- 1College of Veterinary Medicine, Cornell University, Ithaca, NY, United States
- 2South-East Zoo Alliance for Reproduction & Conservation (SEZARC), Yulee, FL, United States
- 3White Oak Conservation, Yulee, FL, United States
- 4Cheetah Conservation Fund, Otjiwarongo, Namibia
- 5Smithsonian National Zoo and Conservation Biology Institute, Front Royal, VA, United States
Zoo managed cheetahs provide an insurance population for wild cheetahs that are under threat of extinction from habitat loss, lack of prey, competition, pet trade and poaching for skin and bones. Assisted reproductive techniques including artificial insemination, in vitro fertilization, and embryo transfer augment natural breeding programs but rely on good quality semen for best results. It is understood that anesthesia can affect semen characteristics such as ejaculate volume, total sperm count, sperm motility, and incidence of urine contamination. Thus, the aim of this study was to conduct a retrospective analysis of 23 years of data to investigate sperm parameters of semen collected under anesthesia using medetomidine in combination with butorphanol and midazolam or Telazol® alone. Electroejaculation records (Medetomidine, Butorphanol, and Midazolam anesthetized n = 59 ejaculates, from 30 cheetahs, Telazol® anesthetized, n= 169 ejaculates, from 72 cheetahs) were evaluated for incidence of urine contamination. Electroejaculation records (Medetomidine, Butorphanol, and Midazolam anesthetized n = 21 ejaculates, from 17 cheetahs, Telazol® anesthetized, n = 143 ejaculates, from 63 cheetahs) were evaluated for total sperm count, total motility, ejaculate volume, and testicle size. Telazol® treated cheetahs had a numerically higher total sperm count (Median ± SD: 42.58 ± 77.8 × 106 spermatozoa) compared to those treated with medetomidine (Median ± SD: 31.2 ±44.58 × 106 spermatozoa), and a significantly (p < 0.05) higher sperm motility (Median ± SD: 70.0 ± 9.71%) compared to medetomidine (Median ± SD: 53.0 ± 16.41%) treated cheetahs. The findings of this study indicate that medetomidine anesthesia results in significantly lower sperm motility and Telazol® anesthesia results in a higher total sperm count and motility, thus resulting in higher quality ejaculate. This information can aid in the veterinary management of the species when involved in genome resource banking and assisted reproductive technologies.
1 Introduction
The International Union for Conservation of Nature’s Red List classifies cheetahs (Acinonyx jubatus) as a “vulnerable” species due to a declining population size resulting from habitat loss or fragmentation, poaching and/or lack of available prey (IUCN/SSC, 2007). Additionally, cheetahs have experienced significant population bottlenecks with subsequent inbreeding which has resulted in a lack of genetic diversity (O’Brien et al., 1983; O’Brien et al., 1985; O’Brien et al., 1986; O’Brien et al., 1987; Menotti-Raymond and O’Brien, 1993). To mitigate further loss of genetic diversity, cheetahs are housed under managed care to act as insurance policies for the declining wild populations. Institutions of the Association of Zoos & Aquariums carefully manage breeding as part of the Species Survival Plan® to maximize genetic diversity.
Assisted reproductive techniques (ART) such as artificial insemination (AI) (Donoghue et al., 1992), in vitro fertilization and embryo transfer (Crosier et al., 2020) are used to augment breeding programs and aim to ensure that genetically underrepresented individuals reproduce and contribute offspring to the managed population. The cryopreservation and storage of spermatozoa in genome resource banks have provided a repository of genetic material that can be used to genetically manage future cheetah populations (Wildt, 1997). However, cheetah semen has been characterized as having low numbers of morphologically normal spermatozoa and low sperm concentrations (Wildt et al., 1983; Crosier et al., 2007; Terrell et al., 2016). The high incidence of abnormally structured cells makes cheetah spermatozoa highly susceptible to cryo-induced damage (Crosier et al., 2006). As a result, the ongoing systematic collection and cryopreservation of spermatozoa is required to support the genetic management of cheetahs through ART.
Semen is collected from cheetahs by a technique known as electroejaculation (EEJ) which requires a surgical plane of anesthesia (Howard, 1993). It has been well documented that analgesics can alter sperm function in a range of species (Agirregoitia et al., 2006; Carrillo et al., 2016; Kottwitz et al., 2017) including the domestic cat (Zambelli et al., 2007) and have anecdotally been reported to influence the success of the semen collection. In zoos, cheetahs were historically anesthetized with a protocol involving drugs such as Telazol®, ketamine, or xylazine for health examinations (Penfold, personal communication). In recent years, this protocol has changed and medetomidine is the more commonly used anesthetic due to better desired physiological effects such as stable heart rate, respiratory rates, and quick recovery (Miller et al., 2003; Williamson et al., 2018). Therefore, the aim of the present study was to retrospectively investigate the effects of anesthesia protocols using Telazol® and medetomidine on semen characteristics.
2 Materials and methods
2.1 Cheetahs and criteria for data selection
Cheetahs were housed at White Oak Conservation (WOC) in Yulee, Florida from 1998-2021 or at the Cheetah Conservation Fund (CCF) in Namibia from 2002-2005. Captive cheetahs at WOC were fed a diet of commercially available beef, horse, venison, and/or pork meat products (Milliken Meat Products Ltd, or Carnivore Diet 10; Natural Balance Pet Foods Inc., Pacoima, CA) seven days a week, bones one day a week, and supplemented with whole rabbits when available. Wild cheetahs at CCF were fed combination of donkey, horse and game species with mineral supplementation as described previously (Crosier et al., 2006; Crosier et al., 2007). Animals had access to ad libitum water. Cheetahs were housed individually or as coalition groups and ranged from one to fifteen years in age. Medical records, stored in Species 360 Zoological Information Management System (ZIMS and reproductive summary reports generated by the South-East Zoo Alliance for Reproduction & Conservation and CCF, were reviewed (228 procedures between the years of 1998 and 2021). Incidence of urine contamination was recorded and compared between the two anesthesia protocols (MBMZ anesthetized n = 59 ejaculates, from 30 cheetahs, Telazol® anesthetized, n= 169 ejaculates, from 72 cheetahs). The effect of age on total sperm count was investigated in each treatment group (MBMZ anesthetized n = 29 ejaculates, from 22 cheetahs, Telazol® anesthetized, n= 162 ejaculates, from 71 cheetahs). To investigate the effect of anesthesia protocols on semen characteristics, ejaculates collected from healthy, sexually mature (≥2 years of age) (Maly et al., 2018) males, that were not contaminated with urine, were included in the data analysis (MBMZ anesthetized n = 21 ejaculates, from 17 cheetahs, Telazol® anesthetized, n= 143 ejaculates, from 63 cheetahs).
2.2 Anesthesia
Males were anesthetized using one of two protocols. Protocol 1 utilized a range of 0.030-0.035 mg/kg of Medetomidine, 0.18-0.22 mg/kg of Butorphanol, and 0.10-0.14 mg/kg of Midazolam (MBMZ) in captive WOC cheetahs. Protocol 2 utilized a range of 4 mg/kg to 6 mg/kg of tiletamine hydrochloride plus zolazepam (Telazol®) (Crosier et al., 2007) in wild CCF cheetahs and two captive WOC cheetahs. While isoflurane gas was used during some cheetah immobilizations, it was not applied until after the EEJ procedure ended.
2.3 Semen collection and characterization
Prior to semen collection, testes were measured with calipers and the total testes volume was calculated (V = L × W2 × 0.524; where V= total volume, L = total length and W = width)14. Investigators conducting semen collections (L.M.P. and A.E.C.) were trained by the same researcher (Dr. Jo-Gayle Howard) and followed the same protocol for semen collection (Howard, 1993). In brief, feces were manually removed from the rectum and the prepuce was rinsed with sterile phosphate-buffered saline. The penis was extended from the prepuce and placed in a sterile collection cup (BD Falcon, Franklin Lakes, NJ, USA). To collect the semen, a Teflon rectal probe (1.6 cm in diameter with three longitudinal electrodes) and electro-stimulator were used to administer 10 stimulations at 2, 3 and 4 volts (Series I), 3, 4, 5 volts (Series II), and 5 and 6 volts (Series III) (Crosier et al., 2006). Semen was characterized as previously described (Crosier et al., 2006; Johnson et al., 2010). Data sheets were reviewed for testicular volume, semen volume, total sperm count, total motility, and presence of urine contamination. In brief, volume was measured using adjustable micropipettes (Pipetman Classic, Gilson Incorporated, Middleton, WI, USA). Sperm concentration was calculated by diluting semen 1:400 with water and counting the number of spermatozoa in a 10 µl volume using a hemocytometer (Daigger Scientific, Inc., Hills, IL, USA). Total sperm count was calculated by multiplying semen volume by the sperm concentration. Sperm motility (%) was subjectively assessed using phase-contrast microscopy (40 ×, Olympus B-Max Microscope, Olumpus Optical Co. Ltd) examining 5 µl aliquot of raw ejaculate at 37°C with a minimum of three separate fields of view examined. The pH was measured using pH indicator strips (EM Science, Gibbstown, NJ, USA). A sample was considered to be contaminated with urine if the pH was <7.5 (Bertschinger et al., 2008).
2.4 Statistical analysis
Statistical analyses were performed using SigmaPlot (V15.0, Systat Software Inc. San Jose, CA, USA). Ejaculate characteristics between anesthesia protocols were analyzed by a one-way ANOVA and nonparametric Kruskal-Wallis Test. Spearman’s correlation was used to evaluate the correlation between testicle size and total sperm count within anesthesia protocols. Incidence of urine contamination between anesthesia protocols was analyzed using a Pearson chi-squared test. Data are reported as the Median ± SD. Data was considered significant if p < 0.05.
3 Results
3.1 Age v. total sperm count
Analysis of n = 29 MBMZ treated ejaculates showed a decline in total sperm count with age (Figure 1A). Three males >10 years of age had a numerically lower total sperm count (mean ± SEM = 1.14 ± 1.21 × 106 spermatozoa) than sexually mature males ≤10 years of age (mean ± SEM = 44.38 ± 9.73 × 106 spermatozoa). Analysis of n = 162 Telazol® treated ejaculates also showed a decline in total sperm count with age (Figure 1B). Three males >10 years of age had a numerically lower total sperm count (mean ± SEM = 32.38 ± 9.69 × 106 spermatozoa) than sexually mature males ≤10 years of age (mean ± SEM = 67.99 ± 6.55 × 106 spermatozoa). These findings contributed to restricting sperm analysis data to sexually mature males ≤10 years of age.
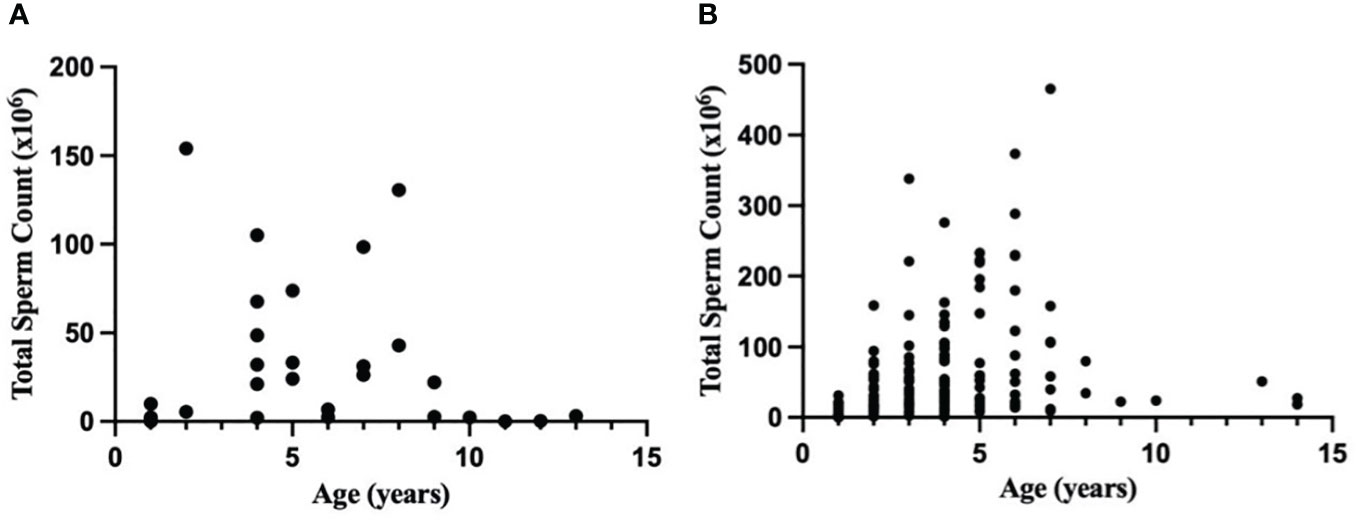
Figure 1 Relationship between age (years) and total sperm count (×106) in a normal ejaculate for healthy cheetahs (Acinonyx jubatus) anesthetized with (A) MBMZ (n = 29 ejaculates, 1-13 years old, three males > 10 years old included) or (B) Telazol® (n = 162 ejaculates, 1-14 years old, three males > 10 years old included).
3.2 Effects of anesthesia on semen collection
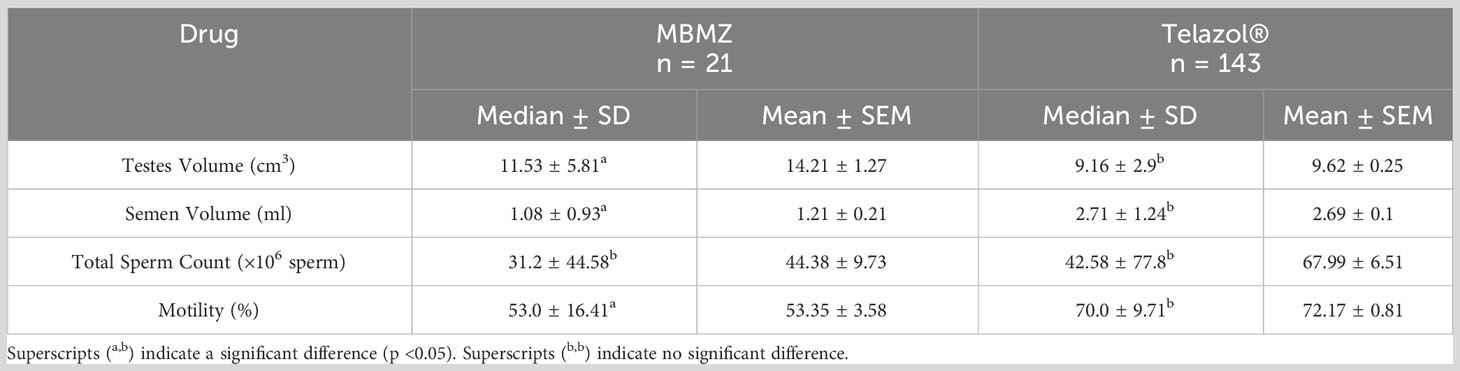
The incidence of urine contamination was greater (p < 0.001) for cheetahs anesthetized using MBMZ (50.85%) than Telazol® (4.14%). Although treatment groups were not statistically different, a numerically higher total sperm count was observed in cheetahs anesthetized with Telazol® (Median ± SD: 42.58 ± 77.8 × 106 spermatozoa) than MBMZ (Median ± SD: 31.2 ± 44.58 × 106 spermatozoa) (Figure 2). Total motility was significantly (p < 0.05) greater in ejaculates collected using Telazol® (Median ± SD: 70.0 ± 9.71%) than MBMZ (Median ± SD: 53.0 ± 16.41%) (Figure 3). There was a significant difference (p < 0.05) in testes size between both treatment groups (Median ± SD MBMZ: 11.53 ± 5.81 cm3, Median ± SD Telazol®: 9.16 ± 2.9 cm3) (Table 1). Testes size and total sperm count were not correlated in the MBMZ treatment group (p > 0.05; R = 0.086) but were weakly correlated in the Telazol® treatment group (p < 0.05; R = 2.1).
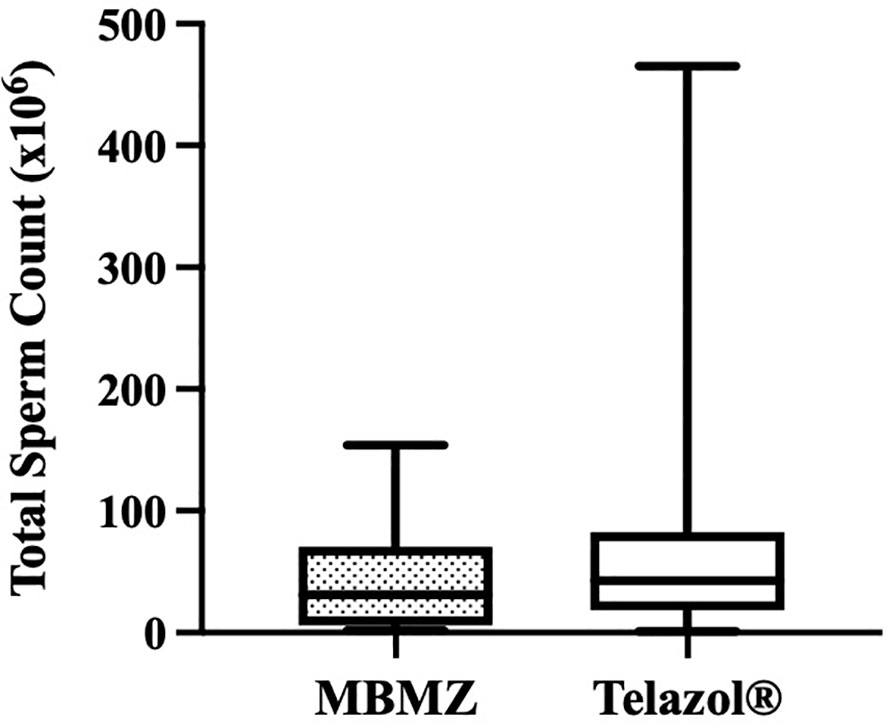
Figure 2 Box and Whiskers plot showing variation of total sperm count in cheetah (Acinonyx jubatus) ejaculates collected by EEJ using MBMZ or Telazol® anesthesia protocols (MBMZ anesthetized n = 21 ejaculates, from 17 cheetahs, Telazol® anesthetized n = 143 ejaculates, from 63 cheetahs). Boxes enclose the 25th and 75th percentiles, the horizontal bars within the boxes are the median values, and the whiskers extend to the minimum and maximum values observed.
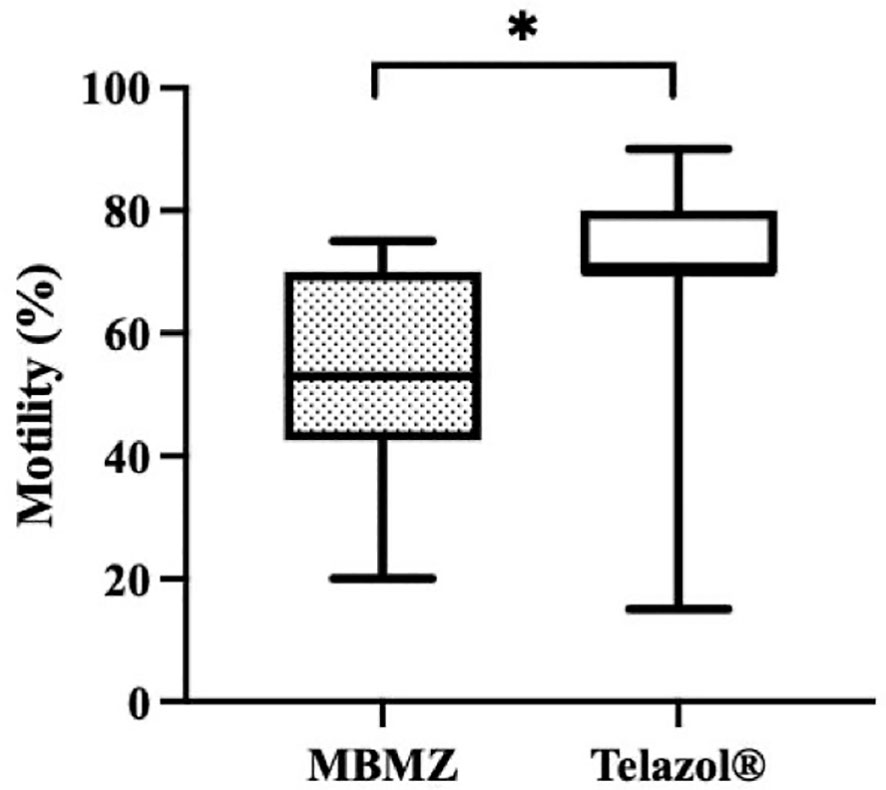
Figure 3 Box and Whiskers plot showing variation of motility in cheetah (Acinonyx jubatus) ejaculates collected by EEJ using MBMZ or Telazo® anesthesia protocols (MBMZ anesthetized n = 21 ejaculates, from 17 cheetahs, Telazol® anesthetized n = 143 ejaculates, from 63 cheetahs). Boxes enclose the 25th and 75th percentiles, the horizontal bars within the boxes are the median values, and the whiskers extend to the minimum and maximum values observed. The asterisk indicates a significant difference in motility (p < 0.05).
4 Discussion
This is the first study to examine the effect of anesthetic drugs on cheetah spermatozoa. The most compelling impact of anesthesia was on sperm motility, with significantly lower motile sperm found in the MBMZ protocol. Although the testes size was larger in cheetahs anesthetized with MBMZ versus Telazol®, and therefore might have been expected to produce more sperm (Olar et al., 1983), the Telazol® treated cheetahs resulted in a greater total sperm count and significantly greater motility. Correlation coefficients reveal that in the MBMZ protocol, testes size was not correlated with total sperm count (p > 0.05; R= 0.086), however, in the Telazol® protocol, testes size and total sperm count were weakly correlated (p < 0.05; R = 2.1). Total sperm count and motility are vital characteristics in determining the quality of sperm samples to be cryopreserved for use in artificial breeding. The incidence of urine contamination was characterized during semen collection and was more commonly observed in the MBMZ protocol.
Medetomidine is an alpha-2-adrenergic agonist commonly used in non-domestic felid anesthesia (Brown et al., 1991). Alpha-2 receptors are found along the male reproductive tract and are required for successful emission and ejaculation (McDonnell, 1992). Studies investigating the effects of medetomidine, dexmedetomidine, or xylazine on the hypothalamic-pituitary axis demonstrated these drugs’ ability to suppress anti-diuretic hormone secretion. Physiologically this limits water re-uptake by the kidneys, leading to increased volume for urination, seen most pronounced with xylazine (Rouch and Kudo, 1996; Cabral et al., 1998; Nuñez et al., 2004; Villela et al., 2005; Murahata and Hikasa, 2012; Uddin et al., 2021). Additionally, medetomidine targets alpha-2 receptors of smooth muscle in the bladder and urethra, possibly leading to conflicting contractions and urine contamination during semen collection (Michel and Vrydag, 2006). Smooth muscle contractions along the male reproductive tract are required to mediate ejaculation, but the reproductive physiology remains complex (McDonnell, 1992). Past studies in domestic species have demonstrated that alpha-2 agonists at higher doses result in increased sperm concentration and motility (Da Silva et al., 2021), but do not stimulate the accessory glands adequately, resulting in a lower volume of semen (McCue, 2021). Incidence of retrograde flow of semen involving alpha-2’s has also been observed (Dooley et al., 1990; Zambelli et al., 2007) though positive effects on sperm concentration were noted with alpha-2’s administered at higher doses. Urine contamination, retrograde flow, and low sperm volume can result in poor sperm quality which is problematic for assisted reproductive techniques and genome resource banking.
The other two components of Protocol 1 were butorphanol (an opioid) and midazolam (a benzodiazepine). When used alone for sedation, butorphanol has been associated with high sperm concentration at collection, yet when combined with an alpha-2 agonist and NMDA antagonist, it has shown a decrease in sperm concentration (Ungerfeld et al., 2022). Midazolam is not commonly used as the sole choice for sedation. In a laboratory setting where rats were administered midazolam twice daily for a week, direct epididymal collections resulted in inferior sperm motility compared to collections from rats administered propofol (Celaleddin and Volkan, 2020). A protocol limiting the use of alpha-2 agonists at low doses might be better for semen collection and genome resource banking and remains a future area of research; additional information on the roles opioids and benzodiazepines play in semen collection is also warranted.
In contrast to medetomidine, Telazol® antagonizes N-methyl-D-aspartate (NMDA) receptors to produce anesthetic effects (Lester, 2012). Multiple studies of domestic and wild felids anecdotally support the notion that sperm motility has a high mean value when Telazol® was used (Brown et al., 1991; Howard et al., 1992; Long et al., 1996). In one study involving six domestic cats, researchers collected semen via EEJ methods using Telazol® anesthesia resulting in an average sperm motility of 82.1% (Long et al., 1996). Similarly, semen also was acquired from six male cheetahs using EEJ methods and Telazol®, with an average motility of 75% (Howard et al., 1992). Lastly, in six adult and four young adult Serengeti-Plains lions under the same conditions (using EEJ and Telazol®), the average motility was 89% and 72%, respectively (Brown et al., 1991). In the present study, spermatozoa were 70.0 ± 9.71% motile in ejaculates from cheetahs treated with Telazol®.
A numerical decline in sperm concentration with age was observed. Males over 10 years old produced a lower total sperm count. These findings are in agreement with other studies where a decrease in litter numbers for males greater than or equal to 13 years of age (Bertschinger et al., 2008) was seen suggesting lower total sperm count as a possible explanation for a decline in sperm quality.
Qualification of cheetahs and population sites were limitations to this study. As previously stated, the original 228 semen samples did not all qualify for final statistical analysis based on male prepubescence, senescence (Durrant et al., 2001; Maly et al., 2018), urine contamination, or disease. Additionally, data from some cheetahs were not considered due to administration of additional drugs or isoflurane gas during semen collection. Some medical records lacked enough information to include as well. Finally, there was a limitation of unbalanced design in treatment groups between populations, as two captive cheetahs from White Oak Conservation were treated with Telazol® and grouped accordingly. In an attempt to present the most vital information, sample sizes were limited. Moving forward, detailed medical records are essential to retrospective analyses that inform reproductive physiologists and veterinarians on protocols conducive to adequate ejaculate quality. Additionally, the two cheetah populations are functionally different. Though unavoidable in retrospect, differences include rangeland (wild vs. captive), diet (carcass vs. frozen and prepared), and management. A difference in testes size was appreciated statistically between the two populations in this study attributed to these differences. In an earlier study, a difference in semen quality was not observed in captive versus wild populations of cheetahs (Wildt et al., 1987); a difference cannot be determined as our design is unbalanced (animals treated with Telazol® were both captive and wild). To confirm a difference, controlled studies using MBMZ in wild and captive populations are necessary to compare.
The present study emphasizes that anesthetic protocol should be considered when semen collection of genome resource banking is the primary reason for sedation of endangered felid species. The results inform veterinarians and reproductive physiologists of the effects alpha-2 agonists have on semen collection procedures in cheetahs. With the availability of reversible medications paired with alpha-2 agonists and smoother recoveries, it is unlikely that Telazol® will be used in the future, but manipulating dosages for medetomidine could be considered. The retrospective analysis of collections in cheetahs adds to the unique history zoological institutions share in managed breeding and warrants continued research to better existing protocols.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because this is a retrospective study on cheetahs.
Author contributions
CB: Conceptualization, Data curation, Investigation, Visualization, Writing – original draft, Writing – review & editing. LP: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. JG: Data curation, Formal Analysis, Investigation, Supervision, Writing – original draft, Writing – review & editing. SC: Investigation, Methodology, Resources, Writing – review & editing. LM: Data curation, Investigation, Methodology, Resources, Writing – review & editing. AC: Data curation, Investigation, Methodology, Resources, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank White Oak Conservation and the Cheetah Conservation Fund for providing medical records and Jack Kottwitz, DVM, PhD, CertAqV for guidance on pharmacological aspects of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agirregoitia E., Valdivia A., Carracedo A., Cases L., Gil J., Subiran N., et al. (2006). Expression and localization of δ-, κ-, and μ-opioid receptors in human spermatozoa and implications for sperm motility. J. Clin. Endocrinol. Metab. 91 (12), 4969–4975. doi: 10.1210/jc.2006-0599
Bertschinger H. J., Meltzer D. G. A., van Dyk A.. (2008). Captive breeding of cheetahs in South Africa 30 years of data from the de wildt cheetah and wildlife centre. Reprod. Domest. Anim. 43, 66–73. doi: 10.1111/j.1439-0531.2008.01144.x
Brown J. C., Bush M., Packer C., Pusey A. E., Monfort S. L., O’Brien S. J., et al. (1991). Developmental changes in pituitary-gonadal function in free-ranging lions (Panthera leo leo) of the Serengeti Plains and Ngorongoro Crater. Reproduction 91 (1), 29–40. doi: 10.1530/jrf.0.0910029
Cabral A. M., Kapusta D. R., Kenigs V. A., Varner K. J. (1998). Central α2-receptor mechanisms contribute to enhanced renal responses during ketamine-xylazine anesthesia. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 275 (6), R1867–R1874. doi: 10.1152/ajpregu.1998.275.6.r1867
Carrillo A. V., Rodriguez M. A., Rodriguez-Martinez H. (2016). The mu (μ) and delta (δ) opioid receptors modulate boar sperm motility. Mol. Reprod. Dev. 83 (8), 724–734. doi: 10.1002/mrd.22675
Celaleddin S., Volkan K. (2020). The effects of propofol and midazolam on rat spermatogenic parameters. Arquivo Brasileiro Medicina Veterinaria E Zootecnia 72 (5), 1727–1730. doi: 10.1590/1678-4162-11909
Crosier A. E., Pukazhenthi B. S., Henghali J. N., Howard J., Dickman A. J., Marker L., et al. (2006). Cryopreservation of spermatozoa from wild-born Namibian cheetahs (Acinonyx jubatus) and influence of glycerol on cryosurvival. Cryobiology 52 (2), 169–181. doi: 10.1016/j.cryobiol.2005.10.011
Crosier A. E., Marker L., Howard J., Pukazhenthi B. S., Henghali J. N., Wildt D. E. (2007). Ejaculate traits in the Namibian cheetah (Acinonyx jubatus): influence of age, season and captivity. Reproduction Fertil. Dev. 19 (2), 370. doi: 10.1071/rd06057
Crosier A. E., Lamy J., Bapodra P., Rapp S., Maly M., Junge R., et al. (2020). First birth of cheetah cubs from in vitro fertilization and embryo transfer. Animals 10 (10), 1811. doi: 10.3390/ani10101811
Da Silva M. C. C., Ullony K. M., Ribeiro de Araujo G., Jorge-Neto P. N., Albuquerque V. B., Caramalac S. M., et al. (2021). Can detomidine replace medetomidine for pharmacological semen collection in domestic cats? Anim. Reprod. 18 (2). doi: 10.1590/1984-3143-ar2021-0017
Donoghue A. M., Howard J., Barone M. A., Goodrowe K. L., Blumer E. S., Snodgrass K., et al. (1992). Successful induction of ovarian activity and laparoscopic intrauterine artificial insemination in the Cheetah (Acinonyx jubatus). J. Zoo Wildlife Med. 23 (3), 288–300. Available at: http://www.jstor.org/stable/20095230.
Dooley M. P., Pineda M. H., Hopper J. G., Hsu W. H. (1990). Retrograde flow of spermatozoa into the urinary bladder of dogs during ejaculation or after sedation with xylazine. PubMed 51 (10), 1574–1579.
Durrant B. S., Millard S. E., Zimmerman D., Lindburg D. G. (2001). Lifetime semen production in a cheetah (Acinonyx jubatus). Zoo Biol. 20 (5), 359–366. doi: 10.1002/zoo.1034
Howard J. (1993). “Semen collection and analysis in nondomestic carnivores,” in Zoo and Wildlife Animal Medicine: Current Therapy III. Eds. Fowler M. E., Miller R. E. (Philadelphia, PA: W.B. Saunders Co), 390–399.
Howard J. G., Barone M. A., Donoghue A. M., Wildt D. E. (1992). The effect of pre-ovulatory anaesthesia on ovulation in laparoscopically inseminated domestic cats. Reproduction 96 (1), 175–186. doi: 10.1530/jrf.0.0960175
IUCN/SSC. (2007). Regional Conservation Strategy for the Cheetah and African Wild Dog in Southern Africa (Gland. Switzerland: IUCN Species Survival Commission).
Johnson W. E., Onorato D. P., Roelke M. E., Land E. D., Cunningham M., Belden R. C., et al. (2010). Genetic restoration of the Florida panther. Science 329 (5999), 1641–1645. doi: 10.1126/science.1192891
Kottwitz J., Stoops M., Reeves J., Harmon R., Wilborn R., Edmondson M., et al. (2017). Negative effects of analgesic and anesthetic drugs on sperm motility: implications for assisted breeding in managed rhinoceros. Proc. Am. Assoc. Zoo Vet., 107–108.
Lester P. A. (2012). “Anesthesia and analgesia,” in The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. Eds. Suckow M. A., Stevens K. A., Wilson R. P. (Boston. Boston: Academic Press), 33–56.
Long J. A., Wildt D. E., Wolfe B. A., Critser J. K., DeRossi R. V., Howard J. (1996). Sperm capacitation and the acrosome reaction are compromised in teratospermic domestic cats1. Biol. Reprod. 54 (3), 638–646. doi: 10.1095/biolreprod54.3.638
Maly M. A., Edwards K. L., Farin C. E., Koester D. C., Crosier A. E. (2018). Assessing puberty in ex situ male cheetahs (Acinonyx jubatus) via fecal hormone metabolites and body weights. Gen. Comp. Endocrinol. 268, 22–33. doi: 10.1016/j.ygcen.2018.07.011
McCue P. M. (2021). Chemical ejaculation. Equine Reprod. Procedures, 455–456. doi: 10.1002/9781119556015.ch121
McDonnell S. M. (1992). Ejaculation: physiology and dysfunction. Vet. Clinics North America-equine Pract. 8 (1), 57–70. doi: 10.1016/s0749-0739(17)30466-2
Menotti-Raymond M., O’Brien S. J. (1993). Dating the genetic bottleneck of the African cheetah. Proc. Natl. Acad. Sci. U. S. A. 90 (8), 3172–3176. doi: 10.1073/pnas.90.8.3172
Michel M. C., Vrydag W. (2006). α1 -, α2 - and β-adrenoceptors in the urinary bladder, urethra and prostate. Br. J. Pharmacol. 147 (S2), S88–S119. doi: 10.1038/sj.bjp.0706619
Miller M. A., Weber M., Neiffer D., Mangold B., Fontenot D., Stetter M. (2003). Anesthetic induction of captive tigers (Panthera tigris) using a medetomidine-ketamine combination. J. Zoo Wildlife Med. 34 (3), 307–308. doi: 10.1638/02-036
Murahata Y., Hikasa Y. (2012). Comparison of the diuretic effects of medetomidine hydrochloride and xylazine hydrochloride in healthy cats. Am. J. Vet. Res. 73 (12), 1871–1880. doi: 10.2460/ajvr.73.12.1871
Nuñez E. E. N., Steffey E. P., Ocampo L., Rodriguez A., Garcia A. A. (2004). Effects of α2-adrenergic receptor agonists on urine production in horses deprived of food and water. Am. J. Vet. Res. 65 (10), 1342–1346. doi: 10.2460/ajvr.2004.65.1342
O’Brien S. J., Wildt D. E., Goldman D., Merril C. R., Bush M. (1983). The cheetah is depauperate in genetic variation. Science 221 (4609), 459–462. doi: 10.1126/science.221.4609.459
O’Brien S. J., Roelke M. E., Marker L., Newman A., Winkler C. A., Meltzer D., et al. (1985). Genetic basis for species vulnerability in the cheetah. Science 227 (4693), 1428–1434. doi: 10.1126/science.2983425
O’Brien S. J., Roelke M. E., Marker L., Newman A., Winkler C.A., Meltzer D., et al. (1986). The cheetah in genetic peril. Sci. Am. 254 (5), 84–95. doi: 10.1038/scientificamerican0586-84
O’Brien S. J., Wildt D. E., Bush M., Caro T., Fitzgibbon C., Aggundey I., et al. (1987). East African cheetahs: evidence for two population bottlenecks? Proc. Natl. Acad. Sci. U. S. A. 84 (2), 508–511. doi: 10.1073/pnas.84.2.508
Olar T. T., Amann R. P., Pickett B. W. (1983). Relationships among testicular size, daily production and output of spermatozoa, and extragonadal spermatozoal reserves of the dog. Biol. Reprod. 29 (5), 1114–1120. doi: 10.1095/biolreprod29.5.1114
Rouch A. J., Kudo L. H. (1996). Alpha 2-adrenergic-mediated inhibition of water and urea permeability in the rat IMCD. Am. J. Physiology-renal Physiol. 271 (1), F150–F157. doi: 10.1152/ajprenal.1996.271.1.f150
Terrell K. A., Crosier A. E., Wildt D. E., O’Brien S. J., Anthony N., Marker L., et al. (2016). Continued decline in genetic diversity among wild cheetahs (Acinonyx jubatus) without further loss of semen quality. Biol. Conserv. 200, 192–199. doi: 10.1016/j.biocon.2016.05.034
Uddin M., Sebastian J., Usama M., Raziq F. I., Saydain G., Rossi N. F. (2021). Dexmedetomidine induced polyuria in the intensive care unit. Case Rep. Crit. Care 2021, 1–3. doi: 10.1155/2021/8850116
Ungerfeld R., Andrews de Moura Fernandes D., Alvarez Balaro M. F., Taira A. R., Gomes de Espirito Santo C., Rodrigues Santos J. D., et al. (2022). Administration of butorphanol with ketamine/xylazine sedation reduces the negative responses to electroejaculation in rams. Theriogenology 191, 96–101. doi: 10.1016/j.theriogenology.2022.08.008
Villela N. R., do Nascimento Junior P., de Carvalho L. R., Teixeira A. (2005). Efeitos da dexmedetomidina sobre o sistema renal e sobre a concentração plasmática do hormônio antidiurético: estudo experimental em cães. Rev. Bras. Anestesiologia 55 (4). doi: 10.1590/s0034-70942005000400007
Wildt D. E. (1997). “Genome resource banking,” in Reproductive Tissue Banking. Eds. Karow A. M., Crtiser J. K. (New York: Elsevier), 399–439.
Wildt D. E., Bush M., Howard J. G., O’Brien S. J., Meltzer D., van Dyk A., et al. (1983). Unique seminal quality in the South African cheetah and a comparative evaluation in the domestic cat. Biol. Reprod. 29 (4), 1019–1025. doi: 10.1095/biolreprod29.4.1019
Wildt D. E., O’Brien S. J., Howard J. G., Caro T. M., Roelke M. E., Brown J. L., et al. (1987). Similarity in Ejaculate-Endocrine characteristics in captive versus Free-Ranging cheetahs of two subspecies1. Biol. Reprod. 36 (2), 351–360. doi: 10.1095/biolreprod36.2.351
Williamson R. H., Muller L. I., Blair C. D. (2018). The use of ketamine-xylazine or butorphanol-azaperone-medetomidine to immobilize American black (Ursus americanus). J. Wildlife Dis. 54 (3), 503–510. doi: 10.7589/2017-10-255
Keywords: semen collection, alpha-2 agonists, electroejaculation, genome resource banking, cheetah (Acinonyx jubatus)
Citation: Baquerizo CI, Penfold LM, Gillis JD, Citino S, Marker L and Crosier AE (2023) A retrospective analysis investigating the effects of Telazol® and medetomidine on ejaculate characteristics in cheetahs (Acinonyx jubatus). Front. Conserv. Sci. 4:1293180. doi: 10.3389/fcosc.2023.1293180
Received: 12 September 2023; Accepted: 09 November 2023;
Published: 28 November 2023.
Edited by:
José Luis Ros-Santaella, Czech University of Life Sciences Prague, CzechiaReviewed by:
Maitê Cardoso Coelho Da Silva, Federal University of Mato Grosso do Sul, BrazilSylwia Prochowska, Wroclaw University of Environmental and Life Sciences, Poland
Copyright © 2023 Baquerizo, Penfold, Gillis, Citino, Marker and Crosier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carolina I. Baquerizo, Y2liMzhAY29ybmVsbC5lZHU=
 Carolina I. Baquerizo
Carolina I. Baquerizo Linda M. Penfold
Linda M. Penfold James D. Gillis
James D. Gillis Scott Citino3
Scott Citino3 Laurie Marker
Laurie Marker Adrienne E. Crosier
Adrienne E. Crosier