
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Conserv. Sci., 28 May 2024
Sec. Animal Conservation
Volume 5 - 2024 | https://doi.org/10.3389/fcosc.2024.1391303
This article is part of the Research TopicAdvances in the Conservation of Neotropical PrimatesView all 6 articles
The database of the IUCN SSC Primate Specialist Group currently (December 2023) registers 218 species and subspecies of Neotropical primates in 24 genera and five families. In the early 1960s, the diversity of Neotropical primates was estimated to be around 200 species and subspecies. From then, through the 1970s to the mid-1990s, however, the perception of the region’s primate diversity dropped, and reached an all-time low at 83 species and subspecies in 1980 (A World List of Mammalian Species, G. B. Corbet and J. E. Hill, British Museum (Natural History), Comstock Publishing, Cornell University Press, London and Ithaca). Interest in taxonomy and primate field research in the Neotropics was subdued up to the late 1970s. Change was sparked by the burgeoning capture of primates for biomedical research in the 1950s and 1960s, and the increasing destruction of the Amazon rainforests from the late 1970s. The numbers increased, at first slowly, but then, in 1995, they leapt back to the 200s in anticipation of a book by C. P. Groves (2001, Primate Taxonomy, Smithsonian Institution Press, Washington, DC). The species’ counts (not including subspecies) rose due to the adoption of the Phylogenetic Species Concept over the Biological Species Concept, the former favoring the category of species over subspecies. In this article, we discuss the changes in species and subspecies numbers in the classification of the Neotropical primates, and report on the taxonomic changes resulting from taxonomic research ongoing since 2012. We emphasize the importance of taxonomic research for an understanding of the diversity of primates, and for conservation planning, not least in identifying the populations that are threatened.
The fundamental importance of a solid understanding of the diversity of life, pursued through the scientific disciplines of systematics and taxonomy, is well emphasized, reviewed, and discussed by Mayr and Ashlock (1991). They cited Elton (1947), who explained that without it “the ecologist is helpless” (p.116). Systematics and taxonomy (the naming of species) (see Simpson, 1961) are the bedrock of both theoretical and applied biology, and Mayr and Ashlock included as examples of the latter medicine, public health, agriculture, the management of natural resources, and conservation.
Here we report on some aspects of the progress and current status of the taxonomy and systematics of the Neotropical primates, emphasizing particularly the preeminent need to identify how many species and subspecies there are and where they occur as the baseline for their conservation. Phylogenetic and phylogenomic analyses are not only an extraordinarily helpful means by which we can distinguish species, but named, they can be identified in evolutionary lineages, providing for conservation strategies based on what have been termed Evolutionarily Significant Units (ESU), prioritizing all taxonomic levels, to subspecies and populations (Ryder, 1986; Avise, 1989; Mello et al., 2018), and allowing for adaptive evolutionary conservation (Fraser and Bernatchez, 2001).
In the case of the Neotropics, concern for primate conservation arose in the 1970s, when Amazonia became a target for development and the exploitation of its natural resources (Goodland and Irwin, 1975). One of those coveted natural resources concerned the use of Neotropical primates for biomedical research (PAHO, 1976; Mittermeier et al., 1993; Rylands and Anzenberger, 2012; Rylands and Mittermeier, 2022). In an address to the Pan American Health Organization (PAHO), Thorington (1976) provided a summary of the incipience and disarray, and, frankly, disregard in the classification of the New World monkeys at that time. Thorington explained that zoological classifications could serve different utilitarian purposes. He suggested that one based on morphology could well serve dental research, while another on serum proteins could “provide better hypotheses for hematological research.” (p.9). The classification of primates in the Neotropics, till then a largely academic pursuit, had found a purpose in biomedicine. In a letter to Science, Hershkovitz (1965) ridiculed the lack of attention given to the taxonomy of the Neotropical primates being, as it is, a fundamental aspect of their use and for the study of primates. In 1976, Colombia, Peru and Brazil banned or restricted primate exports, and PAHO, in desperation, promoted the establishment of in-country primate breeding centers, accompanied by an ambitious program of field surveys to establish ‘stocks’ and evaluate the methods and effects of trapping (cf. Arámbulo III et al., 1993; Rylands and Mittermeier, 2022). These surveys marked the beginning of Neotropical primatology, the focus of which, with the burgeoning exploitation and destruction of the forests of the Amazon basin, quickly changed to the need to document and protect the immense and complex diversity of primates that has been uncovered over the last 50 years. The classification of the Neotropical primates underpins all efforts for their conservation.
William C. Osman Hill, in his three treatises Primates: Comparative Anatomy and Taxonomy of the Platyrrhini in 1957, 1960 and 1962, listed 207 species and subspecies. In 1967, however, John and Prudence Napier published the influential A Handbook of Living Primates (Academic Press, London), which listed 159 species and subspecies. Estimates of the diversity of the Neotropical primates continued to decline from 1970 to 1990. In 1995, however, Rylands et al. published a list more redolent of Hill (1957, 1960, 1962), at least in numbers if not in content. Following the 1970–1990 doldrums, the increase in numbers was quite severely contested, being seen as unnecessarily disruptive to conservation planning, notably the prioritization provided by the Red List of Threatened Species and legislation (Isaac et al., 2004; Mace, 2004). Here, we compare the lists provided by Cabrera (1957) through to Groves (2001) to Rowe and Myers (2016) and that maintained by the IUCN SCC Primate Specialist Group (Rylands and Mittermeier, 2024), among others, to indicate the trends in the overall numbers of the Neotropical primates over the last 110 years and stress the importance of a solid, researched taxonomy, based on morphology, phylogenetics and a consistent, scientific proposition for the delimitation of species. Nicely put by Gutiérrez and Helgen (2013) in a letter to Nature “Mammalogy is beleaguered by a dogmatic regard for mid-twentieth-century propositions, which were seldom based on critical study and lacked phylogenetic information. Species were lumped together and incorporated into influential classification checklists to simplify regional faunas and make them more manageable for non-taxonomists. Modern integrative approaches have shown that this tactic has hidden an incommensurable number of distinctive species from conservation efforts (Morrison et al., 2009), thereby increasing the risk of extinctions.” An example of this is provided by Oates and Ting (2015), who indicated that the demise of Miss Waldron’s Red Colobus, Piliocolobus waldroni, may well have resulted from lack of consideration of its plight due to its classification as a subspecies.
In 2012, Rylands et al. provided a summary update on the, then prevailing, taxonomy and systematics of the platyrrhines, counting 204 species and subspecies in 20 genera. Following on from Rylands et al. (2012), we provide a summary update regarding newly described species, and the changes in the taxonomy and systematics of the Neotropical primates as they stand in our current list of 2024. There have been revisions of the taxonomy of the, now, 24 genera, and 15 new species have been described (Table 1). In March 2024, the database of the IUCN SSC Primate Specialist Group registered 218 species and subspecies of primates for the Neotropical region – 29 of them subspecies (Rylands and Mittermeier, 2024).
The numbers of species and subspecies recognized as comprising the Platyrrhini, the Neotropical primates, has varied considerably since the early 20th century – 110 years from 1913 to 2024. It has ranged from 149 (Elliot, 1913) to a nadir of 59 (Corbet and Hill, 1980) to the current appraisal in 2024 that has 218 (Rylands and Mittermeier (2024). This indecision as to the identity and diversity of primates on the American continent has multiple causes and is also, of course, serious in the sense of being highly consequential, not just in terms of setting up an accurate catalogue, but regarding efforts to conserve their (the primates’) contribution to biodiversity – their genes, the species and their forests.
The reasons for this variation in numbers can be found in the history of taxonomy and systematics, for long a concern principally of museum collections. As mentioned above, field primatology in the Neotropics was incipient in the mid- to late 1960s and 1970s and began in earnest only towards the end of that decade, inspired as it was by the burgeoning destruction of the Amazon rain forests and the massive trade in South America’s primates for biomedical research (Rylands and Anzenberger, 2012; Rylands and Mittermeier, 2022). Field research clearly revealed the need to better understand their occurrence and distributions for species-based conservation programs (Ennos et al., 2005), not least in the discovery of unknown species and subspecies. Figure 1 shows the numbers of Neotropical primate species and subspecies listed in 14 publications. Elliot’s 1913 monograph A Review of Primates counted 142 species and seven subspecies, an increase from Forbes (1896), who listed 88 species. Groves (2001) clarified, however, that Elliot’s review was immensely useful but that “one does not want to take any notice of the taxonomy” (p.43) – Elliot paid little attention to individual or age variation, and there is no indication that he listed anything but the type specimens. Subsequent lists, starting with Cabrera (1957), included numerous subspecies, surpassing even the number of species. Osman Hill in the three volumes dedicated to the platyrrhines (1957, 1960, and 1962) of his encyclopedic series Primates. Comparative Anatomy and Taxonomy listed 70 species and 137 subspecies. Napier and Napier (1967) recognized fewer species (57) and subspecies (102). From there, Corbet and Hill in 1980 recognized just 49 species and 10 subspecies. In 1995, Rylands et al. published a list that, while not increasing the numbers of species to any great extent – 96 compared to 84 listed by Groves (1983) in the second edition of Mammal Species of the World1– recognized 103 subspecies. Rylands et al. (1995), informed by Osman Hill’s volumes and especially by the taxonomic and systematic revisions of Philip Hershkovitz, were aware of the overriding need to emphasize the full gamut of Neotropical primate diversity for its conservation. Four years earlier, Corbet and Hill (1991) had provided a list of a mere 83 species and subspecies.
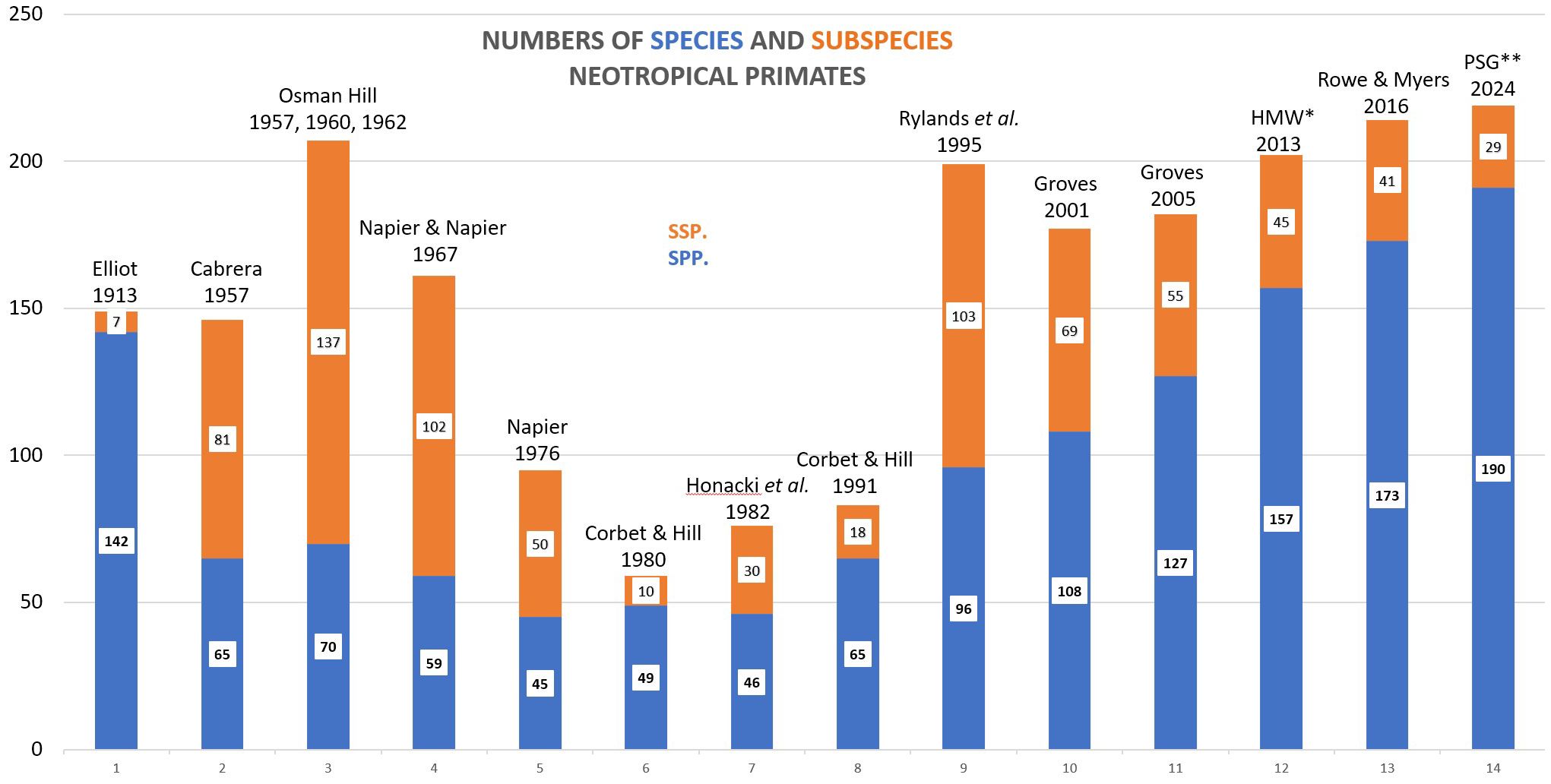
Figure 1 The numbers of species (blue) and subspecies (orange) in 14 lists of Neotropical primates from 1913 to 2024. *HMW 2013 = Mittermeier et al. (2013); ** PSG 2024 = Rylands and Mittermeier (2024).
Ignoring Elliot’s (1913) compilation, the numbers of platyrrhine species were relatively constant for more than 35 years, ranging from a high of 70 (Hill, 1957, 1960, 1962) to a low of 46 (Honacki et al., 1982) but recovering to 65 in 1991 (Corbet and Hill, 1991). The increase in numbers from the 1995 (Rylands et al.) assessment, and those of Groves (2001, 2005) reaching 127 – almost a 50% increase – resulted in accusations of bias and “taxonomic inflation” (Isaac et al., 2004). Isaac et al. (2004) conflated the increase in the number of taxa (from newly discovered species and taxonomic revisions) with the increase in number of species by the elevation of subspecies to species. The outcry arose not just because of the increase in the numbers of platyrrhines but of all the primates. Napier and Napier (1967), the established taxonomy for too many years, counted 180 species worldwide. Groves (2005) listed 376, and today we count 539 (Rylands and Mittermeier, 2024).
The increase in numbers – the inflation – was largely attributed to Groves’ and others’ adoption of the Phylogenetic Species Concept (PSC) over the prevailing Biological Species Concept (BSC), associated with the subjective use of the polytypic principal that assigns look-alikes to subspecies, even those that are allopatric with no intermediates. The main concern of Isaac et al. (2004; see also Mace, 2004) was the importance of lists, such as those of national and international legislation and for conservation initiatives and programs, that need to be stable in length and content but are vulnerable to the chaos of changing taxonomies because of changes in the rules of delimiting species. Mace (2004) advocated for the continued use of the BSC and an independence from the vagaries of taxonomic lists and, most pertinently, their unwanted “inflation.”
Groves’ (2001, 2004, 2012, 2014) cogently defended his use of the PSC, defined by Cracraft (1983) as “an irreducible cluster of organisms that is diagnosably distinct from other such clusters, and within which there is a parental pattern of ancestry and descent.” It is essentially an evolutionary notion of the species as was argued by Simpson (1951). De Queiroz (1998, 2005) proposed a general lineage concept of species that reconciled many of the numerous species concepts (see Mallet, 2001) as just being different methods to delimit species. De Queiroz (2007) emphasized that “One of the most important consequences of a unified species concept is that it clarifies the issue of species delimitation by clearly separating the conceptual problem of defining the species category (species conceptualization) from the methodological problem of inferring the boundaries and numbers of species (species delimitation)” (p.883). Numerous species concepts are not, in fact, concepts, they are merely methods to delimit species. Groves (2014) subsequently outlined his “diagnosability criterion for species delimitation” based on the PSC, in which each species is a testable hypothesis – a species is a population (or aggregation of populations) and differences between species are heritable and fixed (100% of individuals). It being consistently testable distinguishes it from the BSC. The BSC is testable for sexually reproducing organisms when they are sympatric, taking into account that any hybrids produced between them do not have exactly the same reproductive or fitness characteristics as the parental species (Zachos, 2016). It should also be said that the PSC has the practical limitation that, often in order to define the exact lines of descent, it is essential to carry out in-depth genetic analyses on all similar populations. Furthermore, applying this concept of species, very often all of the subspecies of a particular species automatically become distinct species with a consequent notable proliferation in their number (cf. Zachos, 2016).
However, the taxonomic changes and the increase in the numbers of platyrrhine taxa (as opposed to just species) was not entirely due to Groves’ adoption of the PSC and his use of the diagnosability criterion for species delimitation. Hershkovitz’s taxonomic revisions considerably increased the number of taxa without citing any particular species concept. He looked at their morphology, distinguishing characters and their distributions. The renewed interest in taxonomy was stimulated especially by the studies of Hershkovitz, providing, as it did, the wherewithal, the foundation for finding new species, for systematic revisions of species groups and genera, and subsequently for the phylogenetic studies that allowed for extraordinary revelations of their relationships and lineages. Besides this, traditional knowledge of local populations and other indirect testimonies have often contributed to alerting specialists to distinct forms that can then be formally described – from 1980 to 2018, 31 new platyrrhine species were discovered in this way (Rossi et al., 2018).
Eighty-three species and subspecies of Neotropical primates considered valid today have been described since 1913, 34 of them since 1990. An indication of the influence of new species on the authors’ lists since that of Elliot (1913) is given in Table 2. Eliminating the taxa in each list that are today not considered valid and then adding the new species give a hypothetical approximation of the numbers each author might have listed today. For example, Hill (1957, 1960, 1962) listed 207 species and subspecies, 137 of which were subspecies. Fifty-eight of the primates he listed are no longer considered valid (synonyms). Fifty-one taxa that are currently considered valid, were described subsequently. If he were to accept all the newly discovered primates and the synonymy of the 58 no longer recognized, his taxonomic list would today have 200 Neotropical primate species and subspecies – seven less than his total in 1962. This is only hypothetical, of course. It does not include those taxa which Hill, for example, considered to be synonyms that have subsequently been considered valid. It does indicate, however, that the number of Neotropical primate taxa has not changed that much. The concern of Mace (2004) was the elevation of subspecies to species, evident in Figure 1, that would overwhelm the IUCN Red List of Threatened Species. It has not, however. All subspecies are now assessed in the Red List, and taxonomic changes are accounted for. Mace (2004) even suggested a separate, stable conservation-management taxonomy, independent of the changing taxonomic lists from 1995 – perhaps harking back to Napier and Napier (1967) and Thorington’s (1976) suggestions of a menu of classifications to serve different purposes. Gippoliti and Amori (2007) and Gippoliti et al. (2017) argued cogently the importance of robust and unbiased taxonomies and expounded the dangers of conservation management ill-informed by poor taxonomy.
In 2012, Rylands et al. estimated 152 species and 204 species and subspecies (taxa) of Neotropical primates. Today we list 218 taxa (Rylands and Mittermeier, 2024) and here we briefly summarize the taxonomic and systematic changes, including 15 species described for the first time since Rylands et al. (2012).
A number of studies on the phylogenetic affinity of the pygmy marmoset, Cebuella pygmaea, to the Amazonian marmosets (formerly Callithrix, now Mico) indicated that it should be considered congeneric (e.g., Rosenberger, 1981; Barroso et al., 1997; Tagliaro et al., 1997). Schneider and Sampaio (2015) concluded, however, that Cebuella is a valid genus, separate from the Amazonian marmosets (see also Buckner et al., 2015). Garbino (2015) and Garbino and Martins Junior (2018) carried out a comparative study based on genetics, osteology, pelage and vocalizations and also concluded that it should be classified in a genus distinct from Mico.
The white-mouthed tamarin group of Hershkovitz (1977) currently comprises 16 black-mantled and saddle-back tamarins. The phylogenetic studies of Matauschek (2010; Matauschek et al., 2011) and Buckner et al. (2015) showed that they diverged from the tamarin lineage between 9 and 11 million years ago. Matauschek (2010) suggested that this would qualify for a distinct genus. Rylands et al. (2016) placed them in the genus Leontocebus Wagner, 1840. Leontocebus is the earliest name available with a type species that is a member of the nigricollis group—Simia leonina Humboldt, 1805. Humboldt’s “lion marmoset” was re-named by Lesson (1840, p.202) as Leontocebus fuscus. The name Leontocebus derives from the fact that Simia leonina was thought to be a lion marmoset (see Hershkovitz, 1949). In fact, it was a white-mouthed tamarin (see Hershkovitz, 1957). This use of Leontocebus was not a novelty. Cabrera (1957) placed all the tamarins in the genus Leontocebus, with three subgenera: Leontocebus, Oedipomidas (geoffroyi and oedipus) and Marikina (leucopus, bicolor, and martinsi). Garbino and Martins Junior (2018) preferred a subgeneric classification of Saguinus in three tamarin groups: Saguinus (Saguinus); Saguinus (Leontocebus); and Saguinus (Tamarinus). Brcko et al. (2022) followed Garbino and Martins Junior’s (2018) arrangement but placed the groups as genera, not subgenera and added a fourth genus Oedipomidas: Saguinus Hoffmannsegg, 1807 (S. midas, S. niger, S. ursulus, S. bicolor and S. martinsi); Leontocebus Wagner, 1840 (white-mouthed tamarins); Tamarinus Trouessart, 1904) (moustached tamarins, T. mystax, T. kulina, T. labiatus, T. imperator, and T. inustus); and Oedipomidas Reichenbach, 1862 (the northern Colombian and Panamanian tamarins, O. oedipus, O. geoffroyi, and O. leucopus).
Silva-Júnior (2001, 2002) suggested that the tufted capuchins and the untufted capuchins (sensu Hershkovitz, 1949, 1955) are sufficiently distinct in their morphology that they should be considered subgenera or even separate genera. Cebus Erxleben, 1777, refers to the untufted group and Sapajus Kerr, 1792, is the name available for the tufted capuchins. Molecular genetic studies by Lynch Alfaro et al. (2010, 2012a, 2012b) confirmed that the gracile untufted and robust tufted capuchin monkeys should be considered distinct genera, with the divergence being estimated as the Late Miocene, 6.2 mya.
To clarify the evolutionary history of the titi monkeys, Byrne et al. (2016) assembled a large molecular dataset, sequencing 20 nuclear and two mitochondrial loci for 15 species, including representatives from all the then recognized species groups. The results confirmed four distinct clades, for the most part concordant with previously recognized morphological species-groups—the torquatus group, the personatus group, the donacophilus group, and the moloch group (see Hershkovitz, 1990; Kobayashi, 1995; Van Roosmalen et al., 2002). The cupreus and moloch groups were found to be paraphyletic, and Byrne et al. reassigned all the species of the formerly recognized cupreus group to the moloch group. Two of the major divergence events are dated to the Miocene. The torquatus group, the oldest radiation, diverged about 11 mya; and the Atlantic Forest personatus group split from the ancestor of the donacophilus and moloch species groups about 9–8 mya. Taking into account molecular, morphological and biogeographic evidence, Byrne et al. (2016) proposed a new genus level taxonomy: Cheracebus n. gen. in the Orinoco, Negro and upper Amazon basins (torquatus group), Callicebus Thomas, 1903, in the Atlantic Forest (personatus group), and Plecturocebus n. gen. in the Amazon basin and Chaco region (donacophilus and moloch groups).
Groves (2001) placed the Peruvian yellow-tailed woolly monkey in the genus Oreonax Thomas, 1929, distinguishing it from the other woolly monkeys, Lagothrix É Geoffroy St.-Hilaire, 1812. This was resoundingly contested by Matthews and Rosenberger (2008) and more recently by Di Fiore et al. (2015).
Hershkovitz (1977) recognized no subspecific forms for Cebuella but Napier (1976) and Van Roosmalen and Van Roosmalen (1997) argued that the form south of the Rio Solimões-Amazonas, Cebuella niveiventris Lönnberg, 1940, was valid. A phylogenetic study by Boubli et al. (2018) showed that the forms Cebuella pygmaea Spix (from the north of the Solimões-Amazonas) and Cebuella niveiventris Lönnberg (from the south of the Rio Solimões-Amazonas) were distinct species. The type locality for Cebuella pygmaea, as given by Spix, was ambiguous but Garbino et al. (2019) and Boubli et al. (2021) concluded that it is the form to the north of the rios Amazonas-Solimões and Napo, with C. niveiventris being the correct name for the species south of these rivers. Porter et al. (2021) confirmed the conclusions of Garbino et al. (2019) and Boubli et al. (2021) for Ecuador and Peru.
Garbino (2014) reported that Mico manicorensis (Van Roosmalen et al., 2000) was a junior synonym of Mico marcai (Alperin, 1993), previously known only from its type locality on the west bank of the Rio Aripuanã, near the mouth of the Rio Roosevelt. The range of Mico marcai is now known to be between the rios Aripuanã and Marmelos, including the entire basin of the Rio Manicoré, south bank tributaries of the Rio Madeira, south at least to the mouth of the Rio Roosevelt (Silva et al., 2020). A further two marmosets have been described east of the Rio Tapajós. Mico munduruku (Costa-Araújo et al., 2019), occurs in the southwest of the state of Pará, Brazil, from the left margin of the Rio Jamanxim, below the mouth of the Rio Nôvo, possibly up to the right margin of the Tapajós below the mouth of the Rio Cururú. Mico schneideri Costa-Araújo et al. 2021, occurs in the Juruena–Teles Pires interfluvium in the north of the state of Mato Grosso, Brazil.
The nigricollis or white-mouthed tamarin group of Hershkovitz (1977, 1982) included just two species, Saguinus nigricollis (three subspecies) and S. fuscicollis (14 subspecies). Currently, Leontocebus nigricollis is composed of the (same) three subspecies, and the saddle-back tamarin of Hershkovitz is now composed of 13 species and one subspecies. A genetic analysis of the Peruvian members of this group by Matauschek (2010; Matauschek et al., 2011) showed that all but Saguinus fuscicollis melanoleucus should be considered species. Groves (2001) listed S. f. melanoleucus as a species, but Matauschek et al. (2011) found it be genetically very similar to Saguinus weddelli. They maintained it as a subspecies of Saguinus (now Leontocebus) weddelli because of its distinct white coloration. Cropp et al. (1999) showed that Saguinus fuscus, considered a subspecies of fuscicollis by Hershkovitz, was a distinct species. It is the northernmost form of the nigricollis tamarin group and its range is otherwise enveloped by Saguinus nigricollis, south of the Río Putumayo-Içã, and S. nigricollis graellsi to the west of its range in the Japurá-Caquetá interfluvium (Rylands et al., 2011). It has also been confused with S. n. nigricollis (Defler, 1994, 2004; Rylands et al., 2011). Saguinus fuscus is quite probably a member of the nigricollis clade rather than that of fuscicollis.
Of the central Amazonian (Brazilian) forms assigned as subspecies of fuscicollis by Hershkovitz (1977), S. fuscicollis acrensis was found to be a hybrid fuscicollis × melanoleucus (see Peres et al., 1996). The form cruzlimai, its provenance revealed, was shown to be a species by Sampaio et al. (2015). The taxonomic status of the Brazilian saddle-back tamarins, Leontocebus fuscicollis avilapiresi, L. f. mura and L. f. primitivus was reviewed by Röhe (2021), who placed them as full species. Saguinus fuscicollis crandalli Hershkovitz, 1966, known only from a single specimen of unknown provenance, like acrensis, may well be a hybrid. Hershkovitz (1977; p.622) indicated that it might have come from the upper reaches of the Purus and Juruá basins, noting that it was intermediate in coloration between acrensis and the darker cruzlimai from the upper Purus.
Lopes et al. (2023) described the Kulina moustached tamarin, Tamarinus kulina, from the interfluvium of the lower rios Juruá and Tefé, Amazonas, Brazil. In a phylogenetic analysis, Tamarinus mystax was found to be distinct at the species level from its former subspecies T. m. pileatus and T. m pluto. Lopes et al. (2023) placed pluto as a subspecies of pileatus, providing a new taxonomy for the group: Tamarinus mystax, T. pileatus and T. pileatus pluto. The recognition of T. kulina contracts the formerly accepted range of T. mystax (extending east to the Rio Tefé). The eastern limit of T. mystax is now the left (west) bank of the Rio Juruá. Hershkovitz (1979) reviewed the taxonomy and distribution of Saguinus imperator and resurrected Saguinus imperator subgrisescens Lönnberg, 1940. Gregorin et al. (2023) determined that it is a species, not a subspecies, on the basis of a study of the pelage, cranial morphometry, and cytochrome−b divergence. Hybridization is unknown but sampling in target areas is insufficient to determine a contact zone between the two lineages (Gregorin et al., 2023). The two forms are placed in the genus Tamarinus along with the other mustached tamarins and the mottled-faced tamarin Tamarinus inustus that has been shown to belong to the same clade (Buckner et al., 2015; Brcko et al., 2022).
Vallinoto et al. (2006) indicated that the Rio Tocantins may act as a barrier to gene flow for Saguinus niger. This was presaged in a molecular genetic analysis by Tagliaro et al. (2005). The form described as Mystax ursulus umbratus Thomas, 1922, from Cametá, Rio Tocantins, Pará, listed by Groves (2001, 2005) as a junior synonym of S. niger, and by Hershkovitz (1977) as a junior synonym of S. midas niger, was, in this case, considered to be a distinct geographical race or species (J. S. Silva-Júnior, pers. comm., April 2007). A study by Gregorin and de Vivo (2013) revalidated Saguinus ursula Hoffmannsegg, 1807, the type species of Saguinus Hoffmannsegg, 1807, naming a lectotype (one of four syntypes) from the vicinity of Belém, Pará. Its range is delimited in the east by the Rio Tocantins. Saguinus niger occurs west of the Rio Tocantins to the Rio Xingu. Differentiation was based on pelage coloration.
The taxonomy of the squirrel monkeys is unclear. Ruiz-García et al. (2014a) carried out a molecular phylogenetic study of the genus Saimiri. They argued for just two species – S. oerstedii (Central America) and S. sciureus (South America) – and found that genetic distances between the populations were small and recent (Pleistocene). They reported on extensive hybridization, and identified five distinct clades within the range that is currently assigned to S. macrodon and three within the range of S. ustus. They argued that the radiation of South American squirrel monkeys, currently eight species, one with a subspecies (Rylands and Mittermeier, 2024), should be arranged as subspecies of S. sciureus, following the Biological Species Concept. A phylogenetic, phylogeographic study by Lynch Alfaro et al. (2015) found that S. albigena, S. cassiquiarensis and S. macrodon formed a clade, and that, like Ruiz-García et al. (2014a), S. macrodon was paraphyletic, with three macrodon lineages. They termed this group the “cassiquiarensis complex” and suggested that albigena, and the three lineages of macrodon be considered subspecies of cassiquiarensis, which they found to be the oldest named taxon. The biogeographical assessment of Saimiri by Lynch Alfaro et al. (2015) discussed evidence for the ongoing taxonomic research on this complex group. While Ruiz-García et al. (2014a), following Hershkovitz (1984), limited S. collinsi to the island of Marajó in the Amazon estuary, Mercês et al. (2015, 2017) showed that its range extends way south of the Rio Amazonas to the rain forests’ transition to the Cerrado (bush savanna), east to the Maranhão lowlands, and west at least to the Rio Xingu basin.
The capuchin monkeys split into gracile (Cebus) and robust (Sapajus) forms about 6.2. mya (Lynch Alfaro et al., 2012a). The robust capuchins were confined to the Atlantic Forest and Cerrado (bush savanna) until about 700 ka, and their wide-ranging sympatry across the Amazon Basin is the result of a single explosive late Pleistocene invasion by Sapajus apella about 400 ka (Lynch Alfaro et al., 2012a). The species’ recent occupation explains the lack of differentiation throughout the basin even though the pelage varies considerably, something which severely confounded past attempts to document their taxonomy (Hill, 1960; Torres, 1983, 1988; Silva-Júnior, 2001; Rylands et al., 2005). Although not published by Hershkovitz, a glimpse of his prospective taxonomy for the Peruvian robust capuchins at least can be found in Aquino and Encarnación (1994). Today we accept, tentatively, generously, only Sapajus apella margaritae, on the Venezuelan island of Margarita, and S. apella macrocephalus of the upper Amazon, as subspecies. All the non-Amazonian robust capuchins are classified as monotypic species, including the southern black-horned capuchin S. cucullatus (Spix, 1823), from the Atlantic Forest of southern Brazil and northern Argentina, listed as a subspecies by Groves (2001).
An analysis of the mitochondrial DNA of the genus Cebus by Boubli et al. (2012) resulted in the finding that the Venezuelan capuchin, then called Cebus brunneus, was a distinct species with affinities to the white-fronted capuchins (the group that includes Cebus albifrons) as opposed to the weeper or wedge-capped capuchins (the group that includes Cebus olivaceus) as had been thought previously. It was listed in Mittermeier et al. (2013) as such, but examination of the type specimen (the first specimen to which the name had been attributed) revealed that it was in fact Cebus olivaceus, rendering the name brunneus invalid. Boubli et al. (2012) were not wrong, however, in their finding that there is indeed a distinct capuchin, albeit now lacking a name, of the white-fronted group in Venezuela, and studies are underway to describe it, discover its geographic distribution, and give it a name (B. Urbani, J.W. Lynch, pers. comm.).
There have been no taxonomic changes or new species' descriptions since the update of Rylands et al. (2012). The most recent review and appraisal maintains 11 species, one of them, Aotus azarae comprising three subspecies (Fernandez-Duque et al., 2023). These authors emphasize that it is difficult to distinguish species by phenotypical differences. Ruiz-García et al. (2011) indicated that “Aotus azarae and A. a. boliviensis are clearly differentiated forms based on the mtCOII gene and they are extremely divergent with regard to other Aotus,” and that “if Aotus azarae and A. infulatus are related, as suggested by Plautz et al. (2009), this would imply that A. a. azarae and A. a. boliviensis are different species, while A. a. azarae and A. infulatus represent 2 subspecies of the same species.” (p.1232). We are unaware that this proposal has been confirmed.
Six titi monkeys have been described since 2012, five in what is now the genus Plecturocebus and one in the genus Cheracebus. Plecturocebus vieirai (Gualda-Barros et al., 2012) occurs in the Brazilian states of Mato Grosso and Pará, in the interfluvium of the rios Xingu and Irirí, south to the upper reaches of the Rio Teles Pires. Plecturocebus miltoni (Dalponte et al., 2014) was discovered in the Guariba-Roosevelt Extractivist Reserve, between the rios Roosevelt and Aripuanã in the state of Mato Grosso, Brazil. Formerly confused with C. brunneus, Plecturocebus urubambensis was described by Vermeer and Tello-Alvarado (2015) from the ríos Urubamba and Manu, Peru. Plecturocebus brunneus is now confined to the north of the state of Rondônia, Brazil. Vermeer and Tello-Alvarado (2015) also resurrected Plecturocebus toppini (Thomas, 1914), described from the Río Tahuamanu, Peru, and occurring south of the Rio Purus, west from the mouth of its right bank tributary the Rio Ituxí, and south to the north bank of the Río Madre de Dios, an area formerly thought to have been occupied by Plecturocebus dubius. Plecturocebus grovesi was described by Boubli et al. (2019) from Alta Floresta, northern Mato Grosso, Brazil. It occurs between the rios Teles-Pires and Juruena and the Rio Arinos, a right (west) bank tributary of the Juruena. Plecturocebus parecis Gusmão et al., 2019, was described from the central southern part of Brazilian Amazonia in the state of Rondônia and named after the Chapada dos Parecis where it was found. It is closely related to P. cinerascens, described by Spix in 1823 that also occurs in the basin of the upper Rio Madeira but further north. Byrne et al. (2024) reviewed the evidence for P. parecis being a distinct species and concluded that there was a strong argument for it being considered just a cline of P. cinerascens, with gradual variation of pelage coloration from the northern to the southern populations. Gusmão et al. (2019) and Byrne et al. (2024) agree that further study is required.
A collared titi, Cheracebus aquinoi was described by Rengifo et al. (2023) using morphological (cranial and pelage) and molecular (mitochondrial DNA) evidence. It occurs between the ríos Nanay and Tigre, south of the Rio Napo, in Peru. The distribution of the white-collared titi, Cheracebus torquatus (Hoffmannsegg, 1807), had long been a mystery (Hershkovitz, 1990) but Byrne et al. (2020), who reviewed its taxonomic history and studied more than 100 skins across the genus Cheracebus, including the holotype of C. torquatus, concluded that it was a senior synonym of Cheracebus purinus (Thomas, 1927), known from south of the Rio Solimões, between the rios Juruá and Purus in the state of Amazonas, Brazil.
Marsh (2014) revised Hershkovitz’s (1987a) taxonomy of the sakis, Pithecia. Hershkovitz’s (1987a) taxonomy recognized just eight species and subspecies in two species’ groups – Pithecia (one species) and P. monachus (four species). Marsh’s revision was based on examination of the morphology of specimens (skins and skulls) in 36 museums and the pelage patterns of hundreds of photographs of captive and wild sakis. Marsh’s research resulted in a list of 16 species. They included the eight taxa recognized by Hershkovitz, two that he considered to be synonyms (Pithecia hirsuta Spix, 1823, and inusta Spix, 1823), one which was evidently overlooked by Hershkovitz (Pithecia monachus napensis Lönnberg, 1938), and five newly described species (P. cazuzai, P. isabela, P. mittermeieri, P. rylandsi, and P. pissinattii). Pithecia isabela is the saki occurring in the Pacaya-Samiria National Reserve between the lower ríos Ucayali and Marañón in northern Peru. Pithecia cazuzai is found between the lower Rio Japurá and Rio Solimões. Serrano-Villavicencio et al. (2019) reviewed what they referred to as the P. irrorata group that included vanzolinii and three of the species described by Marsh (2014) – mittermeieri, rylandsi, and pissinattii. They concluded that the three new Marsh species are in fact junior synonyms of irrorata Gray, 1843 (not 1842) but that P. irrorata vanzolinii of Hershkovitz (1987a) should, as indicated by Marsh, be considered a species. Genetic research underway may well confirm their conclusions (J. P. Boubli, pers. comm.). Serrano-Villavicencio et al. (2019) also made some important observations concerning the date of authorship of P. irrorata, its nomenclatural types, and the lack of a precise type locality.
Formerly composed of just two species, Chiropotes albinasus (monotypic) and Chiropotes satanas (polytypic), as per Hershkovitz (1985), Groves (2001); Silva-Júnior and Figueiredo (2002), and Silva-Júnior et al., 2013) placed all the bearded sakis as species. Silva-Júnior and Figueiredo (2002) concluded that the name for the bearded sakis in Guyana, Suriname, French Guiana, and east of the Rio Branco in Brazil was correctly Chiropotes sagulatus (Traill, 1821), and restricted Chiropotes chiropotes (Humboldt, 1811) to the west of the Rio Branco and north of the Rio Negro in Brazil, north into Venezuela to the Río Orinoco.
Silva-Júnior and Martins (1999) reported the occurrence of a white uakari along the Rio Jurupari, affluent of the Rio Envira, in the state of Acre, Brazil, that was distinct from Cacajao calvus novaesi of Hershkovitz (1987b) from the Rio Juruá. In 2022, Silva et al. (2022) described it as a new species, the Kanamari white uacari, Cacajao amuna, which occurs along the right bank of the Rio Tarauacá, a south-bank tributary of the Rio Juruá extending to the upper reaches of the Rio Pauiní, an affluent of the Rio Purus. Silva et al. (2022) argued that all the bald uakaris, formerly subspecies of Cacajao calvus, should be considered species.
The current count for howler monkeys is 16 taxa, 11 of them monotypic species (Rylands and Mittermeier, 2024) but some of the subspecies are of doubtfully validity. The mantled howler monkey, Alouatta palliata, has five listed subspecies. A review by Ruiz-García et al. (2017, p.421) concluded that A. palliata mexicana is the most differentiated taxon but that the remaining four – A. p. palliata (Guatemala, Nicaragua, Honduras, and Costa Rica), A. p. aequatorialis (Colombia and Ecuador, west of the Andes), A. p. coibensis (island of Coiba, Panama), and A. p. trabeata (Azuero Peninsula, Panama) – showed no relevant differences among individuals of the different putative taxa (p.421). They suggested just a single subspecies, mexicana, besides the nominate palliata. Rylands et al. (2006) listed coibensis and trabeata as species, which is clearly incorrect. Cortés-Ortiz et al. (2003) found no evidence to justify the validity of coibensis and trabeata as species or subspecies but those concerned with their conservation maintain coibensis as a species with trabeata as its subspecies (for example, Díaz-Ferguson et al., 2024). Cortés-Ortiz et al. (2015) reported on a phylogenetic analysis based on nuclear markers that supported a phylogeographic break between A. p. palliata and A. p. aequatorialis and, like Ruiz-Garcia et al. (2017), a proximity of coibensis and trabeata to aequatorialis. Cortés-Ortiz et al. (2015) indicated the need for further studies of these taxa to better delineate their subspecific taxonomy.
The taxonomic arrangement for the widespread red howler, Alouatta seniculus, currently comprising eight species and subspecies, is also still undecided. In a morphological study, Gregorin (2006) validated two red howler species from western Amazonia, Alouatta juara Elliot, 1910, and Alouatta puruensis Lönnberg, 1941. Their distributions are poorly known and whether they are valid species or subspecies is still undecided. Cortés-Ortiz et al. (2015) maintained them as subspecies until phylogenomic analyses underway can confirm their validity and taxonomic status. The taxonomy of the black belzebul group seems settled with three species – belzebul, ululata and discolor – but genomic analyses may well change that. Research on the long-standing conundrum of whether the Atlantic Forest brown howler, Alouatta guariba Humboldt, 1812, is a monotypic species or comprises two subspecies is underway (L. Oklander, pers. comm).
As pointed out by Morales-Jimenez et al. (2015a), the taxonomy of the spider monkeys is complicated because pelage color patterns are so variable, especially in the Mesoamerican forms, and proposed taxonomies for the genus are very mixed, even using molecular genetics, recognizing from 1–7 species. Ruiz-García et al. (2016), for example, proposed two or three species – Ateles paniscus, A. belzebuth and perhaps A. geoffroyi. Morales-Jimenez et al. (2015a) argued that the taxonomy of Groves’ (2001) reflects the best phylogeny that they had found and confirmed the arrangement of seven species of spider monkeys. They discovered that A. marginatus is basal in the Ateles radiation. Ateles geoffroyi of Mexico and Central America is polytypic. Kellogg and Goldman, 1944, described nine subspecies, but three have been synonymized. Silva-López et al. (1996) argued that Ateles geoffroyi pan Schlegel, 1876, was not valid. Ateles geoffroyi panamensis Kellogg and Goldman, 1944, was considered to be a junior synonym of A. g. ornatus by Napier (1976), Groves (2001) and Morales-Jimenez et al. (2015b). Morales-Jimenez et al. (2015b) concluded that Ateles geoffroyi yucatanensis Kellogg and Goldman, 1944, should be considered a junior synonym of Ateles geoffroyi vellerosus Gray, 1865, but believed that the spider monkeys of the Azuero peninsula, Panama, should continue to be distinguished as A. g. azuerensis (Bole, 1937) until specimens of A. g. ornatus (Gray, 1870) from other locations are available for analysis. Specimens from El Salvador were shown to be distinct from A. g. vellerosus and possibly a new subspecies. Spider monkeys from southwestern Nicaragua and northwestern Costa Rica are aligned with A. g. frontatus, but whether individuals from Nicaragua, currently considered to be A. g. geoffroyi are distinct from those from southwestern Nicaragua and northwestern Costa Rica has yet to be detemined, and the phylogenetic identity of individuals from Honduras, central and western Panama, and eastern Costa Rica is still unknown (Morales-Jiménez et al., 2015b).
The Grizzled Spider Monkey, Ateles geoffroyi grisescens Sclater in Gray, 1866, was described in a manuscript that catalogued the mammals in the London Zoological Gardens in 1865. The type locality, however, is unknown. Kellogg and Goldman (1944) suggested that it might hail from the Río Tuyra (Tuira) basin, Panama, probably extending south-eastward through the Serranía del Sapo in extreme south-eastern Panama and perhaps the Cordillera de Baudó of north-western Colombia (Hernández-Camacho and Cooper, 1976). Méndez-Carvajal and Cortés-Ortiz (2020) affirmed that it had never yet been seen in the wild. Méndez-Carvajal (2021) reported, however, that a group of black spider monkeys with a fringe of whitish hairs on the chin and cheeks had been found on the Pacific side of eastern Panama and believed it to be the spider monkey that Sclater had described. Whitish hairs around the chin and mouth are, however, a diagnostic feature of the Colombian Black Spider Monkey, Ateles rufiventris, the range of which extends into southern Panama (Kellogg and Goldman, 1944 (Hernández-Camacho and Cooper, 1976).
A number of phylogenetic studies have confirmed the taxonomy of the woolly monkeys, Lagothrix, as proposed by Fooden (1963). Although Groves (2001, 2005) classified Lagothrix cana, L. lugens, and L. poeppigii as full species rather than subspecies of Lagothrix lagothricha (Humboldt, 1812), Botero et al. (2011, 2015), Botero and Stevenson (2014), Defler (2014) and Ruiz-García et al. (2014b) argued that they diverged only in the Pleistocene, and that there was much overlap and interbreeding between neighboring taxa, and considerable phenotypic plasticity amongst them. They argued in favor of the classification of Fooden (1963) that has just two species, Lagothrix flavicauda (monotypic) and L. lagothricha (polytypic with four subspecies). Groves (2001, 2005) recognized the form Lagothrix tschudii Pucheran, 1857, from southern Peru and Bolivia as a subspecies L. cana. Fooden (1963) had considered it a junior synonym of L. lagotricha cana. Ruiz-García et al. (2019) tentatively validated Groves’ (2001) recognition of it as a distinct subspecies but of L. lagothricha, not cana.
Groves (2001) listed Lagothrix poeppigii Schinz, 1844 as a species, with Lagothrix castelenaui I. Geoffroy St.-Hilaire and Deville, 1848, a junior synonym because its type locality, as restricted by Fooden (1963) (not by Groves), was in the high altitudes of the western edge of the species’ range, as is that of L. poeppigii. Groves (2001) suggested that there might be an undescribed subspecies in the most easterly, mainly lowland parts of the species’ range. There was evidently a misunderstanding on the part of Ruiz-García et al. (2020) in that they believed that Groves had indicated that L. castelnaui might be a valid subspecies. He had not, so they merely (but importantly) confirmed Groves’ placement of L. castelnaui as a synonym. To what extent they would be able to exclude the possibility of an undescribed western, lowland taxon in the range of poeppigii is unclear.
Vieira (1944) recognized two subspecies of the muriqui, Brachyteles: the southern B. arachnoides (É Geoffroy Saint-Hilaire, 1806) and the northern B. hypoxanthus (Wied, 1829). Groves (2001, 2005) listed them as species, and this arrangement was confirmed by Chaves et al. (2019).
With the extraordinary advances in genetics in recent decades it is now possible to identify evolutionary lineages – which are what we need to conserve. The taxonomies of the younger “explosive”, late Pliocene and Pleistocene radiations, evident in such as Sapajus, Cebus, Saimiri and Aotus, are more difficult to “pin-down”, but hardly indecipherable, and understanding their blurrier lineages, their hybrid zones, is vital. We call them species or subspecies to allow for a descriptive orderliness in understanding diversity. Having the capacity to identify evolutionary lineages, clinging to the BSC – the identification of species and subspecies based on a fixed notion of reproductive isolation and often unfounded opinion of the degree of difference in internal and external morphology – is a sunk-cost fallacy. Groves’ (2004, 2012, 2014) has provided lucid explanations of the importance of the Phylogenetic Species Concept as a scientific proposition for our capacity to identify primate species. Groves’ “diagnosability criterion for species delimitation” is now the key refinement to distinguish evolutionary lineages, providing the insights needed to comprehend, describe, and name biodiversity for its conservation. Even assuming a classification based on the BSC, all taxa with evolutionary or morphologically evident elements, i.e. clear and evident subspecies, must absolutely be taken into consideration from a conservation point of view to safeguard global biodiversity.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
AR: Conceptualization, Writing – original draft, Writing – review & editing. RM: Conceptualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We are most grateful to two reviewers for their incisive and helpful comments.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Arámbulo P. III, Encarnación F., Estupiñán J., Samamé H., Watson C. R., Weller R. E. (Eds.) (1993). Primates of the Americas, Strategies for Conservation and Sustained Use in Biomedical Research (Columbus, OH: Battelle Press).
Avise J. C. (1989). A role for molecular geneticists in the recognition and conservation of endangered species. Trends Ecol. Evol. 4, 279–281.
Barroso C. M. L., Schneider H., Schneider M. P. C., Sampaio I., Harada M. L., Czelusniak J., et al. (1997). Update on the phylogenetic systematics of New World monkeys: further DNA evidence for placing the pygmy marmoset (Cebuella) within the genus Callithrix. Int. J. Primatol. 18, 651–674.
Botero S., Stevenson P. R. (2014). “Coat color is not an indicator of subspecies identity in Colombian woolly monkeys,” in The Woolly Monkey: Behaviour, Ecology, Systematics, and Captive Research. Eds. Defler T. R., Stevenson P. R. (New York: Springer), 17–31.
Botero S., Rengifo L. Y., Bueno M. I., Stevenson P. R. (2011). How many species of woolly monkeys inhabit Colombian forests? Am. J. Primatol. 72, 1131–1140.
Botero S., Stevenson P. R., Di Fiore A. (2015). A primer on the phylogeography of Lagothrix lagotricha (sensu Fooden) in northern South America. Mol. Phylogenet. Evol. 82, 511–517.
Boubli J. P., da Silva M. N. F., Rylands A. B., Nash S. D., Bertuol F., Nunes M., et al. (2018). How many pygmy marmoset (Cebuella Gray, 1870) species are there? A taxonomic re-appraisal based on new molecular evidence. Mol. Phylogenet. Evol. 120, 170–182.
Boubli J. P., Byrne H., da Silva M. N. F., Silva-Júnior J., Costa-Araújo R., Bertuol F., et al. (2019). On a new species of titi monkey (Primates: Plecturocebus Byrne et al., 2016), from Alta Floresta, southern Amazon, Brazil. Mol. Phylogenet. Evol. 132, 117–137.
Boubli J. P., Janiak M. C., Porter L. M., de la Torre S., Cortés-Ortiz L., da Silva M. N. F., et al. (2021). Ancient DNA of the pygmy marmoset type specimen Cebuella pygmaea (Spix 1823) resolves a taxonomic conundrum. Zool. Res. 42, 761–771.
Brcko I. C., Carneiro J., Ruiz-García M., Boubli J. P., Silva-Júnior J. S., Farias I., et al. (2022). Phylogenetics and an updated taxonomic status of the tamarins (Callitrichinae, Cebidae). Mol. Phylogenet. Evol. 173, 10754.
Buckner J. C., Lynch Alfaro J., Rylands A. B., Alfaro M. E. (2015). Biogeography of the marmosets and tamarins (Callitrichidae). Mol. Phylogenet. Evol. 82, 413–425.
Byrne H. M., Costa-Araújo R., Faria I. P., da Silva M. N. F., Messias M., Hrbek T., et al. (2024). Uncertainty regarding species delimitation, geographic distribution, and the evolutionary history of south-central Amazonian titi monkey species (Plecturocebus, Pitheciidae). Int. J. Primatol. 45, 12–34. doi: 10.1007/s10764-021-00249-9
Byrne H. M., Rylands A. B., Carneiro J., Lynch-Alfaro J. W., Bertuol F., da Silva M. N. F. (2016). Phylogenetic relationships of the New World titi monkeys (Callicebus): first appraisal of taxonomy based on molecular evidence. Front. Zool. 13, 10. doi: 10.1186/s12983-016-0142-4
Byrne H. M., Rylands A. B., Nash S. D., Boubli J. P. (2020). On the taxonomic history and true identity of the collared titi, Cheracebus torquatus (Hoffmannsegg, 1807) (Platyrrhini, Callicebinae). Primate Conserv. 34, 13–52.
Cabrera A. (1957). Catalogo de los mamíferos de América del Sur. Rev. Mus. Argentino Cienc. Nat. “Bernardino Rivadavia” 4, 1–307.
Chaves P. B., Magnus T., Jerusalinsky L., Talebi M., Strier K. B., Breves P., et al. (2019). Phylogeographic evidence for two species of muriqui (genus Brachyteles). Am. J. Primatol. 2019, e23066.
Corbet G. B., Hill J. E. (1980). A Word List of Mammalian Species (London and Ithaca: British Museum (Natural History), Comstock Publishing Associates, Cornell University Press).
Corbet G. B., Hill J. E. (1991). A World List of Mammalian Species (Oxford: Natural History Museum Publications, Oxford University Press).
Cortés-Ortiz L., Bermingham E., Rico C., Rodríguez-Luna E., Sampaio I., Ruiz-García M. (2003). Molecular systematics and biogeography of the Neotropical monkey genus, Alouatta. Mol. Phylogenet. Evol. 26, 64–81.
Cortés-Ortiz L., Rylands A. B., Mittermeier R. A. (2015). “The taxonomy of howler monkeys: integrating old and new knowledge from morphological and genetic studies,” in Howler Monkeys: Adaptive Radiation, Systematics, and Morphology. Eds. Kowalewski M. M., Garber P. A., Cortés-Ortiz L., Urbani B., Youlatos D. (New York: Springer), 55–84.
Costa-Araújo R., Melo F. R., Canale G. R., Hernández-Rangel S. M., Messias M. R., Rossi R. V., et al. (2019). The Munduruku marmoset: a new monkey species from southern Amazonia. PeerJ 7, e7019. doi: 10.7717/peerj.7019
Costa-Araújo R., Silva−Júnior J. S., Boubli J. P., Rossi R. V., Canale G. R., Melo F. R., et al. (2021). An integrative analysis uncovers a new, pseudo-cryptic species of Amazonian marmoset (Primates: Callitrichidae: Mico) from the arc of deforestation. Sci. Rep. 11, 15665. doi: 10.1038/s41598-021-93943-w
Cracraft J. (1983). “Species concepts and speciation analysis,” in Current Ornithology, Vol. 1. (New York: Plenum Press), 159–187.
Cropp S. J., Larson A., Cheverud J. M. (1999). Historical biogeography of tamarins, genus Saguinus: the molecular phylogenetic evidence. Am. J. Phys. Anthropol. 108, 65–89.
Dalponte J., Silva F. E., Silva-Júnior J. S. (2014). New species of titi monkeys, genus Callicebus Thomas, 1903 Primates, Pitheciidae), from southern Amazonia, Brazil. Pap. Avuls. Zool. São Paulo 54, 457–472.
Defler T. R. (1994). La conservación de primates en Colombia. Trianea (Acta Cientifica INDERENA) 5, 255–287.
Defler T. R. (2004). Primates of Colombia. Tropical Field Guide Series (Washington, DC: Conservation International).
Defler T. R. (2014). “Colombian Lagothrix: analysis of their phenotypes and taxonomy,” in The Woolly Monkey: Behavior, Ecology, Systematics, and Captive Research. Eds. Defler T. R., Stevenson P. R. (New York: Springer), 32–58.
De Queiroz K. (1998). “The general lineage concept of species, species criteria, and the process of speciation: a conceptual unification and terminological recommendations,” in Endless Forms: Species and Speciation. Eds. Howard D. J., Berlocher S. H. (New York: Oxford University Press), 57–75.
De Queiroz K. (2005). A unified concept of species and its consequences for the future of taxonomy. Proc. California Acad. Sci. Ser. 4 56, 196–215.
Díaz-Ferguson E., Ramos C. W., Pineda Y., Castro-Pérez R. E., Méndez-Carvajal P. G. (2024). Genetic approach of the Coiba Island howler monkey Alouatta coibensis coibensis from Panama, and its conservation implications. Tecnociencia 26, 112–128.
Di Fiore A., Chaves P. B., Cornejo F. M., Schmitt C. A., Shanee S., Cortés-Ortiz L., et al. (2015). The rise and fall of a genus: complete mtDNA genomes shed light on the phylogenetic position of yellow-tailed woolly monkeys, Lagothrix flavicauda, and on the evolutionary history of the family Atelidae (Primates: Platyrrhini). Mol. Phylogenet. Evol. 82, 495–510.
Elliot D. G. (1913). A Review of Primates, Monograph Series (New York: American Museum of Natural History).
Ennos R. A., French G. C., Hollingsworth P. M. (2005). Conserving taxonomic complexity. Trends. Ecol. Evol. 20, 164–168.
Fernandez-Duque E., Corley M. K., Spence-Aizenberg A. (2013). “Family Aotidae (night monkeys),” in Handbook of the Mammals of the World. Volume 3. Primates. Eds. Mittermeier R. A., Rylands A. B., Wilson D. E. (Barcelona: Lynx Edicions), 414–431.
Fernandez-Duque E., Juárez C. P., Defler T. R. (2023). “Morphology, systematics and taxonomy of owl monkeys,” in Owl Monkeys: Biology, Adaptive Radiation and Behavioral Ecology of the Only Nocturnal Primates in the Americas, E (New York: Springer).
Ferrari S. F., Veiga L. M., Pinto L. P., Marsh L. K., Mittermeier R. A., Rylands A. B. (2013). “Family Pitheciidae (titis, sakis, and uacaris),” in Handbook of the Mammals of the World. Volume 3. Primates. Eds. Mittermeier R. A., Rylands A. B., Wilson D. E. (Barcelona: Lynx Edicions), 432–457.
Fraser D. J., Bernatchez L. (2001). Adaptive evolutionary conservation: towards a unified concept for defining conservation units. Mol. Ecol. 10 (12), 2741–2752.
Garbino G. S. T. (2014). The taxonomic status of Mico marcai (Alperin, 1993) and Mico manicorensis (Van Roosmalen et al., 2000) (Cebidae, Callitrichinae) from southwestern Brazilian Amazonia. Int. J. Primatol. 35, 529–546.
Garbino G. S. T. (2015). How many marmoset (Primates: Cebidae: Callitrichinae) genera are there? A phylogenetic analysis based on multiple morphological systems. Cladistics 31, 652–678.
Garbino G. S. T., Casalia D. M., Nascimento F. O., Serrano-Villavicencio J. E. (2019). Taxonomy of the pygmy marmoset (Cebuella Gray, 1866): geographic variation, species delimitation, and nomenclatural notes. Mammal. Biol. 95, 135–142.
Garbino G. S. T., Martins Junior A. M. G. (2018). Phenotypic evolution in marmoset and tamarin monkeys (Cebidae, Callitrichinae) and a revised genus-level classification. Mol. Phylogenet. Evol. 118, 156–171.
Gippoliti S., Amori G. (2007). The problem of subspecies and biased taxonomy in conservation lists: the case of mammals. Folia Zool. 56, 113–117.
Gippoliti S., Cotterill F. P. D., Zinner D., Groves C. P. (2017). Impacts of taxonomic inertia for the conservation of African ungulate diversity: an overview. Biol. Rev. 93, 115–130.
Goodland R. J. A., Irwin H. S. (1975). Amazon Jungle: Green Hell to Red Desert? (Amsterdam: Elsevier Scientific Publishing).
Gregorin R. (2006). Taxonomy and geographic variation of species of the genus Alouatta Lacépède (Primates, Atelidae) in Brazil. Rev. Bras. Zool. 23, 64–144.
Gregorin R., Athaydes D., dos Santos-Júnior J. E., Ayoub T. B. (2023). Taxonomic status of Tamarinus imperator subgrisescens (Lönnberg, 1940) (Cebidae, Callitrichinae). Pap. Avuls. Zool. 63, e202363005.
Gregorin R., de Vivo M. (2013). Revalidation of Saguinus ursula Hoffmannsegg (Primates: Cebidae: Callitrichinae). Zootaxa 3721, 172–182.
Groves C. P. (1983). “Order Primates,” in Mammal Species of the World: A Taxonomic and Geographic Reference, 2nd edition. Eds. Wilson D. E., Reeder D. M. (Washington, D.C: Smithsonian Institution Press), 243–277.
Groves C. P. (2005). “Order primates,” in Mammal Species of the World: A Taxonomic and Geographic Reference, 3rd Edition, Vol. 1 . Eds. Wilson D. E., Reeder D. M. (Johns Hopkins University Press, Baltimore, MD), 111–184.
Gualda-Barros J., do Nascimento F. O., do Amaral M. K. (2012). A new species of Callicebus Thomas, 1903 (Primates, Pitheciidae) from the states of Mato Grosso and Pará, Brazil. Pap. Avul. Zool. São Paulo 52, 261–279.
Gusmão A. C., de Messias M. R., Carneiro J. C., Schneider H., de Alencar T. B., Calouro A. M., et al. (2019). A new species of titi monkey, Plecturocebus Byrne et al., 2016 (Primates, Pitheciideae) from southwestern Amazonian, Brazil. Primate Conserv. 33, 21–35.
Hernández-Camacho J., Cooper R. W. (1976). “The non-human primates of Colombia,” in Neotropical Primates: Field Studies and Conservation. Eds. Thorington R. W. Jr., Heltne P. G. (Washington, DC: National Academy of Sciences), 85–69.
Hershkovitz P. (1949). Mammals of northern Colombia. Preliminary report No. 4: monkeys (Primates), with taxonomic revisions of some forms. Proc. U.S. Natl. Mus. 98, 323–342.
Hershkovitz P. (1957). The systematic position of the marmoset Simia leonina Humboldt (Primates). Proc. Biol. Soc. Washington 70, 17–20.
Hershkovitz P. (1977). Living New World Monkeys (Platyrrhini) with an Introduction to Primates Vol. 1 (Chicago: Chicago University Press).
Hershkovitz P. (1979). Races of the emperor tamarin, Saguinus imperator Goeldi (Callitrichidae, Primates). Primates 20, 277–287.
Hershkovitz P. (1982). Subspecies and geographic distribution of black-mantle tamarins Saguinus nigricollis Spix (Primates: Callitrichidae). Proc. Biol. Soc. Wash. 95, 647–656.
Hershkovitz P. (1984). Taxonomy of squirrel monkeys, genus Saimiri (Cebidae, Platyrrhini): a preliminary report with description of a hitherto unnamed form. Am. J. Primatol. 7, 155–210.
Hershkovitz P. (1985). A preliminary taxonomic review of the South American bearded saki monkeys genus Chiropotes (Cebidae, Platyrrhini), with the description of a new subspecies. Fieldiana Zool. 27, iii + 46.
Hershkovitz P. (1987a). The taxonomy of South American sakis, genus Pithecia (Cebidae, Platyrrhini): a preliminary report and critical review with the description of a new species and new subspecies. Am. J. Primatol. 12, 387–468.
Hershkovitz P. (1987b). Uacaries, New World monkeys of the genus Cacajao (Cebidae, Platyrrhini): a preliminary taxonomic review with the description of a new subspecies. Am. J. Primatol. 12, 1–53.
Hershkovitz P. (1990). Titis, New World monkeys of the genus Callicebus (Cebidae, Platyrrhini): a preliminary taxonomic review. Fieldiana Zool. n.s. 55, 1–109.
Hill W. C. O. (1957). Primates. Comparative Anatomy and Taxonomy III. Pithecoidea Platyrrhini (Families Hapalidae and Callimiconidae) (Edinburgh: Edinburgh University Press).
Hill W. C. O. (1960). Primates Comparative Anatomy and Taxonomy IV. Cebidae Part A (Edinburgh: Edinburgh University Press).
Hill W. C. O. (1962). Primates Comparative Anatomy and Taxonomy V. Cebidae Part B (Edinburgh: Edinburgh University Press).
Honacki J. H., Kinman K. E., Koeppl J. W. (1982). Mammal Species of the World. A Taxonomic and Geographic Reference (Lawrence, KS: Allen Press, Inc., The Association of Systematics Collections).
Isaac N. J. B., Mallet J., Mace G. M. (2004). Taxonomic inflation: its influence on macroecology and conservation. Trends Ecol. Evol. 19, 464–469.
Kobayashi S. (1995). A phylogenetic study of titi monkeys, genus Callicebus, based on cranial measurements: I. Phyletic groups of Callicebus. Primates 36, 101–120.
Lesson R.-P. (1840). Species des mammifères bimanes et quadrumanes; suivi d’un mémoire sur les oryctéropes (Paris: J.-B. Baillière).
Lopes G. P., Röhe F., Bertuol F., Polo E., Lima I. J., Valsecchi J., et al. (2023). Taxonomic review of Saguinus mystax (Spix, 1823) (Primates, Callitrichidae), and description of a new species. PeerJ. 11, e14526. doi: 10.7717/peerj.14526
Lynch Alfaro J. W., Boubli J. P., Olson L. E., Di Fiore A., Wilson B., Gutiérrez-Espeleta G. A., et al. (2012a). Explosive Pleistocene range expansion leads to widespread Amazonian sympatry between robust and gracile capuchin monkeys. J. Biogeog. 39, 272–288.
Lynch Alfaro J. W., Boubli J. P., Paim F. P., Ribas C. C., da Silva M. N. F., Messias M. R., et al. (2015). Biogeography of squirrel monkeys (genus Saimiri): south-central Amazon origin and rapid pan-Amazonian diversification of a lowland primate. Mol. Phylogenet. Evol. 82, 436–454.
Lynch Alfaro J. W., Schwochow D., Santini F., Alfaro M. E. (2010). “Capuchin phylogenetics and statistical phylogeography: implications for behavioral evolution,” in Abstracts and Program: International Primatological Society XXIII Congress Kyoto, 12–18 September 2010. Primate Research. 26, 253. (Abstract).
Lynch Alfaro J. W., Silva-Júnior J. S., Rylands A. B. (2012b). How different are robust and gracile capuchin monkeys? An argument for the use of Sapajus and Cebus. Am. J. Primatol. 74, 273–286.
Mace G. M. (2004). The role of taxonomy in species conservation. Phil. Trans. R. Soc Lond. B. 359, 711–719.
Mallet J. (2001). “Species, concepts,” in Encyclopedia of Biodiversity, Vol. 5 . Ed. Levin S. A. (Academic Press, London), 427–440.
Marsh L. K. (2014). A taxonomic revision of the saki monkeys, Pithecia Desmarest. Neotrop. Primates 21, 1–163.
Matauschek C. (2010). Taxonomy, Phylogeny and Distribution of Tamarins (Genus Saguinus Hoffmannsegg, 1807). Doctoral Dissertation (Göttingen: Georg-August Universität).
Matauschek C., Roos C., Heymann E. W. (2011). Mitochondrial phylogeny of tamarins (Saguinus Hoffmannsegg, 1807) with taxonomic and biogeographic implications for the S. nigricollis species group. Am. J. Phys. Anthropol. 144, 564–574.
Matthews L. J., Rosenberger A. L. (2008). Taxon combinations, parsimony analysis (PAUP*), and the taxonomy of the yellow-tailed woolly monkey, Lagothrix flavicauda. Am. J. Phys. Anthropol. 137, 245–255.
Mayr E., Ashlock P. D. (1991). Principles of Systematic Biology. 2nd edition (Singapore: McGraw Hill).
Mello B., Vilela J. F., Schargo C. G. (2018). Conservation phylogenetics and computational species delimitation of Neotropical primates. Biol. Conserv. 217, 397–406.
Méndez-Carvajal P. G. (2021). El mono araña negro del Darién es encontrado después de 70 años. Imagina 15, 51–52.
Méndez-Carvajal P. G., Cortés-Ortiz L. (2020). Ateles geoffroyi ssp. grisescens. The IUCN Red List of Threatened Species. 2020. e.T2287A17979753. doi: 10.2305/IUCN.UK.2020-2.RLTS.T2287A17979753.en
Mercês M. P., Lynch Alfaro J. W., Ferreira W. A. S., Harada M. L., Silva-Júnior J. S. (2015). Morphology and mitochondrial phylogenetics reveal that the Amazon River separates two eastern squirrel monkey species: Saimiri sciureus and S. collinsi. Mol. Phylogenet. Evol. 82, 426–435.
Mercês M. P., Paula W. S., Silva-Júnior J. S. (2017). New records of Saimiri collinsi Osgood 1916 (Cebidae, Primates), with comments on habitat use and conservation. Mammalia 82, 5pp. doi: 10.1515/mammalia-2017-0050
Mittermeier R. A., Kinzey W. G., Mast R. B. (1993). “Neotropical primate conservation,” in Primates of the Americas, Strategies for Conservation and Sustained Use in Biomedical Research. Eds. Arámbulo P. III, Encarnación F., Estupiñán J., Samamé H., Watson C. R., Weller R. E. (Columbus, OH: Battelle Press), 11–28.
Mittermeier R. A., Rylands A. B., Wilson D. E. (eds.). (2013). Handbook of the Mammals of the World. Volume 3. Primates. (Barcelona: Lynx Edicions).
Morales-Jimenez A. L., Disotell T., Di Fiore A. (2015a). Revisiting the phylogenetic relationships, biogeography, and taxonomy of spider monkeys (genus Ateles) in light of new molecular data. Mol. Phylogenet. Evol. 82, 467–483.
Morales-Jimenez A. L., Cortés-Ortiz L., Di Fiore A. (2015b). Phylogenetic relationships of Mesoamerican spider monkeys (Ateles geoffroyi): molecular evidence suggests the need for a revised taxonomy. Mol. Phylogenet. Evol. 82, 484–494.
Morrison III W. R., Lohr J. L., Duchen P., Wilches R., Trujillo D., Mair M., et al. (2009). The impact of taxonomic change on conservation: does it kill, can it save, or is it just irrelevant? Biol. Conserv. 142, 3201–3206.
Napier P. H. (1976). Catalogue of the Primates in the British Museum (Natural History). Part I. Families Callitrichidae and Cebidae (London: British Museum (Natural History)).
Oates J. F., Ting N. (2015). “Conservation consequences of unstable taxonomies: the case of the red colobus monkeys,” in Taxonomic Tapestries: The Threads of Evolutionary, Behavioural and Conservation Research. Eds. Behie A. M., Oxenham M. F. (Canberra: Australian National University), 321–343.
PAHO (1976). First Inter-American Conference on the Conservation and Utilization of American Nonhuman Primates in Biomedical Research (Lima, Peru, 2–4 June 1975) (Washington, DC: Sci. Publ. 317. Pan American Health Organization (PAHO).
Peres C. A., Patton J. L., da Silva M. N. F. (1996). Riverine barriers and gene flow in Amazonian saddle-back tamarins. Folia Primatol. 67, 113–124.
Plautz H. L., Goncalves E. C., Ferrari S. F., Schneider M. P. C., Silva A. (2009). Evolutionary inferences on the diversity of the genus Aotus (Platyrrhini, Cebidae) from mitochondrial cytochrome c oxidase subunit II gene sequences. Mol. Phylogenet. Evol. 51, 382–387.
Porter L. M., de la Torre S., Pérez-Peña P., Cortés-Ortiz L. (2021). Taxonomic diversity of Cebuella in the western Amazon: Molecular, morphological and pelage diversity of museum and free-ranging specimens. Am. J. Phys. Anthropol. 175, 251–267.
Rengifo E. M., D’Elia G., García G., Charpentier E., Cornejo F. M. (2023). A new species of titi monkey, genus Cheracebus Byrne et al., 2016 (Primates: Pitheciidae), from Peruvian Amazonia. Mammal Study 48 (1), 3–18. doi: 10.3106/ms2022-0019
Röhe F. (2021). Sistemática Molecular e Biogeografia do Gênero Leontocebus Wagner 1839 (Callitrichidae-Primates). PhD thesis (Manaus, AM, Brazil: Instituto Nacional de Pesquisas da Amazônia (INPA).
Rosenberger A. L. (1981). “Systematics: the higher taxa,” in Ecology and Behavior of Neotropical Primates Vol. 1. Eds. Coimbra-Filho A. F., Mittermeier R. A. (Rio de Janeiro: Academia Brasileira de Ciências), 9–27.
Rossi L., Gippoliti S., Angelici F. M. (2018). The role of indirect evidence and traditional ecological knowledge in the discovery and description of new species of monkeys and apes since 1980. Primates 59, 327–337. doi: 10.1007/s10329-018-0667-6
Ruiz-García M., Albino A., Pinedo-Castro M., Zeballos H., Bello A., Leguizamón N., et al. (2019). First molecular phylogenetic analysis of the Lagothrix taxon living in southern Peru and northern Bolivia: Lagothrix lagothricha tschudii (Atelidae, Primates), a new subspecies. Folia Primatol. 90, 215–239.
Ruiz-García M., Cerón A., Sánchez-Castillo S., Rueda-Zozaya P., Pinedo-Castro M., Gutierrez-Espeleta G., et al. (2017). Phylogeography of the mantled howler monkeys (Alouatta palliata; Atelidae, Primates) across its geographical range by means of mitochondrial genetic analyses and new insights about the phylogeny of Alouatta. Folia Primatol. 88, 421–454.
Ruiz-García M., Lichilín N., Escobar-Armel P., Rodríguez G., Gutiérrez-Espeleta G. (2016). “Historical genetic demography and some insights into the systematics of Ateles (Atelidae, Primates) by means of diverse mitochondrial genes,” in Phylogeny, Molecular Population Genetics, Evolutionary Biology and Conservation of the Neotropical Primates. Eds. Ruiz-García M., Shostell J. M. (New York: Nova Science), 1–43.
Ruiz-García M., Luengas-Villamil K., Leguizamon N., de Thoisy B., Gálvez H. (2014a). Molecular phylogenetics and phylogeography of all the Saimiri taxa (Cebidae, Primates) inferred from mt COI and COII gene sequences. Primates 2, 145–161. doi: 10.1007/s10329-014-0452-0
Ruiz-García M., Pinedo-Castro M., Albino A., Arias-Vásquez J. Y., Castellanos A., Shostell J. M. (2020). Invalidation of taxa within the silvery wooly monkey (Lagothrix lagothricha poeppigii, Atelidae, Primates). Mitochondrial DNA Part A. 31, 147–162.
Ruiz-García M., Pinedo-Castro M., Shostell J. M. (2014b). How many genera and species of woolly monkeys (Atelidae, Platyrrhine, Primates) are there? The first molecular analysis of Lagothrix flavicauda, an endemic Peruvian primate species. Mol. Phylogenet. Evol. 79, 179–198.
Ruiz-García M., Vásquez C., Camargo E., Leguizamón N., Gálvez H., Vallejo A., et al. (2011). Molecular phylogenetics of Aotus (Platyrrhini, Cebidae). Int. J. Primatol. 32, 1218–1241.
Ryder O. A. (1986). Species conservation and systematics: the dilemma of subspecies. Trends Ecol. Evol. 1, 9–10.
Rylands A. B., Groves C. P., Mittermeier R. A., Cortés-Ortiz L., Hines J. J. (2006). “Taxonomy and distributions of Mesoamerican primates,” in New Perspectives in the Study of Mesoamerican Primates: Distribution, Ecology, Behavior and Conservation. Eds. Estrada A., Garber P., Pavelka M., Luecke L. (New York: Springer), 29–79.
Rylands A. B., Heymann E. W., Lynch Alfaro J. W., Buckner J., Roos C., Matauschek C., et al. (2016). Taxonomic review of the New World tamarins (Callitrichidae, Primates). Zool. J. Linn. Soc. 177, 1003–1028. doi: 10.1111/zoj.12386
Rylands A. B., Kierulff M. C. M., Mittermeier R. A. (2005). Some notes on the taxonomy and distributions of the tufted capuchin monkeys (Cebus, Cebidae) of South America. Lundiana 6, 97–110.
Rylands A. B., Matauschek C., Aquino R., Encarnación F., Heymann E. W., de la Torre S., et al. (2011). The range of the golden-mantle tamarin, Saguinus tripartitus (Milne Edwards 1878): distributions and sympatry of four tamarins in Colombia, Ecuador, and northern Peru. Primates 52, 25–39.
Rylands A. B., Mittermeier R. A. (2013). “Family Callitrichidae (marmosets and tamarins),” in Handbook of the Mammals of the World. Volume 3. Primates. Eds. Mittermeier R. A., Rylands A. B., Wilson D. E. (Barcelona: Lynx Edicions), 262–346.
Rylands A. B., Mittermeier R. A. (2022). “Primate field research in the Neotropics: early history, systematics, and conservation,” in The Natural History of Primates: A Systematic Study of Ecology and Behavior. Eds. Sussman R. W., Hart D., Colquhoun I. C. (Washington, DC: Rowman & Littlefield), 288–293.
Rylands A. B., Mittermeier R. A. (2024). Taxonomic database (Re:wild, Austin, TX: Primate Program and IUCN SSC Primate Specialist Group (PSG)). December 2023. Unpublished.
Rylands A. B., Mittermeier R. A., Bezerra B. M., Paim F. P., Queiroz H. L. (2013a). “Species accounts of Cebidae,” in Handbook of the Mammals of the World. Volume 3. Primates. Eds. Mittermeier R. A., Rylands A. B., Wilson D. E. (Barcelona: Lynx Edicions), 348–413.
Rylands A. B., Mittermeier R. A., Cornejo F. M., Defler T. R., Glander K. E., Konstant W. R., et al. (2013b). “Family Atelidae (howlers, spider and woolly monkeys and muriquis),” in Handbook of the Mammals of the World. Volume 3. Primates. Eds. Mittermeier R. A., Rylands A. B., Wilson D. E. (Barcelona: Lynx Edicions), 484–542.
Rylands A. B., Mittermeier R. A., Rodríguez-Luna E. (1995). A species list for the New World primates (Platyrrhini): distribution by country, endemism, and conservation status according to the Mace-Lande system. Neotrop. Primates 3, 113–160.
Rylands A. B., Mittermeier R. A., Silva-Júnior J. S. (2012). Neotropical primates: taxonomy and recently described species and subspecies. Int. Zoo Yearb. 46, 11–24.
Sampaio R., Röhe F., Pinho G., Silva-Júnior J. S., Farias I. P., Rylands A. B. (2015). Re-description and assessment of the taxonomic status of Saguinus fuscicollis cruzlimai Hershkovitz, 1966 (Primates, Callitrichinae). Primates 56, 131–144. doi: 10.1007/s10329-015-0458-2
Schneider H., Sampaio I. (2015). The systematics and evolution of New World primates – a review. Mol. Phylogenet. Evol. 82, 348–357.
Serrano-Villavicencio J. S., Hurtado C. M., Vendramel R. L., do Nascimento F. B. (2019). Reconsidering the taxonomy of the Pithecia irrorata species group (Primates: Pitheciidae). J. Mammal. 100, 130–141.
Silva F. E., do Amaral J. V., Roos C., Bowler M., Röhe F., Sampaio R., et al. (2022). Molecular phylogeny and systematics of bald uakaris, genus Cacajao Lesson, 1840 (Primates: Pitheciidae), with the description of a new species. Mol. Phylogenet. Evol. 173, 107509. doi: 10.1016/j.ympev.2022.107509
Silva F. E., el Bizri H. R., Gonçalves J. D., Lemos L. P., Costa-Araújo R., Lima I. J., et al. (2020). The Roosevelt–Rondon expedition marmoset Mico marcai: unveiling the conservation status of a Data Deficient species. Oryx 54, 539–545.
Silva-Júnior J. de S. (2001). Especiação nos Macacos-prego e Caiararas, gênero Cebus Erxleben, 1777 (Primates, Cebidae). Doctoral thesis. Rio de Janeiro, Universidade Federal do Rio de Janeiro.
Silva-Júnior J. de S. (2002). “Sistemática dos macacos–prego e caiararas, gênero Cebus Erxleben, 1777 (Primates, Cebidae),” in Livro de Resumos, X Congresso Brasileiro de Primatologia: Amazônia – A Última Fronteira (Bélém: Sociedade Brasileira de Primatologia), p.35.
Silva-Júnior J. S., Figueiredo W. M. B. (2002). “Previsão sistemática dos cuxiús, genêro Chiropotes Lesson, 1840 (Primates, Pitheciidae),” in Livro de Resumos: X° Congresso Brasileiro de Primatologia “Amazônia: A Última Fronteira“ (Sociedade Brasileira de Primatologia, Belém), p.21.
Silva-Júnior J. S., Martins E. de S. (1999). On a new white bald uakari population in southwestern Brazilian Amazonia. Neotrop. Primates 7, 119–121.
Silva-Júnior J. S., Figueiredo-Ready W. M. B., Ferrari S. F. (2013). “Taxonomy and geographic distribution of the Pitheciidae,” in Evolutionary Biology and Conservation of the Titis, Sakis and Uacaris. Eds. Veiga L. M., Barnett A. A., Ferrari S. F., Norconk M. A. (Cambridge, UK: Cambridge University Press), 31–42.
Silva-López G., Motta-Gill J., Sánchez-Hernández A. I. (1996). Taxonomic notes on Ateles geoffroyi. Neotrop. Primates 4, 41–44.
Tagliaro C. H., Schneider H., Sampaio I., Schneider M. P. C., Vallinoto M., Stanhope M. (2005). Molecular phylogeny of the genus Saguinus (Platyrrhini, Primates) based on the ND1 mitochondrial gene and implications for conservation. Genet. Mol. Biol. 28, 46–53.
Tagliaro C. H., Schneider M. P. C., Schneider H., Sampaio I. C., Stanhope M. J. (1997). Marmoset phylogenetics, conservation perspectives, and evolution of the mtDNA control region. Mol. Biol. Evol. 14, 674–684.
Thorington R. W. Jr. (1976). ”The systematics of New World monkeys,” in First Inter-American Conference on the Conservation and Utilization of American Nonhuman Primates in Biomedical Research (Lima, Peru, 2–4 June 1975) (Washington, DC: Pan American Health Organization (PAHO)), 8–18.
Torres C. (1983). An Ecological Study of the Primates of Southeastern Brazil, with a Reappraisal of Cebus apella Races. PhD thesis. (Edinburgh: University of Edinburgh).
Torres C. (1988). Resultados preliminares de reavaliação das raças do macaco-prego Cebus apella (Primates: Cebidae). Revta. Nordest. Biol. 6, 15–28.
Vallinoto M., Araripe J., Rego. P. S., Tagliaro C. H., Sampaio I., Schneider H. (2006). Tocantins River as an effective barrier to gene flow in Saguinus Niger populations. Genet. Mol. Biol. 12, 823–833.
Van Roosmalen M. G. M., Van Roosmalen T. (1997). An eastern extension of the geographical range of the pygmy marmoset, Cebuella pygmaea. Neotrop. Primates 5, 3–6.
Van Roosmalen M. G. M., Van Roosmalen T., Mittermeier R. A. (2002). A taxonomic review of the titi monkeys, genus Callicebus Thomas, 1903 with the description of two new species, Callicebus bernhardi and Callicebus stephennashi, from Brazilian Amazonia. Neotrop. Primates 10, 1–52.
Van Roosmalen M. G. M., Van Roosmalen T., Mittermeier R. A., Rylands A. B. (2000). Two new species of marmoset, genus Callithrix Erxleben, 1777 (Callitrichidae, Primates), from the Tapajós/Madeira interfluvium, south central Amazonia, Brazil. Neotrop. Primates 8, 2–18.
Vermeer J., Tello-Alvarado J. C. (2015). The distribution and taxonomy of titi monkeys (Callicebus) in central and southern Peru, with the description of a new species. Primate Conserv. 29, 9–29.
Keywords: Platyrrhini, systematics, Phylogenetic Species Concept, Biological Species Concept, taxonomic inflation, new species
Citation: Rylands AB and Mittermeier RA (2024) Taxonomy and systematics of the Neotropical primates: a review and update. Front. Conserv. Sci. 5:1391303. doi: 10.3389/fcosc.2024.1391303
Received: 25 February 2024; Accepted: 15 April 2024;
Published: 28 May 2024.
Edited by:
Carlos R. Ruiz-Miranda, Universidade Estadual do Norte Fluminense, BrazilReviewed by:
Francesco Maria Angelici, National Center for Wildlife Riyadh, Saudi ArabiaCopyright © 2024 Rylands and Mittermeier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anthony B. Rylands, YXJ5bGFuZHNAcmV3aWxkLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.