, Alenazi FM, Alotain AM, Alanzi HD, Alasmari AS. Extrinsic tooth staining potential of high dose and sustained release iron syrups on primary teeth. BMC Oral Health. (2015) 15:90. doi: 10.1186/s12903-015-0072-0
16. Brown F, Diller KR. Calculating the optimum temperature for serving hot beverages. Burns. (2008) 34:648–54. doi: 10.1016/j.burns.2007.09.012
17. Sheen S, Banfield N, Addy M. The effect of unstimulated and stimulated whole saliva on extrinsic staining in vitro—a developmental method. J Dent. (2002) 30:365–9. doi: 10.1016/S0300-5712(02)00053-2
18. Addy M, Moran J, Daviews RM, Beak A, Lewis A. The effect of single morning and evening rinses of chlorhexidine on the development of tooth staining and plaque accumulation. A blind cross-over trial. J Clin Periodontol. (1982) 9:134–140. doi: 10.1111/j.1600-051X.1982.tb01229.x
Keywords: chlorhexidine, stain, temperature, stain assessment, enamel
Citation: Carey CM, Yagudayev A and Font K (2021) Effect of Temperature on Tooth Staining by 0.12% Chlorhexidine Gluconate. Front. Dent. Med. 2:779852. doi: 10.3389/fdmed.2021.779852
Received: 20 September 2021; Accepted: 29 November 2021;
Published: 23 December 2021.
Edited by:
Vanessa Cavalli, State University of Campinas, BrazilCopyright © 2021 Carey, Yagudayev and Font. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Clifton M. Carey, Y2xpZnRvbi5jYXJleUBjdWFuc2NodXR6LmVkdQ==
†These authors have contributed equally to this work and share first authorship
 Clifton M. Carey
Clifton M. Carey Arthur Yagudayev2†
Arthur Yagudayev2†