
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Conserv. Sci., 05 March 2025
Sec. Animal Conservation
Volume 6 - 2025 | https://doi.org/10.3389/fcosc.2025.1504320
This article is part of the Research TopicLong-Term Research on Avian Conservation Ecology in the Age of Global Change and Citizen ScienceView all 8 articles
 Panagiotis Nikolaou1,2,3,4*
Panagiotis Nikolaou1,2,3,4* Billi A. Krochuk2,5
Billi A. Krochuk2,5 Patricia F. Rodrigues2,3,6
Patricia F. Rodrigues2,3,6 Kristin E. Brzeski2,7
Kristin E. Brzeski2,7 Susana L. Mufumu2,8
Susana L. Mufumu2,8 Silvestre E. Malanza2,8
Silvestre E. Malanza2,8 Christopher M. Tonra9
Christopher M. Tonra9 Jacob C. Cooper10,11†
Jacob C. Cooper10,11† Jared D. Wolfe2,7
Jared D. Wolfe2,7 Luke L. Powell1,2,3,4
Luke L. Powell1,2,3,4The Congo Basin rainforest and adjacent Lower Guinea Forest constitute the second largest tract of lowland tropical rainforest in the world. As with the rest of the continent, human population is increasing rapidly and forest degradation is ubiquitous. Forest degradation through logging has pervasive negative effects on ecosystems, but selective logging is considered less impactful than clearcutting. Recent research in Afrotropical forest shows that certain avian species and guilds are more affected by selective logging than others (e.g., specialist insectivores such as followers of Dorylus driver ants); however, the mechanisms behind these patterns are poorly known. In an eight-year mist-netting effort in Equatorial Guinea, we caught 1193 birds in primary forest and high-grade selectively logged forest to determine the effect of disturbance on six demographic and physiological measures on birds. We compared five life history and population traits for ten insectivorous species: proportion of breeding and molting birds, molt-breeding overlap, bird age, and a body mass index. We also analyzed the concentrations of the stress hormone feather corticosterone (fCORT) in five species. All three strict ant-following species (Alethe castanea, Chamaetylas poliocephala, Neocossyphus poensis), and the Muscicapid robin Sheppardia cyornithopsis had a higher proportion of first year birds in secondary forest. Furthermore, two ant-followers, A. castanea and C. poliocephala, had a higher proportion of individuals molting in primary forest. Finally, only Illadopsis cleaveri had higher body condition in secondary forest. We found no differences in breeding status, molt-breeding overlap or fCORT between forest types. Using a long-term mist-netting effort, we use measures taken from birds in-the-hand to go beyond insights from point counts alone; we gain valuable insights into the demography and physiology of Afrotropical birds occupying variably degraded lowland tropical rainforest.
The Afrotropics account for approximately 30% of the world's tropical forest (Malhi et al., 2013); of those forests, 89% are found in Central Africa (Malhi et al., 2013; Mayaux et al., 2013). The Congo Basin accounts for 90% of the carbon stored in Africa’s terrestrial systems (Saatchi et al., 2011; Baccini et al., 2012) and plays a major role in regulating the continent’s climate (Maynard and Royer, 2004).
Deforestation in sub-Saharan Africa is driven primarily by small-scale clearing for rotational agriculture (Tyukavina et al., 2018) and non-mechanized selective logging (Laporte et al., 2007; Rudel, 2013; Curtis et al, 2018; Tyukavina et al., 2018). Clearing for large-scale industrial agriculture, which accounted for approximately 55% of global tropical deforestation between 2000-2005 (Hansen et al., 2008), accounted for under 1% of the deforestation in Central Africa between 2000-2014 (Tyukavina et al., 2018). However, while some estimates have suggested that 84-93% of the deforestation has taken place in Central Africa has occurred because of clearing for small-holder agriculture (Curtis et al., 2018; Tyukavina et al., 2018), others have suggested that logging may contribute as much as 30% of the region’s deforestation after considering the indirect damage from logging operations (e.g., the creation of supporting infrastructure like skid trails and logging roads) (Laporte et al., 2007).
About 10% of all tropical forests are selectively logged on a regular basis (Blaser et al., 2006; Edwards et al., 2014). Some tropical forest nations, including several Central African countries (Puettmann et al., 2015), have introduced selective logging regulations to reduce deforestation through clearcutting while satisfying timber demand more sustainably (Drigo et al., 2009). As a result, large parts of the Afrotropical rainforest have been subject to high-grade selective logging, a practice which involves the harvest of only large, high-value trees such as Okoume (Aucoumea klaineana) (Akindele and Onyekwelu, 2011).
The ecological impact of selective logging on biodiversity differs among tropical regions. In Amazonia, for example, selective logging decreased avian species richness and abundance (Barlow et al., 2006) whereas in Southeast Asia, selective logging had no effect on avian species richness or abundance (Sodhi et al., 2010; Edwards et al., 2011).
Because selective logging is pervasive throughout the tropics and is increasing in extent within the Afrotropics, it is imperative that we understand how this form of forest degradation impacts birds. Birds act as integral components of tropical ecosystems (Sekercioglu et al., 2016; BirdLife International, 2018) and perform functions critical to the integrity of these systems, many of which benefit humans either directly or indirectly, including pest control, pollination, and seed dispersal (Sekercioglu, 2002; BirdLife International, 2018). Although selective logging is demonstrably less detrimental to biodiversity than more intensive forms of timber extraction, there is considerable evidence from across the tropics that suggest it can have negative effects on bird species richness and abundance (Sodhi et al., 2008, Sodhi et al., 2010; Edwards et al., 2011) as well as functional diversity (Bregman et al., 2016). Furthermore, it is now well-established that logging impacts community composition, with a shift in species composition most often resulting in forest interior specialists being replaced by functionally similar forest generalist or gap-/edge-preferring species (Dranzoa, 1998; Sodhi et al., 2008). The implications of changes in forest structure on species are not consistent and depends on various intrinsic characteristics, including diet, foraging mode, habitat and dietary specialization, morphology, and breeding biology (Sodhi et al., 2008; Hamer et al., 2015).
While our understanding of how selective logging impacts tropical avian communities is increasing, gaps still exist (Burivalova et al., 2015; Gray et al., 2007). This is particularly true for the Afrotropics (Cazzolla et al., 2015; Watson et al., 2017), where inconsistencies persist (Gray et al., 2007). Existing research from the Afrotropics suggests that selectively logged forests can support lower species richness and abundance in comparison to undisturbed primary forests (Arcilla et al., 2015; Beier et al., 2002; Dale et al., 2000; Newmark, 2006; Watson et al., 2004); maintain pre-logging levels of richness but prompt shifts in community composition and thus function (Kofron and Chapman, 1995; Dranzoa, 1998; Cordeiro et al., 2015; Arcilla et al., 2015); and/or decrease the relative abundance of forest-dependent species (Newmark, 2006; Dranzoa, 1998; Arcilla et al., 2015; Cordeiro et al., 2015). Avian biodiversity in tropical forests can be an effective indicator of ecosystem health reflecting the long-term impacts of selective logging (De Heer et al., 2005). However, most studies focus on broader patterns of diversity at the community level, whether taxonomic (e.g., Tchoumbou et al., 2020), functional (e.g., Bregman et al., 2016; Mestre et al., 2020), or phylogenetic (e.g., Mestre et al., 2020). While this line of questioning is important, we must also identify patterns within individual species that may be driving community-level patterns. For instance, by considering specific guilds of birds within forest fragments in Kenya, Peters and Okalo (2009) determined that only the most specialized ant-following insectivores had declined. This example reinforces the understanding that obligate or near-obligate ant-following species, i.e., species that depend on predatory driver ants for foraging, are more sensitive than generalist species across the tropics (Peters et al., 2008; Waltert et al., 2005; Barrie et al., in review). Furthermore, in a review of the effects of selective logging across tropical forests, Burivalova et al. (2015) showed that differences in the intra-guild body sizes of species were also influenced by selective logging. In some guilds, larger bodied species were positively impacted by selective logging, whereas in other guilds small-bodied species were negatively affected (Burivalova et al., 2015). Looking at the physiological state of individual birds in selectively logged forests can provide a more holistic understanding of how these species are affected by selective logging (Fefferman and Romero, 2013). There are many aspects that could affect birds’ physiological state in secondary forests; for example, reduced resources, decreased canopy cover, and higher densities of predators (Sekercioglu, 2002; Cosset et al., 2021).
Life history traits can be used to highlight potential effects of selective logging on understory birds. For example, breeding and molting are energy and resource intensive stages in a birds’ life (Salvantes and Williams, 2003; Romero, 2002). They are both dependent on food availability (Wikelski et al., 2000; Marini and Durães, 2001), which can be more limited in selectively logged tropical forests than primary forests (Ross et al., 2018). In a comparative study, Coddington et al. (2023) found a 61% reduction in the proportion of breeding birds following fragmentation, indicating human disturbance can influence the breeding behavior of understory birds. Unlike temperate birds that experience well-defined molting periods and breeding seasons, relative climatic stability in the tropics means that some tropical birds can experience overlap in their molting and breeding seasons (Webster and Handley, 1986). Some birds adjust to the cost of this overlap with slow feather growth rate or molting fewer feathers simultaneously (Echeverry-Galvis and Hau, 2013). A possible scenario is that birds in suboptimal habitat breed and molt at the same time during periods of resource abundance; birds in primary forest are then less likely to breed and molt simultaneously due to a more consistent resource pool (Freed and Cann, 2012).
Population traits such as age ratios can also be used to highlight potential effects of selective logging on understory birds. In territorial bird species, the social dominance hypothesis predicts that age ratios in poor-quality habitats will be young-dominated as older and stronger individuals already occupy and protect territories in better-quality habitats (Hannon and Martin, 2006). These predictions may be exaggerated in the tropics, where territoriality is often experienced year-round (Stutchbury and Morton, 2001) compared to temperate regions where territories are defended during defined breeding seasons. Additionally, tropical songbirds typically have a higher longevity than their temperate counterparts (Snow and Lill, 1974; Williams et al., 2010). However, their mortality rates in disturbed forest are often higher (Ruiz-Gutierrez et al., 2008), creating space for younger birds to move in. In addition to age ratios, body condition can provide insight into an individual bird’s health and thereby the quality of the habitat it occupies (Jakob et al, 1996). Specifically, mass corrected for body size (hereafter ‘body condition’) is a simple condition index that can identify individuals under nutritional stress (Stevenson and Woods, 2006). For example, in Borneo, a study of 55 bird species showed that frugivorous and omnivorous birds that occupied selectively logged habitats had reduced body size compared to conspecifics occupying primary forest (Messina et al., 2021). Finally, poor-quality habitats can cause long-term increases in birds’ stress hormones (e.g., feather corticosteroid (fCORT) Bortolotti et al., 2008), thus one might expect higher fCORT in disturbed secondary forest relative to primary forest.
Here, we tested the hypothesis that high-grade selectively logged (hereafter ‘secondary forests’) have demographic and physiological effects on understory bird species. We tested this hypothesis using life history (molt and breeding) and population traits (age ratios) as well as fCORT and body condition of ten understory insectivores. We selected understory insectivores based on evidence that this guild is particularly vulnerable to anthropogenic disturbance (Dale et al., 2000; Watson et al., 2004; Jarrett et al., 2021; Powell et al., 2015; Barrie et al., in review), especially Afrotropical ant-followers (Peters et al., 2008; Peters and Okalo, 2009, Jarrett et al., 2021; Barrie et al., in review). If secondary forest is indeed a suboptimal habitat relative to primary forests, we predict that birds in secondary forests will have (1) a lower breeding and molting prevalence and a higher prevalence of molt-breeding overlap, (2) a higher proportion of first-year birds and (3) lower mean body condition and higher fCORT concentrations.
Data were collected by Biodiversity Initiative at two locations in Equatorial Guinea: Cuidad de la Paz (hereafter ‘La Paz’) during the sunny dry season (December-January) from 2016 to 2023 and one cloudy dry season in 2022 (1128 captures) and about 65 km south at Altos de Nsork National Park (hereafter ‘Nsork’) in 2014 (65 captures) for a total of eight field seasons. In La Paz, mist nets in the primary forests were over 500 m from the nearest road and at least >1.5 km from the closest settlement; the canopy is closed. The secondary forest site is adjacent to the city; the north and west side are bounded by ~70-m wide paved roads, a ~50-m wide dirt road on the southern portion, and high-tension power lines on the east (30 m wide with vegetation 2-3 m high which is regularly trimmed) effectively isolated the eastern portion (Barrie et al., in review). The canopy of the secondary forest plot remains closed, but it has been commercially logged in the past decades and continues to be regularly selectively logged; it was most recently logged in about 2018. Both forests had similar canopy height (primary: 14.1 m ± 0.5 SE m; secondary 14.1 ± 0.4 SE m). Although primary forests had a higher canopy cover and visibility through the understory when compared to the secondary forests (canopy cover: primary: 88 ± 1.3 SE m; secondary 83 ± 2.2 SE m; visibility: primary: 9.5 ± 0.5 SE m; secondary 7.5 ± 0.4 SE m). At Nsork, primary forest sites were located approximately 800 m into the national park (i.e., north and west) from a road bordering the southern edge of the park. Secondary forest was located <500 m from a main road and had been selectively logged over at least the last three decades. Based on LANDSAT satellite imagery, primary forests at both locations in Nsork were selectively logged as late as the 1980’s but have remained intact since.
We captured birds using mist nets (12 m x 2.5 m); net lanes (Nsork n=6, La Paz n=20) were set up at two sites in each forest type and moved every two days. We opened the nets at about 6:30 AM and closed after six hours. We identified every individual captured to species level when possible, and we recorded standard morphometric measurements (mass [grams], natural wing chord [mm], and tail length [mm]), breeding condition (presence of a brood patch, degree of cloacal protuberance), sex (when possible), and molt status (symmetrical flight feather molt only). We collected a single rectrix (tail feather) from adult birds to be used in fCORT analysis.
We defined the bird’s age following a molt-based aging system (Wolfe-Ryder-Pyle, hereafter ‘WRP’ [Wolfe et al., 2010, Wolfe et al., 2021]). All species captured exhibit a complex basic molt strategy where juveniles undergo a preformative molt into a unique formative (post-juvenile) plumage prior to adopting their adult basic plumage (Howell et al., 2003). Species exhibiting this molt strategy are further divided into two groups: those with a complete preformative molt (where individuals replace all of their juvenile feathers) or a partial-to-incomplete preformative molt (where individuals replace only some of their juvenile feathers). In species that undergo complete preformative molts, the formative and adult basic plumage are virtually indistinguishable. Therefore, we used different age groups for species that undergo complete or partial preformative molts. For species with complete preformative molts, juveniles were defined as individuals that had not undergone their preformative molt (i.e., birds in their juvenile plumage) and the rest were grouped as “adult” birds. For birds with partial preformative molts, we defined first year birds as juvenile birds (juvenile plumage) or adolescent birds (formative plumage) and the rest were defined as adults.
We used data from ten understory insectivorous species, including a subset that are specialized ant-following species, as both groups are particularly vulnerable to anthropogenic disturbance at a Pantropical scale (Peters and Okalo, 2009; Powell et al., 2015). The three specialized ant-followers were Alethe castanea, Chamaetylas poliocephala, and Neocossyphus poensis. Of the African ant-following species, these three have been identified as the specialized ant-followers, depending most heavily on driver ant swarms to forage (Willis, 1986; Peters et al., 2008; Craig, 2022). Furthermore, at our site, these species attended swarms at disproportionally higher rates compare to other regular to occasional ant-following insectivores and displayed behavioral adaptations for tracking and locating ants similar to obligate ant-followers in the Neotropics (Rodrigues, 2024). Understory insectivores included Illadopsis rufipennis, I. fulvescens, I. cleaveri, Hylia prasina, Sheppardia cyornithopsis, Bleda notatus, and B. syndactylus. These species forage for insects and other invertebrates near the forest floor and are similarly vulnerable to anthropogenic disturbance. While these species sometimes also attend ant swarms, they do not specialize on driver ants. We pooled I. rufipennis and I. fulvescens captures as Illadopsis sp. due to their similar ecological niches and challenges in differentiating them in the field.
We measured fCORT concentrations and performed extraction using the protocol described by Bortolotti et al (2008) and ELISA kits (Corticosterone ELISA kit; Neogen Corporation, Ayr, UK), as validated by Carbajal et al. (2014). For A. castanea, B. notatus, and C. poliocephala, we used 20 rectrices (ten from each forest type). For B. syndactylus we used 16 rectrices (six from primary and ten from logged forest forest), and for Illadopsis spp. We used 14 rectrices (eight from primary six in logged forest).
We used generalized linear mixed models (GLMMs) to determine how life history and population traits differed between forest types (R version 4.2.2 and the lme4 package (Bates et al., 2015; R Core Team, 2022). We fit seven models with breeding status (binary), molting stage (binary), breeding-molt overlap (binary), age ratio for species that undergo complete preformative molt (binary), age ratio for species that undergo partial preformative molt (binary), fCORT (numerical) and body condition (numerical) as response variables. The explanatory variables were the individual effects of forest type and species, and their interaction. We included year (i.e., field season) as a random effect. To avoid pseudoreplication, we only included the first capture of each individual per season (individuals are often captured repeatedly in a day or season) leaving us with 792 birds of our focal species in primary forest and 401 in secondary forest. Best-fit models were selected using likelihood ratio tests, starting with the most complex model, followed by subsequent reverse step-wise deletion of non-significant terms. We thought it important to model the age ratio separately depending on the molting strategy as the age groups were different depending on the molt strategy of the species. Models were used to predict (predictMerMod function in the lme4 package, v1.1-26; (Bates et al., 2015) life history and population traits for each species.
We found no effect of forest type on the probability that an individual was breeding (94/792 in primary and 38/401 in secondary) or undergoing molt-breeding overlap (10/325 in primary and 12/190 in secondary) for any of the focal species (LRT vs best-fit: X2 = 8.14, P = 0.52, df = 1192; X2 = 0.44, P = 0.52, df = 514) (Table 1). However, the best fit model showed that forest type had a significant effect on the probability that a bird was molting (337/792 in primary and 178/401 in secondary) (LRT vs best-fit: X2 = 25.69, P < 0.01, df = 1192) (Table 1). Two ant-following species were significantly more likely to be molting in primary forests than in secondary forests: A. castanea and C. poliocephala (Figure 1).
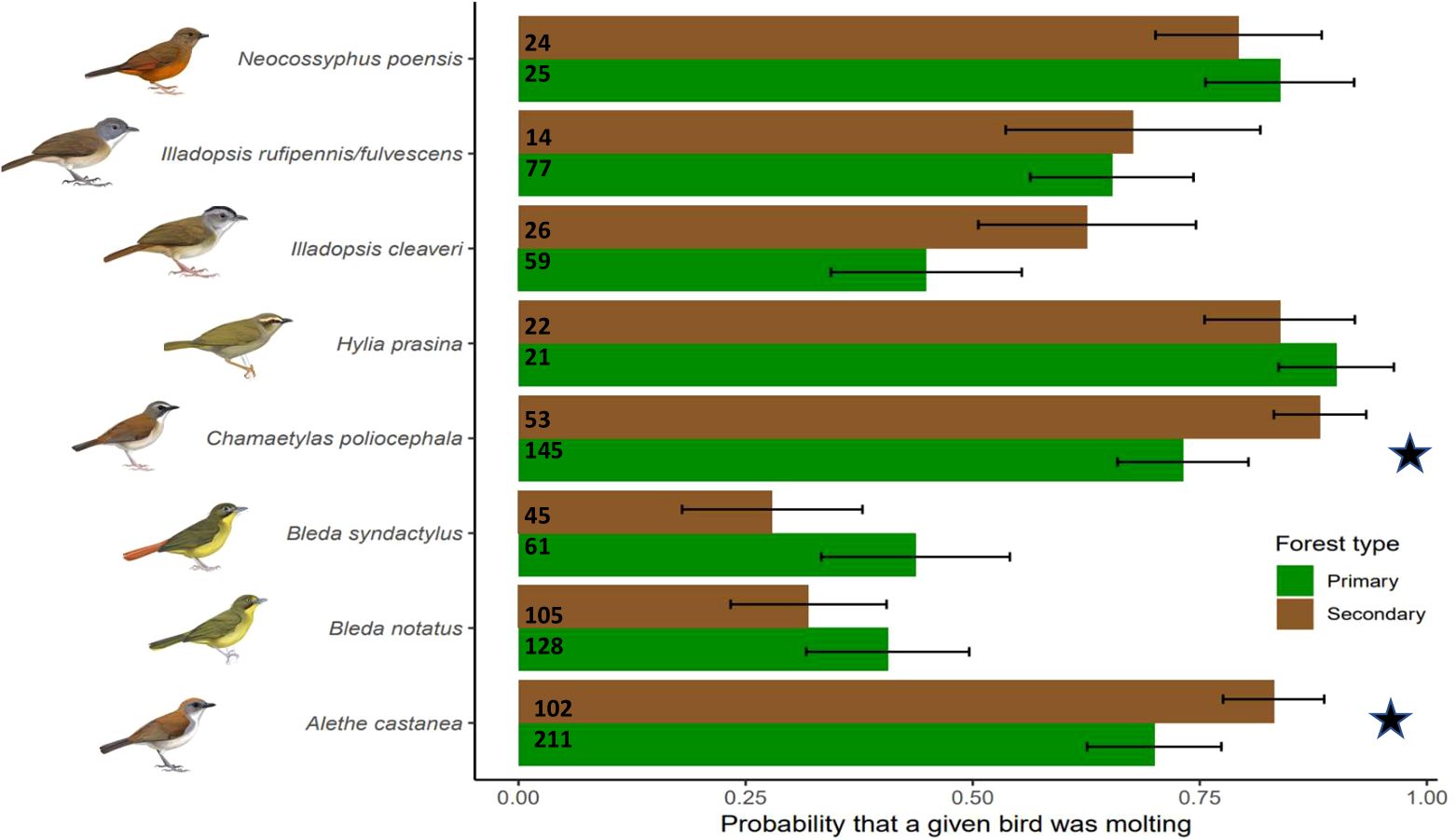
Figure 1. Probability that a given bird was molting in primary (green) and secondary (brown) forest as predicted from the best fit model. Numbers represent the sample size of individual bird captures. Error bars represent 95% confidence intervals. Stars indicate statistical significance at alpha = 0.05. Species illustrations by Faansie Peacock.
For birds that undergo a complete preformative molt, we found no effect of forest type on the probability that a bird was a juvenile bird (22/290 in primary and 17/170 in secondary) (LRT vs best-fit: X2 = 6.26, P = 0.39, df = 459) (Table 1). In contrast, the best fit model for species that undergo a partial preformative molt showed that forest type had a significant effect on the probability of a bird being on its first year (91/369 in primary and 60/139 in secondary) (LRT vs best-fit: X2 = 20.82, P < 0.0001, df = 514) (Table 1). A. castanea, C. poliocephala, N. poensis and S. cyornithopsis had higher probability of a bird being on its first year in secondary forests compared to primary forests (Figure 2).
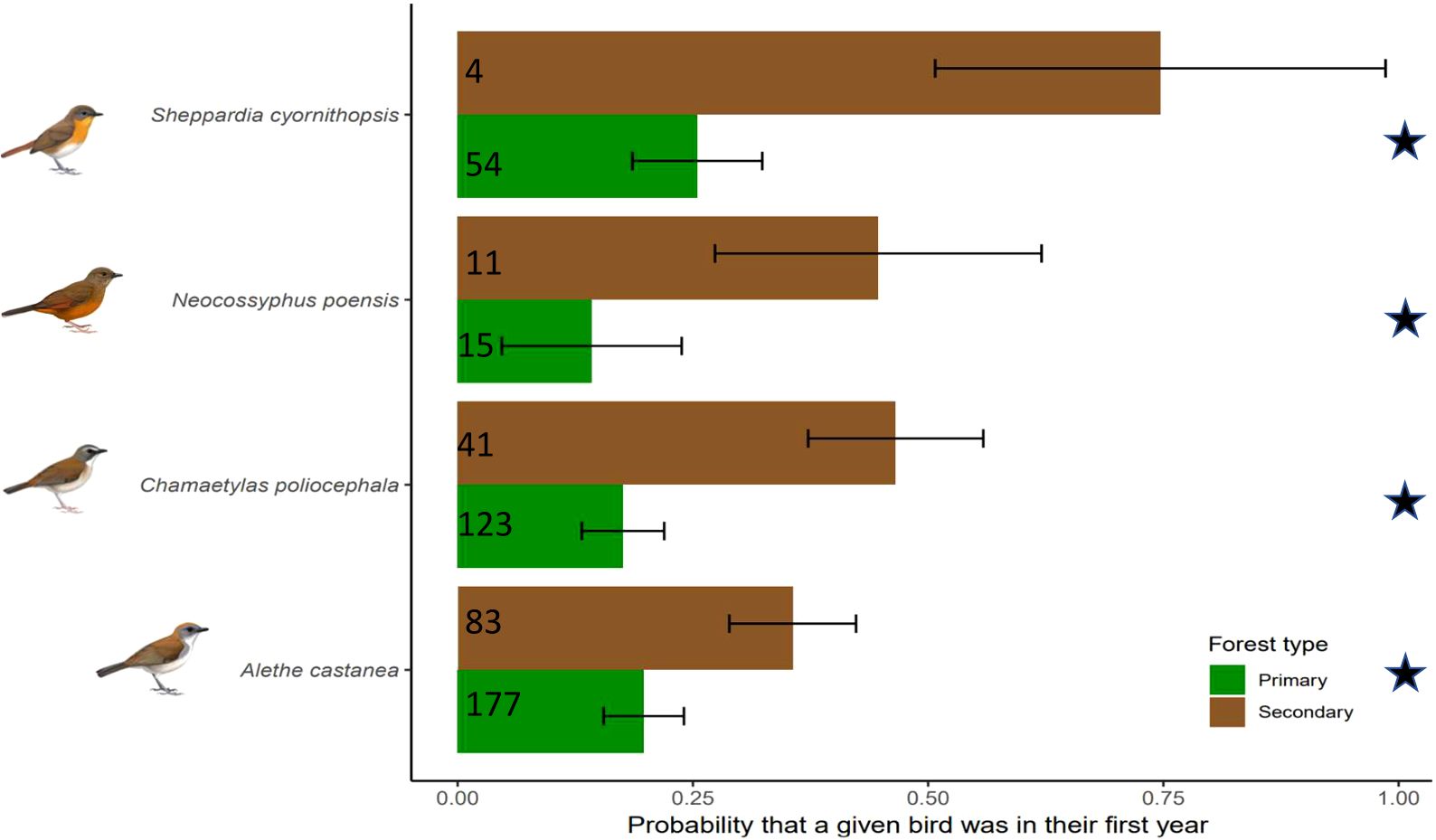
Figure 2. Probability that a given bird was in their first year in primary (green) and secondary (brown) forest as predicted from the best fit model. Numbers represent the sample size of individual bird captures. Error bars represent 95% confidence intervals. Stars indicate statistical significance at alpha = 0.05. Six species are excluded because they have complete pre-formative molts and are thus indistinguishable from adults just weeks after leaving the nest and a different model examined the age ratio. Species illustrations by Faansie Peacock.
The best fit model for body condition showed that forest type had a significant effect (LRT vs best-fit: X2 = 19.84, P < 0.05, df = 1192) (Table 1). The only species that showed a significant difference in body condition was I. cleaveri, which weighed on average 7 g/mm more (6.67%) in secondary forest (Figure 3). We detected no effect of forest type on the amount of fCORT for any species (LRT vs best-fit: X2 = 0.88, P = 0.97, df = 89) (Table 1).
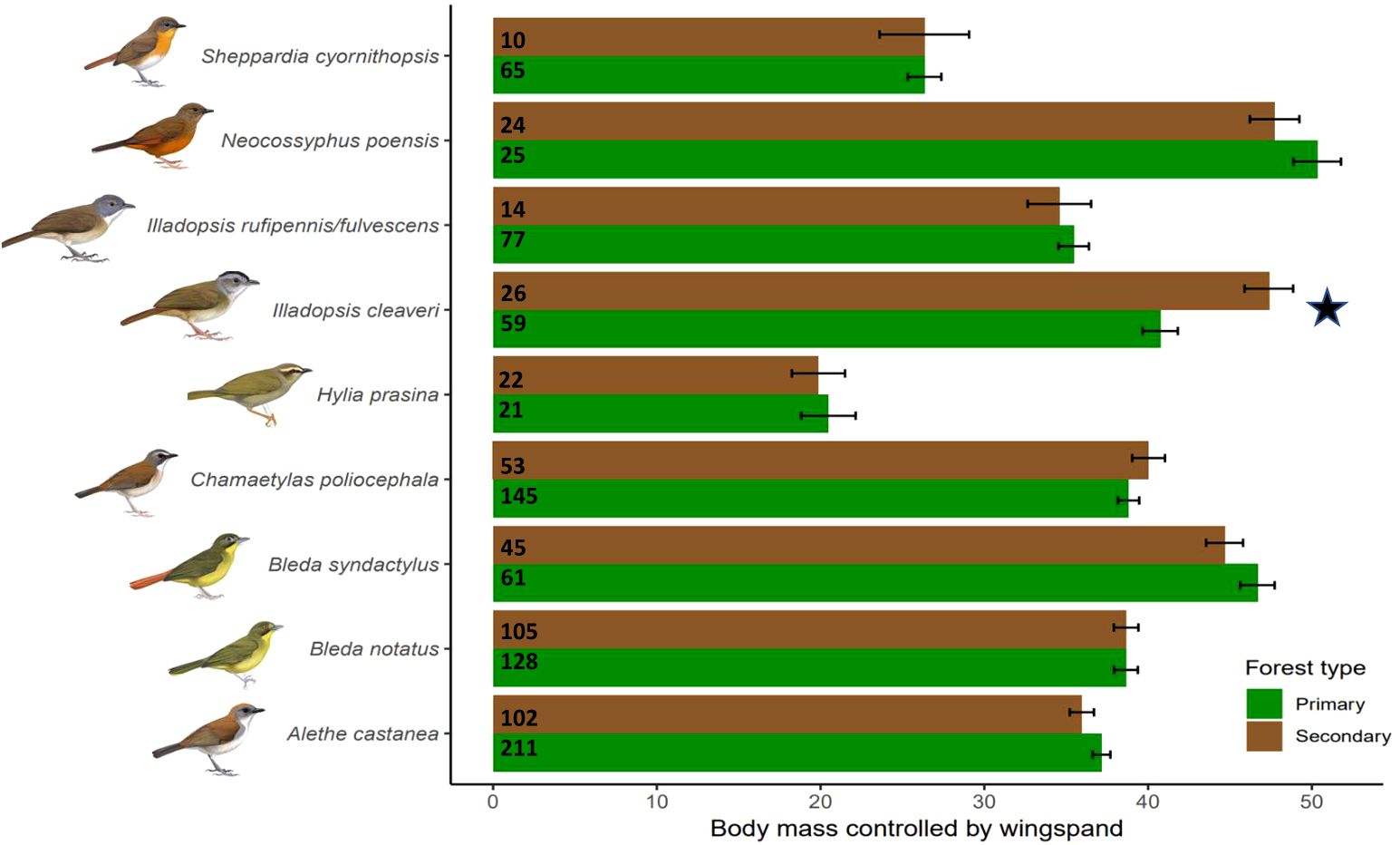
Figure 3. Body condition (body mass corrected by wing length) of birds captured in primary (green) and secondary (brown) forest as predicted from the best fit model. Numbers represent the sample size of individual bird captures. Error bars represent 95% confidence intervals. Stars indicate statistical significance at alpha = 0.05. Species illustrations by Faansie Peacock.
Our study delved into avian responses to selective logging in an Afrotropical lowland rainforest, revealing insights into breeding and molting prevalence, population age ratios, body condition, and stress. Many avian studies rely on point counts or transects to determine some index of abundance or density among habitats, but density can be a misleading index of habitat quality (Van Horne, 1983). By capturing birds and determining their age, mass, molting status, etc., we were able to obtain additional insights on measures that have demographic consequences for bird populations. This work contributes to the broader literature on avian responses to forest disturbance and presents unique findings that enhance our understanding of how birds are affected by anthropogenic disturbance. Noteworthy patterns emerged in age ratio and molting behavior (Table 2).
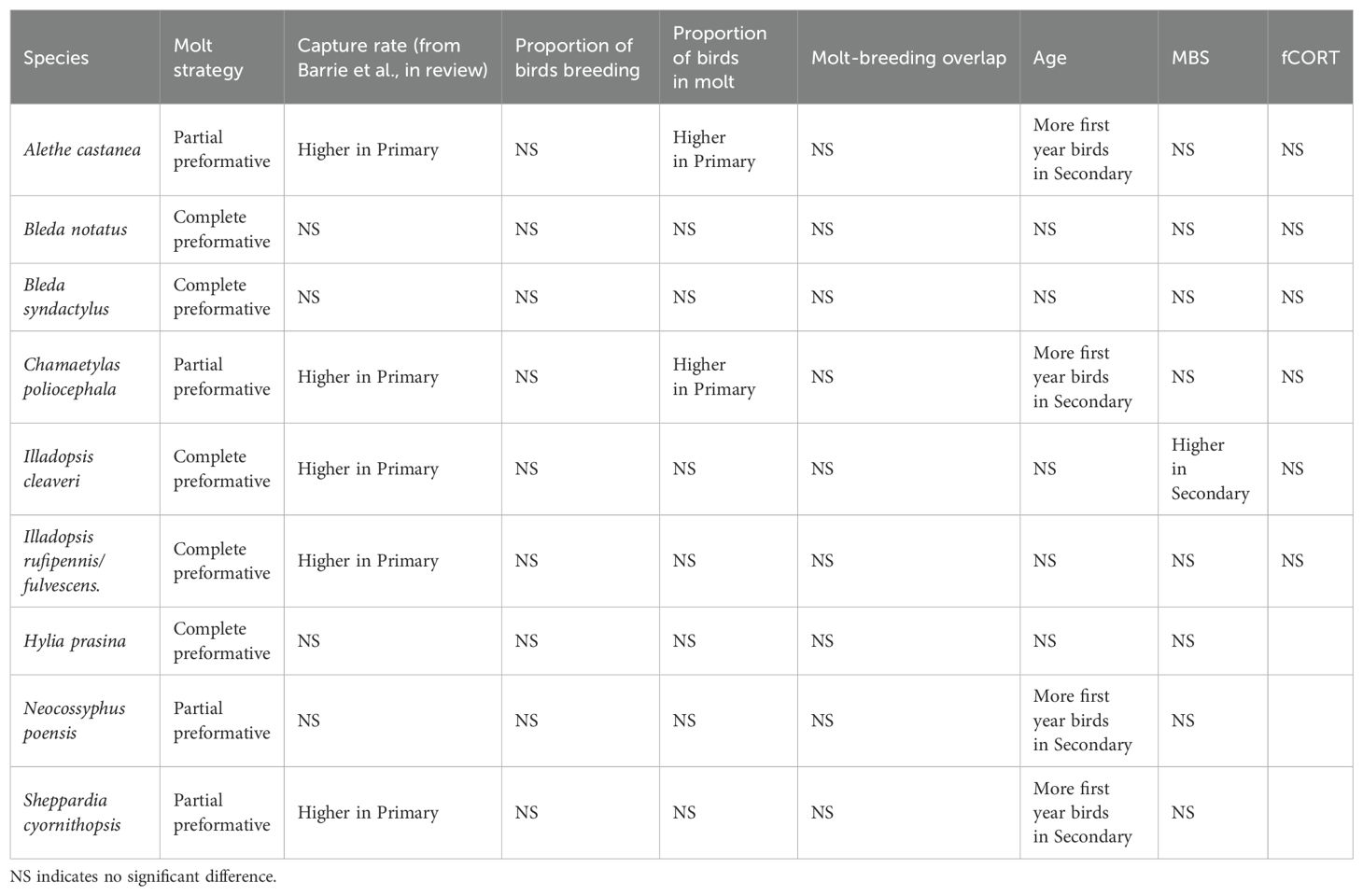
Table 2. Summary of statistically significant differences in life history and population traits between the primary and secondary forest for each bird species.
Breeding and molting are both costly life history traits (Romero, 2002). We expected to find a higher proportion of birds breeding, molting in primary forests as they have access to more food resources, more cover, and lower densities of predators (Sekercioglu, 2002; Cosset et al., 2021). We also expected a higher proportion of birds undergoing molt-breeding overlap in secondary forest. However, we did not identify any differences in the proportion of breeding birds or molt-breeding overlap between the two forest types. This might suggest that forest disturbance does not affect the breeding status of our focal species; in other words, the ability to physiologically mobilize the body into breeding condition per se does not appear to be limiting population growth/maintenance at our study system. This was also the case in tropical rainforests in Malaysia where understory birds in primary and selectively logged forests showed no difference in their breeding status (Yap et al., 2007).
With that said, relatively little is known about the annual cycle of Central African birds. Regional variation may be possible, particularly in species that occupy the entire Congo Basin as well as forests of East Africa and/or West Africa (in the case of the latter species); regional influences on annual life cycles could be present for other more geographically restricted species as climatic influences on seasonality across the Congo Basin and into the Lower Guinea Forest. Though differences may vary subtly, they may appreciably impact the timing and duration of breeding and molting periods. It is possible that our study did not capture the optimum breeding period for these species due to the temporally limited nature of our study. Incorporating data from the cloudy dry season and shoulder seasons where feasible may provide further insights into how the annual life cycle may be influenced by habitat degradation.
Additionally, our study did not address other demographic markers such as hatching or fledging success. With higher densities of predators and fewer resources, it is likely that primary forest specialists relegated to selectively logged forests will have more failed breeding attempts (Thiollay, 1999; Cosset et al., 2021).
In contrast, we found that the proportion of molting birds in two ant-following bird species was higher in primary forests. In Malaysia, species sensitive to forest degradation molted in higher proportions in primary forest compared to secondary forests (Yap et al., 2007). We expected this to be the case, since prior research indicated that species could alter their annual molt in habitats according to food availability (Marini and Durães, 2001). Foster (1975) suggested that suspension of molt could be an environmental adaptation to ensure successful breeding attempts. If secondary forests are indeed more challenging environments for these birds (e.g., fewer Dorylus ants to follow), this may increase the likelihood that a bird would alter their molt cycle to ensure a successful breeding attempt and avoid the consequences of molt-breeding overlap. All of the above could be caused due to reduce raid activity of Dorylus ant activity during the dry season is in secondary forests reducing the foraging opportunities for ant-following birds (Kumar and O’Donnell, 2009). This is likely because secondary forests have a more open canopy and a more unstable microclimate making the birds more vulnerable during the dry season (Cao and Sánchez-Azofeifa, 2017). As a result, these birds would delay their molt cycle to a later season with a higher resource availability, although we are unable to test for that as our data was collected during the dry season. The two ant-following species that had the higher molting probability in primary forests (A. castanea and C. poliocephala) are also captured about twice as often in primary forest at our study site, suggesting that they are sensitive to forest disturbance (Barrie et al., in review).
Among the study species we could age more precisely (due to partial preformative molts creating distinct molt limits), we found a higher proportion of first-year birds in second growth forest. Variation in age ratios across different forest types can be attributed to several mechanisms. First, obligate ant-following birds in the Neotropics hold large, non-exclusive home ranges and although they exhibit low territoriality, they are, however, aggressive and compete for the most profitable regions at swarm fronts (Willson, 2004). Although little is known about their behavior, low territoriality and high competition at swarm fronts have similarly been observed in African ant-followers (Brosset and Erard, 1986). This competition for space at the swarm front intensifies in denser populations (Willson, 2004). Therefore, it is likely that some species may adhere to an ideal despotic distribution where young and potentially dispersing individuals select habitats with fewer antagonistic individuals (Fretwell and Calver, 1969) resulting in young and dispersing birds occurring in second growth forest, habitats characterized by lower densities of ant-following birds (Barrie et al., in review). Regardless of the mechanism, a population with a higher proportion of first year birds is less productive, as first year birds have a lower survival rate and are first-time breeders, which results in lower population growth rate (Pyle et al., 2020).
We did not detect any differences in body condition for all but one species; contrary to our predictions, Illadopsis cleaveri had a higher body condition in secondary forests (Figure 3). We speculate that changes in vegetation structure in the secondary forest may have benefitted I. cleaveri—the smallest-bodied and smallest-billed Illadopisis at our site—perhaps releasing it from competition from the other, larger Illadopsis species that also spend much time on the forest floor looking for arthropods. Indeed, I. fulvescens/rufipennis is nearly absent from the secondary forest; I cleaveri is also quite rare there (Barrie et al., in review) but perhaps is better adapted to the secondary forests’ more cluttered understory. Jones et al. (2022) describe similar results in a study looking at changes in the body condition of 20 understory birds across a gradient of deforestation in tropical forests in Western Andes of Colombia. They found that some species benefited from the changes in the vegetation structure allowing them to increase their body condition, which then had a positive effect on their breeding condition.
We investigated the fCORT levels of six species as an integrated measure of physiological stress aggregated over the period of feather growth (Messina et al., 2020). However, we did not identify any differences in fCORT levels between the forest types for our focal species. Previous work (Bortolotti et al, 2008) has indicated fCORT levels are most closely associated with the frequency or magnitude of a CORT stress response (i.e., acute stress). Therefore, the lack of a difference suggests there is no difference between habitats in physiological stressors, specifically during the period of feather molt. It is possible that differences do exist between habitats at other points in the annual cycle, or in baseline CORT levels, where elevated levels would indicate chronic stress, such as that caused by prolonged food shortage (e.g., Marra and Holberton, 1998). Future work could consider blood CORT levels at multiple timepoints and challenge experiments to both quantify baseline levels and the magnitude of the stress response between habitats, although this approach presents methodological challenges in the field (e.g., Wingfield et al., 1992).
Our study brought to light changes in life history and demographic traits caused by forest degradation in sensitive understory tropical birds. It also highlighted the importance of studies looking beyond the many studies that clump species at the guild level: we demonstrated that only the most sensitive species within guilds displayed changes in their life history and population traits. In the secondary forest, we identified a decrease in the proportion of molting in two ant-following species and detected a higher proportion of first year birds in four species. All of the species that we identified as having alterations in their life history and population traits were also 50% less common in secondary forest (Barrie et al., in review) (Table 2) emphasizing the sensitivity of these species. A comprehensive model of population dynamics could provide further information on the impacts that limit population growth in degraded forests such as juvenile and adult survival rates and nesting success. With this information at hand, we could better explain the patterns that we observed in this study such as the higher proportion of first year birds in secondary forests. Finally, given the keystone nature of Dorylus ants (Peters et al., 2009), and the predicted increase in selective logging across the Afrotropics, it is critical to gain a better understanding of how selective logging and other forms of anthropogentic disturbance impact the behavior and therefore integrity of ant-following bird populations.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was approved by Institutional Animal Care and Use Committee NZP-IACUC Action on Proposal #14-34 at Smithsonian National Zoological Park. The study was conducted in accordance with the local legislation and institutional requirements.
PN: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. BK: Writing – review & editing, Data curation. PR: Writing – review & editing, Data curation. KB: Funding acquisition, Methodology, Project administration, Resources, Writing – review & editing. CT: Investigation, Resources, Writing – review & editing. JC: Writing – review & editing. JW: Data curation, Funding acquisition, Methodology, Project administration, Writing – review & editing. LP: Writing – review & editing, Funding acquisition, Methodology, Project administration, Resources, Supervision. SEM: Data curation, Writing – review & editing. SLM: Writing – review & editing, Data curation.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. LLP was supported Durham University and by a Marie Curie award from the European Union’s Horizon 2020 under grant agreement No. 854248. The publication of this paper was sponsored By University of Glasgow. We also received funding from Stonehill Education, The Polistes Foundation, National Geographic and the U.S. Fish & Wildlife Service.
We would like to thank the government of Equatorial Guinea, Universidad Nacional de Guinea Equatorial, and INDEFOR-AP for allowing us to conduct this study. Special thanks to The Afro American University of Central Africa and UNICON for providing us the facilities necessary to accommodate the personnel and equipment all these years. We would also like to thank Bioko Biodiversity Protection Program for logistical support. Finally, we would like to thank Patricia Guedes, Joris Wiethase, Andrew Weigardt, Laura Torrent, Miguel Angel, Henry Pollock, Phil C Stouffer, George V.N. Powell and the many others that have contributed to the banding efforts of Biodiversity Initiative’s projects. We also thank the Fang people for their permission to access the forest.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Akindele S. O., Onyekwelu J. C. (2011). “Review Silviculture in Secondary Forest,” in Silviculture in the Tropics, eds. Günter S., Weber M., Stimm B., Mosandl R. (Berlin, Heidelberg: Springer), 351–367. doi: 10.1007/978-3-642-19986-8_23
Arcilla N., Holbech L. H., O’Donnell S. (2015). Severe declines of understory birds follow illegal logging in Upper Guinea forests of Ghana, West Africa. Biol. Conserv. 188, 41–49. doi: 10.1016/j.biocon.2015.02.010
Baccini A. G. S. J., Goetz S. J., Walker W. S., Laporte N. T., Sun M., Sulla-Menashe D., et al. (2012). Estimated carbon dioxide emissions from tropical deforestation improved by carbon-density maps. Nat. Climate Change 2, 182–185. doi: 10.1038/nclimate1354
Barlow J., Peres C. A., Henriques L. M. P., Stouffer P. C., Wunderle J. M. (2006). The responses of understorey birds to forest fragmentation, logging and wildfires: an Amazonian synthesis. Biol. Conserv. 128, 182–192. doi: 10.1016/j.biocon.2005.09.028
Bates D., Mächler M., Bolker B., Walker S. (2015). Fitting linear mixed-effects models Using lme4. J. Stat. Software 67, 1–48. doi: 10.18637/jss.v067.i01
Beier P., Drielen M. V., Kankam B. O. (2002). Avifaunal collapse in West African forest fragments. Conserv. Biol. 16, 1097–1111. doi: 10.1046/j.1523-1739.2002.01003.x
BirdLife International (2018). State of Africa's birds 2017: Indicators for our changing environment (Nairobi, Kenya: BirdLife International Africa Partnership).
Blaser J., Poore D., Chandrasekaran C., Hirakuri S., Johnson S., Rubin H., et al. (2006). Status of tropical forest management 2005. Summary report. Int. Forestry Rev. 8, 372–374.
Bortolotti G. R., Marchant T. A., Blas J., German T. (2008). Corticosterone in feathers is a long- term, integrated measure of avian stress physiology. Funct. Ecology. 22, 494–500. doi: 10.1111/j.1365-2435.2008.01387.x
Bregman T. P., Lees A. C., MacGregor H. E. A., Darski B., de Moura N. G., Aleixo A., et al. (2016). Using avian functional traits to assess the impact of land-cover change on ecosystem processes linked to resilience in tropical forests. Proc. R. Soc. B: Biol. Sci. 283 (1844), 20161289. doi: 10.1098/RSPB.2016.1289
Brosset A., Erard C. (1986). Les oiseaux des régions forestières du Nord-Est du Gabon, I. Écologie et comportement des espèces. Rev. d’Écologie (Terre Vie) supplément. 3, 1–297.
Burivalova Z., Lee T. M., Giam X., Sekercioglu Ç.H., Wilcove D. S., Koh L. P. (2015). Avian responses to selective logging shaped by species traits and logging practices. Proc. R. Soc. B: Biol. Sci. 282 (1808), 20150164. doi: 10.1098/RSPB.2015.0164
Cao S., Sánchez-Azofeifa A. (2017). Modeling seasonal surface temperature variations in secondary tropical dry forests. Int. J. Appl. Earth observation geoinformation 62, 122–134. doi: 10.1016/j.jag.2017.06.008
Carbajal A., Tallo-Parra O., Sabes-Alsina M., Mular I., Lopez-Bejar. M. (2014). Feather corticosterone evaluated by ELISA in broilers: A potential tool to evaluate broiler welfare. Poultry science. 93, 2884–2886. doi: 10.3382/ps.2014-04092
Cazzolla R., Castaldi S., Lindsell J. A., Coomes D. A., Marchetti M., Maesano M., et al. (2015). The impact of selective logging and clearcutting on forest structure, tree diversity and above-ground biomass of African tropical forests. Ecol. Res. 30, 119–132. doi: 10.1007/s11284-014-1217-3
Coddington C. P. J., Cooper W. J., Luther D. A. (2023). Effects of forest fragmentation on avian breeding activity. Conserv. Biol. 37 (4), e14063. doi: 10.1111/cobi.14063
Cordeiro N. J., Borghesio L., Joho M. P., Monoski T. J., Mkongewa V. J., Dampf C. J. (2015). Forest fragmentation in an African biodiversity hotspot impacts mixed-species bird flocks. Biol. Conserv. 188, 61–71. doi: 10.1016/j.biocon.2014.09.050
Cosset C. C., Gilroy J. J., Tomassi S., Benedick S., Nelson L., Cannon P. G., et al. (2021). Impacts of tropical selective logging on local-scale movements of understory birds Keywords: Biodiversity Birds Movement Ecology Southeast Asia Sustainable Forest management Production. For. Joint species modelling. Biol. Conserv. 264, 109374. doi: 10.1016/j.biocon.2021.109374
Craig A. J. F. K. (2022). African birds as army ant followers. J. Ornithology 163, 623–631. doi: 10.1007/s10336-022-01987-0
Curtis P. G., Slay C. M., Harris N. L., Tyukavina A., Hansen M. C. (2018). Classifying drivers of global forest loss. Science 361, 1108–1111. doi: 10.1126/science.aau3445
Dale S., Mork K., Solvang R., Plumptre A. J. (2000). Edge effects on the understorey bird community in a logged forest in Uganda. Conserv. Biol. 14, 265–276. doi: 10.1046/j.1523-1739.2000.98340.x
De Heer M., Kapos V., Ten Brink B. J. E. (2005). Biodiversity trends in Europe: development and testing of a species trend indicator for evaluating progress towards the 2010 target. Philos. Trans. R. Soc. B: Biol. Sci. 360, 297–308. doi: 10.1098/rstb.2004.1587
Dranzoa C. (1998). The avifauna 23 years after logging in Kibale National Park, Uganda. Biodiversity Conserv. 7, 777–797. doi: 10.1023/A:1008892419940
Drigo R., Lasserre B., Marchetti M. (2009). Tropical land cover change: patterns, trends and impacts. Plant Biosyst. 143, 311–327. doi: 10.1080/11263500902722618
Echeverry-Galvis M. A., Hau M. (2013). Flight Performance and Feather Quality: Paying the Price of Overlapping Moult and Breeding in a Tropical Highland Bird. PloS One 8, e61106. doi: 10.1371/journal.pone.0061106
Edwards D. P., Larsen T. H., Docherty T. D. S., Ansell F. A., Hsu W. W., Derhé M. A., et al. (2011). Degraded lands worth protecting: The biological importance of Southeast Asia’s repeatedly logged forests. Proc. R. Soc. B: Biol. Sci. 278, 82–90. doi: 10.1098/RSPB.2010.1062
Edwards D. P., Tobias J. A., Sheil D., Meijaard E., Laurance W. F. (2014). Maintaining ecosystem function and services in logged tropical forests. Trends Ecol. Evol. 29, 511–520. doi: 10.1016/J.TREE.2014.07.003
Fefferman N. H., Romero L. M. (2013). Can physiological stress alter population persistence? A model with conservation implications. Conserv. Physiol. 1, cot012. doi: 10.1093/conphys/cot012
Foster M. S. (1975). The overlap of molting and breeding in some tropical birds: cooper ornithological society stable. Condor. 77, 304–314. URL: http://www.jstor.org/stabl/1366226 REFERENCES Linked references are available on JSTOR for this article.
Freed L., Cann R. (2012). Changes in timing, duration, and symmetry of molt of hawaiian forest birds. PloS One 7, 29834. doi: 10.1371/journal.pone.0029834
Fretwell S. D., Calver J. S. (1969). On territorial behavior and other factors influencing habitat distribution in birds ii. Sex ratio variation in the dickcissel. I. Acta Biotheoretica 19, 16–36. doi: 10.1007/BF01601953
Gray M. A., Baldauf S. L., Mayhew P. J., Hill J. K. (2007). The response of avian feeding guilds to tropical forest disturbance. Conserv. Biol. 21, 133–141. doi: 10.1111/j.1523-1739.2006.00557.x
Hamer K. C., Newton R. J., Edwards F. A., Benedick S., Bottrell S. H., Edwards D. P. (2015). Impacts of selective logging on insectivorous birds in Borneo: the importance of trophic position, body size and foraging height. Biol. Conserv. 188, 82–88. doi: 10.1016/j.biocon.2014.09.026
Hannon S. J., Martin K. (2006). Ecology of juvenile grouse during the transition to adulthood. J. Zoology. 269, 422–433. doi: 10.1111/j.1469-7998.2006.00159.x
Hansen M. C., Stehman S. V., Potapov P. V., Loveland T. R., Townshend J. R., DeFries R. S., et al. (2008). Humid tropical forest clearing from 2000 to 2005 quantified by using multitemporal and multiresolution remotely sensed data. Proc. Natl. Acad. Sci. 105, 9439–9444. doi: 10.1073/pnas.0804042105
Howell S. N. G., Corben C., Pyle P., Rogers D. I. (2003). The first basic problem: A review of molt and plumage homologies. Condor 105, 635–653. doi: 10.1650/7225
Jakob E. M., Marshall S. D., Uetz G. W. (1996). Estimating fitness: a comparison of body condition indices. Oikos 77, 61–67. doi: 10.2307/3545585
Jarrett C., Smith T. B., Claire T. T. R., Ferreira D. F., Tchoumbou M., Elikwo M. N. F., et al. (2021). Bird communities in African cocoa agroforestry are diverse but lack specialized insectivores. J. Appl. Ecol. 58, 1237–1247. doi: 10.1111/1365-2664.13864
Jones H. H., Gabriel Colorado Z., Robinson S. K. (2022). Widespread bird species show idiosyncratic responses in residual body mass to selective logging and edge effects in the Colombian Western Andes. Ornithological Appl. 124, 1–18. doi: 10.1093/ornithapp/duac026
Kofron C. P., Chapman A. (1995). Deforestation and bird species composition in Liberia, West Africa. Trop. Zoology 8, 239–256. doi: 10.1080/03946975.1995.10539284
Kumar A., O’Donnell S. (2009). Elevation and forest clearing effects on foraging differ between surface–and subterranean–foraging army ants (Formicidae: Ecitoninae). J. Anim. Ecol. 78 1, 91–97. doi: 10.1111/j.1365-2656.2008.01483.x
Laporte N. T., Stabach J. A., Grosch R., Lin T. S., Goetz S. J. (2007). Expansion of industrial logging in central africa. Science 316, 1451–1451. doi: 10.1126/science.1141057
Malhi Y., Adu-Bredu S., Asare R. A., Lewis S. L., Mayaux P. (2013). African rainforests: past, present and future. Phil. Trans. R. Soc B 368, 20120312. doi: 10.1098/rstb.2012.0312
Marini M.Â., Durães R. (2001). Annual patterns of molt and reproductive activity of passerines in south-central Brazil. Condor 103, 767–775. doi: 10.1650/0010-5422(2001)103[0767:APOMAR]2.0.CO;2
Marra P. P., Holberton R. L. (1998). Corticosterone levels as indicators of habitat quality: effects of habitat segregation in a migratory bird during the non-breeding season. Oecologia 116, 284–292. doi: 10.1007/s004420050590
Mayaux P., Pekel J.-F., Desclée B., Donnay F., Lupi A., Achard F., et al. (2013). State and evolution of the African rainforests between 1990 and 2010. Philos. Trans. R. Soc. B: Biol. Sci. 368, 20120300. doi: 10.1098/rstb.2012.0300
Maynard K., Royer J. F. (2004). Effects of “realistic” land-cover change on a greenhouse-warmed African climate. Climate Dynamics 22, 343–358. doi: 10.1007/s00382-003-0371-z
Messina S., Costantini D., Tomassi S., Cosset C. C. P., Benedick S., Eens M., et al. (2021). Selective logging reduces body size in omnivorous and frugivorous tropical forest birds. Biol. Conserv. 256, 109036. doi: 10.1016/j.biocon.2021.109036
Messina S., Edwards D. P., Marasco V., Canoine V., Cosset C. C. P., Tomassi S., et al. (2020). Glucocorticoids link forest type to local abundance in tropical birds. Funct. Ecol. 34, 1814–1825. doi: 10.1111/1365-2435.13586
Mestre L. A. M., Cosset C. C. P., Nienow S. S., Krul R., Rechetelo J., Festti L., et al. (2020). Impacts of selective logging on avian phylogenetic and functional diversity in the Amazon. Anim. Conserv. 23, 725–740. doi: 10.1111/ACV.12592
Newmark W. D. (2006). A 16-year study of forest disturbance and understory bird community structure and composition in Tanzania. Conserv. Biol. 20, 122–134. doi: 10.1111/j.1523-1739.2005.00273.x
Peters M. K., Fischer G., Schaab G., Kraemer M. (2009). Species compensation maintains abundance and raid rates of African swarm-raiding army ants in rainforest fragments. Biol. Conserv. 142, 668–675. doi: 10.1016/j.biocon.2008.11.021
Peters M. K., Likare S., Kraemer M. (2008). Effects of habitat fragmentation and degradation on flocks of African ant-following birds. Ecol. Appl. 18, 847–858. doi: 10.1890/07-1295.1
Peters M. K., Okalo B. (2009). Severe declines of ant-following birds in African rainforest fragments are facilitated by a subtle change in army ant communities. Biol. Conserv. 142, 2050–2058. doi: 10.1016/J.BIOCON.2009.03.035
Powell L. L., Cordeiro N. J., Stratford J. A. (2015). Ecology and conservation of avian insectivores of the rainforest understory: A pantropical perspective. Biol. Conserv. 188, 1–10. doi: 10.1016/j.biocon.2015.03.025
Puettmann K. J., Wilson S. M., Baker S. C., Donoso P. J., Drössler L., Amente G., et al. (2015). Silvicultural alternatives to conventional even-aged forest management-what limits global adoption. For. Ecosyst. 2, 1–16. doi: 10.1186/s40663-015-0031-x
Pyle P., Foster K. R., Godwin C. M., Kaschube D. R., Saracco J. F. (2020). Yearling proportion correlates with habitat structure in a boreal forest landbird community. PeerJ 8, e8898. doi: 10.7717/peerj.8898
R Core Team (2022). “R: A language and environment for statistical computing,” in R foundation for statistical computing(Vienna, Austria). Available at: https://www.R-project.org/.
Rodrigues P. (2024). The behavioral specialization of African ant-following birds on Dorylus driver ants (Baton Rouge (LA: Louisiana State University).
Romero L. M. (2002). Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. endocrinology. 128, 1–24. doi: 10.1016/S0016-6480(02)00064-3
Ross S. R. P. J., Garcia F. H., Fischer G., Peters M. K. (2018). Selective logging intensity in an East African rain forest predicts reductions in ant diversity. Biotropica 50, 768–778. doi: 10.1111/btp.12569
Rudel T. K. (2013). The national determinants of deforestation in sub-Saharan Africa. Philos. Trans. R. Soc. B: Biol. Sci. 368, 20120405. doi: 10.1098/rstb.2012.0405
Ruiz-Gutierrez V., Gavin T., Dhondt A. (2008). Habitat fragmentation lowers survival of a tropical forest bird. Ecol. applications: Publ. Ecol. Soc. America 184, 838–846. doi: 10.1890/07-1090.1
Saatchi S. S., Harris N. L., Brown S., Lefsky M., Mitchard E. T., Salas W., et al. (2011). Benchmark map of forest carbon stocks in tropical regions across three continents. Proc. Natl. Acad. Sci. 108, 9899–9904. doi: 10.1073/pnas.1019576108
Salvantes K. G., Williams T. D. (2003). Effects of corticosterone on the proportion of breeding females, reproductive output and yolk precursor levels. Gen. Comp. Endocrinology. 130, 205–214. doi: 10.1016/S0016-6480(02)00637-8
Sekercioglu C. H. (2002). Effects of forestry practices on vegetation structure and bird community of Kibale National Park, Uganda. Biol. Conserv. 107, 229–240. doi: 10.1016/S0006-3207(02)00097-6
Sekercioglu Ç. H., Wenny D. G., Whelan C. J., Floyd C. (2016). "Bird ecosystem services promote biodiversity and support human well being," in Why birds matter: Avian ecological function and ecosystem services, (University of Chicago Press) 339–362. doi: 10.7208/chicago/9780226382777.001.0001
Snow D. W., Lill A. (1974). Longevity records for some neotropical land birds. Condor 76, 262–267. doi: 10.2307/1366339
Sodhi N. S., Koh L. P., Clements R., Wanger T. C., Hill J. K., Hamer K. C., et al. (2010). Conserving Southeast Asian Forest biodiversity in human-modified landscapes. Biol. Conserv. 143, 2375–2384. doi: 10.1016/j.biocon.2009.12.029
Sodhi N. S., Posa M. R. C., Lee T. M., Warkentin I. G. (2008). Effects of disturbance or loss of tropical rainforest on birds. Auk 125, 511–519. doi: 10.1525/auk.2008.1708
Stevenson R. D., Woods W. A. Jr. (2006). Condition indices for conservation: new uses for evolving tools. Integr. Comp. Biol. 46, 1169–1190. doi: 10.1093/icb/icl052
Stutchbury B. J. M., Morton E. S. (2001). Behavioral ecology of tropical birds (San Diego: Academic Press).
Tchoumbou M. A., Malange E. F. N., Tiku C. T., Tibab B., Fru-Cho J., Tchuinkam T., et al. (2020). Response of understory bird feeding groups to deforestation gradient in a tropical rainforest of Cameroon. T. ropical Conserv. Sci. 13, 1940082920906970. doi: 10.1177/1940082920906970
Thiollay J. M. (1999). Frequency of mixed species flocking in tropical forest birds and correlates of predation risk: an intertropical comparison. J. Avian Biol. 30, 282–294. doi: 10.2307/3677354
Tyukavina A., Hansen M. C., Potapov P., Parker D., Okpa C., Stehman S. V., et al. (2018). Congo Basin forest loss dominated by increasing smallholder clearing. Sci. Adv. 4, eaat2993. doi: 10.1126/sciadv.aat2993
Van Horne B. (1983). Density as a misleading indicator of habitat quality. J. Wildlife Manage. 47, 893–901. doi: 10.2307/3808148
Waltert M., Bobo K. S., Sainge N. M., Fermon H., Mühlenberg M. (2005). From forest to farmland: habitat effects on afrotropical forest bird diversity. Ecol. Appl. 15, 1351–1366. doi: 10.1890/04-1002
Watson J. E. M., Chapman S., Althor G., Kearney S., Watson J. E. M. (2017). Changing trends and persisting biases in three decades of conservation science. Global Ecol. Conserv. 10, 32–42. doi: 10.1016/j.gecco.2017.01.008
Watson J. E. M., Whittaker R. J., Dawson T.P. (2004). Habitat structure and proximity to forest edge affect the abundance and distribution of forest-dependent birds in tropical coastal forests of southeastern Madagascar. Biol. Conserv. 120, 311–327. doi: 10.1016/j.biocon.2004.03.004
Webster W. D., Handley C. O. Jr (1986). Systematics of Miller’s Long-Tongued bat, Glossophaga longirostris with description of two new subspecies. Occ Pap Mus Texas Tech U. 100, 1–12. doi: 10.5962/bhl.title.142870
Wikelski M., Hau M., Wingfield J. C. (2000). Seasonality of reproduction in a neotropical rain forest bird. Ecology 81, 2458–2472. doi: 10.1890/0012-9658(2000)081[2458:SORIAN]2.0.CO;2
Williams J., Miller R., Harper J., Wiersma P. (2010). Functional linkages for the pace of life, life-history, and environment in birds. Integr. Comp. Biol. 505, 855–868. doi: 10.1093/icb/icq024
Willson S. K. (2004). Obligate army-ant-following birds: a study of ecology, spatial movement patterns, and behavior in Amazonian Peru. Ornithological Monogr. 55, 1–67. doi: 10.2307/40166802
Wingfield J. C., Vleck C. M., Moore M. C. (1992). Seasonal changes of the adrenocortical response to stress in birds of the Sonoran Desert. J. Exp. Zoology 264, 419–428. doi: 10.1002/jez.1402640407
Wolfe J. D., Ryder T. B., Pyle P. (2010). Using molt cycles to categorize the age of tropical birds: An integrative new system. J. Field Ornithology 81, 186–194. doi: 10.1111/j.1557-9263.2010.00276.x
Wolfe J. D., Terrill R. S., Johnson E. I., Powell L. L., Brandt Ryder T. (2021). Ecological and evolutionary significance of molt in lowland Neotropical landbirds. Auk 138, .ukaa073. doi: 10.1093/ornithology/ukaa073
Keywords: Afrotropics, disturbance, first year birds, ant-following birds, understory birds
Citation: Nikolaou P, Krochuk BA, Rodrigues PF, Brzeski KE, Mufumu SL, Malanza SE, Tonra CM, Cooper JC, Wolfe JD and Powell LL (2025) Insights on avian life history and physiological traits in Central Africa: ant-following species have young-dominated age ratios in secondary forest. Front. Conserv. Sci. 6:1504320. doi: 10.3389/fcosc.2025.1504320
Received: 30 September 2024; Accepted: 05 February 2025;
Published: 05 March 2025.
Edited by:
Monte Neate-Clegg, University of California, Los Angeles, United StatesReviewed by:
Kyle Kittelberger, The University of Utah, United StatesCopyright © 2025 Nikolaou, Krochuk, Rodrigues, Brzeski, Mufumu, Malanza, Tonra, Cooper, Wolfe and Powell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Panagiotis Nikolaou, YWVyb3BhbmFnaW90aXNAaG90bWFpbC5jb20=
†Present address: Jacob C. Cooper, Department of Biology, University of Nebraska at Kearney, Kearney, NE, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.