
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Dement., 29 June 2023
Sec. Aging and Risk Factors for Dementia
Volume 2 - 2023 | https://doi.org/10.3389/frdem.2023.1215904
This article is part of the Research TopicHealth conditions outside the brain and the risk of brain aging and dementiaView all 6 articles
 Erin L. Ferguson1*
Erin L. Ferguson1* Eric Vittinghoff1
Eric Vittinghoff1 Adina Zeki Al Hazzouri2
Adina Zeki Al Hazzouri2 Norrina Allen3
Norrina Allen3 Annette Fitzpatrick4
Annette Fitzpatrick4 Kristine Yaffe1,5,6
Kristine Yaffe1,5,6Background: Racial disparities in dementia outcomes persist in the United States. Targeting modifiable risk factors, including cardiovascular risk factors (CVRFs), is a conceivable way to reduce health disparities. Life course CVRFs are often higher in non-White adults and are associated with risk of dementia, but it is unknown whether they contribute to racial disparities in dementia and cognition.
Methods: Using a pooled cohort of 4,159 White and 939 Black participants aged 65–95 years, we conducted a mediation analysis to estimate the proportional effect of race on dementia that is explained by four CVRFs imputed over the life course (20–49, 50–69, and 70–89 years of age): body mass index, fasting glucose, systolic blood pressure, and low-density lipoprotein cholesterol.
Results: Compared to White participants, Black participants had greater risk of dementia (adjusted OR = 1.37; 95% CI: 1.17–1.60). BMI and fasting glucose over the life course were significant mediators of the effect of race on dementia risk, mediating 39.1% (95% CI: 10.5–67.8%) and 8.2% (95% CI: 0.1–16.2%) of the effect, adjusted for sex and age. All four CVRFs together were also significant mediators of the effect of race on scores on global cognition and processing speed, accounting for approximately 11% of the effect.
Conclusions: We found that CVRFs across the life course partially explain disparities in dementia risk and cognition in late-life. Improved prevention and treatment of CVRFs across the life course may be important to reduce health disparities for dementia.
In the US, Black adults have been shown to have consistently higher incidence and prevalence of dementia compared to non-Hispanic White adults (Mayeda et al., 2016; Steenland et al., 2016; Matthews et al., 2019). Previous literature suggests that this disparity can be partially explained by social determinants of health (SDOH), such as socioeconomic status (Yaffe et al., 2013; Samuel et al., 2020), education (Farina et al., 2020), and inequities in access to quality and timely healthcare (Saadi et al., 2017; Kawas et al., 2021; Murchison et al., 2021). These factors are all largely downstream effects of structural and institutionalized racism that interact to contribute to inequities in dementia incidence (Caunca et al., 2020).
These disparities may also stem from differences in cumulative cardiovascular risk factors (CVRFs). CVRFs, such as obesity and hypertension, are known risk factors for cognitive decline and dementia (Whitmer et al., 2005; Alonso et al., 2009; Qiu and Fratiglioni, 2015; Livingston et al., 2020; Yaffe et al., 2021; Zeki Al Hazzouri et al., 2021). CVRFs are very common (Heart Disease and Stroke Statistics–2019 Update: A Report From the American Heart Association | Circulation, n.d.) and can be treated with lifestyle changes (Pan et al., 2018; Rippe, 2018; Lavie et al., 2019) or medical treatment (Field et al., 1995; Jackson et al., 2005), which make them critical targets for intervention. Because the prevalence of CVRFs are higher in Black and Hispanic adults compared to White adults (Lewey and Choudhry, 2014; Howard et al., 2017; Bell et al., 2018), they may also contribute to observed racial disparities in dementia incidence (Chen and Zissimopoulos, 2018). To fully capture the contributions of CVRFs to racial disparities, it is important to consider exposure to CVRFs over early, mid, and late-life. However, most studies of CVRFs and dementia incidence have only examined mid or late-life exposures because of the lack of cohort studies spanning the entire adulthood period.
Using data from a racially/ethnically diverse pooled cohort of Black and White individuals based on four established studies spanning over 7 decades, we investigate whether CVRFs over the adult life course (early adult, mid, and late-life) contribute to racial disparities in dementia risk and cognition. Since prevalence of CVRFs differ observationally by Black or White race (Lewey and Choudhry, 2014; Howard et al., 2017; Bell et al., 2018), we hypothesize that racial disparities in dementia incidence are partially mediated by CVRFs.
Data were pooled from 4 prospective cohorts: the Coronary Artery Risk Development in Young Adults study (CARDIA, an early adult to midlife cohort), the Multi-Ethnic Study of Atherosclerosis (MESA, midlife cohort), the Cardiovascular Health study (CHS, late-life cohort), and the Health, Aging, and Body Composition study (Health ABC, late-life cohort). Participants in each of the four cohorts self-reported their racial identity at enrollment. Additionally, each cohort collected self-reported information on education attainment (less than high school, completion of high school, some college, college graduate, and graduate/professional degree). The pooled cohort included 15,001 adult individuals aged 18–95 at enrollment, with at least 2 repeated measures of each of the measured CVRFs. Details of this pooled cohort are published elsewhere (Zeki Al Hazzouri et al., 2019).
Data from the four prospective cohorts, that together spanned the adult life course: early and midlife data from MESA and CARDIA (n = 9,903), and late-life data from CHS and Health ABC (n = 5,098) were used to model life course CVRF trajectories encompassing early, mid, and late-life. Our late-life cohort participants, n = 5,098, had repeated measures of cognition and dementia outcome data and were the focus of outcome results. We focused on modeling the following four CVRFs: body mass index (BMI), fasting glucose (FG), systolic blood pressure (SBP), and low-density lipoprotein cholesterol (LDL). Using data from all four cohorts, we fit person-specific linear mixed models to estimate flexible trajectories of CVRFs over the life course. This imputation model was adjusted for cohort, demographic information (race, sex, splines in age and birth year, and interactions between age, sex, and race) and time-dependent clinical information (diabetes, hypertension, lipid-lowering and anti- hypertensive medication use, and smoking status). Imputations on these covariates were run prior to imputing CVRF trajectories. The imputation models additionally contained random intercepts and random age splines. As the following analysis depends on the accuracy of these imputation procedures, we conducted a simulation study treating the modeled trajectories of CVRFs as the true values and then used these values to model new trajectories. This analysis found that the imputation errors sometimes induced attenuation bias in regression coefficients, biasing toward the null. Further details about the imputation of CVRFs, its validation, and this simulation study are previously published (Zeki Al Hazzouri et al., 2019).
From these imputations, we estimated CVRF cumulative time-weighted averages (TWAs) for each participant in CHS and Health ABC at the three age ranges: early adulthood (ages 20–49), midlife (ages 50–69), and late-life (ages 70–89) (Table 1). For each age range, average cumulative period-specific CVRF TWAs were categorized into three levels based on standard clinical cutoffs: BMI (<25 kg/m2, 25–30 kg/m2, >30 kg/m2), SBP (<120 mmHg, 120–140 mmHg, >140 mmHg), FG (<100 mg/dL, 100–125 mg/dL, >125 mg/dL), and LDL (<100 mg/dL, 100–130 mg/dL, >130 mg/dL).
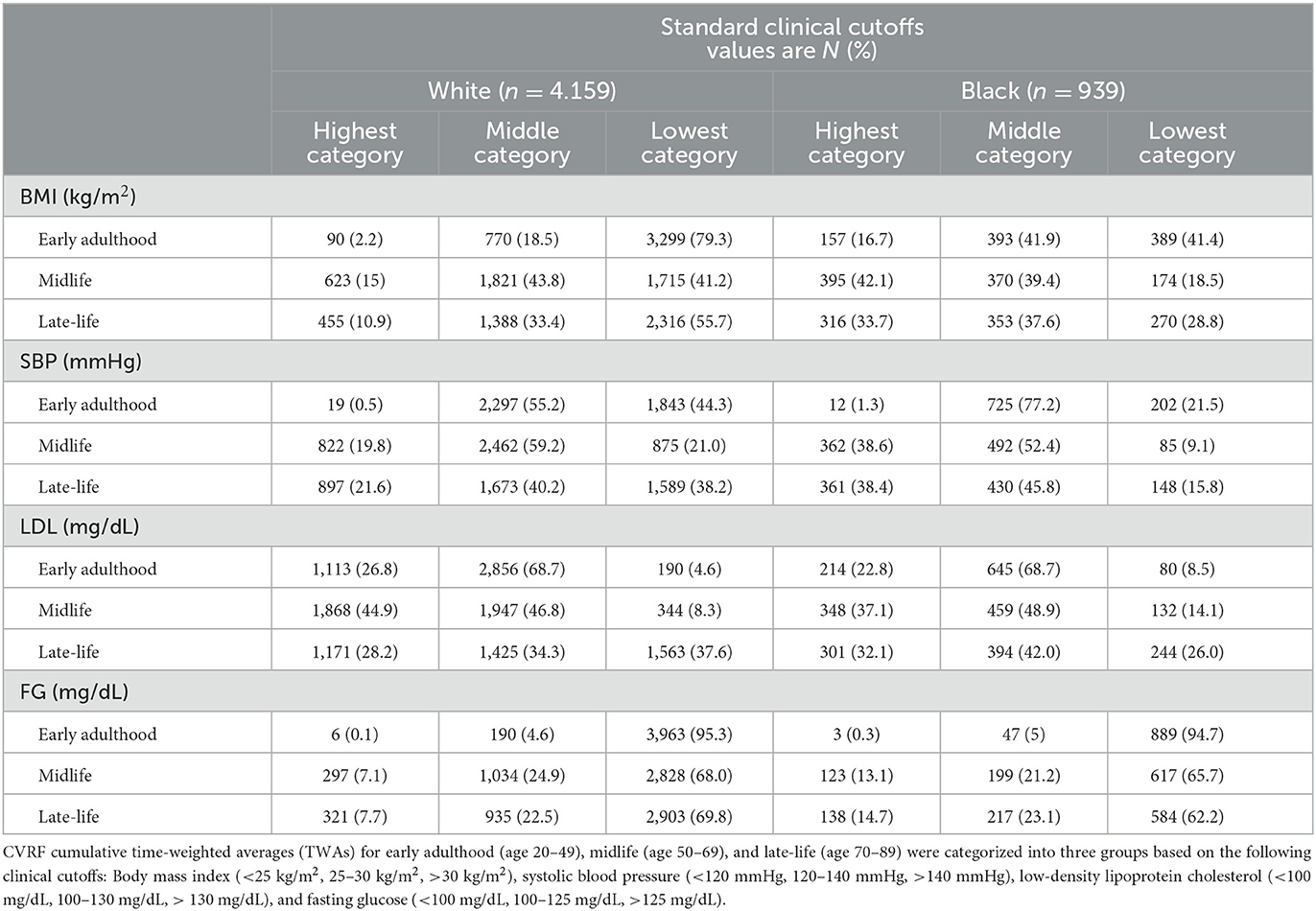
Table 1. Imputed life course Cardiovascular Risk Factor (CVRF) time-weighted averages (TWAs) by race.
Dementia diagnoses were ascertained in the older two cohorts (CHS and Health ABC) by each study protocol. In CHS, participants at risk of dementia were administered a battery of neuropsychological tests, and those who failed the memory test or two other cognitive domains were evaluated by a neurologist. Dementia diagnoses were determined by a clinical adjudication committee (Lopez et al., 2003). In Health ABC, dementia was defined using previously published classifications (Hong et al., 2013; Brenowitz et al., 2021). Over 15 years of follow-up, participants with documented use of dementia medication, a hospitalization with dementia as primary or secondary diagnosis, or clinically meaningful global cognitive decline, defined as a >1.5 SD change, were classified as having dementia (Zeki Al Hazzouri et al., 2021).
Cognition was measured using the Digit Symbol Substitution Test (DSST), a test of processing speed, and the Modified Mini-Mental State Examination (3MS), a test of global cognition, annually in the CHS cohort and every 1–2 years in the Health ABC cohort.
We used Baron and Kenny decomposition methods to quantify mediation by CVRFs (Baron and Kenny, 1986), which is used frequently in the CVRF and cognition literature (Cui et al., 2020; Hu et al., 2022; Wang et al., 2022). First, base pooled logistic regression models adjusting for age and sex were fit for the association between race and dementia risk. Next, augmented pooled logistic regression models were fit for dementia from race, age, and sex, but additionally adjusting for each period-specific CVRF TWAs. In other words, the augmented models are the base models with adjustment for CVRFs in early adulthood, midlife, and late-life together. Mediation by cumulative CVRFs was summarized by the change in the coefficient for race, as adjusting for the mediator should reduce the total effect of race in the base model by blocking the mediating pathway through CVRFs (Baron and Kenny, 1986; Fairchild and McDaniel, 2017). We report the relative change in the coefficient for race as the proportion of the effect mediated by CVRFs, adjusted for age and sex. In subsequent mediation models, we additionally adjusted for education attainment in both base and augmented models (less than high school, completion of high school, some college, college graduate, and graduate/professional degree).
We also conducted mediation analyses using cognition (DSST and 3MS) as our secondary outcome. For these analyses, we used the same Baron and Kenny decomposition approach. We first fit linear regression models for cognition from race, adjusting for age and sex. Then, we fit augmented linear regressions, which were the same as the base model with adjustment for each period-specific CVRF TWAs. Mediation was again summarized by the change in coefficient for race between the base and augmented models. All analyses were conducted using Stata Version 16.1 (Stata LLP, College Station, TX).
Our primary analysis for late-life dementia included 4,159 White and 939 Black participants from CHS and Health ABC. At baseline, Black participants were older (73.1 years vs. 72.5 years), more likely to be female (59.9 vs. 54.5%), and less likely to have completed college-level education (29.5 vs. 50.7%). Estimated average CVRFs by race across the life course are shown in Table 1. All CVRFs increased with age for both White and Black participants. In late-life, Black participants on average had higher values for all four CVRFs compared to White participants (Chi-Square test of independence, all p-values <0.001).
There were 908 incident cases of dementia in the cohorts. Black participants had 1.37 (95% CI: 1.17–1.60) times the odds of developing dementia compared to White participants adjusted for age and sex. When included in the model individually, all CVRFs were statistically significant predictors of dementia. BMI was a notable mediator between race and dementia risk, accounting for 39.1% of the effect (95% CI: 10.5–67.8%). Glucose was also a mediator of this risk, accounting for 8.1% (95% CI: 0.1–16.2%) of the effect (Figure 1); however, SBP and LDL did not mediate the association. When all four CVRFs were included in a single model, they together mediated 29.1% of the association, although this was a trend-level significance (95% CI: −1.8–60%). When we additionally adjusted for education in these models, none of the individual CVRFs were statistically significant mediators. The joint effect of all CVRFs were no longer statistically significant predictors of dementia, but the confidence interval was imprecise, and the joint effect remained consistent with substantial mediation of the relationship between race and dementia risk (Proportion mediated: 47%; 95% CI: −16–110%).
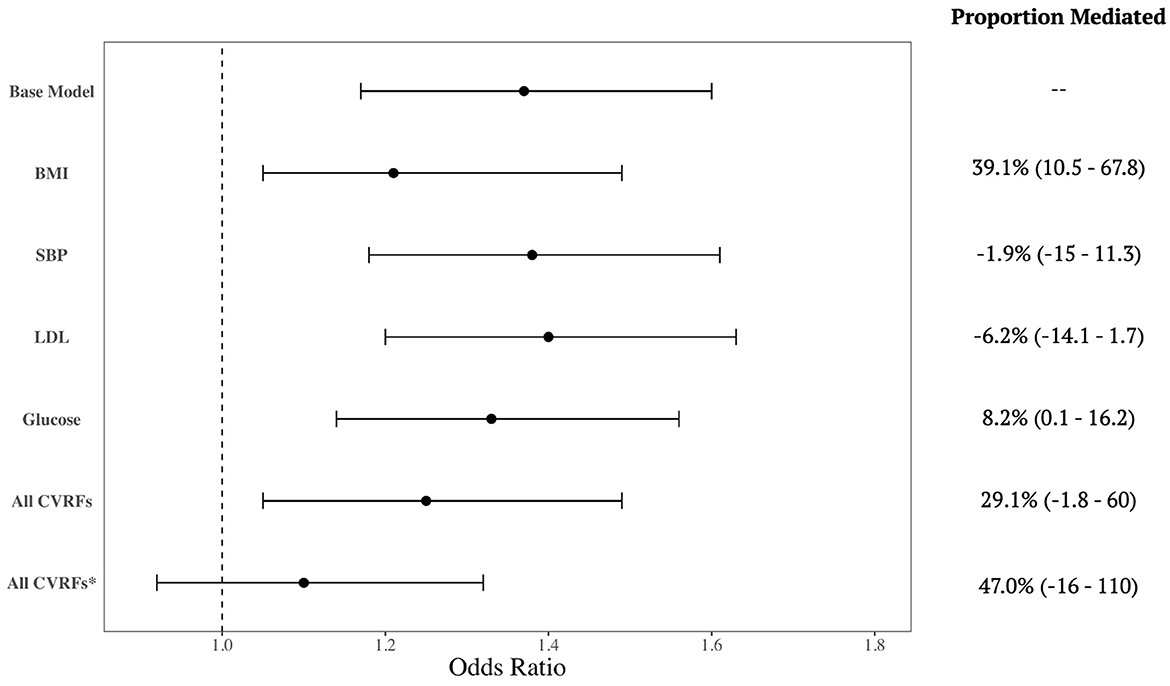
Figure 1. Mediation by cardiovascular risk factors of the relationship between race and dementia incidence. Forest plot shows the crude association and then additionally adjusted for each cardiovascular risk factor (CVRF), separately and all CVRFs together. Mediation effect is summarized by the proportion (%) of the association incidence that is mediated by the CVRF. All models are adjusted for age and sex, except for the last row (*) which is additionally adjusted for education.
In the CHS and Health ABC cohorts, 6,091 individuals had available cognitive scores. On average, Black participants scored 29.3 (14.1) on the DSST and 87.5 (8.65) on the 3MS, while White participants scored 40.2 (12.3) and 92.0 (5.15) respectively. The four CVRF risk factors were significant joint mediators of the effect of race on DSST score, explaining 12.0% of the association (95% CI: 8.5–15.5%, Table 2). Similar results were found for 3MS score, with the four CVRFs mediating 10% of the overall effect (95% CI: 6.2–13.8%, Table 2), mostly due to contributions by BMI and SBP. When additionally adjusting for education, the joint mediation effect of the CVRFs on DSST and 3MS scores were slightly attenuated but remained statistically significant.
Overall, we found that CVRFs contribute to racial disparities in dementia risk, especially BMI and fasting glucose, and the effect of all four CVRFs together accounted for almost a third of the variance, but this was of borderline statistical significance. All four CVRFs together were also mediators of processing speed and global cognition, which is further supportive of an overall mediative effect of CVRFs on dementia. After additional adjustment for education, another potential mediator, the joint contribution of all four CVRFs on cognitive scores remained significant.
The Baron and Kenny mediation framework makes two main assumptions: (1) there is no interaction between the exposure and mediator and (2) there is no unmeasured confounding of the mediator and outcome. First, we hypothesize that it is the difference in prevalence of CVRFs by race that leads to disparities in dementia incidence, rather than differing effects of CVRFs by race. There is prior evidence to support this hypothesis, with previous literature finding no interaction between CVRF and race on cognition (Yaffe et al., 2020; Peterson et al., 2021). Second, as is true with any observational study, there is the potential for unmeasured confounding between CVRFs and dementia. However, we have identified the most important confounders (age, sex, and education) and adjusted for those in our analysis. As such, we believe the assumptions made by this mediation analysis are reasonably met.
These results provide compelling evidence on modifiable risk factors which may contribute to racial disparities in dementia incidence. In this study, we utilized innovative methods to address restrictions on longitudinal data faced by previous studies with limited follow-up and no entire life course observations. Our results are consistent with another pooled cohort study that reported that cumulative lifetime SBP contributes to racial disparities in late-life cognition (Levine et al., 2020). Our conclusions are also supported by previous literature reporting that CVRFs are important risk factors for dementia (Whitmer et al., 2005; Alonso et al., 2009; Qiu and Fratiglioni, 2015; Livingston et al., 2020) and exposure differs by race (Lewey and Choudhry, 2014; Howard et al., 2017; Bell et al., 2018). Various mechanisms have been proposed for the differences in the prevalence of CVRFs between White and Black populations, such as racism (Cozier et al., 2006, 2014; Brondolo et al., 2011; Aaron and Stanford, 2021), chronic stress (Low et al., 2009), and access to timely and quality health care (Churchwell et al., 2020; Yearby et al., 2022). While the specific mechanisms for this pathway are beyond the scope of this report, it is important to recognize the contribution of systemic racism and social determinants of health (SDOH) to these disparities in CVRFs and dementia (Caunca et al., 2020; Zeki Al Hazzouri et al., 2022). Data limitations prevented this analysis from considering the contributions of important SDOH on dementia disparities, such as income and access to healthcare. However, our analysis revealed that education accounted for part of the contribution of CVRFs on dementia and cognition. Since education, and likely also socioeconomic status, play a crucial role in contributing to poor cognitive outcomes, more work is needed to understand the impact of SDOH on these relationships between CVRFs and dementia disparities.
Due to data limitations, we were unable to adjust for genetic factors such as APOE. The APOE ε2 and ε4 alleles are known to be more prevalent among Black individuals, but the association with cognition and AD is weaker among this group (Rajan et al., 2017, 2019; Beydoun et al., 2021). Since the APOE gene is also associated with increased of cardiovascular disease, this genetic risk factor may contribute to the observed mediating role of CVRFs (Mahley, 2016; Rajan et al., 2017). We hope in the future to conduct analyses with complete genetic data in a racially diverse sample to replicate the present analysis with adjustment for APOE.
Our study has important limitations. Most notably, this analysis was restricted to Black and White participants. Disparities in dementia incidence and treatment are documented among other racial groups such as Hispanic and Asian individuals (Mayeda et al., 2016; Chen and Zissimopoulos, 2018; Kawas et al., 2021). Future analyses would benefit from replicating this analysis in cohorts with representation of more diverse racial groups. Additionally, this sample had unbalanced sample sizes of Black and White individuals, meaning that we may have lacked sufficient power to detect all mediating effects of CVRFs. This analysis also uses pooled and imputed data instead of measured longitudinal data which relies on the critical assumption that early life CVRFs were properly and accurately imputed. While error cannot be ruled out, this method was validated using a simulation study and has been used in previous publications (Zeki Al Hazzouri et al., 2019; Brenowitz et al., 2021; Yaffe et al., 2021).
In summary, using novel imputation methods to model life course exposure to CVRFs, our results suggest that CVRFs contribute to racial disparities in dementia risk between Black and White individuals. This work contributes to previous research suggesting that CVRFs are key factors in dementia risk and subsequent disparities (Whitmer et al., 2005; Alonso et al., 2009; Qiu and Fratiglioni, 2015; Levine et al., 2020; Livingston et al., 2020; Yaffe et al., 2021; Zeki Al Hazzouri et al., 2021). Future studies should expand on CVRFs as important targets for reducing racial disparities in dementia and cognitive outcomes.
The data analyzed in this study is subject to the following licenses/restrictions: anonymized data from the cohort studies used in this analysis are available from each study's respective coordinating centers. Specific policies governing each study's data and the process to access data can be found online. Requests to access these datasets should be directed to CARDIA: cardia.dopm.uab.edu/; MESA: mesa-nhlbi.org/; CHS: www.chs-nhlbi.org/; Health ABC: healthabc.nia.nih.gov/.
The current analysis was approved by the Columbia University and University of California San Francisco IRBs approval. The patients/participants provided their written informed consent to participate in this study.
KY, AZ, and EV were involved in conceptualizing and designing the study. KY, NA, and AF obtained the data. EV and EF conducted the statistical analysis. EF drafted the manuscript. All authors commented on subsequent versions and approved the submitted version.
This work was supported by the following grants from the National Institutes of Health, National Institute on Aging (R01AG071916 and RF1AG054443). The funding organization or sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. CARDIA is supported by contracts HHSN268201800003I, HHSN268201800004I, HHSN268201800005I, HHSN268201800006I, and HHSN268201800007I from the National Heart, Lung, and Blood Institute (NHLBI). MESA is supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). CHS is supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, 75N92021D00006, and grants U01HL080295, and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. Health ABC study is supported by National Institute on Aging (NIA) Contracts N01-AG-6–2101; N01-AG-6–2103; N01-AG-6–2106; NIA grant R01-AG028050, and NINR grant R01-NR012459. Health ABC was funded in part by the Intramural Research Program of the NIH, National Institute on Aging.
The authors thank the other investigators, the staff, and the participants of the CARDIA, MESA, CHS, and Health ABC studies for their valuable contributions.
KY serves on DSMBs for Eli Lilly, DIAN, and a National Institute on Aging-sponsored trial, is a board member of Alector, and is a member of the Beeson Scientific Advisory Board and the Global Council on Brain Health.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
KY and NA declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aaron, D. G., and Stanford, F. C. (2021). Is obesity a manifestation of systemic racism? A ten-point strategy for study and intervention. J. Intern. Med. 290, 416–420. doi: 10.1111/joim.13270
Alonso, A., Jacobs, D. R., Menotti, A., Nissinen, A., Dontas, A., Kafatos, A., et al. (2009). Cardiovascular risk factors and dementia mortality: 40 years of follow-up in the Seven Countries Study. J. Neurol. Sci. 280, 79–83. doi: 10.1016/j.jns.2009.02.004
Baron, R. M., and Kenny, D. A. (1986). The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 51, 1173–1182. doi: 10.1037/0022-3514.51.6.1173
Bell, C. N., Thorpe, R. J., Bowie, J. V., and LaVeist, T. A. (2018). Race disparities in cardiovascular disease risk factors within socioeconomic status strata. Ann. Epidemiol. 28, 147–152. doi: 10.1016/j.annepidem.2017.12.007
Beydoun, M. A., Weiss, J., Beydoun, H. A., Hossain, S., Maldonado, A. I., Shen, B., et al. (2021). Race, APOE genotypes, and cognitive decline among middle-aged urban adults. Alzheimers Res. Ther. 13, 120. doi: 10.1186/s13195-021-00855-y
Brenowitz, W. D., Zeki Al Hazzouri, A., Vittinghoff, E., Golden, S. H., Fitzpatrick, A. L., and Yaffe, K. (2021). Depressive symptoms imputed across the life course are associated with cognitive impairment and cognitive decline. J. Alzheimers Dis. JAD 83, 1379–1389. doi: 10.3233/JAD-210588
Brondolo, E., Love, E. E., Pencille, M., Schoenthaler, A., and Ogedegbe, G. (2011). Racism and hypertension: a review of the empirical evidence and implications for clinical practice. Am. J. Hypertens. 24, 518–529. doi: 10.1038/ajh.2011.9
Caunca, M. R., Odden, M. C., Glymour, M. M., Elfassy, T., Kershaw, K. N., Sidney, S., et al. (2020). Association of racial residential segregation throughout young adulthood and cognitive performance in middle-aged participants in the CARDIA Study. JAMA Neurol. 77, 1000–1007. doi: 10.1001/jamaneurol.2020.0860
Chen, C., and Zissimopoulos, J. M. (2018). Racial and ethnic differences in trends in dementia prevalence and risk factors in the United States. Alzheimers Dement. Transl. Res. Clin. Interv. 4, 510–520. doi: 10.1016/j.trci.2018.08.009
Churchwell, K., Elkind, M. S. V., Benjamin, R. M., Carson, A. P., Chang, E. K., Lawrence, W., et al. (2020). Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the american heart association. Circulation 142, e454–e468. doi: 10.1161/CIR.0000000000000936
Cozier, Y., Palmer, J. R., Horton, N. J., Fredman, L., Wise, L. A., and Rosenberg, L. (2006). Racial discrimination and the incidence of hypertension in US Black women. Ann. Epidemiol. 16, 681–687. doi: 10.1016/j.annepidem.2005.11.008
Cozier, Y. C., Yu, J., Coogan, P. F., Bethea, T. N., Rosenberg, L., and Palmer, J. R. (2014). Racism, segregation, and risk of obesity in the black women's health study. Am. J. Epidemiol. 179, 875–883. doi: 10.1093/aje/kwu004
Cui, K., Song, R., Xu, H., Shang, Y., Qi, X., Buchman, A. S., et al. (2020). Association of cardiovascular risk burden with risk and progression of disability: mediating role of cardiovascular disease and cognitive decline. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 9, e017346. doi: 10.1161/JAHA.120.017346
Fairchild, A. J., and McDaniel, H. L. (2017). Best (but oft-forgotten) practices: mediation analysis. Am. J. Clin. Nutr. 105, 1259–1271. doi: 10.3945/ajcn.117.152546
Farina, M. P., Hayward, M. D., Kim, J. K., and Crimmins, E. M. (2020). Racial and educational disparities in dementia and dementia-free life expectancy. J. Gerontol. Ser. B 75, e105–e112. doi: 10.1093/geronb/gbz046
Field, K., Thorogood, M., Silagy, C., Normand, C., O'Neill, C., and Muir, J. (1995). Strategies for reducing coronary risk factors in primary care: which is most cost effective? BMJ 310, 1109–1112. doi: 10.1136/bmj.310.6987.1109
Heart Disease Stroke Statistics–2019 Update: A Report From the American Heart Association | Circulation (n.d.). Available at: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000659 (accessed July 26 2022)
Hong, C. H., Falvey, C., Harris, T. B., Simonsick, E. M., Satterfield, S., Ferrucci, L., et al. (2013). Anemia and risk of dementia in older adults: findings from the Health ABC study. Neurology 81, 528–533. doi: 10.1212/WNL.0b013e31829e701d
Howard, G., Safford, M. M., Moy, C. S., Howard, V. J., Kleindorfer, D. O., Unverzagt, F. W., et al. (2017). Racial differences in the incidence of cardiovascular risk factors in older black and white adults. J. Am. Geriatr. Soc. 65, 83–90. doi: 10.1111/jgs.14472
Hu, H., Meng, L., Bi, Y.-L., Zhang, W., Xu, W., Shen, X.-N., et al. (2022). Tau pathologies mediate the association of blood pressure with cognitive impairment in adults without dementia: The CABLE study. Alzheimers Dement. J. Alzheimers Assoc. 18, 53–64. doi: 10.1002/alz.12377
Jackson, R., Lawes, C. M., Bennett, D. A., Milne, R. J., and Rodgers, A. (2005). Treatment with drugs to lower blood pressure and blood cholesterol based on an individual's absolute cardiovascular risk. The Lancet 365, 434–441. doi: 10.1016/S0140-6736(05)70240-3
Kawas, C. H., Corrada, M. M., and Whitmer, R. A. (2021). Diversity and disparities in dementia diagnosis and care: a challenge for all of us. JAMA Neurol. 78, 650–652. doi: 10.1001/jamaneurol.2021.0285
Lavie, C. J., Ozemek, C., Carbone, S., Katzmarzyk, P. T., and Blair, S. N. (2019). Sedentary behavior, exercise, and cardiovascular health. Circ. Res. 124, 799–815. doi: 10.1161/CIRCRESAHA.118.312669
Levine, D. A., Gross, A. L., Briceño, E. M., Tilton, N., Kabeto, M. U., Hingtgen, S. M., et al. (2020). Association between blood pressure and later-life cognition among black and white individuals. JAMA Neurol. 77, 810–819. doi: 10.1001/jamaneurol.2020.0568
Lewey, J., and Choudhry, N. K. (2014). The current state of ethnic and racial disparities in cardiovascular care: lessons from the past and opportunities for the future. Curr. Cardiol. Rep. 16, 530. doi: 10.1007/s11886-014-0530-3
Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., et al. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet 396, 413–446. doi: 10.1016/S0140-6736(20)30367-6
Lopez, O. L., Kuller, L. H., Fitzpatrick, A., Ives, D., Becker, J. T., and Beauchamp, N. (2003). Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology 22, 1–12. doi: 10.1159/000067110
Low, C. A., Salomon, K., and Matthews, K. A. (2009). Chronic life stress, cardiovascular reactivity, and subclinical cardiovascular disease in adolescents. Psychosom. Med. 71, 927–931. doi: 10.1097/PSY.0b013e3181ba18ed
Mahley, R. W. (2016). Apolipoprotein E: from cardiovascular disease to neurodegenerative disorders. J. Mol. Med. Berl. Ger. 94, 739–746. doi: 10.1007/s00109-016-1427-y
Matthews, K. A., Xu, W., Gaglioti, A. H., Holt, J. B., Croft, J. B., Mack, D., et al. (2019). Racial and ethnic estimates of Alzheimer's disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimers Dement. J. Alzheimers Assoc. 15, 17–24. doi: 10.1016/j.jalz.2018.06.3063
Mayeda, E. R., Glymour, M. M., Quesenberry, C. P., and Whitmer, R. A. (2016). Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 12, 216–224. doi: 10.1016/j.jalz.2015.12.007
Murchison, C. F., Kennedy, R. E., McConathy, J. E., and Roberson, E. D. (2021). Racial differences in Alzheimer's disease specialist encounters are associated with usage of molecular imaging and dementia medications: an enterprise-wide analysis using i2b2. J. Alzheimers Dis. 79, 543–557. doi: 10.3233/JAD-200796
Pan, A., Lin, X., Hemler, E., and Hu, F. B. (2018). Diet and cardiovascular disease: advances and challenges in population-based studies. Cell Metab. 27, 489–496. doi: 10.1016/j.cmet.2018.02.017
Peterson, R. L., George, K. M., Gilsanz, P., Ackley, S., Mayeda, E. R., Glymour, M. M., et al. (2021). Racial/ethnic disparities in young adulthood and midlife cardiovascular risk factors and late-life cognitive domains: the kaiser healthy aging and diverse life experiences (KHANDLE) study. Alzheimer Dis. Assoc. Disord. 35, 99–105. doi: 10.1097/WAD.0000000000000436
Qiu, C., and Fratiglioni, L. (2015). A major role for cardiovascular burden in age-related cognitive decline. Nat. Rev. Cardiol. 12, 267–277. doi: 10.1038/nrcardio.2014.223
Rajan, K. B., Barnes, L. L., Wilson, R. S., McAninch, E. A., Weuve, J., Sighoko, D., et al. (2017). Racial differences in the association between apolipoprotein e risk alleles and overall and total cardiovascular mortality over 18 years. J. Am. Geriatr. Soc. 65, 2425–2430. doi: 10.1111/jgs.15059
Rajan, K. B., McAninch, E. A., Wilson, R. S., Weuve, J., Barnes, L. L., and Evans, D. A. (2019). Race, APOEε4, and long-term cognitive trajectories in a biracial population sample. J. Alzheimers Dis. JAD 72, 45–53. doi: 10.3233/JAD-190538
Rippe, J. M. (2018). Lifestyle strategies for risk factor reduction, prevention, and treatment of cardiovascular disease. Am. J. Lifestyle Med. 13, 204–212. doi: 10.1177/1559827618812395
Saadi, A., Himmelstein, D. U., Woolhandler, S., and Mejia, N. I. (2017). Racial disparities in neurologic health care access and utilization in the United States. Neurology 88, 2268–2275. doi: 10.1212/WNL.0000000000004025
Samuel, L. J., Szanton, S. L., Wolff, J. L., Ornstein, K. A., Parker, L. J., and Gitlin, L. N. (2020). Socioeconomic disparities in six-year incident dementia in a nationally representative cohort of U.S. older adults: an examination of financial resources. BMC Geriatr. 20, 156. doi: 10.1186/s12877-020-01553-4
Steenland, K., Goldstein, F. C., Levey, A., and Wharton, W. (2016). A meta-analysis of Alzheimer's disease incidence and prevalence comparing african-americans and caucasians. J. Alzheimers Dis. JAD 50, 71–76. doi: 10.3233/JAD-150778
Wang, J., Wang, L., Tang, X., Wang, F., Liu, S., Wu, X., et al. (2022). The relationship between cardiovascular disease risk score and postoperative delirium: The PNDABLE study. Front. Aging Neurosci. 14, 851372. doi: 10.3389/fnagi.2022.851372
Whitmer, R. A., Sidney, S., Selby, J., Johnston, S. C., and Yaffe, K. (2005). Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 64, 277–281. doi: 10.1212/01.WNL.0000149519.47454.F2
Yaffe, K., Bahorik, A. L., Hoang, T. D., Forrester, S., Jacobs, D. R., Lewis, C. E., et al. (2020). Cardiovascular risk factors and accelerated cognitive decline in midlife. Neurology 95, e839–e846. doi: 10.1212/WNL.0000000000010078
Yaffe, K., Falvey, C., Harris, T. B., Newman, A., Satterfield, S., Koster, A., et al. (2013). Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ 347, f7051. doi: 10.1136/bmj.f7051
Yaffe, K., Vittinghoff, E., Hoang, T., Matthews, K., Golden, S. H., and Zeki Al Hazzouri, A. (2021). Cardiovascular risk factors across the life course and cognitive decline: a pooled cohort study. Neurology 96, e2212–e2219. doi: 10.1212/WNL.0000000000011747
Yearby, R., Clark, B., and Figueroa, J. F. (2022). Structural racism in historical and modern US health care policy. Health Aff. (Millwood) 41 187–194. doi: 10.1377/hlthaff.2021.01466
Zeki Al Hazzouri, A., Jawadekar, N., Kezios, K., Caunca, M. R., Elfassy, T., Calonico, S., et al. (2022). Racial residential segregation in young adulthood and brain integrity in middle age: can we learn from small samples? Am. J. Epidemiol. 191, 591–598. doi: 10.1093/aje/kwab297
Zeki Al Hazzouri, A., Vittinghoff, E., Hoang, T., Golden, S. H., Fitzpatrick, A. L., Zhang, A., et al. (2021). Body mass index in early adulthood and dementia in late life: Findings from a pooled cohort. Alzheimers Dement. J. Alzheimers Assoc. 17, 1798–1807. doi: 10.1002/alz.12367
Keywords: health disparity populations, risk factors, mediation analysis, imputation, life course, heart disease risk factors
Citation: Ferguson EL, Vittinghoff E, Zeki Al Hazzouri A, Allen N, Fitzpatrick A and Yaffe K (2023) Contribution of life course cardiovascular risk factors to racial disparities in dementia incidence. Front. Dement. 2:1215904. doi: 10.3389/frdem.2023.1215904
Received: 03 May 2023; Accepted: 15 June 2023;
Published: 29 June 2023.
Edited by:
Alexandra Wennberg, Karolinska Institutet (KI), SwedenReviewed by:
Mozhu Ding, Karolinska Institutet (KI), SwedenCopyright © 2023 Ferguson, Vittinghoff, Zeki Al Hazzouri, Allen, Fitzpatrick and Yaffe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erin L. Ferguson, ZXJpbi5mZXJndXNvbkB1Y3NmLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.