- Illinois Natural History Survey, Prairie Research Institute University of Illinois, Champaign, IL, United States
Since 1988, the Cooperative Endangered Species Conservation Fund or “Section 6” fund facilitates partnerships between the U.S. Fish and Wildlife Service and state agencies that aim to provide data pertinent to the recovery of Endangered Species Act (ESA) protected species. Despite the success of these efforts, research for rare plants is chronically underfunded and many species experience long periods of research inactivity that hinders their conservation. One example is Macbridea alba Chapm. (white birds-in-a-nest, Lamiaceae, M. alba from hereon), a federally threatened and state endangered mint endemic to four counties within the Florida panhandle. The species is a candidate for delisting after 30 years of protection under the ESA, however a lack of up-to-date data associated with the species has continually challenged the implementation of effective conservation programs and prolonged the recovery process. The focus of this paper is to review the timeline of recovery goals for M. alba, present a summary of recent research findings (i.e., species distribution models, habitat associations, reproductive ecology), and identify achievements as well as persistent obstacles to recovery and delisting. Our research focused on 5 of 10 recovery actions listed in the recovery plan for M. alba. Our findings provide updated data and make novel contributions to the protection of M. alba that will prioritize and improve management efforts. Overall, our work highlights frequent barriers to the recovery and delisting of rare species, using an endemic plant species as a case-study. Importantly, we outline effective methods for the rapid assessment of at-risk plant species that due to enduring data gaps, face an uncertain future in listing and recovery. We hope our work provides a convincing case demonstrating the critical need for current and expanded ESA funding and encourages a diversity of individuals and institutions to participate in critical rare plant research to swiftly fill research gaps and expedite recovery of some of the rarest plant species across the United States.
1 Introduction
Protecting more than 900 taxa, the Endangered Species Act (ESA) is the strongest existing protection for threatened and endangered species in the United States (Oldfield et al., 2019; CBD, 2021). Comparable to the European Union Habitats Directive adopted in 1992 and the Natura 2000 network, these initiatives act as cornerstones for habitat and species conservation in their respective regions (Fois et al., 2018a; Moreno-Saiz et al., 2021). In the U.S., the ESA primarily extends to federal lands (i.e., public and DoD) and does not extend the protections received by other taxa to plants on private lands (Stein & Gravuer, 2008; Negrón-Ortiz, 2022). After 50 years, the ESA continues to play an essential role in preventing extinction for at-risk species as well as offering potential protection for the critical habitat required for their survival. Once sufficient data is provided to warrant a species protection under the ESA, a recovery plan is established using the best available science that outlines the criteria required to down list or “recover” a species, leading to its eventual delisting, the ultimate goal of the ESA. These criteria address factors outlined in the species listing petition, including current and future threats and the minimum required population size for sustaining the species in the wild (USFWS, 1973). Despite the ESAs many strengths, it has been heavily criticized for low rates of recovery, meaning that measurable criteria have not yet been met to avoid species extinction (Heywood, 2019). Only 21 native plants are documented as recovered and removed from the ESA (Haines et al., 2021 and references therein) with this recovery process taking an average of 27 years (Negrón-Ortiz, 2022). The inability of the ESA to meet recovery and delisting goals is frequently traced to chronic inadequate funding and limited baseline data to support species conservation needs (Clark et al., 2002; Negrón-Ortiz, 2014; Gerber, 2016; Malcom, 2021).
Despite a 300% increase in petitioned species over the last ~50 years, funding allocated to the USFWS has diminished or at best, remained stable (Eberhard et al., 2022), creating a backlog and increasing wait time for petitioned and listed species (Puckett et al., 2016; Eberhard et al., 2022). On average, it can take up to 12 years (10 years longer than the maximum length prescribed by law), for a species to move from petition to listing (Puckett et al., 2016). Meanwhile, species are vulnerable to continued extirpation and possible extinction, particularly for species with extremely low population numbers at the time of petitioning (Schwartz, 2008). Recovery progress is positively correlated with funding; however, funding is often skewed toward specific species or taxonomic groups, most notably vertebrates, often overlooking the recovery of the majority of listed endangered species (Negrón-Ortiz, 2014; Haines et al., 2021). Plants lack adequate protections afforded to other taxa (Kennedy, 2008; Gordon et al., 2020) and are underrepresented in terms of allocated funding (Havens et al., 2014), receiving less than 5% of all endangered species funding (Roberson et al., 2020, and references therein).
Many plant species are understudied which is compounded by funding issues. Much of the literature is focused on common, large-ranged plant species that often offer utility to humans (Lughadha et al., 2020 and references therein). In addition to being innately vulnerable, many rare species and their functional traits and interactions within their environments are undescribed or poorly known causing their risk of extinction to be potentially underestimated (Pimm et al., 2014; Fois et al., 2021). A lack of up-to-date research means that listing is prolonged due to insufficient data, and recovery efforts are often outlined and prioritized based on outdated datasets and information, which further perpetuates mismanagement and hinders plant protection and conservation (Estill and Cruzan, 1999; Le Roux et al., 2019; Molano-Flores and Coons, 2019). Despite most species requiring long periods to reach recovery and delisting, successful recovery has been the result of appropriate population protection, ensuring partnerships and private landowner engagement, and restoration and threat management (Haines et al., 2021). Recovery goals can be achieved for some of our most at-risk species if up-to-date data are available.
In this paper, we review the timeline of recovery goals for M. alba (a species listed for over 30 years), present a synopsis of recent research findings (i.e., species distribution models, habitat associations, reproductive ecology), and identify achievements as well as persistent obstacles to recovery and delisting. Our goal is to answer the following question: is the ESA living to its full potential when it comes to the conservation of M. alba and other rare plants?
2 Materials and methods
2.1 Case study with Macbridea alba
In the biodiversity hotspot of the Florida panhandle, one species currently protected under the ESA is an endemic mint, Macbridea alba. This perennial mint was listed as threatened in 1992 because of concerns around widespread habitat loss and degradation throughout its narrow four county range in the region (Table 1; USFWS, 1992). Since its listing almost 30 years ago, collaborative efforts to survey, monitor, and document species occurrence and abundance have improved our understanding of the species range and ensured protection within select public lands (Negrón-Ortiz, 2020; Wunderlin et al., 2021). Studies have been conducted to inform research needs for recovery, including documenting habitat and species associations (Walker, 1993; Johnson, 2021), pollinator vectors (Pitts-Singer et al., 2002), and demography, specifically focusing on the species relationship to fire disturbance (Walker and White, 1994; referenced in Madsen, 1999; Johnson, 2021). Other studies bring important attention to seed production, germination, and seed longevity (Madsen, 1999; Schulze et al., 2002; Johnson, 2021), as well as genetic diversity (Godt et al., 2004); however, up-to-date data has not been published for almost two decades, meaning that recovery criteria for the species is based on outdated information about the species ecology and conservation needs.
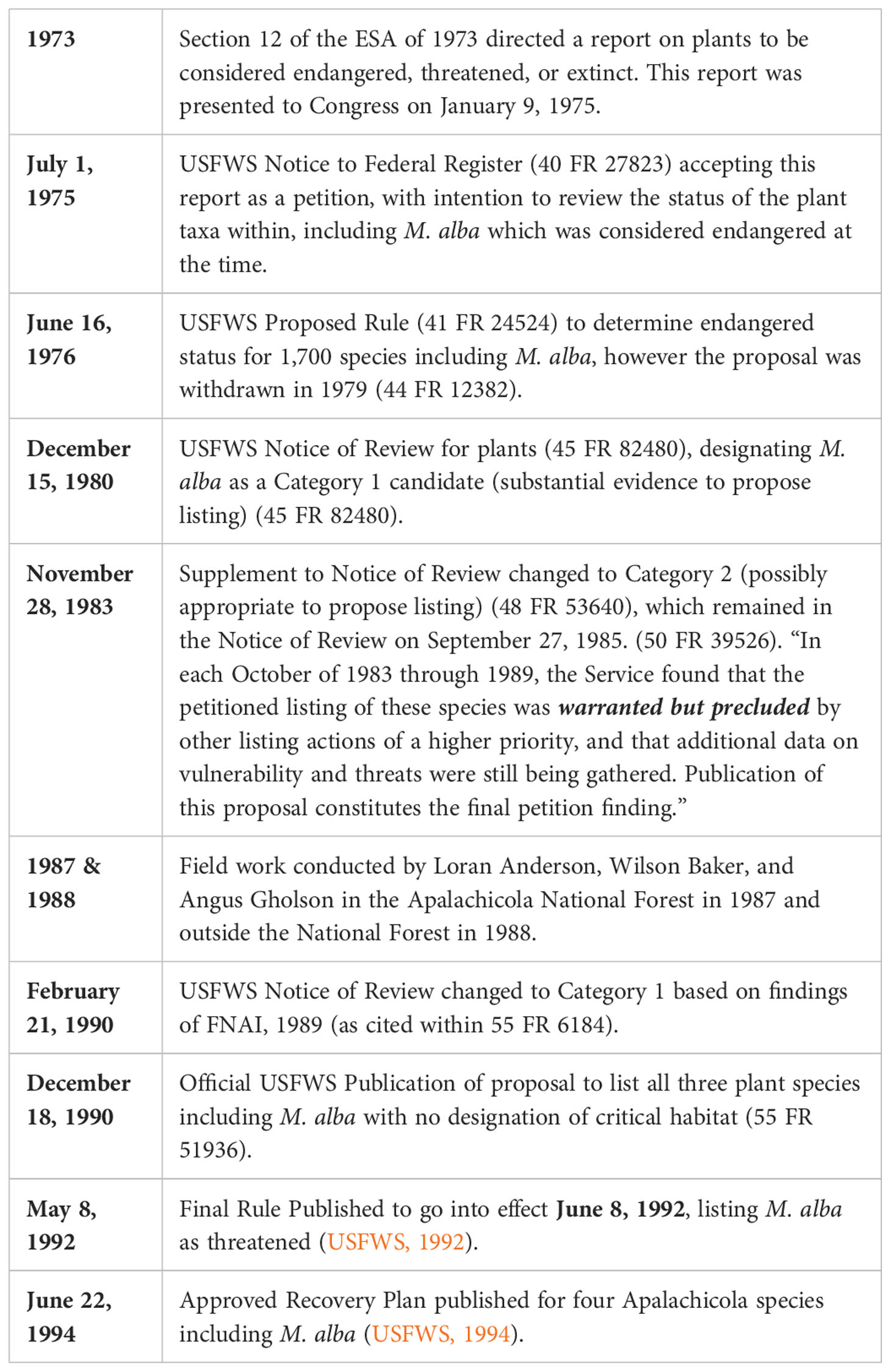
Table 1 Timeline of Petition to Listing for Macbridea alba, an Endangered Species Act (ESA) protected species.
2.2 Recovery criteria
A recovery plan instituted in 1994 (USFWS, 1994) for M. alba outlined the minimum required criteria (Table 2) to consider the species “recovered”. Of the five factors for listing a species, the recovery plan for M. alba specifically addresses Factor 1 (addressing “the present or threatened destruction, modification, or curtailment of the species habitat or range”), as the other factors are either irrelevant to the species or deemed relevant but not addressed in the recovery plan (USFWS, 1992). Recovery action priorities include updating the known distribution and size of M. alba populations within historic occurrence records, securing protections for populations outside of public lands, and developing partnerships with regional authorities and landowners to develop and facilitate appropriate management practices. Further, research that aims to understand the reproduction, genetics, and potential for ex situ and in situ propagation and reintroduction are prioritized conservation efforts for the species. The protection of a minimum of 15 populations throughout the species range for a total of at least 10 years is required to consider delisting the species from the ESA.
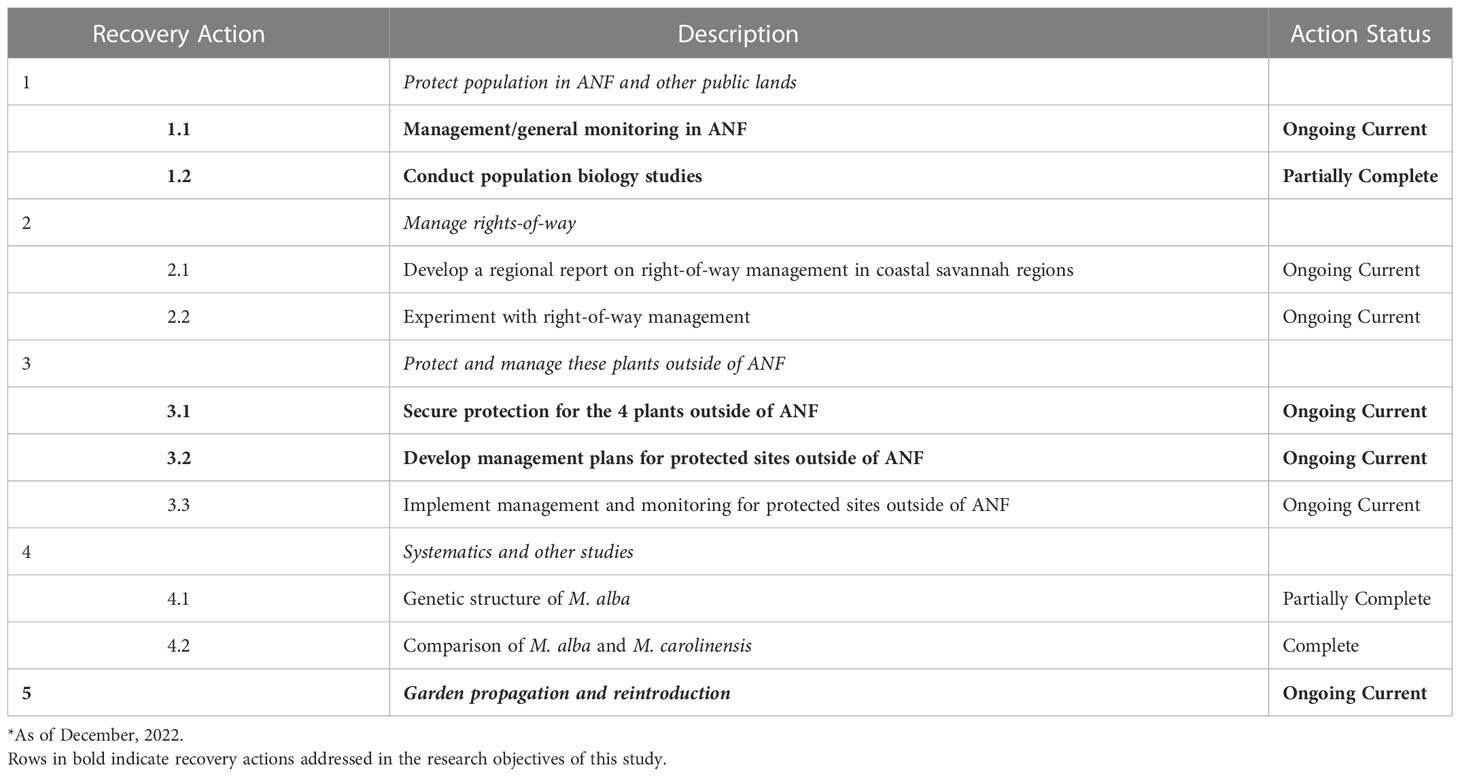
Table 2 Summary of Recovery Plan Implementation Progress (adapted from Report Results (fws.gov)* and Negrón-Ortiz, 2020).
3 Objectives, approach, & results
Basic requirements for species status assessment and recovery planning include documentation of three aspects of a species’ ecology including the following: 1) current abundance and distribution, 2) potential natural causes of variation across populations, and 3) life history strategies that most impact population dynamics (Schemske et al., 1994; Havens et al., 2014). Ongoing monitoring and published research have provided valuable information relevant to these requirements, however the region where M. alba is found has experienced significant change since the completion of this work over 20 years ago; particularly related to land use, management, and development. A reassessment of the species’ status in the context of present conditions is critical for assessing appropriate recovery goals for ongoing protection of the species. In this section, we summarize the results of three objectives and how they relate and contribute to the recovery actions (Table 2) of the species:
Objective 1: Evaluate the accuracy and utility of species distribution models to locate suitable habitat and new populations of M. alba. – Recovery Actions 1.1, 3.1, and 5.
Objective 2: Assess the abundance, distribution, and habitat associations of M. alba populations by conducting field surveys within its prospective range. – Recovery Actions 1.1, 3.1, and 3.2.
Objective 3: Reassess the reproductive ecology of M. alba and evaluate the implications for conservation of the species. – Recovery Actions 1.2 and 5.
Cooperative Endangered Species Conservation Fund or “Section 6” funds in collaboration with the USFWS and the Florida Forest Service, provided funding support for data collection and analyses associated with M. alba recovery actions. Data sets were generated during the flowering and fruiting season (May – September) of 2019 and 2021.
3.1 Objective 1: Species distribution modeling
Species distribution models for M. alba were created with the goal of documenting new occurrences for the species throughout its range, as well as identifying suitable habitat for land preservation or reintroduction initiatives. Historic occurrence records for M. alba were sourced from the Florida Natural Areas Inventory (FNAI, 2018), representing subpopulations within the known four county range (Bay, Franklin, Gulf, Liberty) for the species, however the study area was expanded to eight counties (to include Calhoun, Gadsen, Leon, and Wakulla). A dataset of presence records was used to train species distribution models (SDMs) using Maxent (Phillips et al., 2006), a frequently used methods for presence datasets (Royle et al., 2012; Fois et al., 2018b; Pecchi et al., 2019). Three models were created with varied settings and number of environmental parameters to document variation in overall model performance and predictions. Independent occurrence data was collected during model-based sampling and used to test model accuracy and predictive capacity (Johnson, 2021).
Model-based sampling led to the detection of six new subpopulation records for M. alba adding an additional 500-700 individuals to population numbers with one location found in Bay County outside of protected areas. Records within Apalachicola National Forest (ANF) were near known populations but did contribute to FNAI occurrence record population counts. Additionally, suitable habitat was identified within multiple public lands in the region as potential reintroduction sites. These findings provided data for sections 1.1, 3.1, and 5 of outlined recovery actions for the species (Table 2; USFWS, 1994; Negrón-Ortiz, 2020).
3.2 Objective 2: Population surveys
An urgent recovery action was to provide an update of the current distribution and abundance of M. alba by conducting a revisit to all documented occurrence records throughout the Florida panhandle (FNAI, 2018). Sites were prioritized by filtering those that had not been surveyed since the 1980s to early 2000s, or where M. alba had not been present during the most recent survey. In 2019 and 2021, surveys were conducted across all model-based sampling sites (77 total) in addition to a select number of historic occurrence and long-term monitoring sites for the species (32 of 42 population records [i.e., 175 of 349 subpopulation records]). For each survey, presence or absence of M. alba was recorded and where present, population size was estimated. To assess potential habitat associations for M. alba, percent cover of vegetation at the ground, mid, and canopy layers, class of woody encroachment from low-to-high, and microtopography conditions such upland or slope conditions were recorded for all sites regardless of species presence. Fire disturbance was also evaluated to document trends in species occurrence and abundance within Apalachicola National Forest (USDA, 2020). Vegetation and disturbance data were used to identify potential fine-scale predictors of suitable habitat for M. alba to improve both detection and management efforts.
Surveys confirmed an overall reduction of species distribution outside of protected areas within the panhandle region, with several populations, including two extirpated records, unable to be relocated since surveys conducted in 2008 during the last 5-year review (Negrón-Ortiz, 2009). A total of 4,000 individuals were counted during our survey of occurrence records in 2019. When included in all survey counts gathered from 2010 – 2020, population numbers appear within a stable range of 7,000 to 11,000 individuals, a range similar to previous estimates from surveys conducted between 1963 - 2008 (Negrón-Ortiz, 2020). At present, 26 of the known 42 occurrence records for the species are protected, mostly within Franklin and Liberty counties and at least 65% of known populations occur within ANF, although the large size of populations within ANF make this estimate difficult to assess. The percentage is likely higher than documented however, due to the extirpation of multiple occurrences outside of the National Forest in Bay and Gulf counties and (Negrón-Ortiz, 2020). These areas experience continued fragmentation and competition with land use such as cattle grazing, timber production, and coastal development, which results in the clearing and mismanagement of lands that M. alba previously inhabited in the late 20th century (Negrón-Ortiz, 2020).
Sites with moderate levels (40-60%) of canopy and mid-level (shrub layer) cover, and high levels (60-100%) of ground cover vegetation were most likely to harbor M. alba populations (Anderson et al., 2020; Johnson, 2021). The likelihood of encountering M. alba declined in sites with over 60% mid-level or canopy level cover, and less than 60% ground cover. The species was mostly likely to be found, and in higher abundance, in sloping areas of the landscape as opposed to upland or wetland sites; most frequently where flatwoods gradually slope to depressional wetlands. Lastly, a negative relationship was observed between M. alba presence and increasing time in years since the last burn, however too frequent fire may also inhibit population growth and survival (Anderson et al., 2020; Johnson, 2021). Fire suppression and increased time between prescribed fire; leading to encroachment in the mid and canopy cover layers, leads to decline of M. alba populations over time, even within managed conservation areas (Negrón-Ortiz, 2020). These findings contributed information to recovery actions 1.1, 3.1, and 3.2 for the species (Table 2, USFWS, 1994; Negrón-Ortiz, 2020).
3.3 Objective 3: Reproductive studies
Generating data to assist with both in situ and ex situ propagation, safeguarding, and reintroduction efforts is an ongoing component of the recovery plan for M. alba. This objective aimed to assess M. alba reproduction compared to previous work conducted with the species 20 years ago (Madsen, 1999; Schulze et al., 2002). Based on population size and a range of habitat conditions (i.e., management and vegetation structure) seven sites were targeted for reproductive studies in 2019. At each site, fruit set and seed set were estimated per individual and per population, and any occurrence of vivipary or herbivory (i.e., pre-dispersal ovule/seed predation) were recorded. Ex situ germination trials were conducted over a two-month period in a controlled greenhouse to examine germination success across sampled populations. Seeds per replicate and number of replicates varied by population and seeds were sown on filter paper lined petri dishes moistened with distilled water (Johnson, 2021).
Estimated fruit set and seed set were low across all populations (average fruit set: 4 – 18%/average seed set: 1 – 8%). Vivipary has been previously recorded in M. alba individuals with around 20% of collected seeds germinating in the calyx pre-collection (Schulze et al., 2002). Our results are similar with ~ 25% of all seed collected having germinated within the calyx. In addition, viviparous seedling survival was initially up to 42%, but seedlings did not survive. Ex situ germination was high, with a maximum germination of 83%, similar to the previously reported maximum germination of 85% in Schulze et al. (2002). Germination was considered at the emergence of the radicle and all seedlings were carefully transplanted into pots filled with a soil mix (Johnson, 2021). Seedling survival was as high as 22%, but all seedlings eventually perished. Four of the seven sites exhibited pre-dispersal ovule/seed predation in at least 50% of individuals, with the highest documented pre-dispersal ovule/seed predation in 77.1% of individuals. This pre-dispersal ovule/seed predation was attributed to Endothenia hebesana (Walker) or the Verbena Bud Moth (Tortricidae, Lepidoptera), a previously undocumented herbivore in M. alba populations (Johnson, 2021). Although our data sets are limited to a year for these seven populations, our results document variation in reproductive trends among populations, as well as high germination and low fruit and seed set overall. These findings contributed to recovery actions 1.2 and 5 for the species (USFWS, 1994; Negrón-Ortiz, 2020).
4 Discussion
In the 30 years since listing, there have been major advances in our knowledge of the basic ecology and reproductive biology of M. alba that have led to improved monitoring and management where populations persist, undoubtedly facilitating in the conservation of associate species as well. Thanks to the tireless advocacy of USFWS biologists and their regional conservation partners (e.g., Florida Forest Service), this species has received renewed attention and a call to action after years of stagnation on the Endangered Species list. Our contribution to several recovery actions played a role in updating data critical to the prioritization of future conservation and recovery efforts for the species. While M. alba is presented here as a case-study, this strategy is transferable for other rare species and provides an efficient framework to rapidly assess species status, ensuring that limited conservation resources are employed to their full potential.
Our research confirmed that based on the best available data, there is high conservation and reintroduction potential for M. alba as the species shows high ex situ germination rates and stable population numbers throughout its known range. By confirming the status of historic occurrence records, we have documented a likely shrink in species distribution outside the confines of protected areas while also identifying populations that will likely experience increased decline and extirpation if action is not taken to create partnerships or conservation easements. This work has made novel contributions, such as the discovery of six additional records for M. alba including one outside of conservation lands and has documented potential herbivore pressure from a previously unrecorded seed predator for M. alba. Locating areas for potential reintroduction and actively attempting such introduction will prioritize actions to increase the total number of protected populations that are required to delist the species.
Despite these contributions, recovery is not currently sufficient to warrant the delisting of the species. The most recent 5-year review (Negrón-Ortiz, 2020) compiled results of all research conducted to date, including research conducted by our team. Recovery achievements are at a level 2 with about 26-50% recovery objectives having been achieved; a number unchanged since the 2009 5-year review for the species (Negrón-Ortiz, 2009; Negrón-Ortiz, 2020). The USFWS is doing the best work possible with the available resources they have by facilitating partnerships to fill data gaps that inhibit successful conservation. Unless more resources are allocated to the conservation of the species by the next 5-year review in 2025, it is unlikely that additional recovery goals will be met for the species, and it will remain at its present status.
The persistent challenges faced by the agency to progress species recovery and delisting are outlined in the recent 5-year review (Negrón-Ortiz, 2020), focusing heavily on the difficult task of expanding conservation and improving management within private lands. Despite the overall stability of population numbers over time, there are valid concerns that the few remaining areas harboring populations face increased threats. Almost 70% of all documented occurrences for M. alba are located within ANF, highlighting the destructive potential of stochastic events, both environmental (i.e., devastating impacts of hurricane Michael) and genetic (i.e., further distance between and fragmentation of natural populations). It is of the utmost importance that these lands are protected in perpetuity, but moreover, that new populations are identified and protected as soon as possible. In addition, reintroduction in novel territory that may be habitable for the species in future conditions should be explored (Volis, 2019). Because most populations are protected on public lands, critical habitat was never designated for the species (USFWS, 1992) and damaging practices like slash-and-burn agriculture and poor management of private timberlands and cattle farms (as well as rights-of-ways) have led to the destruction of M. alba habitat and extirpation of populations. Until the minimum of 15 distinct populations are protected, the species must remain under protection, and even then, protection in perpetuity is necessary for species survival due to its inherently narrow range. Future efforts should focus on engagement with private landowners to coordinate protections in a diverse suite of habitats and the implementation of meaningful incentive programs are essential to engage landowners in prescribed burning and sustainable management.
These barriers are not unique to M. alba and for most species, many recovery actions remain unaccomplished or in limbo (Lundquist et al., 2002). Recovery expenditures do not match the need or priorities set in place by recovery plans (Schwartz, 2008) and the development and application of a resource allocation framework (Gerber et al., 2018) is essential to continued success. The utilization of species status assessments in recent years has been an improvement for filling data gaps for listed or petitioned species, helping to expedite a typically arduous timeframe and needs to be applied broadly going forward (Noss et al., 2021). However, as research can be slow to implement once a species is listed, it is important not just to engage a diverse array of institutions and partners to conduct research on listed species, but also incentivize research of at-risk or even currently widespread species so that in the event of petitioning, some information is already in place to assist with these decisions (Molano-Flores, 2021). New funding sources such as the Recovering America’s Wildlife Act (RAWA, if approved by Congress) or other avenues of conservation funding could accelerate and fuel this progress.
5 Conclusion
Plants need priority in funding, listing, and recovery as the foundation of the planet’s biodiverse ecosystems. It is an unignorable fact that the recovery of other wildlife hinges on the success of plant recovery efforts and habitat preservation. In the face of recent reports of devastating habitat loss and increased extinction risk, effective legislation and conservation programs have failed to reflect this need (Pimm et al., 2014; Lughadha et al., 2020). At present, the Endangered Species Act is not living to its full potential in protecting plant species. An increase and more diverse range of funding to the USFWS and their partners is critical to the future success of the ESA but importantly, ensuring that increased funds are allocated in a way that support the mission of efficient listing, recovery planning, and ultimately, delisting species. Fortunately, with the dedicated work of state/federal agencies, and non-government organizations, species like M. alba have some protection in the face of future uncertainty. Our ability to protect plants in the face of current and future change is contingent upon prompt and effective protection for plants and their ecosystems. With limited resources devoted to plant conservation efforts, researchers need efficient methods for rapidly assessing a species’ status, learning about aspects of ecology, distribution, habitat requirements, and potential for reintroduction. Together these results can help those working with species at risk make more informed decisions and further our knowledge of the ecology of rare plants and their environments.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SJ and BM-F conceived conceptual framework presented in the manuscript. SJ wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The authors declare that this study was supported by U.S. Fish and Wildlife Service Section 6 under the Florida Forest Service, FDACS contract number 025436. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
Thanks to the Florida Natural Areas Inventory for providing data, and the Florida Forest Service, St. Joseph Bay State Buffer Preserve, and Tate’s Hell State Forest for access to public lands. This work was supported by U.S. Fish and Wildlife Service Section 6 under the Florida Forest Service, FDACS contract number 025436. Any opinions, findings, conclusions, or recommendations are those of the authors and do not necessarily reflect the views of the U.S. Fish and Wildlife Service. Lastly, we would like to thank the reviewers for the constructive criticism of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anderson C. T., Dietz S., Jenkins A., Drake J. (2020). The effects of fire season, frequency, and forest structure on the flowering abundance of macbridea alba (Tallahassee (FL: Florida Natural Areas Inventory).
CBD (2021) Listing species under the endangered species act. center for biological diversity. Available at: https://www.biologicaldiversity.org/programs/biodiversity/endangered_species_act/listing_species_under_the_endangered_species_act/index.html (Accessed March 30, 2021).
Clark J. A., Hoekstra J. M., Boersma P. D., Kareiva P. (2002). Improving US endangered species act recovery plans: key findings and recommendations of the SCB recovery plan project. Conserv. Biol. 16 (6), 1510–1519. doi: 10.1046/j.1523-1739.2002.01376.x
Eberhard E. K., Wilcove D. S., Dobson A. P. (2022). Too few, too late: U.S. endangered species act undermined by inaction and inadequate funding. PloS One 17 (10), e0275322. doi: 10.1371/journal.pone.0275322
Estill J. C., Cruzan M. B. (1999). Phytogeography of rare plant species endemic to the southeastern united states. Castanea 66 (1-2), 3–23. Available at: https://www.jstor.org/stable/4033879
FNAI (2018) Data from: Macbridea alba element of occurrence spatial data. Available at: https://www.fnai.org/publications/data-requests.
Fois M., Bacchetta G., Cogoni D., Fenu G. (2018a). Current and future effectiveness of the natura 2000 network for protecting plant species in Sardinia: a nice and complex strategy in its raw state? J. Environ. Plann. Manag. 61 (2), 332–347. doi: 10.1080/09640568.2017.1306496
Fois M., Cuena-Lombraña A., Bacchetta G. (2021). Knowledge gaps and challenges for conservation of Mediterranean wetlands: Evidence from a comprehensive inventory and literature analysis for Sardinia. Aquat. Conservat.: Mar. Freshw. Ecosyst. 31 (9), 2621–2631. doi: 10.1002/aqc.3659
Fois M., Cuena-Lombraña A., Fenu G., Bacchetta G. (2018b). Using species distribution models at local scale to guide the search of poorly known species: Review, methodological issues and future directions. Ecol. Modelling. 385, 124–132. doi: 10.1016/j.ecolmodel.2018.07.018
Gerber L. R. (2016). Conservation triage or injurious neglect in endangered species recovery. Proc. Natl. Acad. Sci. 113 (13), 3563–3566. doi: 10.1073/pnas.1525085113
Gerber L. R., Runge M. C., Maloney R. F., Iacona G. D., Drew C. A., Avery-Gomm S., et al. (2018). Endangered species recovery: A resource allocation problem. Science 362 (6412), 284–286. doi: 10.1126/science.aat8434
Godt M. J. W., Walker J., Hamrick J. L. (2004). Allozyme diversity in Macbridea alba (Lamiaceae), an endemic Florida mint. Journal of Heredity 95 (3), 244–249. doi: 10.1093/jhered/esh044
Gordon E. R., Butt N., Rosner-Katz H., Binley A. D., Bennett J. R. (2020). Relative costs of conserving threatened species across taxonomic groups. Conserv. Biol. 34 (1), 276–281. doi: 10.1111/cobi.13382
Haines A. M., Leu M., Costante D. M., Treakle T. C., Parenti C., Miller J. R., et al. (2021). Benchmark for the ESA: Having a backbone is good for recovery. Front. Conserv. Sci. 2 (1). doi: 10.3389/fcosc.2021.630490
Havens K., Kramer A. T., Guerrant E. O. (2014). Getting plant conservation right (or not): The case of the united states. Int. J. Plant Sci. 175 (1), 3–10. doi: 10.1086/674103
Heywood V. H. (2019). Conserving plants within and beyond protected areas – still problematic and future uncertain. Plant Diver. 41 (2), 36–49. doi: 10.1016/j.pld.2018.10.001
Johnson S. A. (2021). A reassessment of the conservation status of a rare Florida endemic mint, macbridea alba (Champaign (IL: University of Illinois at Urbana-Champaign). Available at: http://hdl.handle.net/2142/113172.
Kennedy K. L. (2008). “The center for plant conservation: Twenty years of recovering america’s vanishing flora,” in Saving biological diversity. Eds. Askins R. A., Dreyer G. D., Visgilio G. R., Whitelaw D. M.(Boston, MASpringer Press), 47–58. doi: 10.1007/978-0-387-09565-3_5
Le Roux J. J., Hui C., Castillo M. L., Iriondo J. M., Keet J. H., Khapugin A. A., et al. (2019). Recent anthropogenic plant extinctions differ in biodiversity hotspots and coldspots. Curr. Biol. 29 (17), 2912–2918. doi: 10.1016/j.cub.2019.07.063
Lughadha E. N., Bachman S. P., Leão T. C. C., Forest F., Halley J. M., Moat J., et al. (2020). Extinction risk and threats to plants and fungi. Plants People Planet. 2 (5), 389–408. doi: 10.1002/ppp3.10146
Lundquist C. J., Diehl J. M., Harvey E., Botsford L. W. (2002). Factors affecting implementation of recovery plans. Ecological Applications (Clemson (SC: Clemson University) 12 (3), 713–718. doi: 10.1890/1051-0761(2002)012[0713:FAIORP]2.0.CO2
Madsen D. L. (1999). Seed production and germination studies of macbridea alba (Clemson (SC: Clemson University).
Malcom J. W. (2021). “Consequences of resource limitations on ESA implementation,” in Endangered species act. Eds. Baur D. C., Li Y. W. (Washington, DC: American Bar Association), 417–437. Available at: https://defenders-cci.org/files/ESA_3rd_ch15.pdf.
Molano-Flores B. (2021). The great USA plant conservation challenge. Biodiver. Conserv. 30, 1595–1597. doi: 10.1007/s10531-021-02152-4
Molano-Flores B., Coons J. (2019). Reproductive ecology of Physaria kingii subsp. kaibabensis, an endemic species of the kaibab plateau, USA. Natural Areas J. 40 (4), 345–354. doi: 10.3375/043.040.0407
Moreno-Saiz J. C., Albertos B., Ruiz-Molero E., Mateo R. G. (2021). The European union can afford greater ambition in the conservation of its threatened plants. Biol. Conserv. 261, 109231. doi: 10.1016/j.biocon.2021.109231
Negrón-Ortiz V. (2009). Macbridea alba (White birds-in-a-nest) 5-year review: summary and evaluation (Panama City (FL: U.S. Fish and Wildlife Service). Available at: https://ecos.fws.gov/docs/tess/species_nonpublish/1318.pdf.
Negrón-Ortiz V. (2014). Pattern of expenditures for plant conservation under the endangered species act. Biol. Conserv. 171, 36–43. doi: 10.1016/j.biocon.2014.01.018
Negrón-Ortiz V. (2020). “Macbridea alba (White birds-in-a-nest) 5-year review: summary and evaluation,” (Panama City (FL: U.S. Fish and Wildlife Service). Available at: https://ecos.fws.gov/docs/tess/species_nonpublish/3070.pdf.
Negrón-Ortiz V. (2022). “Plants, science, and the endangered species act. Presidential address at botany 2022,” in The plant science bulletin: A publication of the botanical society of America (St. Louis, MO: Botanical Society of America), 68, 157–164. Available at: https://botany.org/userdata/IssueArchive/issues/originalfile/WebPSB_68:3:2022.pdf.
Noss R. F., Cartwright J. M., Estes D., Witsell T., Elliott G., Adams D., et al. (2021). Improving species status assessments under the US endangered species act and implications for multispecies conservation challenges worldwide. Conserv. Biol. 35 (6), 1715–1724. doi: 10.1111/cobi.13777
Oldfield S. F., Olwell P., Shaw N., Havens K. (2019). “Conservation of plant species,” in Seeds of restoration success (Switzerland: Springer Earth System Sciences). doi: 10.1007/978-3-319-96974-9_4
Pecchi M., Marchi M., Burton V., Giannetti F., Moriondo M., Bernetti I., et al. (2019). Species distribution modelling to support forest management. a literature review. Ecol. Modelling. 411, 108817. doi: 10.1016/j.ecolmodel.2019.108817
Phillips S. J., Anderson R., Schapire R. (2006). Maximum entropy modeling of species geographic distributions. Ecol. Model. 190 (3-4), 231–259. doi: 10.1016/j.ecolmodel.2005.03.026
Pimm S. L., Jenkins C. N., Abell R., Gittleman J. L., Joppa L. N., Raven P. H., et al. (2014). The biodiversity of species and their rates of extinction, distribution, and protection. Science 344 (6187), 1246752. doi: 10.1126/science.1246752
Pitts-Singer T. L., Hanula J. L., Walker J. L. (2002). Insect pollinators of three rare plants in a Florida longleaf pine forest. Florida Entomol. 85 (2), 308–316. doi: 10.1653/0015-4040(2002)085[0308:IPOTRP]2.0.CO;2
Puckett E. E., Kesler D. C., Greenwald D. N. (2016). Taxa, petitioning agency, and lawsuits affect time spent awaiting listing under the US endangered species act. Biol. Conserv. 201, 220–229. doi: 10.1016/j.biocon.2016.07.005
Roberson E. B., Frances A., Havens K., Maschinski J., Meyer A., Ott L. (2020). Fund plant conservation to solve biodiversity crisis. Science 367 (6475), 258. doi: 10.1126/science.aba4360
Royle J. A., Chandler R. B., Yackulic C., Nichols J. D. (2012). Likelihood analysis of species occurrence probability from presence-only data for modelling species distributions. Methods Ecol. Evol. 3 (3), 545–554. doi: 10.1111/j.2041-210X.2011.00182.x
Schemske D. W., Husband B. C., Ruckelshaus M. H., Goodwille C., Parker I. M., Bishop J. G. (1994). Evaluating approaches to the conservation of rare and endangered plants. Ecology 75 (3), 584–606. doi: 10.2307/1941718
Schulze D., Walker J., Spira T. (2002). Germination and seed bank studies of Macbridea alba (Lamiaceae), a federally threatened plant. Castanea 67 (3), 280–289. Available at: https://www.jstor.org/stable/4034350
Schwartz M. W. (2008). The performance of the endangered species act. Annu. Rev. Ecol. Evol. Systemat. 39, 279–299. doi: 10.1146/annurev.ecolsys.39.110707.173538
Stein B. A., Gravuer K. (2008). Hidden in plain sight: the role of plants in state wildlife action plans (Arlington, VA: NatureServe). Available at: https://www.academia.edu/48721216/Hidden_in_Plain_Sight_The_Role_of_Plants_in_State_Wildlife_Action_Plans?from=cover_page.
USDA (2020). Data from: Fire compartment data for Apalachicola national forest (Tallahassee, FL: Florida Forest Service).
USFWS (1973). The endangered species act of 1973, as amended through the 108th congress (Washington, D.C: Department of the Interior: U.S. Fish and Wildlife Service). Available at: https://www.fws.gov/sites/default/files/documents/endangered-species-act-accessible.pdf.
USFWS (1992) Endangered and threatened wildlife and plants; threatened status for three Florida plants. Available at: https://www.govinfo.gov/content/pkg/FR-1992-05-08/pdf/FR-1992-05-08.pdf#page=33.
USFWS (1994). Recovery plan for four plants of the lower Apalachicola region, florida. euphorbia telephioides (Telephus spurge), macbridea alba (white birds-in-a-nest), pinguicula ionantha (Godfrey’s butterwort), scutellaria floridana (Florida skullcap) (Southeast Region, Atlanta, GA: U.S. Fish and Wildlife Service). Available at: https://ecos.fws.gov/docs/recovery_plan/940622.pdf.
USFWS (2021) Press release: U.S. fish and wildlife service proposes delisting 23 species from endangered species act due to extinction. Available at: https://www.fws.gov/press-release/2021-09/us-fish-and-wildlife-service-proposes-delisting-23-species-endangered-species.
Volis S. (2019). Conservation-oriented restoration – a two for one method to restore both threatened species and their habitats. Plant Diver. 41, 50–58. doi: 10.1016/j.pld.2019.01.002
Walker J. (1993). Rare vascular plant taxa associated with the longleaf pine ecosystems: patterns in taxonomy and ecology. In Proc. Annu. Tall Timbers Fire Ecol. Conf. 18, 105–126. Available at: https://talltimbers.org/wp-content/uploads/2018/09/105-Walker1993_op.pdf
Walker J. L., White D. (1994). Morphological and flower production changes with time since burning in Macbridea alba. Abstract retrieved Supplement. Bull. Ecol. Soc. America 75 (2), 240.
Wunderlin R. P., Hansen B. F., Franck A. R., Essig F. B. (2021) Atlas of Florida plants (University of South Florida, Tampa: Institute for Systematic Botany). Available at: http://florida.plantatlas.usf.edu/ (Accessed March 21, 2021).
Keywords: recovery, rare plants, endemic plants, Endangered Species Act, rare plant conservation, Macbridea alba
Citation: Johnson SA and Molano-Flores B (2023) Is the Endangered Species Act living to its full potential? The reassessment of the conservation status and recovery of Macbridea alba Chapm. as a case study. Front. Conserv. Sci. 4:1116848. doi: 10.3389/fcosc.2023.1116848
Received: 05 December 2022; Accepted: 23 January 2023;
Published: 10 February 2023.
Edited by:
Richard T. Corlett, Xishuangbanna Tropical Botanical Garden (CAS), ChinaReviewed by:
Robert Nicholas Trigiano, The University of Tennessee, Knoxville, United StatesMauro Fois, University of Cagliari, Italy
Alan Weakley, University of North Carolina at Chapel Hill, United States
Copyright © 2023 Johnson and Molano-Flores. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Ann Johnson, c2FyYWFqQGlsbGlub2lzLmVkdQ==
 Sara Ann Johnson
Sara Ann Johnson Brenda Molano-Flores
Brenda Molano-Flores