- Clinic of Conservative and Preventive Dentistry, Division of Periodontology and Peri-implant Diseases, Center of Dental Medicine, University of Zurich, Zurich, Switzerland
Purpose: To systemically summarize current knowledge about regeneration of peri-implant defects based on available systematic reviews.
Materials and Methods: A systematic search for review articles published between 2010 and 2020 in four databases was conducted. Only systematic reviews and meta-analyses were included. Based on the available literature, five questions of clinical importance on indication for regenerative approaches, surgical technique, methods of decontamination, outcome of therapy and adjunctive use of biological factors were formulated and answered.
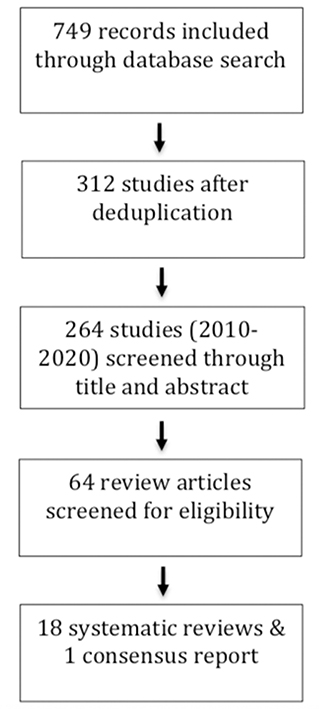
Results: The electronic search resulted in 312 studies, from which 264 studies were published between 2010 and 2020. Finally, 18 systematic reviews and one consensus report were chosen. Data of the included studies were based on 58 to 840 implants. Data on over 4.904 implants were assessed. From the 19 studies that were included, 15 assessed the outcome of regenerative therapy; three, the surgical protocol of regenerative therapy; two, the use of laser in regenerative therapy; and one, the additional use of growth factors in regenerative peri-implant therapy. Three studies assessed more than one topic.
Conclusions: In general, a partial bone fill can be expected in 85% of regenerative procedures. Regeneration leads to a mean of 57% of greater bone fill, compared to open flap surgery only. Defect configuration plays a crucial role in the outcome, whereas the role and extent of benefit of different surgical protocols are still not clear.
Introduction
The placement of dental implants has become a routine procedure for the rehabilitation of patients with one or more missing teeth. High success rates of up to 97 and 75% after 10 (1) and 20 years (2), respectively, have been reported. However, in recent cases of implant placements, mainly biological complications in terms of peri-implant inflammation, known as mucositis and peri-implantitis, are increasingly reported in daily practice (3). Whereas, mucositis is limited to the surrounding soft tissues, peri-implantitis results in progressive peri-implant bone loss (4). Today, peri-implantitis represents the main reason for late implant failure and removal (5). A meta-analysis (MA) estimated a considerable weighted mean prevalence of 22% (confidence interval: 14–30%) for disease development (6).
To treat this respective attachment loss, several treatment approaches are available. Whereas, non-surgical therapy has already been shown to be ineffective (7), open flap surgery seems to have more promising results, if indicated, regenerative procedures seem to have even better outcomes (8). With regard to regenerative procedures, mainly two parameters seem to still be of eminent interest: [1] the effective cleaning and decontamination of the affected implant sites (4), and [2] presence of suitable hard tissue defects allowing for stable augmentation, coverage, and healing (9).
A plethora of different surgical techniques including grafting materials and growth factors, have already been described in the literature (8), and experts agree that overall treatment outcomes improve after 6 months to 10 years, especially when considering clinical probing pocket depth (PPD) and radiographic measurements as primary outcome parameters.
This evidence-based update review was conducted with the aim of stocktaking the available current evidence, with a focus on regenerative peri-implantitis treatment, and highlighting the current and future research perspectives. For this purpose, the authors tried to formulate the most specific clinically relevant questions for this topic based on a systematic literature search.
Materials and Methods
A systematic literature search for reviews and meta-analyses assessing regenerative treatment approaches of peri-implantitis bone defects was conducted in September 2020. It followed the focused question: what is the current knowledge about regeneration of peri-implant defects in human patients?
This review was written in accordance with the PRISMA statement for reporting systematic reviews of studies evaluating healthcare interventions and the methodology for summarizing systematic reviews (10).
The following databases were included: MEDLINE, Embase, Web of Science, and Scopus. The following search terms were applied: “Peri-implantitis” OR “peri-implantitis” OR “peri implantitis” AND “Reconstructive surgery” OR “regeneration” OR “reconstruct” OR “regenerat” OR “guided bone regeneration” OR “GBR” OR “augmentation” OR “augment” AND “Review.”
Based on the available literature, five questions of clinical importance were formulated:
1) Augmentative procedures – for which defects and which patients?
2) (How) can infected implant surfaces be cleaned?
3) How can/should regeneration be achieved?
4) What is the outcome of regenerative therapy in peri-implantitis?
5) Should we apply additional biologicals or stem cells as adjunct measures?
Further, a hand search was conducted, and reference lists of included studies were screened.
Screening and Selection
First, both authors independently assessed the publications by title and abstract. The inclusion and exclusion criteria for the reviews were as follows:
Inclusion criteria: systematic reviews and meta- analyses including human studies published from January 2010 to September 2020.
Exclusion criteria: reviews on animal studies; all other publication forms other than reviews; publication before 2010; and reviews not in English.
Available titles and abstracts were collected and discussed before being finally included or excluded by the two authors.
The quality of included literature was assessed using AMSTAR 2 criteria (11).
Data Extraction
The following key points were collected for all included systematic reviews and summarized in Tables 1, 2: name of authors, year of publication, topic of research, number of included implants, PICO (Population, Intervention, Comparison, Outcome), main results, and conclusions.
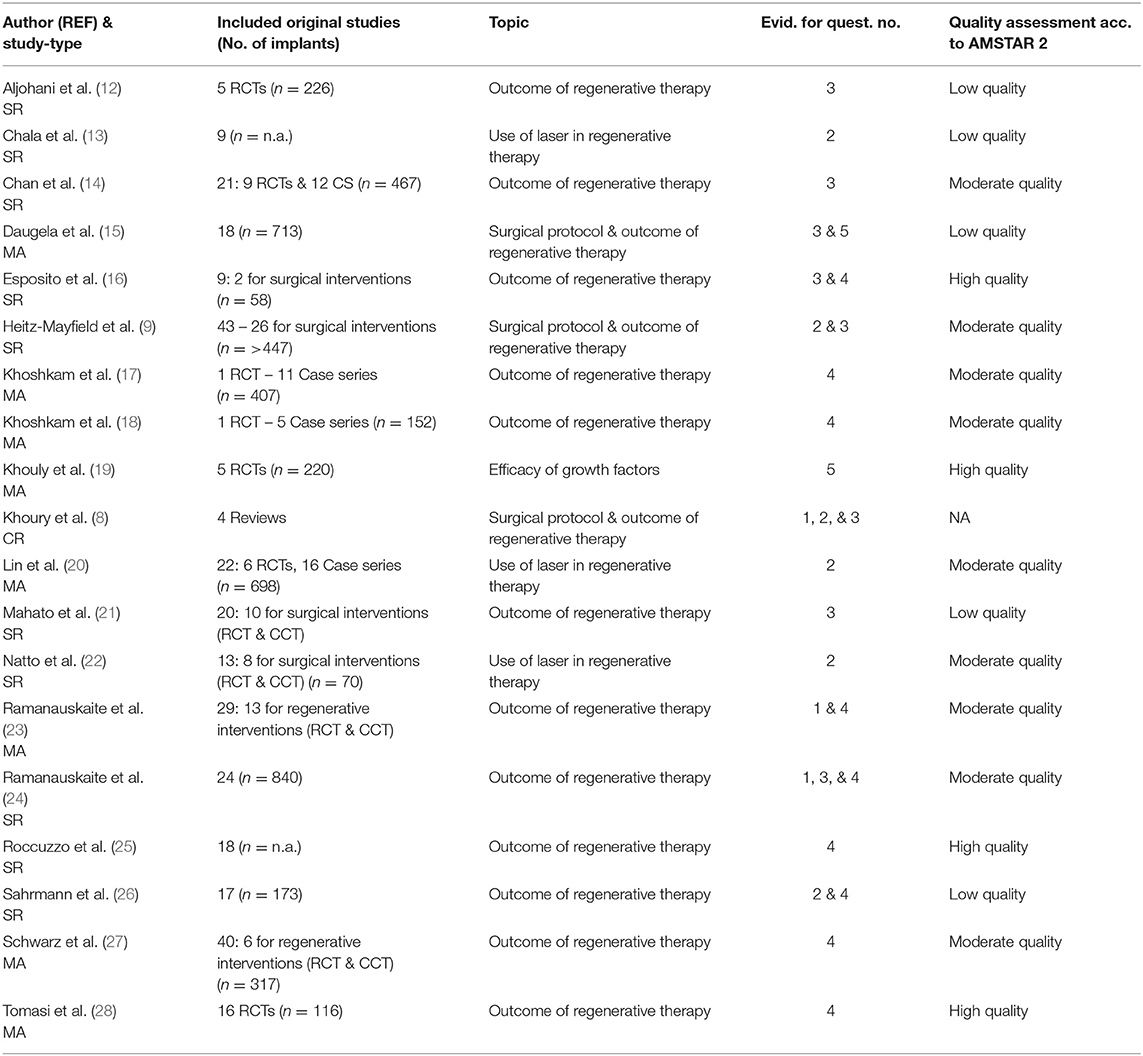
Table 1. Characteristics of the included studies (SR, Systematic Review; MA, Meta-Analysis; RCT, Randomized Clinical Trial; CCT, Case Control Studies).
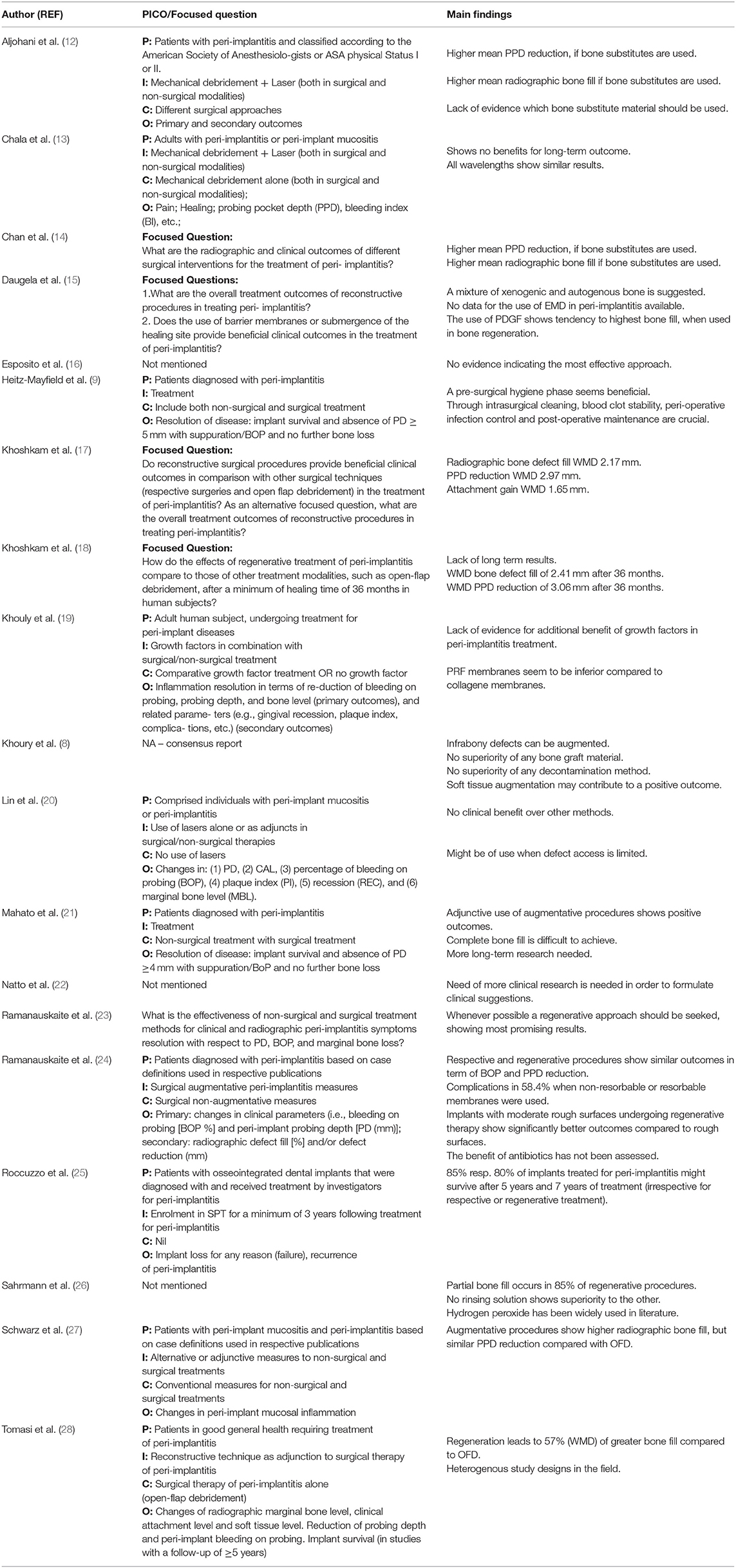
Table 2. Overview over the PICO (Population, Intervention, Comparison, Outcome) question or the formulated focused question with corresponding main findings of each included review.
For each formulated focus question, a specific surrogate parameter or outcome variables were specified as follows:
1) defect configuration and host factors
2) efficacy of available surface cleaning protocols
3) indication and benefit of use of different biomaterials
4) expected outcome of regenerative treatment
5) benefit of use of bio-active materials or cell therapy.
Respective data (if available) and conclusions were collected.
Results
Search
Search results are shown in Figure 1. The electronic search contained 312 articles, which included 264 articles published between 2010 and 2020. Finally, 18 systematic reviews and one consensus report were included. Consequently, 46 studies were excluded, because of their narrative and non-systematic methodology. Inter-examiner agreement of a Cohen's kappa (K) of 0.762 was achieved after the initial screening. The authors discussed discrepancies before reaching a consensus.
Studies
Table 1 describes the 18 included systematic reviews and one consensus report. Table 2 contains the formulated PICO or focus question for each study with their corresponding main conclusions. Among the 19 included studies, 15 studies assessed the outcome of regenerative therapy; three, the surgical protocol of regenerative therapy; two, the use of laser in regenerative therapy; and one, the additional use of growth factors in regenerative peri-implant therapy. Three studies assessed more than one topic. In all, eight studies included a meta-analysis; nine studies, a systematic literature search; and one study, a consensus report. Eight of the 18 reviews included case control trials (CCT) data in addition to randomized control trials (RCT) data. The consensus report was based on the data of four reviews. The data of the included studies were based on 58 to 840 implants. Five studies did not mention the number of included implants. This umbrella review contains data on a total of over 4.904 implants. The quality of the included systematic reviews ranged from low (n = 5), moderate (n = 9), to high (n = 4), according to AMSTAR 2 criteria (11) (Table 1).
Discussion
Question 1: Augmentative Procedures – for Which Defects and Which Patients?
The healing potential of regenerative therapy is mainly determined by the morphology of the underlying bone defects (29). Schwarz and co-workers (30) investigated the impact of defect configuration. Whereas class I defects represent well-defined infrabony defects with an additional sub-classification based on the inflicted lost walls and their configuration, class II defects describe suprabony, i.e., horizontal bone loss. Within class I defects, the Ie-configuration represents a circumferential crater-shaped self-containing defect and was defined as being the most favorable one for a regenerative approach. In addition, with a mean prevalence of 55%, it also represents the most common defect encountered in patients suffering from peri-implantitis (30). In accordance with periodontal defect characteristics, the assumption based on the existing body of literature is that the more walls are present and the more self-containing these defects are, the more promising the potential bone regeneration is (31). In contrast to this optimal situation, regeneration of suprabony class II defects seems—as in line with periodontal defects—not to be a predictably achievable goal yet (8). This means that exposed implant shoulders and threads can only be managed by non-regenerative or maybe respective means, i.e., implantoplasty (32). A combination of a respective approach with guided bone regeneration (GBR) may be considered, if defects show supra- and infrabony components (21, 29). Notably, only 50% of patients treated with such a combined approach of implantoplasty and GBR showed no sign of inflammation after 2 years (32). Of course, with regard to the overall eligibility of a patient for regenerative and augmentative therapy and the overall outcome prognosis, the most relevant systemic and environmental factors, such as smoking, oral hygiene, and medical condition, must be considered (31).
Question 2: (How) Can Infected Implant Surfaces Be Cleaned?
Various decontamination methods, such as mechanical, physical, and chemical approaches and combinations, have been described in the literature (27). All methods commonly aim to remove biofilm and/or calculus and create a biocompatible surface, which conceptually leads to successful re-osseointegration (33). Despite various materials and protocols, none seems capable of complete and reliable decontamination of implant surfaces (29), and no single rinsing solution has shown any superiority over the others (8). Therefore, most protocols represent a combination of mechanical and chemical debridement methods combining the thought advantages of each approach (34).
From a conceptual point of view, any mechanical instrument, such as hand instruments and ultrasonic tips, used for debridement should be softer than the treated implant material to prevent damage to the implant surface (35). For this purpose, tips have been modified or coated with different materials, such as carbon fibers, plastic, and silicon. Unfortunately, most of these instruments have often been judged as ineffective in thoroughly removing biofilm (36). In addition, material remnants and contaminations are possible, which should not be neglected in this context.
Air-polishing devices have, therefore, been suggested for non-surgical and intra-surgical debridement procedures. The efficacy of mechanical and ultrasonic curettage seems to be inferior to that of air-polishing systems (35). However, air-polishing devices have to be applied with care, as a certain risk exists for the generation of an iatrogenic emphysema (8, 26). The extent of re-osseointegration of titanium implants after air polishing therapy has been reported to be between 39 and 46% with increased clinical implant attachment and PPD reduction (35).
Lasers (Diode, Er:YAG, Nd:YAG, Er,Cr:YSGG) have also been suggested for peri-implant therapy (22). Evidence supports the ability of lasers for tissue debridement, cell proliferation (photobiomodulation), bacterial inactivation (antimicrobial photodynamic therapy), and calculus ablation to comparable levels of conventional mechanical instrumentation. In a systematic review and meta-analysis (20), less effectiveness of lasers for surgical treatment of peri-implant diseases was described; i.e., the test group did not show any clinical benefits over the control groups without laser (20). These findings are consistent with those of a recent systematic review showing strong scientific evidence that both treatments, regenerative procedures with or without additional laser disinfection, result in the same outcome in the long-term (13). Although lasers show no significant advantage over other decontamination methods, they might possess some advantages, especially when the access to mechanical instruments is compromised (29).
Regarding chemical agents, different rinsing solutions have been suggested for decontamination of implant surfaces. However, none has been proven to be superior to the other (29). Among the most frequently used and investigated chemicals, the following substances are commonly recommended: hydrogen peroxide, citric acid, sodium chloride, chloramines, tetracycline hydrochloride, and chlorhexidine gluconate. Additionally, based on the available evidence, no single method has been proven to be superior (29), but undoubtedly, the adjunct use of rinsing, as such, is clinically considered an important adjunctive step in cleaning the affected areas. Owing to its availability, efficacy, and safety, hydrogen peroxide, applied on the implant surface for 2 min, has been the substance most widely used for chemical decontamination (26).
Question 3: How Can/Should Regeneration Be Achieved?
The use of bone substitute materials alone or in combination with barrier membranes has been investigated in several studies (12, 15).
With respect to the selection of bone substitute materials, autogenous bone has been used and described as gold standard as in many other augmentative procedures. However, a recent meta-analysis suggested that its use—despite its potential osteoinductive and osteogenic properties—leads to the least bone gain, when compared to synthetic and xenogenic bone grafts, and a resorption of up to 40% of the original volume has been reported (15, 37). Synthetic or xenogenic materials show higher volume stability in the presence of a low or no resorption rate. Most authors, therefore, still suggest a mixture of materials to combine advantages and concurrently overcome disadvantages (15), as mentioned above. However, no recent systematic review still could show any significant superiority of a specific material (12).
Noteworthy, due to limited amounts of human histologies, no final conclusion can be drawn, with respect to re-osseointegration of previously exposed implant surfaces after GBR procedures (32).
When assessing the additional use of a membrane, the studies could not show an improvement of the outcome when membranes were used, especially in self-containing (class-Ie) defects. In contrast, membranes tended to exhibit more complications during healing by developing wound dehiscence. Complications have been reported in 58.4% of all cases, when non-resorbable or resorbable membranes were used (24). Resorbable membranes alone would surely show a lower complication rate, as mostly non-resorbable membranes show a higher rate of wound- dehiscence (38). Membrane fixation and stabilization, in combination with pins, have been suggested for more complex defects, including bone dehiscence, to contain and stabilize the bone substitute material (32). Based on the question, if submerged or unsubmerged healing of the augmented implant site is preferable, evidence showing any superiority of one approach to the other is lacking. However, from a clinical viewpoint, seeking a submerged healing whenever possible to enable an uninterrupted healing seems rational (8). Certainly, due to patients' chewing comfort and other reasons, sometimes this cannot be achieved.
Only little is known about the role of soft tissues in regeneration. Although evidence is lacking, ensuring the availability of adequate amounts of keratinized tissue in the surgical area seems rational. Providing both, stability to the blood clot and enabling the patient to clean the area properly after regeneration (39).
The administration of antibiotics, combined with regenerative procedures, was investigated in several studies, but none actually evaluated the possible positive effect on the regenerative outcome (24).
Question 4: What Is the Outcome of Regenerative Therapy in Peri-Implantitis?
As mentioned already, non-surgical therapy—especially in advanced peri-implantitis defects—seems rather ineffective and unpredictable. However, it should be considered a pre-surgical hygiene phase under any circumstances (9, 26). Similar to regenerative periodontal treatment, thorough intrasurgical cleaning, blood clot stability, peri-operative infection control, and post-operative maintenance seem to be crucial in the regeneration of peri-implant bone defects (9). As compared to periodontitis and inflammations around teeth, peri-implantitis, in general, progresses faster, explaining the need to treat patients without delay to avoid further bone or even implant loss (29).
An early systematic review showed complete bone fill after regenerative peri-implantitis therapy in only roughly 10% of the cases, whereas partial bone fill was described in almost 86% and no bone fill in 4% (26). Therefore, the authors concluded that a complete bone fill represents no predictable therapy outcome, while at least a partial bone fill can be expected (26). In a more recent systematic review, an additional mean bone-level-gain of 1.7 mm and a weighted mean difference (WMD) in bone fill of 57% was observed, when comparing regenerative procedures with open flap surgery (OFD) after 1 year. Surprisingly, no difference in PPD or bleeding on probing (BOP) reduction was observed (28). This was consistent with the observation of other authors stating that augmentative procedures show higher radiographic bone fill but similar PPD and BOP reduction to OFD (24, 27). A recent systematic review showed the highest mean PPD reduction (2.8–3.1 mm) and mean bone defect fill (up to 3.6 mm) in groups using bone substitute materials, compared to OFD (mean PPD reduction of 1.2 mm) alone (12). These findings corroborated the conclusions of other systematic reviews (14, 17, 23).
In spite of an initial successful regeneration of bony defects, cases of implant loss and disease recurrence have been described (24). Furthermore, implants with moderate rough surfaces undergoing regenerative therapy showed significantly better outcomes than rough surfaces (24).
With respect to long-term results, only limited data are available. One systematic review analyzed outcomes after 3 years. A WMD in bone defect fill of 2.4 mm and PPD reduction of 3.1 mm were observed (18). Irrespective of a respective or regenerative approach, Roccuzzo et al. (25) found a survival rate of implants treated for peri-implantitis of 85% resp. Eighty percent after 5 and 7 years of treatment.
In general, available data on peri-implant defect regeneration in humans have been shown to be limited, as a high number of available clinical studies seems to be, unfortunately, case series (28). The mentioned reviews, in addition to the low number of controlled trials, found that study designs and materials used in the different studies were very heterogeneous, leading to difficulties when trying to draw conclusions and formulate clinical suggestions (14, 16, 28).
Question 5: Should We Apply Additional Biologicals or Stem Cells as Adjunctive Measures?
Various bio-active materials or cell therapies, as innovative approaches, are used in many dental and medical fields, and are currently also investigated in the field of peri-implantitis therapy.
Especially, growth factors are frequently used in regenerative dentistry to enhance clinical outcomes. Regeneration stimulating factors include platelet-derived factors, such as platelet-rich fibrin (PRF), plasma rich in growth factors (PRGF), and enamel matrix derivative (EMD). In the following section, the most promising therapeutic options are shortly described (19). Unfortunately, the evidence of systematic reviews is still scarce. Therefore, this part was supplemented with data from narrative reviews to highlight potential future perspectives and the actual status of research.
Autologous Platelet Concentrates
One meta-analysis, including three original studies, observed a higher bone gain when platelet-derived-growth-factor (PDGF) was adjunctively used (15). Nevertheless, it was not proven whether defects treated with adjunctive PDGF actually had more bone fill. Remaining bone substitute materials could not be differentiated from real bone on radiographs.
Platelet-rich plasma (PRP) has been investigated mainly in animal peri-implantitis models, and it shows no additional benefit for the main therapy outcomes (40).
While we can rely on a lot of positive evidence in the use of PRF in periodontology, available data on peri-implant regeneration are very limited (41). PRF-membranes in one randomized controlled clinical trial have been shown to be inferior to collagen membranes (19). The adjunctive use of PRF to OFD showed beneficial outcomes in terms of clinical attachment level (CAL) after 3 and 6 months (19).
Enamel Matrix Derivatives (EMD)
The biologic effect of EMD with its acceleration of wound healing (42), osteopromotive (43), and antibacterial (44) properties has already been shown in several studies, especially in the field of periodontitis and dental traumatology (43). One systematic review revealed no actual clinical benefit in regenerative peri-implantitis therapy (19).
One randomized clinical trial, including 25 patients, is also available and noteworthy (45). This trial revealed a promising benefit of the EMD-group, in terms of bone level changes after 1 year. However, this advantage was not valid after 3 and 5 years of follow-up, respectively (45).
Stem Cells
Stem cells are known to be important for maintenance and regeneration of tissues within the periodontium (46). A similar role for maintenance and regeneration of peri-implant tissues can, therefore, be anticipated (46). Mainly, the use of dental pulp stem cells in bone regeneration therapy around dental implants has been investigated, showing the most osteogenic potential as a source for tissue-engineered bone around implants (47). However, stem cells are not considered an evidence-based treatment protocol currently, but might play a major role in the future.
Experimental Approaches
A recent narrative review also picked up the role of epigenetics in regenerative peri-implantitis therapy (48). Epigenetic modifications, such as methylation and histone modifications of cells, were concluded to have the potential to represent a target for promotion of bone regeneration. MicroRNAs are judged to be promising therapeutic agents, but not safe to be used currently. Scientific evidence for preventing MicroRNAs from interfering with unwanted target genetic pathways, which may cause adverse effects, is still lacking. Further profound investigations are still needed (48).
Future Developments
In the future, improvements should be expected in the field of smart and carrier materials, which may be able to release active substances, such as antibacterial and cell-stimulating substances. Further, the role of soft tissue management in regeneration should be precisely assessed.
More clinical research is needed to verify a possible advantage in adding EMD, autologous platelet concentrates, or stem cells to regenerative procedures.
Most importantly, the cleaning of the implant remains a critical pillar of any successful treatment, and promising developments in this field may also lead to optimized processes. One example, for instance, is the use of electrolytic systems to clean the implant surface (49). However, to date, no evidence-based justification of such treatment is available.
Conclusion
In assessing current literature, the following evidence-based bullet points can be formulated:
• Whereas, infrabony defects can be regenerated, suprabony defects cannot be predictably augmented.
• Air-polishing seems to be more effective than ultrasonic or hand instrumentation, in terms of implant surface debridement.
• No rinsing solution has been proven to be more efficient than another.
• A mixture of xenogenic and autogenous bone is discussed as the most suitable.
• Membranes should be primarily used in complex not self-containing defects.
• Evidence for biologicals and the use of stem cells is still lacking.
• In general, a partial bone fill can be expected in 85% of regenerative procedures.
• Regeneration leads to a mean of 57% of greater bone fill than in open flap surgery only.
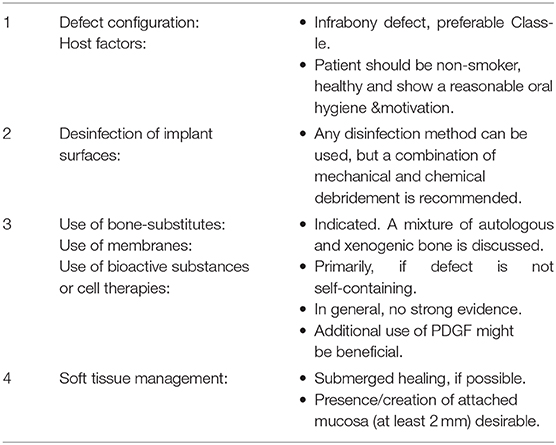
Table 3 shows a step-by-step approach for a regenerative peri-implantitis treatment based on the herein gathered evidence.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Author Contributions
AS and PS conducted the literature research, screened the studies, carefully read, and approved the final text. AS drafted the manuscript. PS validated the manuscript.
Funding
The work was supported by the Clinic of Conservative and Preventive Dentistry, Center of dental Medicine, University of Zurich.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Buser D, Janner SF, Wittneben JG, Brägger U, Ramseier CA, and Salvi GE. 10-year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: a retrospective study in 303 partially edentulous patients. Clin Implant Dent Relat Res. (2012) 14:839–51. doi: 10.1111/j.1708-8208.2012.00456.x
2. Chappuis V, Buser R, Brägger U, Bornstein MM, Salvi GE, and Buser D. Long-term outcomes of dental implants with a titanium plasma-sprayed surface: a 20-year prospective case series study in partially edentulous patients. Clin Implant Dent Relat Res. (2013) 15:780–90. doi: 10.1111/cid.12056
3. Berglundh T, Armitage G, Araujo MG, Avila-Ortiz G, Blanco J, Camargo PM, et al. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 World workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol. (2018) 89(Suppl. 1):S313–8. doi: 10.1002/JPER.17-0739
4. Schwarz F, Derks J, Monje A, and Wang HL. Peri-implantitis. J Clin Periodontol. (2018) 45:S246–66. doi: 10.1111/jcpe.12954
5. Solderer A, Al-Jazrawi A, Sahrmann P, Jung R, Attin T, and Schmidlin PR. Removal of failed dental implants revisited: questions and answers. Clin Exp Dent Res. (2019) 5:712–24. doi: 10.1002/cre2.234
6. Derks J, and Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology J Clin Periodontol. (2015) 42(Suppl. 16):S158–71. doi: 10.1111/jcpe.12334
7. Suárez-López Del Amo F, Yu SH, and Wang HL. Non-surgical therapy for peri-implant diseases: a systematic review. J Oral Maxillofac Res. (2016) 7:e13. doi: 10.5037/jomr.2016.7313
8. Khoury F, Keeve PL, Ramanauskaite A, Schwarz F, Koo KT, Sculean A, et al. Surgical treatment of peri-implantitis - consensus report of working group 4. Int Dent J. (2019) 69(Suppl. 2):18–22. doi: 10.1111/idj.12505
9. Heitz-Mayfield LJ, and Mombelli A. The therapy of peri-implantitis: a systematic review. Int J Oral Maxillofac Implants. (2014) 29:325–45. doi: 10.11607/jomi.2014suppl.g5.3
10. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006
11. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. doi: 10.1136/bmj.j4008
12. Aljohani M, Yong SL, and A Bin R. The effect of surgical regenerative treatment for peri-implantitis: a systematic review. Saudi Dent J. (2020) 32:109–19. doi: 10.1016/j.sdentj.2019.10.006
13. Chala M, Anagnostaki E, Mylona V, Chalas A, Parker S, and Lynch E. Adjunctive use of lasers in peri-implant mucositis and peri-implantitis treatment: a systematic review. Dent J. (2020) 8:68. doi: 10.3390/dj8030068
14. Chan HL, Lin GH, Suarez F, MacEachern M, and Wang HL. Surgical management of peri-implantitis: a systematic review and meta-analysis of treatment outcomes. J Periodontol. (2014) 85:1027–41. doi: 10.1902/jop.2013.130563
15. Daugela P, Cicciù M, and Saulacic N. Surgical regenerative treatments for peri-implantitis: meta-analysis of recent findings in a systematic literature review. J Oral Maxillofac Res. (2016) 7:e15. doi: 10.5037/jomr.2016.7315
16. Esposito M, Grusovin MG, and Worthington HV. Treatment of peri-implantitis: what interventions are effective? A cochrane systematic review. Eur J Oral Implantol. (2012) 5:S21–41. doi: 10.1002/14651858.CD004970.pub5
17. Khoshkam V, Chan HL, Lin GH, MacEachern MP, Monje A, Suarez F, et al. Reconstructive procedures for treating peri-implantitis: a systematic review. J. Dental Res. (2013) 92:131S−8. doi: 10.1177/0022034513509279
18. Khoshkam V, Suarez-Lopez Del Amo F, Monje A, Lin GH, Chan HL, and Wang HL. Long-term radiographic and clinical outcomes of regenerative approach for treating peri-implantitis: a systematic review and meta-analysis. Int J Oral Maxillofac Implants. (2016) 31:1303–10. doi: 10.11607/jomi.4691
19. Khouly I, Pardinas-Lopez S, Ruff RR, and Strauss FJ. Efficacy of growth factors for the treatment of peri-implant diseases: a systematic review and meta-analysis. Clin Oral Invest. (2020) 24:2141–61. doi: 10.1007/s00784-020-03240-5
20. Lin GH, Suárez López Del Amo F, and Wang HL. Laser therapy for treatment of peri-implant mucositis and peri-implantitis: an American academy of periodontology best evidence review. J Periodontol. (2018) 89:766–82. doi: 10.1902/jop.2017.160483
21. Mahato N, Wu X, and Wang L. Management of peri-implantitis: a systematic review, 2010-2015. Springerplus. (2016) 5:105. doi: 10.1186/s40064-016-1735-2
22. Natto ZS, Aladmawy M, Levi PAJr, and Wang HL. Comparison of the efficacy of different types of lasers for the treatment of peri-implantitis: a systematic review. Int J Oral Maxillofac Implants. (2015) 30:338–45. doi: 10.11607/jomi.3846
23. Ramanauskaite A, Daugela P, and Juodzbalys G. Treatment of peri-implantitis: meta-analysis of findings in a systematic literature review and novel protocol proposal. Quintessence Int. (2016) 47:379–93. doi: 10.3290/j.qi.a35131
24. Ramanauskaite A, Obreja K, Sader R, Khoury F, Romanos G, Koo KT, et al. Surgical treatment of periimplantitis with augmentative techniques. Implant Dent. (2019) 28:187–209. doi: 10.1097/ID.0000000000000839
25. Roccuzzo M, Layton DM, Roccuzzo A, and Heitz-Mayfield LJ. Clinical outcomes of peri-implantitis treatment and supportive care: a systematic review. Clin Oral Implants Res. (2018) 29:331–50. doi: 10.1111/clr.13287
26. Sahrmann P, Attin T, and Schmidlin PR. Regenerative treatment of peri-implantitis using bone substitutes and membrane: a systematic review. Clin Implant Dent Relat Res. (2011) 13:46–57. doi: 10.1111/j.1708-8208.2009.00183.x
27. Schwarz F, Schmucker A, and Becker J. Efficacy of alternative or adjunctive measures to conventional treatment of peri-implant mucositis and peri-implantitis: a systematic review and meta-analysis. Int J Implant Dent. (2015) 1:22. doi: 10.1186/s40729-015-0023-1
28. Tomasi C, Regidor E, Ortiz-Vigón A, and Derks J. Efficacy of reconstructive surgical therapy at peri-implantitis-related bone defects a systematic review and meta-analysis. J Clin Periodontol. (2019) 46(Suppl. 21):340–56. doi: 10.1111/jcpe.13070
29. Sinjab K, Garaicoa-Pazmino C, and Wang HL. Decision making for management of periimplant diseases. Implant Dent. (2018) 27:276–81. doi: 10.1097/ID.0000000000000775
30. Schwarz F, Herten M, Sager M, Bieling K, Sculean A, and Becker J. Comparison of naturally occurring and ligature-induced peri-implantitis bone defects in humans and dogs. Clin Oral Implants Res. (2007) 18:161–70. doi: 10.1111/j.1600-0501.2006.01320.x
31. Cortellini P, and Tonetti MS. Clinical concepts for regenerative therapy in intrabony defects. Periodontol. (2015) 68:282–307. doi: 10.1111/prd.12048
32. Renvert S, and Polyzois I. Treatment of pathologic peri-implant pockets. Periodontol. (2018) 76:180–90. doi: 10.1111/prd.12149
33. Madi M, Htet M, Zakaria O, Alagl A, and Kasugai S. Re-osseointegration of dental implants after periimplantitis treatments: a systematic review. Implant Dent. (2018) 27:101–10. doi: 10.1097/ID.0000000000000712
34. Subramani K, and Wismeijer D. Decontamination of titanium implant surface and re-osseointegration to treat peri-implantitis: a literature review. Int J Oral Maxillofac Implants. (2012) 27:1043–54.
35. Smeets R, Henningsen A, Jung O, Heiland M, Hammächer C, and Stein JM. Definition, etiology, prevention and treatment of peri-implantitis–a review. Head Face Med. (2014) 10:34. doi: 10.1186/1746-160X-10-34
36. Fu JH, and Wang HL. Can periimplantitis be treated. Dent Clin North Am. (2015) 59:951–80. doi: 10.1016/j.cden.2015.06.004
37. Levin L, Barbu H, Kurgan S, Comaneanu RM, Referendaru D, and Lorean A. Evaluation of 0.2% delmopinol mouth rinse for prevention of peri-implant mucositis and peri-implantitis: a randomized controlled canine study. Clin Implant Dent Relat Res. (2019) 21:46–51. doi: 10.1111/cid.12692
38. Merli M, Merli I, Raffaelli E, Pagliaro U, Nastri L, and Nieri M. Bone augmentation at implant dehiscences and fenestrations. A systematic review of randomised controlled trials. Eur J Oral Implantol. (2016) 9:11–32.
39. Sculean A, Romanos G, Schwarz F, Ramanauskaite A, Keeve PL, Khoury F, et al. Soft-tissue management as part of the surgical treatment of periimplantitis: a narrative review. Implant Dent. (2019) 28:210–6. doi: 10.1097/ID.0000000000000870
40. Simonpieri A, Del Corso M, Vervelle A, Jimbo R, Inchingolo F, Sammartino G, et al. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin. (PRF) in oral and maxillofacial surgery part 2: bone graft, implant and reconstructive surgery. Curr Pharm Biotechnol. (2012) 13:1231–56. doi: 10.2174/138920112800624472
41. Miron RJ, Zucchelli G, Pikos MA, Salama M, Lee S, Guillemette V, et al. Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin Oral Investig. (2017) 21:1913–27. doi: 10.1007/s00784-017-2133-z
42. Bosshardt DD. Biological mediators and periodontal regeneration: a review of enamel matrix proteins at the cellular and molecular levels. J Clin Periodontol. (2008) 35:87–105. doi: 10.1111/j.1600-051X.2008.01264.x
43. Miron RJ, Sculean A, Cochran DL, Froum S, Zucchelli G, Nemcovsky C, et al. Twenty years of enamel matrix derivative: the past, the present and the future. J Clin Periodontol. (2016) 43:668–83. doi: 10.1111/jcpe.12546
44. Arweiler NB, Auschill TM, Donos N, and Sculean A. Antibacterial effect of an enamel matrix protein derivative on in vivo dental biofilm vitality. Clin Oral Investig. (2002) 6:205–9. doi: 10.1007/s00784-002-0185-0
45. Isehed C, Svenson B, Lundberg P, and Holmlund A. Surgical treatment of peri-implantitis using enamel matrix derivative, an RCT: 3- and 5-year follow-up. J Clin Periodontol. (2018) 45:744–53. doi: 10.1111/jcpe.12894
46. Eggert FM, and Levin L. Biology of teeth and implants: host factors - pathology, regeneration, and the role of stem cells. Quintessence Int. (2018) 49:497–509. doi: 10.3290/j.qi.a40289
47. Marei MK, and El Backly MR. Dental mesenchymal stem cell-based translational regenerative dentistry: from artificial to biological replacement. Front Bioeng Biotechnol. (2019) 6:49. doi: 10.3389/fbioe.2018.00049
48. Asa'ad F, Monje A, and Larsson L. Role of epigenetics in alveolar bone resorption and regeneration around periodontal and peri-implant tissues. Eur J Oral Sci. (2019) 127:477–93. doi: 10.1111/eos.12657
Keywords: peri-implantitis, guided bone regeneration (GBR), regenerative dentistry, dental implant, infrabony defects
Citation: Solderer A and Schmidlin PR (2020) Regenerative Surgical Therapy of Peri-implantitis: An Umbrella Review of Answered/Unanswered Questions and Future Perspectives. Front. Dent. Med. 1:614240. doi: 10.3389/fdmed.2020.614240
Received: 05 October 2020; Accepted: 04 December 2020;
Published: 21 December 2020.
Edited by:
Marcelo Henrique Napimoga, São Leopoldo Mandic School, BrazilReviewed by:
Fawad Javed, University of Rochester, United StatesDaiane Cristina Peruzzo, São Leopoldo Mandic School, Brazil
Copyright © 2020 Solderer and Schmidlin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alex Solderer, YWxleC5zb2xkZXJlckB6em0udXpoLmNo
 Alex Solderer
Alex Solderer Patrick R. Schmidlin
Patrick R. Schmidlin