
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Dent. Med. , 26 March 2021
Sec. Periodontics
Volume 2 - 2021 | https://doi.org/10.3389/fdmed.2021.652605
This article is part of the Research Topic Integrating Oral and Systemic Health: Innovations in Transdisciplinary Science, Health Care and Policy View all 19 articles
 Emily Y. Chu1†
Emily Y. Chu1† Janina Golob Deeb2†
Janina Golob Deeb2† Brian L. Foster3†
Brian L. Foster3† Evlambia Hajishengallis4†
Evlambia Hajishengallis4† Martha J. Somerman1†
Martha J. Somerman1† Vivek Thumbigere-Math1,5*†
Vivek Thumbigere-Math1,5*†The goal of this perspective article is to use multiple idiopathic cervical root resorption (MICRR) as a model to demonstrate the need for transdisciplinary collaborations, from basic science to treatment planning, to improve the quality of health care for all. This is not a review of the literature on the current state of MICRR. Tooth root resorption is a normal physiological process required for resorption and exfoliation of primary teeth; however, root resorption of adult teeth is largely pathological. MICRR is an aggressive form of external root resorption, which occurs near the cemento-enamel junction (CEJ). The cause of MICRR remains elusive, however, it is mediated primarily by osteoclasts/odontoclasts. Accumulating case studies and experiments in animal models have provided insights into defining the etiologies and pathophysiological mechanisms for MICRR, which include: systemic conditions and syndromes, inherited genetic variants affecting osteoclast/odontoclast activity, altered periodontal structures, drug-induced root resorption and rebound effects after cessation of anti-resorptive treatment, chemotherapy, exposure to pets or viral infections, and other factors such as inflammatory conditions or trauma. To determine the causative factors for MICRR, as well as other oral-dental conditions, at minimum, a comprehensive health history should be collected for all patients by dental care providers, discussed with other health care providers and appropriate collaborations established. The examples highlighted in this perspective emphasize the need for transdisciplinary research collaborations coupled with integrated management strategies between medicine and dentistry in order to identify cause(s) early and improve clinical outcomes.
Using MICRR as a model, this perspective underscores the need for researchers and clinicians to adopt transdisciplinary approaches for defining the etiology of unknown oral-dental conditions. In this regard, please see the commentary and short video, 5–7 min, describing the procedure for doing an oral exam for non-dental clinicians published in JAMA, 2018 (1, 2).
While root resorption is a normal physiological process required for resorption and exfoliation of primary teeth (3), root resorption of adult teeth is largely pathological. Pathological root resorption can be broadly classified into internal (i.e., originating within the dental pulp) or external (i.e., attacking the outer root surface) processes, mediated by osteoclasts/odontoclasts (4–7). This perspective focuses on multiple idiopathic cervical root resorption (MICRR), an aggressive form of external root resorption that occurs near the cemento-enamel junction (CEJ) (Figure 1). The CEJ is the area where the crown transitions into root(s) and where gingival fibers attach to a healthy tooth root and surrounding alveolar bone. As we highlight below, there has been progress in identifying etiologies for MICRR, particularly genetic and medication-associated causes, prompting us to reconsider the term “idiopathic” once the cause has been identified.
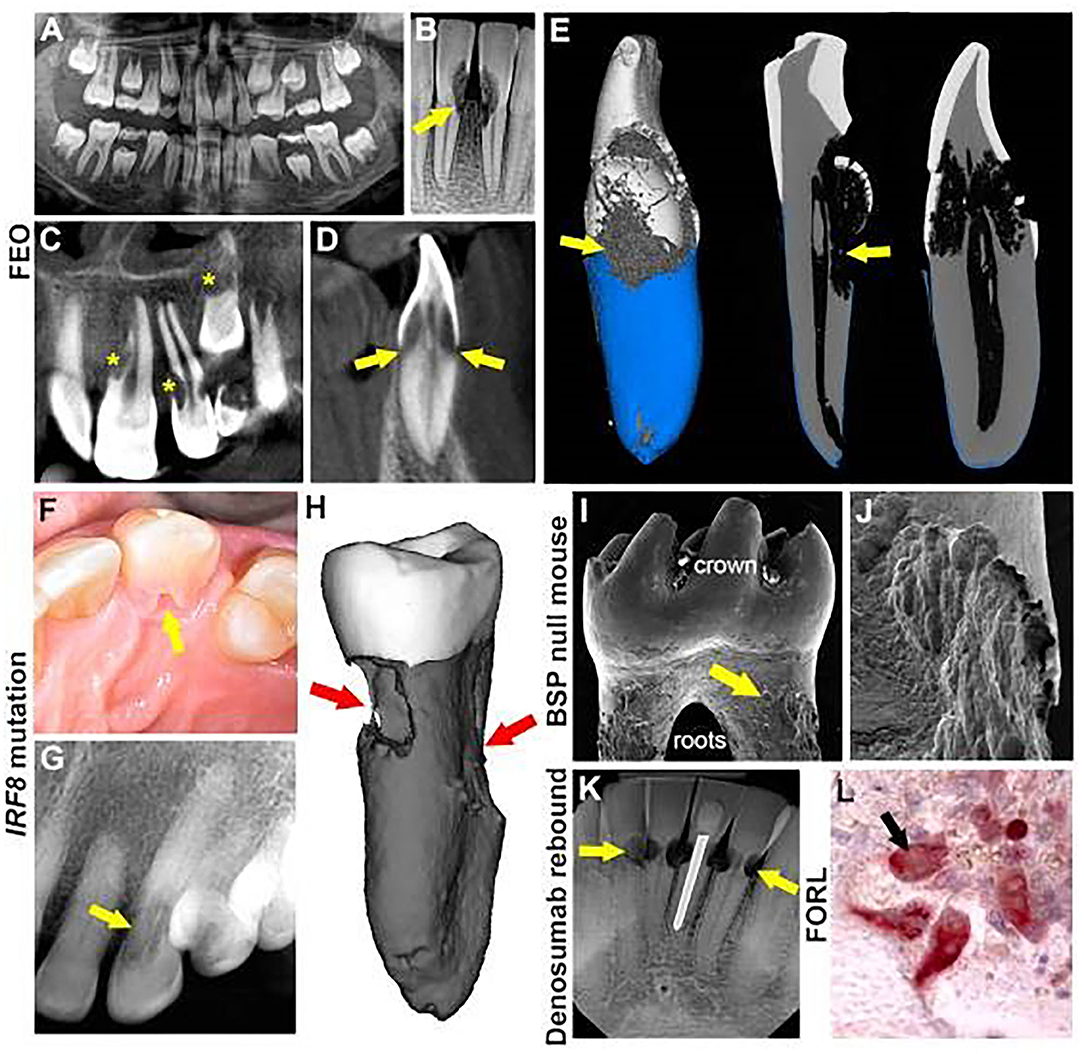
Figure 1. Multiple etiologies of MICRR. (A) Panoramic radiograph of 8-year-old female with familial expansive osteolysis (FEO) associated with TNFRSF11A variant showing cervical root resorption of permanent maxillary and mandibular incisors (yellow arrows). (B) Periapical radiograph of the same patient with FEO, at 9-years-old, showing extent of external resorption (yellow arrows). (C,D) Cone beam computed tomography (CBCT) of the same patient at 9-years-old showing extensive resorption of permanent central and lateral incisors (yellow stars) and unerupted canine (yellow star and yellow arrows). (E) 3D micro-computed tomography (micro-CT) reconstruction of incisor of the same FEO patient at age 9, noting defective cementum formation and root resorption. (F) Intraoral photograph of an advanced resorption lesion (yellow arrow) on the palatal aspect of tooth from affected individual with an inherited IRF8 variant. (G) Radiograph of the lesion (yellow arrow) shown in (F). (H) Micro-CT reconstruction of an extracted tooth exhibiting extensive cervical resorption (red arrows). (I) Scanning electron microscopy (8) image of BSP null mouse molar showing abundant pitting (yellow arrows) at cervical root surfaces. (J) Higher magnification SEM of the tooth in panel I showing details of cervical root resorption in BSP null mouse molar. (K) Periapical radiograph of lower anterior teeth in 69-year-old patient, after discontinuation of denosumab, showing multiple areas of cervical root resorption (yellow areas). (L) Tartrate-resistant acid phosphatase (TRAP) stain of histology section showing multinucleated odontoclasts (red cells, black arrow) on root surfaces of a cat with feline odontoclastic resorption lesions (FORL). (A–D) reproduced with permission from Macaraeg et al. (9). (F,G) reproduced with permission from Neely et al. (10). (I) reproduced with permission from Foster et al. (11). (K) Copyright © 2020 American Academy of Pediatric Dentistry and reproduced with their permission (12).
MICRR affects multiple teeth within the dentition (13–16). MICRR lesions are often asymptomatic, non-carious, and lack overt gingival inflammation, increased pocket depth, or tooth mobility that are associated with classical cases of periodontal disease. Histologically, numerous resorptive areas are noted along root surfaces with evidence of osteoclasts/odontoclasts contained in Howship's lacunae (10, 16, 17). MICRR lesions are frequently aggressive in nature and resistant to interventions, ultimately resulting in tooth loss (10, 16, 17). Often, MICRR is detected as an incidental finding on radiographs or during routine dental examination. Fortunately, with enhanced tools and technologies over the last decade (18, 19), our understanding of MICRR etiology and its course has been improving, which will eventually result in refining clinical management and thus, better outcomes.
While idiopathic root resorption is considered a rare condition, it is one that many dentists encounter over years of practice. The effects are devastating, e.g., loss of dentition, a feeling of helplessness by clinicians and patients due to lack of effective prevention/treatment options, and poor esthetics and function driving patients into isolation, negatively impacting mental health and wellbeing. To date, the prevalence of MICRR remains unknown. Since the first case of MICRR described by Mueller and Rony (20), etiologies of MICRR have been largely speculative. Here, we provide an overview of the cellular and molecular mechanisms mediating root resorption, and provide examples of etiology, including systemic conditions and genetic factors, medications, viral infections, inflammatory conditions, environmental and other proposed causes for MICRR (Table 1) (55).
Histological studies in humans and animals have unequivocally demonstrated that root resorption is mediated by osteoclasts/odontoclasts and is distinct from bacterial-mediated cariogenesis (5, 7, 10, 16, 17, 56). Yet the factors that activate osteoclasts/odontoclasts and recruit them to root surfaces rather than bone surfaces (noted in periodontitis) remain unknown. Bernhard Gottlieb in 1923 noted cases of periodontal disease that were not associated with marked inflammation but rather with perceived defective cementum formation (57–59). He observed that “cementum was the only tissue which connects tooth with the body,” and if not formed correctly, would put individuals at risk for a type of periodontal disease (characterized by gingival recession or pocket formation), which he termed as “marginal cementopathia” (57, 58). Clinicians and researchers have revisited this concept, especially with increased knowledge about conditions that trigger clastic activity, including those associated with defective cementum formation (59, 60).
During initiation of cervical root resorption, the portal of entry is the cementum below the gingival epithelium, and resorption starts with localized destruction and/or removal of PDL (56). Response to PDL injury includes formation of a blood clot and inflammation, followed by granulation tissue and recruitment of macrophages to the affected area (56, 61). Impaired vasculature in the area leads to hypoxia, which promotes osteoclast differentiation and activity (62). As the osteoclastic/odontoclastic resorptive lesions expand toward the pulp space by destroying cementum, dentin, and enamel, several resorption channels and interconnections with PDL (portal of exits) are created, generating a 3D space (52, 56). In most cases, the advancing resorptive lesions are prevented from perforating into the pulp space by the pericanalar resorption resistant (PRSS) sheet (52, 56). This layer consists of predentin, dentin, and occasionally reparative bone-like tissue. In the final stages of the disease, repair and remodeling sometimes occurs through the activity of cementoblast/osteoblast-like cells, resulting in deposition of bone-like tissue into the resorption cavities (52, 56).
Root resorption has been associated with systemic and syndromic conditions, including endocrine disorders. In some of these conditions, dysregulated resorption affects the skeleton, leading to reduced bone mineral density or osteolytic lesions. Examples of conditions associated with root resorption include: hypothyroidism (21), hyperparathyroidism (22, 23), systemic sclerosis (24), Gaucher's disease (25), hereditary hemorrhagic telangiectasia (26), Paget's disease of bone (27, 28), Goltz syndrome (29), Papillon–Lefévre syndrome (30), and Turner syndrome (31). To date, no studies have firmly established causality between these conditions and MICRR.
Familial expansile osteolysis (FEO; OMIM#174810) is caused by mutations in the TNFRSF11A gene (9, 63, 64), which encodes for receptor activator of nuclear factor κ-B (RANK), a receptor found on osteoclasts and their progenitors. Upon binding to RANK ligand (RANKL), RANK promotes osteoclastic formation/function. In FEO, TNFRSF11A mutations affecting its signaling peptide may result in constitutive activation independent of RANK ligand stimulation leading to uncontrolled osteoclast activity (63). Individuals with FEO often present with early-onset deafness, skeletal deformities, and premature loss of teeth (63–65). FEO has been associated with extensive resorption of cervical and apical areas of permanent teeth (32, 33). Recently, Hajishengallis and colleagues reported a case of FEO in a 10-year-old female with missing ossicles and MICRR (9). In addition to MICRR affecting at least 7 erupted permanent teeth, premature atypical root resorption of all primary teeth (started at age 5 and progressed with most of the roots resorbed by age 7) and resorption of an unerupted permanent canine was noted (Figures 1A–D). Genetic testing focusing on missing ossicles at the time of birth was inconclusive and the accelerated root resorption of primary teeth was not well-appreciated. However, when the aggressive root resorption involved permanent teeth, it prompted further endocrinology and genetic testing, which revealed decreased lumbar spine mineral density, high circulating alkaline phosphatase (66) levels, and identification of the TNFRSF11A mutation, which together led to the diagnosis of FEO.
MICRR in this case involved several anterior teeth (maxillary lateral incisors, left central incisor, unerupted left canine, and mandibular central incisors and left canine). Interestingly, cone-beam computed tomography scans of the resorptive defects suggested that the lesions started from a small portal of entry in the cementum and expanded below the bone level inside the tooth, sparing the pulp canal space. Unfortunately, two of the affected teeth (mandibular lower central incisors) showed increased sensitivity and required extraction. Micro-CT analysis of these teeth revealed defective formation of root cementum (Figure 1E). Other potential complications of FEO include progressive osteoclastic resorption that can lead to severe, painful, disabling deformities, and pathologic fractures of bones. Although the patient in this case showed only a possible mineralization disorder, she was placed on intravenous bisphosphonates for management of overall skeletal problems. Ten months later, her biochemical markers of the disease were reversed, and the root resorption lesions appeared stable. The transdisciplinary medical-dental collaboration between Children's Hospital of Philadelphia and University of Pennsylvania School of Dental Medicine generated sufficient diagnostic information to identify the cause of this young patient's condition, leading to appropriate and effective medical and dental treatment.
Genetic variants linked specifically with root resorption, but not overt systemic/syndromic manifestations, have been rare to date. A few reports suggest a genetic predisposition to MICRR based on hereditary patterns (17, 19, 67), and several reports noted MICRR in healthy individuals with apparently non-contributory medical histories (10, 13–16). Neely, Thumbigere-Math, and colleagues reported a familial pattern of MICRR with a 30-year follow-up (10, 17). To the best of our knowledge, this is the only report of inherited MICRR with an extended follow-up. The family included two generations with four MICRR-affected and four unaffected family members (10, 17). The 63-year-old proband presented with a history of MICRR affecting multiple teeth (Figures 1F–H). Over several decades, the resorptive lesions progressed with a total of 19 affected teeth, leading to extraction/exfoliation of 12 teeth. Additionally, the proband's two sons and one daughter developed MICRR during their fourth to sixth decades of life. All affected subjects were asymptomatic, lacked known predisposing factors, and reported a non-contributory medical history. Whole exome-sequencing identified a novel autosomal dominant heterozygous mutation [c.1219 G>A (G388S)] in the interferon regulatory factor 8 (IRF8) gene, which encodes a transcription factor that negatively regulates osteoclast differentiation (19). In vitro and in vivo functional analysis demonstrated that IRF8G388S mutation promoted increased osteoclastogenesis, thus providing a molecular basis for enhanced root resorption. Based on MICRR-associated variants in TNFRSF11A and IRF8, other variants targeting key regulatory steps in the osteoclast/odontoclast pathway might increase predisposition to root resorption. This concept has been borne out by studies using a transgenic mouse model where knockout of Tnfrfsf11b (osteoprotegerin), a decoy receptor for RANKL, promoted extensive molar root resorption (68).
Defective cementum formation has been suggested to predispose to periodontal breakdown, i.e., the concept of “periodontosis” by Gottlieb (58). Reduction or absence of acellular cementum at the cervical root surface theoretically exposes the root to resorption. Mutations in tissue-nonspecific alkaline phosphatase (TNAP), encoded by the ALPL gene, result in the inherited mineralization defect, hypophosphatasia (HPP; OMIM#146300, 241500, 241510) (69–71). Early exfoliation of deciduous teeth and loss of permanent teeth are pathognomonic signs of HPP due to defective cementogenesis. Abnormal root resorption in permanent teeth of some HPP patients has been reported (34, 35), possibly associated with cementum defects. Other inherited cementum defects in humans are rare, but genetically engineered mouse models serve as proof-of-principle examples. Mice deficient in bone sialoprotein (BSP), an extracellular matrix protein critical for cementum mineralization and function, exhibit a lack of acellular cementum and subsequent periodontal breakdown (11, 36). BSP null mice feature dramatic osteoclast/odontoclast mediated root resorption exclusively targeting the cervical regions of all molars (Figures 1I,J). The cementum defect and periodontal destruction in the absence of inflammation illustrate Gottlieb's periodontosis concept and suggest other inherited periodontal structural defects may promote cervical root resorption.
Inherited defects likely intersect with acquired or environmental factors to increase susceptibility to MICRR, possibly explaining delayed onset and diagnosis of root resorption in some cases. We emphasize that not all cases are associated with genetic etiologies, although increased understanding of genetic inputs in MICRR should prompt genetic testing when cases cannot be explained by local etiologic factors or systemic abnormalities.
It is well-recognized that medications have side-effects that can adversely affect oral health. For example, certain medications used to treat epilepsy, hypertension, and heart disease, or immunosuppressants in organ transplant patients, are associated with gingival hyperplasia (72, 73). Several medications cause severe xerostomia (dry mouth) (74, 75). Yet other therapies cause severe oral mucositis requiring treatment alterations. The examples below underscore the importance of collecting detailed medication histories (e.g., prescribed treatments as well as mouth rinses, toothpastes, herbal products, and vitamins) in individuals exhibiting root resorption. Significantly, these situations serve to remind physicians to consider treatment effects on oral health and incorporate dental clinicians in monitoring overall health of patients.
Anti-resorptive therapies are widely prescribed for treatment of osteoporosis and painful osteolytic manifestations of cancer. Several generations of bisphosphonates have served as key anti-resorptive agents for decades, while more recent therapies target regulators of osteoclast, osteocyte and osteoblast differentiation and function. Denosumab is a monoclonal anti-RANKL antibody that inhibits RANK-mediated activation of osteoclasts. Recently, Deeb et al. reported that a 69-year-old patient who discontinued denosumab after 5 years experienced MICRR affecting multiple teeth (Figure 1K), in conjunction with pain and sensitivity, but no alterations in attachment levels (12). A surge in osteoclastic activity may provide an explanation for occurrence and progression of MICRR, i.e., a rebound effect after discontinuing anti-resorptive therapy. After administration of denosumab, osteoclast activity rapidly declines and can drop by over 80% within weeks to months and remain at that level while denosumab treatment is continued (76). Once treatment is discontinued, antibody levels suddenly decline, resulting in transient increases in osteoclastic activity and bone turnover to levels above the starting range, before eventually returning to pretreatment levels (77). Although at this time there is insufficient evidence to support a causality between denosumab and MICRR, a website established to self-report cases of root resorption in patients treated with denosumab revealed 20 cases between 2013 and 2019, affecting predominantly females over 60 years taking denosumab for 2–5 years (https://www.ehealthme.com/ds/prolia/tooth-resorption/). Due to their antiresorptive effects on alveolar bone, systemic bisphosphonate use has been suggested to prevent progression of root resorption (37), and local delivery of bisphosphonates have been explored as potential approach for preventing root resorption of replanted teeth (38). While local use of bisphosphonates appears likely to serve as an effective interventional strategy in these contexts, caution should be taken due to the recent association of systemic anti-resorptives with MICRR, as well as the more established links with medication-related osteonecrosis of the jaw (MRONJ) (78–81).
Llavayol et. al reported that a 16-year-old female who received chemotherapy for ovarian cancer developed MICRR in 12 teeth, 9 years later (39). While the authors discarded other possible etiologies and hypothesized a correlation between chemotherapy and defective cementum and PDL, they were not able to establish the etiology. An alternative interpretation is that the medications affected mineral homeostasis, potentially activating osteoclast activity along with destruction of cementum and PDL.
Cats exhibit a high incidence of external root resorption, termed feline odontoclastic resorptive lesions (FORL), a disorder that strongly resembles MICRR in humans. The prevalence of FORL is around 29–60% (82, 83), and more commonly seen in domestic vs. wild cats, and often in females (84, 85). The etiology of FORL remains unknown, however, the mechanisms and progression of osteoclastic/odontoclastic root resorption appears similar to humans (Figure 1L) (86). Proposed risk factors include increasing age, diet low in magnesium/calcium and higher in vitamin D, and low frequency of teeth cleaning (84). FORL has been associated with Feline herpes virus 1 (FeHV-1), and it has been speculated that transmission of FeHV-1 to humans can initiate MICRR (5, 6, 40–44). Von Arx et al. reported four individuals with MICRR who had extended contact with cats and presented positive titers of neutralizing antibodies against FeHV-1 (40). Similarly, Wu and colleagues reported a case of MICRR in an individual who had contact with cats (14).
Other reports have described patients with herpes zoster/shingles who developed MICRR in corresponding areas of nerve innervation. Solomon et al. reported cervical root resorption in two teeth in a 31-year-old female with a positive history of herpes zoster infection in the corresponding division of the maxillary trigeminal nerve (45). Similarly, Ramchandani and Mellor reported MICRR in a 72-year-old female who presented with a 17 year earlier history of herpes zoster infection of the maxillary division of the trigeminal nerve (46).
Many other examples based on case reports and anecdotal experiences from clinicians and patients are worth mentioning since they may trigger additional thoughts regarding mechanisms mediating purported root resorption. While most examples of MICRR are non-inflammatory, we would be remiss if we did not mention inflammatory conditions associated with root resorption, such as severe periodontal disease, where marked inflammation triggers factors causing both osteoclast-mediated bone and cementum resorption. Additionally, osteoclast inhibitors such as denosumab and bisphosphonates have been associated with an acute-phase response and the release of proinflammatory cytokines, possibly explaining another underlying mechanism between anti-resorptive medications and MICRR (87, 88).
Environmental factors are another area warranting consideration. Examples here relate to Gottlieb's findings discussed above that some types of periodontal disease were associated with perceived defective cementum formation rather than marked inflammation (57–59). Individuals with minor defects in cementogenesis, whether related to genetic factors or exposure to environment toxins during tooth development, when exposed to periodontal pathogens or other local factors may be more susceptible to MICRR. Answers to this proposed mechanism of MICRR will require coordination among transdisciplinary researchers and clinicians as well as patients. Other environmental factors include marked trauma to the oral region, which is known to mediate osteoclast/odontoclast root resorption, usually localized to the affected area. Other examples of environmental factors, but regionally specific vs. MICRR, include playing of wind instruments, parafunctional habits and previous orthodontic treatment, the latter usually limited to apical root resorption.
Several valuable points are to be gained from this perspective:
1. The need for transdisciplinary approaches to improve health outcomes.
The goal of this perspective is to use MICRR as a model for portraying the need for transdisciplinary approaches in order to improve diagnosis and subsequent treatment of diseases/conditions, including the potential to identify medications that may affect oral health. The specific examples of MICRR provided here are used to demonstrate that oral conditions should be considered in the context of the whole body in order to move away from silos, which are unfortunately evident within the disciplines of dentistry and medicine. We must move toward integrated systems approaches for research and treatment.
2. MICRR is associated with multiple conditions.
The complex etiology of MICRR presented here highlights situations where the condition seems to be selective to the dentition, where in other situations it may prove to be a sign i.e., pathognomonic, for the condition. Some groups might consider MICRR as a manifestation associated with multiple conditions involving dysregulation of osteoclast differentiation/activity as the “common denominator” or “central triggering issue,” as opposed to a pathological entity that may have multiple etiologies. Other groups might consider MICRR as a pathological entity as not every individual affected by a myriad of etiologies (e.g., viral infection, use of medications, genetic disorders, etc.) exhibits signs of MICRR. Irrespective of the differing views, the examples provided in this perspective emphasize the need for transdisciplinary collaborations to improve our understanding of the characteristics of a given condition/disease.
3. MICRR remains a puzzle to solve.
Further transdisciplinary research from basic science to clinical studies is needed to define the etiology of MICRR, understand mechanisms honing osteoclasts to tooth root surfaces vs. surrounding bone, and develop treatments to arrest osteoclast activity and help repair tooth root structures vs. extraction.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This work was funded by K99AR073926 (to EC), R01DE027639, R03DE028632, R03DE028411 (to BF), NIAMS intramural research program funding (to MS), and R00DE028439, R03DE029258, and start-up funds from the University of Maryland School of Dentistry (to VT-M).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the subjects and families for their participation in the studies described. We thank Mr. Michael Chavez for micro-CT imaging in Figure 1H, Mr. Erik Holm for scanning electron microscopy imaging in Figures 1I,J, and Dr. Anthony Neely for the clinical photos in Figures 1F,G and his insights into the potential causes of MICRR based on his clinical experiences. We thank Dr. Anthony Randi, Dr. Stephen Russo, and Dr. Thomas Schneider, and their patients for willingness to participate in ongoing studies to advance our knowledge about etiology and risk factors of MICRR.
1. Lee JS, Somerman MJ. The importance of oral health in comprehensive health care. JAMA. (2018) 320:339–40. doi: 10.1001/jama.2017.19777
3. Harokopakis-Hajishengallis E. Physiologic root resorption in primary teeth: molecular and histological events. J Oral Sci. (2007) 49:1–12. doi: 10.2334/josnusd.49.1
4. Andreasen JO. Luxation of permanent teeth due to trauma. A clinical and radiographic follow-up study of 189 injured teeth. Scand J Dent Res. (1970) 78:273–86. doi: 10.1111/j.1600-0722.1970.tb02074.x
5. Fuss Z, Tsesis I, Lin S. Root resorption–diagnosis, classification and treatment choices based on stimulation factors. Dent Traumatol. (2003) 19:175–82. doi: 10.1034/j.1600-9657.2003.00192.x
6. Darcey J, Qualtrough A. Resorption: part 1. Pathology, classification and aetiology. Br Dent J. (2013) 214:439–51. doi: 10.1038/sj.bdj.2013.431
8. Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. (2011) 118:e16–31. doi: 10.1182/blood-2010-12-326355
9. Macaraeg K, Lee SM, Kalra L, Velasco M, Hajishengallis E. Multiple external root resorption in a pediatric patient with familial expansile osteolysis. Pediatr Dent. (2020) 42:62–5.
10. Neely AL, Thumbigere-Math V, Somerman MJ, Foster BL. A familial pattern of multiple idiopathic cervical root resorption with a 30-year follow-up. J Periodontol. (2016) 87:426–33. doi: 10.1902/jop.2015.150536
11. Foster BL, Soenjaya Y, Nociti FH Jr, Holm E, Zerfas PM, Wimer HF, et al. Deficiency in acellular cementum and periodontal attachment in bsp null mice. J Dent Res. (2013) 92:166–72. doi: 10.1177/0022034512469026
12. Deeb JG, Azarnoush K, Laskin DM, Deeb GR. Discontinuation of denosumab as a potential cause of generalized external cervical root resorption: a case report. J Endodont. (2019) 45:640–4. doi: 10.1016/j.joen.2019.02.013
13. Yu VS, Messer HH, Tan KB. Multiple idiopathic cervical resorption: case report and discussion of management options. Int Endodont J. (2011) 44:77–85. doi: 10.1111/j.1365-2591.2010.01820.x
14. Wu J, Lin LY, Yang J, Chen XF, Ge JY, Wu JR, et al. Multiple idiopathic cervical root resorption: a case report. Int Endod J. (2016) 49:189–202. doi: 10.1111/iej.12440
15. Beckett HA, Gilmour AG. Multiple idiopathic cervical root resorption in a male. Br Dent J. (1993) 175:33–4. doi: 10.1038/sj.bdj.4808213
16. Liang H, Burkes EJ, Frederiksen NL. Multiple idiopathic cervical root resorption: systematic review and report of four cases. Dento Maxillo Facial Radiol. (2003) 32:150–5. doi: 10.1259/dmfr/12925020
17. Neely AL, Gordon SC. A familial pattern of multiple idiopathic cervical root resorption in a father and son: a 22-year follow-up. J Periodontol. (2007) 78:367–71. doi: 10.1902/jop.2007.060155
18. Goodell KB, Mines P, Kersten DD. Impact of cone-beam computed tomography on treatment planning for external cervical resorption and a novel axial slice-based classification system. J Endod. (2018) 44:239–44. doi: 10.1016/j.joen.2017.10.001
19. Thumbigere-Math V, Foster BL, Bachu M, Yoshii H, Brooks SR, Coulter A, et al. Inactivating mutation in IRF8 promotes osteoclast transcriptional programs and increases susceptibility to tooth root resorption. J Bone Miner Res. (2019) 34:1155–68. doi: 10.1002/jbmr.3690
20. Mueller E, Rony HR. Laboratory studies of an unusual case of resorption. J Am Dent Assoc. (1930) 17:326–34. doi: 10.14219/jada.archive.1930.0051
21. Becks H, Cowden RC. Root resorptions and their relation to pathologic bone formation: Part II. Classification, degrees, prognosis and frequency. Am J Orthodont Oral Surg. (1942) 28:513–26. doi: 10.1016/S0096-6347(42)90327-2
22. Goultschin J, Nitzan D, Azaz B. Root Resorption - Review And Discussion. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (1982) 54:586–90. doi: 10.1016/0030-4220(82)90199-2
23. Nagaraj E, Kaur RP, Raghuram PH, Kumar PS. Multiple internal resorption in permanent teeth associated with hyperparathyroidism. Indian J Dent Res. (2013) 24:128–31. doi: 10.4103/0970-9290.114917
24. Arroyo-Bote S, Bucchi C, Manzanares MC. External cervical resorption: a new oral manifestation of systemic sclerosis. J Endodont. (2017) 43:1740–3. doi: 10.1016/j.joen.2017.03.040
25. Bender IB, Bender AL. Dental observations in Gaucher's disease: review of the literature and two case reports with 13- and 60-year follow-ups. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (1996) 82:650–9. doi: 10.1016/S1079-2104(96)80440-9
26. Edwards PC, McVaney T. External cervical root resorption involving multiple maxillary teeth in a patient with hereditary hemorrhagic telangiectasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2005) 100:585–91. doi: 10.1016/j.tripleo.2005.02.069
27. Smith NH. Monostotic Paget's disease of the mandible presenting with progressive resorption of the teeth. Oral Surg Oral Med Oral Pathol. (1978) 46:246–53. doi: 10.1016/0030-4220(78)90199-8
28. Monteiro M, Rout J. Multiple root resorption as a presenting sign of Paget's disease of bone. Oral Surg. (2008) 1:53–5. doi: 10.1111/j.1752-248X.2007.00011.x
29. Baxter AM, Shaw MJ, Warren K. Dental and oral lesions in two patients with focal dermal hypoplasia (Goltz syndrome). Br Dent J. (2000) 189:550–3. doi: 10.1038/sj.bdj.4800826
30. Rudiger S, Berglundh T. Root resorption and signs of repair in Papillon-Lefevre syndrome. A case study. Acta Odontol Scand. (1999) 57:221–4. doi: 10.1080/000163599428814
31. Yusof WZ, Ghazali MN. Multiple external root resorption. J Am Dent Assoc. (1989) 118:453–5. doi: 10.14219/jada.archive.1989.0182
32. Olsen CB, Tangchaitrong K, Chippendale I, Graham HK, Dahl HM, Stockigt JR. Tooth root resorption associated with a familial bone dysplasia affecting mother and daughter. Pediatr Dent. (1999) 21:363–7.
33. Mitchell CA, Kennedy JG, Wallace RG. Dental abnormalities associated with familial expansile osteolysis: a clinical and radiographic study. Oral Surg Oral Med Oral Pathol. (1990) 70:301–7. doi: 10.1016/0030-4220(90)90145-I
34. Tangney NJ. Hypophosphatasia: a case report and literature review. Irish Med J. (1979) 72:530–1.
35. Olsson A, Matsson L, Blomquist HK, Larsson A, Sjodin B. Hypophosphatasia affecting the permanent dentition. J Oral Pathol Med. (1996) 25:343–7. doi: 10.1111/j.1600-0714.1996.tb00274.x
36. Foster BL, Ao M, Willoughby C, Soenjaya Y, Holm E, Lukashova L, et al. Mineralization defects in cementum and craniofacial bone from loss of bone sialoprotein. Bone. (2015) 78:150–64. doi: 10.1016/j.bone.2015.05.007
37. Iwamatsu-Kobayashi Y, Satoh-Kuriwada S, Yamamoto T, Hirata M, Toyoda J, Endo H, et al. A case of multiple idiopathic external root resorption: a 6-year follow-up study. Oral Surg Oral Med Oral Pathol Oral Radiol Endodont. (2005) 100:772–9. doi: 10.1016/j.tripleo.2004.11.047
38. Najeeb S, Siddiqui F, Khurshid Z, Zohaib S, Zafar MS, Ansari SA. Effect of bisphosphonates on root resorption after tooth replantation - a systematic review. Dent Traumatol. (2017) 33:77–83. doi: 10.1111/edt.12316
39. Llavayol M, Pons M, Ballester ML, Berastegui E. Multiple cervical root resorption in a young adult female previously treated with chemotherapy: a case report. J Endodont. (2019) 45:349–53. doi: 10.1016/j.joen.2018.12.012
40. von Arx T, Schawalder P, Ackermann M, Bosshardt DD. Human and feline invasive cervical resorptions: the missing link?–Presentation of four cases. J Endodont. (2009) 35:904–13. doi: 10.1016/j.joen.2009.03.044
41. DeLaurier A, Boyde A, Jackson B, Horton MA, Price JS. Identifying early osteoclastic resorptive lesions in feline teeth: a model for understanding the origin of multiple idiopathic root resorption. J Periodont Res. (2009) 44:248–57. doi: 10.1111/j.1600-0765.2008.01123.x
42. Bergmans L, Van Cleynenbreugel J, Verbeken E, Wevers M, Van Meerbeek B, Lambrechts P. Cervical external root resorption in vital teeth. J Clin Periodontol. (2002) 29:580–5. doi: 10.1034/j.1600-051X.2002.290615.x
43. Dahl JE, Pallesen U. Tooth bleaching–a critical review of the biological aspects. Crit Rev Oral Biol Med. (2003) 14:292–304. doi: 10.1177/154411130301400406
44. Southam JC. Clinical and histological aspects of peripheral cervical resorption. J Periodontol. (1967) 38:534–8. doi: 10.1902/jop.1967.38.6_part1.534
45. Solomon CS, Coffiner MO, Chalfin HE. Herpes zoster revisited: implicated in root resorption. J Endodont. (1986) 12:210–3. doi: 10.1016/S0099-2399(86)80157-1
46. Ramchandani PL, Mellor TK. Herpes zoster associated with tooth resorption and periapical lesions. Br J Oral Maxillofac Surg. (2007) 45:71–3. doi: 10.1016/j.bjoms.2005.05.008
47. Heithersay GS. Invasive cervical resorption: an analysis of potential predisposing factors. Quintessence Int. (1999) 30:83–95.
48. Heithersay GS. Clinical, radiologic, and histopathologic features of invasive cervical resorption. Quintessence Int. (1999) 30:27–37.
49. Mavridou AM, Bergmans L, Barendregt D, Lambrechts P. descriptive analysis of factors associated with external cervical resorption. J Endodont. (2017) 43:1602–10. doi: 10.1016/j.joen.2017.05.026
50. Kandalgaonkar SD, Gharat LA, Tupsakhare SD, Gabhane MH. Invasive cervical resorption: a review. J Int Oral Health. (2013) 5:124–30. doi: 10.1155/2013/812323
51. Trope M. Luxation injuries and external root resorption–etiology, treatment, and prognosis. J Calif Dent Assoc. (2000) 28:860–6.
52. Patel S, Mavridou AM, Lambrechts P, Saberi N. External cervical resorption-part 1: histopathology, distribution and presentation. Int Endodont J. (2018) 51:1205–23. doi: 10.1111/iej.12942
53. Chen SS, Greenlee GM, Kim JE, Smith CL, Huang GJ. Systematic review of self-ligating brackets. Am J Orthodont Dentofacial Orthop. (2010) 137:726.e1–6.e18. discussion 726–7. doi: 10.1016/j.ajodo.2009.11.009
54. Gunst V, Huybrechts B, De Almeida Neves A, Bergmans L, Van Meerbeek B, Lambrechts P. Playing wind instruments as a potential aetiologic cofactor in external cervical resorption: two case reports. Int Endodont J. (2011) 44:268–82. doi: 10.1111/j.1365-2591.2010.01822.x
55. George DI Jr, Miller RL. Idiopathic resorption of teeth. A report of three cases. Am J Orthodont. (1986) 89:13–20. doi: 10.1016/0002-9416(86)90108-9
56. Mavridou AM, Hauben E, Wevers M, Schepers E, Bergmans L, Lambrechts P. Understanding external cervical resorption in vital teeth. J Endodont. (2016) 42:1737–51. doi: 10.1016/j.joen.2016.06.007
57. Gottlieb B. Die diffuse atrophie des alveoarknochens. Weitere beitra ge zur kenntnis des alveolarschwundes und dessen wiedergutmachung durch zementwachstum. Z Stomatol. (1923) 21:195–201.
58. Gottlieb B. The new concept of periodontoclasia. J Periodontol. (1946) 17:7–23. doi: 10.1902/jop.1946.17.1.7
59. Fine DH, Cohen DW, Bimstein E, Bruckmann C. A ninety-year history of periodontosis: the legacy of Professor Bernhard Gottlieb. J Periodontol. (2015) 86:1–6. doi: 10.1902/jop.2014.140202
60. Page RC, Baab DA. A new look at the etiology and pathogenesis of early-onset periodontitis. Cementopathia revisited. J Periodontol. (1985) 56:748–51. doi: 10.1902/jop.1985.56.12.748
61. Polimeni G, Xiropaidis AV, Wikesjo UM. Biology and principles of periodontal wound healing/regeneration. Periodontol 2000. (2006) 41:30–47. doi: 10.1111/j.1600-0757.2006.00157.x
62. Arnett TR, Gibbons DC, Utting JC, Orriss IR, Hoebertz A, Rosendaal M, et al. Hypoxia is a major stimulator of osteoclast formation and bone resorption. J Cell Physiol. (2003) 196:2–8. doi: 10.1002/jcp.10321
63. Hughes AE, Ralston SH, Marken J, Bell C, MacPherson H, Wallace RG, et al. Mutations in TNFRSF11A, affecting the signal peptide of RANK, cause familial expansile osteolysis. Nat Genet. (2000) 24:45–8. doi: 10.1038/71667
64. Palenzuela L, Vives-Bauza C, Fernandez-Cadenas I, Meseguer A, Font N, Sarret E, et al. Familial expansile osteolysis in a large Spanish kindred resulting from an insertion mutation in the TNFRSF11A gene. J Med Genet. (2002) 39:E67. doi: 10.1136/jmg.39.10.e67
65. Dickson GR, Shirodria PV, Kanis JA, Beneton MN, Carr KE, Mollan RA. Familial expansile osteolysis: a morphological, histomorphometric and serological study. Bone. (1991) 12:331–8. doi: 10.1016/8756-3282(91)90019-F
66. Madel MB, Ibanez L, Ciucci T, Halper J, Rouleau M, Boutin A, et al. Dissecting the phenotypic and functional heterogeneity of mouse inflammatory osteoclasts by the expression of Cx3cr1. Elife. (2020) 9:e54493. doi: 10.7554/eLife.54493
67. Pinska E, Jarzynka W. Spontaneous resorption of the roots of all permanent teeth as a familial disease. Czas Stomatol. (1966) 19:161–5.
68. Liu Y, Du H, Wang Y, Liu M, Deng S, Fan L, et al. Osteoprotegerin-Knockout mice developed early onset root resorption. J Endodont. (2016) 42:1516–22. doi: 10.1016/j.joen.2016.07.008
69. Foster BL, Nociti FH Jr, Somerman MJ. The rachitic tooth. Endocr Rev. (2014) 35:1–34. doi: 10.1210/er.2013-1009
70. Whyte MP. Hypophosphatasia - aetiology, nosology, pathogenesis, diagnosis and treatment. Nat Rev Endocrinol. (2016) 12:233–46. doi: 10.1038/nrendo.2016.14
71. Whyte MP. Hypophosphatasia: an overview for 2017. Bone. (2017) 102:15–25. doi: 10.1016/j.bone.2017.02.011
72. Brown RS, Beaver WT, Bottomley WK. On the mechanism of drug-induced gingival hyperplasia. J Oral Pathol Med. (1991) 20:201–9. doi: 10.1111/j.1600-0714.1991.tb00419.x
73. Seymour RA, Thomason JM, Ellis JS. The pathogenesis of drug-induced gingival overgrowth. J Clin Periodontol. (1996) 23:165–75. doi: 10.1111/j.1600-051X.1996.tb02072.x
74. Cassolato SF, Turnbull RS. Xerostomia: clinical aspects and treatment. Gerodontology. (2003) 20:64–77. doi: 10.1111/j.1741-2358.2003.00064.x
75. Guggenheimer J, Moore PA. Xerostomia: etiology, recognition and treatment. J Am Dent Assoc. (2003) 134:61–9. doi: 10.14219/jada.archive.2003.0018
76. Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. (2011) 96:972–80. doi: 10.1210/jc.2010-1502
77. Eastell R, Christiansen C, Grauer A, Kutilek S, Libanati C, McClung MR, et al. Effects of denosumab on bone turnover markers in postmenopausal osteoporosis. J Bone Miner Res. (2011) 26:530–7. doi: 10.1002/jbmr.251
78. Wan JT, Sheeley DM, Somerman MJ, Lee JS. Mitigating osteonecrosis of the jaw (ONJ) through preventive dental care and understanding of risk factors. Bone Res. (2020) 8:14. doi: 10.1038/s41413-020-0088-1
79. Zymperdikas VF, Yavropoulou MP, Kaklamanos EG, Papadopoulos MA. Bisphosphonates as supplement to dental treatment: a network meta-analysis. J Dent Res. (2020). doi: 10.1177/0022034520972945. [Epub ahead of print].
80. Thumbigere-Math V, Michalowicz BS, Hodges JS, Tsai ML, Swenson KK, Rockwell L, et al. Periodontal disease as a risk factor for bisphosphonate-related osteonecrosis of the jaw. J Periodontol. (2014) 85:226–33. doi: 10.1902/jop.2013.130017
81. Thumbigere-Math V, Tu L, Huckabay S, Dudek AZ, Lunos S, Basi DL, et al. A retrospective study evaluating frequency and risk factors of osteonecrosis of the jaw in 576 cancer patients receiving intravenous bisphosphonates. Am J Clin Oncol. (2012) 35:386–92. doi: 10.1097/COC.0b013e3182155fcb
82. Ingham KE, Gorrel C, Blackburn J, Farnsworth W. Prevalence of odontoclastic resorptive lesions in a population of clinically healthy cats. J Small Anim Pract. (2001) 42:439–43. doi: 10.1111/j.1748-5827.2001.tb02497.x
83. Lommer MJ, Verstraete FJ. Prevalence of odontoclastic resorption lesions and periapical radiographic lucencies in cats: 265 cases (1995-1998). J Am Vet Med Assoc. (2000) 217:1866–9. doi: 10.2460/javma.2000.217.1866
84. Lund EM, Bohacek LK, Dahlke JL, King VL, Kramek BA, Logan EI. Prevalence and risk factors for odontoclastic resorptive lesions in cats. J Am Vet Med Assoc. (1998) 212:392–5.
85. Scarlett JM, Saidla J, Hess J. Risk factors for odontoclastic resorptive lesions in cats. J Am Anim Hosp Assoc. (1999) 35:188–92. doi: 10.5326/15473317-35-3-188
86. Shigeyama Y, Grove TK, Strayhorn C, Somerman MJ. Expression of adhesion molecules during tooth resorption in feline teeth: a model system for aggressive osteoclastic activity. J Dent Res. (1996) 75:1650–7. doi: 10.1177/00220345960750090601
Keywords: periodontal disease, root resorption, osteoclast, odontoclast, genetics, medications, bone resorption
Citation: Chu EY, Deeb JG, Foster BL, Hajishengallis E, Somerman MJ and Thumbigere-Math V (2021) Multiple Idiopathic Cervical Root Resorption: A Challenge for a Transdisciplinary Medical-Dental Team. Front. Dent. Med. 2:652605. doi: 10.3389/fdmed.2021.652605
Received: 12 January 2021; Accepted: 11 February 2021;
Published: 26 March 2021.
Edited by:
Guliz N. Guncu, Hacettepe University, TurkeyReviewed by:
Victor Montalli, São Leopoldo Mandic School, BrazilCopyright © 2021 Chu, Deeb, Foster, Hajishengallis, Somerman and Thumbigere-Math. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vivek Thumbigere-Math, dnRodW1iaWdlcmVAdW1hcnlsYW5kLmVkdQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.