- 1Chester Zoo, North of England Zoological Society, Chester, United Kingdom
- 2School of Environmental and Natural Sciences, Bangor University, Bangor, United Kingdom
- 3Centre for Ecology and Conservation, Biosciences, College of Life and Environmental Sciences, University of Exeter, Penryn, United Kingdom
The modern zoo relies on the persistence of genetically and physiologically healthy populations of endangered species, which is enabled through breeding programmes globally and regionally. Many species commonly held in zoos are poorly studied in the wild, leading to a lack of in-depth knowledge surrounding breeding behaviours and subsequent parental behaviours and early life development of young. Knowledge of this information is critical to make informed management decisions which promote successful rearing of young in zoos. While the critically endangered Sumatran tiger is popular in zoos, rates of cub survival in the first 5 months are lower than 50%, highlighting the need for scientific evidence driving management decisions. This study monitors nursing and cub grooming behaviours in a first-time mother Sumatran tiger (Panthera tigris sumatrae) and her cubs throughout four stages of cub development. The social proximity of cubs and dam were recorded to describe social interactions within the group throughout cub aging. A series of Friedman tests and post-hoc tests found significant decreases in both cub grooming (χ2 (3, N = 96) = 14.20, p < 0.01) and nursing (χ2 (3, N = 96) = 25.77, p < 0.001) behaviours between the birth and weaning of the cubs, as well as within different phases of cub development between those times. Cub-to-cub proximity was maintained from birth to weaning, with cubs spending significant amounts of time in close proximity (within one adult body length) of each other (χ2 (3, N = 96) = 15.231, p = 0.001) throughout the study. The dam was found to spend significantly less time with the cubs as they reached weaning age (χ2 (3, N = 96) = 27.88, p < 0.001). These results are thought to be the first of their kind to detail timings of cub development and early life socialisation, providing evidence for timing of first food provision to young and promote the provision of space for the dam to spend time away from the cubs, while allowing the cubs to become confident, mobile, and independent.
1 Introduction
The global decline of biodiversity has transformed the role of zoos, shifting the focus from the collecting and displaying of exotic species, to a focus on education, conservation, engagement, and research (Greenwell et al., 2023). The implementation of captive breeding programmes supports these goals by aiming to maintain genetic diversity and demographic stability in ex-situ populations (Greenwell et al., 2023; Kurniawan et al., 2021; Semiadi and Nugraha, 2006), by making breeding recommendations between specific compatible individuals (Martin-Wintle et al., 2017; Saunders et al., 2014). These programmes are particularly valuable for endangered species, such as Panthera sp. where maintaining healthy populations is critical.
Tigers (Panthera tigris) are a popular species in zoos (Carr, 2016; Powell et al., 2024) representing their wild counterparts which are threatened by habitat loss (Imron et al., 2011; Luskin et al., 2017; McKay et al., 2018), poaching (McKay et al., 2018), and a reduction in prey and dense vegetation (Patana et al., 2021; Pusparini et al., 2018). While there are some wider discussions surrounding the taxonomy of tiger subspecies (Stubbington et al., 2023), the CATnews Special Issue (Kitchener et al., 2017) currently describes 8 extant subspecies of Panthera tigris. Indonesia was once home to three subspecies, two of which have been declared extinct (the Javanese tiger, P. t. sondaica and the Bali tiger, P. t. balica; Kurniawan et al., 2021). Sumatran tigers are the only remaining extant species in this area, endemic to the forests of the island of Sumatra (Patana et al., 2021), where they are difficult to survey due to low numbers. A meta-regression of existing capture-recapture studies of Sumatran tigers has found that fragmented habitats have led to an increase in population density, but a reduction in overall population numbers since 1996 (Luskin et al., 2017). Luskin et al. (2017) estimated there to be two existing robust populations, each with fewer than 30 breeding females and fewer than 400 adults (Stubbington et al., 2023), and as such, the species has been classified as Critically Endangered by the International Union for the Conservation of Nature (IUCN; Luskin et al., 2017; Semiadi and Nugraha, 2006). While numbers of Sumatran tigers are declining in the wild, they are commonly found in zoos (Saunders et al., 2014), with 295 individuals currently recorded globally (Species360 Zoological Information Management System, 2024).
The breeding programme for Sumatran tigers in zoos across the world is supported by the Global Species Management Plan (GSMP) developed for this species (Wilting et al., 2015), which allows zoos to cooperate with each other to maintain healthy ex-situ populations. Successful reproduction is vital to the progress of breeding programmes (Stubbington et al., 2023), and understanding the factors which influence this is critical (Holland et al., 2023; Saunders et al., 2014). A review of the Tiger International Studbook in 2006 found that captive cub mortality at less than 5 months reached 59% (Semiadi and Nugraha, 2006), while a more recent figure in the Sumatran Tiger European Endangered species breeding Programme (EEP) Long-term Monitoring Plan reports juvenile mortality to be 42% in the first few weeks of life (Stubbington et al., 2023). This highlights a need for scientific, evidence-based decisions to be made to effectively manage populations of this species (Holland et al., 2023; Saunders et al., 2014). Saunders et al. (2014) found that certain demographic and population management factors such as age, female breeding experience and whether the male and female were previously housed at the same institution, may influence breeding success (Stubbington et al., 2023). However, little information describing the behaviours exhibited by Sumatran tigers during parturition and subsequent raising of cubs in zoos currently exists (Pastorino et al., 2022).
Building upon this knowledge, increasing rates of successful cub weaning will help to maintain healthy global zoo populations of Sumatran tigers, providing stable insurance populations (Saunders et al., 2014). Tigers are one of the most charismatic endangered species, receiving significant conservation attention (Armstrong et al., 2021; Bullock et al., 2021; Carr, 2016; Wilting et al., 2015), and act as flagships to provide opportunities for education, public awareness and fundraising for wider conservation issues (Christie, 2010); by attracting visitors to zoos, they allow the funding of other conservation endeavours. As part of a wider project, we recorded a range of behaviours to document the dam’s activity budget following the birth of her cubs continuously over 24-hour periods. In this study, we report on changes in two key maternal behaviours (grooming of cubs and nursing cubs), as well as the changes in proximity of cubs to each other and the dam from birth through to weaning age; critical behaviours which are vital for cub survival (Holland et al., 2023; Yachmennikova et al., 2018). Although not the main aim of the paper, we also report on the presence of a male during birth, which has rarely been described in the literature to date (Holland et al., 2023). Typical husbandry for this species includes housing males and females separately, except for breeding purposes (Galardi et al., 2021; Pastorino et al., 2022), however, there are increasing reports of collections keeping a male and female mixed throughout their lives (Holland et al., 2023), with suggestions that male Sumatran tigers may join females when mating and when females rear their cubs (Holland et al., 2023; Pastorino et al., 2022). We predict that as time passes, the dam will spend less time engaging in cub-directed behaviours (grooming, nursing), and the cubs will spend more time apart from the dam and each other as they become increasingly independent.
2 Methods
2.1 Study population
A breeding recommendation for Kasarna, an eight-year-old female Sumatran tiger born at Chester Zoo, led to the arrival of Dash, a three-year-old male Sumatran tiger, in July 2022. Both individuals were included in this study, along with the cubs born on 7th January 2023. Three cubs were born, but one did not survive beyond the first development phase. The study began on the day of the birth of the cubs. The group were housed in a custom-built habitat with four dens (104 m2 total) and two outdoor areas (1800 and 210 m2 respectively) which enables individuals to be physically separated from each other for husbandry purposes as required. The cubs and the dam had access to three dens and were given access to the smaller off-show paddock when the cubs became independently mobile and started following the adult female around the study area. The temperature in the dens was maintained at a minimum of 18°C, with the lights switched on between 07:30 and 17:00. The keepers minimised access to the dens throughout the study period, with a black curtain installed between the keeper corridor and the dens to reduce visual contact between keepers and the animals. When accessing the dens to provide feed, keepers checked a remotely accessible live CCTV feed to ensure the group was calm before entering the house. Using slides to place food in the dens with as little disturbance as possible, keepers would then opportunistically spot clean the enclosure, before leaving and monitoring the group’s reaction on the CCTV. Visual contact between keepers and cubs was initiated by the cubs when they gained mobility and started exploring the dens when keepers were present.
Prior to the birth, the dam’s diet was increased by 30% (by mass) and this was maintained throughout the study period. The cubs were provided with their own food after they started showing interest in the dam’s food by sniffing and picking it up. Food was provided in the dens as part of the regular husbandry routine, by being placed on the floor for the individuals to find. They were provided with a rabbit alongside the dam’s feed, which allowed them to explore and practice feeding on solid food. The dam and the cubs were weighed opportunistically every other month for the first few months of the cubs’ lives, and keepers used body scoring to monitor their condition. This allowed keepers to ensure the cubs were increasing in weight as expected, and that the dam’s weight did not decrease excessively. At both 8 weeks and 11 weeks, the cubs were microchipped, vaccinated, and weighed by the veterinary team.
The sire was present for 100 minutes during and following the birth of the cubs but was subsequently separated from the group to provide the dam and the cubs with time to bond and was reintroduced 48 hours post-birth for 90 minutes, giving him access to outdoor areas, as recommended in the long-term management plan for the species (Stubbington et al., 2023).
2.2 Data collection
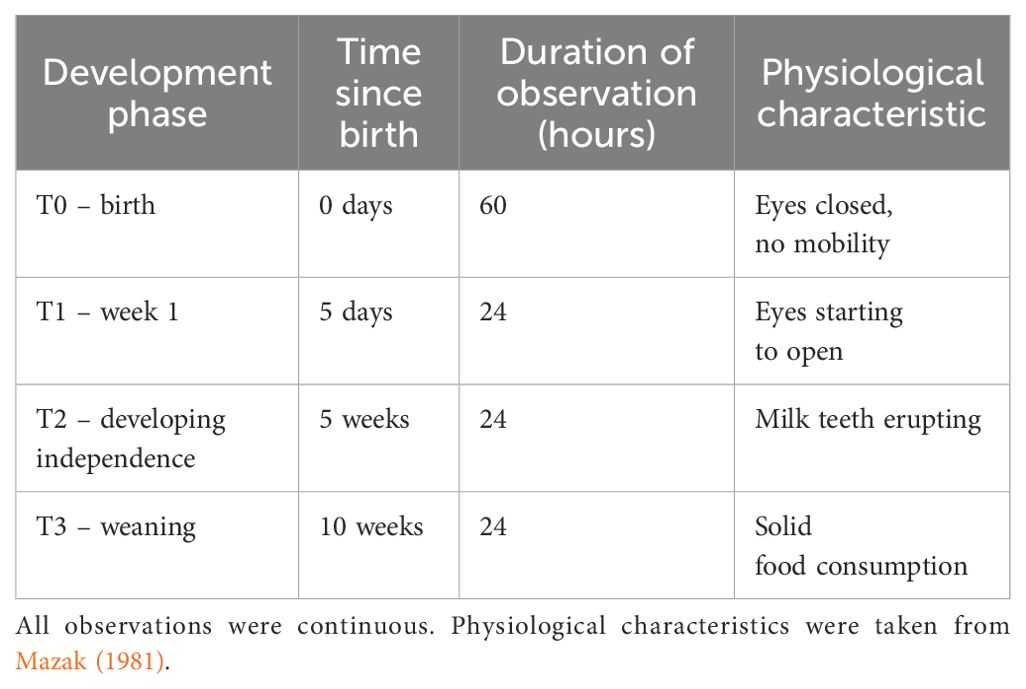
We observed the newborns and their parents via a remotely accessible CCTV cameras (Axis IP Camera, North West Security Group) which recorded the footage and automatically uploaded and stored it onto a server from which the observers could access remotely for up to one month after capture (Milestone XProtect video management software). CCTV cameras were fitted during the construction of the habitat, with the aim of covering as much of the areas available to the animals as possible (Figure 1). Only the outdoor areas and the inside of the tunnels linking rooms did not have CCTV coverage. Footage was reviewed from the moment of the cubs' birth to their weaning age at 10 weeks using continuous focal animal observations for defined periods within each development phase (see Table 1), totalling 132 hours. The location and social proximity of all individuals was continuously recorded during the observation periods, along with the behaviours of the sire and dam when visible in the study area, following the ethogram described in Table 2. Cub behaviours were not recorded as it was not possible to individually identify them.
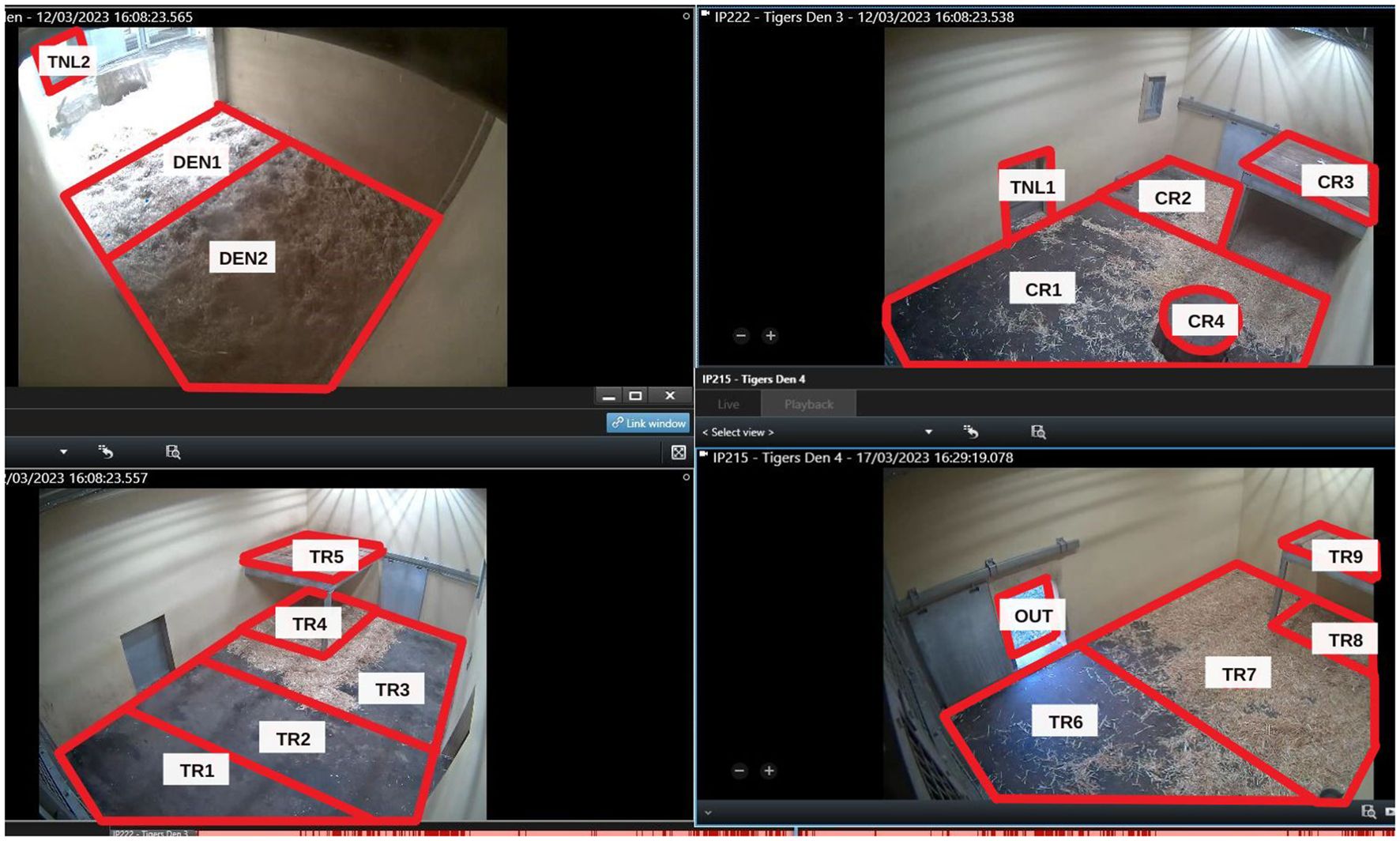
Figure 1. The study area. The red lines represent the delimitations of the areas as defined in BORIS.
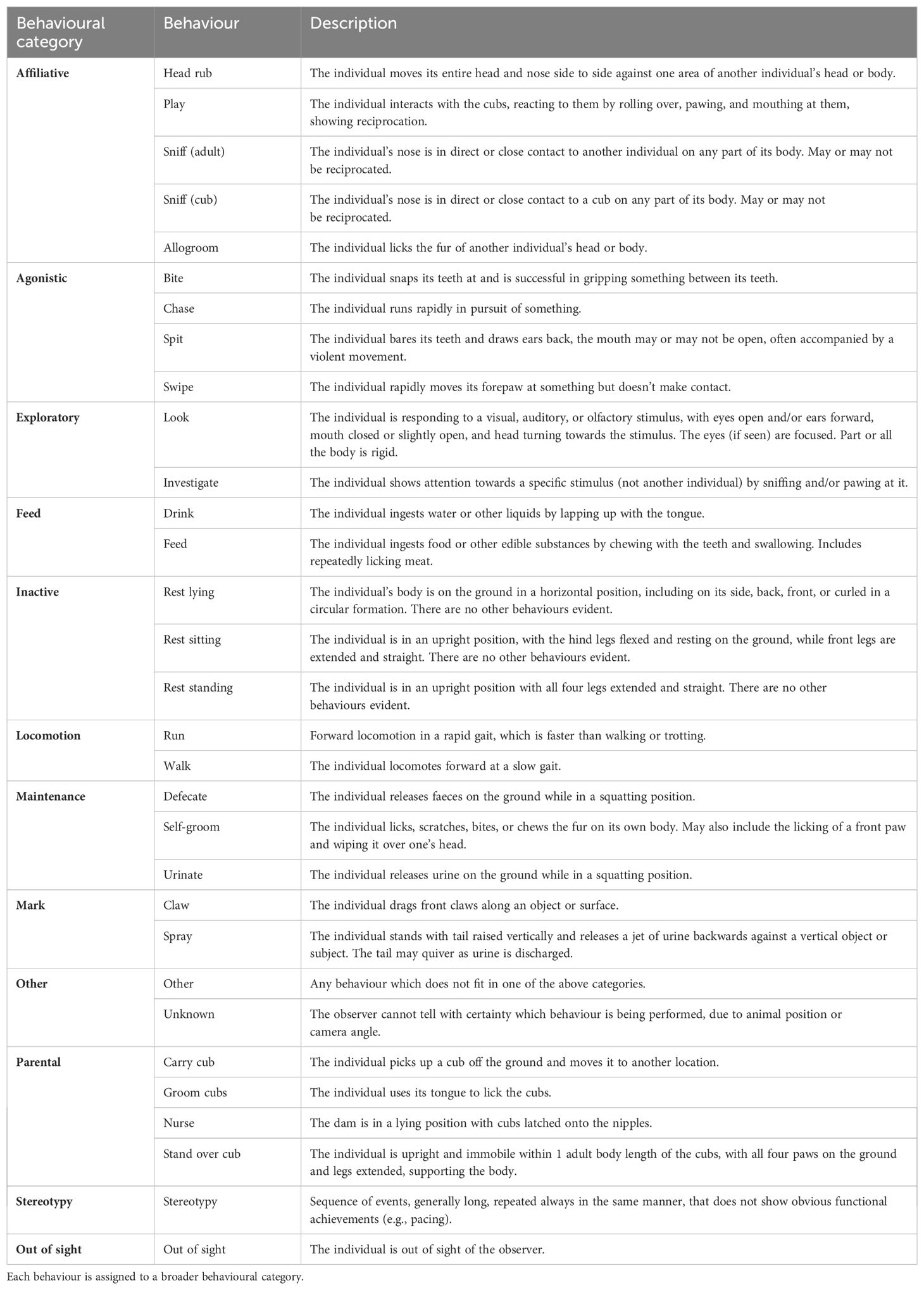
Table 2. Ethogram adapted from Stanton et al. (2015) and developed to describe maternal and social behaviours in adult Sumatran tigers (P. t. sumatrae) following the birth of cubs.
Cameras were fitted with infrared lighting for night-time visibility enabling 24-hour sampling. The study period was divided into four distinct phases, as described in Table 1 below. This method resulted in 132 hours of footage spread over a period of 70 days.
Data were collected using BORIS (Behavioural Observation Research Interactive Software), a free and open-source software which allows the user to code behaviours from pre-recorded video clips (Friard and Gamba, 2016). It has previously been used to record the behaviours of Sumatran tigers in Ragunan Zoological Park, Jakarta (Kusmarani et al., 2019). This software increases the accuracy and efficiency of data collection by automatically noting the timestamp for each behaviour, with an accuracy of up to 0.001 seconds, making the data collection process faster and more reliable. Coding the behaviours from pre-recorded CCTV footage allows for re-winding and slowing down of footage to ensure accuracy of timings and behaviours, and for the observation of several individuals simultaneously (Kusmarani et al., 2019). The study subjects were marked as ‘Out of sight’ when they were outdoors with limited CCTV coverage (OUT on Figure 1), or inside a tunnel between rooms (TNL1 and TNL2 on Figure 1), and therefore out of sight of the cameras.
A map of the study area was created using screenshots taken from the CCTV images and uploaded into BORIS (Figure 1), allowing observers to record the location of each individual. The areas were divided by physical limitations (e.g. platform at a height), but also by aspects such as the line of sight of an individual in an area (an area where an individual could see through a tunnel to the next room was separated into its own area, e.g. TR2, which could see into CR1 through TNL2).
Prior to the commencement of the data collection, a series of inter-observer reliability tests were performed on 10-minute samples of the footage to ensure all observers were coding behaviours uniformly. This was repeated until all observers (LN, CG, JD and EG) had a rate of agreement of over 90% when using a 5 second window (range from 91% to 96% agreement between observer pairs, with a Kohen Cappa’s score ranging from 0.89 to 0.95, controlling for the likelihood of observers coding the same behaviour at the same time by chance). The observations collected in BORIS were exported in a CSV format and uploaded into RStudio version 4.3.0 (“Already Tomorrow”; R Core Team, 2023) for tidying and analysis.
2.3 Analysis
We conducted all analyses in RStudio, and the data were formatted using the “tidyverse” package (Wickham et al., 2019) before analysis.
While we recorded behaviours following an extensive ethogram as part of a long-term monitoring project, we chose to focus this study on maternal behaviours as an indication of parental investment in the species (“parental” behavioural category described in Table 2). As such, we calculated the proportion of time the dam spent nursing and grooming the cubs by determining the total number of seconds spent performing each behaviour within each hour of each studied development phase. Nursing and grooming the cubs are key behaviours that play a vital function in the development of cubs in Felidae. Data were also collected on sire parental behaviour such as grooming, sniffing and guarding when mixed with the dam and cubs for short periods during the birth (100 minutes) and 48 hours after the birth (90 minutes). Other parental behaviours recorded as part of the ethogram were not analysed. For the T0 phase, we used the 60 hours of footage to determine an average proportion of each behaviour for each hour of the day, resulting in 24 data points for each phase.
The continuous location of each individual was used to describe social proximity of individuals within the group. The proportion of time individuals spent apart and together was calculated in the same way as the behavioural data.
To examine the changes in both chosen key behaviours and time spent together between individuals between each phase, we conducted a series of Friedman tests as the data was not normally distributed, and to account for repeated measures. As the results from all tests were statistically significant, we performed a series of post-hoc tests in the form of pairwise Wilcoxon rank sum tests with Bonferroni correction, which is the appropriate post-hoc test for a Friedman test. In all Friedman tests, the grouping variable was the development phases (categorical; “T0”, “T1”, “T2”, “T3”), and the hour slots (continuous; 0-23) were used as the blocking variable.
To examine the changes in mean proportion of time spent in key parental behaviours between phases, we used the proportion of time spent grooming and nursing the cubs respectively as response variables (F1 and F2).
To describe the proportion of time the cubs spent in close proximity to each other, we calculated the duration (total number of seconds) of time the cubs spent in the same location and within one adult body length of each other (as divided in Figure 1), the same room but different location, and in a different room to one another for each phase. The proportion of time the cubs spent in the exact same location and within one adult body length of each other was used as a response variable in the Friedman test (F3).
To describe the proportion of time the dam spent in proximity to the cubs, we calculated the total number of seconds the dam spent with the cubs in the first development phase. In subsequent phases, we recorded the number of seconds the dam spent with zero, one or two cubs. The proportion of time the adult female spent in the same location as cubs was used as a response variable in the Friedman test (F4).
3 Results
A total of 26 of the 31 behaviours described in the ethogram were observed at least once throughout the study period and used for a long-term monitoring study. The results reported below focus on parental behaviours (as defined in Table 2) proximity of the cubs to the dam and each other.
3.1 Parental behaviours
As predicted, the proportion of time the dam spent in parental behaviours decreased steadily throughout the development phases. Grooming and nursing made up the majority of parental behaviours throughout (Figure 2).
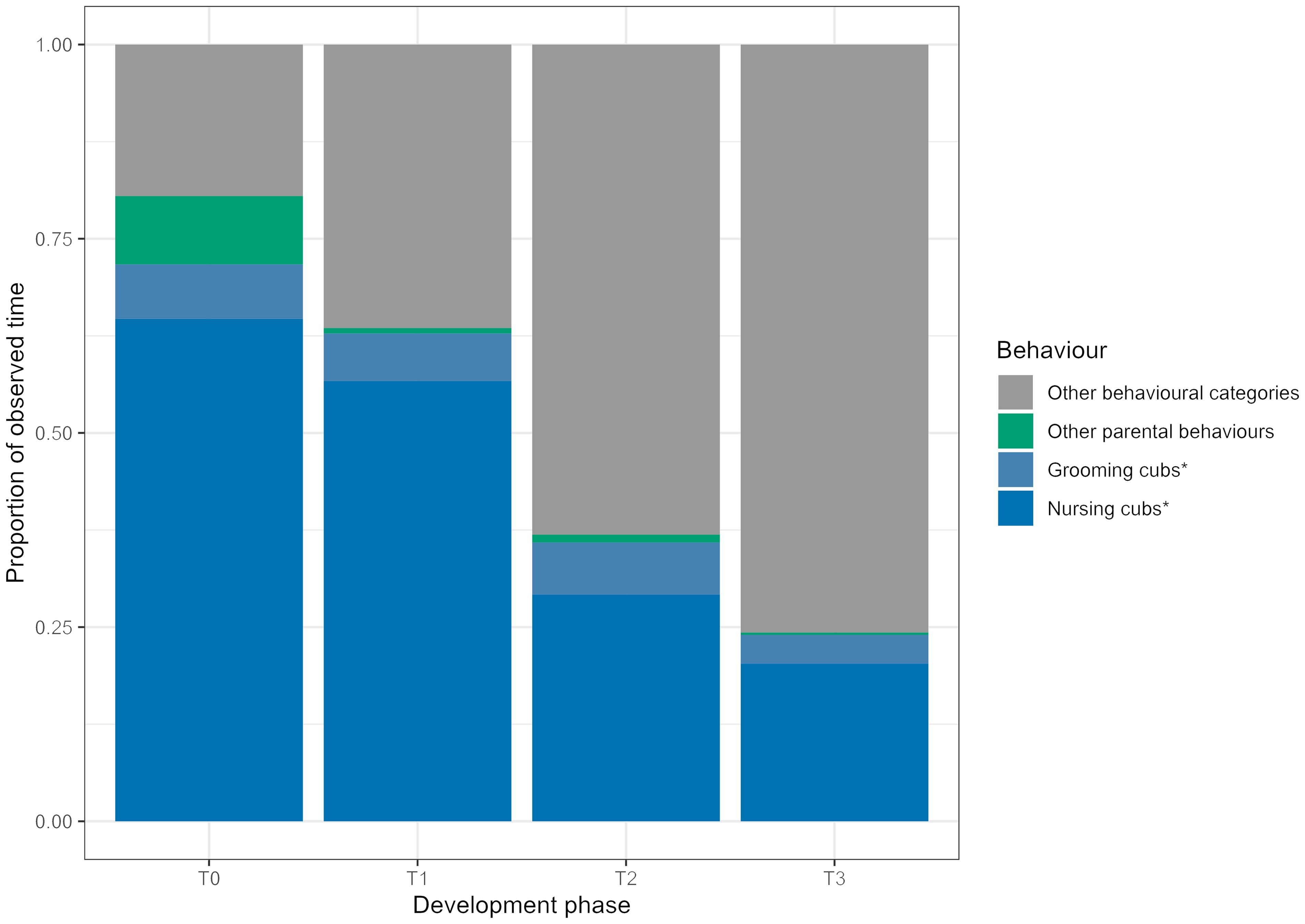
Figure 2. Across the development phases, the proportion of time the dam spent in parental behaviours decreased. Nursing was the most prominent parental behaviour. Parental behaviours are represented by the coloured areas and other behavioural categories, as defined in the ethogram, are in grey. The parental behaviours have been separated into the studied behaviours (denoted by an asterisk) and other parental behaviours which were observed, but not analysed.
3.1.1 Grooming cubs
A Friedman test revealed a statistically significant difference in cub grooming durations across developmental phases (Figure 3; F1; χ2 (3, N = 96) = 14.20, p < 0.01). Pairwise Wilcoxon rank sum tests with Bonferroni corrections detected a significant decrease in mean proportions of time spent grooming between phases T0 and T3, and T2 and T3 (Table 3).
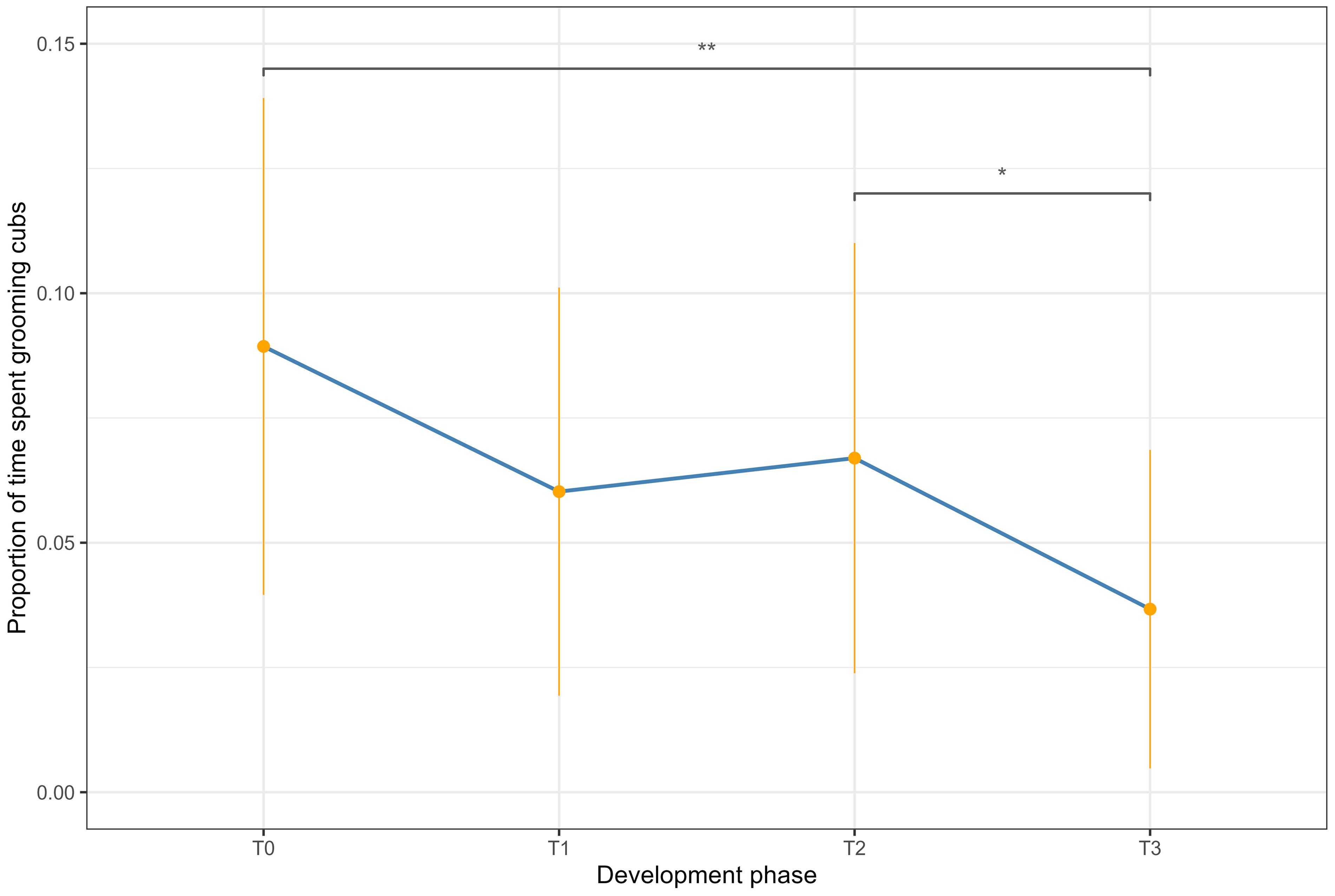
Figure 3. Proportion of time the dam spent grooming the cubs over the four development phases. The orange dots represent the mean proportion observed over each hour of the day, while the orange lines represent the standard deviation. T0 represents the 60 hours following the birth of the cubs, T1 is 5 days following the birth, T2 is 5 weeks following the birth and T3 is 10 weeks following the birth. The asterisks represent statistically significant results from the Wilcoxon rank sum test with Bonferroni correction.
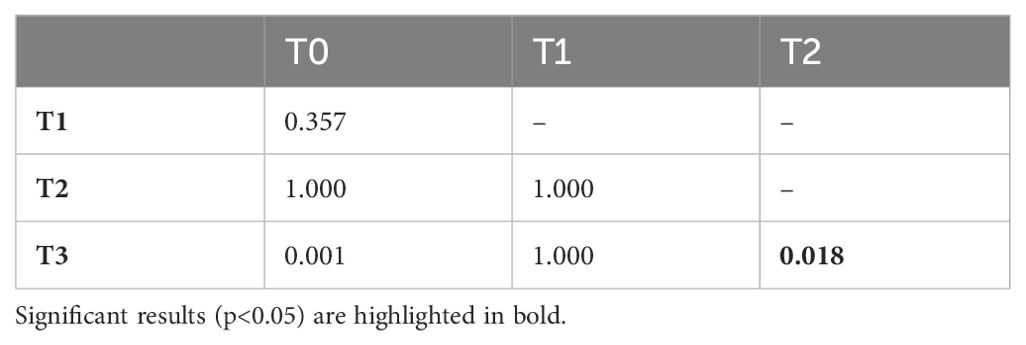
Table 3. p-values of the Wilcoxon rank sum test with Bonferroni correction when investigating the proportion of time the dam spent grooming the cubs.
3.1.2 Nursing cubs
Throughout the study period, the mean proportion of time the dam spent nursing her cubs steadily decreased. A Friedman test found that the difference in mean cub nursing behaviours between developmental phases was statistically significant (Figure 4; F2; χ2 (3, N = 96) = 25.77, p < 0.001). Pairwise Wilcoxon rank sum tests with Bonferroni corrections detected significant decreases in mean proportions of time spent nursing the cubs between all phases, except for T0 and T1, and T2 and T3 (Table 4).
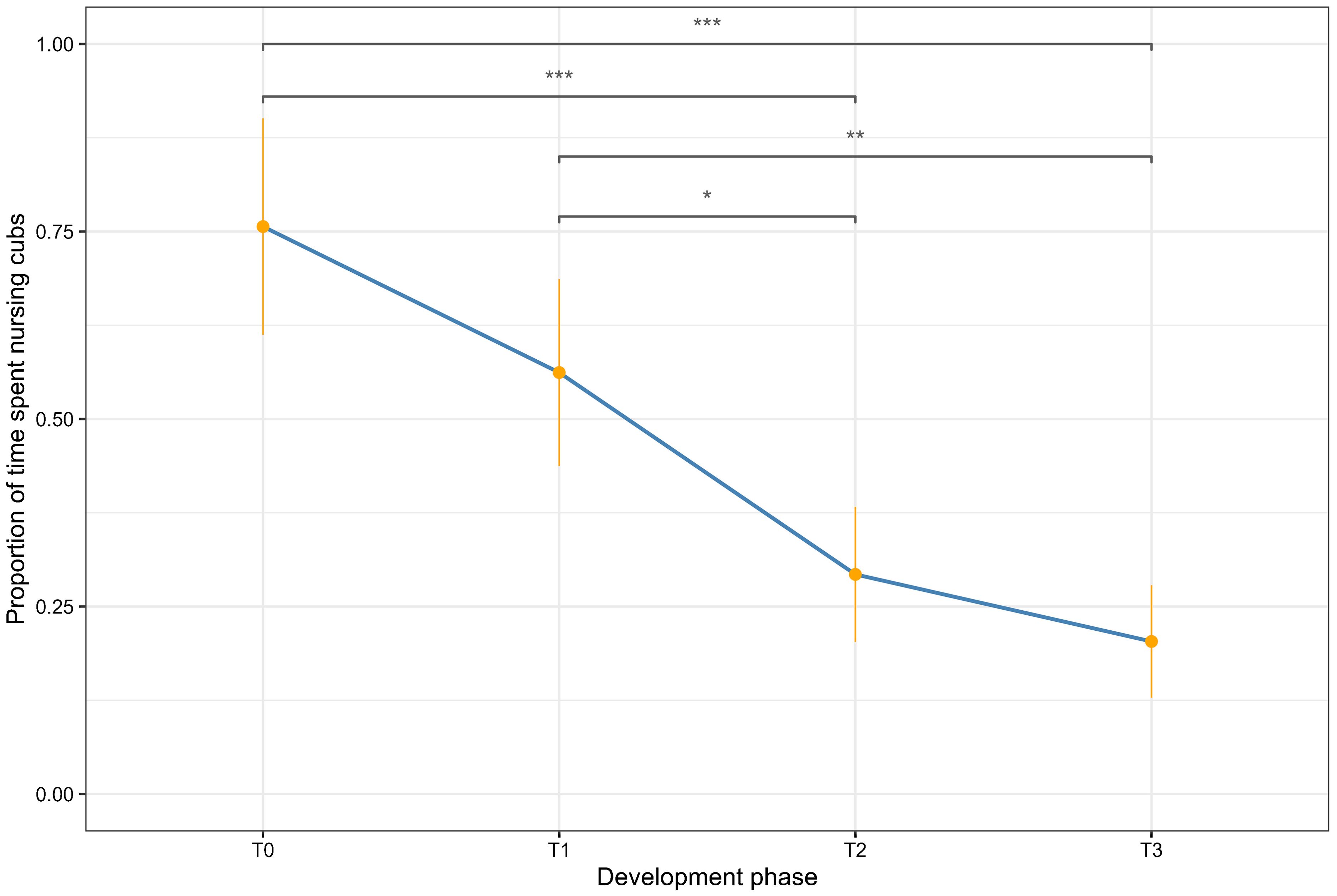
Figure 4. Proportion of time the dam spent nursing the cubs over the four development phases. The orange dots represent the mean proportion observed over each hour of the day, while the orange lines represent the standard deviation. T0 represents the 60 hours following the birth of the cubs, T1 is 5 days following the birth, T2 is 5 weeks following the birth and T3 is 10 weeks following the birth. The asterisks represent statistically significant results from the Wilcoxon rank sum test with Bonferroni correction.
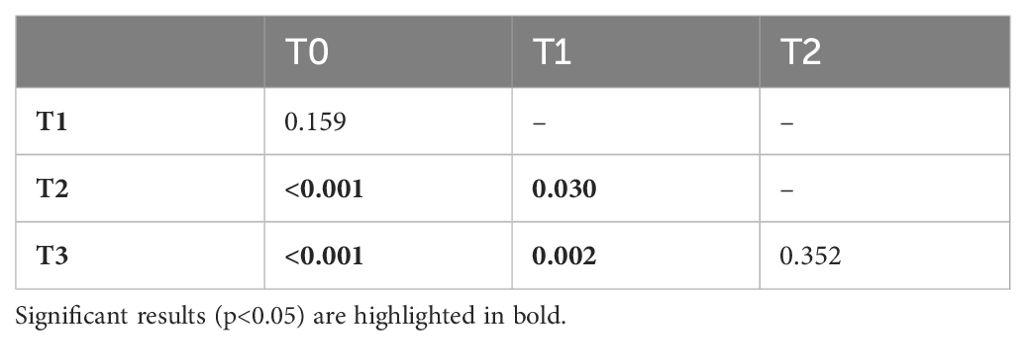
Table 4. p-values of the Wilcoxon rank sum test with Bonferroni correction when investigating the proportion of time the dam spent nursing the cubs.
3.2 Social proximity
3.2.1 Proximity of the cubs to each other
The proportion of time cubs spent in proximity to each other was divided into three categories: same location (within one adult body length), same room (different location; more than one adult body length but within visual contact), and different room (likely no visual contact).
The mean proportion of time the cubs spent in the same location was significantly different between phases (F3; χ2 (3, N = 96) = 15.231, p = 0.001), however post-hoc tests revealed no significant differences in social proximity between the phases. There was, however, a near-significant visible trend for the cubs spending less time in the same location during phase T2 (Figure 5; Table 5).
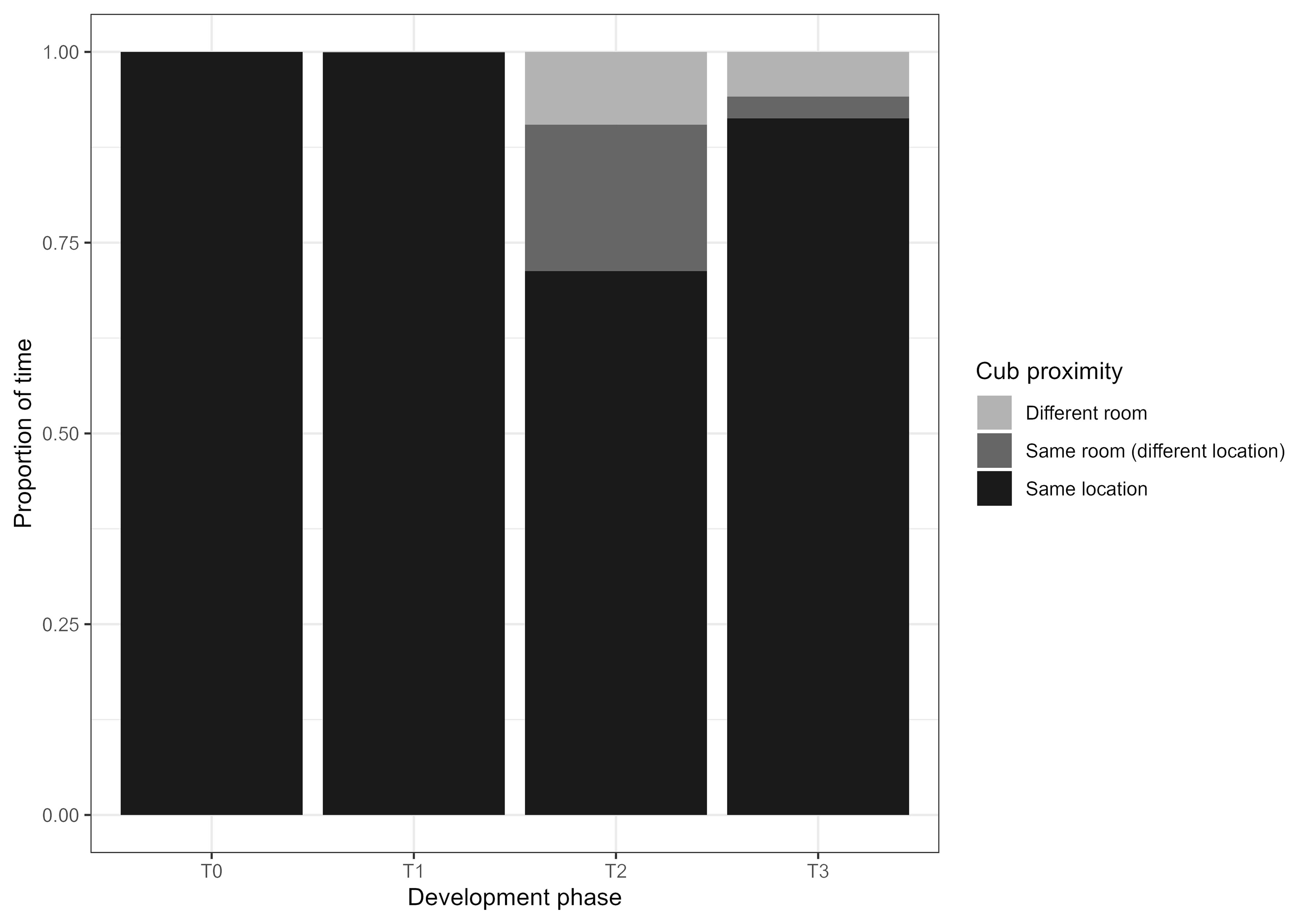
Figure 5. Across the developmental phases the cubs spent similar amounts of time in close proximity to each other. The increase of “Same room (different location)” in T2 (when the cubs are 5 weeks old) highlights a phase in their development where one cub started showing signs of independence before the other one. ‘Same location’ represents times when the cubs were in the exact same study area and within one adult body length. ‘Same room (different location)’ indicates the cubs were in the same room but further than one adult body length. ‘Different room’ indicates the cubs were not in the same room and likely had no visual contact with each other.
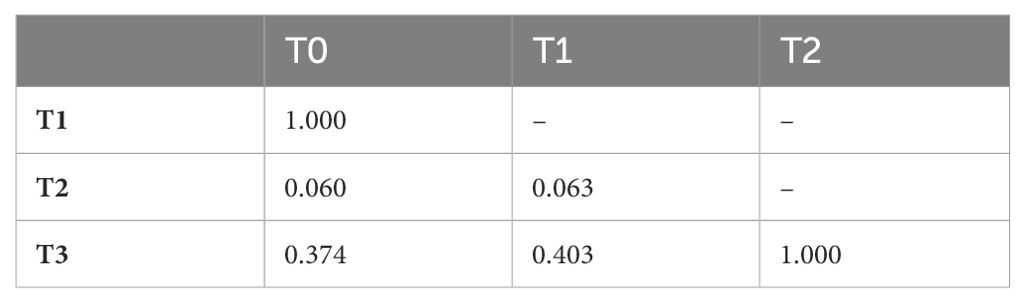
Table 5. p-values of the Wilcoxon rank sum test with Bonferroni correction when investigating the proportion of time cubs spent in the exact same location as each other.
3.2.2 Proximity of the dam to the cubs
The proportion of time the dam spent in proximity to the cubs was divided into three categories: no cubs, one cub and two cubs. Throughout the study period, there was a steady decline in the proportion of time the dam spent with cubs, instead spending time mostly with no cubs (Figure 6).
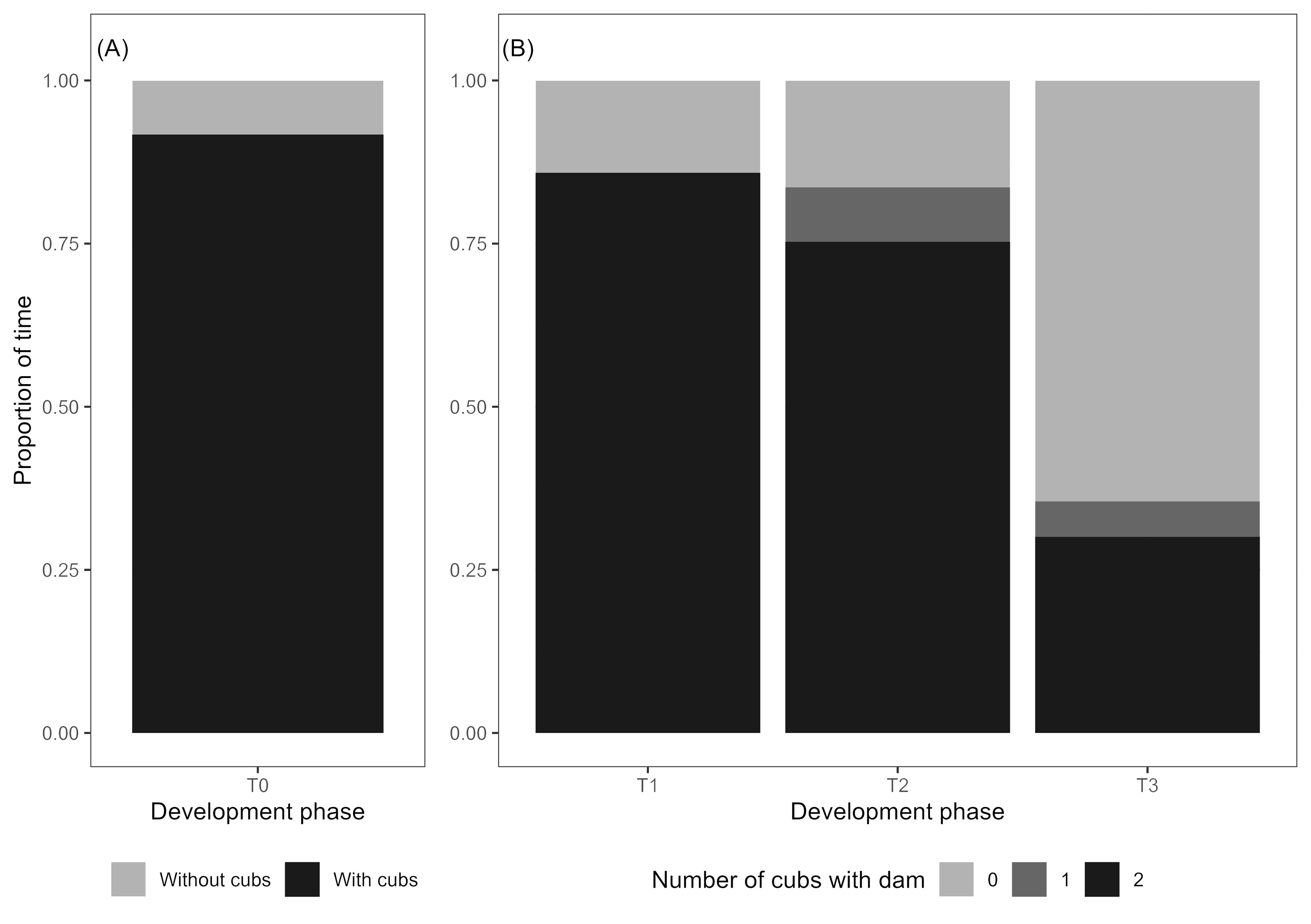
Figure 6. Across the development phases the dam spent significantly less time in proximity to the cubs (p < 0.001). (A) In development phase T0 all cubs were present but remained in close proximity to each other and were relatively immobile, with the dam spending 91.7% of her time with them. (B) The proportion of time the dam spent in close proximity to 1 or 2 cubs significantly decreased in development phase T3.
The mean proportion of time the dam spent in the exact same location as the cubs was significantly different across the study periods (F4; χ2 (3, N = 96) = 27.88, p < 0.001). Pairwise Wilcoxon rank sum tests with Bonferroni corrections revealed that the dam spent significantly less time with the cubs in phase T3 compared to all other phases (Table 6).
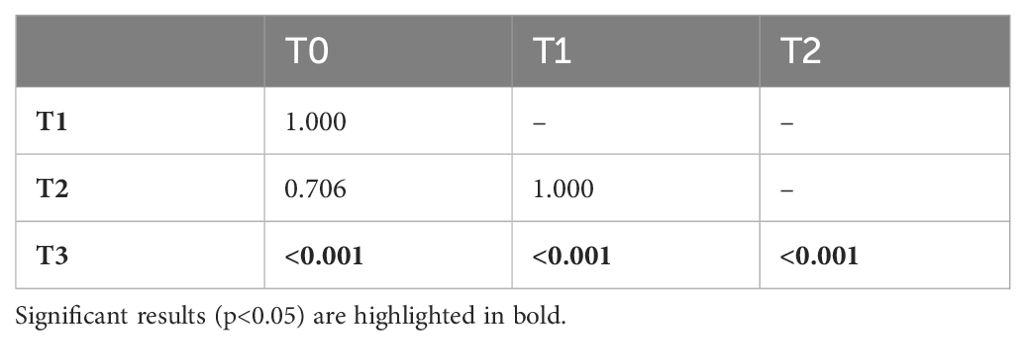
Table 6. p-values of the Wilcoxon rank sum test with Bonferroni correction when investigating the proportion of time the dam spent in the same location as the cubs.
3.2.3 Male parental behaviour
The sire spent 19.5% of observed time when mixed in with the group engaging in parental behaviours (predominantly sniffing and grooming the cubs). Additionally, he spent 59% of observed time inside the den with the cubs (within one adult body length), and 16.5% of observed time on top of the den, suggesting a chosen proximity to the cubs and possibly indicating guarding behaviour. He was out of sight for 2.5% of total observed time, highlighting the proximity he kept to the group throughout the time he was present.
4 Discussion
This study aimed to quantify key parental behaviours during the development of Sumatran tiger cubs, as well as document changes in interactions between the cubs and the dam in their early life as they reach independence. We found that the dam decreased the amount of time she spent in proximity with the cubs, particularly from approximately five weeks of age, with parental care also decreasing as a result. Understanding the behavioural changes at different stages of cub development is crucial to provide effective husbandry, which will not only ensure optimal welfare for the species, but increase the likelihood of successful breeding, boosting numbers for the breeding programme (Yachmennikova et al., 2018).
4.1 Parental behaviours
The parental care strategy in big cats suggests that females will halt breeding and provide maternal care until their existing cubs are independent. This is also thought to play a role in the subsequent breeding success of their offspring (Balme et al., 2017). In the wild, the duration of parental investment can vary markedly due to factors such as litter size, prey availability, environmental stressors, and sex allocation (Balme et al., 2017; Johansson et al., 2021), but little is known about how it varies ex-situ.
Allogrooming in carnivores maintains group stability and redirects aggression and is also thought to be a way of affiliating with higher-ranking individuals (Van Den Bos, 1998). In a mother-cub relationship, grooming may serve a mostly hygienic function of keeping the cubs clean before they are able to do it themselves (Modena et al., 2023; Van Den Bos, 1998). However, it is also possible that investing affiliation in the cubs may provide them with the skills necessary to successfully bond with conspecifics in later life, increasing their chances of successful reproduction (Massen et al., 2010). This, in turn indirectly benefits the mother by increasing the chances of her genes getting passed on (Dunbar and Shultz, 2010). As predicted, our results found that the proportion of time the dam spent grooming the cubs significantly decreased from the start of the study period (the day of the birth) to weaning. This decrease may be explained by the fact that the cubs had gained enough independence to self-groom for hygiene reasons. Despite this, the cub grooming behaviour did not disappear altogether, suggesting that the behaviour fulfils a social function and that the group were well affiliated. Further, grooming may contribute to the reduction of stress in cubs by providing an olfactory “safe space” created from saliva, urine and faeces (Behnke et al., 2021; Soini et al., 2012). The pheromones produced by the dam and deposited on the young through the action of grooming, are detected by olfactory processes (Shreve and Udell, 2017) and have been found to reduce distress vocalisations in domestic kittens by conveying information on familiar conspecific presence.
The increased levels of oxytocin in the dam following the birth promote pair-bonding and parental care in mammals, which is a crucial stage for maternal bonding. But the release of oxytocin following a birth also promotes nursing behaviours: studies have found the peptide not only stimulates lactation but in turn increases the amount of food being provided to the young (Montgomery et al., 2018). Milk feeding is crucial for the early-life development of cubs (Alekseeva et al., 2020): it provides the cub with the necessary nutrients and microbiome to promote successful development to independence (Alekseeva et al., 2020; De Jonge et al., 2022; Zhang et al., 2022). The benefits of nursing may in turn also influence that individual’s breeding success, emphasising the impact of this early-life behaviour on an entire lifespan (Balme et al., 2017). The proportion of time the dam spent nursing the cubs in this study steadily decreased with the development of the cubs. While the behaviour represented over 50% of the dam’s activity budget throughout the week following the birth of the cubs, she spent significantly less time engaging in this behaviour at 5 weeks and 10 weeks following the birth. Nursing cubs is energetically expensive for the dam: a study in domestic cats found that cortisol levels were highest in females four weeks after the birth of their cubs, presumably due to the energetic requirements of producing milk as the cubs begin to be weaned (Alekseeva et al., 2020). The adult female in this study spent the highest observed proportion of time resting five weeks after the birth of her cubs (T2; 37% of observed time), supporting the suggestion that felids experience high physiological stress during lactation. The increase in proportion of time spent resting may also be due to the additional time available as she starts spending less time grooming and nursing the cubs, as the cubs gain their milk teeth and start investigating solid food. As the cubs age, the concentration of oxytocin in the dam will decrease, influencing the amount of time she chooses to spend in parental behaviours, especially nursing (Romero, 2015).
4.2 Social proximity and male presence during parturition
4.2.1 Social proximity
Close proximity between individuals is usually related to a level of tolerance and association to another individual, and our results suggest that the cubs showed a high level of association with each other as they consistently spent high proportions of time in close proximity throughout the study period. These results highlight the difference in cub sociality in Sumatran tigers compared to other felids, such as the Iberian lynx (Lynx pardinus), where sibling fights cause high rates of first-year mortality (Antonevich and Naidenko, 2009). While the tiger cubs maintained a high level of social proximity throughout, there were times during their development, notably at five weeks old (T2), where the cubs spent slightly less time in close proximity. This was due to one of the cubs showing more interest in exploration than the other. The behaviourally ‘bolder’ cub would follow the dam around the study area, while the other would remain mostly in the room near the birthing den. Keepers anecdotally mentioned that these differences in personalities were not apparent ten weeks after their birth as the less ‘bold’ cub had become independent and confident enough to follow their sibling. As the cubs became more independent, the adult female spent increasing amounts of time solitary in the habitat, mostly outdoors. A study tracking wild pumas (Puma concolor) using accelerometers estimated that females with kittens were away from their young for over 80% of studied time (Laundré and Hernández, 2008). While the motivation for spending time away from cubs may differ in the wild (e.g. for hunting), our results found that the dam spent 65% of her time in a different room and therefore, out of sight from the cubs 10 weeks after their birth.
4.2.2 Male presence during parturition
Although not a focus in this study, we recorded behaviours of the sire, who was present during the birth of the cubs and for a short duration later in phase T0. He spent 20% of his time engaged in parental care behaviours, supporting the suggestion that male tigers may have the capacity to form social bonds with related individuals (Holland et al., 2023). Similar findings have been reported in other groups: in 2016, a male Sumatran tiger mixed with two newborn cubs showed no cub-directed aggression, and regularly performed cub-guarding and affiliative behaviours towards the cubs (Pastorino et al., 2022). In 2020, a pair of Malayan tigers (Panthera tigris jacksoni) and their cubs were housed together, with evidence of affiliative behaviours performed by the male towards not only the adult female, but also the cubs (Holland et al., 2023). Another study found evidence of males regularly engaging in affiliative behaviours when left alone with cubs (Pastorino et al., 2022), as well as no observed instances of male aggression towards the cubs, even during 24-hour continuous monitoring (Holland et al., 2023; Pastorino et al., 2022). There may, therefore, be higher social flexibility than previously thought in big cat species (Holland et al., 2023; Pastorino et al., 2022). Following the study period, when the cubs were 11 weeks old, the male was re-introduced to the dam and cubs, following a period of visual contact through mesh. When the group was reunited, the sire showed no signs of aggression towards the cubs, instead showing affiliative behaviours with the dam, as well as curiosity and affiliation towards the cubs in the week following the mixing (personal communication, Dave Hall).
Mixing the male and keeping the group together may prove beneficial for several reasons – all individuals have access to the totality of the available habitat and have contact with each other, which has been found to lead to higher behavioural diversity with lower frequencies of stereotypies (De Rouck et al., 2005). Bullock et al. (2021) found that cubs and young tigers spent more time engaging in social behaviours than older tigers, emphasising the importance of early life socialisation. In groups, cubs can develop appropriate species-specific social behaviours (De Rouck et al., 2005) by playing with their siblings (Antonevich et al., 2019) and learning social skills from observing and interacting with their parents (De Rouck et al., 2005). This may increase their chances of being confident around conspecifics and breeding successfully when reaching adulthood, a vital trait for Critically Endangered species in a breeding programme (Ianni, 2015; Kelling et al., 2013).
The decision to mix the group should, however, be considered on a case-by-case basis, utilising keepers’ expertise in the species and the specific individuals in their care. In this case study, both parents were first-time breeders who had been mixed six months before the birth of the cubs. Due to this relatively short period of social housing, the team felt that the female would benefit from more time to develop her experience as a mother and enable the young group to bond before permitting ad libitum access to the male (personal communication, Dave Hall). When mixed, keepers anecdotally noted that the male wanted to access the cubbing den, as he had previously been spending time in there before the birth of the cubs. However, by separating the male, they provided him access to his own resources, and, importantly, ad libitum outdoor access. For future breeding attempts, keepers aim to construct another den area to increase opportunities for the male to be mixed in from parturition until weaning (personal communication, Dave Hall).
5 Conclusion
We believe that the findings in this study are the first of their kind to quantify and describe changes in maternal behaviours and social proximity from birth to weaning in a group of Sumatran tigers across 24-hour time periods. We found significant decreases in key maternal behaviours (grooming and nursing the cubs) between T0, the 60 hours immediately following the birth of the cubs, and T3, when the cubs were 10 weeks old. We also found a significant decrease in total duration of time the dam spent with the cubs between these two phases, however, the cubs were found to spend most of the observed time in close proximity to each other throughout the entire study period. Male parental care was consistent with previously published studies, whereby males showed high levels of interaction and curiosity towards the cubs, with no instances of agonistic behaviours observed. This provides additional evidence to support the presence of a sire during birth as a viable breeding strategy. Minimal keeper disturbance of the group during the first few weeks of life was thought to promote bond strength between parents and cubs, and to enable a natural decrease in maternal behaviour frequency and independence of the cubs, while CCTV access provided keepers with the ability to conduct welfare checks on the animals in their care and ensure the success of such important captive breeding programmes. These results document parental care behaviours while highlighting the timelines of cub development from birth to independence. This information can provide a practical evidence base for zoo managers to ensure that husbandry and management promotes ample space for the dam to spend time with and away from the cubs, opportunities for sire interaction, and opportunities for cubs to develop mobility, confidence, and independence in the lead up to weaning age.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by North of England Zoological Society. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
LN: Conceptualization, Data curation, Formal Analysis, Methodology, Visualization, Writing – original draft, Investigation, Supervision, Validation. CG: Investigation, Writing – review & editing. JD: Investigation, Writing – review & editing. EG: Investigation, Writing – review & editing. ND: Writing – review & editing, Conceptualization, Resources. DH: Writing – review & editing, Resources. JW: Conceptualization, Methodology, Writing – review & editing, Data curation, Formal Analysis, Validation. LH: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing, Project administration, Resources.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funded by the North England Zoological Society, with no other external funding sources.
Acknowledgments
The authors would like to thank members of the Carnivore Team and Animal Managers at Chester Zoo for their support during this study and vital information on husbandry practice for the species.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alekseeva G. S., Loshchagina J. A., Erofeeva M. N., Naidenko S. V. (2020). Stressed by maternity: Changes of cortisol level in lactating domestic cats. Animals 10 (5), 903. doi: 10.3390/ani10050903
Antonevich A. L., Alekseeva G. S., Vasilieva N. A., Pavlova E. V., Loshchagina J. A., Duplyakina S. Y., et al. (2019). Social play changes reflect differences in biology and development of three felids. Russian J. Theriology 18, 80–90. doi: 10.15298/rusjtheriol.18.2.02
Antonevich A. L., Naidenko S. (2009). “A comparative note on early sibling aggression in two related species: The iberian and the eurasian lynx,” In book: Iberian Lynx Ex-situ Conservation: An Interdisciplinary Approach.Chapter: A Comparative Note on Early Sibling Aggression in Two Related Species: The Iberian and the Eurasian Lynx. (Fundación Biodiversidad, Madrid), 156–163. doi: 10.13140/2.1.4513.8880
Armstrong E. E., Khan A., Taylor R. W., Gouy A., Greenbaum G., Thiéry A., et al. (2021). Recent evolutionary history of tigers highlights contrasting roles of genetic drift and selection. Mol. Biol. Evol. 38, 2366–2379. doi: 10.1093/molbev/msab032
Balme G. A., Robinson H. S., Pitman R. T., Hunter L. T. B. (2017). Flexibility in the duration of parental care: Female leopards prioritise cub survival over reproductive output. J. Anim. Ecol. 86, 1224–1234. doi: 10.1111/1365-2656.12713
Behnke A. C., Vitale K. R., Udell M. A. R. (2021). The effect of owner presence and scent on stress resilience in cats. Appl. Anim. Behav. Sci. 243, 105444. doi: 10.1016/j.applanim.2021.105444
Bullock N., James C., Williams E. (2021). Using keeper questionnaires to capture zoo-housed tiger (Panthera tigris) personality: considerations for animal management. J. Zoological Botanical Gardens 2, 650–663. doi: 10.3390/jzbg2040047
Carr N. (2016). Ideal animals and animal traits for zoos: General public perspectives. Tourism Manage. 57, 37–44. doi: 10.1016/j.tourman.2016.05.013
Christie S. (2010). “Why keep tigers in zoos?,” in Tigers of the World: The Science, Politics and Conservation of Panthera tigris, 2nd ed. Eds. Tilson R., Nyhus P. J. (New York: William Andrew Publishing), 205–214. doi: 10.1016/B978-0-8155-1570-8.00015-3
De Jonge N., Carlsen B., Christensen M. H., Pertoldi C., Nielsen J. L. (2022). The gut microbiome of 54 mammalian species. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.886252
De Rouck M., Kitchener A. C., Law G., Nelissen M. (2005). A comparative study of the influence of social housing conditions on the behaviour of captive tigers (Panthera tigris). Anim. Welfare 14, 229–238. doi: 10.1017/s0962728600029390
Dunbar R. I. M., Shultz S. (2010). Bondedness and sociality. Behaviour 147, 775–803. doi: 10.1163/000579510X501151
Friard O., Gamba M. (2016). BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 7, 1325–1330. doi: 10.1111/2041-210X.12584
Galardi E. G., Fabbroni M., A Rausa F., Preziosi R., Brereton J. E., Pastorino G. Q. (2021). Sociality and enclosure use of group-housed lions and tigers. J. Vet. Med. Anim. Sci. 4, 1068. Available at: http://meddocsonline.org/.
Greenwell P. J., Riley L. M., Lemos de Figueiredo R., Brereton J. E., Mooney A., Rose P. E. (2023). The societal value of the modern zoo: A commentary on how zoos can positively impact on human populations locally and globally. In J. Zoological Botanical Gardens 4, 53–69. doi: 10.3390/jzbg4010006
Holland A., Galardi E. G., Fabbroni M., Hashmi A., Catinaud J., Preziosi R., et al. (2023). Exploration of social proximity and behavior in captive malayan tigers and their cubs. Animals 13 (6), 1040. doi: 10.3390/ani13061040
Ianni D. F. (2015). Welfare of a pair of Captive Tigers: a Hand-Reared Female and a Parent-Reared Male. J. Adv. Agric. 5, 545–556. Available at: www.cirjaa.com.
Imron M. A., Herzog S., Berger U. (2011). The influence of agroforestry and other land-use types on the persistence of a Sumatran tiger (Panthera tigris sumatrae) population: An individual-based model approach. Environ. Manage. 48, 276–288. doi: 10.1007/s00267-010-9577-0
Johansson Ö., Ausilio G., Low M., Lkhagvajav P., Weckworth B., Sharma K. (2021). The timing of breeding and independence for snow leopard females and their cubs. Mamm. Biol. 101, 173–180. doi: 10.1007/s42991-020-00073-3
Kelling A. S., Bashaw M. J., Bloomsmith M. A., Maple T. L. (2013). Socialization of a single hand-reared tiger cub. J. Appl. Anim. Welfare Sci. 16, 47–63. doi: 10.1080/10888705.2013.741000
Kitchener A. C., Breitenmoser-Würsten C., Eizirik E., Gentry A., Werdelin L., Wilting A., et al. (2017). A revised taxonomy of the Felidae: The final report of the Cat Classification Task Force of the IUCN Cat Specialist Group. Cat News.
Kurniawan B., Ningsih S., Susanti T., Farikhatin F. (2021). Behavior Analysis of Sumatran tiger (Panthera tigris sumatrae, Pocock 1929) in Taman Rimba Zoo Jambi. IOP Conf. Series: Materials Sci. Eng. 1098, 52–76. doi: 10.1088/1757-899x/1098/5/052076
Kusmarani F. M., Sjahfirdi L., Sunarto S. (2019). Application of digital ethogram in Sumatran tiger (Panthera tigris sondaica) behavioral observation at Ragunan Zoological Park. AIP Conf. Proc. 2168, 020082. doi: 10.1063/1.5132509
Laundré J. W., Hernández L. (2008). The amount of time female pumas Puma concolor spend with their kittens. Wildlife Biol. 14, 221–227. doi: 10.2981/0909-6396(2008)14[221:TAOTFP]2.0.CO;2
Luskin M. S., Albert W. R., Tobler M. W. (2017). Sumatran tiger survival threatened by deforestation despite increasing densities in parks. Nat. Commun. 8, 1783. doi: 10.1038/s41467-017-01656-4
Martin-Wintle M. S., Shepherdson D., Zhang G., Huang Y., Luo B., Swaisgood R. R. (2017). Do opposites attract? Effects of personality matching in breeding pairs of captive giant pandas on reproductive success. Biol. Conserv. 207, 27–37. doi: 10.1016/j.biocon.2017.01.010
Massen J. J. M., Sterck E. H. M., De Vos H. (2010). Close social associations in animals and humans: Functions and mechanisms of friendship. In Behav. 147, 1379–1412. doi: 10.1163/000579510X528224
McKay J. E., St John F. A. V., Harihar A., Martyr D., Leader-Williams N., Milliyanawati B., et al. (2018). Tolerating tigers: Gaining local and spiritual perspectives on human-tiger interactions in Sumatra through rural community interviews. PLoS One 13 (11), e0201447. doi: 10.1371/journal.pone.0201447
Modena P. Z., Adania C. H., Lopez V. M., Guillermo-Ferreira R. (2023). Maternal behavioural analysis during a successful captive breeding of jaguars Panthera onca. Theriogenology Wild 2, 100027. doi: 10.1016/j.therwi.2023.100027
Montgomery T. M., Pendleton E. L., Smith J. E. (2018). Physiological mechanisms mediating patterns of reproductive suppression and alloparental care in cooperatively breeding carnivores. Physiol. Behav. 193, 167–178. doi: 10.1016/j.physbeh.2017.11.006
Pastorino G. Q., Dougal C., Sanders K., Stubbington T., Brereton J. E., Preziosi R. (2022). Patterns of diurnal and nocturnal cub-directed social interaction and guarding behaviour in sumatran tigers. Acta Sci. Veterinary Sci. 4 (9), 70–78. doi: 10.31080/asvs.2022.04.0499
Patana P., Winda Saputri M., Marpatasino K. (2021). The occurrence of Sumatran Tiger (Panthera tigris sumatrae) in an industrial plantation forest area, North Sumatra, Indonesia. Indonesian J. Appl. Environ. Stud. 2, 47–51. doi: 10.33751/injast.v2i1.3079
Powell D. M., Meyer T. G., Dorsey C., Vernon R. (2024). What types of animals should be in the future zoo? Thoughts from United States residents and zoo and aquarium staff. J. Zoological Botanical Gardens 5, 157–178. doi: 10.3390/jzbg5020011
Pusparini W., Batubara T., Surahmat F., Ardiantiono A., Sugiharti T., Muslich M., et al. (2018). A pathway to recovery: The Critically Endangered Sumatran tiger Panthera tigris sumatrae in an “in danger“ UNESCO World Heritage Site. ORYX 52, 25–34. doi: 10.1017/S0030605317001144
R Core Team (2023). R: A Language and Environment for Statistical Computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org/.
Romero T. (2015). Proximate mechanisms underlying cooperation in carnivores. Japanese J. Animal Psychol. 65 (1), 35–43.
Saunders S. P., Harris T., Traylor-Holzer K., Beck K. G. (2014). Factors influencing breeding success, ovarian cyclicity, and cub survival in zoo-managed tigers (Panthera tigris). Anim. Reprod. Sci. 144, 38–47. doi: 10.1016/j.anireprosci.2013.11.006
Semiadi G., Nugraha T. P. (2006). Reproductive profile of captive Sumatran tiger (Panthera tigris sumatrae). Biodiversitas J. Biol. Diversity 7 (4), 368–371. doi: 10.13057/biodiv/d070413
Shreve K. R. V., Udell M. A. (2017). Stress, security, and scent: The influence of chemical signals on the social lives of domestic cats and implications for applied settings. Appl. Anim. Behav. Sci. 187, 69–76. doi: 10.1016/j.applanim.2016.11.011
Soini H. A., Linville S. U., Wiesler D., Posto A. L., Williams D. R., Novotny M. V. (2012). Investigation of scents on cheeks and foreheads of large felines in connection to the facial marking behavior. J. Chem. Ecol. 38, 145–156. doi: 10.1007/s10886-012-0075-0
Stanton L. A., Sullivan M. S., Fazio J. M. (2015). A standardized ethogram for the felidae: A tool for behavioral researchers. Appl. Anim. Behav. Sci. 173, 3–16. doi: 10.1016/j.applanim.2015.04.001
Stubbington T., Reed L., Fienieg E., Corlay M. (2023). Sumatran tiger (Panthera tigris sumatrae) EEP Long-term Management Plan 2023. European Association of Zoos and Aquaria, Amsterdam, Netherlands.
Van Den Bos R. (1998). The function of allogrooming in domestic cats (Felis silvestris catus); A study in a group of cats living in confinement. J. Ethology 16, 1–13. doi: 10.1007/BF02896348
Wickham H., Averick M., Bryan J., Chang W., McGowan L. D., François R., et al. (2019). Welcome to the tidyverse. J. Open Source Software 4, 1686. doi: 10.21105/joss.01686
Wilting A., Courtiol A., Christiansen P., Niedballa J., Scharf A. K., Orlando L., et al. (2015). Planning tiger recovery: Understanding intraspecific variation for effective conservation. Sci. Adv. 1 (5). doi: 10.1126/sciadv.1400175
Yachmennikova A. A., Rozhnov V. V., Blidchenko E., Poyarkov A. D., Korenkova A. A., Shteiman A. A. (2018). Data integration for the general-purpose scale of tiger cubs ontogenesis. Biol. Bull. Rev. 8, 245–255. doi: 10.1134/s2079086418030106
Keywords: zoo, parental care, breeding programmes, tiger, animal behaviour
Citation: Naidenov L, Grindle C, Duke J, Gough EJ, Davis N, Hall D, Waterman JO and Holmes L (2024) From birth to weaning: maternal investment, cub development and behaviour in Sumatran tigers (Panthera tigris sumatrae). Front. Conserv. Sci. 5:1460238. doi: 10.3389/fcosc.2024.1460238
Received: 05 July 2024; Accepted: 19 August 2024;
Published: 04 September 2024.
Edited by:
Anton Baotic, Austrian Academy of Sciences (OeAW), AustriaReviewed by:
Paul Rose, University of Exeter, United KingdomJames Edward Brereton, Sparsholt College, United Kingdom
Copyright © 2024 Naidenov, Grindle, Duke, Gough, Davis, Hall, Waterman and Holmes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Naidenov, bC5uYWlkZW5vdkBjaGVzdGVyem9vLm9yZw==
 Laura Naidenov
Laura Naidenov Chris Grindle1
Chris Grindle1 Jonathon Duke
Jonathon Duke Elena J. Gough
Elena J. Gough Dave Hall
Dave Hall Lisa Holmes
Lisa Holmes