- 1Warnell School of Forestry and Natural Resources, University of Georgia, Athens, GA, United States
- 2Faculty of Natural and Agricultural Sciences, Mammal Research Institute, University of Pretoria, Pretoria, South Africa
The increasing global emergence of pathogens transmitted between wildlife and domestic animals are critically important conservation and economic concerns. International organizations, such as the World Organization for Animal Health (OIE), have called for cross-jurisdictional government investment in defensible, reliable surveillance systems and biosecurity measures to prevent pathogen transmission at the wildlife-domestic animal interface. A classic example of a pathogen that transmits across the wildlife-domestic animal interface is rabbit hemorrhagic disease virus 2 (RHDV2), which has spread to five continents in the 11 years since its discovery. RHDV2 is a highly contagious virus that infects wild and domestic rabbits and hares (lagomorphs). Globally, RHDV2 has resulted in population declines of wild lagomorphs, with associated biodiversity and hunting impacts, as well as economic losses for commercial rabbit industries. To assess the degree to which government agencies are positioned to engage in cross-jurisdictional approaches to mitigate pathogen spillover, we conducted the first study of how agricultural and wildlife agencies in the United States of America (U.S.) have responded to RHDV2 since it was detected in wild and domestic lagomorphs in March 2020. We surveyed and interviewed animal health personnel at 95 state wildlife and agricultural agencies, thereby accounting for all 50 states. Agencies have primarily responded to RHDV2 through disease investigations of potential RHDV2 cases, vaccinations, and education and outreach with the public and stakeholder groups. However, agencies' inconsistent jurisdiction within and across states over lagomorph populations and industries, limited knowledge of wild lagomorph populations and the composition of the domestic rabbit industry, and resource constraints have hindered management efforts. Improved understanding of the domestic lagomorph trade and transport routes is urgently needed to mitigate the risks associated with human-mediated movement of rabbits and RHDV2 across the U.S. Greater flexibility in agency funding and increased allocation of discretionary funds to agencies for management of animal diseases would allow agencies to respond more rapidly and effectively to emerging pathogens such as RHDV2. Federal leadership is needed to engage state agencies in collaborative, proactive interagency disease management across the U.S.
Introduction
Pathogen spillover between wildlife and domestic animals (pets, livestock) is a global conservation and economic problem. The growing international trade in domestic animals and wildlife has exacerbated disease risks through the introduction of foreign animal pathogens into naïve animal populations, resulting in wildlife population declines, substantial financial losses to agriculture and the commercial animal trade, and costly disease control efforts (Pepin et al., 2014; Kao et al., 2018). The World Organization for Animal Health (OIE), the Convention on Biological Diversity, European Union Regulation 2016/429 on transmissible animal diseases (Animal Health Law), and international trade agreements require cross-jurisdictional government investment in defensible and reliable surveillance systems and biosecurity measures to prevent pathogen transmission at the wildlife-domestic animal interface (Ryser-Degiorgis, 2013; Portier et al., 2019; Stephen et al., 2019; also referred to as the livestock-wildlife interface). Unfortunately, federal, state, and provincial government agencies that are responsible for biosecurity often have limited knowledge of how to mitigate pathogen transmission at this interface (Miller et al., 2013), lack the jurisdictional authority and funding to implement appropriate surveillance systems and biosecurity measures (Siemer et al., 2012), and have failed to communicate effectively with key stakeholders and the public about disease risks (Pedersen et al., 2012). Although pathogen transmission at the wildlife-domestic animal interface is often bi-directional, legislative directives, and institutionalized habits of thought that govern agencies' actions (e.g., the definition of animals and diseases as “livestock” vs. “wildlife”) have undermined effective disease management because agencies view certain categories of animals as irrelevant to their mission (Jerolmack, 2013). The rapid rise in pathogen spillover at the wildlife-domestic animal interface (e.g., avian influenza, brucellosis, chronic wasting disease, bovine tuberculosis) and associated conservation and economic impacts highlight the importance of implementing collaborative disease management activities by agricultural and wildlife agencies (Carmichael, 2012; Jerolmack, 2013).
Animal health surveillance is critical to provide early warning of emerging infectious diseases that may threaten agriculture and wildlife (Ryser-Degiorgis, 2013; Stephen et al., 2019). The OIE defines surveillance as “the systematic ongoing collection, collation, and analysis of information related to animal health and the timely dissemination of information so that action [e.g., restrictions on live animal trade] can be taken” (World Organization for Animal Health [OIE], 2021). Continued data collection and data sharing across government agencies is crucial to detect and effectively manage diseases (Pedersen et al., 2012; Ryser-Degiorgis, 2013). However, surveillance performance standards that apply to domestic animal health are difficult to apply to wildlife health surveillance, which is typically based on convenience sampling of dead or visibly sick animals (Ryser-Degiorgis, 2013; Stephen et al., 2019; also referred to as general, passive, or scanning surveillance). Agencies responsible for wildlife health surveillance are hampered by a number of factors, including incomplete ecological knowledge of wildlife populations at risk, limited ability to determine the true disease status of wildlife populations, pathogen persistence or transmission outside of vertebrate hosts (i.e., vector-borne pathogens), insufficient diagnostic facilities and staff, regulatory restrictions, and funding constraints (Siemer et al., 2012; Ryser-Degiorgis, 2013; Portier et al., 2019; Stephen et al., 2019). Targeted (active) surveillance of wildlife that appear healthy is uncommon because high sample sizes are required to provide reliable pathogen prevalence estimates that account for relevant biological, spatial, and temporal variables (Ryser-Degiorgis, 2013). Wildlife health surveillance programs typically lack consistent performance standards and diagnostic protocols, which undermines the accuracy, efficiency, and comparability of surveillance across different regions or states. These challenges make it difficult to demonstrate the need for, and value of, surveillance programs to policymakers, industry, and the public (Ryser-Degiorgis, 2013; Stephen et al., 2019).
Additionally, management actions to control or prevent pathogen transmission in domestic animals (e.g., isolation, vaccination, culling) cannot be readily applied to free-ranging wildlife that interact with an array of other species and are difficult to capture, handle, or vaccinate (Portier et al., 2019). The inherent challenges of disease management in wildlife are exacerbated by political backlash from the agricultural and live animal trade sectors that have faced harsh disease control measures (e.g., culling of their animals) and would prefer wildlife to be culled or contained to prevent pathogen transmission threats to their industries (Siemer et al., 2012; Portier et al., 2019). Concurrently, management actions may be illegal for species of conservation concern (e.g., threatened or endangered species), and wildlife disease management may result in political backlash from environmental groups, hunters, and the public (Siemer et al., 2012; Portier et al., 2019). These socio-cultural, economic, and political pressures have contributed to conflicting agency missions, program goals, and cultural differences that preclude comprehensive, collaborative disease management across agencies, states, and countries (Jerolmack, 2013; Miller et al., 2013).
In recognition of the obstacles to effective disease management, international working groups of wildlife managers, veterinarians, public health officers, microbiologists, ecologists, and government officials have identified measures by which pathogen control at the wildlife-domestic animal interface may be improved (Portier et al., 2019). They recommend that descriptive studies to define the epidemiological role of affected species and pathogen monitoring should be prioritized and implemented as soon as possible after a disease outbreak (Portier et al., 2019). Next, risk assessments are required to evaluate the biological and economic impacts of pathogen transmission and to identify uncertainties pertaining to management interventions. Emergency, practical actions (e.g., reinforcement of existing biosecurity measures) should be taken while risk assessments are being conducted (Ryser-Degiorgis, 2013; Portier et al., 2019). Following risk assessments, an overall management goal should be implemented for the complete host-pathogen system with appropriate management actions to be executed by different agencies (Portier et al., 2019). Concurrently, social sciences studies are required to ascertain what messaging and communication strategies are needed to engage key stakeholders in participatory disease management and engender their political support for disease management (Ryser-Degiorgis, 2013).
To assess the degree to which government agencies are positioned to engage in cross-jurisdictional approaches to mitigate pathogen transmission at the wildlife-domestic animal interface, we conducted a study of how agricultural and wildlife agencies in the United States of America (U.S.) have responded to a novel foreign animal pathogen, rabbit hemorrhagic disease virus 2 (RHDV2).
RHDV2 is a non-enveloped, icosahedral, single-stranded RNA virus of the genus Lagovirus, family Caliciviridae (Asin et al., 2021). This highly contagious virus infects lagomorphs (rabbits and hares), typically has a 3–9-day incubation period, and can cause fatal disease within 2–4 days of infection (Le Gall-Reculé et al., 2013; World Organization for Animal Health [OIE], 2019). Infected animals often show no obvious signs of disease before death (Williams et al., 2021), and mortality rates may be as high as 80% (Le Gall-Reculé et al., 2013; World Organization for Animal Health [OIE], 2019). RHDV2 spreads through direct or indirect contact with infected lagomorphs (including oculonasal secretions, urine, feces, and blood), lagomorph carcasses or carcass parts, insect vectors, and environments or materials contaminated by infected lagomorphs (e.g., bedding, forage). The virus may remain viable up to 15 weeks in dry conditions and over 90 days in decaying animal tissue outdoors (World Organization for Animal Health [OIE], 2019). As such, RHDV2 is difficult to contain in wild lagomorph populations and is easily spread to new regions by people.
The virus was first detected in France in 2010 and has spread globally across Europe and the United Kingdom into Africa, China, Israel, Japan, New Zealand, and North America (Canada, Mexico, U.S.) due to human-mediated movement of lagomorphs (Rouco et al., 2019; Ramsey et al., 2020; Katayama et al., 2021). RHDV2 resulted in population declines (60–70%) of wild lagomorphs in Europe, triggering ecological disruptions, trophic cascades, and declines of rabbit-specialist predators, such as the endangered Iberian Lynx pardinus and Spanish Imperial eagle Aquila adalberti (Monterroso et al., 2016). Intensive, costly management efforts may be required to recover ecosystems that are impacted by RHDV2 due to significant alterations to ecosystem structure and function (Guerrero-Casado et al., 2013; Delibes-Mateos et al., 2014). The virus has also resulted in substantial economic losses for the commercial rabbit trade and hunting industries in Europe (Rouco et al., 2019).
In the U.S., RHDV2 is classified as a foreign animal pathogen. It was first detected in domestic rabbits in Ohio in 2018 (Williams et al., 2021). In March 2020, a phylogenetically distinct RHDV2 was confirmed in domestic and wild lagomorphs in New Mexico (U. S. Department of Agriculture [USDA], 2019). As of March 2022, RHDV2 has been detected in wild and/or domestic lagomorphs in 19 states (Figure 1; U. S. Department of Agriculture [USDA], 2022). RHDV2 poses a risk to the 15 native lagomorph species in the U.S., including several threatened and endangered species or species of conservation concern (e.g., New England cottontail Sylvilagus transitionalis).
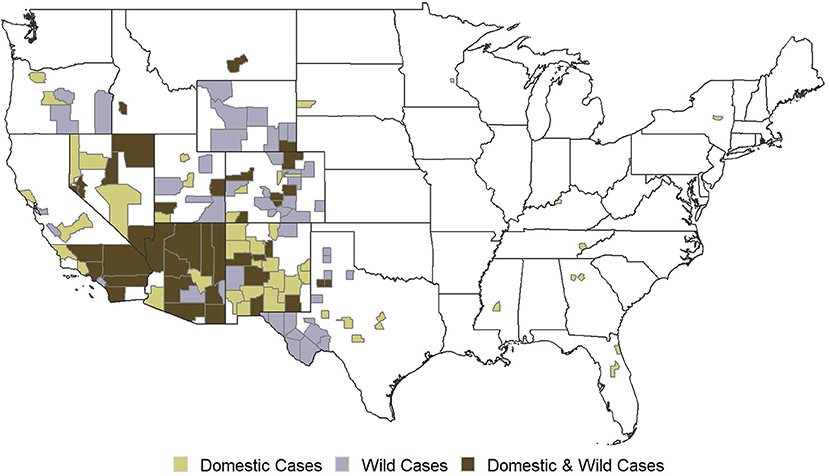
Figure 1. Occurrence of RHDV2 in the United States as of February 2022. The first case of RHDV2 detection in domestic rabbits in the eastern United States occurred in Lake County, Florida on December 30, 2020. Data source: USDA Animal and Plant Health Inspection Service (APHIS).
RHDV2 also poses a threat to the domestic rabbit industry in the U.S. (rabbit breeders and exhibitors, rabbit rescues, meat and fur producers), as RHDV2 is an important cause of disease in pet rabbits (Marschang et al., 2018). The risk that RHDV2 will continue to spread rapidly across the U.S. is exacerbated by the size and decentralized nature of the domestic rabbit trade, and deliberate and accidental releases of domestic rabbits. In 2017, almost 500,000 rabbits were sold nationally for commercial use (U. S. Department of Agriculture [USDA], 2019), and rabbits are the third most common household pet in the U.S. (House Rabbit Society, 2014; American Veterinary Medical Association, 2018; >2 million pet rabbits nationally).
Both agricultural and wildlife agencies in the U.S. have a mandate to mitigate RHDV2 transmission. Per the USDA Foreign Animal Disease Preparedness and Response Plan, state agricultural agencies are required to control foreign animal diseases that impact agricultural systems by implementing biosecurity measures to mitigate pathogen transmission, engaging in diagnostics and surveillance to detect pathogens, and educating key stakeholders and the public about pathogen transmission risks (Pepin et al., 2014). Under the Public Trust Doctrine, North American Model of Wildlife Conservation, and Endangered Species Act (ESA) of 1973, state and federal wildlife agencies are expected to protect, conserve, and restore wildlife populations by mitigating disease risks to wildlife (Siemer et al., 2012).
Given existing mandates for agricultural and wildlife agencies to prevent or control RHDV2 transmission, we surveyed agency staff to ascertain what progress agencies have made toward a coordinated RHDV2 response in the U.S. We focused on agencies' management of lagomorphs prior to the 2020 RHDV2 outbreak and their current management responses to RHDV2. We investigated inter- and intra-state jurisdictional constraints to collaborative RHDV2 management, as well as other limitations to agencies' capacity for RHDV2 control (e.g., funding, program management). Our study is novel in two regards: (1) to the best of our knowledge, it is the first national study pertaining to both agricultural and wildlife agency efforts to manage a pathogen at the wildlife-domestic animal interface; and (2) it is the first study of how agencies are responding to RHDV2 in the U.S.
Methods
We initially administered an online survey to animal health contacts in state wildlife and agricultural agencies for all 50 U.S. states from February to August 2021. We invited 100 agency staff (one wildlife and one agricultural agency representative for each state) to participate in this research. We elicited information about agencies' (1) jurisdiction over different lagomorph populations or industries, (2) RHDV2 management at the state level, (3) collaborations with other agencies to cooperatively mitigate RHDV2 transmission, and (4) communication with stakeholders about RHDV2 (Appendix S1). We invited survey respondents to participate in follow-up, in-depth semi-structured interviews to obtain more detailed qualitative insights on agencies' knowledge of lagomorph populations and industries, lagomorph management, responses to RHDV2, and engagement with the public and key stakeholders (Appendix S2). We conducted these interviews from March to August 2021. Interviews ranged from 20 to 96 min in duration.
The two lead authors used open coding to analyze transcripts of the semi-structured interviews (Strauss and Corbin, 1994). Each author identified multiple codes to capture as many topics as possible, reviewed and refined the codes (Berg, 2001), grouped codes that shared a commonality into categories (Graneheim and Lundman, 2004), and assessed the underlying meanings of these categories to identify overall themes raised by research participants. Both authors analyzed the data independently to ensure consistency and validity of findings.
We pretested the online and semi-structured questionnaires with four state agency representatives and four subject experts (social sciences, wildlife disease, wildlife ecology) prior to implementation. The University of Georgia Institutional Review Board reviewed all research materials and protocols and characterized our study as non-human subject research because we elicited no identifiable or sensitive private information from research participants.
Results
We received 95 completed online quantitative surveys from agricultural and wildlife agencies (95% response rate, Figure 2). We conducted semi-structured interviews with 27 agricultural agencies (77.1% response rate; 35 agencies were invited for an interview if they responded to the survey before August 2021) and 46 wildlife agencies (95.8% response rate; Alaska and Hawaii were omitted because respondents referred us to their agricultural agency counterparts). The interviews provided detailed insights into agencies' management of RHDV2, but the online surveys provided the core information for our study. The numerical results presented below apply to information gathered from the online surveys unless otherwise specified. Four key themes emerged from the surveys and interviews, which demonstrated that agencies had mutually exclusive, often incomplete jurisdiction over lagomorphs and insufficient resources to engage in a comprehensive, integrated approach to RHDV2 mitigation.
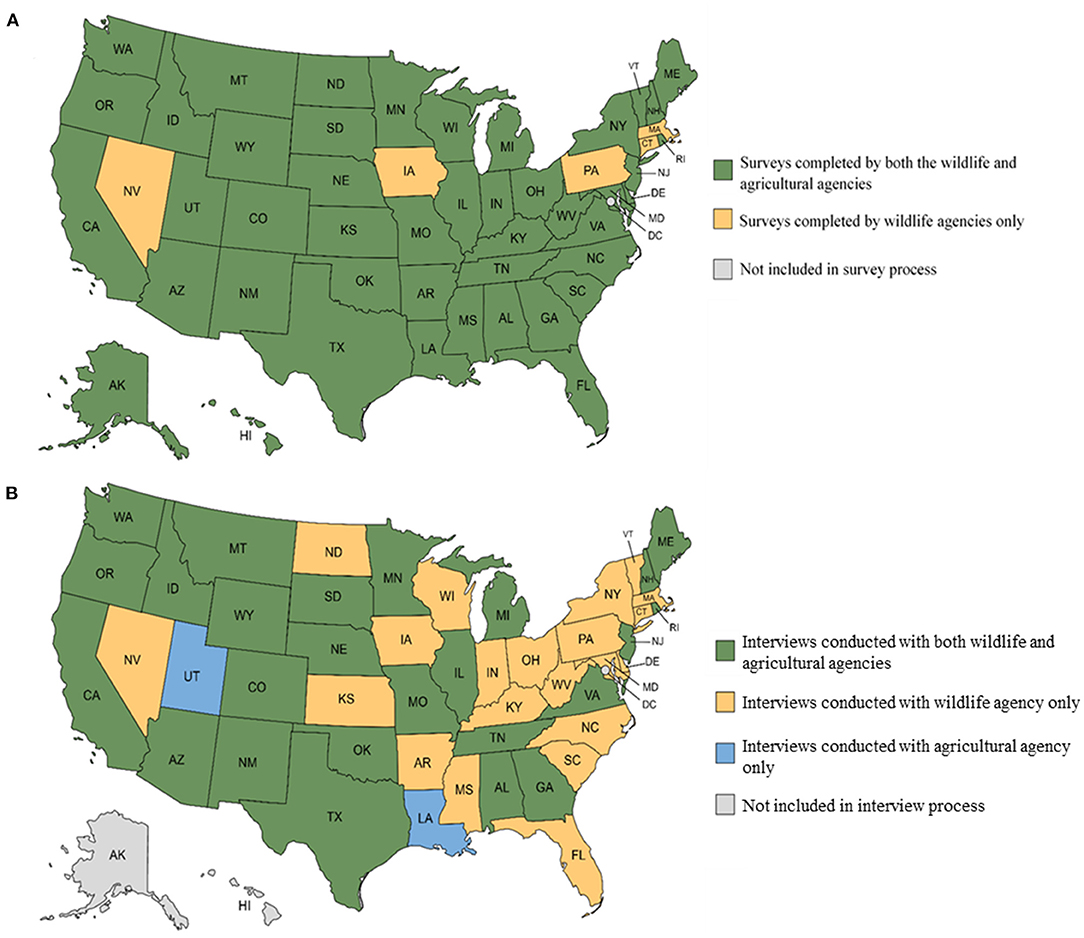
Figure 2. Distribution of completed (A) online surveys and (B) semi-structured interviews of wildlife and agricultural agencies across the United States, 2021. Created with mapchart.net.
Theme 1: Divides, Gaps, and Inconsistencies in Agency Classification and Management of Lagomorphs Preclude a Comprehensive Approach to RHDV2 Mitigation
Management of Wild Lagomorphs
All 50 state wildlife agencies had regulatory authority over wild lagomorph populations, 47 agencies (94% of wildlife agencies) had regulatory authority over wildlife rehabilitation facilities, and 22 agencies (44% of wildlife agencies) had regulatory authority over rabbit hunting preserves. Wildlife rehabilitation facilities treat and care for injured, sick, and orphaned wildlife, with the objective of releasing wildlife once they have recovered from disease or injury (Duncan et al., 2008). Hunting preserves are enclosed areas where people release domestic or wild rabbits to train hunting dogs.
Wildlife agencies focused their management efforts on protected species (n = 30; 60% of wildlife agencies) such as the riparian brush rabbit (Sylvilagus bachmani riparius) and game species (e.g., eastern cottontail Sylvilagus floridanus; snowshoe hare Lepus americanus). Wild lagomorph species that are classified as unprotected, pests, or predatory were not protected, managed, or monitored by wildlife agencies. Wild lagomorph management before the RHDV2 outbreak primarily encompassed hunting regulations, general habitat management that benefited lagomorphs, and enforcement of rules for specialty stakeholder groups (e.g., wildlife rehabilitators, rabbit preserve operators). Forty-nine states allowed lagomorph hunting, but hunting regulations (defined vs. year-round hunting) varied considerably across states depending on how lagomorphs were classified. States with protected lagomorph species or subspecies often engaged in actions specific to the species of concern, including targeted habitat management, recording occupancy and population trends, and establishing captive populations. Wildlife agencies issued specialty permits (e.g., the trap and release of wild lagomorphs), provided guidance, and enforced rules that apply to wildlife rehabilitators, rabbit preserve operators, and other stakeholders who interact with wild lagomorphs (e.g., falconers, researchers). Wildlife rehabilitation facilities altered rehabilitation practices in response to agency guidance on RHDV2.
Wildlife agencies often conduct hunter harvest or small game hunter surveys to estimate lagomorph harvests, and during interviews, 14 agency staff specifically mentioned lagomorph monitoring efforts (e.g., rural mail carrier surveys, roadside surveys). However, despite these efforts, 24 wildlife agency personnel stated during interviews that they were unsure of wild lagomorph population trends unless species were protected, e.g., “Currently [population estimates are] all anecdotal. We don't do any population modeling of eastern cottontails. I'd imagine that they're stable or increasing.”
Management of Domestic Rabbits
Twenty-nine agricultural agencies (64.4% of the 45 agricultural agencies that completed the survey) had regulatory authority over pet rabbits and 26 agencies had regulatory authority over rabbit breeders (57.8% of surveyed agricultural agencies). However, fewer agricultural agencies had jurisdiction over the rabbit meat industry (n = 24; 53.3% of surveyed agricultural agencies), rabbit rescue groups (n = 19; 42.7%), or laboratories that use rabbits in research (n = 10; 22.2%). Rabbit rescue groups take in abandoned rabbits, neuter these animals before adopting them out, and educate the public about owning domestic rabbits.
Agricultural agencies' jurisdiction over, and management of, domestic rabbits was largely dictated by how rabbits were classified. Thirty-three states (73.3% of states for which we surveyed agricultural agencies) classified domestic rabbits as companion animals, whereas only 20 states (44.4% of states for which we surveyed agricultural agencies) classified domestic rabbits as livestock. Respondents noted that companion animals are not actively regulated or managed because agricultural agencies focus effort and resources on traditional livestock industries (e.g., cattle). In follow-up interviews, 10 individuals clarified that, despite rabbits being classified as livestock in their state, rabbits were not included in traditional livestock regulations or were exempt from laws requiring livestock operations to register the location and size of their operation with the state agricultural agency (i.e., rabbits were not included in larger efforts to trace disease and contact between infected animals in the livestock supply chain). Nonetheless, agricultural agencies were expected to respond to RHDV2 because it is classified as a foreign animal disease and is reportable to the OIE. As noted by one interviewee, “[Rabbits] aren't a species that is considered livestock. We don't regulate them, but we're tied into [RHDV2 management] because we do foreign animal disease investigations.” The inconsistent classifications of rabbits and exemptions from certain agricultural rules likely impacted agency knowledge of the domestic rabbit industry, as during interviews, 54 individuals (both agricultural and wildlife agencies) noted that their agency had little to no knowledge of domestic rabbit operations or the number of domestic rabbits in their state. No agency knew the locations or scale of all domestic rabbit operations.
Agricultural agencies also had little to no interaction with the rabbit industry prior to the RHDV2 outbreak. Some agencies implemented animal health and welfare, and/or animal importation regulations that encompassed rabbit industries, depending on how the state classified domestic rabbits. Thirty-three states (73.3% of states for which we surveyed agricultural agencies) had a certificate of veterinary inspection (CVI) requirement for all domestic animals or livestock being transported into the state, but during interviews, 10 individuals noted that few people submit CVIs for their rabbits. CVIs are health certificates issued by federal, state, tribal or accredited veterinarians that certify that animals listed on the certificate have been inspected and were found to satisfy the regulations pertaining to their intended movement. One interviewee stated “I will say that our enforcement [of CVIs] is difficult and our compliance is pretty poor with rabbits. They've never really been a target species.”
Management of Feral Domestic Rabbits
Feral domestic rabbits were typically overlooked by both agricultural and wildlife agency regulations, which is concerning because there were several RHDV2 outbreaks in domestic and feral rabbits after the virus was detected in Ohio in 2018 and prior to detection of RHDV2 in wild lagomorphs in New Mexico in 2020 (Williams et al., 2021). During interviews, 10 individuals claimed that their agency has jurisdiction over feral rabbit populations and 23 were unsure which (if any) agency had jurisdiction, with one interviewee stating, “So we're under the impression that feral rabbits are under the jurisdiction of [the department of] agriculture. But when we had the [RHDV2] outbreak they weren't very interested in doing much about it, so we ended up kind of addressing the issue.” Despite lack of clear authority, during interviews, eight individuals stated that their agencies would work together to manage feral rabbit populations to mitigate RHDV2 transmission.
RHDV2 Response
Agency response to RHDV2 varied greatly between states, but most management focused on responding to reported potential RHDV2 cases (Theme 2), outreach (Theme 3), and vaccinations. At the time of this study (Feb-Aug 2021), the USDA only allowed state veterinarians in states with confirmed RHDV2 cases to import vaccines from Europe. Fourteen state veterinarians had approved the import and use of the vaccine by private veterinarians for treating domestic rabbits. Additionally, four wildlife agency staff stated that their agency or working group was granted permission to import the vaccine for treatment of their endangered or threatened lagomorph populations. In September 2021, a RHDV2 vaccine produced in the U.S. received emergency use authorization from the USDA (Medgene Labs, 2021).
During interviews, 17 individuals from agricultural and wildlife agencies discussed efforts to prevent the anthropogenic spread of RHDV2 through emergency or temporary regulatory and permit changes to restrict the movement of live and dead lagomorphs. Agricultural agencies had shortened the issuance time for rabbit CVIs from 30 days to 72 h, canceled rabbit shows, or prohibited the movement of lagomorphs and associated products from areas with confirmed RHDV2 cases. Wildlife agencies had prevented the take of lagomorphs for research or prohibited the import of live or dead wild lagomorphs from areas with confirmed RHDV2 cases. A subset of interviewees mentioned the development of a risk assessment or response plan (n = 9) and increased intra-agency awareness and biosecurity (n = 5; e.g., new protocols for people who own rabbits and work with threatened/endangered lagomorphs).
During interviews, 34 agricultural and wildlife agency staff (six agencies from states with RHDV2 cases at the time of the interview) stated that their agency's management of lagomorphs or regulatory response had not changed since detection and spread of RHDV2, with one individual stating “RHDV has run its course completely unmanaged in [this state].” Interviewees noted that limited management was driven by low prioritization of lagomorphs and RHDV2 based on the location of the disease, greater emphasis on other animal or wildlife health issues (notably, chronic wasting disease), or lack of public interest in or awareness of RHDV2. For example, an individual from a wildlife agency stated “[There have been no management interventions] because we haven't had [RHDV2] yet. I think once we get the sledgehammer to the head with detection, we would maybe do something... Even after the first case of RHDV2, I don't think there would be much change.” During interviews, lack of agency jurisdiction over lagomorphs (n = 20), limited resources or staff (n = 23), and a lack of effective management options at the agency's disposal (n = 16) were cited as key reasons for not implementing measures to mitigate RHDV2 spread. Seventeen interviewees from both agricultural and wildlife agencies added that they needed more support from federal agencies, in particular from the USDA, to better manage RHDV2. These interviewees suggested that states would be better positioned to mitigate the spread of RHDV2 if the USDA clearly defined rabbits as livestock, included rabbits on the list of domestic animals that are tracked when they are moved across state borders, and limited inter-state movement of domestic rabbits. Interviewees also wanted relevant federal agencies to provide clear guidelines on how state agencies should contain or prevent the spread of RHDV2, and to help coordinate an inter-agency response to RHDV2.
Theme 2: Agencies Have Primarily Relied on Stakeholder Reports of Lagomorph Mortalities to Monitor for RHDV2
Agencies relied on opportunistic, passive disease surveillance in the form of public and stakeholder reports of lagomorph mortalities to detect the presence and spread of RHDV2. Most commonly, interviewees noted that they received reports of a suspicious lagomorph death via phone calls to the agencies' customer service number, a local office, or an animal/wildlife disease hotline (n = 50). After receiving a mortality report, an agency staff member typically contacted the reporting party for more information to determine whether the agency would conduct a disease investigation. During interviews, 26 individuals stated that their agency would investigate any suspicious rabbit death, while 19 others stated that their agencies would only investigate if several rabbits were found dead on the landscape or if multiple criteria for investigation were met (e.g., dead rabbit on the landscape in an unaffected county). Interviewees from agricultural agencies stated they would quarantine a property (n = 18) or issue verbal hold orders (n = 3) until the cause of a rabbit mortality was determined. The quarantine orders would remain in effect if the rabbit died from RHDV2, and the state's wildlife agency would often conduct targeted surveillance around the property to determine if RHDV2 had spread to wild lagomorphs.
Wildlife agencies relied on the public, hunters, falconers, and rehabilitation centers to report mortalities because they do not have the resources to prioritize active RHDV2 monitoring. For example, “We're really relying on the public [to report rabbit deaths]... We've talked a little bit about surveying for mortalities but [dead rabbits] just don't last long on the landscape, and so trying to survey for mortalities is an exercise in futility, [especially trying to find a carcass] that would be fresh enough to get a PCR [polymerase chain reaction] positive result… The biggest [obstacle to RHDV2 control] is [lack of] surveillance … and we don't have the manpower to say let's go do rabbit surveys every month.”
Theme 3: Agencies Have Relied on Press Releases and Posting Information on Their Websites to Disseminate Information About RHDV2 to the Public and Their Stakeholders
Both agricultural and wildlife agencies relied on outreach to inform stakeholders about RHDV2 and recommended biosecurity measures (e.g., handling and disposal of carcasses, maintaining barriers between domestic and wild rabbits) for limiting the anthropogenic spread of the disease. Most agricultural and wildlife agency staff (n = 84; 88.4% of all surveyed agencies) stated that they had disseminated information since the advent of RHDV2, with most wildlife agencies targeting hunters, falconers, and wildlife rehabilitation centers and agricultural agencies primarily targeting rabbit owners, rabbit breeders, and state extension specialists (Figure 3). Most commonly, respondents (n = 51; 53.7% of all surveyed agencies) indicated that their agency had communicated with stakeholders two to five times since March 2020 (when RHDV2 was detected in the southwestern U.S.), primarily using the agency website, press releases, and email (Figure 4). Sixteen agencies had only used press releases to disseminate information about RHDV2 at the time of their interview, often only once in early-to-mid 2020. During interviews, agency staff recognized the limitations of this strategy, with one stating “The news packet…it's unidirectional. We are sending out the information and we don't know what the public does with it.”
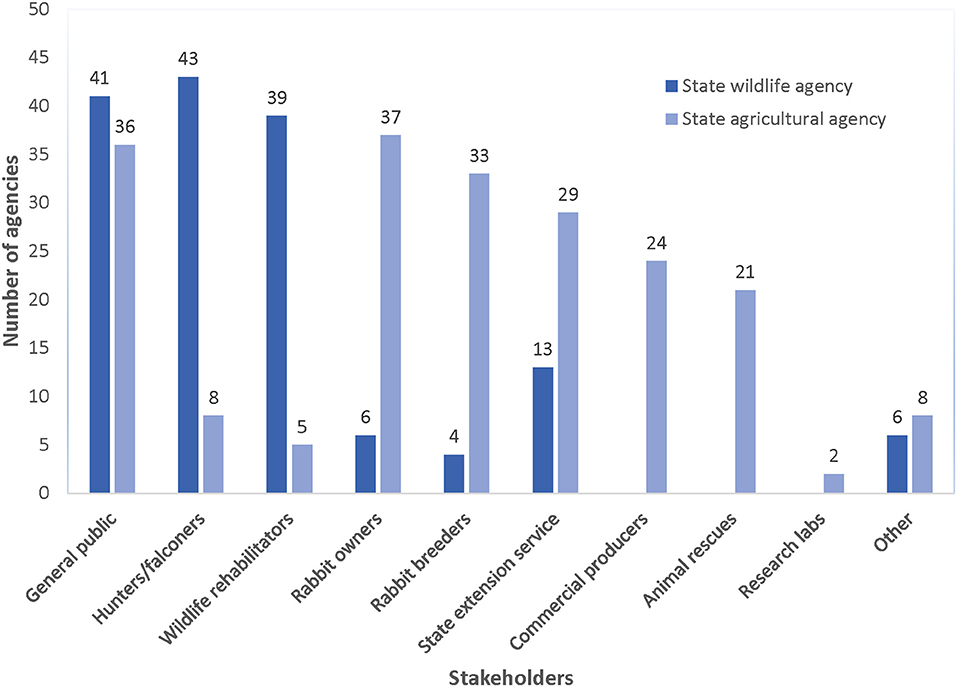
Figure 3. Number of state agricultural (n = 45) and wildlife agencies (n = 50) that engage in education and outreach efforts targeted toward different stakeholder groups, 2021.
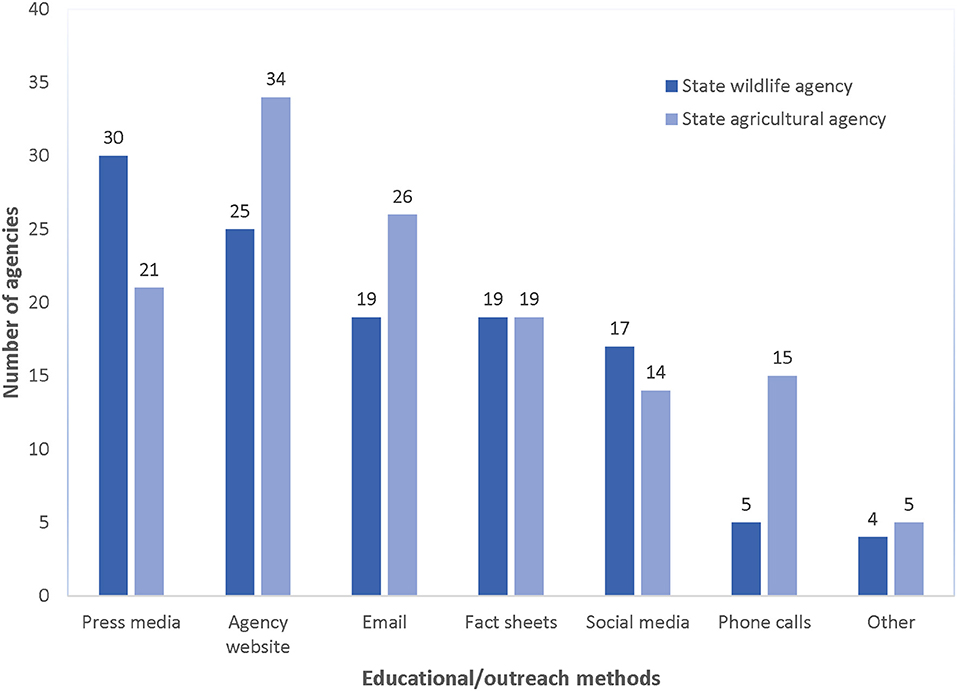
Figure 4. Education strategies implemented by state agricultural (n = 45) and wildlife agencies (n = 50), 2021.
Agencies used email listservs to inform stakeholders about recommended biosecurity measures. However, most agencies did not have contact information for rabbit owners or breeders. Thirteen individuals had attempted to communicate with rabbit owners and breeders by reaching out to organized breeder groups, joining rabbit breeder social media groups, or creating a list of rabbit breeders through online searches. Ten agencies in states without RHDV2 (eight wildlife and two agricultural agencies) had not disseminated any information about RHDV2 at the time of completing the online survey. During interviews, individuals stated that rabbit mortality reports to their agency increased following outreach efforts, albeit temporarily in states without RHDV2. Interviewees further noted that, despite outreach efforts, the non-rabbit owning public were likely still unaware of RHDV2.
Theme 4: Collaborative Agency Interactions Mainly Encompass Working Groups
Less than half of wildlife (n = 21; 42% of wildlife agencies) and agricultural agencies (n = 17; 37.8% of surveyed agricultural agencies) had an in-house working group or team addressing RHDV2 prevention or management. Wildlife (n = 25; 50%) and agricultural agency staff (n = 22; 48.9%) were more likely to be part of an interagency working group or team, commonly composed of state and federal agency staff (e.g., USDA). Agency staff participated in these working groups to gather information about RHDV2, typically sourced from situation reports provided by the USDA. Agricultural agency staff also received information from the National Assembly of Animal Health Officials (n = 20; 44.4%) and wildlife agency staff received information from regional Association of Fish and Wildlife Agencies groups (n = 16; 32%). Thirty interviewees discussed how both wildlife and agricultural agencies collaborated within state to ensure RHDV2 reports and information about potential RHDV2 cases were directed to the appropriate agency staff. Thirty-four agencies also worked together to create outreach materials, send out joint press releases, or ensure that both agencies' contact information was included on outreach materials.
Discussion
RHDV2 is a classic example of a highly contagious pathogen transmitted at the wildlife-domestic animal interface that threatens biodiversity (Asin et al., 2021), imperiled and native species (Monterroso et al., 2016), ecosystem structure and function (Guerrero-Casado et al., 2013; Delibes-Mateos et al., 2014), and culturally and economically important industries (hunting, the live rabbit trade, agricultural production) across multiple regions of the world (Rouco et al., 2019). Internationally and in the U.S., RHDV2 control requires collaborative management interventions by agricultural and wildlife agencies to mitigate pathogen spillover and dissemination (Carmichael, 2012; Miller et al., 2013). We found that state agricultural and wildlife agencies in the U.S. have worked together within and across states to investigate potential RHDV2 cases, create interagency RHDV2 working groups, and participate in joint outreach efforts. These are important first steps to enabling inter-agency coordination, which clarifies management authority, legitimizes agency management actions, and facilitates more efficient use of agency funds for RHDV2 detection and response (Siemer et al., 2012). Current management efforts are consistent with international and national recommendations on initial steps toward cross-jurisdictional, defensible, and reliable surveillance systems and biosecurity measures to prevent pathogen transmission at the wildlife-domestic animal interface (see Ryser-Degiorgis, 2013; Portier et al., 2019; Stephen et al., 2019). However, in common with other pathogens and contexts, this study identified how U.S. agencies have been hampered by restrictive jurisdictional and regulatory frameworks, insufficient resources and funding, and incomplete information about pathogen transmission pathways and disease impacts (Siemer et al., 2012; Ryser-Degiorgis, 2013; Portier et al., 2019; Stephen et al., 2019). For example, base funding for wildlife agencies depends on sales of hunting and fishing licenses and federal Pittman-Robertson funds, with some additional grant funding for efforts to control pathogens of political concern (most frequently, chronic wasting disease; Siemer et al., 2012). Greater flexibility in agency funding and increased allocation of discretionary funds to agencies for management of multiple diseases would allow agencies to respond more rapidly and effectively to emerging pathogens (Siemer et al., 2012).
One of the greatest obstacles to establishing regional or national strategies for managing RHDV2 in the U.S. is agencies' inconsistent jurisdiction over wild and domestic lagomorphs, owing to different classifications of lagomorphs across states (e.g., game vs. non-game species, companion animals vs. livestock), and unclear jurisdiction over feral rabbits. The USDA Foreign Animal Disease Preparedness and Response Plan, the Public Trust Doctrine, and the ESA require agencies to control foreign animal diseases by implementing pathogen surveillance and biosecurity measures and educating the public about pathogen transmission risks (Siemer et al., 2012; Pepin et al., 2014). However, despite the fact that RHDV2 is a foreign animal pathogen, the USDA has not provided clear leadership on RHDV2 control. To date, the USDA has focused on diagnostics and has engaged in some outreach. But both agricultural and wildlife agency respondents suggested that the USDA has not provided critical leadership that is needed to catalyze cross-jurisdictional RHDV2 response because the virus affects lagomorphs rather than cattle, swine, or poultry.
In the absence of clear federal leadership, state agencies have relied on state-level classification of lagomorphs and directives from their internal leadership to determine what resources and management actions to apply to RHDV2 control (Carmichael, 2012; Siemer et al., 2012). Accordingly, state wildlife agencies have largely focused their terrestrial disease management efforts on chronic wasting disease and white nose syndrome (Siemer et al., 2012). State agricultural agencies have not prioritized RHDV2 mitigation because they place higher priority on other domestic animals and livestock. Federal agencies (e.g., the USDA) or national organizations representing state agencies (e.g., the Association of Fish and Wildlife Agencies) should develop best management practices for state agencies to control RHDV2.
Ideally, adaptive management would be used to manage RHDV2, whereby agencies engage in risk assessment, surveillance (investigating wild and domestic lagomorph mortalities to rule out RHDV2), monitoring (assessment of infected lagomorph populations to detect spatial and temporal trends in infection and mortality), and pathogen control and management (Miller et al., 2013; Gortazar et al., 2015). However, currently, both agricultural and wildlife agency staff are fettered in their efforts to control RHDV2 because they have incomplete information on the disease status of lagomorphs (e.g., limited knowledge of the size of the domestic rabbit industry, biological and logistical constraints in conducting pathogen surveillance in wild lagomorph populations). Owing to funding constraints and the sheer volume of species they are required to manage, wildlife agencies have limited data on the spatial distribution, movement, population structure, and population density of wild lagomorphs, which is important for determining population level disease risks (Siemer et al., 2012; Miller et al., 2013). Agricultural agency staff are also constrained in efforts to control RHDV2 by lack of information on the size and composition of the domestic rabbit trade and the nature and frequency of contact between lagomorphs that could result in RHDV2 transmission (e.g., omission of rabbits from disease tracing in the livestock supply chain, limited enforcement of CVIs, lack of information about the movement of domestic rabbits and rabbit-related products within and across states; see also Langwig et al., 2015). This is concerning because agencies' disease preparedness and the effectiveness of their response plans depends on quantification of spatio-temporal pathogen prevalence in wildlife and domestic animal populations, knowledge of how the structure of different agricultural operations and rabbit trade organizations impact pathogen transmission within domestic animals, and understanding of the mechanisms and rates of transmission between different agricultural and domestic rabbit operations (Pepin et al., 2014). The role of the rabbit trade in long-distance virus transmission is evidenced by the fact that most recently, RHDV2 was confirmed in domestic rabbits in New York (New York State Department of Agriculture Markets, 2021) and Tennessee (Tennessee Department of Agriculture, 2022), well outside the range of infected wild lagomorphs. Improved understanding of the domestic lagomorph trade and transport routes is urgently needed to mitigate the risks associated with human-mediated movement of rabbits and RHDV2 across the U.S.
Both agricultural and wildlife agencies have worked to improve risk mitigation and control of RHDV2 by recommending biosecurity measures to stakeholders and the public (e.g., disinfection procedures, secure barriers between domestic and wild lagomorphs), using emergency regulations to temporarily restrict the movement of live and dead lagomorphs across state lines, reducing the issuance time for CVIs, facilitating vaccination of lagomorphs, and creating response plans. These measures are appropriate for mitigating RHDV2 transmission. However, funding, staffing, and technological limitations (e.g., the absence of an oral vaccination that would allow wildlife agencies to control RHDV2 in lagomorph populations over large geographical areas; Gortazar et al., 2015), combined with jurisdictional differences across states and agencies, complicate the process of these strategies being implemented regionally or nationally.
Resource and technological constraints have limited the development of monitoring and surveillance systems for early detection of RHDV2 in wildlife populations. At the time of this study, RHDV2 surveillance and monitoring efforts largely relied upon passive approaches involving investigations of lagomorph mortality events and agencies had limited knowledge of wild lagomorph populations. Opportunities for active surveillance of live animals is limited because of the difficulty of antemortem sampling in wild lagomorphs, apparent high case fatality rates limiting some survey approaches, and lack of validated commercial diagnostic assays (e.g., antibody test kits; Strive et al., 2020). Although public, hunter, and rehabilitation facility reports of suspected RHDV2 mortalities are a useful form of passive surveillance (Langwig et al., 2015; Lawson et al., 2015), overreliance on these reports may not lead to early pathogen detection in non-game species (Miller et al., 2013) and may decrease detection probability in infected lagomorph populations (Duncan et al., 2008; Belsare et al., 2020). Passive surveillance for RHDV2 is further complicated by the fact that wild lagomorphs are difficult to find in underbrush habitat, lagomorphs may die in their burrows, and predators and scavengers quickly remove carcasses from the landscape (Artois et al., 2009). It is therefore encouraging that there are efforts to develop tools for rapid diagnosis of the disease in the field and detection of the virus in urine, feces, and respiratory secretions (Fresco-Taboada et al., 2022). These efforts should be enhanced by surveys of hunters, recreationists, and landowners to ascertain whether the health and populations of wild lagomorphs have changed, a participatory approach that has been applied successfully to sarcoptic mange and alopecia surveillance in Europe (Ryser-Degiorgis, 2013).
Although we have noted limitations in current efforts to control RHDV2 across both agricultural and wildlife agencies, we recognize that RHDV2 control also depends critically on voluntary behavior changes by diverse stakeholders (e.g., hunters, falconers, pet owners, rabbit breeders, rabbit rescues, individuals who transport rabbits). Agencies play an important role in encouraging voluntary behavior change through well-designed outreach strategies (Ryser-Degiorgis, 2013; Gortazar et al., 2015). Current RHDV2 educational and outreach materials often adhere to the knowledge deficit model, which assumes that increased knowledge of RHDV2 and biosecurity measures will result in behavior changes by key stakeholders. However, this approach is ineffective for some audiences because it does not account for socio-psychological determinants of people's behavior (e.g., attitudes, beliefs, emotions; Abrahamse and Matthies, 2018; Nabi et al., 2018). Agencies should develop risk communication materials that appeal to diverse stakeholder groups with narrow goals and objectives. One communication strategy worth exploring is providing tailored information that is designed to engage specific stakeholders based on characteristics unique to those groups (e.g., concern about the continued economic viability of the domestic rabbit industry) or social norms (e.g., group behaviors to maintain game populations; Abrahamse and Matthies, 2018). Improved communication between agencies and key stakeholders may enhance participatory RHDV2 surveillance and control, and catalyze political will for disease management, especially if agencies provide regular feedback to stakeholders and partners (Ryser-Degiorgis, 2013).
Data Availability Statement
The deidentified quantitative data collected through the implementation of the online survey will be made available by the corresponding author, upon reasonable request. Participants in the semi-structured interviews did not agree for transcripts of their interviews to be shared publicly, so supporting qualitative data is not available.
Ethics Statement
The studies involving human participants were reviewed and approved by University of Georgia Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Funding was acquired by EP and MK. HS and EP conceived and designed the survey, interview instruments, analyzed and interpreted the data, and were responsible for writing the manuscript. HS conducted all data collection. EP supervised the project and was responsible for project administration. All authors provided feedback and helped shape the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Association of Fish and Wildlife Agencies (AFWA) in the U.S. Department of the Interior under the Multistate Conservation Grant Program (award number: F21AS00004).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Dr. Gino D'Angelo, Dr. Mark Ruder, and Dr. Dave Stallknecht for helpful guidance and comments during this research.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2022.857678/full#supplementary-material
References
Abrahamse, W., and Matthies, E. (2018). “Informational strategies to promote pro-environmental behaviour: changing knowledge, awareness, and attitudes,” in Environmental Psychology: An Introduction, eds L. Steg and J. I. M. de Groot (Chichester: John Wiley and Sons), 261–272. doi: 10.1002/9781119241072.ch26
American Veterinary Medical Association (2018). AVMA - Pet Ownership and Demographic 2018. Available online at: https://ebusiness.avma.org/ProductCatalog/product.aspx?ID=1529 (accessed November 15, 2021).
Artois, M., Bengis, R., Delahay, R. J., Duchêne, M. J., Duff, J. P., Ferroglio, E., et al. (2009). “Wildlife disease surveillance and monitoring,” in Management of Disease in Wild Mammals, eds. R. J. Delahay, G. C. Smith, and M. R. Hutchings (Tokyo: Springer), 187–213. doi: 10.1007/978-4-431-77134-0_10
Asin, J., Nyaoke, A. C., Moore, J. D., Gonzalez-Astudillo, V., Clifford, D. L., Lantz, E. L., et al. (2021). Outbreak of rabbit hemorrhagic disease virus 2 in the southwestern United States: first detections in southern California. J. Vet. Diagn. 33, 728–731. doi: 10.1177/10406387211006353
Belsare, A. V., Gompper, M. E., Keller, B., Sumners, J., Hansen, L., and Millspaugh, J. J. (2020). An agent-based framework for improving wildlife disease surveillance: a case study of chronic wasting disease in Missouri white-tailed deer. Ecol. Modell. 417, 108919. doi: 10.1016/j.ecolmodel.2019.108919
Berg, B. L. (2001). Qualitative Research Methods for the Social Sciences (4th edition). Boston, MA: Pearson.
Carmichael, C. (2012). Coordinating an effective response to wildlife diseases. Wildl. Soc. Bull. 36, 204–206. doi: 10.1002/wsb.123
Delibes-Mateos, M., Ferreira, C., Carro, F., Escudero, M. A., and Gortázar, C. (2014). Ecosystem effects of variant rabbit hemorrhagic disease virus, Iberian Peninsula. Emerging Infect. Dis. 20, 2166–2168. doi: 10.3201/eid2012.140517
Duncan, C., Backus, L., Lynn, T., Powers, B., and Salman, M. (2008). Passive, opportunistic wildlife disease surveillance in the Rocky Mountain Region, USA. Transbound. Emerg. Dis. 55, 308–314. doi: 10.1111/j.1865-1682.2008.01039.x
Fresco-Taboada, A., Montón, M., Tapia, I., Soria, E., Bárcena, J., Guillou-Cloarec, C., et al. (2022). Development and evaluation of a duplex lateral flow assay for the detection and differentiation between rabbit haemorrhagic disease virus lagovirus europaeus/GI. 1 and/GI. 2. Biology 11, 401. doi: 10.3390/biology11030401
Gortazar, C., Diez-Delgado, I., Barasona, J. A., Vicente, J., De La Fuente, J., and Boadella, M. (2015). The wild side of disease control at the wildlife-livestock-human interface: a review. Front. Vet. Sci. 1, 27. doi: 10.3389/fvets.2014.00027
Graneheim, U. H., and Lundman, B. (2004). Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ. Today 24, 105–112. doi: 10.1016/j.nedt.2003.10.001
Guerrero-Casado, J., Carpio, A. J., Ruiz-Aizpurua, L., and Tortosa, F. S. (2013). Restocking a keystone species in a biodiversity hotspot: recovering the European rabbit on a landscape scale. J. Nat. Conserv. 21, 444–448. doi: 10.1016/j.jnc.2013.07.006
House Rabbit Society (2014). How Many Pet Rabbits Are There in the U.S.? Available online at: https://rabbit.org/how-many-pet-rabbits-are-there-in-the-u-s/ (accessed November 10, 2021)
Jerolmack, C. (2013). Who's worried about turkeys? How ‘organisational silos' impede zoonotic disease surveillance. Sociol. Health Illn. 35, 200–212. doi: 10.1111/j.1467-9566.2012.01501.x
Kao, S. Y. Z., VanderWaal, K., Enns, E. A., Craft, M. E., Alvarez, J., Picasso, C., et al. (2018). Modeling cost-effectiveness of risk-based bovine tuberculosis surveillance in Minnesota. Prev. Vet. Med. 159, 1–11. doi: 10.1016/j.prevetmed.2018.08.011
Katayama, A., Miyazaki, A., Okazaki, N., Nakayama, T., and Mikami, O. (2021). An outbreak of rabbit hemorrhagic disease (RHD) caused by Lagovirus europaeus GI. 2/rabbit hemorrhagic disease virus 2 (RHDV2) in Ehime, Japan. J. Vet. Med. Sci. 83, 931–934. doi: 10.1292/jvms.21-0128
Langwig, K. E., Voyles, J., Wilber, M. Q., Frick, W. F., Murray, K. A., Bolker, B. M., et al. (2015). Context-dependent conservation responses to emerging wildlife diseases. Front. Ecol. Environ.13, 195–202. doi: 10.1890/140241
Lawson, B., Petrovan, S. O., and Cunningham, A. A. (2015). Citizen science and wildlife disease surveillance. Ecohealth 12, 693–702. doi: 10.1007/s10393-015-1054-z
Le Gall-Reculé, G., Lavazza, A., Marchandeau, S., Bertagnoli, S., Zwingelstein, F., Cavadini, P., et al. (2013). Emergence of a new lagovirus related to rabbit haemorrhagic disease virus. Vet. Res. 44, 81. doi: 10.1186/1297-9716-44-81
Marschang, R. E., Weider, K., Erhard, H., Klas, E. M., and Laik-Schandelmaier, C. (2018). Rabbit hemorrhagic disease viruses detected in pet rabbits in a commercial Laboratory in Europe. J. Exot. Pet Med. 27, 27–30. doi: 10.1053/j.jepm.2017.11.003
Medgene Labs (2021). RHDV2 Vaccine. Available online at: https://medgenelabs.com/rhdv2-vaccine/ (accessed December 13, 2021)
Miller, R. S., Farnsworth, M. L., and Malmberg, J. L. (2013). Diseases at the livestock–wildlife interface: status, challenges, and opportunities in the United States. Prev. Vet. Med. 110, 119–132. doi: 10.1016/j.prevetmed.2012.11.021
Monterroso, P., Garrote, G., Serronha, A., Santos, E., Delibes-Mateos, M., Abrantes, J., et al. (2016). Disease-mediated bottom-up regulation: an emergent virus affects a keystone prey, and alters the dynamics of trophic webs. Nature 6, 36072. doi: 10.1038/srep36072
Nabi, R. L., Gustafson, A., and Jensen, R. (2018). Framing climate change: exploring the role of emotion in generating advocacy behavior. Sci. Commun. 40, 442–468. doi: 10.1177/1075547018776019
New York State Department of Agriculture and Markets (2021). State Agriculture Department Confirms Case of Rabbit Hemorrhagic Disease Virus 2 in New York. Available online at: https://agriculture.ny.gov/news/state-agriculture-department-confirms-case-rabbit-hemorrhagic-disease-virus-2-new-york (accessed January 10, 2021)
Pedersen, K., Baroch, J. A., Nolte, D. L., Gidlewski, T., and Deliberto, T. J. (2012). “The role of the National Wildlife Disease Program in wildlife disease surveillance and emergency response,” in Proceedings of the 14th Wildlife Disease Management Conference (USDA National Wildlife Research Center - Staff Publications). Available online at: https://digitalcommons.unl.edu/icwdm_usdanwrc/1176 (accessed March 31, 2022).
Pepin, K. M., Spackman, E., Brown, J. D., Pabilonia, K. L., Garber, L. P., Weaver, J. T., et al. (2014). Using quantitative disease dynamics as a tool for guiding response to avian influenza in poultry in the United States of America. Prev. Vet. Med. 113, 376–397. doi: 10.1016/j.prevetmed.2013.11.011
Portier, J., Ryser-Degiorgis, M. P., Hutchings, M. R., Monchâtre-Leroy, E., Richomme, C., Larrat, S., et al. (2019). Multi-host disease management: the why and the how to include wildlife. BMC Vet. Res. 15, 295. doi: 10.1186/s12917-019-2030-6
Ramsey, D. S., Cox, T., Strive, T., Forsyth, D. M., Stuart, I., Hall, R., et al. (2020). Emerging RHDV2 suppresses the impact of endemic and novel strains of RHDV on wild rabbit populations. J. Appl. Ecol. 57, 630–641. doi: 10.1111/1365-2664.13548
Rouco, C., Aguayo-Adán, J. A., Santoro, S., Abrantes, J., and Delibes-Mateos, M. (2019). Worldwide rapid spread of the novel rabbit haemorrhagic disease virus (GI. 2/RHDV2/b). Transbound. Emerg. Dis. 66, 1762–1764. doi: 10.1111/tbed.13189
Ryser-Degiorgis, M. P. (2013). Wildlife health investigations: needs, challenges and recommendations. BMC Vet. Res. 9, 223. doi: 10.1186/1746-6148-9-223
Siemer, W. F., Lauber, T. B., Decker, D. J., and Riley, S. J. (2012). Agency traits that build capacity to manage disease. Hum. Dimens. Wildl. 17, 376–388. doi: 10.1080/10871209.2012.709309
Stephen, C., Zimmer, P., and Lee, M. (2019). Is there a due diligence standard for wildlife disease surveillance? A Canadian case study. Can. Vet. J. 60, 841–847.
Strauss, A., and Corbin, J. (1994). “Grounded theory methodology,” in Handbook of Qualitative Research, eds. N. K. Denzin and Y. S. Lincoln (Thousand Oaks, CA: Sage), 273–285.
Strive, T., Piper, M., Huang, N., Mourant, R., Kovaliski, J., Capucci, L., et al. (2020). Retrospective serological analysis reveals presence of the emerging lagovirus RHDV2 in Australia in wild rabbits at least five months prior to its first detection. Transbound. Emerg. Dis. 67, 822–833. doi: 10.1111/tbed.13403
Tennessee Department of Agriculture (2022). Rabbit Disease Confirmed in Tennessee. Available online at: https://www.tn.gov/agriculture/news/2022/1/28/rabbit-disease-confirmed-in-tennessee.html (accessed March 31, 2022).
U. S. Department of Agriculture [USDA] (2019). 2017 Census of Agriculture. https://www.nass.usda.gov/Publications/AgCensus/2017/index.php (accessed October 22, 2021)
U. S. Department of Agriculture [USDA] (2022). 2020-22 Rabbit Hemorrhagic Disease Affected Counties. Available online at: https://www.aphis.usda.gov/aphis/maps/animal-health/rhd (accessed March 20, 2022)
Williams, L. B., Edmonds, S. E., Kerr, S. R., Broughton-Neiswanger, L. E., and Snekvik, K. R. (2021). Clinical and pathologic findings in an outbreak in rabbits of natural infection by rabbit hemorrhagic disease virus 2 in the northwestern United States. J. Vet. Diagn. 33, 732–735. doi: 10.1177/10406387211022466
World Organization for Animal Health [OIE] (2019). Rabbit Hemorrhagic Disease. Available online at: https://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/Disease_cards/RHD.pdf (accessed October 22, 2021)
World Organization for Animal Health [OIE] (2021). Terrestrial Animal Health Code. Available online at: https://www.oie.int/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access (accessed October 22, 2021)
Keywords: human dimensions of wildlife conservation, lagomorphs, rabbit hemorrhagic disease virus 2 (RHDV2), qualitative analysis, jurisdictional barriers, state wildlife agencies, state agricultural agencies
Citation: Shapiro HG, Pienaar EF and Kohl MT (2022) Barriers to Management of a Foreign Animal Disease at the Wildlife-Domestic Animal Interface: The Case of Rabbit Hemorrhagic Disease in the United States. Front. Conserv. Sci. 3:857678. doi: 10.3389/fcosc.2022.857678
Received: 18 January 2022; Accepted: 04 April 2022;
Published: 12 May 2022.
Edited by:
David J. Duffy, University of Florida, United StatesReviewed by:
Peter Stuart, Munster Technological University, IrelandAnnie Page-Karjian, Florida Atlantic University, United States
Michael Gunn, Department of Agriculture Food and the Marine, Ireland
Copyright © 2022 Shapiro, Pienaar and Kohl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elizabeth F. Pienaar, ZWxpemFiZXRoLnBpZW5hYXJAdWdhLmVkdQ==
†These authors have contributed equally to this work and share first authorship
 Hannah G. Shapiro
Hannah G. Shapiro Elizabeth F. Pienaar
Elizabeth F. Pienaar Michel T. Kohl
Michel T. Kohl