- 1Department of Entomology and Great Lakes Bioenergy Research Center, Michigan State University, East Lansing, MI, United States
- 2Department of Entomology, University of Kentucky, Lexington, KY, United States
- 3Science and Mathematics Division, Lorain County Community College, Elyria, OH, United States
The adoption of biomass crops grown for energy is a likely source of major landscape change in coming decades during the transition from fossil fuels. There are a wide range of cropping systems that have not been widely deployed yet but could become commonplace, and our knowledge of their ecological attributes and biodiversity impacts is limited. Ants are prominent and functionally important components of grassland and agricultural ecosystems. Given their outsized influences on ecosystem structure and function, we sought to understand how ant communities are likely to be shaped by a range of bioenergy cropping systems. We characterized ant communities in a long-term experimental array in Michigan, USA containing ten dedicated bioenergy crops including annual monocultures, simple monoculture or near-monoculture perennial grasses, and complex polyculture systems. Community composition differed strongly among cropping systems, and ants were more abundant, species-rich, and functionally diverse in complex systems than in simpler systems, particularly annual crops. Our results illustrate the divergent effects that bioenergy crop adoption could have for ant communities and the important functions they carry out in agroecosystems.
Introduction
Shifts in agricultural land use and management can drive changes in biodiversity and the supply of ecosystem services. As we seek alternative energy sources in response to climate change, landscapes are likely to increasingly contain bioenergy crops that are grown for biomass used to make ethanol or other fuels (IPCC (Intergovernmental Panel on Climate Change), 2018). The biodiversity impacts of widespread bioenergy adoption are mostly negative and can be large when they encroach on natural areas (Immerzeel et al., 2014; Núñez-Regueiro et al., 2021). However, there are a wide variety of cropping systems that can be used for bioenergy and considerable variation in the amounts of diversity they host (Haan et al., 2023). This means net effects on biodiversity will depend on the nature and management of the cropping systems that are used and the types of habitats they replace. For example, strategically replacing annual crops with biodiverse perennial biofuel feedstocks using precision conservation could provide a pathway for adding biodiversity to intensified agricultural landscapes (Basso and Antle, 2020). Thus, in general, we need much more information about how different types of bioenergy cropping systems shape biotic communities and their functions.
Ants (Hymenoptera: Formicidae) are among the most abundant, active and functionally important groups of organisms in agroecosystems and grasslands, and they strongly influence ecosystem patterns and processes through a number of direct and indirect pathways (Folgarait, 1998; Del Toro et al., 2012; Wills and Landis, 2018). They are also useful indicator species for evaluating management practices (Underwood and Fisher, 2006). Ants are both predators and competitors of herbivores, including crop pests (Grieshop et al., 2012; Wills et al., 2019; Helms et al., 2020; Helms et al., 2021). They also form mutualisms with phloem-feeding herbivores in which they exchange protection for honeydew; in these cases they guard against both herbivores and predators causing complex indirect effects on plants that can be either positive or negative (Wills and Landis, 2018). Ants also serve as plant mutualists offering protection in exchange for carbohydrates from extrafloral nectaries (Bentley, 1976; Bentley, 1977), but if they focus on floral nectar they can interfere with pollination (Lach, 2005; Cembrowski et al., 2014). Still other ant species are primarily seed dispersers (Leal et al., 2015), or are thieves or social parasites that exploit other ant species (Buschinger, 1986; Buschinger, 2009). Below ground, they are ecosystem engineers that alter soil porosity, nutrient distribution, pH, and microbial communities (Bruyn and Conacher, 1990; Cammeraat and Risch, 2008; Frouz and Jilková, 2008). Literature syntheses suggest that ants’ overall effects on plants, while complex, tend to be positive (Styrsky and Eubanks, 2006; Rosumek et al., 2009). In general, while the roles of ants in crops and grasslands are well-studied, their community composition and functional roles in the various types of bioenergy cropping systems that could become widespread in coming years are still underexplored (Helms et al., 2020).
We asked how ant communities differ across ten bioenergy crop types. Most of these cropping systems (except for corn) have not been deployed at large scales yet but could soon become mainstay crops that are common components of agricultural landscapes. We used a long-term replicated experimental array in Michigan, United States containing ten dedicated bioenergy cropping systems. These included annual crops (corn, two sorghum systems), simple perennial systems (two switchgrass systems, Miscanthus, and a native grass mix), and plant-diverse perennial systems (reconstructed prairie, successional volunteer vegetation, and a short-rotation coppicing system with poplar trees). We sampled the ant community throughout this experimental array using pitfall traps. Patterns of ant species richness in this array are described by Haan et al. (2023) alongside those for several other invertebrate groups, plants, and microbes. This study showed ant richness was low in annual systems, and across the perennial systems it increased linearly with plant species richness (Haan et al., 2023). Thus, in the present study we focus on the taxonomic and functional dimensions of ant community composition in these crops.
Our overarching hypothesis was that ant community attributes would be shaped by the types of bioenergy cropping systems they occur in. Our specific predictions were as follows: First, we expected that ant community composition would differ across cropping systems. We expected these differences would correspond to the gradient of management intensity and plant diversity these cropping systems express, with communities ± aligning within the annual systems, simple perennial systems, and plant-diverse perennial systems, but differing strongly between these groups. From a functional perspective, we expected the complex and plant-diverse cropping systems to host ant communities that were associated with more functions and for some of the functions to be carried out by a larger set of species.
Methods
Overview of experimental setup
Data from this study were collected in 2021 in the Bioenergy Cropping Systems Experiment (BCSE), a long-term experimental array located at Kellogg Biological Station, Michigan State University, USA. The BCSE follows a complete blocked design with 5 replicates of each of 10 bioenergy cropping system types. Plots measure 28×40 m and are embedded in a matrix of turfgrass with 15 m spacing between plots. For details on the array see https://lter.kbs.msu.edu/research/long-term-experiments/glbrc-intensive-experiment/. The BCSE contains 3 annual monoculture systems, including continuous corn (Zea mays L.), continuous sorghum (Sorghum bicolor L. Moench photoperiod-sensitive hybrid ES5200), and a second sorghum variety (photoperiod-insensitive hybrid TAM17900) with a fall-planted cereal rye cover crop (Secale cereale L. var. Wheeler). It also contains four perennial systems maintained as monocultures or near-monocultures. These include Miscanthus x giganteus, a sterile hybrid grass that produces bamboo-like thickets, and two treatments containing switchgrass (Panicum virgatum L. var. Cave-in-rock), which is a native North American perennial grass that has been cultivated for use as a biomass crop. One of the switchgrass treatments was seeded into cover crop residue and established during the data collection period; the other was a mature stand established in 2008. There was also a treatment containing five native prairie grass species (Panicum virgatum L., Andropogon gerardii Vitman, Sorghastrum nutans (L.) Nash, Elymus canadensis L., and Schizachyrium scoparium (Michx.) Nash). The last group of treatments, all of which were complex perennial polycultures with higher plant diversity, included reconstructed prairie (18 species; 6 grasses, 9 forbs, 3 legumes, plus volunteers), successional volunteer vegetation (dominated by warm and cool season grasses, grassland/prairie forbs, and a variety of volunteer herbaceous and woody species), and a short-rotation poplar coppicing system (Populus ‘NM6’, a hybrid between P. nigra and P. maximowiczii). The poplar treatment contained a diverse understory of volunteer vegetation. It was planted in 2008, coppiced in 2014, and replaced in 2019; thus, the stand was in its third year of growth during the study with stems ~2–3 m tall. Lists of species seeded into each treatment can be found at https://lter.kbs.msu.edu/wp-content/uploads/2012/05/GLBRC-Species.pdf.
Ant community sampling
Field sampling methods are described in Haan et al. (2023). We placed pitfall traps in 150 locations throughout the experimental array, each corresponding to one of the 3 sampling stations within each of 50 plots. Traps were placed approximately 1.4 m NW of each station and were separated by distances of 14-21 m. In each location we placed PVC sockets (5.08 cm diameter) in the soil so we could move traps in and out with minimal soil disturbance. Sockets were covered with plastic mailing tube caps or capped pitfall cups when traps were not deployed. Traps were 120 mL plastic cups filled with ~60mL 95% ethanol with unscented dish soap to break surface tension (ethanol refilled on hot days as needed). Each trap was sheltered from rainfall with a 15x15 cm plexiglass square held in place with lawn staples. Traps were set to be flush with the soil. Traps were set for 48 h periods six times throughout summer 2021, during the weeks of 31 May, 7 June, 12 July, 19 July, 23 August, and 30 August. This resulted in 900 trapping events distributed across the 150 trapping locations. We omit 16 trapping events because of labeling errors or because traps were destroyed by management activities in the plots. We identified trapped ants using Ellison et al. (2012) and Coovert (2005). Ants were preserved in ethanol and will be housed in the Albert J. Cook arthropod research collection at Michigan State University.
Statistical analysis
For most analyses we considered all individuals of the same species collected at a given sampling location to be from a single colony (Ellison et al., 2007; Gotelli et al., 2011). We took this conservative approach because multiple workers from the same colony are likely to be found in each trapping location. We used these estimates of colony density as abundance metrics in all compositional analyses.
Data analyses were conducted in R version 4.2.1. We tested for community differences among crops using Permutational Multivariate Analysis of Variance (PERMANOVA) using the adonis2 function in vegan (Oksanen et al., 2022). We included both cropping system and replicate (i.e., block) as fixed effects, the latter to detect positional differences within the array if they occurred. We used a Bray-Curtis dissimilarity index and 9999 permutations. We visualized these community differences using Nonmetric Multidimensional Scaling (NMDS) using the metaMDS function in vegan. The final solution had 3 dimensions and a stress value of 0.13.
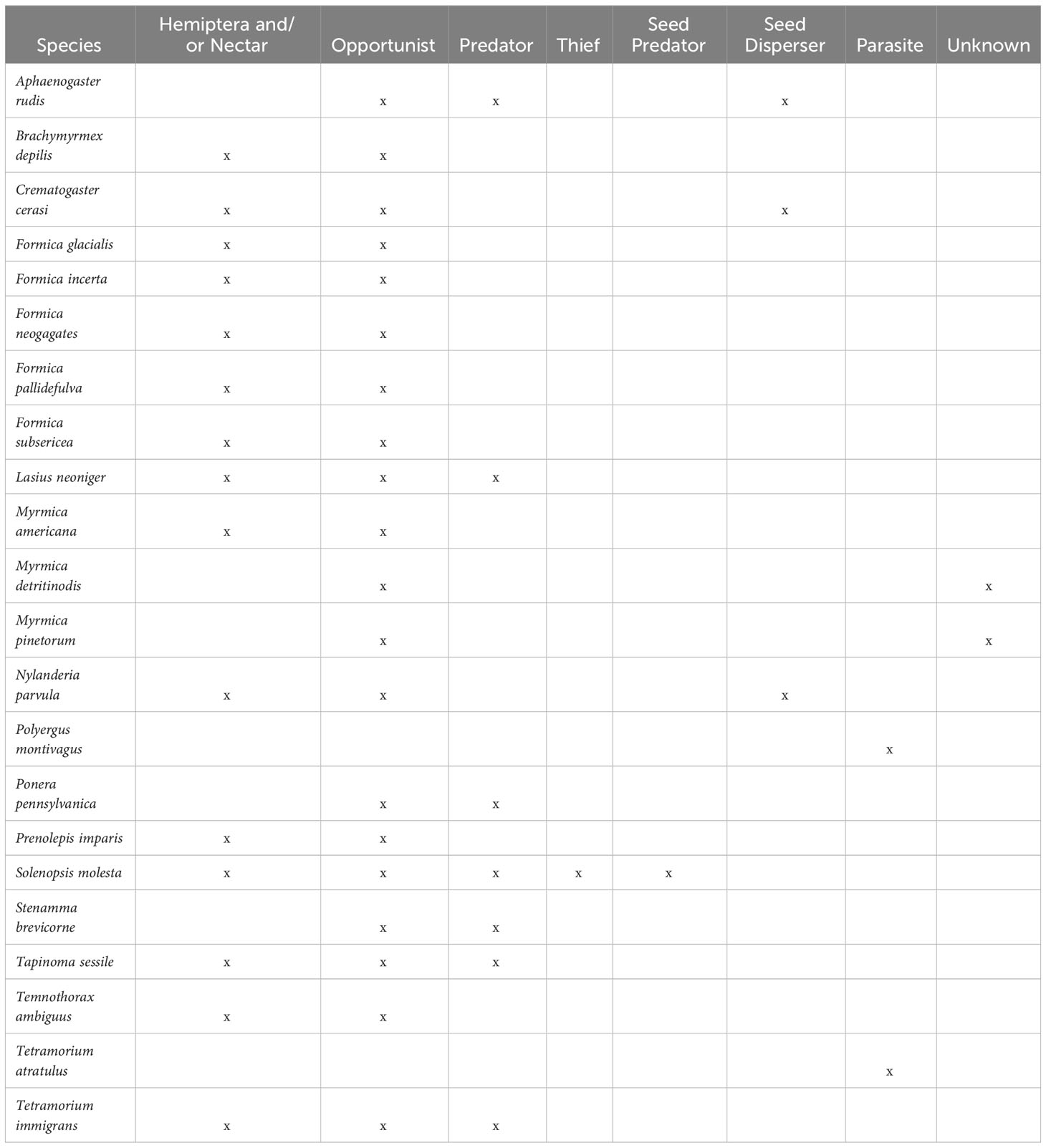
We collected information on the types of functional roles each species carries out using regional guides (AntWiki, n.d; Coovert, 2005; Ellison et al., 2012). The categories we included were opportunist, predator, seed disperser, seed predator, thief, social parasite, and Hemiptera- or nectar-focused (we assumed species known to harvest honeydew also drink nectar directly from plants and vice versa). This is not an exhaustive list of functions (e.g., it is biased toward above-ground activities), but it approximates the types of functions that are readily observed and are described in available sources. We placed ants into one or more of these functional categories (range 1-5, mean 2.2 categories per species). All species except the social parasites were included as opportunists (Polyergus montivagus feeds on food provided by its host or on its host directly, while Tetramorium atratulum is workerless). Two species, Myrmica detritinodis and Myrmica pinetorum, had no available information on the functions they carry out; we listed these as belonging to an additional placeholder category, ‘unknown’, in addition to being (presumably) opportunists.
Most ant species perform multiple functions (e.g., predator and aphid mutualist) and their net contributions to one role relative to the other are unknown. Therefore, we quantified functional dimensions of the community as follows. First, we tabulated the number of individual workers collected per plot that are affiliated with each function (regardless of species). Second, we calculated species richness per function (i.e., functional redundancy), which we defined as the number of species detected in each plot that are known to perform each function. Finally, within each function we calculated Hill’s number (exponent of Shannon diversity), a diversity estimate that equals species richness when the community is perfectly even and decreases with unevenness. Evenness was calculated using the number of workers captured, rather than the estimated number of colonies (i.e., we assumed the number of workers has a more direct bearing on a function being carried out).
Results
We captured and identified 9993 individual ants belonging to 22 species (Tables 1, 2; we also include a Tetramorium atratulum queen as this species has no worker caste). The most common species were Lasius neoniger and Tetramorium immigrans, workers of which made up 61% and 21% of the overall catch, respectively. These were the two most abundant species in every cropping system in the array after summing across replicates within a treatment. All species we detected were native except T. immigrans and its obligate parasite T. atratulum (Helms et al., 2019).
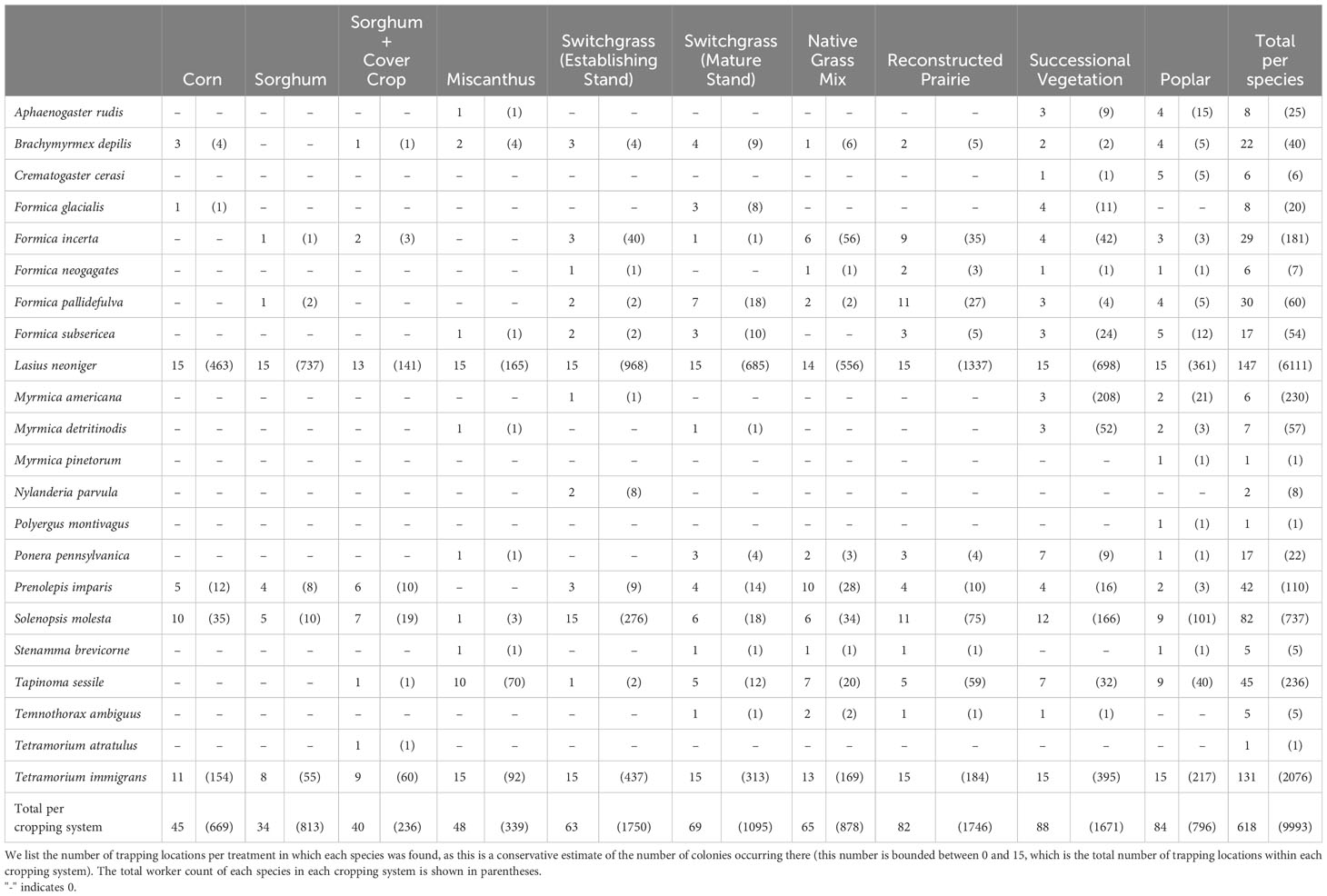
Table 1 Numbers of colonies and individuals of each species detected across each of ten bioenergy cropping systems.
Species richness differed strongly across cropping systems with mean richness varying by a factor of 2.7 between the least and most species-rich treatments. (see Haan et al., 2023). Richness was lowest in corn, sorghum, and miscanthus, all of which averaged < 5 species per plot. Richness was intermediate in other simple perennial systems (means = 6.4 species per plot in both the establishing switchgrass and native grass mix; 7.4 in mature switchgrass). Richness was highest in poplar, successional vegetation, and reconstructed prairie (mean 10.4, 9.8, and 7.8 species/plot, respectively).
Ant community composition differed strongly among cropping systems (PERMANOVA; F[df = 9] = 4.32, p < 0.001) but not by replicate (F[df = 4] = 0.93, p = 0.56). Visualizing community differences with NMDS revealed that communities in each cropping system clustered together to varying degrees, but consistent with our expectations there was a general pattern in which communities in annual crops and complex perennial systems differed strongly from each other while those in simple perennial systems were intermediate (Figure 1).
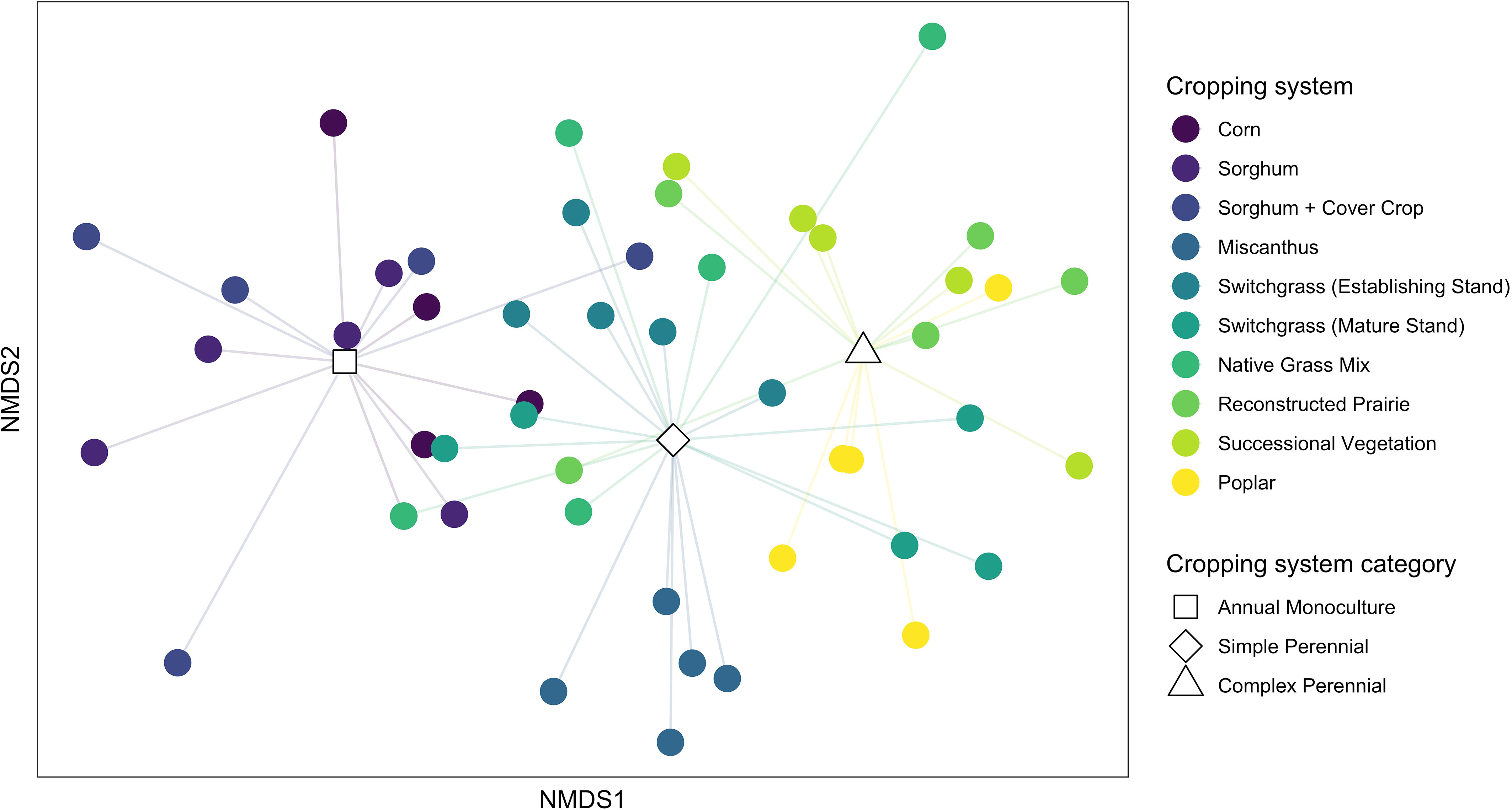
Figure 1 NMDS depicting ant community dissimilarity among cropping systems (3-dimensional solution; stress = 0.13). Each point represents the community in one plot (18 48-h trapping events), and distance between points is ± proportional to Bray-Curtis community dissimilarity. Points are color coded by treatment. Lines connect points from each treatment to the mean axis scores for broad cropping system categories, illustrating that communities in annual crops were similar to one another and contrasted with those in complex perennial systems, while composition in simple perennial systems was intermediate between the two.
Functional profiles differed across cropping systems, with patterns differing depending on whether one considers ant abundance or diversity (Figure 2). Reconstructed prairie, successional vegetation, and establishing switchgrass stands contained very large numbers of predatory, opportunist, nectar-feeding/hemiptera-tending individuals; this occurred in part because the numerically dominant L. neoniger belonged to all of these groups and was especially abundant in these crops (Tables 1, 2). Abundance within most functional groups was lowest in the sorghum system with a cover crop and in Miscanthus. The number of species performing each function differed across cropping systems and was highest in plant-diverse perennial polycultures. This pattern was consistent through the lenses of both species richness and Hill’s number (Figure 2).
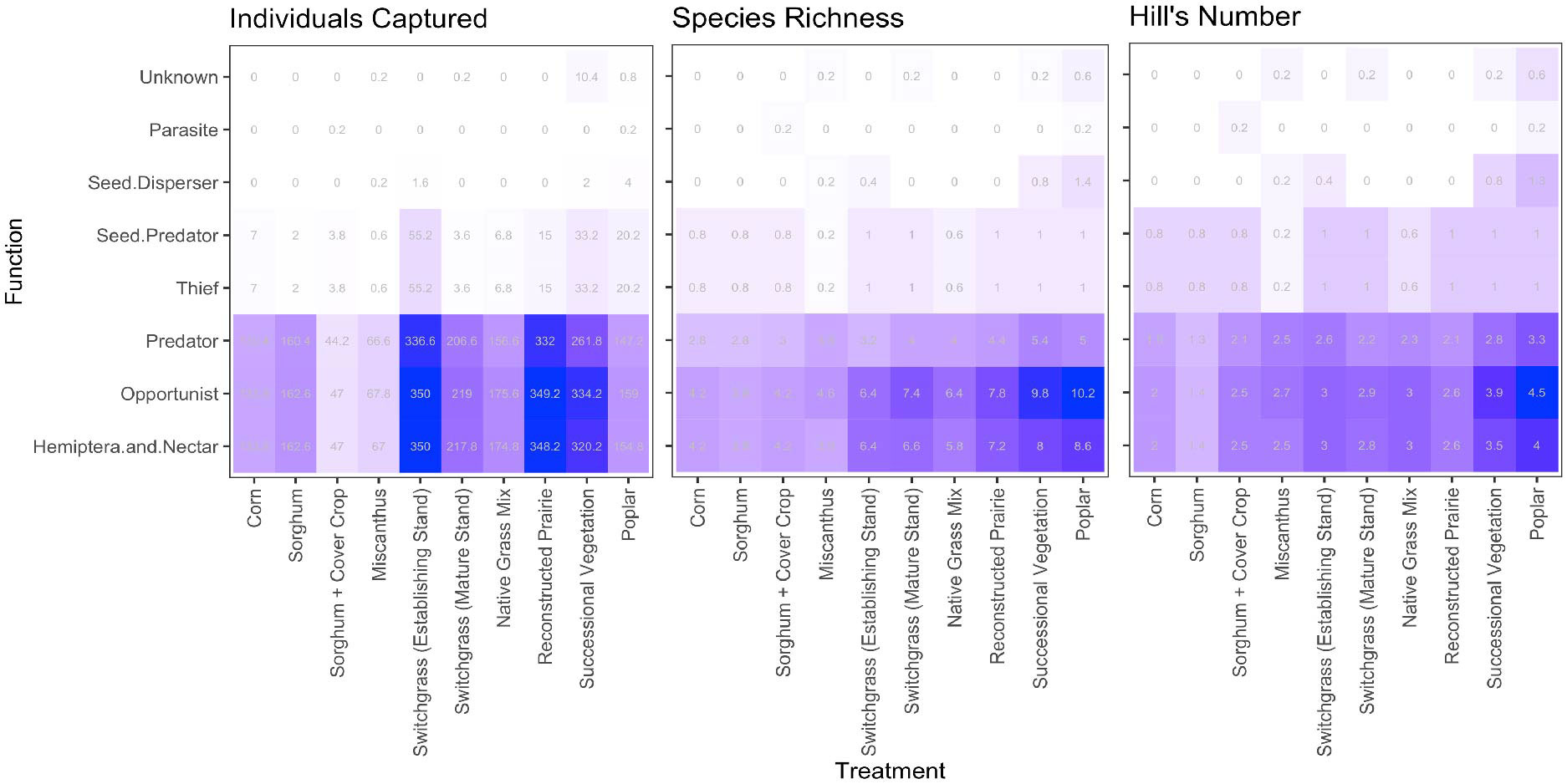
Figure 2 Functional profiles of ant communities across ten bioenergy cropping systems. Cropping systems are arranged from left to right in approximate order of decreasing management intensity and increased biodiversity (Haan et al., 2023). Numbers and color spectrum show the mean captured individuals (left), species richness (center), and Hill’s number (right) associated with each function in each cropping system. Means were calculated across the five replicates per cropping system. Some species contribute to more than one functional group; see Table 2.
Discussion
Our findings illustrate the wide range of effects that expansion of bioenergy cropping systems could have on ant communities and their functions. Complex perennial systems host diverse ant communities that are functionally and compositionally different from those found in annual crops, while the assemblages found in simple perennial systems are generally intermediate between those in intensified annual crops and complex perennial systems. Ecologically simple bioenergy systems, particularly those using intensified annual cropping systems, support species-poor ant communities with fewer functions and low functional redundancy. This pattern is consistent with patterns of ant abundance, richness, and function documented in other studies in candidate bioenergy crops, including a subset of the crops used in this study (Helms et al., 2020). It is also corroborated by a wide variety of other taxa beyond ants (Haan et al., 2023) and a number of previous studies and syntheses showing diversified bioenergy crops may be able to achieve conservation and ecosystem service goals but intensified and/or annual systems are likely to undermine them (Werling et al., 2011; Immerzeel et al., 2014; Werling et al., 2014; Núñez-Regueiro et al., 2021). In general, land use decisions around bioenergy should consider beta diversity carefully, as different crops could make unique contributions to landscape species pools and focusing exclusively on the most-diverse crops could reduce gamma diversity. However, in this study ant communities in the array were quite nested (Haan et al., 2023); the only species unique to annual systems was a single Tetramorium atratulus, an exotic ant that parasitizes T. immigrans. Similarly, only one species (Nylanderia parvula) was unique to the perennial monoculture systems (Table 1).
From an agronomic standpoint, the most important function of ant communities in bioenergy systems is likely to be predation of crop pests. A contemporaneous study in the same experimental array quantified predation intensity in each cropping system using moldable plasticine sentinel caterpillars (Haan & Landis, 2023). These herbivore mimics record imprints from predator attacks, allowing investigators to measure attack rates and gather coarse taxonomic information on attackers by the imprints they leave (e.g., mammal teeth, bird beak, arthropod mandibles, arthropod proboscis). This study found attack rates on sentinel caterpillars were higher in perennial than in annual systems, and differences were largely driven by arthropods that attack with mandibles. These types of marks were infrequent (and at times entirely absent) in annual crops but often made up the majority of attacks in perennial systems. While Haan and Landis (2023) did not distinguish between marks left by ants and those from other arthropods that attack using mandibles, most marks were of a size and placement consistent with those of ants (N. Haan, personal observation) and a video surveillance study has shown ants are dominant predators in corn and grassland systems in the region (Grieshop et al., 2012).
We found a clear pattern in which more functions occurred and were carried out by a more diverse group of ants in complex perennial bioenergy habitats (Figure 2). However, we do not know the relative contributions of each species to the different functions they perform nor which species are more important for furthering a given function. Since ants’ activities are multifaceted, they may simultaneously have indirect positive and negative effects on other organisms that are hard to tease apart. For example, an ant may tend aphids by guarding against other natural enemies (negatively impacting the plant) but also removing non-aphid herbivores (positively impacting the plant); without experiments the net effect on the plant is not known. The effects of a given species depend on context and can vary seasonally (Philpott et al., 2014). Seasonal patterns may also depend on management; for example, (Helms et al., 2021) compared ant activity in organic and conventional cropping systems and found that while both systems contained similar ant communities, they were active at different times within the growing season. Finally, we note that some of the species we collected have better-known natural histories than others. Poorly-studied species are likely to have additional roles in ecosystems that have escaped notice and could not be included in our study.
This study used an experimental array with plots buffered by 15 m of mowed turfgrass. Most ants we collected will have originated from nests in the same plots where they were trapped, but in some cases they could have visited from turfgrass outside the plots (minimum 8 m from traps) or possibly from other plots altogether (requiring foraging distance of >23 m). Worker density is likely to decrease with distance from nests, but some species may forage at scales larger than that of the experimental array (Carroll and Janzen, 1973; Traniello, 1989). If spillover from one plot to another influenced our findings it would have had a homogenizing effect, reducing our ability to detect community differences between treatments. Thus we view the differences detected within the experimental array to be conservative; they could be stronger if measurements had been taken at field-realistic scales.
A given crop type can be subject to a range of management systems, with important implications for biotic communities residing there. For example, ant communities within row-crops can differ between conventional and organic systems (Helms et al., 2021). In a bioenergy context, short-rotation coppicing systems in particular can be managed in a variety of styles (Vanbeveren and Ceulemans, 2019) and grasses like switchgrass can be managed as near monocultures (as in this study) or with volunteer plant species tolerated or through intentional management as a polyculture. We stress that each crop is not a monolith and that intensity of management will shape biotic communities in addition to crop type per se.
Ant communities and their functions in future agricultural landscapes are likely to be shaped by policies and choices around bioenergy production. The current bioenergy portfolio in the US relies heavily on corn; currently ~40% of the US annual corn harvest goes to ethanol (USDA ERS (United States Department of Agriculture Economic Research Service) US Bioenergy Statistics, 2022). As demand for bioenergy increases, evidence shows expanding the footprint of corn will result in a number of undesirable outcomes including nutrient pollution, landscape simplification, increased insecticide use, and biodiversity loss (Donner and Kucharik, 2008; Meehan et al., 2011; Lark et al., 2020; Prokopy et al., 2020; Lark et al., 2022; Haan et al., 2023). However, opportunities also exist to incorporate biodiverse perennial bioenergy systems into simplified agricultural landscapes, providing pathways to enhance biodiversity and associated services (Werling et al., 2014). As functionally important and numerically dominant organisms in crops and grasslands, ants exemplify the range of outcomes that could come to bear as the result of future bioenergy policies and the land use change that follows.
Data availability statement
The ant community dataset analyzed in this study is available at doi: 10.5281/zenodo.8215581. The functional information applied to each species, along with R code used to generate analyses, figures, and tables, is available at doi:10.5281/zenodo.10125287.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
NH: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing. JH: Investigation, Methodology, Writing – review & editing. DL: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Great Lakes Bioenergy Research Center, US Department of Energy, Office of Science, Office of Biological and Environmental Research under award numbers DE-SC0018409 and DE-FC02-07ER64494, the National Science Foundation Long-Term Ecological Research Program at Kellogg Biological Station DEB 1832042, and Michigan State University AgBioResearch. NLH was supported during analysis and writing by the University of Kentucky Martin-Gatton College of Agriculture, Food, and Environment.
Acknowledgments
C. Fiser, A. Krudy, H. Eberhard, O. Eschedor, and S. Zhou contributed to field and lab work. B. Wills provided valuable advice on ant identification. We thank J. Smith and N. Haddad for sharing supplies and guidance on trapping methods.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
AntWiki. Available at: https://www.antwiki.org/wiki/Welcome_to_AntWiki.
Basso B., Antle J. (2020). Digital agriculture to design sustainable agricultural systems. Nat. Sustainability 3 (4), 4. doi: 10.1038/s41893-020-0510-0
Bentley B. L. (1976). Plants bearing extrafloral nectaries and the associated ant community: interhabitat differences in the reduction of herbivore damage. Ecology 57 (4), 815–820. doi: 10.2307/1936195
Bentley B. L. (1977). Extrafloral nectaries and protection by pugnacious bodyguards. Annu. Rev. Ecol. Systematics 8 (1), 407–427. doi: 10.1146/annurev.es.08.110177.002203
Bruyn L. L., Conacher A. J. (1990). The role of termites and ants in soil modification—A review. Soil Res. 28 (1), 55–93. doi: 10.1071/sr9900055
Buschinger A. (1986). Evolution of social parasitism in ants. Trends Ecol. Evol. 1 (6), 155–160. doi: 10.1016/0169-5347(86)90044-3
Buschinger A. (2009). Social parasitism among ants: A review (Hymenoptera: Formicidae). Myrmecological News 12, 219–235.
Cammeraat E. L. H., Risch A. C. (2008). The impact of ants on mineral soil properties and processes at different spatial scales. J. Appl. Entomology 132 (4), 285–294. doi: 10.1111/j.1439-0418.2008.01281.x
Carroll C. R., Janzen D. H. (1973). Ecology of foraging by ants. Annu. Rev. Ecol. Systematics 4 (1), 231–257. doi: 10.1146/annurev.es.04.110173.001311
Cembrowski A. R., Tan M. G., Thomson J. D., Frederickson M. E. (2014). Ants and ant scent reduce bumblebee pollination of artificial flowers. Am. Nat. 183 (1), 133–139. doi: 10.1086/674101
Del Toro I., Ribbons R. R., Pelini S. L. (2012). The little things that run the world revisited: A review of ant-mediated ecosystem services and disservices (Hymenoptera: Formicidae). Myrmecological News 17 (0), 133–146.
Donner S. D., Kucharik C. J. (2008). Corn-based ethanol production compromises goal of reducing nitrogen export by the Mississippi River. Proc. Natl. Acad. Sci. 105 (11), 4513–4518. doi: 10.1073/pnas.0708300105
Ellison A. M., Gotelli N. J., Farnsworth E. J., Alpert G. D. (2012). A field guide to the ants of new england (New Haven, CT: Yale University Press).
Ellison A. M., Record S., Arguello A., Gotelli N. J. (2007). Rapid inventory of the ant assemblage in a temperate hardwood forest: species composition and assessment of sampling methods. Environ. Entomology 36 (4), 766–775. doi: 10.1093/ee/36.4.766
Folgarait P. J. (1998). Ant biodiversity and its relationship to ecosystem functioning: A review. Biodiversity Conserv. 7 (9), 1221–1244. doi: 10.1023/A:1008891901953
Frouz J., Jilková V. (2008). The effect of ants on soil properties and processes (Hymenoptera: Formicidae). Myrmecological News 11, 191–199.
Gotelli N. J., Ellison A. M., Dunn R. R., Sanders N. J. (2011) Counting ants (Hymenoptera: Formicidae): Biodiversity sampling and statistical analysis for myrmecologists. Myrmecological News 15, 13–19.
Grieshop M. J., Werling B., Buehrer K., Perrone J., Isaacs R., Landis D. (2012). Big brother is watching: studying insect predation in the age of digital surveillance. Am. Entomologist 58 (3), 172–182. doi: 10.1093/ae/58.3.172
Haan N. L., Benucci G. M. N., Fiser C., Bonito G., Landis D. A. (2023). Contrasting effects of bioenergy crops on biodiversity. Sci. Adv. 9, eadh7960. doi: 10.1126/sciadv.adh7960
Haan N. L., Landis D. A. (2023). Pest suppression potential varies across 10 bioenergy cropping systems. GCB Bioenergy 9, eadh7960. doi: 10.1111/gcbb.13053
Helms J. A., Ijelu S. E., Haddad N. M. (2019). Range expansion in an introduced social parasite-host species pair. Biol. Invasions 21 (8), 2751–2759. doi: 10.1007/s10530-019-02011-y
Helms J. A., Ijelu S. E., Wills B. D., Landis D. A., Haddad N. M. (2020). Ant biodiversity and ecosystem services in bioenergy landscapes. Agriculture Ecosyst. Environ. 290, 106780. doi: 10.1016/j.agee.2019.106780
Helms J. A., Smith J., Clark S., Knupp K., Haddad N. M. (2021). Ant communities and ecosystem services in organic versus conventional agriculture in the U.S. Corn belt. Environ. Entomology 50 (6), 1276–1285. doi: 10.1093/ee/nvab105
Immerzeel D. J., Verweij P. A., van der Hilst F., Faaij A. P. C. (2014). Biodiversity impacts of bioenergy crop production: A state-of-the-art review. GCB Bioenergy 6 (3), 183–209. doi: 10.1111/gcbb.12067
IPCC (Intergovernmental Panel on Climate Change). (2018). Special Report: Global warming of 1.5 C. Available at: https://www.ipcc.ch/sr15/.
Lach L. (2005). Interference and exploitation competition of three nectar-thieving invasive ant species. Insectes Sociaux 52 (3), 257–262. doi: 10.1007/s00040-005-0807-z
Lark T. J., Hendricks N. P., Smith A., Pates N., Spawn-Lee S. A., Bougie M., et al. (2022). Environmental outcomes of the US renewable fuel standard. Proc. Natl. Acad. Sci. 119 (9), e2101084119. doi: 10.1073/pnas.2101084119
Lark T. J., Spawn S. A., Bougie M., Gibbs H. K. (2020). Cropland expansion in the United States produces marginal yields at high costs to wildlife. Nat. Commun. 11 (1), 1. doi: 10.1038/s41467-020-18045-z
Leal I. R., Leal L. C., Andersen A. N. (2015). The benefits of myrmecochory: A matter of stature. Biotropica 47 (3), 281–285. doi: 10.1111/btp.12213
Meehan T. D., Werling B. P., Landis D. A., Gratton C. (2011). Agricultural landscape simplification and insecticide use in the Midwestern United States. Proc. Natl. Acad. Sci. 108 (28), 11500–11505. doi: 10.1073/pnas.1100751108
Núñez-Regueiro M. M., Siddiqui S. F., Fletcher R. J. (2021). Effects of bioenergy on biodiversity arising from land-use change and crop type. Conserv. Biol. 35 (1), 77–87. doi: 10.1111/cobi.13452
Oksanen J., Simpson G., Blanchet F., Kindt R., Legendre P., Minchin P., et al. (2022). _vegan: Community Ecology Package_. R package version 2.6-4. Available at: https://CRAN.R-project.org/package=vegan.
Philpott S. M., Perfecto I., Vandermeer J. (2014). Behavioral diversity of predatory arboreal ants in coffee agroecosystems. Environ. Entomology 37 (1), 181–191. doi: 10.1093/ee/37.1.181
Prokopy L. S., Gramig B. M., Bower A., Church S. P., Ellison B., Gassman P. W., et al. (2020). The urgency of transforming the Midwestern U.S. landscape into more than corn and soybean. Agric. Hum. Values 37 (3), 537–539. doi: 10.1007/s10460-020-10077-x
Rosumek F. B., Silveira F. A. O., de S. Neves F., de U. Barbosa N. P., Diniz L., Oki Y., et al. (2009). Ants on plants: A meta-analysis of the role of ants as plant biotic defenses. Oecologia 160 (3), 537–549. doi: 10.1007/s00442-009-1309-x
Styrsky J. D., Eubanks M. D. (2006). Ecological consequences of interactions between ants and honeydew-producing insects. Proc. R. Soc. B: Biol. Sci. 274 (1607), 151–164. doi: 10.1098/rspb.2006.3701
Traniello J. F. A. (1989). Foraging strategies of ants. Annu. Rev. Entomology 34 (1), 191–210. doi: 10.1146/annurev.en.34.010189.001203
Underwood E. C., Fisher B. L. (2006). The role of ants in conservation monitoring: If, when, and how. Biol. Conserv. 132 (2), 166–182. doi: 10.1016/j.biocon.2006.03.022
USDA ERS (United States Department of Agriculture Economic Research Service) US Bioenergy Statistics (2022). Available at: https://www.ers.usda.gov/data-products/u-s-bioenergy-statistics/.
Vanbeveren S. P. P., Ceulemans R. (2019). Biodiversity in short-rotation coppice. Renewable Sustain. Energy Rev. 111, 34–43. doi: 10.1016/j.rser.2019.05.012
Werling B. P., Dickson T. L., Isaacs R., Gaines H., Gratton C., Gross K. L., et al. (2014). Perennial grasslands enhance biodiversity and multiple ecosystem services in bioenergy landscapes. Proc. Natl. Acad. Sci. 111 (4), 1652–1657. doi: 10.1073/pnas.1309492111
Werling B. P., Meehan T. D., Robertson B. A., Gratton C., Landis D. A. (2011). Biocontrol potential varies with changes in biofuel–crop plant communities and landscape perenniality. GCB Bioenergy 3 (5), 347–359. doi: 10.1111/j.1757-1707.2011.01092.x
Wills B. D., Kim T. N., Fox A. F., Gratton C., Landis D. A. (2019). Reducing native ant abundance decreases predation rates in midwestern grasslands. Environ. Entomology 48 (6), 1360–1368. doi: 10.1093/ee/nvz127
Keywords: ants, bioenergy, climate change, ecosystem service, grassland
Citation: Haan NL, Helms JA and Landis DA (2024) Bioenergy cropping systems shape ant community composition and functional roles. Front. Conserv. Sci. 4:1283225. doi: 10.3389/fcosc.2023.1283225
Received: 25 August 2023; Accepted: 15 November 2023;
Published: 23 January 2024.
Edited by:
Elena D. Concepción, Spanish National Research Council (CSIC), SpainReviewed by:
Aurele Toussaint, University of Tartu, EstoniaJens Dauber, Johann Heinrich von Thünen Institute, Germany
Copyright © 2024 Haan, Helms and Landis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nathan L. Haan, bmF0ZS5oYWFuQHVreS5lZHU=
 Nathan L. Haan
Nathan L. Haan Jackson A. Helms3
Jackson A. Helms3 Douglas A. Landis
Douglas A. Landis