
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Conserv. Sci. , 13 June 2024
Sec. Conservation Social Sciences
Volume 5 - 2024 | https://doi.org/10.3389/fcosc.2024.1383611
Many critically endangered species persist in remnant populations so small that ecological information required to assist recovery, such as species-typical demographic parameters and habitat preferences, can be difficult to acquire based on science alone. Traditional ecological knowledge (TEK) or local ecological knowledge (LEK) can fill information gaps and provide additional understanding, though this expertise is not everlasting and often overlooked. We report on research focused on a species survival plan for mountain bongo (Tragelaphus eurycerus isaaci), a critically endangered antelope endemic to Kenya, persisting in the wild with fewer than 80 individuals in four separated montane forests. In preparation for a potential conservation translocation of captive-bred bongos into one or more forests, extensive camera trapping yielded limited results, suggesting that data were based on the activities of just a few individuals. Moreover, additional information critical to translocations, such as typical group size and sex ratios, could neither be observed nor obtained from the literature. This knowledge gap was largely resolved using expert interviews conducted with eight former Kenyan hunters, along with historical range and browse mapping, enriching understanding of behavioral characteristics rendering bongo particularly vulnerable to exploitation. Consistently similar responses from observations spanning a 50-year period (1950s to 1990s) across four ecosystems added certainty to responses. This study endorses a combination of data sources when dealing with remnant populations, and specifically recommends making use of this documented mountain bongo TEK/LEK to inform decisions about potential bongo reintroductions in Kenya.
Concerns over the declining numbers of wildlife species in diverse ecosystems worldwide have led efforts from conservationists to protect species at risk of extinction. To assist with this, wildlife managers often draw on documented, scientific knowledge of animal behavior to better manage their survival. However, when a species is critically endangered, the opportunities to observe them in the wild are minimal, which can limit the data collected. Even when remnant wild populations can be observed and studied, questions arise of possible behavioral shifts that have made the survival of the limited representatives remaining possible (Sheppard et al., 2022). This lack of understanding of the behavioral ecology of critically endangered species creates a problem with management decisions. To fill this information gap, the opportunity to access traditional ecological knowledge (TEK) or local ecological knowledge (LEK) about a species or an ecosystem may provide another useful point of reference.
TEK describes Indigenous and other traditional knowledge of local natural resources. Berkes et al. (2000) define it as “a cumulative body of knowledge, practice and belief evolving by adaptive processes and passed down through generations by cultural transmission about the relationship of living beings (including humans) with one another and with their environment”. The wealth of knowledge within local and Indigenous communities about the natural environment and its resources is expected to be vast and varied. It has developed over thousands of years and been passed down across generations in oral teachings (Berkes et al., 2000). TEK has received growing attention within western science in recent years, for improved results with complex natural resource management problems. Current and important examples can be found in climate change studies (Wyllie de Echeverria and Thornton, 2019; Hosen et al., 2020), Inuit/Arctic studies (Pearce et al., 2015; Henri et al., 2018; Eerkes-Medrano and Huntington, 2021), and biodiversity/species-specific studies (Turvey et al., 2018; Bessesen and Gonzalez-Suarez, 2021). The extractive nature of acquiring TEK must be tempered with the researcher’s understanding of colonialism, histories of oppression, and contemporary challenges facing Indigenous populations (Kovach, 2010; Kelly, 2022).
Stern and Humphries (2022) conducted a systemic review of 49 studies that included TEK into wildlife analyses, pointing out that differences in the form and function of this wildlife information created challenges for integration. Experiential wildlife knowledge acquired over time through the personal observations of knowledge holders was reportedly documented through point observations, semi-structured interviews, questionnaires, facilitated workshops, participatory mapping and, ideally, collaborative field projects that enabled knowledge co-production (Stern and Humphries, 2022). The gathering of TEK should be coupled with other forms of data collection where possible, as challenges with access and reliability may limit its usefulness. As with all anthropological studies, concerns arise due to the fallibility of memory, the accuracy of interpretation across languages, and the ability to locate and engage with key informants (Sharma, 2010; Thomson, 2011; Hodge and Costa, 2021). For example, ecosystem and species-specific knowledge, shared in the form of folktales and parables, might be lost or eroded since older community members are typically the cultural repositories of oral history, and this information is seldom well-known or shared with or by the youth (Turvey et al., 2018). Limitations can be mitigated by using trusted sources as interpreters, sourcing and engaging with the knowledge brokers themselves (i.e., elders, traditional herbalists, honey harvesters, hunters, etc.), and advancing the strength of the TEK provided by gathering separate testimony from additional informants.
While TEK refers to traditional knowledge passed down through generations, new knowledge is created all the time, and it is not only generated by Indigenous people. LEK is a term referring to more recently acquired local ecological knowledge. Charnley et al. (2007) define LEK as knowledge, practices, and beliefs regarding ecological relationships that are gained through extensive personal observation and interaction with local ecosystems (Charnley et al., 2007).
Hunters, fishers and trappers make a living in knowing the landscape and the wildlife living within it. To be successful, an intimate understanding of the behavioral ecology of the target species is advantageous. Though the goals are different, the skills of field conservationists and game hunters are very similar, and these TEK/LEK experiences have been widely recognized for the contribution they can make to wildlife management decisions (McPherson et al., 2016; Raftogianni et al., 2022; Stern and Humphries, 2022). The phenomenon known as “shifting baselines syndrome”, defined as a gradual change in the accepted norms for ecological conditions due to human influence whereby people begin to accept degraded states as the norm (Pauly, 1995), highlights the importance of historic observations by hunters. As their recollections can provide baseline data that reduce the risk of overlooking historical species ecological patterns, such as abundance and distribution, historical TEK/LEK thereby plays a pivotal role in framing current species recovery efforts and addressing gaps in natural history and behavioral ecological knowledge (Pauly, 1995; Sousa et al., 2020).
Legal forms of sport and trophy hunting, when properly regulated, can support wildlife management by increasing funding for conservation at multiple scales (Paulson, 2012), providing an alternative to culling for population control of big-game species (Mysterud et al., 2020), and maintaining cultural significant practices Indigenous groups (Ronoh et al., 2016). And at one time, the great wildlife herds of East and Southern Africa were believed to be so strong that hunting activity would have little impact on them. Reaching its height of popularity in the 1800s, commercial trophy hunting in Africa was an early form of tourism, largely carried out by men from Europe and North America, who were commonly dubbed the Great White Hunters (Capstick, 1991; Steinhart, 2006; Prettejohn, 2012; Pinnock, 2019; Hurt, 2020). Trophy hunters kept and displayed parts of the hunted animal to remember the hunting experience, and most often left the meat and other body parts for scavengers, or local members of the hunting party, to consume. However, as species declines became apparent, various African nations took steps to end big game trophy hunting. For example, the East African Professional Hunters Association (EAPHA), established in 1934 and comprised of gentlemen hunters who conducted their activities with an understood code of ethics, was dismantled when the ban on hunting was made into law in Kenya in May 1977 (Hurt, 2020).
Conversely, illegal hunting, or poaching, occurs unregulated on both local and international scales. Locally, illegal hunting often aims to supplement diets or local markets, and historically, its impact was minimal when human and wildlife populations were balanced. However, the unregulated nature of such activities can lead to the local extinction of species and, if widespread, can push them towards endangerment. Internationally, poaching is wildlife crime functioning in dangerous international networks, similar to the trafficking of illegal drugs and arms. Well known examples include the poaching of elephants for ivory, and tigers for their skins and bones (World Wildlife Fund, 2022). Interpol reports that the illegal trade in wildlife is estimated to be worth up to USD 20 billion per year (Nellemann et al., 2016) and involves additional crimes such as money laundering, corruption, and document fraud, necessitating an international response (Interpol, 2022).
In addition to the ‘Big Five’ – buffalo (Syncerus caffer), elephant (Loxodonta spp.), leopard (Panthera pardus), lion (P. leo), and rhinoceros (Diceros bicornis and Ceratotherium simum), who have been given this name as those considered most challenging and therefore prized to kill, in Kenya the endemic mountain bongo (Tragelaphus eurycerus isaaci) was also to be found on early government hunting permits (Supplementary Figure S1) and was equally sought out locally by poachers.
The mountain bongo is listed as Critically Endangered, with their population decline and extirpation from various parts of the antelope range attributed to hunting pressure coupled with forest loss and degradation (IUCN SSC Antelope Specialist Group, 2017). In 2017, an estimated 60 to 100 individuals were thought to remain in separate mountain ecosystems in smaller sub-populations within the upland forests of Kenya (Svengren et al., 2017). Contributing to their endangerment has been the live-trapping of the species for export to foreign zoos and other captive breeding facilities. By the end of 2023, 415 mountain bongo were recorded in accredited facilities worldwide (Species 360 Zoological Information Management System (ZIMS), 2023), although non-accredited facilities, lacking collection demographic obligations, would push this number substantially higher. Within Kenya, approximately 70 mountain bongo are found at the Mount Kenya Wildlife Conservancy (R. Aruho, personal communication, March 2, 2024). Knowledge about the behavioral ecology of mountain bongo is largely derived from studies carried out on captive bongo populations due to the limited number of individuals persisting in the wild.
This paper provides the results of TEK/LEK interviews with historical mountain bongo hunters in Kenya. It documents rapidly disappearing knowledge held by elders who existed during an era when herds of African wildlife were so abundant that people assumed they could never disappear. Formal approaches for documenting and incorporating these early accounts have been largely absent, and historical perspectives have mostly been lost to time. By employing a framework to maximize the use of hunting history, it can be possible to overcome in part the shifting baselines syndrome (Pauly, 1995). This paper does not set out to defend the practice of trophy hunting but recognizes that vast ecological knowledge is frequently possessed by game hunters. In a world where ongoing environmental degradation at local, regional and global scales invites the continual lowering of accepted thresholds in environmental norms, wildlife conservation science benefits from the development of frameworks incorporating historical knowledge for improved change-over-time reflections. The contribution this knowledge provides to our understanding of wild mountain bongo behavior, thereby closing the knowledge gap for this critically endangered species, is explored within. Information is gathered by the authors from key informant interviews leading to the mapping of historical bongo ranges and identification of preferred bongo browse species.
The bongo (Tragelaphus eurycerus) is the largest forest antelope found in equatorial Africa. With imposing horns on both males and females, its distinct large size and striking reddish-brown coat, with thick white stripes, makes the species an appealing symbol for forest conservation (Figure 1). For the same reasons, the species has also been prized by trophy hunters (Koopmans et al., 2021). There are two existing bongo subspecies: the lowland or western bongo (T. e. eurycerus), which is found across Central and West Africa (Guinea, Togo, Cameroon, and South Sudan and the Republic of Congo (Koopmans et al., 2021), and the mountain or eastern bongo (T. e. isaaci), which is endemic to Kenya.

Figure 1 Mountain bongo (Tragelaphus eurycerus isaaci) have unique sets of 8–12 white stripes on each side of their body, a nose chevron, enormous ears, and spiraled horns with one twist. Males (left; Credit: John David) darken as they age from a deep russet red to nearly black. The horns of females (right; Credit: Donna J Sheppard) are thinner and more parallel than the massive horns of males.
Male and female mountain bongo have a unique set of 8 to 12 white stripes on each side of the body (S. Njuki, Mount Kenya Wildlife Conservancy, personal communication, July 18, 2022), a nose chevron, enormous ears, and spiraled horns with one twist growing up to 75 cm to 99 cm (Estes, 1999). The horns of females are thinner and more parallel, whereas the horns of males are massive. These stocky forest antelopes are deep russet red in color, with males darkening as they age until nearly black (Kingdon, 2015) (Figure 1).
Primarily browsing on low-level shrubs, young trees, herbs and vines, mountain bongo habitat is found in Kenya’s highland forests, with the species preferring to range in slopes between 2000 m and 3000 m (Kingdon, 2015). Bongo are known to be inactive during the day, typically resting in thickets. They move and feed at night, moving through dense cover with horns laid back (Estes, 1999; Kingdon, 2015; Koopmans et al., 2021). Historically, healthy mountain bongo populations were found in Kenya’s mountain forests including the Cherangani Hills, Mt. Londiani, Chepalungu Hills, the Mau Complex of forests including the Mau Eburu Forest, the Aberdare ranges, and the Mt. Kenya National Forest Reserve (Gibbon et al., 2015; IUCN SSC Antelope Specialist Group, 2017; Kenya Wildlife Service, 2019) (Figure 2).
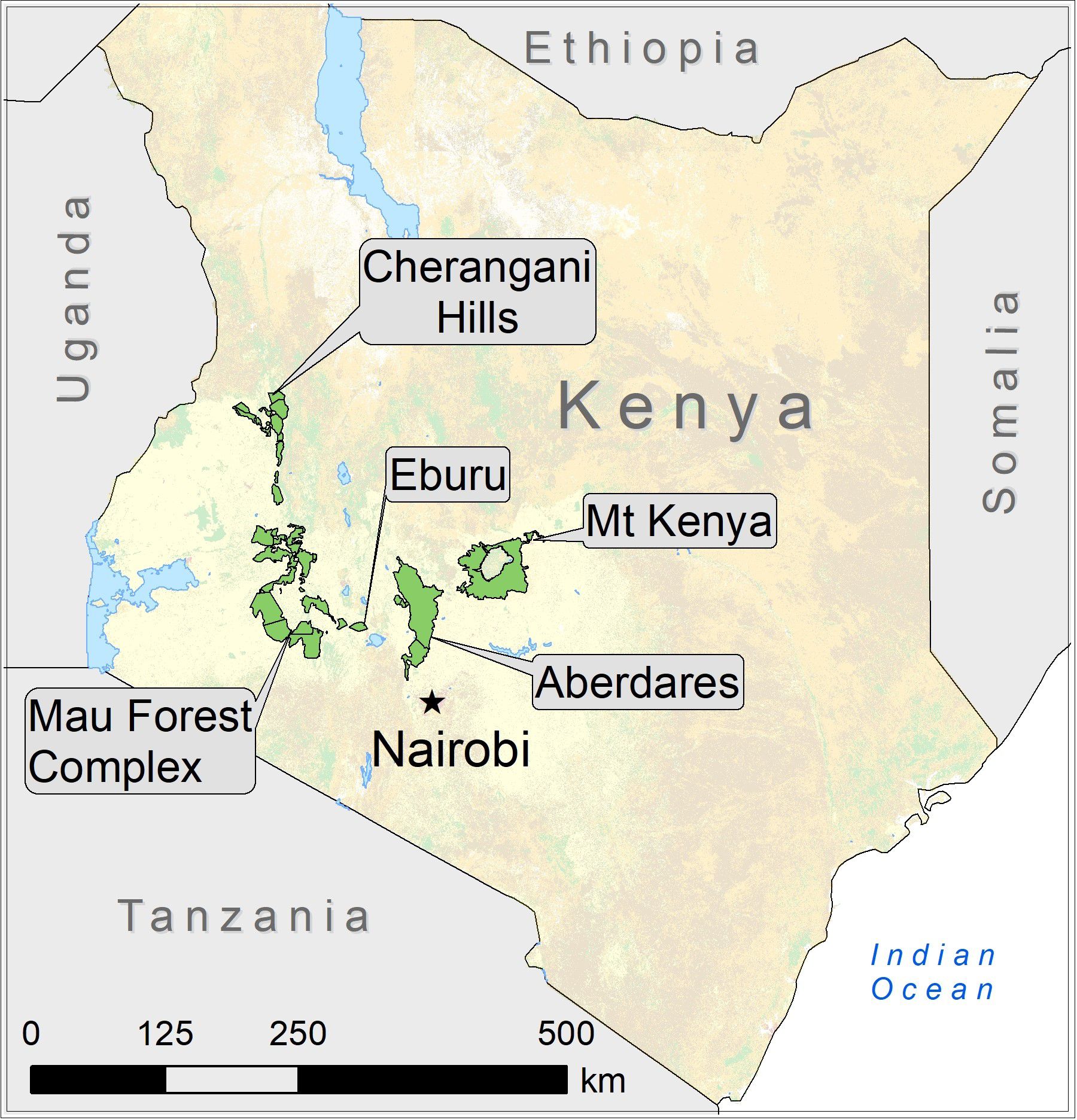
Figure 2 Mountain forests in Kenya where healthy mountain bongo populations were historically found.
Mountain bongo were first classified as critically endangered in 2008 and updated in 2016 (IUCN SSC Antelope Specialist Group, 2017). At the time of the 2016 IUCN assessment, bongo were considered to survive in four unconnected, isolated montane forests (Elkan and Smith, 2013). Though accurate population estimates of this cryptic, mostly nocturnal species inhabiting dense, montane forest have proved challenging, attempts have been made using camera trapping technology.
Since 2006, estimates have been derived from camera trapping activities of the Bongo Surveillance Project (BSP) whose cameras were installed in mountain forest locations containing secondary mountain bongo signs (Prettejohn et al., 2020). In 2015, the BSP estimated that the mountain bongo population had declined to around one hundred individuals (Shears, 2015). In 2017, this figure was re-forecasted downwards, deemed to be as low as 73 individuals (Svengren et al., 2017). By 2018 however, despite one extensive camera trapping study at Eburu Forest for example, only five bongo events were observed from the 182,781 wildlife images analyzed across a 21-month period suggesting the survival of just one or two individuals in that ecosystem (Sheppard et al., 2022). By 2019, the populations in Eburu Forest and Mt. Kenya Forest were deemed functionally extinct (Prettejohn et al., 2020; Sheppard et al., 2022), with survival hopes pinned on viable populations within the Salient section of the Aberdare National Park, and the Maasai Mau section of the Mau Forest Complex (Prettejohn et al., 2020).
The remaining wild population is joined by captive-bred populations found in Kenya at Mt. Kenya Wildlife Conservancy (MKWC), and further afield in the Americas, Europe, and Australasia. In 2017, MKWC bordering the Mt. Kenya National Forest Reserve, held 63 mountain bongo, including 25 bulls and 38 cows (Svengren et al., 2017). Five years on from that, in 2022, the population had grown to as estimated 70 individuals (S. Njuki, Mount Kenya Wildlife Conservancy, personal communication, July 18, 2022).
Kenya’s five main mountain forests – the Mau Forest complex (including Eburu Forest), the Aberdare Mountains, Mount Kenya, the Cherangani Hills, and Mount Elgon are referred to as Kenya’s water towers as they capture most of the country’s rains (Pearce, 2015; Figure 2). Kenya’s montane forests store and release water, ensuring the year-round flow of major rivers, including the 1000 km long Tana River. They substantially contribute to the economy, supplying more than 75% of the renewable surface water resources of Kenya (United Nations Environmental Programme (UNEP), 2012). As Mount Elgon is the only forest among Kenya’s water towers that has neither historically nor currently supported mountain bongo populations, it will not be specifically discussed.
The climate of these montane forests, occupying elevations above 1500 m AMSL and into moorland and subalpine habitat in some cases, is wetter than the surrounding lowlands. Nighttime temperatures fall below 10°C in the cold season (June through August) and daytime temperatures rise above 30°C during the warm season (January through March) (Martin and Burgess, 2022). Frosts are possible at higher elevations, and freezing rain occurs.
Within these water towers, various vegetation zones can be distinguished including the closed-canopy forest, the bamboo zone, the sub-alpine and alpine vegetation. Within the forest belt, at the lower attitudes (between 1500 m and 3350 m), common hardwood tree species include camphor (Ocotea usambarensis), cedar (Juniperus procera), and podo (Podocarpus milanjianus) (Lambrechts et al., 2003; Luke and Beentje, 2020). These species give way to mountain bamboo (Oldeania alpina; also known as Arundinaria alpina, or four other synonyms) (POWO, 2024) and trees such as East African rosewood (Hygenia abyssinica in the higher attitudes up to 3,600 m (Luke and Beentje, 2020).
Kenyan mountain forests offer shelter to faunal species uniquely adapted to high elevation forest ecosystems. In addition to the endemic, critically endangered mountain bongo, Kenya’s mountain forests support the Jackson’s mongoose (Bdeogale jacksoni) (Lambrechts et al., 2003), and many amphibians and birds are also endemic to the montane areas (BirdLife International, 2022). Other large, threatened mammals of international conservation interest include the giant forest hog (Hylochoerus meinertzhageni), black rhinoceros (D. bicornis), forest elephant (L. africana), and leopard (P. pardus) (Lambrechts et al., 2003). The presence of mountain bongo in these forests could be viewed as an indicator of a healthy mountain ecosystem, suggesting not only that the faunal assemblage is complete but also may play a role in balancing the abundance or distribution of other species in the ecosystem.
Kenya’s montane forests face decline through regularly occurring human activities such as deforestation, cattle grazing, infrastructural development (Ayuyo and Sweta, 2014), selective logging, charcoal burning, Cannabis sativa fields, landslides, quarries, and human encroachment (Lambrechts et al., 2005), and, in some cases, sand harvesting (Olang and Kundu, 2011). Kenya mountain forests with current and/or historical mountain bongo populations include Mau Forest Complex including Eburu Forest, the Aberdare ecosystem, Mt. Kenya National Park and National Reserve, and the Cherangani Hills Forest (Figure 2).
Historical mountain bongo hunters, known through mountain forest conservation networks in Kenya, were invited to share their knowledge on successful bongo hunting and live-trapping practices, and the behavioral ecology of the species. The selection of respondents was based on two main criteria. First, individuals with confirmed knowledge of the mountain bongo and former hunting practices were identified through snowball sampling. This technique, useful when potential participants are hard to find, involves research participants recruiting others for a study, allowing researchers to use their judgement in choose participants with specific expertise (Atkinson and Flint, 2001; Nikolopoulou, 2022). Second, individuals from multiple mountain ecosystems where the mountain bongo historically existed were selected. This approach provided diverse perspectives and allowed for comparisons across different ecosystems, enhancing the reliability and depth of the information gathered.
Interviews were conducted in the respondent’s language of choice. Interviews conducted in languages other than English were interpreted with the use of one expert translator, who was known to the interviewer. A total of eight men – between the ages of 60 and 90 years – were interviewed with the use of a questionnaire, and all responses written down in English. As documented in other exploratory, qualitative studies, small, purposeful samples can provide valuable insights on targeted themes (Rust et al., 2017; Poor et al., 2021). Each interview took two to five hours to complete. All respondents signed a written consent, adapted from the ethics protocol of the University of Guelph, Ontario, Canada, providing their information-sharing permission, prior to the commencement of the interview.
The interviews used a pre-determined list of questions and themes; however, by using the semi-structured approach, each interviewee had the flexibility to respond more broadly, and were invited to recount stories and memories spontaneously. Semi-structured interviews provide relevancy to the topic while remaining responsive to the participant (Bartholomew et al., 2000). The interviewer was able to learn more about each respondent’s specific information and to probe further for answers. By having a consistent structure within each questionnaire, coupled with an open-ended questioning format, respondents were able to provide responses rich with additional information.
Responses provided by the eight former hunters led to additional data collection following the interviews. Bongo location site names and details were documented where possible (further detailed in Section 2.4.2), as were details on observed bongo browse. With the help of local guides, browse species named by interviewees in the local languages of Ogiek, Kiswahili and Kikuyu were later identified and photographed. Samples were collected and stored in a plant press for further identification by a trained Kenyan botanist, Francis Norman Muruga Gachathi, Kenya Forestry Research Institute (KEFRI).
Using the information shared in the interviews, an experience-centered narrative analysis was chosen to interpret the practices and understandings of several people about the same phenomena (Squire, 2013). This method focuses on personal narratives that reveal the lived experience and individual perspectives and was chosen for its ability to capture complex human elements often overlooked by traditional, quantitative approaches. These narratives may refer to singular events but also may refer to a culmination or lifetime of events – flexible in time and defined by theme rather than structure (Squire et al., 2008). In this study, each key informant was able to tell their story of thematic, meaningful aspects of their lived experiences with mountain bongo hunting and/or trapping.
During key informant interviews, all named locations of former bongo hunting activities were documented, and efforts were made to cross-reference statements with information gathered from site surveys. These areas were visited with the help of local guides knowledgeable of the forest terrain. Former bongo areas were documented using a handheld Garmin GPSMAP 64S. To ensure safety, armed government forest security officers were also recruited to join the team. GPS points of former bongo areas identified by local guides were mapped over the RCMRD 2016 Land Use Land Cover map derived from Sentinel 2 Global Land Cover data (RCMRD-SERVIR, 2017). Ellipses were drawn over these points to indicate the approximate areas bongo used to exist for each of the mountain forests in Kenya with current and/or historical mountain bongo populations since the 1950s. The size of each ellipse represents the relative number of individuals thought to exist in each forest at the time, as approximated by the former hunters. As the hunters tended to hunt in only one or two areas, the historical population estimates are not complete for all forest regions over all decades.
Results from bongo hunter interviews are clustered into the themes of hunter demographics, hunter knowledge of bongo behavioral ecology, information about historical bongo hunting practices and information about historical bongo live-trapping practices. Corroborating statements are indicated by the number of respondents out of eight and does not imply the remaining former hunters did not agree with the statement, they just did not comment on that specific note. Along with the number of corroborating former hunters, some statements are credited to a specific former hunter as well, as indicated by their initials following the statement.
Interviews completed with eight former bongo hunters resulted in the provision of information from four different mountain forest ecosystems including four accounts from the Aberdare, three from the Mau Complex, and two each from Eburu and Mt. Kenya (Figure 3).
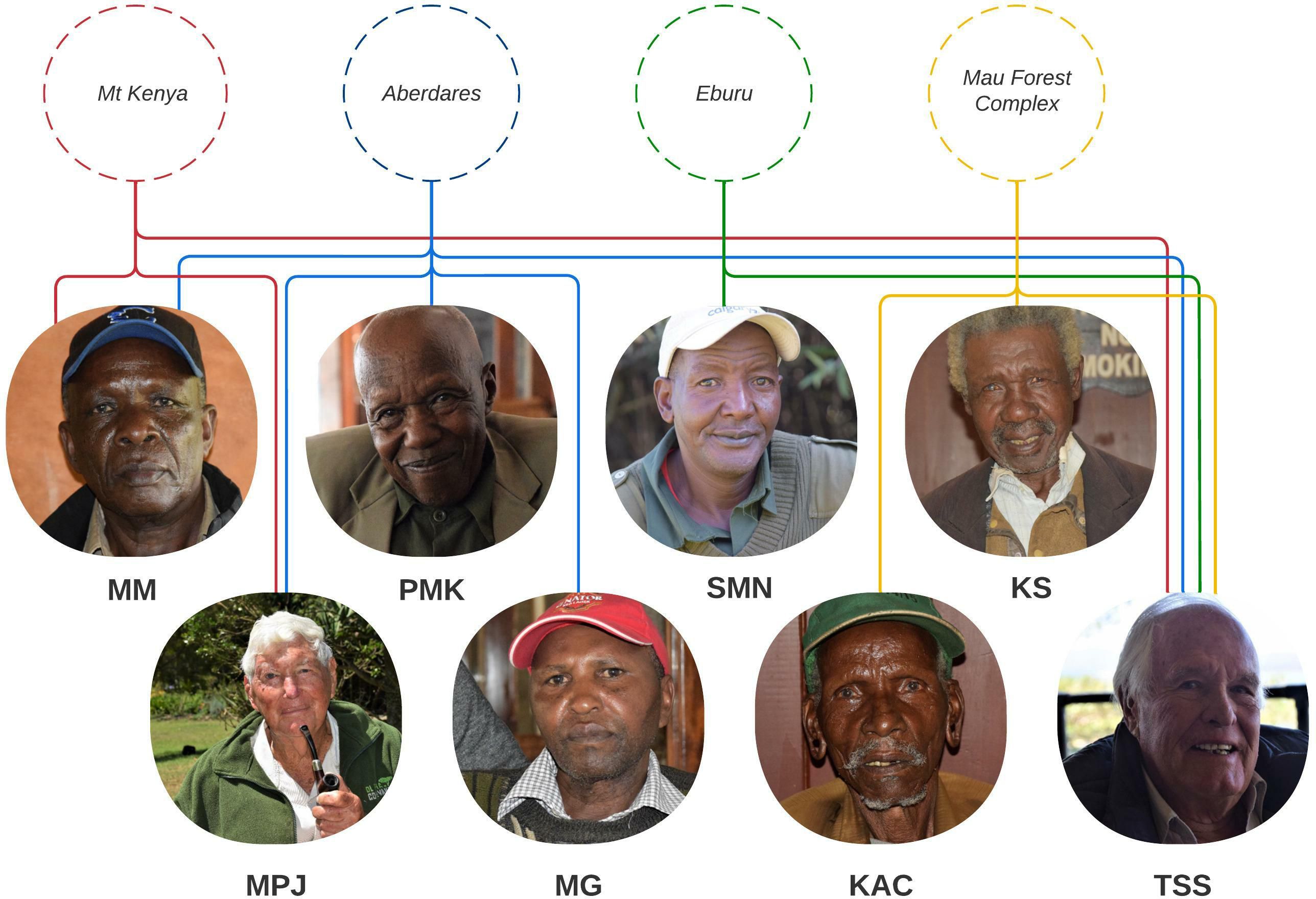
Figure 3 Former bongo hunters that participated in the interviews and the ecosystems they had hunted in.
Respondents reported on experiences over a roughly 40-year timeframe, from the early 1950s to 1992, including both legal and illegal hunting experiences. Prior to the nation-wide hunting ban in Kenya in 1977, under Legal Notice 120, the Wildlife (Conservation and Management) (Prohibition on Hunting of Game Animals) Regulations 1977 (Attorney-General, Republic of Kenya, 2012), hunting was legal and widespread. During this time, trophy hunting safaris were a leading form of tourism. Six of the eight former bongo hunters were involved with this side of the industry; two of the six also hunted for meat using local methods during this time. With the Government of Kenya’s policy making hunting illegal in the 1970s, the other two key respondent interviews were done with former poachers, active during the years after the wildlife hunting ban came into effect.
Respondents possessed a diverse hunting experience, with one, for example, retaining hunting licenses for seven African countries, and active across 50 years of trophy hunting in East and Central Africa, while another played the role of scout in advance hunting parties in the South West Mau, and never shot bongo. Combined, the total years of hunting experience documented was at least 120 years for the eight men.
General consensus among the eight historical bongo hunters indicates that mountain bongo live in single male, multi-female social groups in which the male stays slightly separated from the main herd (mentioned by 7 of the 8 hunters) (see Supplementary Table S1 for a summary of bongo behavioral ecology information shared by bongo hunters). Females were known to separate from the herd during estrous periods to mate with a breeding bull for three to four days (4/8). Females also separate from the herd when giving birth, returning several days later with the calf (2/8). Old males were chased out of the herd, and other out-group males could be found alone or in pairs (5/8).
The earliest memories from the eight respondents took place in the 1950s, when herd sizes were reportedly observed up to 50 strong, particularly in the Aberdare and Mau ecosystems. During those times, large bongo herds of 20 to 30 were common in the Aberdare, Mau, and Mt. Kenya forests, interspersed with smaller herds of 4–7 individuals. In Eburu Forest, group sizes were not as big, with hunters reportedly tracking groups of 4–6 individuals at most.
Over the following decades, single male, multi-female social structures remained intact and home range sizes remained relatively consistent. However, the herd sizes gradually declined over time. By the 1960s and 1970s, observed group sizes dropped to between 10 and 20 individuals, and by the present day, remnant populations are known to be surviving in narrowly defined locations within wider ecosystems. Figure 4 offers a change-over-time diagram of declining bongo populations in two documented ecosystems: the South Western Mau and the southeastern slopes of the Aberdare. The columns illustrate the observed typical herd composition and size over the various time windows from the oral reports. The main herds, comprised of females and calves, declined over time through over-hunting, while the single breeding males continued to be represented in the declining populations, and continued to be observed on the periphery of the main herds of females and calves.
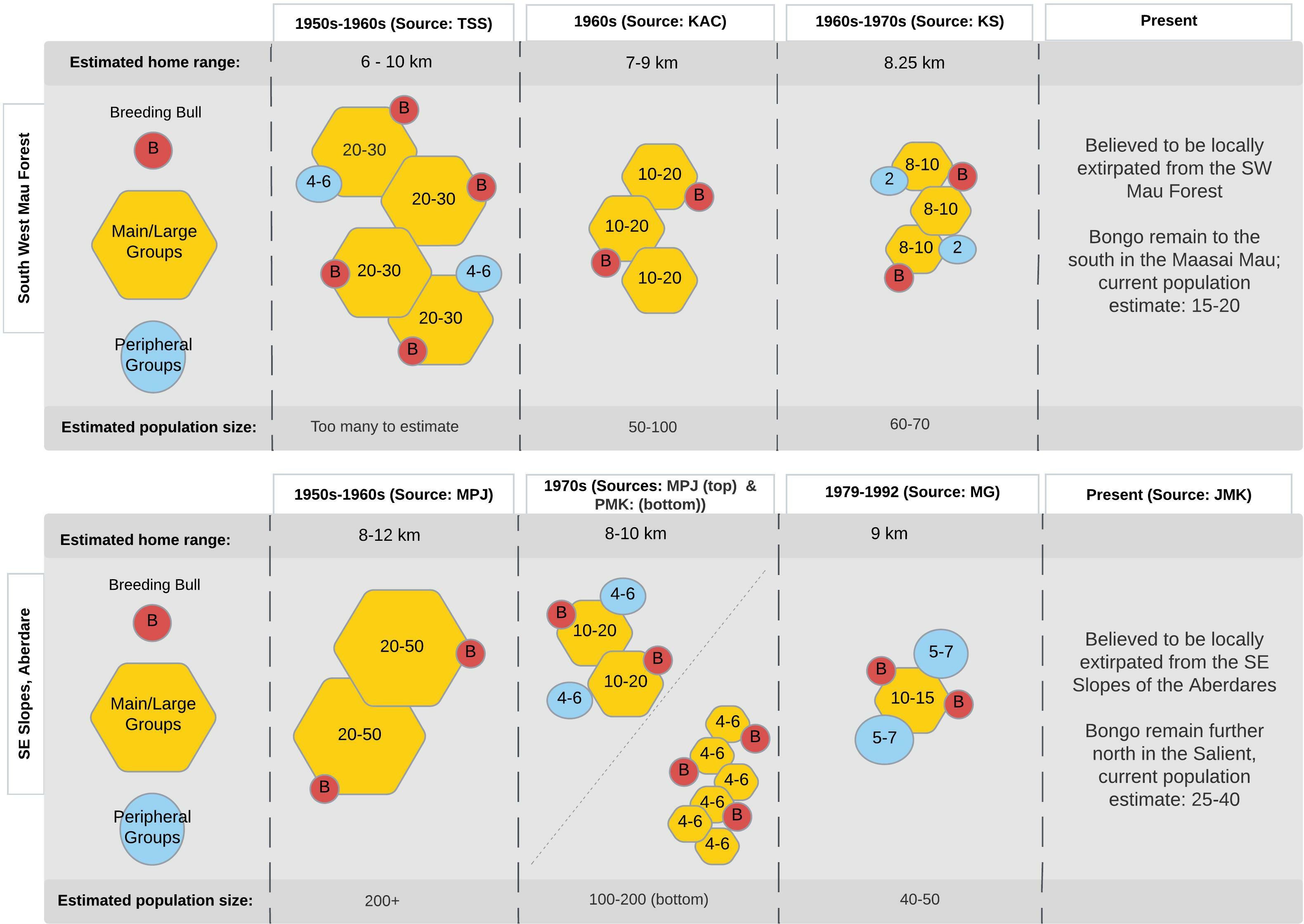
Figure 4 Changes in estimated home range (top row), bongo population size (bottom row), and typical herd size and composition (middle row) over time in the South Western Mau (upper panel) and the Southeastern slopes of the Aberdare (lower panel). The changes in the observed size of the main herds (yellow hexagons) and smaller peripheral groups (blue circles) are depicted by size and numbers within these shapes. The small red circles represent single breeding males observed on the periphery of the main herds, with overlapping shapes illustrating the spatial relation of these smaller groups and single males to the main herds.
Respondents suggested that bongo densities were ecosystem dependent. Predictions surmised that in the 1970s, when bongo populations were still healthy, the eastern Aberdares and the Mau Forest comprised densities in the hundreds, or densities that were too many to estimate (5/8). The three hunters who did not report on bongo density knowledge across different ecosystems were limited in exposure to a singular ecosystem and lacked travel experience. Figure 5 illustrates historical mountain bongo ranges across Kenya, as reported by bongo hunters.
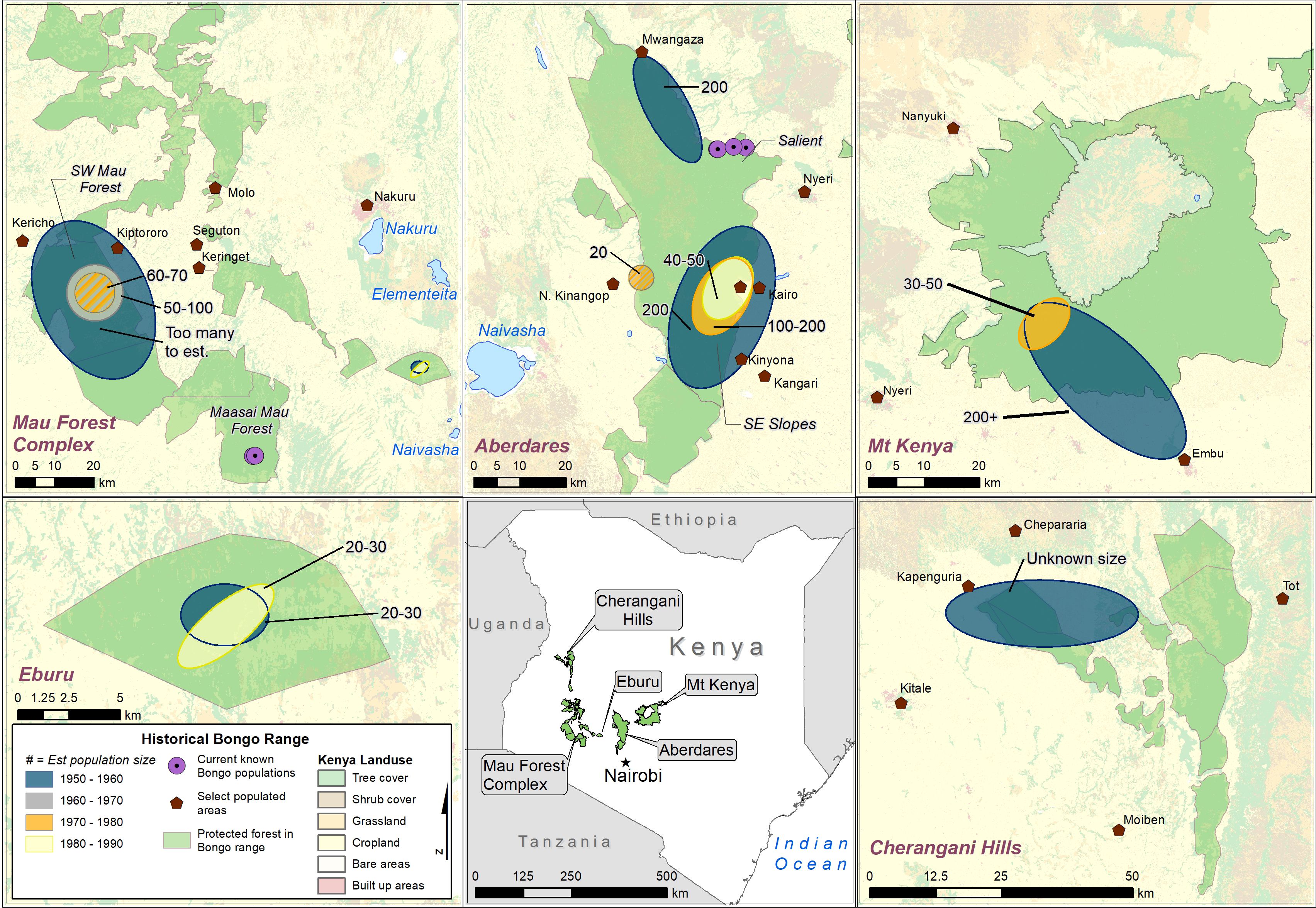
Figure 5 Historical mountain bongo ranges across Kenya, as reported by bongo hunters. The ellipses represent estimated changes in population size by decade, however, this information was not provided for all locations or by all hunters. The purple circles represent locations of currently known bongo populations (i.e., in the Salient of the Aberdare and in the Maasai Mau Forest Block of the Mau Forest Complex).
Bongo populations were not found across the entirety of these mountain forests but favored specific forest sections with bongo-preferred features. Respondents indicated that bongo were found in the same habitat as giant forest hog (H. meinertzhageni) (7/8), with bongo reportedly eating similar foods to giant forest hog but at different heights; in the vicinity of salt licks or sources of salty water (6/8); and in the same forest zone where mountain bamboo was dominant (5/8), between 2,150 and 3,300 m (Luke and Beentje, 2020).
Prior to the 1950s, mountain bongo stretched from Limuru (50 km from Nairobi) in the south to the Cherangani Hills in the north (MPJ). In the 1950s, within the Aberdare, bongo were primarily found along the eastern flanks, with large populations in the northeastern and southeastern Aberdare (Maganjo, Thuti, and North Mathioya), and a third population in the Salient. Bongo were found on these eastern slopes throughout the year. Seasonally, small bongo populations were detected in the western Aberdare below the moorland at Chania River/Gura Falls (PMK).
At Mt. Kenya, bongo were only found in the southwestern slopes from Ragati to Embu. One hunter reported tracking bongo regularly in the Biruiru Hills of Ragati, where each herd established a home range around a particular salt lick (MM). Another hunter, based at Embu in the 1950s, witnessed a bongo coming down to the river to drink right beside their camp (TSS). A third respondent went on hunting expeditions in the 1960s at Meru but learned that bongo were not known there (KS).
Formerly the entire Mau Complex was thought to house mountain bongo, and the population so ubiquitous that it was impossible to estimate size (TSS). In the South Western Mau Forest, bongo were found primarily from Bosta to Kabbilat on one side, and from Emitik and Chemosit on the other. By the 1970s however, respondents claimed they could go a year without seeing a live bongo, only locating dead, snared individuals at salt licks (KS).
Within living memory, Eburu Forest was not thought to have a large bongo population. Total number was estimated to be between 20 and 30 during the 1960s and 1970s (PMK; SMN; TSS) and dropping to less than 15 individuals by the 1990s (SMN).
Respondents uniformly reported on known features of bongo activity patterns, with consensus scored on features including nocturnality, with a heightened period of activity at dawn and dusk (8/8); relatively small home ranges, of 1 km to 12 km suggested (8/8), and staying close to their food patches; the habit of spending several days in a single area feeding on a preferred browse species (6/8); and the behavior of following the same predictable trails and tracks (4/8). Similar to elephants (L. africana), bongo were observed to repeatedly follow the same trails through the forest.
Though differences in features of bongo ranging patterns were observed between rainy and dry seasons, these behaviors were inconsistently reported by former bongo hunters. Some felt that bongo ranged more during the dry seasons (2/8), and some felt that ranging increased during the rainy seasons (2/8). Others reported that food choices differed with the changing seasons (3/8), such as, ‘bongo moved with the season, chasing the food they eat’ (PMK).
Bongo were believed to have no competitors for browse. Though bushbuck (Tragelaphus scriptus) and giant forest hog (H. meinertzhageni) were observed feeding from the same climbers and other plants, they would be using resources at different levels. Bongo are thought to have more limited diets and spend longer periods feeding from one source than the bushbuck or the giant forest hog.
Predation, beyond human hunting activities, was thought to be rare, but it was witnessed. Leopard (P. pardus) and large groups of spotted hyena (Crocuta crocuta) opportunistically snatch youngsters. In the 1960s and 70s, it was still possible to see larger-sized bongo cows up in trees after being killed and dragged up there by the oversized leopard of the Mau Forest (TSS), and African crowned eagle (Stephanoaetus coronatus) have been known to collect days-old baby calves (MG).
Prior to 1977, when wildlife hunting was legal and widespread in Kenya, mountain bongo, like other prized faunal trophies, were on a quota for licensed hunters only. For example, in 1957 professional hunters were able to purchase a license to kill one male mountain bongo per year for 20 Kenya shillings (Supplementary Figure S2; TSS). To do this, foreign hunters hired the services of local guides and trackers.
When working with international clients, Kenyan trackers developed techniques to improve bongo hunting success by going out in advance parties in preparation for the arrival of international trophy hunters (Table 1). They would start at a naturally occurring salt or mineral lick (7/8), close to water sources (4/8) within the mountain bamboo (Oldeania alpina) vegetation layer, and follow the largest tracks (4/8), assuming that these tracks were made by males. As trophy hunters only shot the largest males, the fact that male bongo had a habit of separating themselves from the rest of the herd made them prime hunting targets. Reportedly, hunters would also seek out signs of giant forest hog (2/8), or locate a favorite bongo browse species, such as the locally named ‘setiot’ or ‘thunguya’ (7/8). Table 2 provides a list of species routinely named by hunters as important bongo browse and photos of those browse species are presented in Supplementary Figure S3.
Bongo were difficult to locate without the help of dogs, and therefore these advance parties would use dogs to ease the work. Even if hunters did not use dogs, everyone reported that hunting with dogs was the easiest way to locate bongo (8/8). Those who did not use dogs at all experienced diminished success. For example, in 1960 there were 30 hunting licenses issued and just two bongo shot, and in 1961 there were 41 licenses taken out with five mountain bongo shot (Anderson, 1963). Unlike other antelope species, when being chased by dogs, bongo would eventually turn to face the dog pack and aggressively confront them. While the trophy hunters were there for the impressive horns of the biggest breeding bulls, illegal hunters were eating the meat with their families, and selling the magnificent, and much-sought, skins to curios traders in Nairobi (MG). Instead of guns, poachers used dogs and spears.
None of the eight men interviewed engaged in nighttime hunting, when bongo are active. Hunting was only done at dawn and dusk. Bongo rest during the day and are known to be excellent at hiding in dense forest. One prolific hunter claimed that in all his 50 years in the bush, he had never seen a bongo in the day (PMK), and could only find them with dogs. When asked, former hunters were able to report on the total number of bongo they had killed. One prolific poaching team, active in the Eastern Aberdares until 1992, killed 27 bongo over 23 years of hunting. The majority of hunters reported on killing far fewer bongo, ranging between 3 and 12 individuals across a span of 5 to 20 years (see Table 1 for a complete listing).
After the 1977 hunting ban in Kenya, the mountain bongo became a target for live export to international zoos, primarily through the Mount Kenya Game Ranch (now Mt. Kenya Wildlife Conservancy, MKWC) (Powers, 2010). Pitfall traps, bamboo fencing and net traps were three of the live-trapping methods employed for bongo, with pitfall traps proving most effective; detailed descriptions and diagrams of these methods and their success rates can be found in Data Sheet 1.
In an effort to recover the critically endangered mountain bongo to former, healthy wild populations in Kenya, ecosystem-by-ecosystem conservation translocations have been recommended (Kenya Wildlife Service, 2019). A successful reintroduction program hinges on anthropogenic, genetic and ecological factors. Eliminating illicit forest activities is essential to provide enhanced assurance of site security for released individuals and the broad support for a bongo re-introduction from neighboring stakeholder communities (Sheppard et al., 2022). Additionally, understanding the elements of bongo behavioral ecology such as herd structure, preferred browse species, ranging patterns, and habitat preferences informs the suitable selection of release site locations and ensures greatest chance of survival for released individuals. Though captive-bred bongo populations have provided a source for some of this information, data gaps remain, and wild bongo behaviors may not be the same as those observed in captive populations.
Knowledge uncovered during this research provides additional information about the known and anticipated behavior of wild mountain bongo. Foremost knowledge provided by historical hunter TEK/LEK to be used in the current effort to better protect and recover mountain bongo populations include: a) the discovery that bongo herds were substantially larger in the past and re-introduction management plans can use those herd sizes to inform normative population recovery; and b) the clarification of locations within mountain forest ecosystems where bongo historically resided or did not reside to be used as building blocks upon which suitable locations for bongo re-introduction sites can be selected. The use of dogs to search for released bongo would not be implemented in re-introduction pilots, however the knowledge of the extreme effort required to locate wild bongo informs decision making for monitoring strategies to be implemented in the case of released, reintroduced bongo.
The eight former hunters who lived in diverse parts of Kenya and once hunted in various forest ecosystems, were largely in agreement over a number of features of mountain bongo behavior. Key highlights include the fact that mountain bongo ranged over small areas (1–12 km); repeatedly used the same game trails; were nocturnal and crepuscular; demonstrated a preference for naturally occurring salt licks and ranged within the elevation layer dominated by mountain bamboo (2,150 m to 3,300 m). Furthermore, bongo spent multiple days repeatedly feeding on preferred browse in single male, multi-female social groups with the male preferring to separate slightly from the herd. Although small herds of four to six individuals were observed, mountain bongo of the 1950s and 1960s were also comfortable existing in much larger herds. Group sightings of 20–50 animals were once a common occurrence. These massive herds did not range throughout the Aberdare, the Mau Complex nor Mt. Kenya forests, but were existing in sub-sections of those larger ecosystems including, for example, primarily the eastern/southeastern Aberdares, and the south/southwestern Mt. Kenya forests.
Also emerging from the data is evidence of the gradual decline into endangerment of a species left unchecked over a 50-year period. Key influences across this period included a major effort to harvest live bongo from the Aberdare for export to international zoos in the 1970s; the heavy price paid for over a decade of unregulated and illegal shooting in the 1970s and 1980s when the elephant population was decimated from 165,000 to 15,000 (TSS); and the fact that poachers knew the bongo behavior well, aware that animals resting quietly in the dense undergrowth were difficult to locate during daylight hours but easy to find with hunting dogs.
TEK/LEK as a knowledge base has been criticized for its lack of testability (Usher, 2000). However, the repetition of similar details provided across informants in this study adds strength to the individual claims, increasing confidence levels in the most consistent facts provided. It is clear from the duplication of responses, that historical bongo hunter ecological knowledge provides a useful source of information about wild bongo behavior and offers clues to the decline of the species over time. It is recommended therefore, in this specific case with mountain bongo in Kenya, that knowledge shared by these bongo experts be applied to the design of any future captive-bred mountain bongo re-introduction plans.
The value to bongo recovery of the information shared in this study illustrates the potential application of TEK/LEK in situations where scientific inference is limited. When a species decline occurs before their behavioral ecology has been categorically documented, historical ecological knowledge offers a glimpse into a world that is no longer accessible, and one that can inform decisions for the present and future survival of a species. Furthermore, along with countering knowledge gaps in animal behavior, population size and ranging patterns, TEK, LEK and historic observations can, in part, surmount a shifting baseline in what we know about these factors.
Yet, TEK and LEK appear to be environmental decision-making considerations not widely utilized. At the federal level in Canada for example, TEK is officially recognized as a legitimate information source for wildlife management legislation, yet it is not widely used by administrators when writing regulations (Beaulieu-Guay, 2022).
Integrating different types of knowledge in environmental management projects has reportedly been inherently complex (Raymond et al., 2010), even though Indigenous knowledge systems are considered to be essential for sustainable development and environmental management (Athayde et al., 2017). A developing trend in current studies is hybridization, where traditional knowledge, practices, and beliefs are merged with novel forms of knowledge and technologies to create new knowledge systems (Gomez-Baggethun et al., 2013).
The challenges with TEK/LEK usage appear considerable, and include communication barriers, arising from the use of different languages; conceptual barriers, stemming from differing worldviews and relationships with nature; and political barriers, resulting from an unwillingness to acknowledge TEK that may conflict with industry or government agendas (Ellis, 2005). Others have noted that there is a lack of appropriate methods for organizing and presenting it for assessment and management purposes (Usher, 2000).
While it may be true that interviewing people successfully can prove difficult – especially with interviewees using different languages from one’s own, or coming from challenging sub-groups of society such as the elderly, researchers who hope to work with traditional keepers of ecological knowledge must shed the perception of intellectual superiority and seek ways to collaborate for the benefit of everyone involved. Allotting sufficient time and personnel for meetings and interviews can go a long way in addressing these challenges. Allowing the conversation to be structured in a way best suited to the respondent will also help keep thoughts and memories clear. Respondents may have challenges with hearing and seeing properly and are not always able to grasp the intentions of the interviewer. Semi-structured interviewing enables the tailoring of questions for each respondent’s understanding (Berg, 2008). Also, where possible, return meetings should be arranged to limit the length of time required at any given session. Confidence in the information gathered can be buttressed through cross-referencing measures such as interviews with additional informants and/or the completion of ground truthing surveys, and other field data collection methods.
There are social processes underpinning different perspectives on nature, and these value systems shift over time leading to tensions within a society over natural resource management, usage, and markets. These differing perspectives come about when the meaning of items and subjects change over time. Perspectives on trophy hunting have shifted considerably in the last century. Big game hunters, who once captured the imagination of many with their African hunting exploits, have been replaced by tourists shooting photos instead. In modern times, there is a great debate over the role and value of trophy hunting and hunters in conservation (Sas-Rolfes, 2017). Yet, in a recent systemic review of 49 studies interweaving TEK into quantitative wildlife analysis, the most common descriptor of knowledge holders was hunters/trappers (Stern and Humphries, 2022).
For the most part, conservation challenges are complex in character and there is a need to widen perspectives to embrace an extensive variety of collaborators, both conventional and unconventional. It is sometimes in the unexpected that the solutions are found. Reformed poachers, for example, make exceptional camera trapping team members (Sheppard et al., 2022). Having spent countless hours traveling in it, they know the terrain and the routes the animals take, and therefore have insight into the prime locations for camera traps (pers. obs.). By incorporating a multitude of perspectives, it may be possible to better tackle some of the conservation challenges plaguing the planet. Though the inclusion and integration of local, expert, or hunter knowledge about wildlife populations and their habitats can be problematic, it is possible to co-produce research to meaningfully include TEK/LEK into analysis (Castello, 2004; Berkes, 2012). Moreover, when ecological information about critically endangered species is difficult to acquire, traditional and historical knowledge holders provide a reservoir of experiential knowledge and a vital source of additional data.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
DJS: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. DS: Visualization, Writing – original draft, Writing – review & editing. SM: Investigation, Writing – original draft, Writing – review & editing. PM: Investigation, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding was provided by the Calgary Zoo Foundation.
We first extend our heartfelt appreciation to the former bongo hunters (KAC, KS, MG, MM, MPJ, PMK, SMN, TSS) who participated in the interviews, without whom this study would not have been possible. We would like to remember Mathew Gichuri, who lost his life shortly after the study was completed and was not able to witness his contribution to mountain bongo knowledge documented in print. Thanks to colleagues at Rhino Ark Kenya Charitable Trust for their useful guidance and perspectives, to the local guides and government forest security officers from Kenya Forest Service and Kenya Wildlife Service who assisted in visiting the bongo locations indicated by the hunters, and to Francis Norman Muruga Gachathi from the Kenya Forestry Research Institute for his valuable assistance in identifying bongo browse. We are grateful for feedback from Typhenn Brichieri-Colombi, Christian Lambrechts and Tara Stephens, and three reviewers, whose comments and suggestions helped improve the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2024.1383611/full#supplementary-material
Anderson M. (1963). “Bongo on a family safari,” in Karatasi Yenye Habari Summer, vol. 1963. (Shikar Safari Club, Cary, Illinois), 6–11.
Athayde S., Silva-Lugo J., Schmink M., Kaiabi A., Heckenberger M. (2017). Reconnecting art and science for sustainability: Learning from Indigenous knowledge through participatory action-research in the Amazon. Ecol. Soc. 22, 36. doi: 10.5751/ES-09323-220236
Atkinson R., Flint J. (2001). Accessing hidden and hard-to-reach populations: Snowball research strategies. Soc. Res. Update 33, 1-4.
Attorney-General, Republic of Kenya (2012). Wildlife (Conservation and Management) Act; Chapter 376 (Nairobi: National Council for Law Reporting). Revised Edition 2012 [1985].
Ayuyo I. O. A., Sweta L. (2014). Land cover and land use mapping and change detection of mau complex in Kenya using geospatial technology. Int. J. Sci. Res. 3, 767–778.
Bartholomew K., Henderson A. J. Z., Marcia J. E. (2000). “Coding semi-structured interviews in social psychological research,” in Handbook of research methods in social and personality psychology. Eds. Reis H., Judd C. M. (Cambridge University Press, Cambridge), 286–312.
Beaulieu-Guay L. R. (2022). The many faces of knowledge: Do science and traditional ecological knowledge coexist in federal assessments. Can. Public Administration 65, 403–420. doi: 10.1111/capa.12491
Berg B. L. (2008). Qualitative research methods for the social sciences (7th ed.) (London, England: Pearson).
Berkes F. (2012). Sacred Ecology (3rd ed.) (Abingdon-on-Thames, U.K: Routledge). doi: 10.4324/9780203123843
Berkes F., Colding J., Folke C. (2000). Rediscovery of traditional ecological knowledge as adaptive management. Ecol. Appl. 10, 1251–1262. doi: 10.1890/1051-0761(2000)010[1251:ROTEKA]2.0.CO;2
Bessesen B. L., Gonzalez-Suarez M. (2021). The value and limitations of local ecological knowledge: Longitudinal and retrospective assessment of flagship species in Golfo Dulce, Costa Rica. People Nat. 3, 627–638. doi: 10.1002/pan3.10219
BirdLife International (2022) Important Bird Areas Factsheet: Aberdare Mountains. Available online at: http://www.birdlife.org.
Capstick P. H. (1991). Death in the Long Grass (New York, New York, United States of America: St. Martin’s Press).
Castello L. (2004). A method to count Pirarucu Arapaima gigas: Fishers, Assessment, and Management. North Am. J. Fisheries Manage. 24, 379–389. doi: 10.1577/M02-024.1
Charnley S., Fischer A. P., Jones E. T. (2007). Integrating traditional and local ecological knowledge into forest biodiversity conservation in the Pacific Northwest. For. Ecol. Manage. 246, 14–28. doi: 10.1016/j.foreco.2007.03.047
Eerkes-Medrano L., Huntington H. P. (2021). Untold stories: Indigenous knowledge beyond the changing Arctic cryosphere. Front. Climate 3. doi: 10.3389/fclim.2021.675805
Elkan P. W., Smith J. L. D. (2013). Mammals Of Africa: Volume VI. Kingdon, J. and Hoffmann, M. (eds). 1st ed., London: A & C Black Publishers Ltd.
Ellis S. C. (2005). Meaningful consideration? A review of traditional knowledge in environmental decision making. Arctic 58, 66-77. doi: 10.14430/arctic390
Encyclopedia of Life (2018). "Basketgrass Oplismenus hirtellus (L.) P. Beauv." Available from Encyclopedia of Life v3, https://eol.org/pages/1115094. Accessed 3 Jan 2023.
Estes R. D. (1999). The Safari Companion: A guide to watching African mammals (White River Junction, Vermont: Chelsea Green Publishing Company).
Gachathi M. (2007). Kikuyu Botanical Dictionary: A guide to plant names, uses and cultural values (2nd ed) (Nairobi, Kenya: English Press Ltd).
Gibbon G. E. M., Bindemann M., Roberts D. L. (2015). Factors affecting the identification of individual mountain bongo antelope. PeerJ 3, e1303. doi: 10.7717/peerj.1303
Gomez-Baggethun E., Corbera E., Reyes-Garcia V. (2013). Traditional ecological knowledge and global environmental change: Research findings and policy implications. Ecol. Soc. 18, 72. doi: 10.5751/ES-06288-180472
Henri D. A., Jean-Gagnon F., Gilchrist H. G. (2018). Using Inuit traditional ecological knowledge for detecting and monitoring avian cholera among Common Eiders in the eastern Canadian Arctic. Ecol. Soc. 23, 22. doi: 10.5751/ES-09289-230122
Hodge P. A., Costa A. (2021). Oral history and organizational research: challenges of building knowledge about the past. Organizacoes Sociedade J. 28, 722–756. doi: 10.1590/1984-92302021v28n9901en
Hosen N., Nakamura H., Hamzah A. (2020). Adaptation to climate change: Does traditional ecological knowledge hold the key? Sustainability 12, 676. doi: 10.3390/su12020676
Hurt R. (2020). A hunter’s hunter: A lifetime of African safari (CA, Safari Press: Huntington Beach).
Interpol (2022) Wildlife Crime. Available online at: https://www.interpol.int/en/Crimes/Environmental-crime/Wildlife-crime.
IUCN SSC Antelope Specialist Group (2017). Tragelaphus eurycerus ssp isaaci. The IUCN Red List of Threatened Species. IUCN: Online resource. Access date: 3 Jan 2023. doi: 10.2305/IUCN.UK.2017-2.RLTS.T22057A50197212.en
Kelly D. (2020). Traditional Ecological Knowledge and Western Science: Projections, Problems, and Potential (Indigenous NH Collaborative Collective). Available at: https://indigenousnh.com/2020/01/31/traditional-ecological-knowledge-and-western-science-projections-problems-and-potential/.
Kenya Wildlife Service (2019). National recovery and action plan for the mountain bongo (Tragelaphus eurycerus isaaci) in Kenya, (2019-2023) (Nairobi, Kenya: Kenya Wildlife Service).
Kingdon J. (2015). The Kingdon Field Guide to African Mammals (2nd ed.) (London, U.K: Bloomsbury Publishing Plc).
Koopmans M., Stokes E. J., Opepa C. K., Mouele A. M., Abea G., Strindberg S., et al. (2021). Wild bongo density estimation and population viability analysis improves conservation management. Global Ecol. Conserv. 28, e01661. doi: 10.1016/j.gecco.2021.e01661
Kovach M. (2010). Indigenous methodologies: Characteristics, conversations, and contexts (Toronto, Canada: University of Toronto Press).
Lambrechts C., Gachanja M., Woodley B. (2005). Maasai Mau Forest Status Report (Nairobi, Kenya: Department for International Development (DFID) through Kenya Forests Working Group).
Lambrechts C., Woodley B., Church C., Gachanja M. (2003). Aerial Survey of the Destruction of the Aberdare Range Forests. (Nairobi, Kenya: United Nation’s Environment Programme, Division of Early Warning and Assessment).
Luke Q., Beentje H. (2020). 100 Trees to See on Safari; Easy ID Guide for East Africa (South Africa: Struik Nature).
Martin E., Burgess N. (2022). “East African montane forests,” in One Earth. Online resource. Access date: 3 Jan 2023. Available at: https://www.oneearth.org/ecoregions/east-african-montane-forests/.
McPherson J. M., Sammy J., Sheppard D. J., Mason J. J., Brichieri-Colombi T. A., Moehrenschlager A. (2016). Integrating traditional knowledge when it appears to conflict with conservation: lessons from the discovery and protection of sitatunga in Ghana. Ecol. Soc. 21, 24. doi: 10.5751/ES-08089-210124
Mysterud A., Rauset G. R., Van Moorter B., Andersen R., Strand O., Rivrud I. M. (2020). The last moves: the effect of hunting and culling on the risk of disease spread from a population of reindeer. J. Appl. Ecol. 57, 2509–2518. doi: 10.1111/1365-2664.13761
Nellemann C., Henriksen R., Kreilhuber A., Stewart D., Kotsovou M., Raxter P. (2016). The Rise of Environmental Crime: A Growing Threat to Natural Resources, Peace, Development and Security. RHIPTO. Available online at: www.rhipto.org. Accessed on 3 Jan 2023.
Nikolopoulou K. (2022) What is snowball sampling? Definition & examples. Scribbr. Available online at: https://www.scribbr.com/methodology/snowball-sampling/.
Olang L. O., Kundu P. M. (2011). “Land degradation of the Mau Forest Complex in Eastern Africa: A review for management and restoration planning,” in Environmental Monitoring. Ed. Ekundayo E. (InTech, Rijeka, Croatia), 245–262, ISBN: ISBN: 978-953-307-724-6
Paulson N. (2012). The place of hunters in global conservation advocacy. Conserv. Soc. 10, 53–62. doi: 10.4103/0972-4923.92195
Pauly D. (1995). Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol. Evol. 10, 430. doi: 10.1016/S0169-5347(00)89171-5
Pearce F. (2015). “In Kenya’s mountain forests, a new path to conservation,” in The Yale School of the Environment: Yale Environment 360. Available at: https://e360.yale.edu/features/in_Kenyas_mountain_forests_a_new_path_to_conservation. Online resource. Access date: 3 Jan 2023.
Pearce T., Ford J., Cunsolo Willox A., Smit B. (2015). Inuit traditional ecological knowledge (TEK), subsistence hunting and adaptation to climate change in the Canadian Artic. Arctic 68, 233–245. doi: 10.14430/arctic4475
Pinnock D. (2019). Trophy Hunting Part One: The nasty colonial sport of shooting wild animals (South Africa: The Maverick). Available at: https://www.dailymaverick.co.za/article/2019-04-25-trophy-hunting-part-one-the-nasty-colonial-sport-of-shooting-wild-animals/.
Poor E. E., Imron M. A., Novalina R., Shaffer L. J., Mullinax J. M. (2021). Increasing diversity to save biodiversity: Rising to the challenge and supporting Indonesian women in conservation. Conserv. Sci. Pract. 3, e395. doi: 10.1111/csp2.395
POWO (2024). Taxon: Oideania alpina (K.Schum) stapleton. Plants of the World Online. Royal Botanic Gardens, Kew. Available online at: http://www.plantsoftheworldonline.org/. Accessed on 3 Jan 2023.
Prettejohn M. (2012). Endless Horizons: 100 years of the Prettejohn family in Kenya (Kijabe, Kenya: Old Africa Books).
Prettejohn M., Shears J., Comport P. (2020). Bongo Surveillance Project 2020 Review (UK: Lulu Press Inc).
Raftogianni G., Kontsiotis V. J., Liordos V. (2022). Wildlife knowledge and attitudes toward hunting: A comparative hunter–non-hunter analysis. Sustainability 14, 14541. doi: 10.3390/su142114541
RCMRD-SERVIR. (2017). Kenya Sentinel2 Land Use Land Cover. Available at: http://geoportal.rcmrd.org/layers/servir%3Akenya_sentinel2_lulc2016. Accessed 4 October 2019
Raymond C. M., Fazey I., Reed M., Stringer L., Robinson G., Evely A. C. (2010). Integrating local and scientific knowledge for environmental management. J. Environ. Manage. 91, 1766–1777. doi: 10.1016/j.jenvman.2010.03.023
Ronoh T. K., Makori G., Ayub M. (2016). Hunting apprenticeship as Indigenous form of educaiton for sustainable conservation of wildlife in Mau Forest of Kenya. Eur. J. Educ. Stud. 1, 1-29. doi: 10.6084/m9.figshare.3118432
Rust N. A., Abrams A., Challender D. W., Chapron G., Ghoddousi A., Glikman J. A., et al. (2017). Quantity does not always mean quality: The importance of qualitative social science in conservation research. Soc. Natural Resour. 30, 1304–1310. doi: 10.1080/08941920.2017.1333661
Sas-Rolfes M. (2017). African wildlife conservation and the evolution of hunting institutions. Environ. Res. Lett. 12, 115007. doi: 10.1088/1748-9326/aa854b
Sharma B. (2010). “Language and cultural barriers,” in Encyclopedia of case study research. Eds. Mills A. J., Durepos G., Wiebe E. (SAGE Publications, Inc, Thousand Oaks, CA), 519–521. doi: 10.4135/9781412957397
Shears J. (2015). ““Report for bongo studbook”,” in International Studbook for Eastern/Mountain Bongo. Ed. Bosley L. F. (The Oregon Zoo, Portland), 14–18.
Sheppard D. J., Brichieri-Colombi T. A., Stark D. J., Lambrechts C., Moehrenschlager A., McPherson J. M. (2022). When ecological analysis reveals hidden human dimensions: Building on long-term community participation to enable a conservation translocation of mountain bongo in Kenya. Front. Conserv. Sci. 2. doi: 10.3389/fcosc.2021.788267
Sousa R., Nogueira J. G., Miranda F., Teixeira A. (2020). Time travelling through local ecological knowledge regarding an endangered species. Sci. Total Environ. 739, 140047. doi: 10.1016/j.scitotenv.2020.140047
Species 360 Zoological Information Management System (ZIMS) (2023) Species holding report for: Tragelaphus eurycerus/Bongo. Available online at: https://Species360.org.
Squire C. (2013). “From experience-centred to socioculturally-oriented approaches to narrative,” in Doing Narrative Research. Eds. Andrews M., Squire C., Tamboukou M. (Sage, London), 47–71.
Squire C., Andrews M., Tamboukou M. (2008). “Introduction: what is narrative research?,” in Doing narrative research. Eds. Andrews M., Squire C., Tamboukou M. (Sage, London), 1–21.
Steinhart E. I. (2006). Black Poachers, White Hunters: A social history of hunting in colonial Kenya (Oxford, U.K: James Currey Publishers).
Stern E. R., Humphries M. M. (2022). Interweaving local, expert, and Indigenous knowledge into qualitative wildlife analyses: A systematic review. Biol. Conserv. 266, 109444. doi: 10.1016/j.biocon.2021.109444
Svengren H., Prettejohn M., Bunge D., Fundi P., Björklund M. (2017). Relatedness and genetic variation in wild and captive populations of mountain bongo in Kenya obtained from genome-wide single-nucleotide polymorphism (SNP) data. Global Ecol. Conserv. 11, 196–206. doi: 10.1016/j.gecco.2017.07.001
Thomson A. (2011). “Memory and remembering in oral history,” in The Oxford Handbook of Oral History. Ed. Ritchie D. A. (Oxford University Press, Oxford, U.K), 77–95.
Turvey S. T., Bryant J. V., McClune K. A. (2018). Differential loss of components of traditional ecological knowledge following a primate extinction event. R. Soc. Open Sci. 5, 172352. doi: 10.1098/rsos.172352
United Nations Environmental Programme (UNEP) (2012). The Role and Contribution of Montane Forests and Related Ecosystem Services to the Kenyan Economy. (Nairobi, Kenya: United Nations Environment Programme).
Usher P. (2000). Traditional ecological knowledge in environmental assessment and management. Arctic 53, 183-193. doi: 10.14430/arctic849
World Wildlife Fund (2022) Illegal Wildlife Trade: Overview. Available online at: https://www.worldwildlife.org/threats/illegal-wildlife-trade.
Wyllie de Echeverria V. R., Thornton T. F. (2019). Using traditional ecological knowledge to understand and adapt to climate and biodiversity change on the Pacific coast of North America. Ambio 48, 1447–1469. doi: 10.1007/s13280-019-01218-6
Keywords: conservation re-introduction, critically endangered species, historical knowledge, informants, mountain bongo, semi-structured interviews
Citation: Sheppard DJ, Stark DJ, Muturi SW and Munene PH (2024) Benefits of traditional and local ecological knowledge for species recovery when scientific inference is limited. Front. Conserv. Sci. 5:1383611. doi: 10.3389/fcosc.2024.1383611
Received: 07 February 2024; Accepted: 24 May 2024;
Published: 13 June 2024.
Edited by:
Jean Hugé, Open University of the Netherlands, NetherlandsReviewed by:
L. Jen Shaffer, University of Maryland, College Park, United StatesCopyright © 2024 Sheppard, Stark, Muturi and Munene. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Donna J. Sheppard, ZG9ubmFzQGNhbGdhcnl6b28uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.