- 1Department of Endodontics and Restorative Dental Medicine, School of Dental Medicine Zagreb, Zagreb, Croatia
- 2Health Center Zagreb, Zagreb, Croatia
- 3Radiotherapy Unit, Department of Oncology, University Hospital Centre Zagreb, Zagreb, Croatia
Introduction: The aim of this study was to evaluate the effects of radiation and tooth bleaching on the physical and morphological properties of enamel and dentin on permanent teeth.
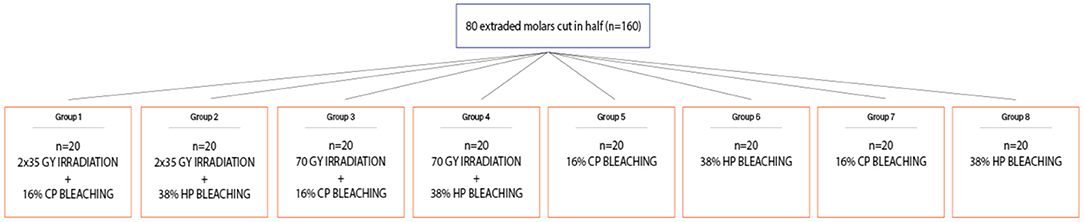
Materials and Methods: Eighty fresh, non-carious third molars were used in this study. Before cutting the crown in half, the teeth samples were randomly allocated to treatment and control groups by using a lottery method. The first group (n = 20) underwent standard radiation protocol (2 Gy/fraction/day, 5 days/week) with bleaching treatment afterward using 16% carbamide peroxide gel, the second group (n = 20) underwent standard radiation protocol with afterward bleaching treatment using 38% hydrogen peroxide, the third group (n = 20) underwent a short, one strong, experimental dose of 70 Gy with afterward bleaching treatment using 16% carbamide peroxide gel, and the fourth group (n = 20) underwent one strong, experimental dose of 70 Gy with afterward bleaching treatment using 38% hydrogen peroxide gel. Groups 5–8 (n = 20) served as control as they underwent only bleaching treatment. Vickers microhardness and surface roughness were performed before (initial) and after irradiation and before bleaching or after only bleaching. The effects of irradiation and bleaching on microhardness (or roughness) of enamel and dentin were analyzed in the repeated-measures ANOVA model.
Results: Enamel microhardness after experimental single 70-Gy irradiation or after standard radiation protocol and bleaching with 16 or 38% gel was not statistically significant from microhardness in the control group (p > 0.05). There was a statistically significantly greater reduction in the average microhardness of enamel and dentin during bleaching with 38% gel compared to 16% for both radiation protocols (p < 0.001). After experimental 70-Gy irradiation and bleaching, a 16% statistically significant increase in surface roughness was found for enamel (p = 0.006) and dentin (p = 0.018), while this was not recorded for 38% gel. There was a statistically significantly greater increase in the average roughness of enamel and dentin during bleaching with 38% gel compared to 16% (p < 0.001) for both radiation protocols.
Conclusions: Directly induced radiation leads to potential damage of hard dental tissues, which can be further damaged by additional bleaching. If teeth whitening is necessary after irradiation, it is suggested to use lower concentrations of whitening gels.
Introduction
Head and neck cancers are malignant diseases that involve soft and hard tissues of the head and neck, including the oral cavity (1). Compared to other malignancies, the incidence of these cancers has been increasing in the last decade, and cancers of the tongue, salivary glands, and hypopharynx constitute a significant share (2, 3). In addition to chemotherapy, radiotherapy is used to treat head and neck cancer, and the consequences of these procedures are pain, inflammation of the skin and mucous membranes in the area of radiation, reduced salivation, which later leads to poor oral hygiene, caries, periodontal disease, and reduced quality of life (4, 5). Saliva has a number of protective functions, so hyposalivation leads to a higher incidence of caries due to the inability to maintain a favorable pH, increased acidity in the composition of plaque, and failure to maintain the natural microflora in the mouth. Also, radiation can lead to direct damage to hard tooth structures, and this is considered to be one of the reasons for the faster development of caries in irradiated persons (6). Lack of saliva and increased accumulation of plaque, in addition to caries, also encourages the development of periodontal disease, which leads to premature extraction and loss of teeth. Thus for these patients, it is very important to practice good oral hygiene (7). Radiation-induced tooth decay begins to occur in the first year after radiotherapy and becomes more severe over time (6). Minimal tooth damage occurs below 30 Gy radiation, while two to three times increased risk of tooth damage and caries development occurs between doses of 30 and 60 Gy, which is probably associated with reduced salivation, and 10 times increased risk of tooth and salivary damage occurs when the dose is >60 Gy (8). Doses >60 Gy reported changes in hard dental tissues including decreased microhardness, change in elastic modulus, and tensile strength, as well as an increased possibility of enamel fracture (9–11). So, it can be concluded that the direct effect of radiation on hard dental structures amplifies with increasing total radiation dose.
According to the guidelines for patients suffering from head and neck cancer, they should be informed about possible oral complications and the need for prophylactic and therapeutic procedures. Dental examinations are, therefore, recommended to be performed more frequently, while oral hygiene must be carried out seriously and thoroughly, and daily topical application of high fluoride concentrations during and after radiotherapy is recommended. Fluoridation can be carried out using splints, pastes with high fluorine concentrations of 5,000 ppm, or fluorine solutions (12, 13). Periodontitis must also be addressed promptly, and frequent visits to the dentist are, therefore, recommended (14).
Teeth whitening is considered to be the simplest and easiest procedure to change tooth color. To date, studies on the effects of products based on hydrogen peroxide and carbamide peroxide on the structure of the tooth are still not final. While some authors describe that it does not lead to any harmful effects, others argue that the use of such products may be associated with many side effects, which include surface changes of enamel and dentin such as reduced surface microhardness, reduced calcium and phosphate levels with the loss of organic components from the treated tooth, gingival irritation, modifications in surface morphology, and tooth hypersensitivity, which are usually greater when using higher concentrations of bleaching gels without some remineralizing effects of saliva or other post-treatment agents, which can neutralize such effect (15–18). Changes in the surface integrity of the enamel crystals with a higher susceptibility to demineralization can also occur after bleaching (19), which can be prevented by the use of remineralizing agents (20, 21). Bleaching agents also affect the chemical structure of hard dental tissues. The main reaction of the bleaching process is oxidation. Decreases in microhardness or changes in chemical structure are primarily the result of oxidation processes in enamel and dentin, whereby organic matter and inorganic matter are involved (18, 22). Surface changes after bleaching can be assessed by microhardness tests, scanning electron microscopy (SEM), and measuring surface roughness (15, 16).
It is important to study the effect of radiation on hard dental tissues and to develop effective strategies to prevent radiation caries, dry mouth syndrome, and possible later discoloration and to maintain better overall oral health. Dry mouth increases the risk of dental staining and discoloration as a result of enamel erosion and increased levels of plaque and food debris on the teeth. The problem of xerostomia after irradiation includes moderate to a severe loss of tooth structure, erosive pitting lesions, and also the use of chlorhexidine to prevent the formation of early caries and periodontal disease, which can also lead to severe tooth discoloration (23–25), so the need for bleaching after irradiation treatment is sometimes required. There is also an increase in the number of young people with head and neck cancer who want to still have healthy and white teeth even after the radiotherapy and to maintain a quality life.
The aim of this study was to evaluate the combined effect of different irradiation protocols with later bleaching treatments on the mechanical and morphological properties of enamel and dentin on permanent teeth. The null hypothesis of this study is that (I) there is no statistically significant difference between the non-irradiated teeth and the teeth exposed to radiation and afterward bleaching in terms of microhardness and surface roughness and that (II) there is no statistically significant difference between the different bleaching systems in terms of microhardness and surface roughness.
Materials and Methods
Sample Preparation
The teeth samples were prepared by one examiner who was trained and calibrated. Eighty fresh, non-carious third molars were extracted from non-irradiated individuals and were cleaned and stored in 1% chloramine solution at room temperature immediately after extraction. The use of extracted human teeth has been approved by the Ethics Committee of the School of Dental Medicine, University of Zagreb, and University Hospital Center Zagreb, Croatia. The roots of the teeth were cut from the crown part using a diamond saw (Isomet, Buehler Dusseldorf, Germany) about 2 mm below the enamel–cement joint. The crowns of the teeth were stored in deionized water at 4 degrees. The pulp chamber was cleaned of the remaining pulp tissue. The tooth crown was embedded in an acrylic resin (AcryFix Kit; Struers, Ballerup, Denmark) and then cut in half, and the round side parts were also cut to get a flat surface using the same Isomet saw. Before cutting the crown in half, the teeth samples were randomly allocated to treatment and control groups by using a lottery method (Figure 1).
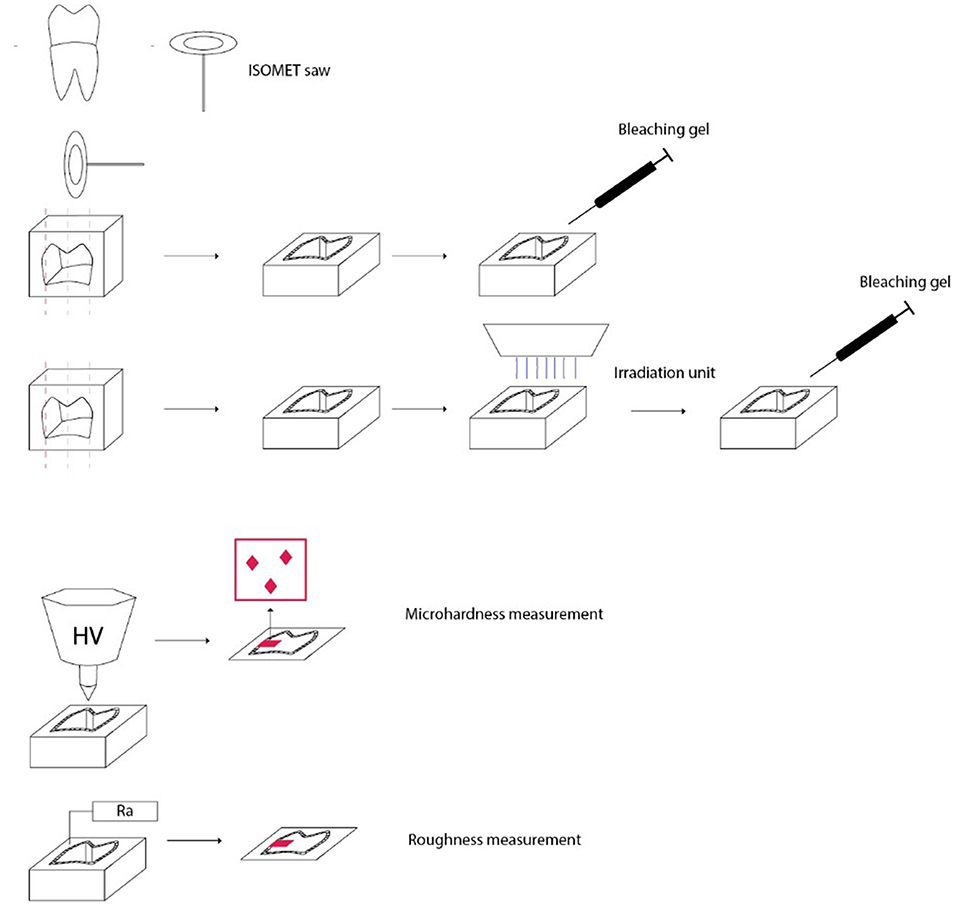
Figure 1. Preparation of teeth samples, irradiation and bleaching procedures, and different measurement protocols.
The first group (n = 20) underwent the standard radiation protocol (2 Gy/fraction/day, 5 days/week) with afterward bleaching treatment using 16% carbamide peroxide gel, the second group (n = 20) underwent the standard radiation protocol (2 Gy/fraction/day, 5 days/week) with afterward bleaching treatment using 38% hydrogen peroxide, the third group (n = 20) underwent a short, one strong, experimental dose of 70 Gy with afterward bleaching treatment using 16% carbamide peroxide gel, and the fourth group (n = 20) underwent one strong, experimental dose of 70 Gy with afterward bleaching treatment using 38% hydrogen peroxide gel. The other four groups served as control as they underwent only bleaching treatment, without irradiation first, so the fifth group (n = 20) was bleached using 16% carbamide peroxide gel, and the sixth group (n = 20) was bleached using 38% hydrogen peroxide gel and was used for control and comparison to groups 1 and 2. The seventh group (n = 20) was bleached using 16% carbamide peroxide gel, and the eighth group (n = 20) was bleached using 38% hydrogen peroxide gel and was used for control and comparison to groups 3 and 4 (Figure 2). Teeth were stored in deionized water at 37°C (Cultura Incubator, Ivoclar Vivadent, Schaan, Liechtenstein) in between the measurements.
To measure the surface microhardness and roughness, tooth halves that were embedded in an acrylic resin with the vestibular or oral surfaces facing upward and free of acrylate resin, and parallel to the table surface, were used. Samples of the labial or oral surface were polished using water-cooled disks (Water Proof Silicon Carbide Paper, 4000 grit; Buehler, Dusseldorf, Germany) and 1.0-, 0.3-, and 0.05-μm powder particle sizes for polishing (Buehler, Dusseldorf, Germany). The entire process on the polishing machine (Minitech 250, Presi, France) was carried out by a single operator. All specimens were rinsed ultrasonically with deionized water for 5 min.
Irradiation Procedure
To simulate oral cancer radiotherapy, teeth sections in groups 1 and 2 were exposed to 2-Gy fractions, 5 days a week for 7 weeks for a total of 35 fractions equal to 70 Gy (a common dose for oral cancer). During the weekend, the samples were not irradiated (26). Prepared samples in groups 3 and 4 were irradiated with one single experimental dose of 70 Gy during only one irradiation fraction. Irradiation was performed at the Department of Oncology, University Hospital Center Zagreb, with a linear accelerator Siemens Primus (Siemens Healthineers AG, Erlangen, Germany) radiotherapy unit. A 6 MV radiation beam was used with SSD (source to surface distance) of 100-cm setup for sample irradiation. Two centimeters of buildup material was placed above and below samples to ensure sufficient buildup and scatter conditions. The groups 5–8 were kept in deionized water without irradiation exposure. Between the radiation cycles, the fragments were stored in deionized water in an incubator at 37°C, which was renewed daily.
Bleaching Procedure
In groups 1, 3, 5, and 7, 16% I-Smile carbamide peroxide gel (Siauliai, Lithuania) was applied on the enamel and dentin surface and was in contact for 1 h; then, the gel was removed and another day new gel was applied for again 1 h, and this procedure was repeated for 7 days in total. In groups 2,4, 6, and 8, enamel and dentin were bleached with 38% hydrogen peroxide gel BMS White (Pisa, Italy) for 15 min, and then, the old gel was removed and the new gel was again applied for another 15 min and the procedure was repeated for one more session of 15 min (45 min in total). In both procedures, using 16% CP or 38% HP, bleaching gel was applied directly from the syringe in a 2-mm-thick layer. The materials used are described in Table 1. During the bleaching treatment, the specimen was on a cotton pellet soaked with deionized water. After the bleaching procedure, the bleaching gel was removed with a Heidemann spatula and cotton pellet, and the surface was cleaned with deionized water, dried with compressed air and cotton tissues, and another layer of bleaching gel was put on the surface. When the specimens were not being bleached, they were placed in deionized water at 37 degrees in an incubator.
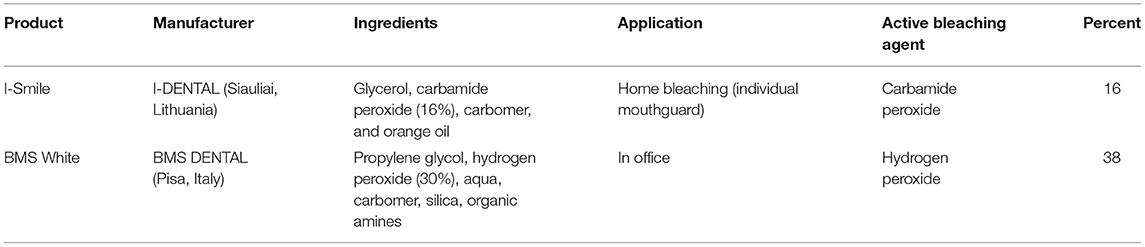
Table 1. Summarized bleaching products (data given by the manufacturer) including ingredients, application, active bleaching agent, and percentage concentration of hydrogen or carbamide peroxide.
pH Measurements
For pH measurements, a pH meter (Pinnacle 555 pH/ion meter, Corning, Tewksbury, United States) was used. The pH meter was initially calibrated. The bleaching gels were placed in 30-ml graduated plastic cups. The pH electrode was immersed inside the gel to allow a uniform contact with the electrode tip. The bleaching gels were in contact with the pH electrode for 20 min at room temperature (24°C). The electrode was thoroughly washed between samples. The measured pH of I-Smile was 6.75, while the pH of BMS White was 5.7.
Surface Microhardness Analysis
The microhardness of enamel and dentin was measured in groups 1–4 before (initial measurement) and at the end of irradiation and afterward bleaching treatment and in groups 5–8 before (initial measurement) and at the end of bleaching treatment. Measurements were carried out using the Vickers microhardness test (ESI Prüftechnik GmbH, Germany) with a force of 100 g for 10 s (27, 28). A diamond pyramid applied to the sample surface was used for measurement. Vickers measurements (HV) were performed at three different points on both enamel and dentin: surface, middle, and deep parts (at 50-μm intervals). The average of all three Vickers hardness values obtained from enamel and dentin was recorded as the total value of enamel and dentin hardness (29, 30).
Surface Roughness Analysis
Surface roughness (Ra μm) of enamel and dentin was measured in groups 1–4 before (initial measurement) and at the end of irradiation and afterward bleaching treatment and in groups 5–8 before (initial measurement) and at the end of bleaching treatment using Mitutoyo Surftest SJ-210 Series 178-Portable Surface Roughness Tester (Houston, United States) calibrated at a 0.25-mm cutoff and 0.2 mm/s speed. Three measurements were performed, and the mean value for each sample was calculated (29, 31, 32).
Statistical Analysis
The effects of irradiation and bleaching on microhardness (or roughness) of enamel and dentin were analyzed in the repeated-measures ANOVA model, which included two factors: type of irradiation and type of bleaching gel. The unit of analysis was the change score from baseline microhardness (or roughness) measurements after irradiation and after additional bleaching treatment. Normality of residuals was satisfactory, with indicators of asymmetry and kurtosis within the acceptable limits. Heterogeneity in variance was detected between different bleaching treatments and was accommodated by adding the separate error variance parameters for different bleaching treatments into the ANOVA model. Bonferroni–Holm multiplicity adjustment was used for planned pairwise comparisons between different treatments. The results were analyzed at a significance level of 0.05. The analysis was performed in the SAS System software package (SAS Institute Inc., North Carolina, United States).
Results
The distributions of the measured values of microhardness (Figures 3, 4) and roughness (Figures 5, 6) after different treatments are graphically shown in Box-plot diagrams.
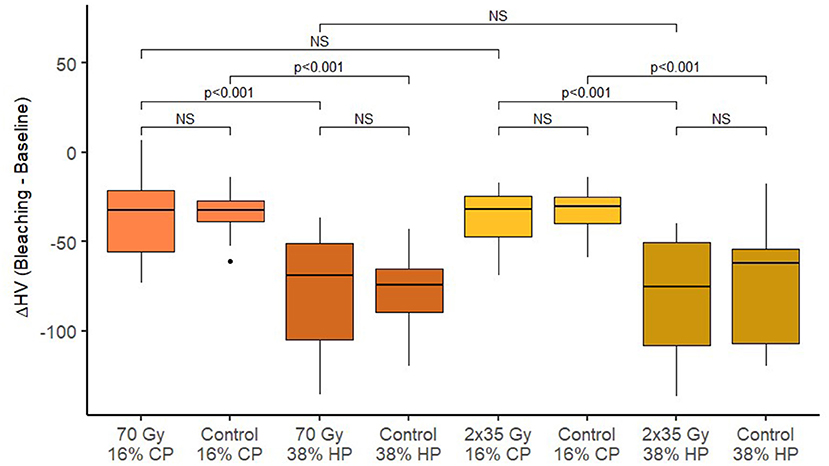
Figure 3. Box-plots of changes in enamel Vickers microhardness after bleaching across different treatment groups.
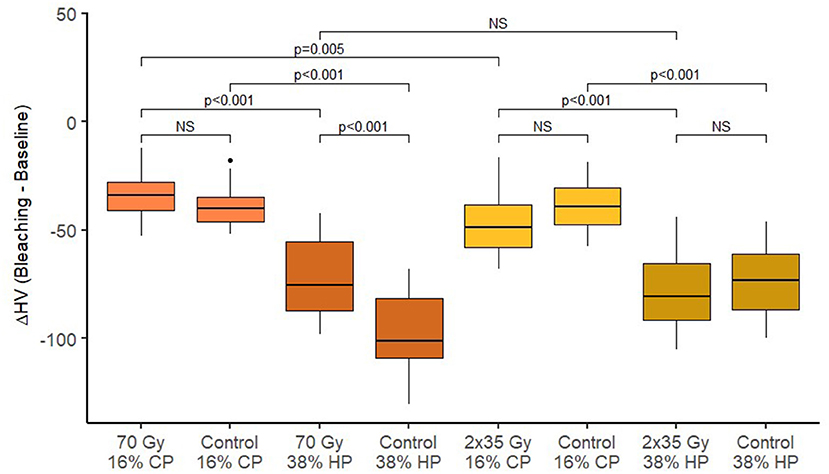
Figure 4. Box plots of changes in dentin Vickers microhardness after bleaching across different treatment groups.
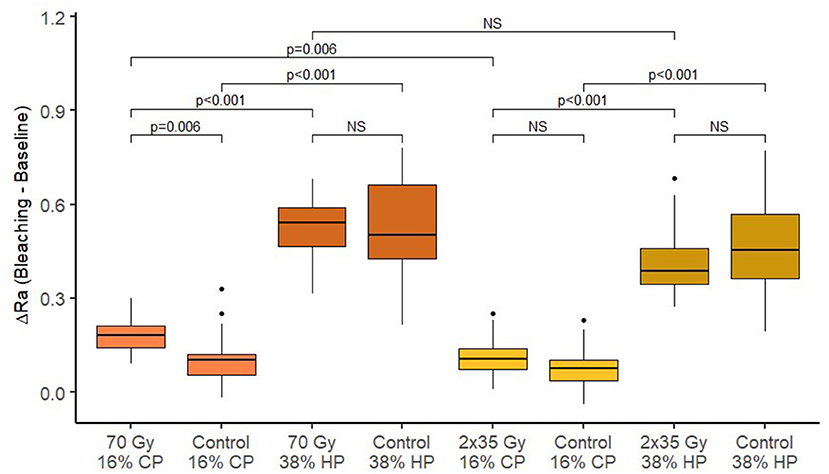
Figure 5. Box-plots of changes in enamel roughness after bleaching across different treatment groups.
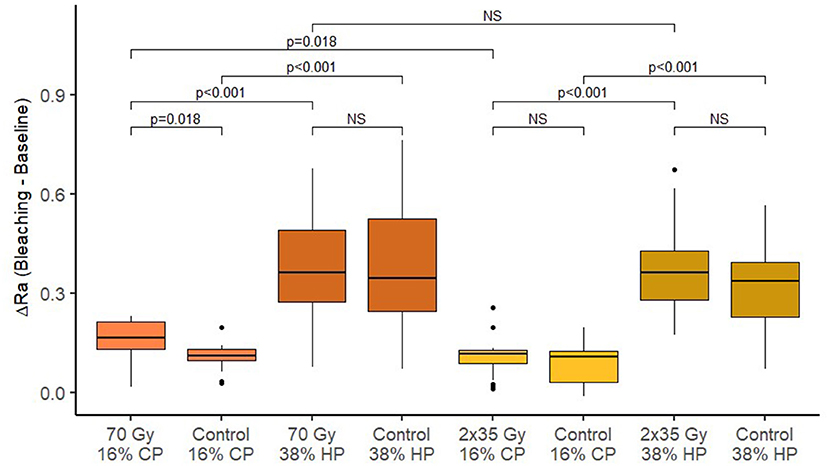
Figure 6. Box-plots of changes in dentin roughness after bleaching across different treatment groups.
Vickers Microhardness Measurements
After irradiation, a statistically significant reduction of microhardness of both enamel and dentin was observed for both irradiation methods (p < 0.001). Table 2 shows the summary of microhardness (HV) measurements. There is no significant difference between microhardness reduction for 2 Gy/fraction/day, 5 days/week irradiation method, and 70-Gy irradiation method (p = 0.856 for enamel and p =0.889 for dentin). After different bleaching treatments, an additional reduction in microhardness was observed for enamel and dentin (p < 0.001 for all combinations of irradiation and bleaching gels).
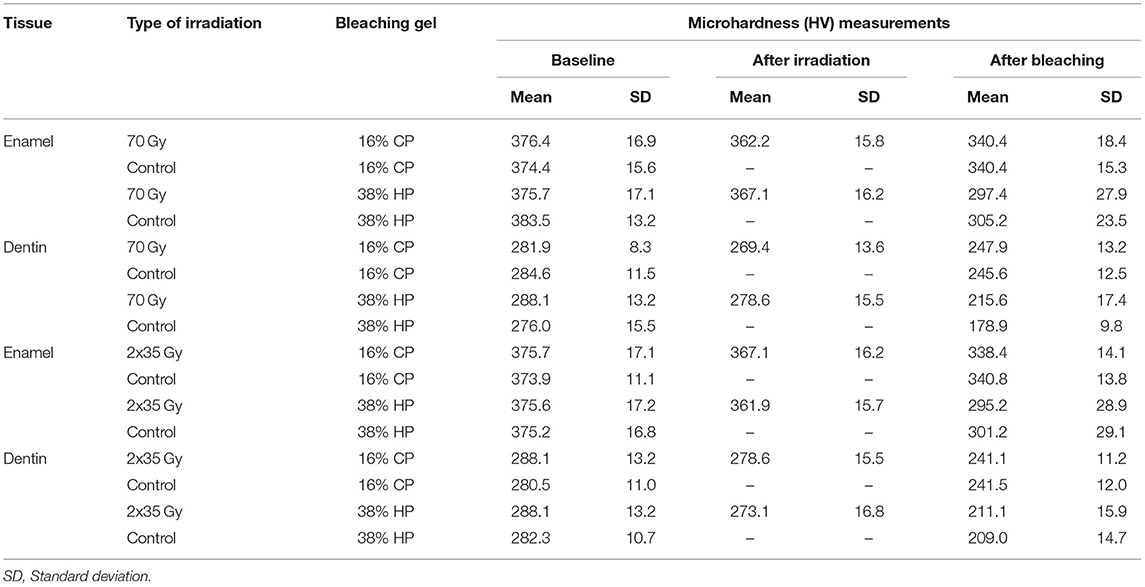
Table 2. Summary of baseline, irradiation, and bleaching measurements of enamel and dentin Vickers microhardness (HV) across different treatment groups.
Enamel Microhardness
Enamel microhardness after experimental single 70-Gy irradiation and bleaching with 16% gel did not statistically significantly differ from the average change in enamel microhardness in the control group (without irradiation) (p = 1.000). The change in the microhardness of the enamel after bleaching with 38% gel was also not affected by the treatment with experimental single 70-Gy irradiation (p = 1.000). In the control group, the reduction in microhardness of enamel after bleaching with 38% gel was statistically significant compared to 16% (p < 0.001). In the experimental group with radiation, there was also a statistically significantly greater reduction in microhardness of enamel during bleaching with 38% gel compared to 16% (p < 0.001).
Enamel microhardness after the standard radiation protocol (2 Gy/fraction/day, 5 days/week) and bleaching with 16% gel did not differ statistically significantly from the average change in enamel microhardness in the control group (p = 1.000). The change in the microhardness of the enamel after bleaching with 38% gel was also not affected by the treatment with the standard radiation protocol (p = 1.000). In the experimental group with the standard radiation protocol, a statistically significant reduction in enamel microhardness was recorded during bleaching with 38% gel compared to 16% (p < 0.001).
Dentin Microhardness
Dentin microhardness after experimental single 70-Gy irradiation and whitening with 38% gel was statistically significantly lower than the dentin microhardness in the control group (without radiation) (p < 0.001). The change in dentin microhardness after bleaching with 16% gel was not affected by treatment with experimental single 70 Gy (p = 0.742). In the control group, without radiation, dentin microhardness after bleaching was significantly reduced when bleaching with 38% gel compared to 16% (p < 0.001). In the experimental group with radiation, there was also a statistically significantly greater reduction in microhardness of dentin during bleaching with 38% gel compared to 16% (p < 0.001).
Dentin microhardness after the standard radiation protocol (2 Gy/fraction/day, 5 days/week) and bleaching with 16% gel did not differ statistically significantly from dentin microhardness in the control group (without radiation) (p = 0.174). Dentin microhardness after bleaching with 38% gel was not affected by the standard radiation treatment (p = 1.000). In the control group, without radiation, dentin microhardness after bleaching was significantly reduced when bleaching with 38% gel compared to 16% (p < 0.001). In the experimental group with standard radiation, there was also a statistically significantly greater reduction in microhardness of dentin during bleaching with 38% gel compared to 16% (p < 0.001).
Roughness Measurements
After the 2 Gy/fraction/day, 5 days/week irradiation method, and 70-Gy irradiation, there was a significant increase in surface roughness of enamel and dentin (p < 0.001). Table 3 shows the summary of roughness (Ra) measurements. There is no significant difference in average surface roughness between 70 Gy irradiation and 2 Gy/fraction/day, 5 days/week (p = 0.842 for enamel and p = 0.899 for dentin). After different bleaching treatments, an additional increase in surface roughness was observed for enamel and dentin (p < 0.05 for all combinations of irradiation and bleaching gels), except on enamel using 16% CP after 2 Gy/fraction/day, 5 days/week irradiation protocol (p = 0.545).
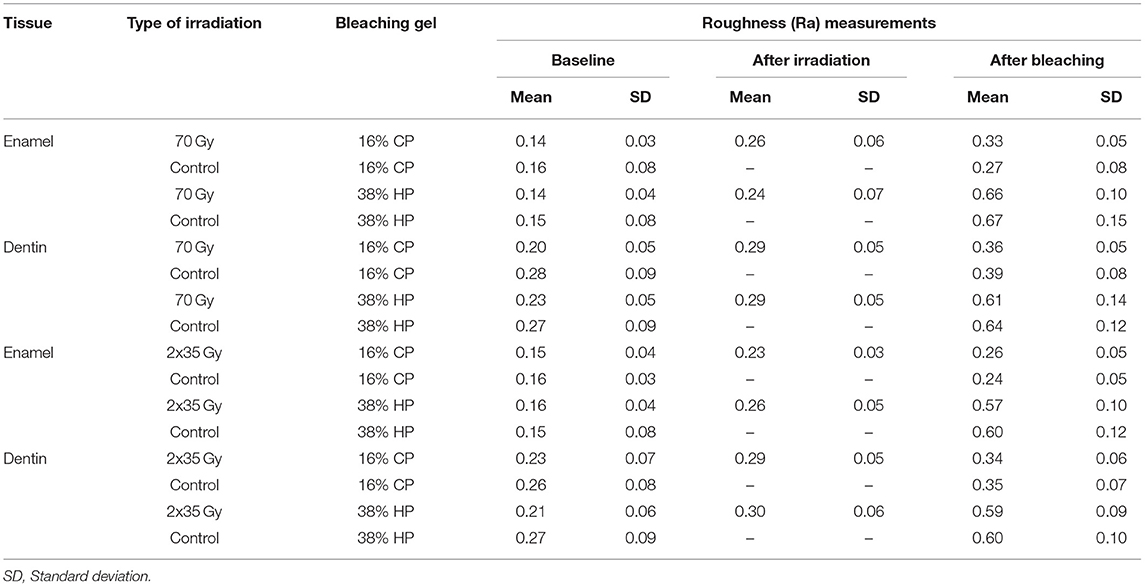
Table 3. Summary of baseline, irradiation, and bleaching measurements of enamel and dentin roughness across different treatment groups.
Enamel Surface Roughness
Enamel roughness after experimental single 70-Gy irradiation and bleaching with 16% gel was statistically significantly different from the average change in enamel roughness in comparison with the control group (without radiation) (p = 0.006). Enamel roughness after bleaching with 38% gel was not affected by the experimental single 70-Gy treatment (p = 0.938). In the control group, without radiation, a reduction in enamel roughness after bleaching was significantly increased for 38% gel compared to 16% (p < 0.001). In the experimental group with radiation, there was also a statistically significantly greater increase in the average enamel roughness during bleaching with 38% gel compared to 16% (p < 0.001).
Enamel roughness after the standard radiation protocol (2 Gy/fraction/day, 5 days/week) and bleaching with 16% gel did not statistically significantly differ from the average change in enamel roughness in comparison with control group (without radiation) (p = 0.676). The change in enamel roughness after bleaching with 38% gel was also not affected by the standard radiation protocol (p = 0.842). In the control group, without radiation, a reduction in enamel roughness after bleaching was significantly more increased when bleaching with 38% gel compared to 16% (p < 0.001). In the experimental group with radiation, there was also a statistically significantly greater increase in the average enamel roughness during bleaching with 38% gel compared to 16% (p < 0.001).
Dentin Surface Roughness
Dentin roughness after experimental single 70-Gy irradiation and bleaching with 16% gel was statistically significantly different from the average change in dentin roughness in comparison with the control group (without radiation) (p = 0.018). The change in dentin roughness after whitening with 38% gel was not affected by the treatment with experimental single 70-Gy irradiation (p = 1.000). In the control group, without radiation, an increase in dentin roughness after bleaching was significantly more intense when bleaching with 38% gel compared to 16% (p < 0.001). In the experimental group with radiation, there was also a statistically significant increase in the average roughness of dentin during whitening with 38% gel compared to 16% (p < 0.001).
Dentin roughness after the standard radiation protocol (2 Gy/fraction/day, 5 days/week) and bleaching with 16% gel did not statistically significantly differ from the average change in dentin roughness in comparison with the control group (without radiation) (p = 1.000). The change in dentin roughness after bleaching with 38% gel was also not affected after the standard radiation protocol (p = 1.000). In the control group, without radiation, dentin roughness after bleaching was significantly more increased when bleaching with 38% gel compared to 16% (p < 0.001). In the experimental group with radiation, there was also a statistically significant increase in average dentin roughness during whitening with 38% gel compared to 16% (p < 0.001).
Discussion
Nowadays, high-energy radioactive elements and particle accelerators are used for head and neck radiation procedures. These elements act directly by stimulating the breaking of DNA strands or indirectly by causing the effect of cell necrosis in the production of hydrogen peroxide resulting from the physical effect of free radicals and gamma radiation in the void (33). During radiation to head and neck cancers, healthy surrounding tissues such as bones, mucous membranes, teeth, and salivary glands are unfortunately not well-protected and their damage occurs. Most oncologists suggest treating their patients with radiation called “conventional fractionation.” This treatment consists of a total number of doses of 65–72 Gy of high-energy radiation, which means that it is divided into daily fractions (a series of treatments) of 1.8–2 Gy. These fractions are given over a period of 7 weeks, 5 days per week (34, 35). Radiation in the head and neck area can lead to direct damage of hard tooth structures such as changes in crystal composition, increased enamel solubility, and decreased microhardness, but unfortunately the mechanism of radiation-related caries has not been accurately described (14). In the study by Lu et al., a reduction in microhardness was noted after exposure to 30 Gy, while increasing the dose to 60 Gy, the damage was even greater (36), which is consistent with the reports of other previous studies who find that exposure of 30 Gy resulted in a reduction of microhardness and elastic modulus of enamel near the dentin–enamel junction, but no significant change was found at the sites of middle enamel, middle dentin, and the dentin–enamel junction after exposure of 60 Gy (37, 38). Also, reduced microhardness and elastic modulus at the dentin–enamel junction could decrease the ability of tooth deformation during mastication (39), leading to potential enamel exfoliation several months after radiation (40). Radiation of 60 Gy caused a decrease in microhardness of enamel and dentin, and the elemental analysis observed that there were decreases in all elements compared to the control group (26). Many other studies report potential changes in radiation effects, and while some report an increase (37, 38), some have reported a reduction (10, 41) in the total microhardness of enamel and dentin after radiotherapy of the head and neck. Goncalves et al. (37) observed that enamel microhardness values decreased in superficial depth up to 30 Gy cumulative dose but increased with doses higher than that. In the middle enamel, microhardness did not differ significantly compared with the non-irradiated enamel after cumulative radiation doses of 10, 30, 40, 50, and 60 Gy, while in deeper layers of enamel, there was no change in microhardness. Dentin microhardness decreased after 10, 20, 30, 50, and 60 Gy cumulative radiation doses compared with non-irradiated dentin.
Bleaching agents have an effect on the chemical/physical and morphological structure of enamel and dentin that must be taken into account when this therapy is used. Hydrogen peroxide is an oxidative agent with the ability to produce highly reactive peroxide and superoxide ions. Even though bleaching is a complex process, the main reaction is based on the oxidation process. As a result of oxidation, a change in microhardness and morphological characteristics of hard dental tissue can be observed (42). Bleaching agents in direct contact with enamel and dentin can lead to a reduction in microhardness, loss or damage of organic and inorganic components, increased roughness, and other potential damage, which is usually in correlation with concentration or application time (15–18, 29, 30).
Studies comparing the effect of irradiation and afterward bleaching are lacking, so these results could not be compared to other findings. This is the first study in which bleaching after the irradiation process was examined together. This gave us the opportunity to compare the responses of enamel and dentin to different bleaching agents after different applications of radiation within the same study protocol. Furthermore, there is no study in the literature examining the combined effect of radiation and bleaching, which makes this study original. Microhardness and surface roughness of hard dental tissues can be changed due to the effects of direct irradiation, but also by bleaching action. In our study, enamel microhardness after experimental single 70-Gy irradiation or after the standard radiation protocol (2 Gy/fraction/day, 5 days/week) and bleaching with 16 or 38% gel was not statistically significant from microhardness in the control group, while dentin microhardness after experimental single 70-Gy irradiation and whitening with 38% gel was statistically significantly lower in comparison with lower radiation dose treatment. After experimental 70-Gy irradiation and bleaching, a 16% statistically significant increase in surface roughness was found for enamel and dentin, while this was not recorded for 38% gel, so the first null hypothesis of this study is that there is no difference between the non-irradiated teeth and the teeth exposed to radiation and afterward bleaching in terms of microhardness and surface roughness was partially accepted. This could perhaps be the result of the more inorganic structure of enamel, which is more resistant to both bleaching and irradiation in comparison with dentin. Gülsüm et al. (39) reported that microhardness of all layers of the enamel of permanent teeth decreased (from surface to deeper layers) with an increase in irradiation dose from 20 to 60 Gy, while other studies indicate a decrease in surface microhardness of dentin (43–45), which is further explained by high water content in dentin (10%), obliteration of dentinal tubules, degeneration of collagen fibers, and greater effect of free radicals released after radiation (46). One detailed study confirmed that after cumulative radiation of 30 and 60 Gy, no morphological alteration in the prismatic enamel structure was observed, but the interprismatic portion became more evident with the increase in the radiation dose. The enamel of non-irradiated teeth still had well-organized prisms surrounded by interprismatic portions, while the prismatic structure of irradiated enamel remained unaltered even after the application of the different radiation doses. A small morphological change was observed in the interprismatic region after the 30-Gy radiation dose. The dentin of non-irradiated teeth presented well-defined dentinal tubules and collagen fibers, but after radiation there was an increase in the morphological alterations after 30- and 60-Gy radiation doses (37). Our finding of the greater effect of higher irradiation dose can be explained by the strong irradiation effect, which can damage the inorganic and organic structure of hard dental tissue and can be seen from the study by Ferraz et al., who determined that low salivary flow, which in this case can be connected to irradiation in the head area, had less capacity for remineralization of bleached enamel compared to normal flow (47).
Several studies estimated the relationship between concentrations of hydrogen peroxide or carbamide peroxide and the decrease in enamel and dentin microhardness (48, 49). The second null hypothesis is that there is no difference between the different bleaching systems in terms of microhardness and that surface roughness was rejected because there was a statistically significantly greater increase in the average roughness of enamel and dentin during bleaching with 38% gel compared to 16% for both radiation protocols. This finding was similar to other studies where bleaching with a higher concentration of bleaching agents led to a significant surface microhardness reduction in comparison with lower concentrations with mild or slight alterations with no loss of superficial structure found on SEM (27, 28). The impact of bleaching agents on the possible reduction in microhardness and change in surface roughness also depends on the pH of the agent and on the overall quality of hard dental tissues. Bleaching agents with higher acidity can produce more alterations of the enamel structure and reduce enamel microhardness (50, 51). Both bleaching gels had pH below neutral (pH = 7.0): pH of I-Smile was 6.75, while pH of BMS White was 5.7, but both were above critical demineralization level for enamel, which is in the range of 4.5–5.5. In our study, 38% HP caused a greater reduction in microhardness and surface roughness compared to 16% CP, which is probably the result of a greater concentration of bleaching agent, rather than lowering pH. The results of this study are in agreement with the results of Lewinstein et al., since they report a significant reduction in enamel microhardness after treatment with 35% HP (52).
Study conditions can also have an effect on the results of change in microhardness and roughness of hard dental tissues. A dry environment can affect the mechanical properties of dental specimens due to dehydration (53). In this study, the dental samples were stored in deionized water. In contrast, other studies have reported that there was no significant change in the mechanical properties (54) or chemical composition of enamel and dentin after irradiation to sterilize extracted teeth (55). Moreover, there is great variability within the experimental methods used in various studies, including differences in retention time and storage of tooth samples that could affect results (34, 56) as well as differences in how and where a particular measurement was carried out on each tooth (57). Finally, another important source of variability is the difference between teeth of different patients and even within the same patient (58).
The literature also shows that the fractionated doses are used to avoid alterations in the salivary glands and soft tissues and the dose is cumulative (59); thus, our model of using one single dose of 70 Gy is not clinically approved but showed us the potential effect of one high dose on hard dental tissue and cannot be used in vivo. Additionally, previous in vitro studies, without the involvement of cells or soft tissues, did not use fractionated dose either (60). In a clinical situation, this method of using a linear accelerator for radiation therapy presents great advantages, mainly from the use of 360° rotation radiation, which allows the primary target to receive the total amount of radiation necessary for treatment, while the adjacent structures and organs are at a limited risk (61). Despite the advantages of this method, it is quite expensive and still not the method of choice in all countries. Clinically, even with the use of this method and fractioned doses, the teeth are located close to the targeted area and exposure of hard dental tissue still cannot be prevented (61). That is why fractioned doses as commonly indicated in clinical situations were compared with one single dose. Munoz et al. (62) also found that cobalt irradiation unit at different doses (0, 20, 40, and 70 Gy) radiation does also significantly decreased microhardness, so this is still one of the problems concerning irradiation in the head and neck region and should be studied further. Post-treatment with artificial saliva and ACP showed a significant increase in surface microhardness, improved surface roughness, and enhanced remineralization of the hard dental tissues (28, 63). Treatment with other remineralizing agents like ACP-CPP or fluoride solution can increase the microhardness of the bleached enamel afterward (28), so this is something that should be also examined in our future studies dealing with irradiated and bleached hard dental tissues.
This study is based on in vitro research that attempts to differentiate the direct effects of radiation on hard dental tissue. Under the limitations of this in vitro study, it can be concluded that direct irradiation in combination with bleaching induced potential hard tooth tissue damage. Both types of bleaching agents with different concentrations of hydrogen peroxide have a significant influence on the surface microhardness of human enamel and dentin. However, there are some limitations to this research. The mechanical properties of hard dental tissues are influenced not only by the region of the tooth but also by the orientation of enamel crystals and dentin tubules. Teeth in the oral cavity are presumably exposed to lower doses of radiation during clinical treatment than to the experimental setup. Based on this and other studies, we are still far away from the consensus for the best clinical approach, restorative material materials, and strategies for those patients. Therefore, in vivo studies are needed to better investigate the direct effects of radiation on teeth and surrounding soft tissues.
Conclusions
This study has shown that directly induced radiation leads to potential damage to hard dental tissues, which can be further damaged by an additional bleaching process. According to the findings from our in vitro study, considering also the limitations (no artificial saliva, extracted teeth, etc.) but in accordance with previously mentioned studies, if teeth whitening is necessary, especially after irradiation, it is suggested to use lower concentrations of carbamide peroxide over a longer period of time than to use high concentrations of hydrogen peroxide, which further reduces surface microhardness and leads to increased roughness. The effect of radiation on the potential damage of hard dental tissue is also based on free radicals as in the bleaching process, so this negative effect can be combined. Different findings in the previous studies indicate that there are still no clear data on the subject of radiation and hard dental tissues and combination with different concentrations of bleaching agents in the literature and that similar studies especially using potential remineralization agents and other conditions are needed for the future.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The use of extracted human teeth has been approved by the Ethics Committee of the Faculty School of Dental Medicine, University of Zagreb and University Hospital Center Zagreb, Croatia.
Disclosure
EK, the corresponding author, involved in scientific and technical contribution. AT involved in scientific and technical contribution. MS involved in scientific and technical contribution. TG involved in scientific and technical contribution.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This study was supported by Grant of University of Zagreb, year 2020/2021.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. (2008) 371:1695–709. doi: 10.1016/S0140-6736(08)60728-X
2. Suh JD, Cho JH. Trends in head and neck cancer in South Korea between 1999 and 2012. Clin Exp Otorhinolaryngol. (2016) 9:263–9. doi: 10.21053/ceo.2015.01123
3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre L. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Canc J Clinic. (2018) 68:394–424. doi: 10.3322/caac.21492
4. Specht L. Oral complications in the head and neck radiation patient. Introduction and scope of the problem. Support Care Canc. (2002) 10:36–9. doi: 10.1007/s005200100283
5. Kelly C, Paleri V, Downs C, Shah R. Deterioration in quality of life and depressive symptoms during radiation therapy for head and neck cancer. Otolaryngol Head Neck Surg. (2007) 136:108–11. doi: 10.1016/j.otohns.2006.06.1278
6. Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. (2003) 14:199–212. doi: 10.1177/154411130301400305
7. Kielbassa AM, Hinkelbein W, Hellwig E, Meyer-Luckel H. Radiation-related damage to dentition. Lancet Oncol. (2006) 7:326–35 doi: 10.1016/S1470-2045(06)70658-1
8. Walker MP, Wichman B, Cheng A-L, Coster J, Williams KB. Impact of radiotherapy dose on dentition breakdown in head and neck cancer patients. Pract Radiat Oncol. (2011) 1:142–8. doi: 10.1016/j.prro.2011.03.003
9. Franzel W, Gerlach R, Hein H-J, Schaller H-G. Effect of tumor therapeutic irradiation on the mechanical properties of teeth tissue. Z Med Phys. (2006) 16:148–54. doi: 10.1078/0939-3889-00307
10. Franzel W, Gerlach R. The irradiation action on human dental tissue by X-rays and electrons–a nanoindenter study. Z Med Phys. (2009) 19:5–10. doi: 10.1016/j.zemedi.2008.10.009
11. Soares CJ, Castro CG, Neiva NA, Soares PV, Santos-Filho PCF, Navez LZ, et al. Effect of gamma irradiation on ultimate tensile strength of enamel and dentin. J Dent Res. (2010) 89:159–64. doi: 10.1177/0022034509351251
12. Schiødt M, Hermund NU. Management of oral disease prior to radiation therapy. Support Care Canc. (2002) 10:40–3. doi: 10.1007/s005200100284
13. Dholam KP, Somani PP, Prabhu SD, Ambre SR. Effectiveness of fluoride varnish application as cariostatic and desensitizing agent in irradiated head and neck cancer patients. Int J Dent. (2013) 2013:824982. doi: 10.1155/2013/824982
14. Parahoo RS, Semple CJ, Killough S, McCaughan E. The experience among patients with multiple dental loss as a consequence of treatment for head and neck cancer: a qualitative study. J Dentistry. (2019) 82:30–7. doi: 10.1016/j.jdent.2019.01.010
15. Thariat J, Ramus L, Darcourt V, Marcy P-Y, Guevara N, Odin G, et al. Compliance with fluoride custom trays in irradiated head and neck cancer patients. Support Care Canc. (2012) 20:1811–4. doi: 10.1007/s00520-011-1279-5
16. Attin T, Vollmer D, Wiegand A, Attin R, Betke H. Subsurface microhardness of enamel and dentin after different external bleaching procedures. Am J Dentistry. (2005) 18:8–12.
17. Pintado-Palomino K, Peitl Filho O, Zanotto ED, Tirapelli C. A clinical, randomized, controlled study on the use of desensitizing agents during tooth bleaching. J Dent. (2015) 43:1099–105. doi: 10.1016/j.jdent.2015.07.002
18. Rodríguez-Martínez J, Valiente M, Sánchez-Martín M-J. Tooth whitening: from the established treatments to novel approaches to prevent side effects. J Esthet Restor Dent. (2019) 31:431–40. doi: 10.1111/jerd.12519
19. Shi X-C, Ma H, Zhou J-L, Li W. The effect of cold-light-activated bleaching treatment on enamel surfaces in vitro. Int J Oral Sci. (2012) 4:208–13. doi: 10.1038/ijos.2012.70
20. Rodrigues JA, Basting TR, Serra MC, Rodrigues AL. Effect of 10% carbamide peroxide bleaching materials on enamel microhardness. Am J Dentistry. (2011) 14:67–71.
21. Carrasco-Guerisoli LD, Schiavoni RJ, Barroso JM, Guerisoli DM, Pe'cora JD. Effect of different bleaching systems on the ultrastructure of bovine dentin. Dent Traumatol. (2009) 25:176–80. doi: 10.1111/j.1600-9657.2008.00644.x
22. Kwon SR, Wertz PW. Review of the mechanism of tooth whitening. J Esthet Restor Dent. (2015) 27:240–57. doi: 10.1111/jerd.12152
23. Avondoglio DM. Rehabilitation of a head and neck cancer survivor with a functionally and biomechanically challenged dentition. Compend Contin Educ Dent. (2020) 41:42–9.
24. Chiu YH, Tseng WH, Ko JY, Wang TG. Radiation-induced swallowing dysfunction in patients with head and neck cancer: a literature review. J Formos Med Assoc. (2021) S0929-6646(21)00300-4. doi: 10.1016/j.jfma.2021.06.020
25. Hasani E, Baghban AA, Sheikh-Al-Eslamian SM, Sadr A. Share effect of bleaching on color change of composite after immersion in chlorhexidine and coffee. J Conserv Dent. (2019) 22:529–32. doi: 10.4103/JCD.JCD_37_19
26. Reed R, Xu C, Liu Y, Gorski JP, Wang Y, Walker MP. Radiotherapy effect on nano-mechanical properties and chemical composition of enamel and dentine. Arch Oral Biol. (2016) 60:690–7. doi: 10.1016/j.archoralbio.2015.02.020
27. Klarić E, Marciuš M, Ristić M, Sever I, Prskalo K, Tarle Z. Surface changes of enamel and dentine after two different bleaching procedures. Acta Clin Croat. (2013) 52:413–428.
28. Klarić E, Rakić M, Sever I, Milat O, Par M, Tarle Z. Enamel and dentin microhardness and chemical composition after experimental light-activated bleaching. Operat Dentistry. (2015) 40:132–41. doi: 10.2341/14-148-L
29. Wijetunga C, Otsuki M, Abdou A, Luong M, Feng Q, Tagam J. The effect of in-office bleaching materials with different pH on the surface topography of bovine enamel. Dent Mater J. (2021). doi: 10.4012/dmj.2021-010. [Epub ahead of print].
30. Priscila C, Liporoni S, Zaripah W, Bakar W, Zanatta F, Ambrosano G. Influence of erosion/abrasion and the dentifrice abrasiveness concomitant with bleaching procedures. Clin Cosmet Investig Dent. (2020) 1:101–9. doi: 10.2147/CCIDE.S234716
31. Bala O, Arisu HD, Yikilgan I, Arslan S, Gullu A. Evaluation of surface roughness and hardness of different glass ionomer cements. Eur J Dent. (2012) 6:79–86. doi: 10.1055/s-0039-1698934
32. Trentino AC, Soares AF, Duarte MAH, Ishikiriama SK, Mondelli RFL. Evaluation of pH levels and surface roughness after bleaching and abrasion tests of eight commercial products. Photomed Laser Surg. (2015) 33:372–7. doi: 10.1089/pho.2014.3869
33. Walter F, Freislederer P, Belka C, Heinz C, Sohn M, Roeder F. Evaluation of daily patient positioning for radiotherapy with a commercial 3D surface imaging system (catalyst). Radiat Oncol. (2016) 11:154. doi: 10.1186/s13014-016-0728-1
34. White JM, Goodis HE, Marshall SJ, Marshall GW. Sterilization of teeth by gamma radiation. J Dent Res. (1994) 73:1560–7. doi: 10.1177/00220345940730091201
35. Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. (2011) 12:127–36. doi: 10.1016/S1470-2045(10)70290-4
36. Lu H, Zhao Q, Guo J, Zeng B, Yu X, Yu D, et al. Direct radiation-induced effects on dental hard tissue. Radiat Oncol. (2019) 14:5 doi: 10.1186/s13014-019-1208-1
37. Gonçalves LM, Palma-Dibb RG, Paula-Silva FW, Oliveira HF, Nelson-Filho P, Silva LA, et al. Radiation therapy alters microhardness and microstructure of enamel and dentin of permanent human teeth. J Dent. (2014) 42:986–92. doi: 10.1016/j.jdent.2014.05.011
38. de Siqueira MT, Palma-Dibb RG, de Oliveira HF, Garcia Paula-Silva FW, NelsonFilho P, da Silva RA, et al. The effect of radiation therapy on the mechanical and morphological properties of the enamel and dentin of deciduous teeth—an in vitro study. Radiat Oncol. (2014) 9:30. doi: 10.1186/1748-717X-9-30
39. Gülsüm D, Burçin A, Öztun T. Effect of different doses of radiation on morphological, mechanical and chemical properties of primary and permanent teeth-an in vitro study. BMC Oral Health. (2020) 20:242. doi: 10.1186/s12903-020-01222-3
40. Seyedmahmoud R, Wang Y, Thiagarajan G. Oral cancer radiotherapy affects enamel microhardness and associated indentation pattern morphology. Clin Oral Investig. (2018) 22:1795–803. doi: 10.1007/s00784-017-2275-z
41. Qing P, Huang S, Gao S, Qian L, Yu H. Effect of gamma irradiation on the wear behavior of human tooth enamel. Sci Rep. (2015) 5:11568. doi: 10.1038/srep11568
42. Rodrigues JA, Basting T, Serra MC, Rodrigues. Effect of 10% carbamide peroxide bleaching materials on enamel microhardness. Am J Dent. (2001) 14:67–71.
43. de Barros da Cunha SR, Fonseca FP, Ramos P, Haddad CMK, Fregnani ER, Aranha ACC. Effects of different radiation doses on the microhardness, superficial morphology, and mineral components of human enamel. Arch Oral Biol. (2017) 80:130–5. doi: 10.1016/j.archoralbio.2017.04.007
44. Kielbassa AM, Munz I, Bruggmoser G, Schulte-Monting J. Effect of demineralization and remineralization on microhardness of irradiated dentin. J Clin Dent. (2002) 13:104–10.
45. Wu L, Geng K, Gao Q. Effects of different anti-caries agents on microhardness and superficial microstructure of irradiated permanent dentin: an in vitro study. BMC Oral Health. (2019) 19:113. doi: 10.1186/s12903-019-0815-4
46. Kielbassa AM, Wrbas KT, Schulte-Mönting J, Hellwig E. Correlation of transversal microradiography and microhardness on in situ induced demineralization in irradiated and non-irradiated human enamel. Arch Oral Biol. (1999) 44:243–51. doi: 10.1016/S0003-9969(98)00123-X
47. Ferraz LN, Isabele V, Gláucia M, Ambrosano B, Lopes M, Alves D. Effect of tooth bleaching and application of different dentifrices on enamel properties under normal and hyposalivation conditions: an in situ study. Clin Oral Investig. (2021). doi: 10.1007/s00784-021-03899-4. [Epub ahead of print].
48. Araujo FD, Baratieri lN, Araújo E. In situ study of in-office bleaching procedures using light sources on human enamel microhardness. Oper Dent. (2010) 35:139–46. doi: 10.2341/08-033-C
49. Rodrigues JA, Marchi GM, Ambrosano GM. Microhardness evaluation of in situ vital bleaching on human dental enamel using a novel study design. Dent Mater. (2005) 21:1059–67. doi: 10.1016/j.dental.2005.03.011
50. Sun l, Liang S, SA Y. Surface alteration of human tooth enamel subjected to acidic and neutral 30% hydrogen peroxide. J Dent. (2011) 39:686–92. doi: 10.1016/j.jdent.2011.07.011
51. Sa Y, Sun l, Wang Z. E?ects of two in-office bleaching agents with di?erent pH on the structure of human enamel: an in situ and in vitro study. Oper Dent. (2013) 38:100–10. doi: 10.2341/11-173-L
52. Lewinstein I, Fuhrer N, Churaru N, Cardash H. Effect of different peroxide bleaching regimens and subsequent fluoridation on the hardness of human enamel and dentin. J Prosthet Dent. (2004) 92:337–42. doi: 10.1016/j.prosdent.2004.07.019
53. Lewis G, Nyman JS. The use of nanoindentation for characterizing the properties of mineralized hard tissues: state-of-the art review. J Biomed Mater Res B Appl Biomater. (2008) 87:286–301. doi: 10.1002/jbm.b.31092
54. Brauer DS, Saeki K, Hilton JF, Marshall GW, Marshall SJ. Effect of sterilization by gamma radiation on nano-mechanical properties of teeth. Dent Mater. (2008) 24:1137–40. doi: 10.1016/j.dental.2008.02.016
55. Gellrich NC, Schramm A, Bockmann R, Kugler J. Follow-up in patients with oral cancer. J Oral Maxillofac Surg. (2002) 60:380–6. doi: 10.1053/joms.2002.31224
56. Habelitz S, Marshall GW Jr, Balooch M, Marshall SJ. Nanoindentation and storage of teeth. J Biomech. (2002) 35:995–8. doi: 10.1016/S0021-9290(02)00039-8
57. Sultana S, Nikaido T, Asafujjoha M, Tagami J, Matin K. Storage media to preserve dentin and their effects on surface properties. Int Chin J Dent. (2006) 6:123–9.
58. Park S, Wang DH, Zhang D, Romberg E, Arola D. Mechanical properties of human enamel as a function of age and location in the tooth. J Mater Sci. (2008) 19:2317–24. doi: 10.1007/s10856-007-3340-y
59. Hoebers F, Yu E, Eisbruch A, Thorstad W, O'Sullivan B, Dawson LA, et al. A pragmatic contouring guideline for salivary gland structures in head and neck radiation oncology: the MOIST target. Am J Clin Oncol. (2013) 36:70–6. doi: 10.1097/COC.0b013e31823a538e
60. Pioch T, Golfels D, Staehle HJ. An experimental study of the stability of irradiated teeth in the region of the dentinoenamel junction. Endod Dent Traumatol. (1991) 8:241–4. doi: 10.1111/j.1600-9657.1992.tb00251.x
61. Lieshout HF, Bots CP. The effect of radiotherapy on dental hard tissue—a systematic review. Clin Oral Investig. (2014) 18:17–24. doi: 10.1007/s00784-013-1034-z
62. Muñoz MA, Garín-Correa C, González-Arriagada W, Davila X, Häberle P, Bedran-Rusel A, et al. The adverse effects of radiotherapy on the structure of dental hard tissues and longevity of dental restoration. Int J Radiat Biol. (2020) 96:910–8. doi: 10.1080/09553002.2020.1741718
Keywords: radiotherapy, tooth bleaching, microhardness, surface roughness, enamel, dentin
Citation: Klarić Sever E, Tarle A, Soče M and Grego T (2021) Direct Radiotherapy-Induced Effects on Dental Hard Tissue in Combination With Bleaching Procedure. Front. Dent. Med. 2:714400. doi: 10.3389/fdmed.2021.714400
Received: 25 May 2021; Accepted: 16 August 2021;
Published: 10 September 2021.
Edited by:
Vanessa Cavalli, State University of Campinas, BrazilReviewed by:
Priscila Melo, Campinas State University, BrazilRayssa Ferreira Zanatta, Universidade de Taubaté, Brazil
Copyright © 2021 Klarić Sever, Tarle, Soče and Grego. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eva Klarić Sever, ZWtsYXJpY0BzZnpnLmhy
 Eva Klarić Sever
Eva Klarić Sever Andro Tarle2
Andro Tarle2 Majana Soče
Majana Soče Timor Grego
Timor Grego