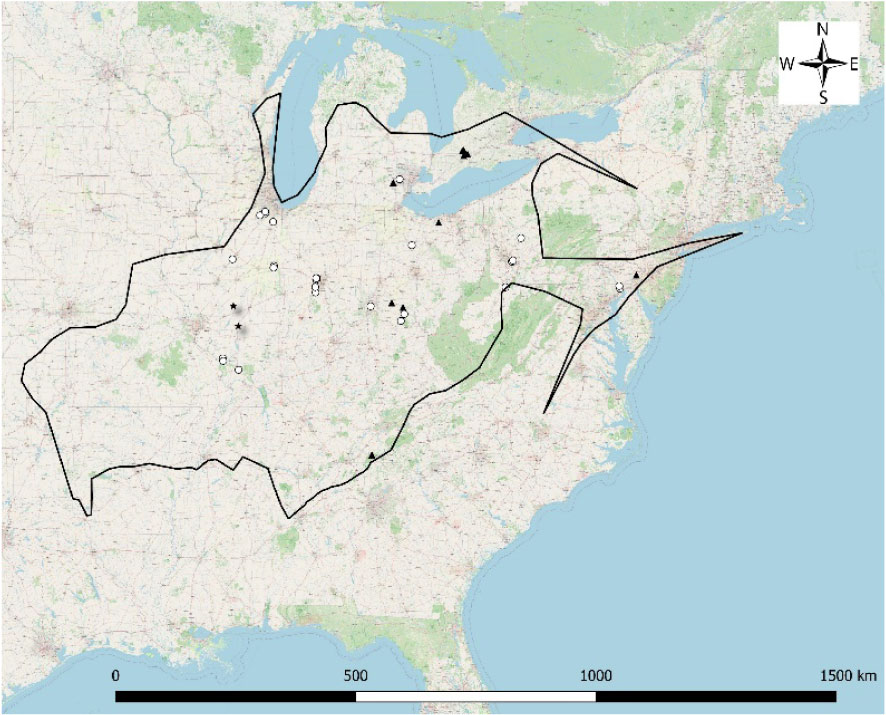
- 1Illinois Natural History Survey, University of Illinois, Champaign, IL, United States
- 2Department of Botany, Wisconsin State Herbarium, University of Wisconsin, Madison, WI, United States
Funding for rare plant conservation is limited. In addition, many aspects of the biology and ecology of rare plants are unknown. Therefore, low-cost data generation approaches to fill these gaps should be pursued. Herbarium specimens can be used as a low-cost alternative to learn about the basic biology and ecology of rare plant species. The information provided on herbarium labels has dramatically increased in recent decades to include precise locality (i.e., latitude/longitude), exact dates, habitat, associated species, and substrate. In addition, herbarium specimens are being digitized and the resulting images and data are available via clearinghouses such as GBIF and SEINet. Already, herbarium specimens of rare plants have been used to develop habitat suitability models, predict range shifts, and assess changes in flower phenology due to climate change. Herbarium specimens can also provide a wealth of information about the reproductive biology and biotic interactions of rare plants. In this paper, we will demonstrate how this information can be accessed and present a practical application for using this information to populate an important federal listing document in the USA, Species Status Assessments (SSA). We will provide examples from the literature, as well as case studies from our own research, to demonstrate how this information can be collected from herbarium specimens and how and where to incorporate this information into SSAs. More generally, data gleaned from herbarium specimens can become part of a conservationist’s tool kit to further our knowledge of past, present, and future trends for rare plants. Additional knowledge of a species’ biology and ecology allows land managers and conservationists to make more informed decisions and allows for greater protection of listed species.
Introduction
In the United States approximately 27,000 plant species are native and about 8,500 have been estimated to be rare (Table 1; NatureServe, 2022a). Approximately 11% of these rare plants are listed under the Endangered Species Act (ESA), and thereby have protection (Table 1). Many extrinsic (e.g., habitat loss/degradation, invasive species/pathogens/or pests, climate change) and intrinsic (e.g., habitat specialization, mutualistic interactions) factors (Ravikanth et al., 2018) have led to the decline of plant species in the United States (and worldwide) making recovery a challenge. In addition, lack of funding in the United States hinders the conservation efforts for rare plants (Molano-Flores, 2021), although this is also a global issue (e.g., Adamo et al., 2022). Negrón-Ortiz (2014) noted that although plants make up more than 50% of all organisms listed under the ESA, they receive less than 5% of the funding allocated for species recovery from state and federal agencies. Regardless of these limitations, active collaborations between agencies and researchers have and continue to generate data to inform state and federal documents that are key to the protection and conservation of rare plant species. At the federal level, these include Recovery Plans, Five-Year Reviews, and Species Status Assessments (SSA). In particular, the development of an SSA is crucial for the listing process and requires the analysis of past and current conditions and the prediction of future trends regarding the organism in question. An SSA has three sections (or stages): 1) Species’ Ecological Needs, 2) Current Species’ Condition, and 3) Future Species’ Condition and Status. See Figure 1 and Smith et al. (2018) for further explanations. Overall, an SSA is a biological risk assessment with an inclusive scientific review of the most current information and projected future trends available for a species with the purpose of informing decision-makers (U.S. Fish and Wildlife Service, 2022; Noss et al., 2021).
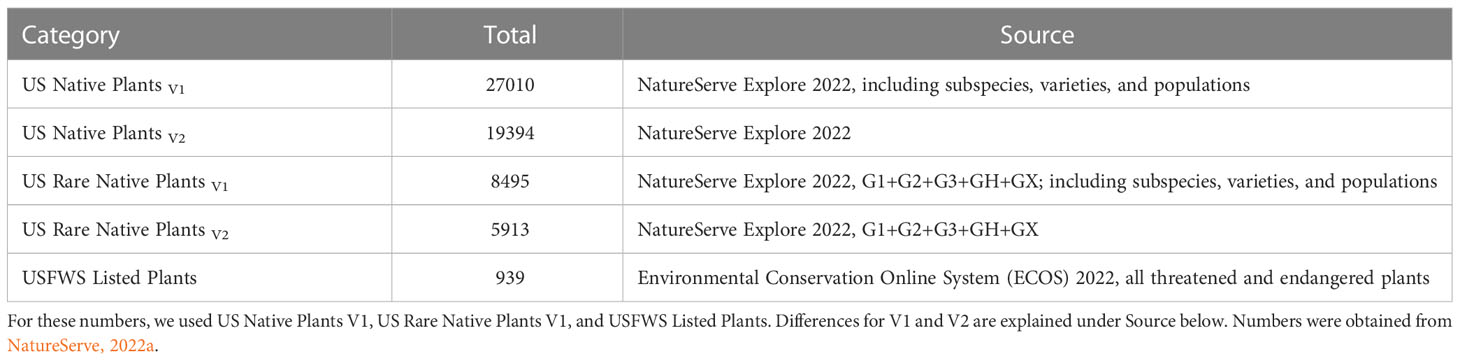
Table 1 Summary of the total number of US Native Plants, US Rare Native Plants, and USFWS Listed Plants.
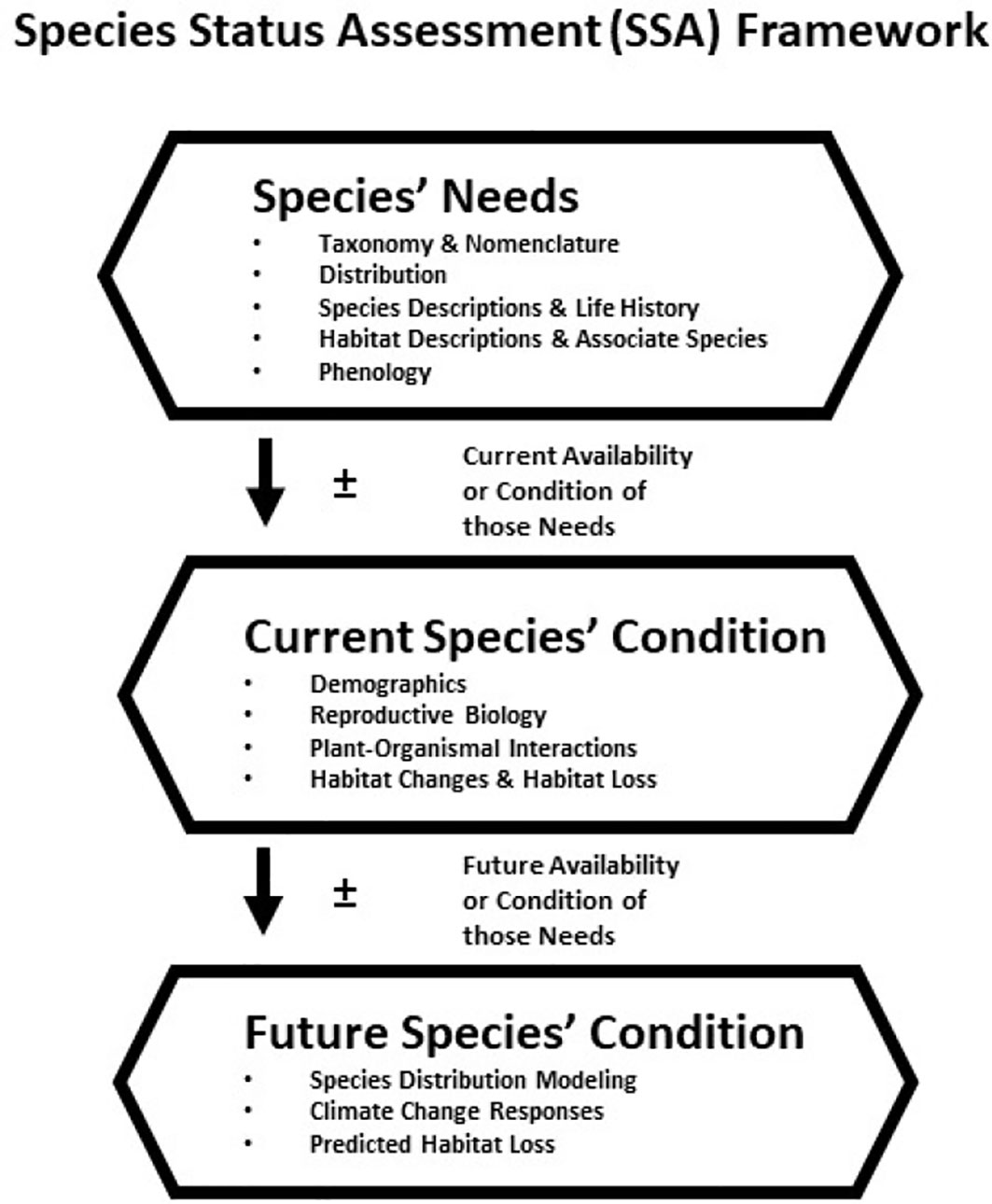
Figure 1 Species Status Assessment (SSA) framework and herbarium specimen contribution to the assessment stage (not all are listed): 1) Species’ Needs – This SSA stage provides the best biological information associated with a species, and its ecological requirements at the individual, population and/or species level; 2) Current Species’ Conditions – This SSA stage describes the current condition of the species’ habitat and demographics to explain past and current demographic and distribution changes; and 3) Future Species’ Conditions – This SSA stage predicts, based on the best available information, a species’ response to plausible future scenarios of environmental conditions and capability to persist in the wild over time. Image and text modified from U.S. Fish and Wildlife Service (2016).
Thorough data collection is not practical for most rare species due to challenges including significant demands of time, funding, and personnel, as well as access and permit requirements. Herbarium specimen data are generally open source and available through large clearinghouses such as GBIF and SEINet, though for rare plants locality data may be obscured to protect populations and an extra step may be needed (i.e., contact herbarium curator or database manager) to get access to this information. Several papers have already been published using herbaria and herbarium specimens as “big data sources” and several of these papers have focused on rare plants (e.g., Lughadha et al., 2005; Lavoie, 2013; Nualart et al., 2017; Marsico et al., 2020; Heberling, 2022 and citations therein). For these reasons, the intent of our paper as a perspective is to highlight the value of utilizing herbarium specimens as a cost-effective way to generate information that can be included in the three sections of an SSA for the listing of rare plants. By taking this approach, we are providing a practical application of the data and the role that herbarium specimens can play in the listing and conservation of rare species. For each section of the SSA, we provide examples from the literature and from our own research that have used herbarium specimens to gather data on rare plants. Integration of herbarium specimen data into an SSA is possible as high-quality images of many rare plants are now accessible online and available at no cost, making this an important cost-benefit approach to data gathering for rare plant research. Lastly, we want to note that the approach presented here can be extended to incorporate new information into Recovery Plans and Five-Year Reviews. This same information could also be utilized by conservationists at state and local levels to inform their work on the preservation of rare plants.
Species Status Assessments - species’ ecological needs
This section of the SSA focuses on a species’ background information including its taxonomy, classification, life history, and ecological relationships. It includes a detailed species description, distribution/range maps, a description of habitat, and other basic material regarding its biology and ecology. Herbarium specimens can be a source for much of these data, especially the taxonomy, distribution, and habitat. For example, the SSAs for Ocmulgee skullcap (Scutellaria ocmulgee; U.S. Fish and Wildlife Service, 2021) and bracted twistflower (Streptanthus bracteatus; U.S. Fish and Wildlife Service, 2020) mention herbarium specimens and their associated labels as the source for this type of data.
One of the greatest contributions of herbarium specimens under this section is the proper and verifiable identification of the taxonomic unit of conservation and its vouchering. This is extremely important as it can change the geographical range of a species and the scope of the area to be evaluated by an SSA. For example, in the case of Scutellaria ocmulgee (Ocmulgee skullcap), a species that has been confused with S. mellichampii (another rare southeastern United States skullcap), recent field and herbarium work has allowed for the reassessment of its taxonomy and distribution (Weakley et al., 2020). Due to such work, the SSA for Ocmulgee skullcap is now focused on a much narrower geographical range (U.S. Fish and Wildlife Service, 2016). Having a clear understanding of the nomenclature and the changes it has undergone in the past is also key to the legal protection of any rare species (Mace, 2004), including plants (Standley, 1992; Schatz, 2002). For example, if an SSA was to be developed for Minuartia patula, which has undergone multiple changes of nomenclature and delimitation (Kartesz, 1994; Kartesz, 1999; Dillenberger and Kadereit, 2014; Schilling et al., 2022), under which sense [or taxon concept (Franz et al., 2008; Sigovini et al., 2016)] would it be recognized? In cases such as these, consulting herbarium specimens, taxonomic databases and literature, or even taxonomic experts to determine the taxonomic unit of conservation is prudent. Practitioners should also be aware that the name that a species is listed under may not be its currently accepted name. Once a species has been listed, changing its legally recognized name can be a slow and difficult process. Although Harperella nodosa is now the currently accepted name for Ptilimnium nodosum (Feist et al., 2012) and has been for a decade, Ptilimnium nodosum is still the name listed and protected under the ESA. Herbarium specimens and taxonomic databases are crucial for species verification during the listing, protection, and management of a rare plant, as correctly identifying a species will determine or influence many aspects of its conservation status including its critical habitat designation.
As mentioned above, specimen labels can be a source of ecological information including habitat type, associated species, flowering/fruiting time, and relative abundance. Although this information might be scarce on historical specimens, in recent decades it has become common to include it in some detail. Figure 2 showcases two herbarium specimens for kittentails (Synthyris bullii) that provide information on habitat, associated species, and even population estimates, and demographics (i.e., life stages, reproductive vs. non-reproductive). Utilizing herbarium specimens that capture the various life stages (i.e., seedling, juvenile, adult) of a rare plant and including them in an SSA can assist with population demographic studies and facilitate species identification in the field. Also, locality information from herbarium specimens can assist in the creation of historic range maps that provide insights beyond existing Element of Occurrence Records (EORs) available via Natural Heritage programs. Again, although this information might be scarce on historic records, more recent specimens often include detailed locality information including GPS coordinates. With georeferencing techniques, locality descriptions from older specimens can be converted into coordinates with estimates of uncertainty. An example of how herbarium specimens can offer an important supplement or source of occurrence data to help fill gaps in our understanding of the distribution of rare plant species, is given in Box 1 and Supplement 1. Lastly, Hardisty et al. (2022) advocate for the implementation of the Digital Extended Specimen (DES). In this scenario, any data associated with an herbarium specimen would be housed together with it as a DES digital object. Such data could include morphological descriptions, measurements of traits, chemical analyses, DNA sequences, extensive habitat information, field photos, links to literature including species descriptions, and more. This would thereby centralize the data to be used for SSAs and make herbarium specimens even more useful.
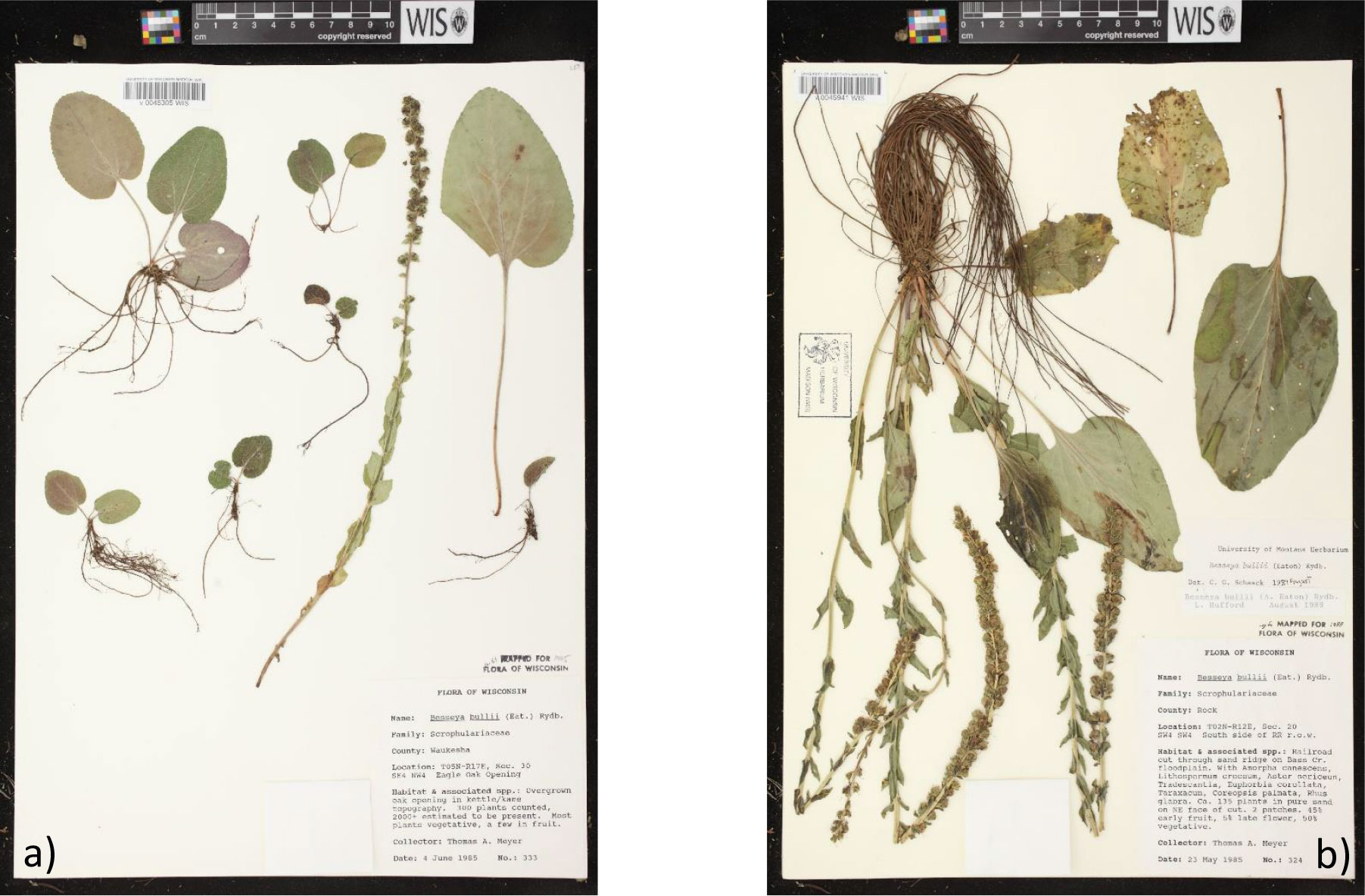
Figure 2 Herbarium specimens for kittentails (Synthyris bullii). The label of each specimen has information on habitat, associated species, and population estimates. In addition, both specimens (A, B) are capturing different life stages (e.g., seedling development, reproductive vs. non-reproductive individuals). Images from the Wisconsin State Herbarium (WIS).
Box 1.
Herbaria offer an important supplement or source of occurrence data for understanding rare plant species distribution, as well as providing a building block for species searches and range assessment. Species Distribution Models (SDMs) were built using Maxent for the state-threatened mint White Birds in-a-nest (Macbridea alba) of the Florida panhandle and the endangered Zapata Bladderpod (Physaria thamnophila) native to South Texas. A total of 353 and 98 unique occurrences records for Macbridea alba and Physaria thamnophila respectively, were obtained from the BIOTICS database with permission from the Florida Natural Areas Inventory and the Texas National Diversity Database. For comparison, coordinate data were also obtained from GBIF and SEINet from herbarium specimens and observations from iNaturalist for a total 15 and 16 total occurrences, respectively for each species.
In the case of Macbridea alba, the SDM built with BIOTICS occurrence data was similar to the known distribution of the species (Figure 1A). However, the SDM built with herbaria records for this species was unspecific (Figure 1B). In the case of Physaria thamnophila model predictions improved with the use of herbaria data over the use of BIOTICS data (Figures 2A and 2B). When seeking to find additional conservation lands or populations within the known range of the species, a combination of model predictions and user expertise should be used to guide searches and confidence in model predictions. For both species, combining both herbaria and BIOTICS data for the model only slightly increased predicted suitable area compared to the model built with BIOTICS data alone (Figure 1c and Figure 2c). A large difference in the number of records sourced from BIOTICS data compared to herbaria records for Macbridea alba created vastly different modeling outcomes compared to Physaria thamnophila, which had fewer BIOTICS records.
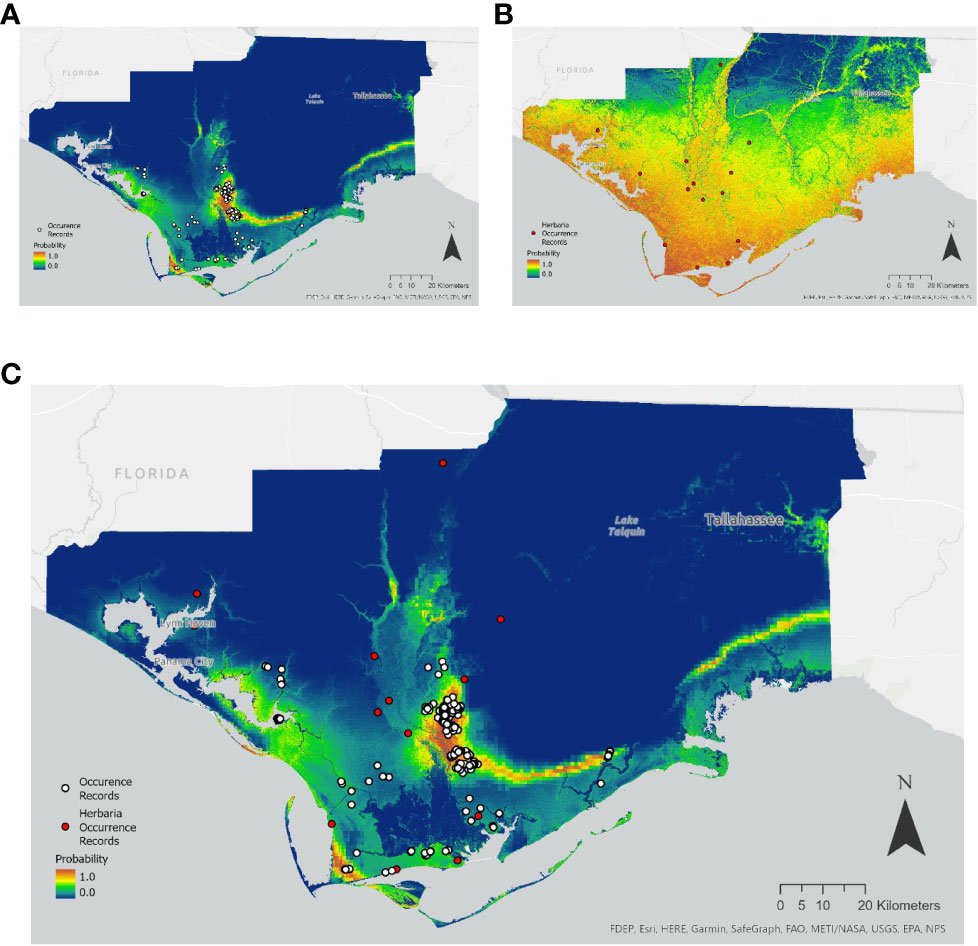
Figure 1 Maxent model output for the average of five cross-validated models trained using: (A) BIOTICS records, (B) Herbaria records, or (C) both BIOTICS and Herbaria records for Macbridea alba. Probability is colored on a scale of 0.0 to 1.0 (blue to red). Scale 1:900,000. BIOTICS occurrence records are shown as black-outlined white circles and Herbaria occurrence records are shown as black-outlined red circles.
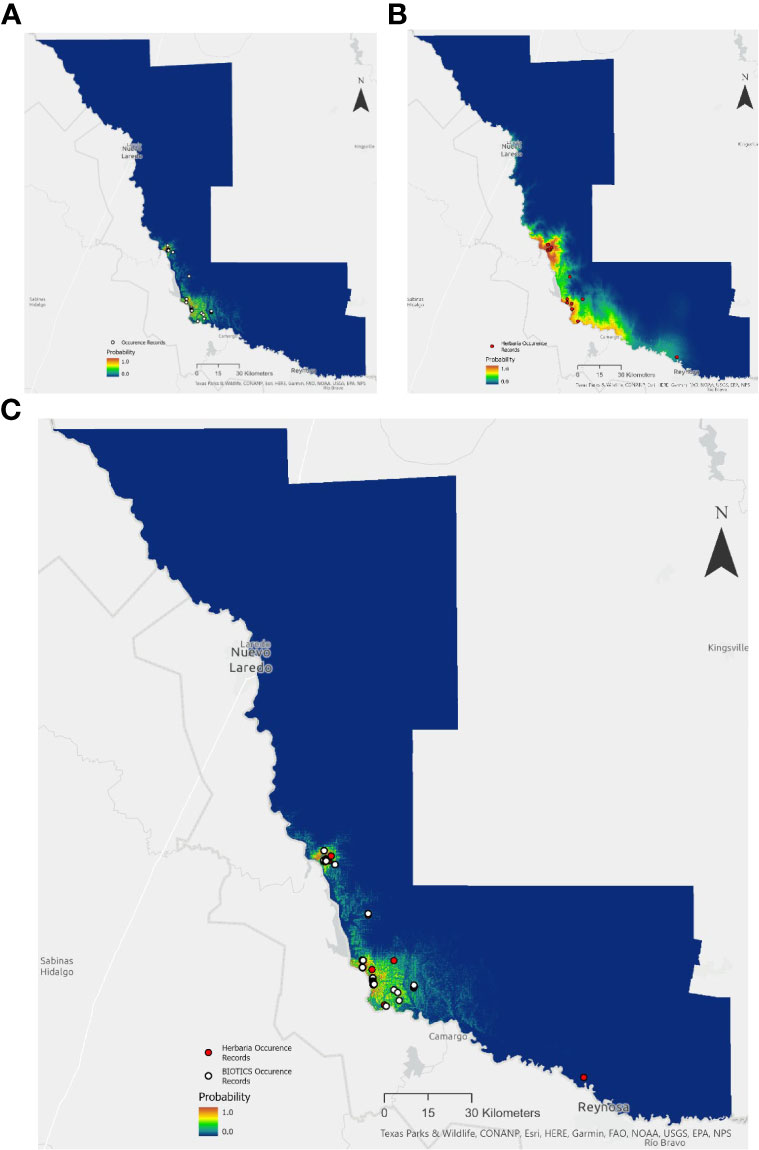
Figure 2 Maxent model output for the average of five cross-validated models trained using: (A) BIOTICS records, (B) Herbaria records, or (C) both BIOTICS and Herbaria records for Physaria thamnophila. Probability is colored on a scale of 0.0 to 1.0 (blue to red). Scale 1:200,000. BIOTICS occurrence records are shown as black-outlined white circles and Herbaria occurrence records are shown as black-outlined red circles.
Box 2.
For the harbinger of spring rust fungus, an autoecious rust that parasitizes harbinger of spring (Figure 1), MycoPortal documents 44 specimens, all from the eastern part of the range of the host plant. No specimens were known from states in the western half of the host plant’s range. On April 3, 2021, the harbinger of spring rust was first observed in Illinois with the first Illinois specimen collected April 9, 2021 (Marcum #7719 at ILLS). This discovery prompted additional searches throughout the Illinois range of the host plant and since the rust fungus is an obligate parasite, it was suspected that it would be sympatric with the host plant. Field searches have been initiated but trying to cover the entirety of the state of Illinois can be overwhelming. Examination of herbarium specimens provided a rapid and inexpensive way to assess the distribution of the rust in Illinois. All herbarium specimens at ILL and ILLS herbaria were thoroughly examined and the harbinger of spring rust was observed on two specimens, one from 1967 and another from 2008 (Figure 1C). These records along with iNaturalist observations have helped to establish a more complete picture of the rust fungus’ Illinois distribution (Figure 2).

Figure 1 (A) Erigenia bulbosa (harbinger-of-spring), (B) Puccinia erigeniae (harbinger-of-spring rust), and (C) Herbarium specimen from ILLS with arrows pointing to rust.
Species Status Assessments - current species’ conditions
This section of the SSA focuses on the current conditions of a species’ habitat and demographics. It also seeks to explain past and current changes in a species’ distribution and population patterns. Extensive field-based data exist for very few rare plants and SSAs rely on peer-reviewed papers, thesis work, reports, and personal observations to populate this section. Due to a lack of research on many rare plants, even utilizing these varied resources may not provide sufficient data to evaluate accurately the status of a species. We propose that utilizing herbarium specimens could provide a cost-effective way to supplement the available information and we present several examples below.
The Midwestern endemic species kittentails (Synthyris bullii) was once considered for federal protection in the 1980s and is still vulnerable across its geographical range (U.S. Fish and Wildlife Service, 1985; NatureServe, 2022b). A wealth of field-based data have been generated for kittentails including breeding system (McKone et al., 1995), population size (Chi and Molano-Flores, 2014; Leja et al., 2015), plant-pollinator interactions (Chi & Molano-Flores, 2015), pre-dispersal seed predation (Leja et al., 2015), habitat degradation (Curtis et al., 2013; Chi and Molano-Flores, 2015; Leja et al., 2015), floral display (Chi and Molano-Flores, 2014), flower morphology (Chi and Molano-Flores, 2016), and seed ecology (Curtis et al., 2013; Janssen et al., 2020). Having this volume of data is the exception, as for most rare plants we lack information about their basic biology and ecology (Molano-Flores, 2021). From our field data it was concluded that habitat structure (i.e., open, semi-shaded, shaded) does not affect the size of kittentails populations and that there is a greater percent of reproductive individuals in open vs. shaded habitats (Figure 3). We used herbarium specimens to generate similar datasets and compared them to the field-based datasets to see if we would get similar results. We found that herbarium specimen labels for kittentails had adequate data associated with habitat type and quality to facilitate a series of analyses demonstrating that habitat structure did not affect population size but did affect the percent of reproductive individuals in a population (Figure 3). These results were similar to those from our field-based studies, and both supported the conclusion that habitat degradation and the associated changes are a threat to this species and could affect its reproductive potential. See Figures 3, 4 for additional information on protocols and citations.
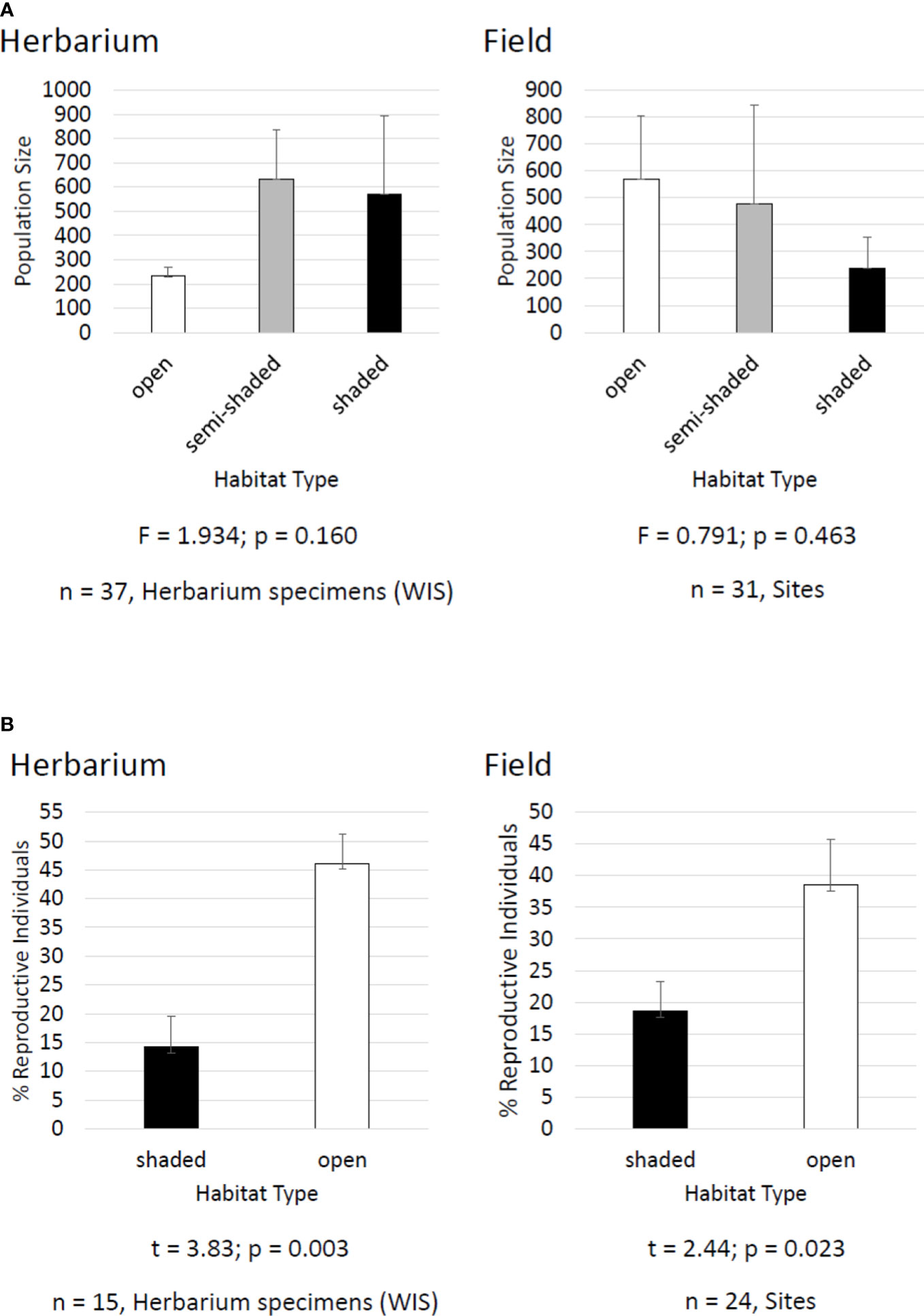
Figure 3 Comparison of kittentails (Synthyris bullii) herbarium and field datasets for: (A) population size and habitat type and (B) percent of reproductive individuals in a population and habitat type. Datasets were based on herbarium specimen labels from the Wisconsin State Herbarium (WIS) and combined unpublished and published datasets (e.g., Leja et al., 2015). Herbarium specimen labels had data on population size and % of reproductive individuals. Habitat type was based on Chi and Molano-Flores (2015). Herbarium specimen labels had information on associated species and habitat descriptions that could be translated into habitat type. Data analyses (ANOVA and t-test) were conducted using SigmaPlot 13.0.
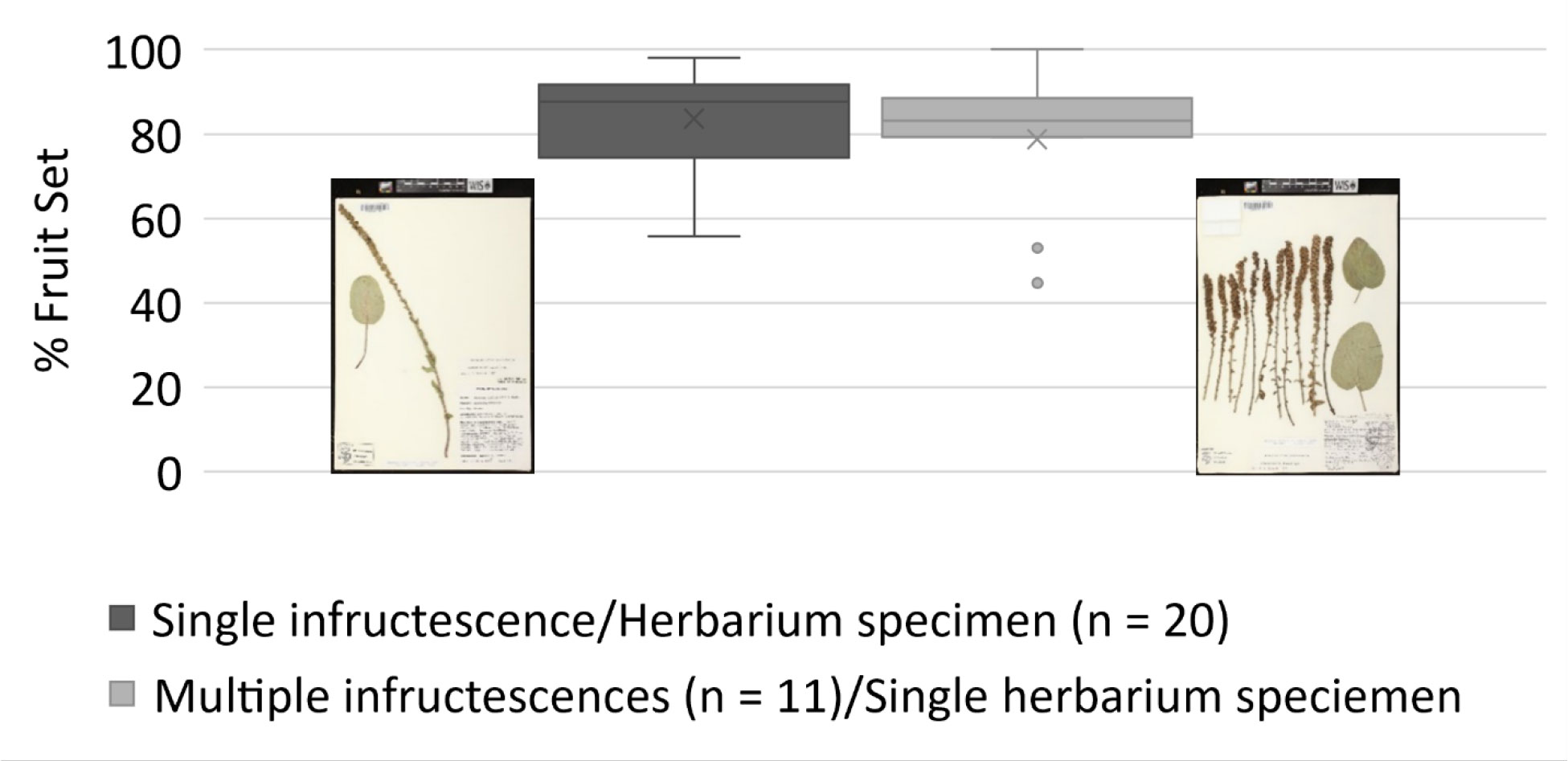
Figure 4 Fruit set estimates for kittentails (Synthyris bullii) at the individual level (i.e., per herbarium specimens) and population level (i.e., multiple infructescences per herbarium specimens per site). Fruit set estimates followed Chi and Molano-Flores (2014). Images from the Wisconsin State Herbarium (WIS).
A similar approach of using herbarium and field data was taken by Beauvais et al. (2017) to assess the impact of deer on white trillium (Trillium grandiflorum). Approximately 700 herbarium specimens collected in Quebec (Canada) were used to evaluate leaf area of flowering individuals and compared with contemporary field-based data. The study found that flowering individuals in contemporary sites with deer had a lower leaf area than historic herbarium specimens and flowering individuals in contemporary sites without deer. These studies demonstrate that information from herbarium specimen labels can be analyzed to address questions associated with habitat change due to biotic or abiotic factors and their impacts on rare plants. Studies utilizing herbarium specimens, therefore, could be used in lieu of more intensive field-based studies.
The use of herbarium specimens to study reproductive biology has been somewhat limited (e.g., Heberling, 2022 and citations therein), but it is gaining more attention in the reproductive and conservation biology fields. Several papers have been published focusing on the development of Machine Learning approaches to document the presence of reproductive structures on herbarium specimens (e.g., Lorieul et al., 2019; Davis et al., 2020; Goëau et al., 2020; Pearson et al., 2020; Triki et al., 2021). Bontrager and Angert (2016) used herbarium specimens to explore how climate contributes to variation in reproductive output and herkogamy of Clarkia pulchella. In the case of rare plants, Krosnick et al. (2022) used herbarium specimens to explore the relationship between taproot size and number of inflorescences per plant for Physaria globosa. The use of herbarium specimens to assess the reproductive output of rare plants continues to be highly underutilized, although in the SSA for Leavenworthia texana [Texas golden gladecress] (U.S. Fish and Wildlife Service, 2022) herbarium specimens were used to determine ranges of fruit set. Depending on the species, it is possible to estimate reproductive output at the individual level or even at the population level using herbarium specimens (Figure 4). Here we present two examples assessing the reproductive output of kittentails and Physaria thamnophila, (Zapata bladderpod), a rare Texas endemic. For kittentails we have determined fruit set using herbarium specimens at the individual and population levels (Figure 4). Our results are very similar to fruit set estimates for this species obtained from field-based studies, ~80% (Chi and Molano-Flores, 2015; Leja et al., 2015). However, one thing that herbarium specimens were not be able to capture, is the entire range of fruit set production for kittentails (0% to 100%; Figure 4).
For the Zapata bladderpod we found that fruit set estimates in herbarium specimens were higher than those found in several of the field-based study populations (Figure 5). Based on these two examples, herbarium specimens could, at a minimum, capture the upper end of fruit production for a rare species. A similar approach can be used to estimate seed set from herbarium specimens. For example, for Texas golden gladecress herbarium specimens showed mostly three seeds per silique but with a range of 1 to 5 seeds, although the species has been described to have 4 to 14 ovules per ovary (Flora of North America Editorial Committee, 2010) and produce up to 11 seeds per silique (U.S. Fish and Wildlife Service, 2022). Overall, herbarium specimens can provide an estimate of reproductive potential for a rare plant based on the number of reproductive structures, inflorescences, and/or infructescences as part of the species description.
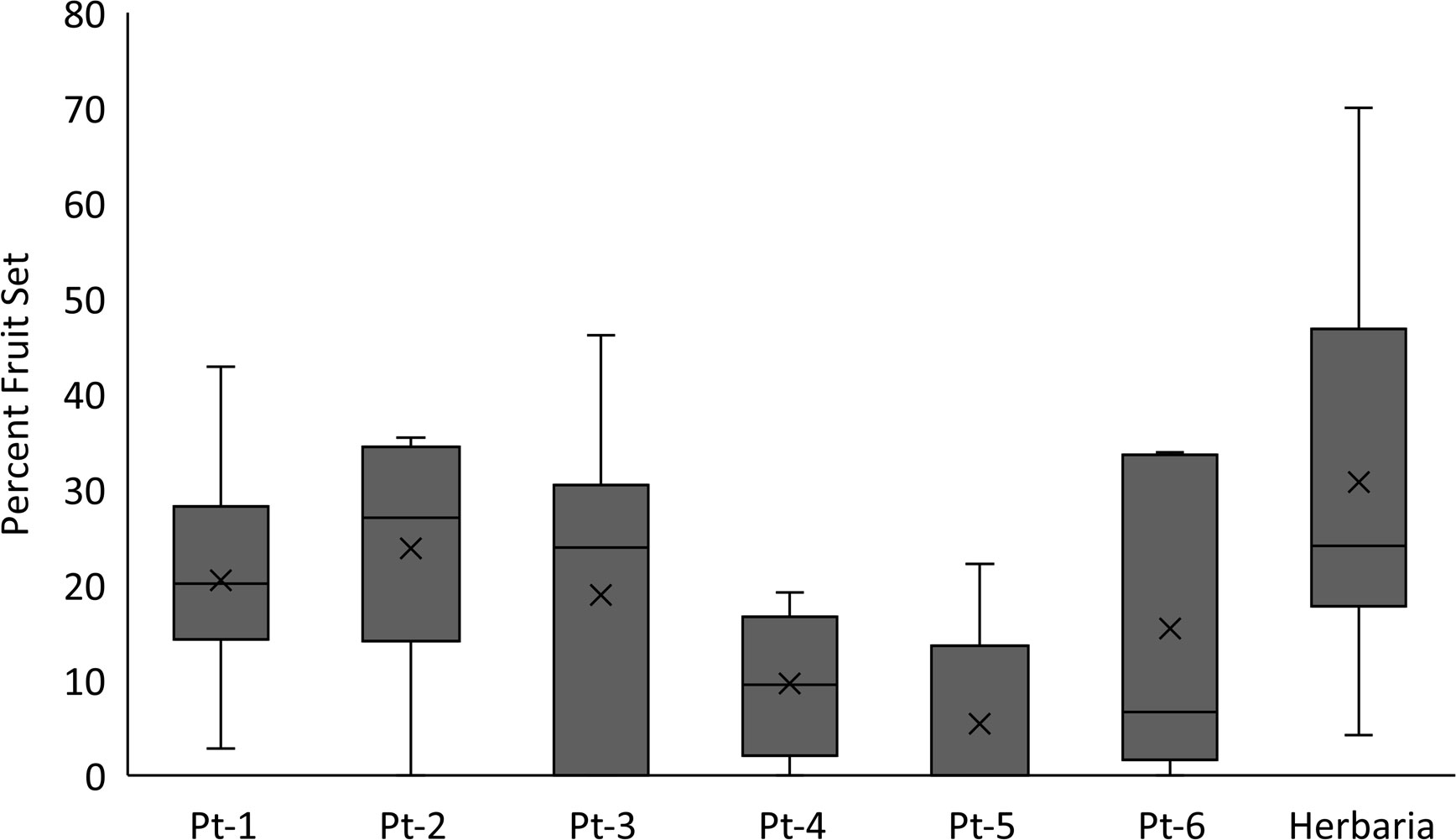
Figure 5 Percent fruit set comparison between populations of Physaria thamnophila (Zapata bladderpod) and specimens from herbaria. Fruit set was defined as number of fruits/total number of pedicels (e.g., empty and bearing fruit, flower, and flower buds). Significant differences were found for fruit set among groups (ANOVA: F = 4.16; p = 0.001). Material from herbaria had greater fruit set than Pt-4 and Pt-5, but no other comparisons were significant (Tukey test p values > 0.05). Data analyses were conducted using SigmaPlot 13.0.
Another piece of reproductive biology missing for many rare plants is their breeding system. Herbarium specimens can help us describe dichogamy (i.e., temporal separation between male and female flowers [protrandy vs. protogyny]), and herkogamy (i.e., spatial separation between male and female flowers [e.g., distyle, dioecy]) for many rare plants without doing any fieldwork. For example, for kittentails, McKone et al. (1995) described dichogamy based on field observations but examining herbarium specimens would have resulted in the same outcome (Figure 6). In the case of the SSAs for Ocmulgee skullcap (U.S. Fish and Wildlife Service, 2020) and bracted twistflower (U.S. Fish and Wildlife Service, 2021), there is no mention that these species have acropetal flower development although this is visible in herbarium specimens. Even this basic observation could explain lower fruit set and/or seed set within inflorescences of a rare plant, as it has been shown in other species (Diggle, 1995).

Figure 6 (A) Herbarium specimen of kittentails (Synthyris bullii) showing dichogamy and (B) Herbarium specimen showing evidence of historic leaf herbivory associated with kittentails (Synthyris bullii). Images from the Wisconsin State Herbarium (WIS).
In addition, it is possible to determine whether a species is autogamous, xenogamous, or has a mixed breeding system based on their pollen to ovule ratio (Cruden, 1977). It is important to note that the pollen to ovule ratios should be viewed as a conservative and indirect indicator of breeding systems and, when feasible, field-based studies should be conducted for confirmation (e.g., Kozurahova & Richards, 2016; Atasagun et al., 2021). Many SSAs allude to aspects of the breeding system based on congener work [e.g., Leavenworthia texana; Texas golden gladecress (U.S. Fish and Wildlife Service, 2022)], but herbarium specimens can be used to estimate pollen to ovule ratios as a proxy for field-based studies. In addition, based on the number of individuals found on the herbarium specimen or through the use of duplicates, differences within and across populations can be explored as has been done for natural populations [e.g., Erbar and Langlotz, 2005; Pearion and Molano-Flores, 2015). Knowing this information is important as it can tell us something about gene flow and the need for pollinators. However, it is important to note that if using herbarium specimens to determine the breeding system of a rare species, particular attention should be placed on using anthers that have not dehisced as this could result in the misidentification of the breeding system.
Two major areas of research needed for rare plants are seed germination and population genetics as they can guide the collection of seeds and reintroduction efforts (Molano-Flores, 2021). Although many rare plants lack this information from extant populations, herbarium specimens can provide a window into historic genetic diversity and, if seeds can be germinated, highlight the loss of genotypes (Nakahama et al., 2015; Nualart et al., 2017; Albani Rocchetti et al., 2021). In the United States examples of successful seed germination from herbarium specimens include Bowles et al. (1993) germinating seeds from Asclepias meadii and Astragalus tennesseensis and Wolkis et al. (2022) germinating extinct or critically endangered Hawaiian plants. However, storage conditions, seed maturity, seed dormancy, and seed longevity play a role in the germination of seeds from herbarium specimens (Godefroid et al., 2011). Going a step further, herbarium specimens can provide material for tissue cultures (Muller et al., 2021) assisting with the propagation of rare plants. In the development of SSAs, Recovery Plans, and 5-year Reviews, exploring the use of material from herbarium specimens should be mentioned to restore genetic diversity of rare plants if needed.
Another area where herbarium specimens can provide additional information for SSAs is in the case of plant-organismal interactions. For example, plant-herbivore interactions (e.g., leaf herbivory, florivory, and pre-dispersal seed predation) could be explored using herbarium specimens as they can negatively impact rare plant population sizes and vital rates (Ancheta and Heard, 2011). Already, studies using herbarium specimens have been conducted to assess leaf herbivory. For example, Lees et al. (2011) explored the invasion patterns of the horse-chestnut leaf-mining moth Cameraria ohridella in Europe by searching for leaf mining damage in herbarium specimens, placing such invasion decades earlier than previous thought. Also, Meineke et al. (2019) used herbarium specimens of four species in the northeastern United States to determine “chewing” (i.e., leaf removal by herbivores) and demonstrated temporal changes of insect damage due to climate (i.e., temperature) and urbanization. Gathering this information from herbarium specimens can determine whether these antagonistic interactions have always existed or are a new occurrence for a rare plant. We were able to find evidence of leaf herbivory and florivory in herbarium specimens of kittentails and Zapata bladderpod respectively and field-based visits confirmed these observations (Figures 6, 7). Lastly, Rakosy et al. (2022) highlight the value of herbarium specimens to further explore plant-pollinator interactions. For example, it is possible to examine stigmas of rare plants from herbarium specimens and contemporary field collections to determine the quantity of conspecific vs. heterospecific pollen, which could suggest pollen limitation or compositional changes of the habitat. Already, Johnson et al. (2019) have demonstrated that this is a viable strategy using six common native Hawaiian species.

Figure 7 Herbarium specimens of Physaria thamnophila (Zapata bladderpod). Evidence of historic florivory can be found on herbarium specimens [ovals denote florivory on online specimens Catalog #:TEX00030311 (A) and Catalog #:TEX00199372 (B)] and photos showing contemporary florivory (C–E). Images from the University of Texas at Austin Herbarium (TEX). Photos by Sara Johnson.
Other plant-organismal interactions that could be explored using herbarium specimens for rare plants are microbial interactions. Already, Daru et al. (2018a) have developed a protocol for capturing the diversity of endophytic fungi preserved in the leaves of herbarium specimens. For example, work by Adams et al. (2021) on the Canadian rare plant Geum peckii determined that this species can host pathogens (i.e., endophytic fungi) of its competitor plant species. At a minimum, herbarium specimens or seed bank repositories (e.g., Hill et al., 2021) could be preserving the microbial communities associated with rare plants. Furthermore, herbarium specimens can be used to find evidence and/or patterns of diseases associated with rare plants. Ristaino (2020) has demonstrated this with both crop and non-crop plants. Two additional examples can be seen on the leaves of bracted twistflower with powdery mildew and harbinger of spring (Erigenia bulbosa) with rust (Puccinia erigeniae; Box 2). Already in the case of powdery mildew, herbarium specimens have been used to sequence this pathogen and could assist in the exploration of the origin and spread of the pathogen and be used to assess its virulence structure (Bradshaw and Tobin, 2020). Similar examples of recent studies using herbarium specimens to track the identity and origin of pathogens are Campos et al. (2021) with citrus canker and Rieux et al. (2021) with cassava mosaic geminivirus. Understanding these interactions can provide a window into the decline of rare plants due to pathogens and could aid in the development of propagation and reintroduction protocols for rare plant species if key microbial/fungi communities are needed to ensure the successful survivorship of rare plants as part of these efforts.
Species Status Assessments - future species’ conditions
This section of the SSA focuses on predicting how the species will respond to future environmental scenarios due to anthropogenic and environmental influences such as development, natural resource extractions, and invasive species. Material and data from herbarium specimens have been used to explore how these anthropogenic impacts and environmental changes affect common and rare plants (Meineke et al., 2018 and citations therein, Albani Rocchetti et al., 2021 and citations therein). For example, in the case of Macbridea alba, a federally listed species in the Florida Panhandle, examination of locality data from herbarium specimens using historic and current aerial images has shown the rapid loss of habitat for this species (Figure 8). A similar approach can be used to predict future patterns of anthropogenic and environmental change for other rare plants. Two areas where herbarium specimens have been used considerably are species distribution modeling (SDM) and phenology.

Figure 8 Macbridea alba locations (diamonds) within Bay County, Florida showing change of habitat from managed timberlands (2014) to cleared land converted to cattle pasture (2019). At these locations, Macbridea alba populations were last recorded in the 1980’s and are now considered extirpated. Aerial imagery from ArcGIS World Imagery Wayback.
Herbarium specimens from online portals (e.g., iDigBio, GBIF, SEINet, SERNEC), are rich sources of data to fill gaps in the distribution of rare plants. Throughout the SDM literature, such repositories are frequently cited as sources of occurrence data for modeled species (Elith et al., 2006; Gaubert et al., 2006; Oleas et al., 2019; Ribeiro et al., 2022). Online portals are more readily available and accessible than official repositories of rare species data (e.g., Natural Heritage or BIOTICS data) which typically require a permit to access. The convenience and range of these data, in addition to a variety of integrated novel resources (e.g., ArcGIS tools and R packages) contribute to their usefulness, however, they are not without limitations. These data can be subject to multiple sources of bias including opportunistic and unbalanced data collection (Feeley and Silman, 2011; Stolar and Nielsen, 2015), sampling from an unrepresentative range of habitats or climate regions (Loiselle et al., 2008), potential misidentification of the target species (Graham et al., 2004; Oleas et al., 2019), and missing or inaccurate locality and coordinate information (Soberon and Peterson, 2004; Graham et al., 2008). These concerns are not unique to open access data however, as similar issues arise across the majority of available occurrence data, unless strategic sampling is conducted across the known range of a species. In regard to SSAs, localities sourced from herbaria can offer an important substitute or supplement for modeling species distributions to prioritize field surveys and find new populations (de Queiroz et al., 2012; McCune, 2016), explore current and future range changes (i.e., expansions or contractions [e.g., Greve et al., 2016; Erfanian et al., 2021; Shay et al., 2021]), assess the impacts of climate change (e.g., Abbott et al., 2017; Dangremond et al., 2022), and even assist with the conservation planning and strategies for rare species (Qazi et al., 2022). Box 1 illustrates SDMs based on herbarium specimens and EOR data for Macbridea alba and Physaria thamnophila (Supplement 1).
Herbarium specimens have been used to study the phenological responses of plants to climate change (Calinger et al., 2013; Nualart et al., 2017; Willis et al., 2017; Gallinat et al., 2018). A lot of papers have captured the fact that plants are blooming earlier as a result of climate change using herbarium specimens (e.g., Park et al., 2018; Miller et al., 2021). For example, Munson and Sher (2015) found that rare plant species in the Rocky Mountains have accelerated their blooming date by over a month since the late 1800s. A similar pattern of early blooming was found for several rare species from the New Jersey Pine Barrens (Fertakos and Clement, 2021). Recently, Dangremond et al. (2022), also utilizing herbarium specimens, found that for Trientalis borealis, a species that is rare in portions of its range, blooming periods have changed for northern populations, but not for southern populations. These papers note that these changes in flowering phenology could result in disruptions in plant-insect interactions affecting the reproductive output of rare plants. Herbarium specimens can facilitate the inclusion of SDMs and the exploration of the impact of climate change on phenology and other aspects of the biology and distribution of rare plants in an SSA.
Limitations (for now)
In the past, herbarium specimens were not collected with the primary objective of assisting with rare plant conservation and as such, limitations exist in their usefulness and the interpretation of the data generated from them. Several papers have been published highlighting the issues associated with herbarium specimens ranging from informational gaps (i.e., incomplete locality information) to collection biases associated with location (e.g., more specimens collected near roads), specimen appearance (e.g., size, color), and timing (Roberts et al., 2016; Willis et al., 2017; Daru et al., 2018b; Meineke et al., 2019; Kozlov et al., 2021; and citations therein). Misidentifications are also an issue that could affect the development of species distribution models (Oleas et al., 2019). In addition, removal of material from the specimens might be ill advised as it might diminish the overall historical and biological value of the specimen. However, Albani Rocchetti et al. (2022) conducted a survey of herbarium curators noting their inclination to allow the removal of small amounts of material as long as the specimen’s integrity was preserved. There was less support for the use of type specimens and/or specimens with historical value. However, new nondestructive techniques are being developed for herbarium specimens. For example, Shepherd (2017) was able to remove material from herbarium specimens for the extraction of DNA by rubbing them with a Staedtler “Mars Plastic” eraser. Also, the field of environmental DNA (eDNA) is working to develop nondestructive protocols for the sampling of plant and other biotic material from the surfaces of herbarium specimens to explore plant-organismal interactions. Already eDNA has been shown to be an effective tool to detect plant-animal interactions, including pollinators (Banerjee et al., 2022; Harper et al., 2022). Regardless of the sampling approach, it is important that any destructive sampling must be carried out under the umbrella of a robust policy (see Rabeler et al, 2019 for a more thorough discussion of herbarium policies and destructive sampling). Lastly, as with any data gathering exercise, time is an issue, but machine learning is facilitating the extraction of information from herbarium specimens such as phenological stages including buds, flowers, and fruits (Lorieul et al., 2019; Albani Rocchetti et al., 2021; Love et al., 2021; Hussein et al., 2022). Regardless of some of these limitations, herbarium specimens can generate data at the individual, population, and species level.
Conclusion
The goal of this paper as a perspective was to demonstrate that herbarium specimens can be used to generate data for the documents that will be used to determine the federal listing of plant species (i.e., SSA) and can provide additional data for documents concerned with the status and preservation of listed species (i.e., Recovery Plans and Five-Year Reviews). Figure 1 highlights the different ways that herbarium specimens can be incorporated as part of an SSA. It is important to note that people writing SSA, or any other listing document should do their best to find as many herbarium specimens as possible of the species of conservation concern and acknowledge how many herbarium specimens were examined and used to generate data and to run analyses. As continued and increasing anthropogenic changes exacerbate habitat change and increase the decline of plant diversity, more plant species will become vulnerable and, unfortunately, will be considered for listing under the ESA. Thorough data collection is implausible for most rare species due to a range of challenges including significant demands of time, funding, and personnel, as well as access and permit requirements. Herbarium specimens can provide a wealth of information at a low cost for a user with limited resources and thereby assist with the conservation of rare plants. To further our knowledge of past, present, and future conditions for rare plants, herbarium specimens should be utilized as a part of a conservationist’s tool kit. With the information that can be gathered from herbarium specimens about a species’ biology and ecology, land managers and conservationists will be able to make more informed decisions, and in turn, implement improved protections and conservation practices for these species.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
BMF, PBM, SAJ, & MAF conceived the conceptual framework presented in the manuscript. BMF wrote the first draft of the manuscript. SAJ wrote the first draft of the supplement and one box. PBM wrote the first draft of one box. MAF, PBM, and SAJ provided data, revisions, and edits for the manuscript. All authors read and approved the final version of the manuscript.
Funding
The authors declare that no external funding was used for the purpose of this manuscript. However, the authors declare that we used data generated from funding received from the U. S. Fish and Wildlife Service, Florida Forest Service, and Texas Parks and Wildlife Department. The funding agencies were not involved in any aspect of manuscript development or the decision to submit it for publication.
Acknowledgments
We thank the Society of Herbarium Curators for organizing a special session at Botany 2022 titled “For Biodiversity at the brink: leveraging herbaria for conservation!” This manuscript emerged as the result of giving an oral presentation at this special session.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2023.1144593/full#supplementary-material
References
Abbott R. E., Doak D. F., DeMarche. M. L. (2017). Portfolio effects, climate change, and the persistence of small populations: Analyses on the rare plant Saussurea weberi. Ecology 98 (4), 1071–1081. doi: 10.1002/ecy.1738
Adamo M., Sousa R., Wipf S., Correia R. A., Lumia A., Mucciarelli M., et al. (2022). Dimension and impact of biases in funding for species and habitat conservation. Biol. Conserv. 272 (5), 109636. doi: 10.1016/j.biocon.2022.109636
Adams S. J., Robicheau B. M., LaRue D., Browne R. D., Walker A. K. (2021). Foliar endophytic fungi from the endangered Eastern Mountain avens (Geum peckii, Rosaceae) in Canada. Plants 10, 1026. doi: 10.3390/plants10051026
Albani Rocchetti G., Armstrong C. G., Abeli T., Orsenigo S., Jasper C., Joly S., et al. (2021). Reversing extinction trends: New uses of (old) herbarium specimens to accelerate conservation action on threatened species. New Phytol. 230, 433–450. doi: 10.1111/nph.17133
Albani Rocchetti G., Davis C., Caneva G., Bacchetta G., Fabrini G., Fenu G., et al. (2022). A pragmatic and prudent consensus on the resurrection of extinct plant species using herbarium specimens. Taxon 71, 168–177. doi: 10.1002/tax.12601
Ancheta J., Heard S. B. (2011). Impacts of insect herbivores on rare plant populations. Biol. Conserv. 144, 2395–2402. doi: 10.1016/j.biocon.2011.06.019
Atasagun B., Aksoy A., Güllü İ.B., Albayrak S. (2021). Reproductive biology of Astragalus argaeus (Fabaceae), a critically endangered endemic species. An. Acad. Bras. Cienc. 93 (suppl 3), e20201613. doi: 10.1590/0001-3765202120201613
Banerjee P., Stewart K. A., Antognazza C. M., Bunholi I. V., Deiner K., Barnes M. A., et al. (2022). Plant–animal interactions in the era of environmental DNA (eDNA)-A review. Environ. DNA 4 (5), 987–999. doi: 10.1002/edn3.308
Beauvais M.-P., Pellerin S., Dubé J., Lavoie C. (2017). Herbarium specimens as tools to assess the impact of large herbivores on plant species. Botany 95 (2), 153–162. doi: 10.1139/cjb-2016-0206
Bontrager M., Angert A. L. (2016) Effects of range-wide variation in climate and isolation on floral traits and reproductive output of Clarkia pulchella. Am. J. Bot. 103, 10–21 doi: 10.3732/ajb.1500091
Bowles M. L., Betz R. F., DeMauro M. M. (1993). Propagation of rare plants from historic seed collections: Implications for species restoration and herbarium management. Restor. Ecol. 1, 101–106. doi: 10.1111/j.1526-100X.1993.tb00015.x
Bradshaw M., Tobin P. C. (2020). Sequencing herbarium specimens of a common detrimental plant disease (powdery mildew). Phytopathology 110 (7), 1248–1254. doi: 10.1094/PHYTO-04-20-0139-PER
Calinger K. M., Queenborough S., Curtis P. S. (2013). Herbarium specimens reveal the footprint of climate change on flowering trends across north-central North America. Ecol. Lett. 16 (8), 1037–1044. doi: 10.1111/ele.12135
Campos P. E., Groot Crego C., Boyer K., Gaudeul M., Baider C., Richard D., et al. (2021). First historical genome of a crop bacterial pathogen from herbarium specimen: Insights into citrus canker emergence. PloS Pathog. 17 (7), e1009714. doi: 10.1371/journal.ppat.1009714
Chi K., Molano-Flores B. (2014). Can floral display size compensate for Allee effects caused by low population abundance and density in (Plantaginaceae), a rare species? Am. J. Bot. 101, 428–436. doi: 10.3732/ajb.1300457
Chi K., Molano-Flores B. (2015). Habitat degradation disrupts plant-pollinator interactions for a rare, self-compatible prairie species. Plant Ecol. 216, 1275–1283. doi: 10.1007/s11258-015-0507-3
Chi K., Molano-Flores B. (2016). Reproductive morphology of a rare endemic Midwestern species in association with habitat degradation. J. Torrey Bot. Soc. 143, 169–179. doi: 10.3159/TORREY-D-14-00086.1
Cruden R. W. (1977). Pollen-Ovule Ratios: A conservative indicator of breeding systems in flowering plants. Evolution 31, 32–46. doi: 10.2307/2407542
Curtis M., Chi K., Molano-Flores B. (2013). Seed ecology of Synthris bullii (Plantaginaceae), a rare endemic of the Midwestern USA. Botany 91, 884–889. doi: 10.1139/cjb-2013-0024
Dangremond E. M., Hill C. H., Louaibi S., Muñoz I. (2022). Phenological responsiveness and fecundity decline near the southern range limit of Trientalis borealis (Primulaceae). Plant Ecol. 223 (1), 41–52. doi: 10.1007/s11258-021-01190-w
Daru B. H., Bowman E. A., Pfister D. H., Arnold A. E. (2018a). A novel proof of concept for capturing the diversity of endophytic fungi preserved in herbarium specimens. Philos. Trans. R. Soc Lond. B Biol. Sci. 374, 20170395. doi: 10.1098/rstb.2017.0395
Daru B. H., Park D. S., Primack R. B., Willis C. G., Barrington D. S., Whitfeld T. J. S., et al. (2018b). Widespread sampling biases in herbaria revealed from large-scale digitization. New Phytol. 217, 939–955. doi: 10.1111/nph.14855
Davis C. C., Champ J., Park D. S., Breckheimer I., Lyra G. M., Xie J., et al. (2020). A new method for counting reproductive structures in digitized herbarium specimens using Mask R-CNN. Front. Plant Sci. 11, 1129. doi: 10.3389/fpls.2020.01129
de Queiroz T. F., Baughman C., Baughman O., Gara M., Williams N. (2012). Species distribution modeling for conservation of rare, edaphic endemic plants in White River Valley, Nevada. Nat. Area J. 32, 149–158. doi: 10.3375/043.032.0203
Diggle P. K. (1995). Architectural effects and the interpretation of patterns of fruit and seed development. Annu. Rev. Ecol. Syst. 26, 531–552. doi: 10.1146/annurev.es.26.110195.002531
Dillenberger M. S., Kadereit J. W. (2014). Maximum polyphyly: Multiple origins and delimitation with plesiomorphic characters require a new circumscription of Minuartia (Caryophyllaceae). Taxon 63, 64–88. doi: 10.12705/631.5
Elith J., Graham C. H., Anderson R. P., Dudík M., Ferrier S., Guisan A., et al. (2006). Novel methods improve prediction of species' distributions from occurrence data. Ecography 29, 129–151. doi: 10.1111/j.2006.0906-7590.04596.x
Erbar C., Langlotz M. (2005). Pollen to ovule ratios: Standard or variation — a compilation. Bot. Jahrb. Syst. 126, 71–132. doi: 10.1127/0006-8152/2005/0126-0071
Erfanian M. B., Sagharyan M., Memariani F., Ejtehadi H. (2021). Predicting range shifts of three endangered endemic plants of the Khorassan-Kopet Dagh floristic province under global change. Sci. Rep. 11 (1), 9159. doi: 10.1038/s41598-021-88577-x
Feeley K. J., Silman M. R. (2011). Keep collecting: Accurate species distribution modelling requires more collections than previously thought. Divers. Distrib. 17 (6), 1132–1140. doi: 10.1111/j.1472-4642.2011.00813.x
Feist M. A. E., Downie S. R., Magee A. R., Liu M. (2012). Revised generic delimitations of Oxypolis and Ptilimnium (Apiaceae) based on leaf morphology, comparative fruit anatomy, and phylogenetic analysis of nuclear rDNA ITS and cpDNA trnQ-trnK intergeneric spacer sequence data. Taxon 61, 402–418. doi: 10.1002/tax.612011
Fertakos M. E., Clement W. L. (2021). Tracking phenology over 125 years among native flora of the New Jersey Pine Barrens. J. Torrey Bot. Soc. 148 (4), 253–265. doi: 10.3159/TORREY-D-20-00046.1
Flora of North America Editorial Committee (2010). Flora of North America North of Mexico. Vol. 7. Magnoliophyta: salicaceae to brassicaceae. Leavenworthia texana,” (New York: Oxford University Press), 487.
Franz N., Peet R. K., Weakley A. S. (2008). “On the use of taxonomic concepts in support of biodiversity research and taxonomy The new taxonomy,”. Ed. Wheeler Q. D. (Boca Raton, FL, USA: CRC Press), 61–84.
Gallinat A. S., Russo L. E. K., Melaas E. K., Willis C. G., Primack R. B. (2018). Herbarium specimens show patterns of fruiting phenology in native and invasive plant species across New England. Am. J. Bot. 105 (1), 31–41. doi: 10.1002/ajb2.1005
Gaubert P., Papeş M., Peterson A. T. (2006). Natural history collections and the conservation of poorly known taxa: Ecological niche modeling in central African rainforest genets (Genetta spp.). Biol. Conserv. 130 (1), 106–117. doi: 10.1016/j.biocon.2005.12.006
Godefroid S., Van de Vyer A., Stoffelen P., Robbrecht E., Vanderborght T. (2011). Testing the viability of seeds from old herbarium specimens for conservation purposes. Taxon 60, 565–569. doi: 10.1002/tax.602022
Goëau H., Mora-Fallas A., Champ J., Love N. L. R., Mazer S. J., Mata-Montero E., et al. (2020). A new fine-grained method for automated visual analysis of herbarium specimens: A case study for phenological data extraction. Appl. Plant Sci. 8 (6). doi: 10.1002/aps3.11368
Graham C. H., Elith J., Hijmans R. J., Guisan A., Townsend Peterson A., Loiselle B. A., et al. (2008). The influence of spatial errors in species occurrence data used in distribution models. J. Appl. Ecol. 45 (1), 239–247. doi: 10.1111/j.1365-2664.2007.01408.x
Graham C. H., Ferrier S., Huettman F., Moritz C., Peterson A. T. (2004). New developments in museum-based informatics and applications in biodiversity analysis. TREE 19 (9), 497–503. doi: 10.1016/j.tree.2004.07.006
Greve M., Lykke A. M., Fagg C. W., Gereau R. E., Lewis G. P., Marchant R., et al. (2016). Realising the potential of herbarium records for conservation biology. S. Afr. J. Bot. 105, 317–323. doi: 10.1016/j.sajb.2016.03.017
Hardisty A. R., Ellwood E. R., Nelson G., Zimkus B., Buschbom J., Addink W., et al. (2022). Digital Extended Specimens: Enabling an extensible network of biodiversity data records as integrated digital objects on the internet. BioScience 72 (10), 978–987. doi: 10.1093/biosci/biac060
Harper L. R., Niemiller M. L., Benito J. B., Paddock L. E., Knittle E. (2022). BeeDNA: Microfluidic environmental DNA metabarcoding as a tool for connecting plant and pollinator communities. Environ. DNA 5, 191–211. doi: 10.1002/edn3.370
Heberling J. M. (2022). Herbaria as big data sources of plant traits. Int. J. Plant Sci. 183 (2), 87–118. doi: 10.1086/717623
Hill R., Llewellyn T., Downes E., Oddy J., MacIntosh C., Kallow S., et al. (2021). Seed banks as incidental fungi banks: Fungal endophyte diversity in stored seeds of banana wild relatives. Front. Microbiol. 12, 643731. doi: 10.3389/fmicb.2021.643731
Hussein B. R., Malik O. A., Ong W.-H., Slik J. W. F. (2022). Applications of computer vision and machine learning techniques for digitized herbarium specimens: A systematic literature review. Ecol. Inform. 69, 101641. doi: 10.1016/j.ecoinf.2022.101641
Janssen E., Zaya D. N., Molano-Flores B., Yao L. (2020). Assessment of seed germinability after prolonged seed storage for Synthyris bullii (Plantaginaceae), a rare endemic of the Midwestern U.S.A. Am. Midl. Nat. 183, 115–128.
Johnson A. L., Rebolleda-Gómez M., Ashman T.-L. (2019). Pollen on stigmas of herbarium specimens: A window into the impacts of a century of environmental disturbance on pollen transfer. Am. Nat. 194 (3), 405–413. doi: 10.1086/704607
Kartesz J. T. (1994). “A synonymized checklist of the vascular flora of the United States, Canada, and Greenland (Second edition), vol. 1, checklist,” (Inc., Portland, OR: Timber Press).
Kartesz J. T., Meacham C. A. (1999). Synthesis of the North American Flora, Version 1.0. North Carolina Botanical Garden, Chapel Hill, N.C.
Kozlov M. V., Sokolova I. V., Zverev V., Zvereva E. L. (2021). Changes in plant collection practices from the 16th to 21st centuries: Implications for the use of herbarium specimens in global change research. Ann. Bot. 27 (7), 865–873. doi: 10.1093/aob/mcab016
Kozurahova E., Richards A. G. (2016). Breeding systems of rare and endemic Asytagalus, Oxytropis, and Onobrychis species (Fabaceae) tested with alternative methods. C. R. Acad. Bulg. Sci. 69 (12), 1571–1580.
Krosnick S. E., Thacker J. H., Mattingly H. T., Call G. P., Maynord S. C., Adams D. S., et al. (2022). Ecological correlates of reproductive output in a Tennessee population of Short's bladderpod, Physaria globose (Brassicaceae). Castanea 87 (1), 20–38. doi: 10.2179/0008-7475.87.1.20
Lavoie C. (2013). Biological collections in an ever changing world: Herbaria as tools for biogeographical and environmental studies. Perspect. Plant Ecol. Evol. Syst. 15, 68–76. doi: 10.1016/j.ppees.2012.10.002
Lees D. C., Lack H. W., Rougerie R., Hernandez-Lopez A., Raus T., Avtzis N. D., et al. (2011). Tracking origins of invasive herbivores through herbaria and archival DNA: The case of the horse-chestnut leaf miner. Front. Ecol. Environ. 9 (6), 32–328. doi: 10.1890/100098
Leja M., Chi K., Molano-Flores B. (2015). Presence and intensity of pre-dispersal seed predation in a rare plant in response to habitat quality and population metrics. Nat. Areas J. 35, 542–549. doi: 10.3375/043.035.0406
Loiselle B. A., Jørgensen P. M., Consiglio T., Jiménez I., Blake J. G., Lohmann L. G., et al. (2008). Predicting species distributions from herbarium collections: Does climate bias in collection sampling influence model outcomes? J. Biogeogr. 35 (1), 105–116. doi: 10.1111/j.1365-2699.2007.01779.x
Lorieul T., Pearson K. D., Ellwood E. R., Goëau H., Molino J. F., Sweeney P. W., et al. (2019). Toward a large-scale and deep phenological stage annotation of herbarium specimens: Case studies from temperate, tropical, and equatorial floras. Appl. Plant Sci. 7 (3), e01233. doi: 10.1002/aps3.1233
Love N. L. R., Bonnet P., Goëau H., Joly A., Mazer S. J. (2021). Machine learning undercounts reproductive organs on herbarium specimens but accurately derives their quantitative phenological status: A case study of Streptanthus tortuosus. Plants 10, 2471. doi: 10.3390/plants10112471
Lughadha E. N., Baillie J., Barthlott W., Brummitt N. A., Cheek M. R., Farjon A., et al. (2005). Measuring the fate of plant diversity: Towards a foundation for future monitoring and opportunities for urgent action. Philos. Trans. R. Soc Lond. B Biol. Sci. 360, 359–372. doi: 10.1098/rstb.2004.1596
Mace G. M. (2004). The role of taxonomy in species conservation. Philos. Trans. R. Soc Lond. B Biol. Sci. 359 (1444), 711–719. doi: 10.1098/rstb.2003.1454
Marsico T. D., Krimmel E. R., Carter J. R., Gillespie E. L., Lowe P. D., McCauley R., et al. (2020). Small herbaria contribute unique biogeographic records to county, locality, and temporal scales . Am. J. Bot. 107 (11), 1577–1587. doi: 10.1002/ajb2.1563
McCune J. L. (2016). Species distribution models predict rare species occurrences despite significant effects of landscape context. J. Appl. Ecol. 53, 1871–1879. doi: 10.1111/1365-2664.12702
McKone M. J., Ostertag R., Rauscher J. T., Heiser D. A., Russell F. L. (1995). An exception to Darwin’s syndrome: Floral position, protogyny, and insect visitation in Besseya bullii (Scrophulariaceae). Oecologia 101, 68–74. doi: 10.1007/BF00328902
Meineke E. K., Classen A. T., Sanders N. J., Jonathan Davies T. (2019). Herbarium specimens reveal increasing herbivory over the past century. J. Ecol. 107, 105–117. doi: 10.1111/1365-2745.13057
Meineke E. K., Davis C. C., Davies T. J. (2018). The unrealized potential of herbaria for global change biology. Ecol. Monogr. 88, 505–525. doi: 10.1002/ecm.1307
Miller T. K., Gallinat A. S., Smith L. C., Primack R. B. (2021). Comparing fruiting phenology across two historical datasets: Thoreau's observations and herbarium specimens. Ann. Bot. 128 (2), 159–170. doi: 10.1093/aob/mcab019
Molano-Flores B. (2021). The great USA plant conservation challenge. Biodivers. Conserv. 30, 1595–1597. doi: 10.1007/s10531-021-02152-4
Muller S., Priolet V., Badel É., Buord S. (2021). Herbaria, the last resort for extinct plant species. In Natural History Collections in the Science of the 21st Century Ed. Pellens R. doi: 10.1002/9781119882237.ch10
Munson S. M., Sher A. A. (2015). Long-term shifts in the phenology of rare and endemic Rocky Mountain plants . Am. J. Bot. 102, 1268–1276. doi: 10.3732/ajb.1500156
Nakahama N., Hirasawa Y., Minato T., Hasegawa M., Isagi Y., Shiga T. (2015). Recovery of genetic diversity in threatened plants through use of germinated seeds from herbarium specimens. Plant Ecol. 216, 1635–1647. doi: 10.1007/s11258-015-0547-8
NatureServe. (2022a). NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer (Arlington, Virginia: NatureServe). Available at: https://explorer.natureserve.org/. (Accessed December 27, 2022).
NatureServe. (2022b). Besseya bullii. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer (Arlington, Virginia: NatureServe). Available at: https://explorer.natureserve.org/. (Accessed December 27, 2022).
Negrón-Ortiz V. (2014). Pattern of expenditures for plant conservation under the Endangered Species Act. Biol. Conserv. 171, 36–43. doi: 10.1016/j.biocon.2014.01.018
Noss R. F., Cartwright J. M., Estes D., Witsell T., Elliott G., Adams D., et al. (2021). Improving species status assessments under the U.S. Endangered Species Act and implications for multispecies conservation challenges worldwide. Conserv. Biol. 35 (6), 1715–1724. doi: 10.1111/cobi.13777
Nualart N., Ibáñez N., Soriano I., López-Pujol J. (2017). Assessing the relevance of herbarium collections as tools for conservation biology. Bot. Rev. 83, 303–325. doi: 10.1007/s12229-017-9188-z
Oleas N. H., Feeley K. J., Fajardo J., Meerow A. W., Gebelein J., Francisco-Ortega J. (2019). Muddy boots beget wisdom: Implications for rare or endangered plant species distribution models. Diversity 11 (1), 10. doi: 10.3390/d11010010
Park D. S., Breckheimer I., Williams A. C., Law E., Ellison A. M., Davis C. C. (2018). Herbarium specimens reveal substantial and unexpected variation in phenological sensitivity across the eastern United States. Philos. Trans. R. Soc Lond. B Biol. Sci. 374, 20170394. doi: 10.1098/rstb.2017.0394
Pearion M., Molano-Flores B. (2015). The reproductive ecology of Mononeuria patula (Michx.) Dillenb. and Kadereit (Caryophyllaceae). J. Torrey Bot. Soc. 142, 38–50. doi: 10.3159/TORREY-D-14-00016.1
Pearson K. D., Nelson G., Aronson M. F. J., Bonnet P., Brenskelle L., Davis C. C., et al. (2020). Machine learning using digitized herbarium specimens to advance phenological research. BioScience 70 (7), 610–620. doi: 10.1093/biosci/biaa044
Qazi A. W., Saqib Z., Zaman-ul-Haq M. (2022). Trends in species distribution modelling in context of rare and endemic plants: A systematic review. Ecol. Process. 11, 40. doi: 10.1186/s13717-022-00384-y
Rabeler R. K., Svoboda H. T., Thiers B., Prather L. A., Macklin J. A., Lagomarsino L. P., et al. (2019). Herbarium practices and ethics, III. Syst. Bot. 44 (1), 7–13. doi: 10.1600/036364419X697840
Rakosy D., Ashman T.-L., Zoller L., Stanley A., Knight T. M. (2022). Integration of historic collections can shed light on patterns of change in plant–pollinator interactions and pollination service. Funct. Eco. 37, 218–233. doi: 10.1111/1365-2435.14211
Ravikanth G., Jagadish M. R., Vasudeva R., Uma Shaanker R., Aravind N. A. (2018). Recovery of critically endangered plant species in India: Need for a comprehensive approach. Curr. Sci. 114 (3), 504–511. doi: 10.18520/cs/v114/i03/504-511
Ribeiro B. R., Guidoni-Martins K., Tessarolo G., Velazco S. J. E., Jardim L., Bachman S. P., et al. (2022). Issues with species occurrence data and their impact on extinction risk assessments. Biol. Conserv. 273, 109674. doi: 10.1016/j.biocon.2022.109674
Rieux A., Campos P., Duvermy A., Scussel S., Martin D., Gaudeul M., et al. (2021). Contribution of historical herbarium small RNAs to the reconstruction of a cassava mosaic geminivirus evolutionary history. Sci. Rep. 11 (1), 21280. doi: 10.1038/s41598-021-00518-w
Ristaino J. B. (2020). The importance of mycological and plant herbaria in tracking plant killers. Front. Ecol. Evol. 7, 521. doi: 10.3389/fevo.2019.00521
Roberts D. L., Taylor L., Joppa L. N. (2016). Threatened or Data Deficient: Assessing the conservation status of poorly known species. Diversity Distrib. 22, 558–565. doi: 10.1111/ddi.12418
Schatz G. E. (2002). Taxonomy and herbaria in service of plant conservation: Lessons from Madagascar’s endemic families. Ann. Missouri Bot. Gard. 89 (2), 145–152. doi: 10.2307/3298559
Schilling E. E., Floden A. J., Weakley A. S., Winder C., Small R. L. (2022). Molecular barcoding reveals unexpected diversity in eastern North American stitchworts (Caryophyllaceae). Bot. J. Linn. Soc. 200, 75–84. doi: 10.1093/botlinnean/boac001
Shay J. E., Pennington L. K., Mandussi Montiel-Molina J. A., Toews D. J., Hendrickson B. T., Sexton J. P. (2021). Rules of plant species ranges: Applications for conservation strategies. Front. Ecol. Evol. 9, 700962. doi: 10.3389/fevo.2021.700962
Shepherd L. D. (2017). A non-destructive DNA sampling technique for herbarium specimens. PloS One 12 (8), e0183555. doi: 10.1371/journal.pone.0183555
Sigovini M., Keppel E., Tagliapietra D. (2016). Open Nomenclature in the biodiversity era. Methods Ecol. Evol. 7, 1217–1225. doi: 10.1111/2041-210X.12594
Smith D. R., Allan N. L., McGowan C. P., Szymanski J. A., Oetker S. R., Bell, et al. (2018). Development of a species status assessment process for decisions under the U.S. Endangered Species Act. J. Fish Wildl. Manage. 9, 302–320. doi: 10.3996/052017-JFWM-041
Soberon J., Peterson T. (2004). Biodiversity informatics: Managing and applying primary biodiversity data. Philos. Trans. R. Soc B: Biol. Sci. 359, 689–698. doi: 10.1098/rstb.2003.1439
Stolar J., Nielsen S. E. (2015). Accounting for spatially biased sampling effort in presence-only species distribution modelling. Divers. Distrib. 21 (5), 595–608. doi: 10.1111/ddi.12279
Triki A., Bouaziz B., Gaikwad J., Mahdi W. (2021). A deep learning-based approach for segmenting and counting reproductive organs from digitized herbarium specimen images using refined Mask Scoring R-CNN. CEUR Workshop Proc. 3067, 138–148.
U.S. Fish and Wildlife Service (1985). Endangered and threatened wildlife and plants; review of plant taxa for listing as endangered or threatened species. Fed. Reg. 50, 39534–39583.
U.S. Fish and Wildlife Service (2016). USFWS Species Status Assessment Framework: An integrated analytical framework for conservation. Version 3.4. 21 pp.
U.S. Fish and Wildlife Service (2020). Species status assessment report for the Scutellaria ocmulgee (Ocmulgee skullcap), Version 1.2. (Atlanta, GA: U.S. Fish and Wildlife Service, South Atlantic-Gulf Coast and Mississippi Basin Regions), 81 pp.
U.S. Fish and Wildlife Service (2021). Species status assessment of bracted twistflower (Streptanthus bracteatus A. Gray). March 2021 (Version 1.0). (New Mexico: U.S. Fish and Wildlife Service Southwest Region, Albuquerque), 75 pp.
U.S. Fish and Wildlife Service (2022). Species Status Assessment, Leavenworthia texana (Texas golden gladecress): Version 1. 80 pp.
Weakley A., Medford H. C., Sorrie B. A., McCormick C. A., Bridges E. L., Orzell S. L., et al. (2020). Studies in the vascular flora of the Southeastern United States. J. Bot. Res. Inst. 14 (2), 199–240. doi: 10.17348/jbrit.v14.i2.1004
Willis C. G., Ellwood E. R., Primack R. B., Davis C. C., Pearson K. D., Gallinat A. S., et al. (2017). Old plants, new tricks: Phenological research using herbarium specimens. TREE 32 (7), 531–546. doi: 10.1016/j.tree.2017.03.015
Keywords: herbarium specimens, fruit set, rare plants, plant-insect interactions, Species Status Assessments
Citation: Molano-Flores B, Johnson SA, Marcum PB and Feist MA (2023) Utilizing herbarium specimens to assist with the listing of rare plants. Front. Conserv. Sci. 4:1144593. doi: 10.3389/fcosc.2023.1144593
Received: 14 January 2023; Accepted: 10 July 2023;
Published: 28 August 2023.
Edited by:
David W. Inouye, University of Maryland, College Park, United StatesReviewed by:
Bonnie Isaac, Carnegie Museum of Natural History, United StatesKatelin Pearson, Arizona State University, United States
Copyright © 2023 Molano-Flores, Johnson, Marcum and Feist. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brenda Molano-Flores, bW9sYW5vMUBpbGxpbm9pcy5lZHU=
 Brenda Molano-Flores
Brenda Molano-Flores Sara A. Johnson
Sara A. Johnson Paul B. Marcum1
Paul B. Marcum1