
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Conserv. Sci. , 04 February 2025
Sec. Animal Conservation
Volume 6 - 2025 | https://doi.org/10.3389/fcosc.2025.1520857
This article is part of the Research Topic Long-Term Research on Avian Conservation Ecology in the Age of Global Change and Citizen Science View all 8 articles
Organisms in mountainous areas are frequently exposed to climatic extremes and are among the most vulnerable to climate change. Long-term studies on birds along elevational gradients, which are vital in understanding species dynamics, are rare in tropical mountains, which limits the ability to understand their population trends in the face of climate change. We modelled local abundances of understorey bird species (N=18) over a 13-year period (2011–2023) in Mt. Kasigau, Kenya, using mist netting data collected along an elevational gradient. Our models show relatively stable bird abundances in the study period. However, we found two distinct population crashes that affected most species in 2015 and 2022, suggesting that changes in local dynamics may lead to heavy declines of bird populations in mountainous regions. Most species had stable local abundances in the study period, but parametric bootstrapping revealed a declining trend for a few species, including an endemic, threatened species. We highlight the importance of mountainous regions in maintaining relatively stable populations in the face of global environmental transformation such as posed by climate change, and the dynamism of bird species populations across relatively small spatial-temporal variations. While mountain ecosystems are viewed as potential refugia for biodiversity in the face of a warming climate, further studies are needed to understand the drivers of short and long-term declines in bird populations at higher elevations, especially in tropical Africa.
Biodiversity declines in the Anthropocene continue to increase compared to the presumed prehuman background rate, with profound effects on ecosystem functioning and services (Loss et al., 2015; Rosenberg et al., 2019). Tropical ecosystems are important biodiversity reservoirs compared to other biomes, but their integrity continues to be threatened by existential anthropogenic threats such as habitat loss, climate change, unregulated harvest, and other forms of human-caused mortality (Barlow et al., 2018a; Ceballos et al., 2015; Gardner et al., 2009; Pollock et al., 2022; Rosenberg et al., 2019). Increasing temperatures coupled with changing rainfall patterns in the tropics are expected to impact species’ distribution patterns and population dynamics (Freeman et al., 2018; Freeman and Class Freeman, 2014; Magurran et al., 2010; Toms et al., 2012), in addition to driving upslope range shifts of lowland tropical species across taxa (Freeman et al., 2018; Freeman and Class Freeman, 2014). There is strong theoretical and empirical evidence indicating that tropical biotas are more strongly affected by anthropogenic ecosystem changes than their temperate counterparts (Colwell et al., 2008; Sekercioglu et al., 2008). Due to the global importance of tropical forests as carbon sinks and biodiversity, mitigating anthropogenic impacts on ecosystems and conserving tropical biodiversity has become an increasingly urgent research priority (Barlow et al., 2018b; Pollock et al., 2022). Despite general consensus that loss of montane forest habitat can lead to a decline of these small and isolated populations that are already elevationally constrained (Guo et al., 2013; Lomolino, 2001; McCain, 2009), few studies exist along elevational gradients especially in tropical Africa (Kittelberger et al., 2021). Furthermore, few elevational studies in Africa have focused on species trends despite the continent having steep elevation gradients many of which are unprotected (Elsen et al., 2018; La Sorte et al., 2014; Sheldon, 2019).
Baseline data are urgently needed to document how tropical species along elevational gradients have responded and will respond to rising temperatures, deforestation, and other anthropogenic threats (Freeman and Beehler, 2018b). However, there is a scarcity of baseline data in the tropics on species’ distributions (Collen et al., 2008; van der Hoek et al., 2020). There are few published elevational baselines of raw data that include the number of individuals of each species detected (Freeman et al., 2018a; Freeman and Beehler, 2018b; Pagaduan and Afuang, 2012). Here, we used a 13-year (2011 to 2023) population study of understory birds along an elevation gradient of a tropical montane forest – the longest study of its kind in the Eastern Arc Mountains – to evaluate long-term population trends from an isolated but intact forest reserve in southern Kenya. We used the number of unique individuals captured as an index of abundance (Pollock et al., 2022) and modelled the populations of 18 out of the 56 resident bird species, with the goal of determining how their abundances had changed over the 13-year sampling period.
We conducted our study along an elevational gradient of Mt. Kasigau (Figure 1), the northeastern most mountain of the Eastern Arc and coastal forests biodiversity hotspot (Myers et al., 2000). These ancient crystalline mountains are characterized by high species richness and the higher concentration of endemic species richness compared to other hotspot locations, but face among the highest degrees of habitat fragmentation and loss (Newmark, 1998). The climate in the surrounding lowlands is semiarid, with average annual rainfall in the 300–500 mm range that is largely irregular and prone to fail. However, the mountain itself receives relatively higher rainfall owing to its higher elevation and forest cover, which captures cloud precipitation brought in by southeast trade winds originating from the Indian Ocean (Aerts et al., 2011). There are typically two rainy seasons, in November and April, known as the grass rains and the long rains, respectively. The rainfall pattern is bimodal alternated with a long (June–September) and a short (January–March) dry period (Aerts et al., 2011). The mountain slopes are fairly steep, rising from 600 to 1641 m within 6km. Floristically, two distinct vegetation types characterize the mountain. The lower elevations are characterized by the Vachellia-Commiphora Dryland Savannah, which transitions into patches of grassland and open shrubs in mid-elevations. Dominant species include Vachellia tortilis, V. nilotica, V. busseio, Commiphora africana, C. campestris, and C. confusa. A few emergent hardwoods include Terminalia spinosa, Melia volkensii, and Boscia coriacea, with the occasional V. zanzibarica. The higher elevations are characterized by cloud montane forest, with characteristic trees including Cola greenwayi, Newtonia buchananii, Sysygium sp. and Diospyros sp. (Birdlife International, 2025).
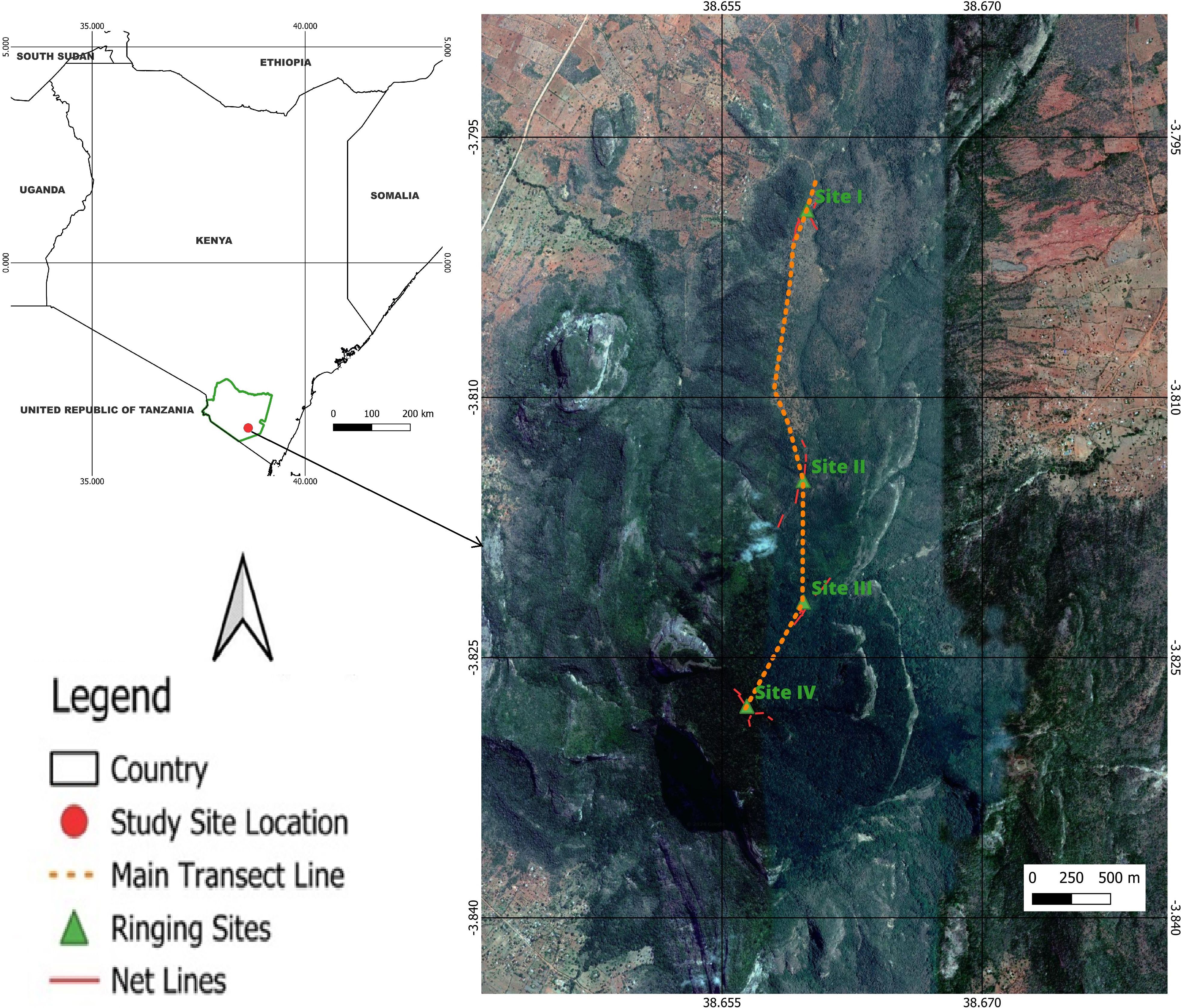
Figure 1. Location of Mt. Kasigau in Taita Taveta County, Southern Kenya. We conducted our study along a transect traversing the mountain’s elevation gradient with four ringing stations. The mist net configuration is also shown.
We established mist net lines at four sites, each approximately 230m in elevational difference, along a 3.5km long transect on Mt. Kasigau. We set the transect on the northern side of the mountain which is relatively uniform in gradient and is more accessible and practical for ringing owing to fewer cliffs and rock faces (Figure 1). We designated the sites numbers I–IV from the lowest to the highest, with the basic vegetation type, composition and net line characteristics as described below (Amakobe, 2020).
● Site I (858m asl) is characterized by bushland vegetation type, with numerous dryland species. Dominant trees include Vachellia hockii, Euphorbia quinquecostata and Commiphora baluensis, while dominant shrubs include Grewia bicolor and Catunaregam nilotica. There is evidence of human disturbance, albeit minimal, through firewood collection, livestock grazing and isolated incidences of logging on this site. There are three net lines on this site (96m, 84m and 120m).
● Site II (1104m asl) is characterized by woodland vegetation type, with tall trees up to 20m in height and an open canopy cover of above 20%. Bushes and shrubs dominate the ground layer. Dominant trees include Dombeya kirkii, Olea africana and Manilkara sp with Croton pseudopuchellus, Combretum exalatum and Searsia natalensis. There are five net lines on this site (48m, 48m, 60m, 36m and 108m).
● Site III (1321m asl) is characteristic of evergreen forest vegetation type, largely consisting of tall, broad-leaved trees, shrubs and climbers. Trees and shrubs in this site are largely evenly distributed. Typical trees include Rawsonia lucida, Sorindeia madagascariensis, Tabernaemontana stapfiana, Dracaena steudneri, Garcinia volkensii among others. There are two main shrub species at this site, Piper capense and Diospyros natalensis. There are three net lines on this site (96m, 72m and 132m).
● Site IV (1547m asl) consists of montane cloud forest of trees, shrubs and climbers with several species endemic to the area (including the larger Taita Hills Forests). Dominant trees are mainly Myrica salicifolia and Psychotria lauracea, with several other trees present in lower frequencies, including Newtonia buchananii, Turraea holstii, Xymalos monospora, Sorindeia madagascariensis, Tabernaemontana stapfiana among others. The main shrubs on this site are Dracaena steudneri, Diospyros natalensis and Piper capense. There are four net lines on this site (60m, 60m, 84m and 96m).
We ringed birds using standard mist netting procedures (Karr, 1981), using permanent mist net lines (positions) established at the four elevations (Figure 1). We undertook 1 to 4 sampling sessions a year, making as much effort as possible to sample birds in the one dry (January–February or July) and one wet (April–May or September–October) season annually. Sampling was suspended in 2016, 2017 and 2018 due to funding constraints, resuming again in 2019. During a sampling session, we undertook ringing at all 4 sites along the transect, each for two days, making a sampling session 8 days in total. Net lines were evenly spaced on each site, and a daily trapping effort of a total of 300 meters of mist nets per site operated for 6 hours (6:00 am to 12:00pm) maintained constant in all the 4 sites. We checked mist nets at 1-hour intervals to ensure prompt removal, processing, and release of captured birds. We restricted our assessment of species richness to understorey bird communities, i.e., 0–4m above the forest floor (Derlindati and Caziani, 2005) as these are the most reliably caught by mist nets (Karr, 1981). During periods of inclement weather, especially heavy rains, we temporarily closed the nets to pause sampling, resuming after weather conditions improved. We marked all individual birds with uniquely numbered aluminum rings and identified them to the species level using Birds of Kenya and Northern Tanzania (Zimmerman et al., 1999) field guide and expert knowledge.
We performed all analyses in R 4.4.0 (R Core Team, 2024) loaded into RStudio 2024.04.1 Build 748 for Windows. Our analysis aimed to determine the long-term trends of bird species abundance between 2011 and 2023. Despite evidence of idiosyncratic variations in bird abundances over long timescales in this population (see Amakobe, 2020; Wambugu et al., 2024), the focus of this study was to estimate long-term trends for individual bird species. We modelled the local abundance for all the species from which we captured at least 20 individuals (N=18 species). We used the number of unique individuals captured from the same species in each sampling session and site as a proxy for their local abundance (e.g. Blake and Loiselle, 2015a; Pollock et al., 2022). We chose this index because our sampling protocol is designed to minimize behavioral avoidance of mist nets by sampling at each site for only two days per session, and this index is expected to be reliable with this type of dataset (Remsen and Good, 1996). When we contrasted the number of captures obtained on the first and second day of each sampling session per site, the paired Wilcoxon signed-rank test indicated a statistically significant difference (W = 1998, p = 9.74 × 10−6) in the number of samples collected on the first sampling day of each session at each site (median = 14) compared to the second day (median = 10). Specifically, the number of samples collected on the second day was significantly lower than the first day (Supplementary Figure S1), which indicates a slight but consistent net avoidance within sessions. Nevertheless, we did not observe systematic declines in captures between sessions (Supplementary Figure S1) or consistent increases in captures after prolonged periods without sampling, suggesting that the number of unique individuals captured was a reliable index of abundance in the long-term.
We completed our local abundance dataset post-fieldwork by including absences whenever a given species was not recorded in a season-site combination in which it was detected at some point during the study period. We modelled local abundance as a function of the year and included site and season as covariates to account for their potential influence. We did not include net hours as an offset to account for differing sampling efforts, since they remained fairly stable across the study, with 6h per day and 2 sampling days per session at each site. There was a maximum of one session per season each year of study.
We first fitted a generalized linear model using Poisson distribution for all 18 species, using the function glmmTMB from the homonymous R package, v. 1.1.9 (Brooks et al., 2017). We verified the overdispersion of the models with the function overdisp.test included in the script diagnostic_fcns.r (Fischer et al., 2024). For those species whose models showed evidence of overdispersion (i.e., the variance is greater than the mean as indicated by the dispersion parameter being significantly greater than 1) (N=16), we fitted 3 additional models: Poisson distribution corrected for zero-inflation, negative binomial type 1 and negative binomial type 2. Negative binomial type 1, also known as quasi-Poisson, has a linear parameterization and is particularly useful when the variability of the data grows proportionally to the mean. Type 2 presents quadratic parameterization, and thus, it is more suitable when the variability increases exponentially (Ver Hoef and Boveng, 2007). We used Akaike’s Information Criterion (AIC) to select the best-fitting model for each species. If the model with the lowest AIC presented overdispersion, then the model with the second lowest AIC was selected for the species. We verified the deviance and Pearson residuals of the selected, as well as the simulated residuals derived with the function simulateResiduals of the package DHARMa v.0.4.6 (Hartig and Lohse, 2022), to ensure the absence of systematic deviances of the assumptions.
Since the impact of losing a certain number of individuals depends on the initial population size, we aimed to account for this to ensure meaningful comparisons across species. Following Pollock et al. (2022), we computed two metrics derived from the slopes associated with the year (βyear), which is the rate of change in the predicted number of unique individuals captured each year (see Supplementary Table S1 in the Supplementary material for raw βyear estimates). The first metric was the annualized proportional change in abundance (APC) (i.e., eβ−1), which informs about the expected yearly change in the local abundance and, as such, conveys the speed of the change, with greater absolute values representing quicker changes in the abundance. The second metric was the total proportional change (TPC) (i.e. eβt−1), which represents the total local abundance variation over the full study period of 13 years. For both metrics, we calculated 95% confidence intervals by substituting βyear with its corresponding confidence interval values. We categorized the local abundance trends as “increasing” if the 95% confidence interval only contained positive values, “decreasing” if the interval was negative and “stable” if the interval contained zero. We calculated an additional measurement of the total local abundance change by subtracting the predicted number of individuals at the end of the study (2023) according to the best-fitted model from those predicted at the beginning (2011) (Δn). In order to obtain a confidence interval of the predicted local abundance increment, we performed parametric bootstrapping. This consisted of repeating 100 times the process of simulating data for each species based on their best-fitting model, refitting the model with the new sample and using it to calculate the corresponding increment. This measure captures the number of individuals expected to have been gained or lost in the local abundance over the study period.
The trends in the number of species captured at each site over time in Mt. Kasigau elevational transect are shown in Figure 2. We registered a total of 2149 bird captures constituting 1755 unique individuals from 61 species in 30 families over the 19 sampling sessions that took place in 9 years, i.e. 2011–2015, 2019 and 2021–2023 (Figure 2; Supplementary Table S1). The total sampling time amounted to approximately 912 net hours within the 13-year period. Out of the 61 species registered in the study period, 18 species had at least 20 individual captures and were used in our models (Figure 3).
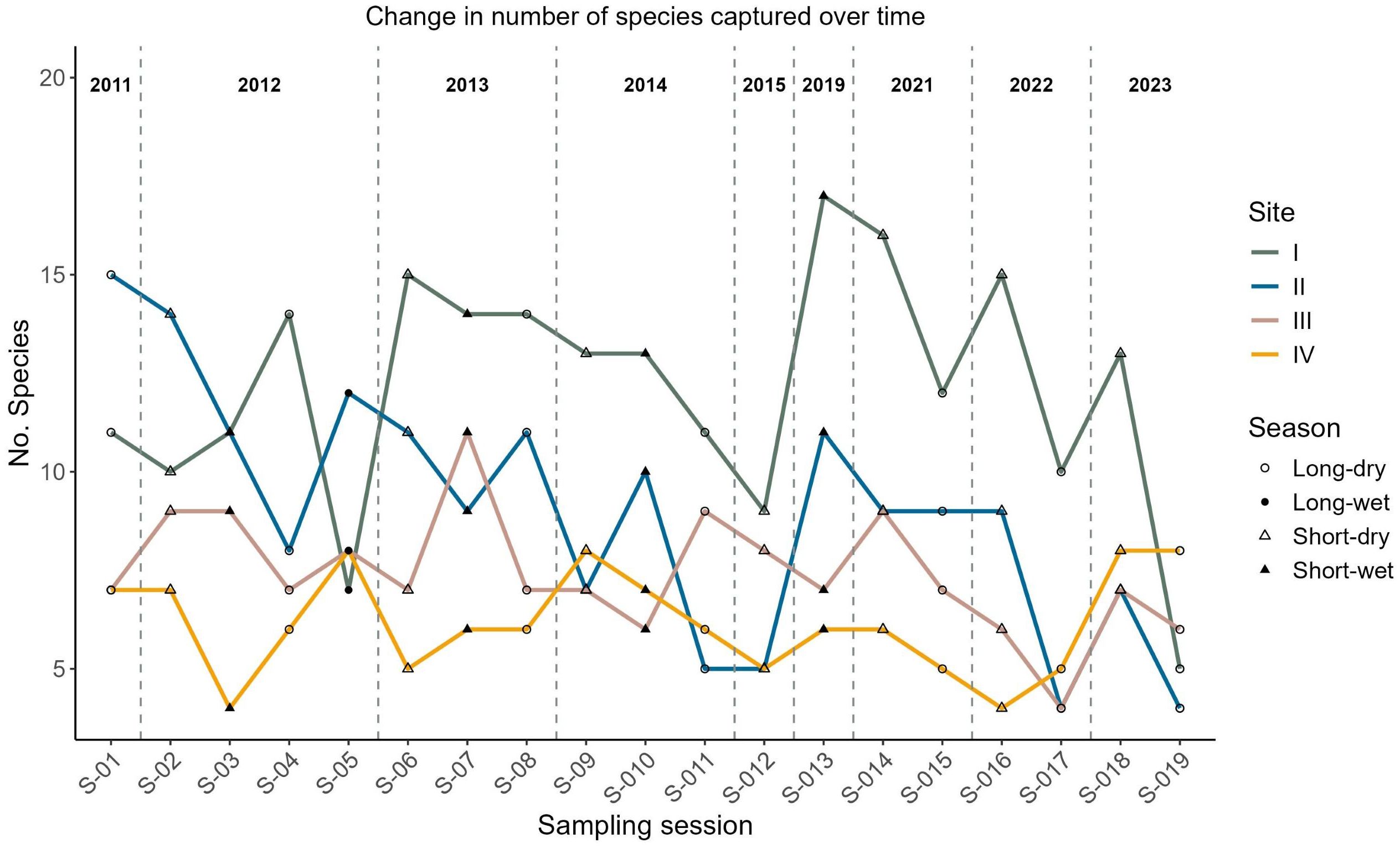
Figure 2. Variation in the number of species captured per site over time in Mt. Kasigau elevational transect and number of ringing sessions conducted.
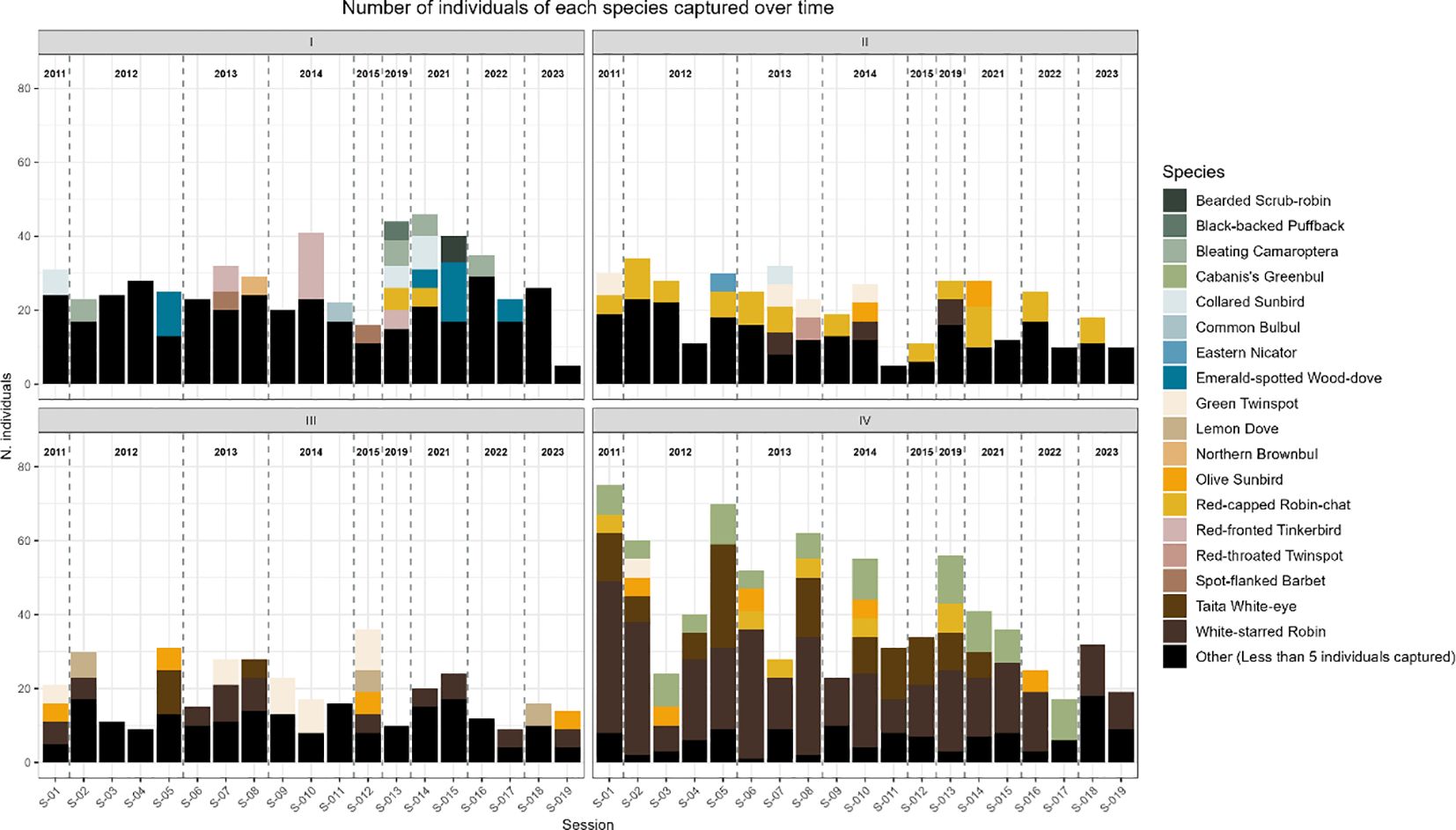
Figure 3. Number of individuals captured for each species yearly at each site, showing the 18 species modelled. All other species were reclassified in the graphs as “other” to simplify the graph.
Overall, the models indicated that 15 of the 18 species analyzed (83.33%) had stable local abundances between 2011 and 2023 (Figure 4; Supplementary Table S1). The exceptions were the Green twinspot (Mandingoa nitidula, APC= −0.16, APC95%CI = [−0.23, −0.09]; TPC= −0.90, TPC95%CI= [−0.97, −0.69]), and the Taita White-eye (Zosterops silvanus, APC=−0.11, APC95%CI=[−0.18, −0.04]; TPC=−0.78, TPC95%CI= [−0.92,−0.40]), whose declines were statistically significant, and the Eastern Nicator (Nicator gularis, APC= 0.07, APC95%CI= [0.02, 0.14]; TPC=1.54, TPC95%CI=[0.23, 4.23]) whose increase was statistically significant.
When we compared the estimated increments in local abundance obtained through parametric bootstrapping (Δn) between 2011 and 2023 (Figure 5; Supplementary Table S2), our results differed slightly. In this case, besides the Green twinspot (Δn= −12.31; Δn95%CI= [−19.81, −7.65]) and the Taita White-eye (Δn=−2.89; Δn95%CI= [−7.53, 0.4]), the Red-throated Twinspot (Hypargos niveoguttatus) also showed a decreasing trend in this analysis (Δn= −9.61; Δn95%CI= [−2268.93, −1.74]. According to theparametric bootstrap on the local abundance increment, four species showed a local abundance increase. As in the previous analysis, Eastern Nicator (Nicator gularis, Δn=4.77; Δn95%CI= [0.92. 8.1]) was one of them. The other three were White-starred Robin (Pogonocichla stellata, Δn= 3.26; Δn95%CI= [1.31, 5.04]), Bleating Camaroptera (Camaroptera brachyura, Δn= 3.26; Δn95%CI= [1.19, 5.13]), and Bearded Scrub-robin (Cercotrichas quadrivirgata, Δn= 2.54; Δn95%CI= [0.27, 4.79]).
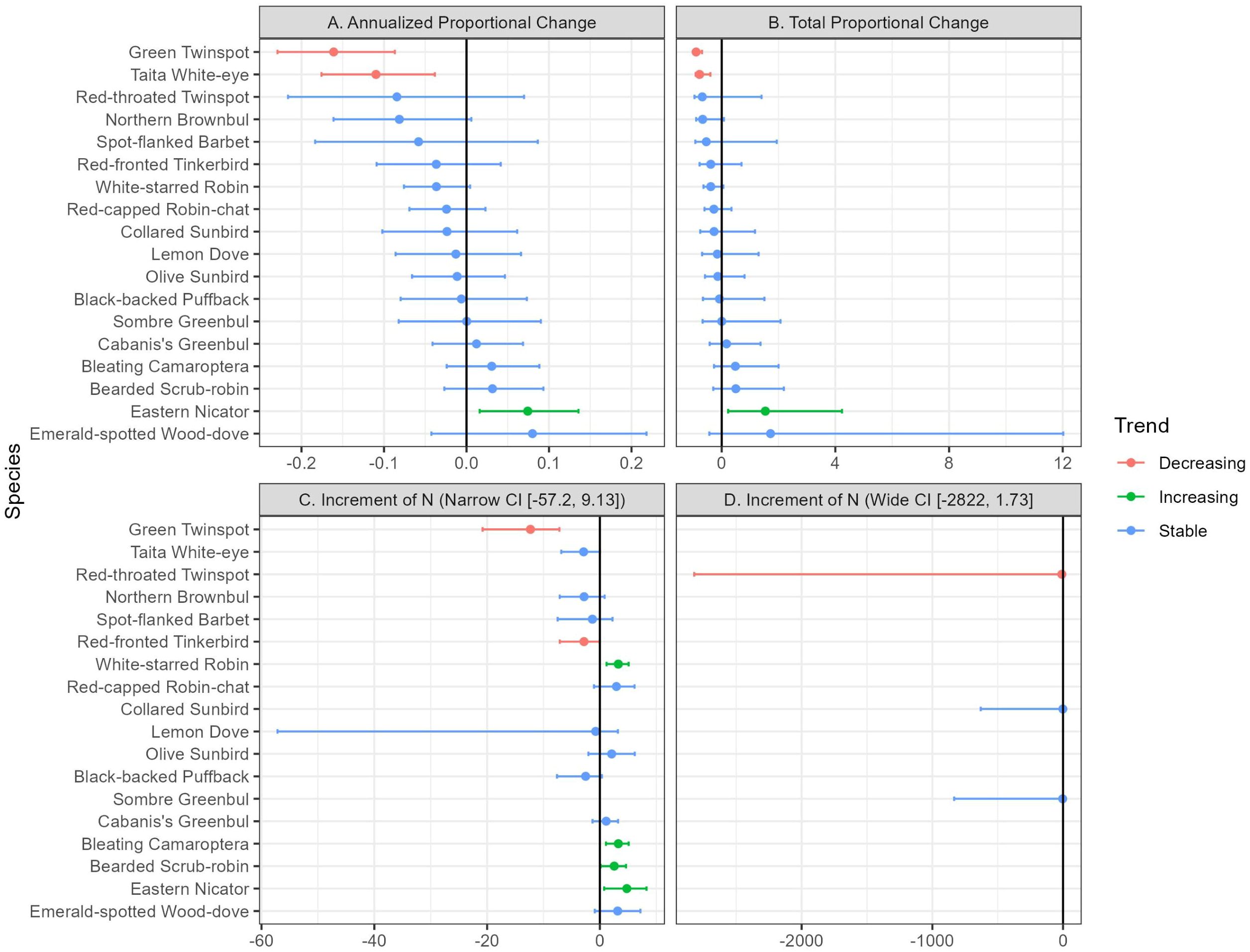
Figure 5. Local abundance change metrics between 2011 and 2023 for the 18 bird species modelled, from Mt. Kasigau, Kenya. (A) Annualized proportional change (i.e., eβ−1) represents the expected yearly change in the local abundance and, as such, conveys the speed of the change, with greater absolute values representing quicker changes in the abundance. (B) Total proportional change (i.e. eβt−1) represents the total local abundance variation over the full study period of 13 years. In both cases, β represents the slope of the covariate year (βyear) in the model chosen for each species. (C) Increment of N represents the number of individuals expected to have been gained or lost in the local abundance over the study period, estimating confidence intervals by bootstrapping. Due to their large confidence intervals, three species were depicted on a separate graph (i.e., D), employing a larger scale than the graphs on the left (C).
Our results show a stable population trend for most studied bird species in Mt. Kasigau, despite inter-seasonal and inter-year fluctuations (Figure 5). These results are in line with those in Manu National Park (Peru), where bird populations and rainfall were found to remain reasonably stable in a 40-year period (Martínez et al., 2023). However, our models are in contrast with those of other recent studies on montane bird population trends in tropical and Paleartic region that report generalized declines over the last decades (Blake and Loiselle, 2024; Brown et al., 2019; Lehikoinen et al., 2014; Neate-Clegg et al., 2021; Pollock et al., 2022; Riegert et al., 2021; Zamora and Barea-Azcón, 2015). Many tropical mountains have experienced changes in their climatic conditions and vegetation structure, implying changes in species niches that are likely to be driving the population fluctuations (de la Fuente et al., 2023; Dulle et al., 2016; Riegert et al., 2021). In contrast, the vegetation cover in Mt. Kasigau has remained largely stable over the study period, as have the temperatures at the sampling sites and the rainfall at the county level (Nyambariga et al., 2023). This general intactness of the montane forest habitat in Mt. Kasigau, which is largely devoid of human activities besides small scale firewood collection in the lower altitudes, livestock grazing in dry conditions and ecotourism, may explain the largely stable bird populations. Further, tropical climates are assumed to have constant environmental conditions which lead to constancy of resources and, hence, more stable populations especially in areas unaffected by significant human activities (Blake and Loiselle, 2015a; Sigel et al., 2006).
Despite this apparent stability, the declining trend for a globally threatened endemic species Taita white-eye, Zosterops silvanus, alongside Green twinspot Mandingoa nitidula (Figures 4, 5) may be indicative of changing habitat parameters or species-specific life history factors that do not favour these species. These declines are not likely to be caused by habitat changes as there has been minimal human disturbance in our study area during the course of the study. Besides natural tree-falls and isolated cases of selective removal of high-quality timber trees, small scale firewood collection and ecotourism are unlikely to have caused these declines. An increase in ecotourism may lead to negative impacts on birds due to behavioural changes caused by noise (Canaday, 1996), but there has not been a substantial increase in this activity during the study to affect bird populations. This suggests that other factors besides habitat changes may be driving these declines and may include but are not limited to disease and/or climate change. There have been earlier sentiments regarding the declining Taita White-eye when the species was suspected to have experienced a population crash in Mt. Kasigau (BirdLife International, 2022). The decrease in the Taita White-eye’s abundance is particularly concerning since Mt. Kasigau was identified as the species’ stronghold in the late 1990s, estimated to harbour 78% of the world’s population of this threatened species (Mulwa et al., 2007). A potential cause contributing to the Taita White-eye’s abundance declines could be inbreeding, which can cost lifetime fitness in birds (Harrisson et al., 2019). Supporting this hypothesis is that the gene flow between Mt. Kasigau and Taita Hills populations is scarce owing to isolation of the former, and the percentage of heterozygosity is relatively low (Habel et al., 2014). Inbreeding has been shown to have a lifetime fitness cost in birds (Harrisson et al., 2019). Restricted dispersal and frequent inbreeding within “sky island” systems, such as Mt. Kasigau for the Taita White-eye, can occur even in highly mobile bird species (Ceresa et al., 2024). In contrast, the Eastern Nicator showed remarkable increases in its abundance in the same period. Further studies are needed to understand the underlying causes of these population changes despite relatively stable rainfall and temperature conditions across the study period. Moreover, other species in the same diet guilds similar to these species have not experienced similar population changes.
Despite overall stability in bird populations, our analyses show short term dips and peaks in bird population trends. These dips may be attributable to the number of sampling sessions undertaken: there was only one sampling session undertaken in 2011, 2015 and 2019 while there were 2–4 sessions in all other years. However, periodic dips and peaks in bird abundances have been observed in other studies and have been hypothesised to be the effect of large-scale climatic cycles, such as El Niño-Southern Oscillation (ENSO) which can affect bird populations in different ways across geographical regions (Ballard et al., 2003; Blake and Loiselle, 2015a; LaManna et al., 2012). These ENSO events may not only influence the breeding success on birds both positively and negatively but could also affect their foraging behaviour and diet. In our study area, periodic changes in weather parameters were suggested as driving these periodic declines in an earlier study (see Wambugu et al., 2024). Data from the Kenya Meteorological Department (Kenya Meteorological Department, n.d) reveals that both 2011 and 2015 corresponded to La Niña and El Niño extreme weather periods, respectively, which may partly explain these dips in bird population trends in our study. It is however worth noting that many of the species in our study were only captured in one or two years at each site. Thus, our dataset only provides an indication of the general trend in bird community over time but it’s unclear regarding the turnover between species.
Several species were consistently present in the first half of the study (2011–2015) but absent in the second half (notably Common bulbul and Lesser Honeyguide in Site I; Spotted Flycatcher in Site II; and African Pygmy Kingfisher and African Goshawk in Site III). Likewise, other species appeared in the second half such as the Red-throated Twinspot and the Variable Sunbird in Site I. Other studies have termed similar observations as winner–loser species replacements, which may be triggered by changes in habitat parameters (Lees and Peres, 2006; Tabarelli et al., 2012). Winner–loser patterns are clearer in human–modified landscapes due to widespread habitat changes (e.g. Filgueiras et al., 2021) but less so in more intact habitats. In our study, these changes appear to have occurred immediately after population collapse, thought to be due to changes in resource availability as a result of unconfirmed events (Figure 2).
Our study shows that most bird species populations in Mt. Kasigau remained stable despite reported declines in similar locations elsewhere in the tropics. This stability indicates the role of montane areas as refugia for birds in the face of a warming climate, along with other anthropogenic pressures. Our study further emphasizes that relatively small mountain regions can play an important role in maintaining stable bird populations as global environmental transformation continues to escalate. However, future studies should focus on species-specific life history aspects to better understand population trends of Afromontane birds, especially those that appear to be undergoing declines.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The animal study was approved by National Commission for Science, Technology and Innovation (NACOSTI, Kenya) and the East African Bird Ringing Scheme. The study was conducted in accordance with the local legislation and institutional requirements.
MW: Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LM: Data curation, Formal analysis, Methodology, Resources, Software, Visualization, Validation, Writing – original draft, Writing – review & editing. BA: Data curation, Investigation, Project administration, Resources, Supervision, Writing – review & editing. MG: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors declare that this study received funding from Wildlife Works PLC. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that Generative AI was used in the creation of this manuscript. Technical support for R code writing was obtained from ChatGPT (GPT-4, OpenAI, https://openai.comb, October 2023), Gemini (Large language model, Google AI, https://gemini.google.com/, 2023) and Microsoft Copilot (GPT-4 Conversational AI Model. Microsoft, https://copilot.cloud.microsoft/, 2024).
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2025.1520857/full#supplementary-material
Supplementary Table 1 | Number of unique individuals captured each year in each site for each species. Site I: 858m asl, Site II: 1104m asl, Site III: 1321m asl, Site IV: 1547m asl.
Supplementary Table 2 | Summary of Results for Modelled species (n=18) along the Mt. Kasigau Elevational transect.
Supplementary Figure 1 | Number of captures in days 1 and 2 of each sampling session and site
Aerts R., Thijs K. W., Lehouck V., Beentje H., Bytebier B., Matthysen E., et al. (2011). Woody plant communities of isolated Afromontane cloud forests in Taita Hills, Kenya. Plant Ecol. 212, 639–649. doi: 10.1007/s11258-010-9853-3
Amakobe B. A. (2020). Bird species composition and diversity along an ecological gradient on Mount Kasigau in Taita-Taveta county, south east Kenya. Nairobi, Kenya: African Nazarene University.
Ballard G., Geupel G. R., Nur N., Gardali T. (2003). Long-term declines and decadal patterns in population trends of songbirds in western north america 1979–1999. Condor 105, 737. doi: 10.1650/7131
Barlow J., França F., Gardner T. A., Hicks C. C., Lennox G. D., Berenguer E., et al. (2018a). The future of hyperdiverse tropical ecosystems. Nature 559, 517–526. doi: 10.1038/s41586-018-0301-1
Barlow J., França F., Gardner T. A., Hicks C. C., Lennox G. D., Berenguer E., et al. (2018b). The future of hyperdiverse tropical ecosystems. Nature 559, 517–526. doi: 10.1038/s41586-018-0301-1
Birdlife International (2025). Important Bird Area factsheet: Mount Kasigau forest (Kenya). Available online at: https://datazone.birdlife.org/site/factsheet/mount-kasigau-forest-iba-kenya on 23/01/2025.
BirdLife International (2022). Zosterops silvanus. The IUCN Red List of Threatened Species 2022: e.T22713957A188598582. Available online at: https://www.iucnredlist.org/species/22713957/188598582 (Accessed on September 23, 2024).
Blake J. G., Loiselle B. A. (2015a). Enigmatic declines in bird numbers in lowland forest of eastern Ecuador may be a consequence of climate change. PeerJ 3, e1177. doi: 10.7717/peerj.1177
Blake J. G., Loiselle B. A. (2024). Sharp declines in observation and capture rates of Amazon birds in absence of human disturbance. Global Ecol. Conserv. 51, e02902. doi: 10.1016/j.gecco.2024.e02902
Brooks M. E., Kristensen K., Benthem K. J., Magnusson A., Berg C. W., Nielsen A., et al. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378. doi: 10.32614/RJ-2017-066
Brown J. A., Lockwood J. L., Avery J. D., Curtis Burkhalter J., Aagaard K., Fenn K. H. (2019). Evaluating the long-term effectiveness of terrestrial protected areas: a 40-year look at forest bird diversity. Biodiversity Conserv. 28, 811–826. doi: 10.1007/s10531-018-01693-5
Canaday C. (1996). Loss of insectivorous birds along a gradient of human impact in Amazonia. Biol. Conserv. 77, 63–77. doi: 10.1016/0006-3207(95)00115-8
Ceballos G., Ehrlich P. R., Barnosky A. D., García A., Pringle R. M., Palmer T. M. (2015). Accelerated modern human-induced species losses: Entering the sixth mass extinction. Sci. Adv. 1, e1400253. doi: 10.1126/sciadv.1400253
Ceresa F., Brambilla M., Kvist L., Vitulano S., Pes M., Tomasi L., et al. (2024). Restricted dispersal and inbreeding in a high-elevation bird across the ‘sky islands’ of the European Alps. J. Biogeography 51, 853–868. doi: 10.1111/jbi.14787
Collen B., Ram M., Zamin T., McRae L. (2008). The tropical biodiversity data gap: addressing disparity in global monitoring. Trop. Conserv. Sci. 1, 75–88. doi: 10.1177/194008290800100202
Colwell R. K., Brehm G., Cardelús C. L., Gilman A. C., Longino J. T. (2008). Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science 322, 258–261. doi: 10.1126/science.1162547
de la Fuente A., Navarro A., Williams S. E. (2023). The climatic drivers of long-term population changes in rainforest montane birds. Global Change Biol. 29, 2132–2140. doi: 10.1111/gcb.16608
Derlindati E. J., Caziani S. M. (2005). Using canopy and understory mist nets and point counts to study bird assemblages in chaco forests. Wilson Bull. 117 (1), 92–99. doi: 10.1676/03-063
Dulle H. I., Ferger S. W., Cordeiro N. J., Howell K. M., Schleuning M., Böhning-Gaese K., et al. (2016). Changes in abundances of forest understorey birds on Africa’s highest mountain suggest subtle effects of climate change. Diversity Distributions 22, 288–299. doi: 10.1111/ddi.12405
Elsen P. R., Monahan W. B., Merenlender A. M. (2018). Global patterns of protection of elevational gradients in mountain ranges. Proc. Natl. Acad. Sci. 115, 6004–6009. doi: 10.1073/pnas.1720141115
Filgueiras B. K. C., Peres C. A., Melo F. P. L., Leal I. R., Tabarelli M. (2021). Winner–loser species replacements in human-modified landscapes. Trends Ecol. Evol. 36, 545–555. doi: 10.1016/j.tree.2021.02.006
Fischer J., Rathke E.-M., Mundry R. (2024). Data and Code for “Older Barbary macaques show limited capacity for self-regulation to avoid hazardous social interactions. doi: 10.17605/OSF.IO/VJEB3
Freeman B. G., Beehler B. M. (2018b). Limited support for the “abundant centre” hypothesis in birds along a tropical elevational gradient: implications for the fate of lowland tropical species in a warmer future. J. Biogeography 45, 1884–1895. doi: 10.1111/jbi.13370
Freeman B. G., Class Freeman A. M. (2014). Rapid upslope shifts in New Guinean birds illustrate strong distributional responses of tropical montane species to global warming. Proc. Natl. Acad. Sci. 111, 4490–4494. doi: 10.1073/pnas.1318190111
Freeman B. G., Lee-Yaw J. A., Sunday J. M., Hargreaves A. L. (2018a). Expanding, shifting and shrinking: The impact of global warming on species’ elevational distributions. Global Ecol. Biogeography 27, 1268–1276. doi: 10.1111/geb.12774
Freeman B. G., Scholer M. N., Ruiz-Gutierrez V., Fitzpatrick J. W. (2018). Climate change causes upslope shifts and mountaintop extirpations in a tropical bird community. Proc. Natl. Acad. Sci. 115, 11982–11987. doi: 10.1073/pnas.1804224115
Gardner T. A., Barlow J., Chazdon R., Ewers R. M., Harvey C. A., Peres C. A., et al. (2009). Prospects for tropical forest biodiversity in a human-modified world. Ecol. Lett. 12, 561–582. doi: 10.1111/j.1461-0248.2009.01294.x
Guo Q., Kelt D. A., Sun Z., Liu H., Hu L., Ren H., et al. (2013). Global variation in elevational diversity patterns. Sci. Rep. 3 (1), 3007. doi: 10.1038/srep03007
Habel J. C., Mulwa R. K., Gassert F., Rödder D., Ulrich W., Borghesio L., et al. (2014). Population signatures of large-scale, long-term disjunction and small-scale, short-term habitat fragmentation in an Afromontane forest bird. Heredity 113, 205–214. doi: 10.1038/hdy.2014.15
Harrisson K. A., Magrath M. J. L., Yen J. D. L., Pavlova A., Murray N., Quin B., et al. (2019). Lifetime fitness costs of inbreeding and being inbred in a critically endangered bird. Curr. Biol. 29, 2711–2717.e4. doi: 10.1016/j.cub.2019.06.064
Hartig F., Lohse L. (2022). DHARMa: residual diagnostics for hierarchical (Multi-level / mixed) regression models. Available online at: https://cran.r-project.org/web/packages/DHARMa/index.html (Accessed October 3, 2024).
Karr’ J. R. (1981). “Surveying birds with mist nets,” in Studies in Avian Biology (Illinois, USA: University of Illinois, Champaign), 6, 62–67.
Kenya Meteorological Department. (n.d.). Extreme climate events in Kenya between 2011 to 2020. (Nairobi: Kenya Meteorological Department).
Kittelberger K. D., Neate-Clegg M. H. C., Buechley E. R., Hakkı Şekercioğlu Ç. (2021). Community characteristics of forest understory birds along an elevational gradient in the Horn of Africa: A multi-year baseline. Ornithological Appl. 123 (2). doi: 10.1093/ornithapp/duab009
LaManna J. A., George T. L., Saracco J. F., Nott M. P., DeSante D. F. (2012). El Niño–Southern Oscillation influences annual survival of a migratory songbird at a regional scale. Auk 129, 734–743. doi: 10.1525/auk.2012.12017
La Sorte F. A., Butchart S. H. M., Jetz W., Böhning-Gaese K. (2014). Range-wide latitudinal and elevational temperature gradients for the world’s terrestrial birds: implications under global climate change. PloS One 9, e98361. doi: 10.1371/journal.pone.0098361
Lees A. C., Peres C. A. (2006). Rapid avifaunal collapse along the Amazonian deforestation frontier. Biol. Conserv. 133, 198–211. doi: 10.1016/j.biocon.2006.06.005
Lehikoinen A., Green M., Husby M., Kålås J. A., Lindström Å. (2014). Common montane birds are declining in northern Europe. J. Avian Biol. 45, 3–14. doi: 10.1111/j.1600-048X.2013.00177.x
Lomolino M. V. (2001). Elevation gradients of species-density: historical and prospective views. Global Ecol. Biogeography 10 (1), 3–13. doi: 10.1046/j.1466-822x.2001.00229.x
Loss S. R., Will T., Marra P. P. (2015). Direct mortality of birds from anthropogenic causes. Annu. Rev. Ecology Evolution Systematics 46, 99–120. doi: 10.1146/annurev-ecolsys-112414-054133
Magurran A. E., Baillie S. R., Buckland S. T., Dick J., Elston D. A., Scott E. M., et al. (2010). Long-term datasets in biodiversity research and monitoring: assessing change in ecological communities through time. Trends Ecol. Evol. 25, 574–582. doi: 10.1016/j.tree.2010.06.016
Martínez A. E., Ponciano J. M., Gomez J. P., Valqui T., Novoa J., Antezana M., et al. (2023). The structure and organisation of an Amazonian bird community remains little changed after nearly four decades in Manu National Park. Ecol. Lett. 26, 335–346. doi: 10.1111/ele.14159
McCain C. M. (2009). Global analysis of bird elevational diversity. Global Ecol. Biogeography 18 (3), 346–360. doi: 10.1111/j.1466-8238.2008.00443.x
Mulwa R. K., Bennun L. A., Ogol C. K. P. O., Lens L. (2007). Population status and distribution of Taita White-eye Zosterops silvanus in the fragmented forests of Taita Hills and Mount Kasigau, Kenya. Bird Conserv. Int. 17, 141–150. doi: 10.1017/S0959270907000664
Myers N., Mittermeier R. A., Mittermeier C. G., da Fonseca G. A. B., Kent J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858. doi: 10.1038/35002501
Neate-Clegg M. H. C., Jones S. E. I., Tobias J. A., Newmark W. D., Şekercioǧlu Ç.H. (2021). Ecological correlates of elevational range shifts in tropical birds. Front. Ecol. Evol. 9. doi: 10.3389/fevo.2021.621749
Newmark W. D. (1998). Forest area, fragmentation, and loss in the eastern arc mountains: implications for the conservation of biological diversity. J. East Afr. Natural History 87, 29–36. doi: 10.2982/0012-8317(1998)87[29:fafali]2.0.co;2
Nyambariga F. K., Opere A. O., Kituyi E., Amwata D. A. (2023). Climate change scenario projections and their implications on food systems in taita taveta county, kenya. PloS Climate 2 (6), e0000114. doi: 10.1371/journal.pclm.0000114
Pagaduan D. C., Afuang L. E. (2012). Understorey bird species diversity along elevational gradients on the northeastern slope of Mt. Makiling, Luzon, Philippines. (Laguna, Philippines: The Asian International Journal of Life Sciences).
Pollock H. S., Toms J. D., Tarwater C. E., Benson T. J., Karr J. R., Brawn J. D. (2022). Long-term monitoring reveals widespread and severe declines of understory birds in a protected Neotropical forest. Proc. Natl. Acad. Sci. 119 (16), e2108731119. doi: 10.1073/pnas.2108731119
R Core Team (2024). R: A language and environment for statistical computing. (4.4.0). Available online at: https://www.R-project.org/ (Accessed October 3, 2024).
Remsen J. V., Good D. A. (1996). Misuse of data from mist-net captures to assess relative abundance in bird populations. Auk 113 (2), 381–398. doi: 10.2307/4088905
Riegert J., Chmel K., Vlček J., Hrázský Z., Sedláček O., Grill S., et al. (2021). Alarming declines in bird abundance in an Afromontane global biodiversity hotspot. Biodiversity Conserv. 30, 3385–3408. doi: 10.1007/s10531-021-02252-1
Rosenberg K. V., Dokter A. M., Blancher P. J., Sauer J. R., Smith A. C., Smith P. A., et al. (2019). Decline of the North American avifauna. Available online at: http://science.sciencemag.org/ (Accessed March 4 , 2024).
Sekercioglu C. H., Schneider S. H., Fay J. P., Loarie S. R. (2008). Climate change, elevational range shifts, and bird extinctions. Conserv. Biol. 22, 140–150. doi: 10.1111/j.1523-1739.2007.00852.x
Sheldon K. S. (2019). Climate change in the tropics: ecological and evolutionary responses at low latitudes. Annu. Rev. Ecology Evolution Systematics 50, 303–333. doi: 10.1146/annurev-ecolsys-110218-025005
Sigel B. J., Sherry T. W., Young B. E. (2006). Avian community response to lowland tropical rainforest isolation: 40 years of change at la selva biological station, Costa Rica. Conserv. Biol. 20, 111–121. doi: 10.1111/j.1523-1739.2005.00293.x
Tabarelli M., Peres C. A., Melo F. P. L. (2012). The ‘few winners and many losers’ paradigm revisited: Emerging prospects for tropical forest biodiversity. Biol. Conserv. 155, 136–140. doi: 10.1016/j.biocon.2012.06.020
Toms J. D., Faarborg J., Arendt W. J. (2012). Climate change and birds in the forgotten tropics: the importance of tropical dry forests. Ibis 154, 632–634. doi: 10.1111/j.1474-919X.2012.01248.x
van der Hoek Y., Faida E., Musemakweli V., Tuyisingize D. (2020). Living the high life: remarkable high-elevation records of birds in an East African mountain range. Ecology 101 (1). doi: 10.1002/ecy.2866
Ver Hoef J. M., Boveng P. L. (2007). Quasi-poisson vs. Negative binomial regression: how should we model overdispersed count data? Ecology 88, 2766–2772. doi: 10.1890/07-0043.1
Wambugu G. M., Amakobe B., Şekercioğlu Ç.H., Githiru M. (2024). Elevational patterns of species richness and community structure of understorey birds in an East African montane forest. Afr. J. Ecol. 62 (1). doi: 10.1111/aje.13235
Zamora R., Barea-Azcón J. M. (2015). Long-term changes in mountain passerine bird communities in the sierra nevada (Southern Spain): A 30-year case study. Ardeola 62, 3. doi: 10.13157/arla.62.1.2015.3
Keywords: elevational gradient, Afrotropical, understorey birds, climate change, Mount Kasigau, refugia
Citation: Wambugu M, Martínez-Íñigo L, Amakobe B and Githiru M (2025) Many winners, few losers: stable bird populations on an Afrotropical mountain amidst climate change. Front. Conserv. Sci. 6:1520857. doi: 10.3389/fcosc.2025.1520857
Received: 01 November 2024; Accepted: 13 January 2025;
Published: 04 February 2025.
Edited by:
Monte Neate-Clegg, University of California, Los Angeles, United StatesReviewed by:
Kyle Kittelberger, The University of Utah, United StatesCopyright © 2025 Wambugu, Martínez-Íñigo, Amakobe and Githiru. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mwangi Wambugu, d2FtYnVndS5nZW9mZnJleUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.