- 1Department of Arctic and Marine Biology, UiT the Arctic University of Norway, Tromsø, Norway
- 2Norwegian Polar Institute, Fram Centre, Tromsø, Norway
- 3Department of Biological Sciences, University of Alberta, Edmonton, AB, Canada
- 4Conservation Science and Wildlife Health, San Diego Zoo Wildlife Alliance, San Diego, CA, United States
Wildlife harvest remains a conservation concern for many species and assessing patterns of harvest can provide insights on sustainability and inform management. Polar bears (Ursus maritimus) are harvested over a large part of their range by local people. The species has a history of unsustainable harvest that was largely rectified by an international agreement that required science-based management. The objective of our study was to examine the temporal patterns in the number of polar bears harvested, harvest sex ratios, and harvest rates from 1970 to 2018. We analyzed data from 39,049 harvested polar bears (annual mean 797 bears) collected from 1970 to 2018. Harvest varied across populations and times that reflect varying management objectives, episodic events, and changes based on new population estimates. More males than females were harvested with an overall M:F sex ratio of 1.84. Harvest varied by jurisdiction with 68.0% of bears harvested in Canada, 18.0% in Greenland, 11.8% in the USA, and 2.2% in Norway. Harvest rate was often near the 4.5% target rate. Where data allowed harvest rate estimation, the target rate was exceeded in 11 of 13 populations with 1–5 populations per year above the target since 1978. Harvest rates at times were up to 15.9% of the estimated population size suggesting rare episodes of severe over-harvest. Harvest rate was unrelated to a proxy for ecosystem productivity (area of continental shelf within each population) but was correlated with prey diversity. In the last 5–10 years, monitored populations all had harvest rates near sustainable limits, suggesting improvements in management. Polar bear harvest management has reduced the threat it once posed to the species. However, infrequent estimates of abundance, new management objectives, and climate change have raised new concerns about the effects of harvest.
Introduction
Among mammals, habitat loss and harvest are the main conservation threats to the majority of assessed species (Schipper et al., 2008; Vié et al., 2009). Unsustainable harvest of wildlife is a significant threat to global biodiversity (Weinbaum et al., 2013; Benitez-Lopez et al., 2017) and many large mammal species have been depleted (Pistorius et al., 2011; Rocha et al., 2015). Unregulated harvest remains a significant obstacle to population recovery (Wittemyer et al., 2014). However, a sustainable harvest is possible if population size and vital rates are known and harvest is maintained at levels that do not lead to population decrease (Caughley, 1977; McCullough, 1996). It is therefore useful to monitor demographic parameters along with information on the numbers and locations of individuals harvested to assess sustainability (Law, 1979; Kokko et al., 2001). Population estimates, information on past yields, and harvest sex- and age-composition may, however, provide sufficient information for harvest management even if detailed information on demographic parameters are unavailable (e.g., Harris and Metzgar, 1987; Beston and Mace, 2012; Vajas et al., 2021). However, the information available on harvest often varies widely. For many Arctic large mammals, their large ranges, low population densities, remoteness, and use for subsistence raise challenges for management.
Arctic marine mammals have a history of unsustainable harvest with depletion occurring in walrus (Odobenus rosmarus, Bockstoce and Botkin, 1982), bowhead whales (Balaena mysticetus, Woodby and Botkin, 1993), belugas (Delphinapterus leucas, Alvarez-Flores and Heide-Jorgensen, 2004), and polar bears (Ursus maritimus, Prestrud and Stirling, 1994). Currently, 78% of the Arctic marine mammal populations are legally harvested (Laidre et al., 2015). However, Laidre et al. also found that only 35% of these populations had some assessment of trend suggesting that harvest sustainability is often unknown. Therefore, lack of adequate monitoring leaves Arctic marine mammals vulnerable to over-harvest and other stressors. Sea-ice dependent species are further challenged by sea ice declines that result in habitat loss that can reduce carrying capacity (Tynan and DeMaster, 1997; Stirling et al., 1999; Bromaghin et al., 2015; Laidre et al., 2015; Heide-Jorgensen et al., 2020). Many Arctic marine mammal populations are small (Laidre et al., 2015), and such populations in an increasingly stochastic environment present management challenges (Caughley, 1994; Lande, 1998).
The history of polar bear exploitation by humans dates back ~10,000 years (Makeyev et al., 1993), and has continued at varying intensity to modern times (Prestrud and Stirling, 1994; Regehr et al., 2017). Even though harvest was high in some regions during the first half of the 1900s (e.g., 200–900 annually in Svalbard, Norway), there was little concern about the harvest (Loughrey, 1956; Lønø, 1965). This lack of concern partly stemmed from the remoteness of the Arctic, lack of population monitoring, and limited exchange of information between polar bear jurisdictions (Prestrud and Stirling, 1994). However, during the 1950s, concerns arose that over-harvest and lack of information on population status were putting the species at risk (Lentfer, 1976; Amstrup et al., 1986). Such concerns resulted in a ban on polar bear harvest in the Soviet Union in 1956 that has largely continued to date (Prestrud and Stirling, 1994).
Polar bears are distributed over ~24 million km2 across the Arctic, from about 51°N in Canada, north to the North Pole, within 19 subpopulations (Figure 1; Polar Bear Specialist Group, 2010). The delineation of polar bear subpopulations is based on telemetry data, recapture of tagged bears, and returns of tags from bears harvested by Inuit hunters, genetics, sea ice, and geographic barriers to movement (Bethke et al., 1996; Paetkau et al., 1999; Mauritzen et al., 2002; Peacock et al., 2015). The term “subpopulation” is the term used by the International Union for Conservation of Nature (IUCN) Species Survival Commission for “geographically and otherwise distinct groups in the population between which there is little demographic or genetic exchange” (IUCN, 2012). We use the term “population” to describe the separate polar bear units used for management.
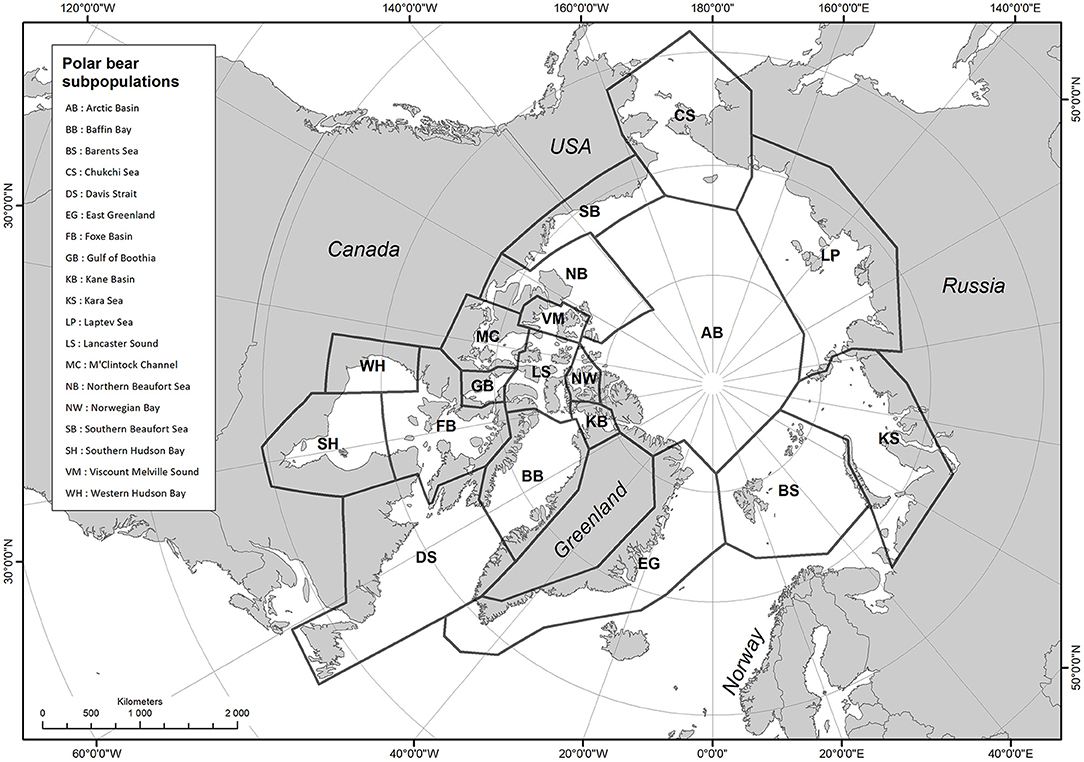
Figure 1. Polar bear populations as delineated by the IUCN/SSC Polar Bear Specialist Group (after Polar Bear Specialist Group, 2018).
Through the 1960s, 1,100–1,500 polar bears were harvested across their range annually, but without estimates of abundance or population status that could be used to determine sustainability (Prestrud and Stirling, 1994). The lack of population estimates and ongoing harvest resulted in a call for international cooperation on research and management in the early 1960s (Tovey and Scott, 1957; Scott et al., 1959; Harington, 1964). Concerns about over-harvest eventually led to the Agreement on the Conservation of Polar Bears in 1973 (Agreement) signed between the five nations within the range of polar bears (Lentfer, 1974; Larsen and Stirling, 2009). The Agreement implemented a harvest ban with exceptions for traditional subsistence harvest. Most polar bear harvest since the Agreement is by local people, although Canada has allowed a limited sport hunt (Wenzel, 2011). Use of harvested polar bears varies across jurisdictions, but they are generally used for food and commercial trade in hides (Dowsley, 2010).
After the Agreement was ratified in 1976, overall harvest levels decreased (Prestrud and Stirling, 1994). There have nevertheless been concerns about possible over-harvest and unbalanced population composition in several populations (Taylor et al., 1987, 2006b; Prestrud and Stirling, 1994; Derocher et al., 1997). For example, in Canada, the Viscount-Melville Sound is managed conservatively due to concerns about depletion (Taylor et al., 2002) and the M'Clintock Channel population has been managed for recovery due to over-harvest that occurred between 1974 and 1992 that resulted in a significant population decline (Taylor et al., 2006b). Management concerns about the sustainability of polar bear harvest remain but more broadly, climate change is viewed as the dominant long-term threat to the species (Stirling and Derocher, 1993; Derocher et al., 2004; Regehr et al., 2016). The interaction between climate change and harvest is likely to result in new challenges (Regehr et al., 2017, 2021a,b). The main challenge is that resilience to exploitation depends on survival and reproduction, yet declines in both parameters for polar bears have been associated with climate change (Derocher et al., 2004; Molnár et al., 2010, 2014; Regehr et al., 2010; Rode et al., 2010).
Additional challenges to harvest sustainability levels are likely associated with ecological differences between populations with higher productivity areas supporting more polar bears than low productivity areas. Several habitat-related variables affect polar bear distribution and densities (Durner et al., 2009; Hamilton and Derocher, 2018) with prey density, especially ringed seals (Pusa hispida), influencing polar bear abundance (Stirling and Oritsland, 1995). More available prey and more favorable habitat can support more polar bears, thus facilitating higher levels of sustainable harvest.
To maximize harvest, polar bear harvest in Canada has been male-biased, where the number of males harvested is two or three times higher that of females (Derocher et al., 1997; Taylor et al., 2008c). Other nations do not have sex-specific harvest objectives. Male-biased harvesting is founded on the assumption that population growth rate is determined by females within a polygynous mating system, as long as there are enough males to mate available females (Caughley, 1977). However, such sex-biased harvest may have unintended demographic effects, and a skewed adult sex ratio might also have consequences for mating behavior and reproductive rates (Schacht et al., 2017). Further, male depletion could cause an Allee effect through reduced mating success and lower reproductive output, which could increase extinction risk (Lindstrom and Kokko, 1998; Molnár et al., 2008). Based on a sustainable yield of <1.6% adult females in a population, and a 2M:1F target sex ratio in the harvest, a harvest rate of 4.5% has been considered sustainable (Taylor et al., 1987, 2008c; Regehr et al., 2017). While our study does not address the age of harvested bears, regulations pertaining to the protection of adult females accompanied by offspring and the dependent offspring from harvest varied over time and between jurisdictions (Nageak et al., 1991; Calvert et al., 1993; Taylor et al., 2008c). Therefore, such regulations would have influenced the sex ratio of harvest to different degrees across populations.
The objectives of this study were to assess the temporal patterns in the number of polar bears harvested, harvest sex ratios, and harvest rates from the legal take of polar bears in the circumpolar Arctic 1970–2018. Further, we also assessed possible over-harvest, whether harvest rates varied with ecosystem productivity, and the extent to which having, or not having, estimates of population size may have influenced harvest management decisions. We predicted that the number of polar bears harvested would reflect the intent of the Agreement to manage using the best available scientific data, and that the incidences of over-harvest would be limited.
Materials and Methods
Harvest Data
We collated as complete data as possible on harvest from government agencies in all populations with legal harvest and known take (16 of the 19 populations), 1970–2018. Consequently, three populations were excluded due to lack of legal harvest: the Kara Sea, Laptev Sea, and the Arctic Basin. We note that there are no population estimates in East Greenland, Chukchi Sea has incomplete harvest data, and harvest ceased in the Barents Sea in 1973. Data for West Greenland before 2006 and for Québec in Canada before 1989 were taken from the IUCN Species Survival Commission Polar Bear Specialist Group (Polar Bear Specialist Group, 1976, 1980, 1985, 1986, 1991, 1993, 1998). Data from West Greenland before 1993 were reported by municipality and were allocated to a population. For West Greenland, harvest for Qaanaaq (Avanersuaq/Thule) was assigned to Kane Basin, Uummannaq-Nuuk harvest was assigned to Baffin Bay, and Paamiut and south harvest was assigned to Davis Strait. Data from Québec relates to the Davis Strait, Foxe Basin and Southern Hudson Bay populations. Quebec data before 1989 are given as a sum for all three populations, therefore, harvest was assigned to each of these three populations based on the mean proportion of the harvest taken from each in 1989–2018. Information on quotas was collected from various sources (Polar Bear Specialist Group, 1991, 1993, 1998, 2002, 2006, 2010, 2018; Brower et al., 2002; Government of Nunavut, 2021). To our knowledge all harvest numbers include kills in defense of life and property, but do not include unreported harvest, poaching, and struck or lost bears and thus represent a minimum removal.
Time series changes and trends in annual harvest were initially smoothed using a Generalized Additive Model (GAM) with a Poisson distribution for the counts, fitted with the gam() function in the mgcv package in R version 3.5.0 (Wood, 2017; R Core Team, 2019). Smoothing terms are introduced to explore both linear and non-linear relationships. In the GAM, the harvest as a function of time is included as a spline s(Year), using the default penalized regression splines (Wood, 2017). As we were interested in long-term changes over time, and not short-term variation, we constrained the basis dimension used in each smooth by changing the k-parameter in the spline function s() from the default of 10 to 6 when investigating single populations. Choosing a different k or the default values had limited influence of the model fits (Supplementary Figure 1). Autocorrelation and partial autocorrelation function (ACF/PACF) plots were used to assess autocorrelation of residuals, and an AR1 model using a random effect specification was fitted to time series by utilizing the generalized additive mixed model formulation [function gamm()] in the mgcv package in R (Wood, 2017). An AR1 model is a first-order autoregressive model that adjusts for correlation between 1 year and the previous year's random residual term (see Saveliev et al., 2009 for an example). As counts showed evidence of overdispersion, we also used a quasi-likelihood approach to model counts, by using the “family = quasipoisson” argument in the function gamm. The models were fitted independently for all subpopulations. The R script for the GAM modeling and plotting of harvest numbers is provided in the Supplementary Material 2.
Sex Ratio
Sex ratio is defined as the number of males to females in the harvest (M:F) as reported by hunters or recorded by management personnel. Time series change and trends in annual sex ratio of harvested animals of known sex in each population were examined using a GAM with a binomial distribution with the mgcv package in R version 3.5.0 (R Core Team, 2019). Autocorrelation and partial autocorrelation function (ACF/PACF) plots of residuals were checked for autocorrelation.
To summarize long-term changes in population harvest sex ratio, data were pooled into four approximately equal periods that were long enough to provide adequate sample sizes: 1970–1981 (12 years), 1982–1993 (12 years), 1994–2005 (12 years), and 2006–2018 (13 years) using a generalized linear model with a binomial distribution with period as the predictor variable and using quasi-likelihood to account for overdispersion.
Harvest Rate
We use abundance estimates to assess harvest rate and management responses to new estimates. Annual harvest rate was defined as the ratio of recorded harvest and population abundance estimate (Table 1). We used capture-recapture or aerial survey estimates, and applied them for 10 years after the last year of data collection. This 10-year period was used by managers in some jurisdictions before switching to abundance estimates from population simulation models based on vital rates. We elected to not use population simulation estimates because most were unavailable or unpublished, and we had concerns about accuracy of projections. If a revised estimate was produced, we used the year of publication as the initial year for the revised estimate, to separate this from the original estimate. This approach provided a standardization across populations because the details of management actions are often unavailable or unrecorded. If two estimates were based on the same data, and the periods overlapped, we used the lower estimate as a precautionary approach. Overlapping estimates only occurred in two populations, so this issue had minimal effect on the study. Annual population estimates were available for some populations, but managers have used a single pooled abundance estimate from each inventory and have not used these annual estimates. Therefore, we followed a similar procedure in our analyses and used the point estimates. Over-harvest was defined as an annual harvest rate > 4.5% following Taylor et al. (1987).
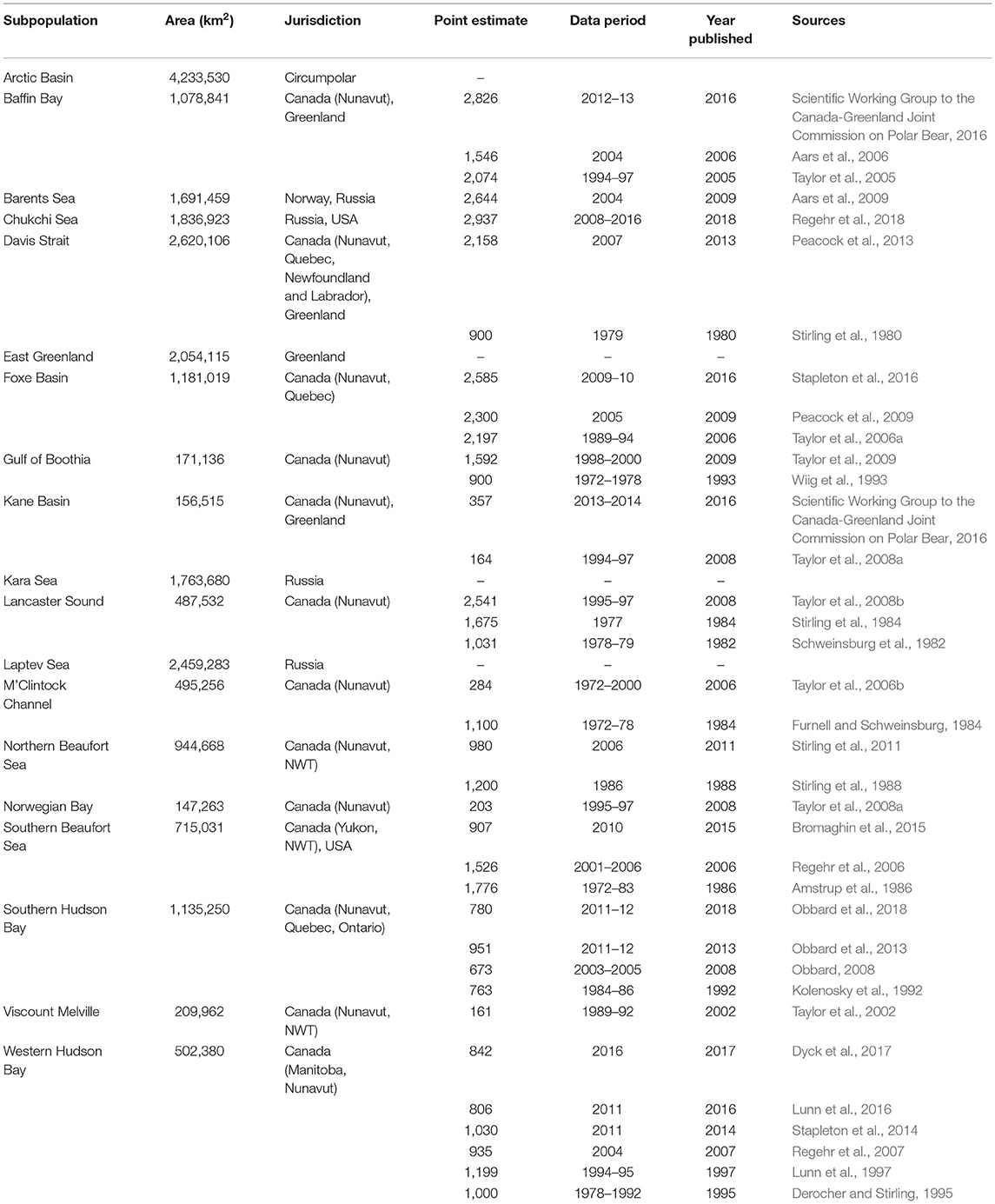
Table 1. Information about polar bear populations (Polar Bear Specialist Group, 2021), their area, jurisdictions, and population estimates, including source references.
Six of 19 populations were excluded from harvest rate estimates: three due to lack of both population estimates and existing harvest (Arctic Basin, Kara Sea, and Laptev Sea), one due to lack of harvest (Barents Sea), one due to lack of population estimates (East Greenland), and one (Chukchi Sea) due to lack of Russian data.
We used 34 published population estimates (Table 1; Supplementary Figure 3) for 13 populations to calculate harvest rates. Of the 34 estimates, seven were aerial survey estimates and 27 were mark-recapture estimates, and 31 were estimates of total population size, including COYs (cubs of the year), yearlings and dependent young. There was a mean of 5 years (SE = 0.6, range 0–15) between the last year of data collection and the estimate being published. In the 11 populations with more than one estimate, the mean interval between population estimates was 11.4 years (SE = 1.9, range: 0–28), calculated from the last year of data collection, and 10.4 years (SE = 2.0, range: 1–33) when calculated from the year of publication. As of 2021, estimates in the two populations with only one available estimate, Norwegian Bay and Viscount Melville, were 13 and 19 years old, respectively, calculated from the year of publication. Nine of 15 legally harvested populations did not have recent (≤ 10 years old) population estimates at the time of our analyses. New estimates, however, are available most years for some populations as part of their respective inventory cycle, which varies across jurisdictions.
Harvest Rate and Area Productivity
To examine the relationship between harvest rates and ecosystem productivity we assumed that total population area (land and sea included), the proportion of shallow (<300 m) continental shelf area within each total population area, and/or the number prey species available within each population could be used as proxies for ecosystem productivity, following Hamilton and Derocher (2018). We used linear regressions to investigate the relation between overall mean harvest rate for each population and the three ecosystem productivity proxies. We converted the proportion of shallow shelf water (in percent) to actual area of shelf water for the analyses. The data used in these analyses were not available for all populations. Therefore, the small sample size required the use of simple models. There's no a priori reason to expect anything other than a linear relationship.
Results
Harvest Numbers
Between 1970 and 2018, 39,049 polar bears (annual mean 797 bears, SE = 16, range: 629–1,285) were harvested in 16 populations, excluding Russia, but including the US portion of the Chukchi Sea population. Of those harvested, 19,611 were male (annual mean: 400 bears, SE: 9, range: 253–557), 10,663 were female (annual mean: 218 bears, SE: 5, range: 146–294), and 8,775 were of unknown sex (mean: 179, SE: 17, annual range: 7–688), resulting in an overall M:F sex ratio of 1.84. Of the total harvested bears, 68.0% (26,570) were harvested in Canada, 18.0% (7,018) in Greenland, 11.8% (4,591) in the USA, and 2.2% (870) in Norway.
The total harvest numbers summed for all jurisdictions dropped right after 1970 due to the strong decrease in the Barents Sea harvest, and showed a weak decreasing trend after 1971 (Figure 2). Numbers, however, increased from ~650 bears/year to ~900 bears/year between about 1975 and the early 1980s. Since the mid-1980s, harvest had a negative trend with some fluctuations at both yearly and ca. 5-year intervals. Between 1970 and 2018, Davis Strait and Gulf of Boothia had monotonic increasing trends, Chukchi Sea (US data only) showed monotonic decreasing trend and the 12 remaining populations had varying harvest annual numbers. Between 2008 and 2018, East Greenland and Northern Beaufort Sea had an increasing trend.
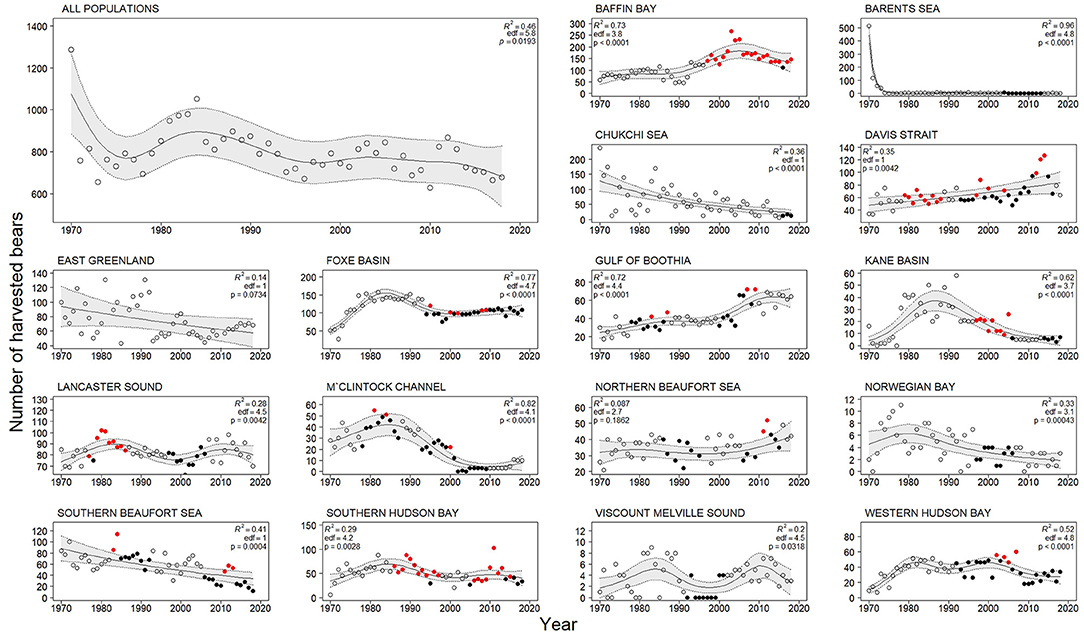
Figure 2. Total annual harvest of polar bears summing all jurisdictions, except Russia (large panel top left), and annual harvest in 16 of 19 polar bear populations (small panels), in the period 1970–2018. No Russian data were available for Chukchi Sea and Barents Sea. Open circles represent years with populations estimates > 10 years old, black circles years with population estimate ≤ 10 years and harvest rate ≤ 4.5%, and red circles represent years with population estimate ≤ 10 years and harvest rate > 4.5%. Trend lines are fitted with a Generalized Additive Model (GAM, k = 6). Gray bands indicate the 95% confidence intervals.
Sex Ratio
Reporting of sex in harvested bears increased from 1970 to 2018, and after 2006 sex was reported in 97.9% of the harvest (7,293 of 7,447 bears), except for 2017 and 2018, when the proportion of known sex in harvest was 75.7% (1,018 of 1,344 bears; Supplementary Figure 4). The proportion of harvest where sex was recorded varied greatly between populations (Supplementary Figure 4).
The circumpolar sex ratio for all data over all years was 1.84M:1F. The sex ratio appeared to be lower for some years before 1990 than the years after. After an initial decline in the proportion of harvested males, the sex ratio increased from 1980 to 1995, after which the sex ratio trend line flattened at 2M:1F (Figure 3).
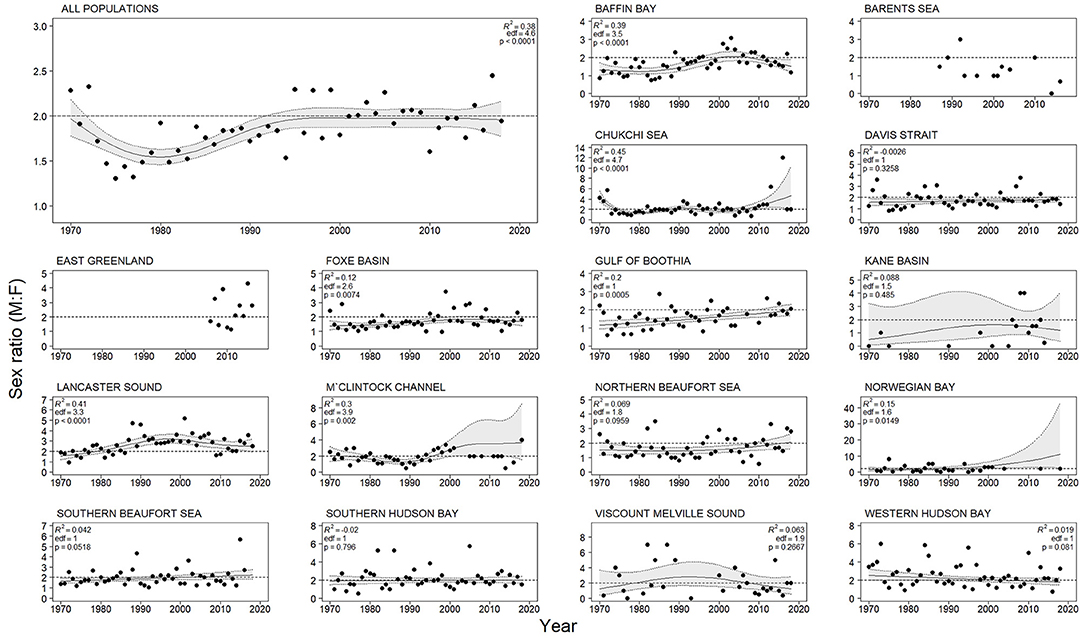
Figure 3. Sex ratio of all harvested bears of known sex (large panel top left), and in harvest for 16 of 19 polar bear populations (small panels), in the period 1970–2018. Dotted horizontal line represents the 2M:1F sex ratio, and gray bands indicate the 95% confidence intervals. Trend lines are fitted with a Generalized Additive Model (GAM, k = 6). Trend lines not shown for Barents Sea and East Greenland populations due to data not covering the entire period.
Divided into four periods, the difference in sex ratio over these four periods was small (range: 1.65–2.00). However, sex ratio was significantly higher in 1994–2005 and 2006–2018 than in 1970–1981 [difference on logit scale 1994–2005 vs. 1970–1981 0.19 (SE = 0.05), 2006–2018 vs. 1970–81 0.17 (SE = 0.05)] but there was no evidence for a difference between 1994–2005 and 2006–2018 (difference on logit scale −0.02, SE = 0.05).
The sex ratio over time for the 16 populations varied widely, from 0 to 12.0 (Figure 3). GAMs for sex ratio were not fitted for Barents Sea and East Greenland because data on sex of harvest was unavailable for the entire period. GAMs did not provide strong evidence (i.e., P > 0.05, see Figure 3 for population specific P-values) for changes for Davis Strait, Kane Basin, Northern Beaufort Sea, Southern Beaufort Sea, Southern Hudson Bay, Viscount Melville Sound, and Western Hudson Bay. The data suggest an increase in sex ratio (i.e., more males) in Gulf of Boothia, and a variable sex ratio in Baffin Bay, Chukchi Sea, Foxe Basin, Lancaster Sound, and M'Clintock Channel. Sex ratio in M'Clintock Channel increased from 1990 for approximately a decade. In Norwegian Bay there seems to be a significant change, but harvest numbers are < 10 in all years but two, resulting in low statistical power.
In years where there were harvest of males, but no harvest of females, the sex ratio (M:F) could not be calculated. This was recorded in 6 of 16 populations, in 18 of 49 years in Norwegian Bay, 15 of 49 years in the Barents Sea, 6 of 49 years in M'Clintock Channel, 5 of 49 years in Kane Basin and Viscount Melville, and 1 of 49 years in Southern Hudson Bay.
Harvest Rates
Harvest rates varied over time within most populations and between populations (Figure 4). Based on mean annual harvest rates for all 13 populations with population estimates the mean harvest rate for all populations over time (calculated by first averaging by population and then over all populations) was 4.2%, not very different from the raw average of harvest rates (4.5%) or the abundance weighted average (4.6%). Of the 255 annual rates calculated, 93 were higher than the sustainable level of 4.5%, and 17 were >10%. The highest harvest rates were 15.0, 15.3, and 15.9%, recorded in Baffin Bay in 2005, Southern Hudson Bay in 2011, and Kane Basin in 2005, respectively.
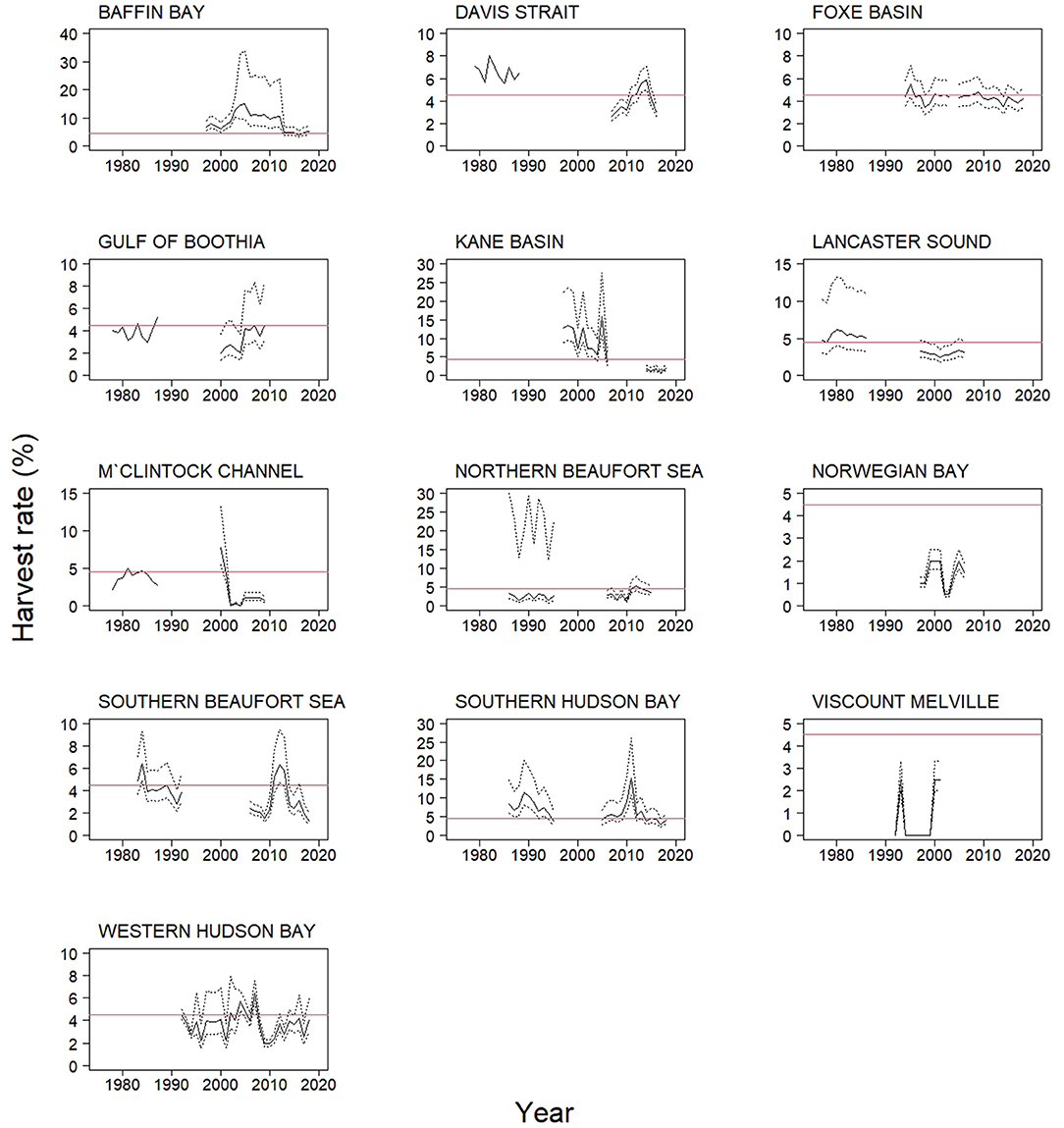
Figure 4. Harvest rates for 13 of 19 polar bear populations in 1970–2018, calculated from total harvest and population estimate ≤ 10 years old reported with 95% confidence intervals shown by dotted lines. Solid red line is the 4.5% harvest level, which has been suggested as the maximum sustainable harvest level. Six populations are excluded: Arctic Basin, Kara Sea, and Laptev Sea have no population estimate or legal harvest, Barents Sea has no harvest, East Greenland has no population estimate, and Chukchi Sea due to lack of Russian data.
Over-harvest occurred in all 13 populations at some point except Norwegian Bay and Viscount Melville. In these 13 populations, over-harvest occurred for a mean of 8.5 years (SE = 0.6, range: 2–21), representing a mean 36.5% of years with known harvest rates.
Harvest Rates and Area Productivity
Linear regression analysis between total population area, population shelf area, prey diversity, and overall mean harvest rate for each population found only one significant relationship (Supplementary Figures 5A–C). There was no significant linear relation between mean harvest rate and total population area (df = 11, t = 1.17, p = 0.27), or between mean harvest rate and area of shelf ≤ 300 m (df = 11, t = 0.86, p = 0.41). The relationship between mean population harvest rates and prey diversity for 1970–2018 was positive (df = 11, t = 3.74, p = 0.003, R2 = 0.52).
Discussion
Unsustainable harvest is a conservation concern for Arctic marine mammals, and limited data exists to examine long-term trends in harvest to assess species management. We examined 49 years of polar bear harvest data representing the best estimate of legal take in the circumpolar Arctic. As predicted, the overall number of harvested polar bears declined over time. While the sex ratio in the harvest varied across populations, it trended toward two males for every female at the circumpolar level in the last 20 years, a fundamental underpinning of a 4.5% harvest rate being sustainable. Contrary to predictions, incidents of over-harvest occurred in nearly all populations that could be assessed for it, and in some cases lasted for several years.
When examining harvest data spanning 49 years, variation in reporting and data quality may result in inaccuracies in both the number and sex of harvested bears. Reported harvest may underestimate total removals to varying degrees due to failure to report, inaccuracy of reporting, poaching, and struck and lost. However, the extent of each possible bias is unknown. Because there are no data with which to correct such a bias across all the data, we assumed the data are representative of the actual harvest. It is noteworthy that based on DNA analyses, sex of bears was misidentified in 12% of harvest returns in Alaska, resulting in an underreporting of females (Schliebe et al., 1999). In the Southern Beaufort Sea between 1980 and 1998, 2.5% (12/479) of the harvested bears had no sex data in Canada and 22.4% (147/656) lacked sex data in Alaska (Brower et al., 2002). Reasons for not reporting sex are unclear but, in a worst-case scenario, if 12% of all bears recorded as male were female and if all harvested bears of unknown sex were female, the sex ratio of the harvest in the Southern Beaufort Sea would be 1.02M:1F, as opposed to the reported ratio of 1.88M:1F. If calculated across all years and populations this bias would give a sex ratio of 0.79M:1F, as opposed to the reported ratio of 1.84M:1F. If such a bias in reporting occurred, harvest impacts on populations could have been more severe.
There are no uniform characteristics or trends in the number of polar bears being harvested in the 16 populations. Some populations had an increase in harvest, others a decline, and the remaining populations showed variation over time. Polar bear populations have low intrinsic growth rates, annually at ca. 4.5% at the maximum net productivity level (Regehr et al., 2017), and are thus easy to deplete and slow to recover. How harvest will affect a polar bear population will depend on several factors including abundance, age and sex structure, possible Allee effects (Molnár et al., 2008), distribution (Stirling and Andriashek, 1992), and genetic structure (Coltman et al., 2003). Our study, focusing on harvest numbers, cannot assess such changes. It is, however, possible that harvest contributed to the decline in population abundance in the Western Hudson Bay (Regehr et al., 2007), and in the Southern Beaufort Sea (Bromaghin et al., 2015) populations. It is also likely that harvest was unsustainable at times in Kane Basin, Southern Hudson Bay, and Baffin Bay. Nonetheless, across the monitored populations, harvest rates seem to have been within sustainable limits in the 5–10 years before 2018.
The sustainability of a specific harvest level is challenging to assess. In most cases, we do not have data to determine if harvest is additive, or whether there are compensatory mechanisms. However, based on population modeling, a harvest rate of 4.5% should be sustainable if the sex ratio of the harvest was 2M:1F (Taylor et al., 1987; Regehr et al., 2017). In populations with infrequent abundance estimates, harvest levels may be set above sustainable levels and cause a population decline. Such declines might not be detected before new inventories are available, which on average take more than a decade and, for some populations, more than two decades. Using the 4.5% harvest threshold, our data show potential over-harvest issues in 11 out of 13 populations where we have relevant data. Higher harvest rates might be acceptable where environmental conditions are being monitored (Regehr et al., 2017). However, population estimates are often infrequent and have wide confidence intervals, so harvest rate estimates are affected by possible errors in both of its constituents: harvest numbers and population estimates. Our study has used the point estimates for our presentation of harvest rates. A more conservative approach would have been to use the lower 95% confidence interval, which would have indicated more frequent and much higher levels of over-harvest.
The sex ratio of harvested animals can vary due to sex-selective harvesting as a management objective, protection of females with offspring, sex-specific vulnerability, population sex composition, environmental conditions, or hunter preference. A rule in Nunavut to protect females was implemented in 2004 with the goal of harvesting two males per harvested female (e.g., Anonymous, 2004). Interestingly, harvest data show that sex ratio of harvested animals, while variable, has been around 2M:1F for most populations in most years, despite male-biased harvest only having been a management objective since 2004, and only in Nunavut. Females with offspring have been protected in some populations (e.g., Northwest Territories—Lee and Taylor, 1994), and may contribute to a larger proportion of males in the harvest (Derocher et al., 1997). The long-term effect of a skewed sex ratio is unknown, however, reducing the number of males might eventually lead to reduced reproduction through a component Allee effect (Molnár et al., 2014). However, it is impossible to conclude anything about potential demographic impacts of a sex-selective harvest without information of the age of harvested animals. In black bears (U. americanus), age and sex data have been used to calculate harvest rates of both sexes, as this enables the study of cohorts in the population when certain assumptions are met (Paloheimo and Fraser, 1981; Fraser et al., 1982; Harris and Metzgar, 1987). The lack of such data for polar bears suggests a need for additional analyses for each population, especially as paternity varies with age (Richardson et al., 2020).
The Agreement has been regarded as the main driver for the establishment of a sound management regime on polar bear conservation through a coordinated international cooperation on research and management (Prestrud and Stirling, 1994), and for ending over-harvest. The Agreement has been acknowledged both for its simplicity and for being one of the first international regimes to be based on ecological principles (Fikkan et al., 1993). However, the chronology of events before and after the signing of the Agreement shows that the annual total take of polar bears was reduced by >50% when the Agreement was being negotiated (1965–1973; Fikkan, 1990; Prestrud and Stirling, 1994), and after an immediate drop in harvest numbers after 1970, from 1,100 to 1,500 in the 1960s (Prestrud and Stirling, 1994), to about 600 in 1973, the harvest increased in the decade up to 1984, when 1,040 bears were harvested. Harvest quotas were implemented in a precautionary, knowledge-driven framework in most populations, to aid long-term harvest sustainability. However, this has not always been the case for polar bears (Prestrud and Stirling, 1994). Harvest quotas were implemented in Canada in 1968 (except Québec, which has not implemented a quota), in USA in 1971, and in Greenland in 2006. The increase in harvest numbers in the years after the signing of the Agreement was largely due to increased harvest within Canada, particularly in six populations (i.e., Foxe Basin, Kane Basin, Lancaster Sound, M'Clintock Channel, Southern Hudson Bay, and Western Hudson Bay). However, reasons behind this increase are unknown and were not based on increased estimates of population abundance.
Accurate and precise population abundance estimates along with information about age-specific survival and reproduction rates are required as a basis for quotas (Vongraven et al., 2012). Before 1980, most populations had not been surveyed, and only five populations had estimates with confidence intervals (i.e., Southern Beaufort Sea, Gulf of Boothia, Lancaster Sound, M'Clintock Channel, and Davis Strait). As environmental change happens, the need for population surveys increases if a sustainable harvest is to be maintained, and this is a significant challenge for most population management authorities (Peacock et al., 2012). Excluding three populations that were regularly monitored (Southern Beaufort Sea, Northern Beaufort Sea, and Western Hudson Bay), the mean interval between consecutive estimates was 10.9 years (range: 1–36 years), with only six populations having estimates ≤ 10 years old (Hamilton and Derocher, 2018). This study also reported delays up to 12 years in release of population estimates following completion of the inventory. The combination of long inventory cycles and delays in publishing results is a challenge to sustainable harvest management.
Biased population estimates in Baffin Bay and Davis Strait were likely the cause of the high harvest rate estimates. Inconsistent sampling efforts, low mark-recapture sample sizes, and few recoveries can result in unreliable population estimates. In 2016, it was concluded that estimates, published in 2005 and 2006, were biased low (Scientific Working Group to the Canada-Greenland Joint Commission on Polar Bear, 2016). Therefore, our harvest estimates in Baffin Bay of 10–15% and even higher in 2003–2012 were likely a result of underestimated population sizes, and not necessarily indicative of a severe over-harvest. Nonetheless, management did not reduce harvest in response to a potential over-harvest. Co-management boards kept quotas at levels identified as unsustainable based on existing data (Peacock et al., 2011). Also, contributing to the estimated high harvest rate was high and unreported harvest in the Greenland part of Baffin Bay (Stirling and Parkinson, 2006). Another example of biased population estimates occurred in Davis Strait where the 1979 estimate of 900 bears was likely biased low as the entire population was not sampled (Peacock et al., 2013). Nevertheless, management decisions made in the 1990s and early 2000s were not based on a precautionary approach. Again, of further concern, harvest monitoring in these populations was lacking because it was not coordinated between Greenland and Canada before 2009, and although Greenland established a quota system in 2006, harvest was largely unreported before this. This situation improved in 2009 through a Memorandum of Understanding between the two jurisdictions on polar bears harvest (Environment Climate Change Canada, 2009). Similar issues were evident in Kane Basin in the same period.
Despite long-standing concerns about over-harvest reported in Foxe Basin in 1991 (Lunn et al., 1998), our data suggests a sustainable harvest after 1994. In 1997, the Polar Bear Specialist Group stated that the Foxe Basin harvest quota from 1970 onwards was too high, and they believed that the population was reduced from about 3,000 to 2,300 (Lunn et al., 1998). Our data show an increasing harvest number from 1970 until the mid-1980s, as opposed to stable high harvest from 1970 onwards. Due to concerns of over-harvest the harvest quotas were reduce ca. 30%, from the mid-1990s, to a level that has been maintained since.
Only three populations (i.e., Gulf of Boothia, Davis Strait and Baffin Bay) had increasing harvest over the 49 years. In the Gulf of Boothia population, harvest rates for the most part seemed sustainable in the early 1980s and 2000s, and the proportion of males in the harvest was increasing. In 2005 quotas almost doubled, from 40 bears per year to 74 bears per year, based on traditional ecological knowledge and new capture data (Polar Bear Specialist Group, 2010) and harvest rates increased from ca 2.5% to ca 4.5%. Population growth rate was at the time 2.5% (Taylor et al., 2009), and if harvest numbers continue to increase over-harvest might be an issue. A new population estimate based on genetic mark-recapture data collected in 2015–17 indicates that the population has remained stable after the 1998–2000 estimate (Dyck et al., 2020). This population had a density that is many times higher than other populations across the Arctic (Hamilton and Derocher, 2018) suggesting the harvest may be sustainable.
Our data suggests that there has been limited responses from management authorities to new information related to harvest in some populations. In Southern Hudson Bay, spikes in harvest rates in 2010 and 2011 were not acted upon by management. This population is harvested by Ontario, Québec, and Nunavut yet only Ontario reduced their quotas in response to the high harvest (Polar Bear Specialist Group, 2010, 2018) despite a 17% decline in the population from 2011 to 2016 (Obbard et al., 2018). Duration of sea ice cover was thought to be the main factor in the decline (Obbard et al., 2018) but the role of harvest remains unclear. In Western Hudson Bay there were reports of a population decline (Lunn et al., 2016; Dyck et al., 2017) and lower survival of juveniles and subadults (Regehr et al., 2007), but the quota was increased three times after 2010 (Government of Nunavut, 2021). The Southern Beaufort Sea population declined 25–50% from 2004 to 2007 (Bromaghin et al., 2015) and we found that the harvest of this population decreased but it was not due to management actions as there was no change in quotas. Consequently, the harvest decline may be associated with fewer bears being available, a change in the hunting effort by the communities that has resulted in lower hunting pressure, or sea ice changes that affected the bears or hunters. We suggest that input from local hunters may provide additional insight on the declining harvest.
Traditional ecological knowledge has become an integral part of polar bear harvest co-management within most of harvested polar bear populations (Dowsley and Wenzel, 2008). Ongoing changes in management practices suggest a divergence between science-based management and management based on traditional ecological knowledge. For example, from 2019, Nunavut changed from a male-selective 2M:1F harvest to a non-selective 1M:1F harvest without a corresponding change in total allowable harvest nor scientific assessment. The goal in the new co-management plan for the populations in Baffin Bay, Davis Strait, Foxe Basin, and Western Hudson Bay is to manage for a decrease if population size “is stable or increasing and public safety becomes a major concern” (Government of Nunavut, 2019). This change adds conservation concern about how ongoing harvest may affect polar bear populations. There has also been concern raised when harvest quotas has been increased based on traditional ecological knowledge alone, without the support from scientific studies (Wiig, 2005).
There are 19 populations in the circumpolar Arctic and three have recorded sea-ice related declines in abundance (Bromaghin et al., 2015; Lunn et al., 2016; Obbard et al., 2018) although more are predicted to decline (de la Guardia et al., 2013; Regehr et al., 2016; Hamilton and Derocher, 2018). Whether harvest can amplify population fluctuations caused by climate change is debated (Gamelon et al., 2019). Our analyses suggest that polar bear harvesting appears sustainable across most populations yet there are trends in some populations that require a detailed assessment. Our finding that harvest was related to the prey diversity of the various populations suggests that ecosystem processes are important (e.g., Sciullo et al., 2017; Galicia et al., 2021), but these relationships remain unclear. Ringed seals, the main prey of polar bears (Thiemann et al., 2008), fluctuate in abundance (Stirling, 2002; Nguyen et al., 2017). We suggest that prey switching may occur in those polar bear populations with greater prey diversity and thus, their abundance may be less affected by variation in ringed seals and thus sustain higher harvest levels. In a rapidly changing Arctic, precautionary harvest management is required to avoid unnecessary risk to polar bear populations. Other stressors add to the management challenges. Known risk factors associated with future viability of Arctic marine mammal populations include disturbance from tourism and development, increasing human population/increased accessibility (i.e., higher demand for wildlife), commercial trade, and pollution (Schipper et al., 2008; Laidre et al., 2015; Avila et al., 2018; Nelms et al., 2021).
For sound and knowledge-based management of polar bear populations, and to allow sustainable harvest, we recommend that population inventories are done more frequently, that the results are reported promptly, and that projected effects of climate change are integrated into harvest management. There is a need for an improved monitoring of the sex and age composition of harvested animals, and of coordinated effort across jurisdictions (Vongraven et al., 2012). Further, retention of a 2M:1F sex ratio of harvest, confirmed and reliable sex information of all harvested bears, and with changing environmental conditions, managers could elect to reduce the 4.5% target harvest rate, or manage on the lower confidence interval of a population estimate. Present changes in polar bear harvest management, in concert with rapid change in Arctic ecosystems, emphasizes the need to manage polar bear populations with a higher degree of precaution than we see at present.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: Polar bear harvest data belongs to the jurisdictions and are legally used for this study only as a part of a signed agreement between the authors and the jurisdictions. Requests to access these datasets should be directed to Manitoba, Canada: Government of Manitoba, email: bWdpQGdvdi5tYi5jYQ== Newfoundland & Labrador, Canada: Department of Fisheries, Forestry and Agriculture, email: aW5mb0Bnb3YubmwuY2E= Northwest Territories, Canada: Environment and Natural Resources, Government of Northwest Territories, email: RU5SX0NvbW11bmljYXRpb25zQGdvdi5udC5jYQ== Nunavut, Canada: Nunavut Wildlife Management Board, email: cmVjZXB0aW9uaXN0QG53bWIuY29t Ontario, Canada: Ministry of Northern Development, Mines, Natural Resources and Forestry, email: am9zZXBoLm5vcnRocnVwQG9udGFyaW8uY2E= Quebec, Canada: Ministère des Forets, de la Faune et des Parcs, Quebec, email: c2VydmljZXMuY2xpZW50ZWxlQG1mZnAuZ291di5xYy5jYQ== Yukon, Canada: Yukon Fish and Wildlife Management Board, Whitehorse, email: b2ZmaWNlbWFuYWdlckB5ZndtYi5jYQ== Greenland: Ministry of Fisheries, Hunting and Agriculture, Nuuk, Greenland, email: YW1hbGllQG5hbm9xLmds Norway: website: https://www.mosj.no/en/influence/hunting-trapping/polar-bear-bag.html USA: US Fish and Wildlife Service Alaska, email: aW5mb0Bmd3MuZ292.
Ethics Statement
Ethical review and approval was not required for the animal study because the study analyses and discusses information from harvest data collected by others (polar bear harvest management jurisdictions).
Author Contributions
DV conceived the original idea of the study, collected the data, and wrote the first draft. DV and AD developed the design of the study. DV, AD, NP, and NY contributed to the statistical analyses. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
Norwegian Polar Institute have paid travels between Tromsø, Norway and University of Alberta, Edmonton, and logistics related to accomodation when abroad and work place when in Tromsø, Norway.
Conflict of Interest
NP was employed by San Diego Zoo Wildlife Alliance.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We dedicate this paper to the memory of Markus Dyck who died tragically in April 2021 while conducting polar bear research in the Canadian Arctic. Markus provided Nunavut harvest data and had committed to contribute to the study. We thank the authorities and people in various jurisdictions that provided harvest data, and special thanks to Dr. Marsha Branigan, Northwest Territories, Canada. Thanks to students and scientists at the Derocher Lab for all inspired discussions, and special thanks to Dr. Jody Reimer for her frequent input on all kinds of statistical enigmas. We would like to thank Dr. Ian Stirling for commenting on a late draft that improved the final manuscript, and Dr. Sterling Miller and Dr. Erik Andersen for their constructive reviews of the manuscript. Finally, we thank the Norwegian Polar Institute for logistic support and for funding necessary travel.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2022.836544/full#supplementary-material
References
Aars, J., Marques, T. A., Buckland, S. T., Andersen, M., Belikov, S., Boltunov, A., et al. (2009). Estimating the Barents Sea polar bear subpopulation size. Marine Mammal Sci. 25, 35–52. doi: 10.1111/j.1748-7692.2008.00228.x
Alvarez-Flores, C. M., and Heide-Jorgensen, M. P. (2004). A risk assessment of the sustainability of the harvest of beluga (Delphinapterus leucas (Pallas 1776)) in West Greenland. Ices J. Marine Sci. 61, 274–286. doi: 10.1016/j.icesjms.2003.12.004
Amstrup, S. C., Stirling, I., and Lentfer, J. W. (1986). Past and present status of Polar Bears in Alaska. Wildl. Soc. Bull. 14, 241–254.
Anonymous (2004). Memorandum of Understanding for the Management of the “Baffin Bay” Polar Bear Population. Agreement Between Qikiqtarjuaq Nativak, Clyde River Namautaq, Pond Inlet Mittimatalik Hunters' and Trappers' Organization, Qikiqtaaluk Wildlife Board, and the Department of Environment, 25p.
Avila, I. C., Kaschner, K., and Dormann, C. F. (2018). Current global risks to marine mammals: taking stock of the threats. Biol. Conserv. 221, 44–58. doi: 10.1016/j.biocon.2018.02.021
Benitez-Lopez, A., Alkemade, R., Schipper, A. M., Ingram, D. J., Verweij, P. A., Eikelboom, J. A. J., et al. (2017). The impact of hunting on tropical mammal and bird populations. Science 356, 180–183. doi: 10.1126/science.aaj1891
Beston, J. A., and Mace, R. D. (2012). What can harvest data tell us about Montana's black bears? Ursus 23, 30–41. doi: 10.2192/URSUS-D-11-00012.1
Bethke, R., Taylor, M., Amstrup, S., and Messier, F. (1996). Population delineation of polar bears using satellite collar data. Ecol. Appl. 6, 311–317. doi: 10.2307/2269574
Bockstoce, J. R., and Botkin, D. B. (1982). The harvest of pacific walruses by the pelagic whaling industry, 1848 to 1914. Arctic Alpine Res. 14, 183–188. doi: 10.2307/1551150
Bromaghin, J. F., McDonald, T. L., Stirling, I., Derocher, A. E., Richardson, E. S., Regehr, E. V., et al. (2015). Polar bear population dynamics in the southern Beaufort Sea during a period of sea ice decline. Ecol. Appl. 25, 634–651. doi: 10.1890/14-1129.1
Brower, C. D., Carpenter, A., Branigan, M. L., Calvert, W., Evans, T., Fischbach, A. S., et al. (2002). The polar bear management agreement for the southern Beaufort Sea: an evaluation of the first ten years of a unique conservation agreement. Arctic 55, 362–372. doi: 10.14430/arctic720
Calvert, W., Taylor, M., Stirling, I., Kolenosky, G. B., Kearney, S., Crête, M., et al. (1993). “Polar bear management in Canada 1988-92,” in Proceedings of the 11th Working Meeting of the IUCN/SSC Polar Bear Specialist Group 25-27 January 1993, Copenhagen, Denmark, eds Ø. Wiig, E.W. Born, and G.W. Garner (Gland; Cambridge: IUCN), 61–79.
Caughley, G. (1994). Directions in conservation biology. J. Anim. Ecol. 63, 215–244. doi: 10.2307/5542
Coltman, D. W., O'Donoghue, P., Jorgenson, J. T., Hogg, J. T., Strobeck, C., and Festa-Bianchet, M. (2003). Undesirable evolutionary consequences of trophy hunting. Nature 426, 655–658. doi: 10.1038/nature02177
de la Guardia, L. C., Derocher, A. E., Myers, P. G., van Scheltinga, A. D. T., and Lunn, N. J. (2013). Future sea ice conditions in Western Hudson Bay and consequences for polar bears in the 21st century. Glob. Chang. Biol. 19, 2675–2687. doi: 10.1111/gcb.12272
Derocher, A. E., Lunn, N. J., and Stirling, I. (2004). Polar bears in a warming climate. Integr. Comp. Biol. 44, 163–176. doi: 10.1093/icb/44.2.163
Derocher, A. E., and Stirling, I. (1995). Estimation of polar bear population-size and survival in Western Hudson-Bay. J. Wildl. Manage. 59, 215–221. doi: 10.2307/3808933
Derocher, A. E., Stirling, I., and Calvert, W. (1997). Male-biased harvesting of polar bears in western Hudson Bay. J. Wildl. Manage. 61, 1075–1082. doi: 10.2307/3802104
Dowsley, M. (2010). The value of a polar bear: evaluating the role of a multiple-use resource in the Nunavut mixed economy. Arctic Anthropol. 47, 39–56. doi: 10.1353/arc.0.0035
Dowsley, M., and Wenzel, G. (2008). “The time of the most polar bears”: a co-management conflict in Nunavut. Arctic 61, 177–189. doi: 10.14430/arctic56
Durner, G. M., Douglas, D. C., Nielson, R. M., Amstrup, S. C., McDonald, T. L., Stirling, I., et al. (2009). Predicting 21st century polar bear habitat distribution from global circulation models. Ecol. Monogr. 79, 25–58. doi: 10.1890/07-2089.1
Dyck, M., Campbell, M., Lee, D., Boulanger, J., and Hedman, D. (2017). “2016 aerial survey of the Western Hudson Bay polar bear subpopulation,” in Status Report 2017-xx, Nunavut Department of Environment, Wildlife Research Section, Igloolik, Nunavut, 82p.
Dyck, M., Regehr, E. V., and Ware, J. V. (2020). “Assessment of abundance for the Gulf of Boothia polar bear subpopulation using genetic mark-recapture,” in Final Report to the Department of Environment, Government of Nunavut, 75p.
Environment and Climate Change Canada (2009). “Memorandum of understanding between the Government of Canada, the Government of Nunavut, and the Government of Greenland for the Conservation and Management of Polar Bear Populations,” in Report M-BD-10/EN From the Governments of Canada, Nunavut, and Greenland.
Fikkan, A., Osherenko, G., and Arikainen, A. (1993). “Polar bears: the importance of simplicity,” in Polar Politics: Creating International Environmental Regimes, eds O. R. Young and G. Osherenko (Ithaca, London: Cornell University Press), 96–151.
Fraser, D., Gardner, J. F., Kolenosky, G. B., and Strathearn, S. (1982). Estimation of harvest rate of black bears from age and sex data. Wildl. Soc. Bull. 10, 53–57.
Furnell, D. J., and Schweinsburg, R. E. (1984). Population-dynamics of central Canadian Arctic island polar bears. J. Wildl. Manage. 48, 722–728. doi: 10.2307/3801419
Galicia, M. P., Thiemann, G. W., Dyck, M. G., Ferguson, S. H., and Stirling, I. (2021). Prey selection of polar bears in Foxe Basin, NU, Canada: evidence of dietary flexibility in a specialized predator. Oxford Open Clim. Change 1, kgab002. doi: 10.1093/oxfclm/kgab002
Gamelon, M., Sandercock, B. K., and Sæther, B.-E. (2019). Does harvesting amplify environmentally induced population fluctuations over time in marine and terrestrial species? J. Appl. Ecol. 56, 2186–2194. doi: 10.1111/1365-2664.13466
Government of Nunavut (2019). Nunavut Polar Bear Co-management Plan. Downloaded from https://www.gov.nu.ca/sites/default/files/nwmb_approved_polar_bear_comanagement_plan_sept_2019_eng.pdf June 2, 2020.
Government of Nunavut (2021). Nunavut Polar Bear Harvest Reports 2000-2018. Department of Environment, Government of Nunavut. Annual harvest tables. Available online at: https://www.gov.nu.ca/environnement/information/wildlife-research-reports#polarbear (accessed December 1, 2021).
Hamilton, S. G., and Derocher, A. E. (2018). Assessment of global polar bear abundance and vulnerability. Anim. Conserv. 22, 83–95. doi: 10.1111/acv.12439
Harington, C. R. (1964). Polar bears and their present status. Canadian Audubon Magazine Jan-Feb, 3–10.
Harris, R. B., and Metzgar, L. H. (1987). Estimating harvest rates of bears from sex-ratio changes. J. Wildl. Manage. 51, 802–811. doi: 10.2307/3801745
Heide-Jorgensen, M. P., Garde, E., Hansen, R. G., Tervo, O. M., Sinding, M. H. S., Witting, L., et al. (2020). Narwhals require targeted conservation. Science 370, 416–416. doi: 10.1126/science.abe7105
IUCN (2012). IUCN Red List Categories and Criteria: Version 3.1 Second Edition. Gland; Cambridge: IUCN.
Kokko, H., Lindström, J., and Ranta, E. (2001). “Life histories and sustainable harvesting,” in Conservation of Exploited Species, eds J. D. Reynolds, G. M. Mace, K. H. Redford and J. G. Robinson (Cambridge: Cambridge University Press), 301–322.
Kolenosky, G. B., Abraham, K. F., and Greenwood, C. J. (1992). Polar bears of Southern Hudson Bay. Final report, Polar Bear Project 1984-88, October 1992, Ontario Ministry of Natural Resources, Maple, Ontario.
Laidre, K. L., Stern, H., Kovacs, K. M., Lowry, L., Moore, S. E., Regehr, E. V., et al. (2015). Arctic marine mammal population status, sea ice habitat loss, and conservation recommendations for the 21st century. Conserv. Biol. 29, 724–737. doi: 10.1111/cobi.12474
Lande, R. (1998). Anthropogenic, ecological and genetic factors in extinction and conservation. Res. Popul. Ecol. 40, 259–269. doi: 10.1007/BF02763457
Larsen, T. S., and Stirling, I. (2009). The agreement on the conservation of polar bears - its history and future. Norwegian Polar Inst. Rep. Series 127, 16p.
Law, R. (1979). Harvest optimization in populations with age distributions. Am. Nat. 114, 250–259. doi: 10.1086/283472
Lee, L. J., and Taylor, M. K. (1994). Aspects of the polar bear harvest in the Northwest Territories. Int. Conf. Bear Res. Manage. 9, 237–243. doi: 10.2307/3872707
Lentfer, J. (1974). Agreement on conservation of polar bears. Polar Record 17, 327–330. doi: 10.1017/S0032247400032071
Lentfer, J. (1976). Polar bear management in Alaska. Bear Biol. Manage. 3, 209–213. doi: 10.2307/3872768
Lindstrom, J., and Kokko, H. (1998). Sexual reproduction and population dynamics: the role of polygyny and demographic sex differences. Proc. R. Soc. B Biol. Sci. 265, 483–488. doi: 10.1098/rspb.1998.0320
Lønø, O. (1965). The catches of polar bears in Arctic regions in the period 1945-1963. On Svalbard Fauna 6, 151–155.
Loughrey, A. G. (1956). The polar bear and its protection. Oryx 3, 233–239. doi: 10.1017/S0030605300038825
Lunn, N. J., Servanty, S., Regehr, E. V., Converse, S. J., Richardson, E., and Stirling, I. (2016). Demography of an apex predator at the edge of its range: impacts of changing sea ice on polar bears in Hudson Bay. Ecol. Appl. 26, 1302–1320. doi: 10.1890/15-1256
Lunn, N. J., Stirling, I., Andriashek, D., and Kolenosky, G. B. (1997). Re-estimating the size of the polar bear population in western Hudson Bay. Arctic 50, 234–240. doi: 10.14430/arctic1105
Lunn, N. J., Taylor, M., Calvert, W., Stirling, I., Obbard, M., Elliott, C., et al. (1998). “Polar bear management in Canada 1993-1996,” in Proceedings of the 12th Working Meeting of the IUCN/SSC Polar Bear Specialist Group 3-7 February 1997, Oslo, Norway. Occasional Paper of the IUCN Species Survival Commission No. 19, eds A.E. Derocher, G.W. Garner, N.J. Lunn & Ø. Wiig. (Gland; Cambridge: IUCN), 51–68.
Makeyev, V. M., Pitul'ko, V. V., and Kasparov, A. K. (1993). The natural environment of the de long archipelago and ancient man in the late Pleistocene and early Holocene. Polar Geogr. Geol. 17, 55–63. doi: 10.1080/10889379309377503
Mauritzen, M., Derocher, A. E., Wiig, O., Belikov, S. E., Boltunov, A. N., Hansen, E., et al. (2002). Using satellite telemetry to define spatial population structure in polar bears in the Norwegian and western Russian Arctic. J. Appl. Ecol. 39, 79–90. doi: 10.1046/j.1365-2664.2002.00690.x
McCullough, D. R. (1996). Spatially structured populations and harvest theory. J. Wildl. Manage. 60, 1–9. doi: 10.2307/3802033
Molnár, P. K., Derocher, A. E., Lewis, M. A., and Taylor, M. K. (2008). Modelling the mating system of polar bears: a mechanistic approach to the Allee effect. Proc. R. Soc. B Biol. Sci. 275, 217–226. doi: 10.1098/rspb.2007.1307
Molnár, P. K., Derocher, A. E., Thiemann, G. W., and Lewis, M. A. (2010). Predicting survival, reproduction and abundance of polar bears under climate change. Biol. Conserv. 143, 1612–1622. doi: 10.1016/j.biocon.2010.04.004
Molnár, P. K., Lewis, M. A., and Derocher, A. E. (2014). Estimating Allee dynamics before they can be observed: polar bears as a case study. PLoS ONE 9, e85410. doi: 10.1371/journal.pone.0085410
Nageak, B. P., Brouwer, C. D., and Schliebe, S. L. (1991). Polar bear management in the Southern Beaufort Sea: an agreement between the inuvialuit game council and the north slope borough fish and game committee. Trans. North Am. Wildl. Nat. Resources Conf. 56, 337–343.
Nelms, S. E., Alfaro-Shigueto, J., Arnould, J. P. Y., Avila, I. C., Nash, S. B., Campbell, E., et al. (2021). Marine mammal conservation: over the horizon. Endanger. Species Res. 44, 291–325. doi: 10.3354/esr01115
Nguyen, L., Pilfold, N. W., Derocher, A. E., Stirling, I., Bohart, A. M., and Richardson, E. (2017). Ringed seal (Pusa hispida) tooth annuli as an index of reproduction in the Beaufort Sea. Ecol. Indic. 77, 286–292. doi: 10.1016/j.ecolind.2017.02.003
Obbard, M. E. (2008). “Southern Hudson Bay polar bear project 2003-05,” in Final Report, Wildlife Research and Development, Section, Ontario Ministry of Natural Resources, Peterborough, Ontario, Canada, 64p.
Obbard, M. E., Middel, K. R., Stapleton, S., Thibault, I., Brodeur, V., and Jutras, C. (2013). “Estimating abundance of the Southern Hudson Bay polar bear subpopulation using aerial surveys, 2011 and 2012,” in Wildlife Research Series 2013-01, Ontario Ministry of Natural Resources, Science and Research Branch, 33p.
Obbard, M. E., Stapleton, S., Szor, G., Middel, K. R., Jutras, C., and Dyck, M. (2018). Re-assessing abundance of Southern Hudson Bay polar bears by aerial survey: effects of climate change at the southern edge of the range. Arctic Sci. 4, 634–655. doi: 10.1139/as-2018-0004
Paetkau, D., Amstrup, S. C., Born, E. W., Calvert, W., Derocher, A. E., Garner, G. W., et al. (1999). Genetic structure of the world's polar bear populations. Mol. Ecol. 8, 1571–1584. doi: 10.1046/j.1365-294x.1999.00733.x
Paloheimo, J. E., and Fraser, D. (1981). Estimation of harvest rate and vulnerability from age and sex data. J. Wildl. Manage. 45, 948–958. doi: 10.2307/3808102
Peacock, E., Derocher, A. E., Thiemann, G. W., and Stirling, I. (2011). Conservation and management of Canada's polar bears (Ursus maritimus) in a changing Arctic. Can. J. Zool. 89, 371–385. doi: 10.1139/z11-021
Peacock, E., Laake, J., Laidre, K. L., Born, E. W., and Atkinson, S. N. (2012). The utility of harvest recoveries of marked individuals to assess polar bear (Ursus maritimus) survival. Arctic 65, 391–400. doi: 10.14430/arctic4237
Peacock, E., Sahanatien, V., Stapleton, S., Derocher, A., and Garshelis, D. (2009). Foxe Basin Polar Bear Project 2009 Interim Report. Wildlife Research Section, Department of Environment, Goverment of Nunavut, 58p.
Peacock, E., Sonsthagen, S. A., Obbard, M. E., Boltunov, A., Regehr, E. V., Ovsyanikov, N., et al. (2015). Implications of the circumpolar genetic structure of polar bears for their conservation in a rapidly warming Arctic. PLoS ONE 10, e112021. doi: 10.1371/journal.pone.0112021
Peacock, E., Taylor, M. K., Laake, J., and Stirling, I. (2013). Population ecology of polar bears in Davis Strait, Canada and Greenland. J. Wildl. Manage. 77, 463–476. doi: 10.1002/jwmg.489
Pistorius, P. A., de Bruyn, P. J. N., and Bester, M. N. (2011). Population dynamics of southern elephant seals: a synthesis of three decades of demographic research at Marion Island. Afr. J. Mar. Sci. 33, 523–534. doi: 10.2989/1814232X.2011.637357
Polar Bear Specialist Group (1976). Proceedings of the 5th Working Meeting of the Polar Bear Specialist Group, 3-5 December 1974, St. Prex, Switzerland. Morges, Switzerland: IUCN.
Polar Bear Specialist Group (1980). Proceedings of the 6th and 7th Working Meeting of the IUCN/SSC Polar Bear Specialist Group, 7-10 December 1976, IUCN Headquarters, Switzerland, and 30 Jan- 1 Feb 1979. Copenhagen, Denmark. IUCN.
Polar Bear Specialist Group (1985). Proceedings of the 8th working meeting of the IUCN/SSC Polar Bear Specialist Group, 15-19 January 1981. Oslo, Norway. IUCN.
Polar Bear Specialist Group (1986). Proceedings of the 9th working meeting of the IUCN/SSC Polar Bear Specialist Group 9-11 August 1985. Edmonton, Canada. IUCN.
Polar Bear Specialist Group (1991). Proceedings of the 10th working meeting of the IUCN/SSC Polar Bear Specialist Group 25-29 October 1988. Sochi, USSR. IUCN.
Polar Bear Specialist Group (1993). Proceedings of the 11th working meeting of the IUCN/SSC Polar Bear Specialist Group 25-27 January 1993, Copenhagen, Denmark. IUCN.
Polar Bear Specialist Group (1998). Proceedings of the 12th working meeting of the IUCN/SSC Polar Bear Specialist Group 3-7 February 1997, Oslo, Norway. Gland, Switzerland and Cambridge, UK: IUCN.
Polar Bear Specialist Group (2002). Proceedings of the 13th working meeting of the IUCN/SSC Polar Bear Specialist Group 23-28 June 2001, Nuuk, Greenland. Gland, Switzerland and Cambridge, UK: IUCN.
Polar Bear Specialist Group (2006). Proceedings of the 14th working meeting of the IUCN/SSC Polar Bear Specialist Group 20-24 June 2005, Seattle, USA. Gland, Switzerland and Cambridge, UK: IUCN.
Polar Bear Specialist Group (2010). Proceedings of the 15th working meeting of the IUCN/SSC Polar Bear Specialist Group 29 June - 3 July 2009, Copenhagen, Denmark. Gland, Switzerland and Cambridge, UK: IUCN.
Polar Bear Specialist Group (2018). Proceedings of the 18th working meeting of the IUCN/SSC Polar Bear Specialist Group 7-11 June 2016, Anchorage, Alaska. Gland, Switzerland and Cambridge, UK: IUCN.
Polar Bear Specialist Group (2021). Status Report on the World's Polar Bear Subpopulations. Available online at: http://www.iucn-pbsg.org (accessed January 15, 2022).
Prestrud, P., and Stirling, I. (1994). The International Polar Bear Agreement and the current status of polar bear conservation. Aquatic Mammals 20, 113–124.
R Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: http://www.r-project.org (accessed June 11, 2019).
Regehr, E. V., Amstrup, S. C., and Stirling, I. (2006). “Polar bear population status in the Southern Beaufort Sea,” in US Geological Survey Open-File Report 2006-1337, 20p.
Regehr, E. V., Dyck, M., Iverson, S., Lee, D. S., Lunn, N. J., Northrup, J. M., et al. (2021a). Incorporating climate change in a harvest risk assessment for polar bears Ursus maritimus in Southern Hudson Bay. Biol. Conserv. 258, 109128. doi: 10.1016/j.biocon.2021.109128
Regehr, E. V., Hostetter, N. J., Wilson, R. R., Rode, K. D., St Martin, M., and Converse, S. J. (2018). Integrated population modeling provides the first empirical estimates of vital rates and abundance for polar bears in the Chukchi Sea. Sci. Rep. 8, 16780. doi: 10.1038/s41598-018-34824-7
Regehr, E. V., Hunter, C. M., Caswell, H., Amstrup, S. C., and Stirling, I. (2010). Survival and breeding of polar bears in the southern Beaufort Sea in relation to sea ice. J. Anim. Ecol. 79, 117–127. doi: 10.1111/j.1365-2656.2009.01603.x
Regehr, E. V., Laidre, K. L., Akçakaya, H. R., Amstrup, S. C., Atwood, T. C., Lunn, N. J., et al. (2016). Conservation status of polar bears (Ursus maritimus) in relation to projected sea-ice declines. Biol. Lett. 12, 20160556. doi: 10.1098/rsbl.2016.0556
Regehr, E. V., Lunn, N. J., Amstrup, S. C., and Stirling, L. (2007). Effects of earlier sea ice breakup on survival and population size of polar bears in western Hudson bay. J. Wildl. Manage. 71, 2673–2683. doi: 10.2193/2006-180
Regehr, E. V., Runge, M. C., Von Duyke, A., Wilson, R. R., Polasek, L., Rode, K. D., et al. (2021b). Demographic risk assessment for a harvested species threatened by climate change: polar bears in the Chukchi Sea. Ecol. Appl. 31, e02461. doi: 10.1002/eap.2461
Regehr, E. V., Wilson, R. R., Rode, K. D., Runge, M. C., and Stern, H. L. (2017). Harvesting wildlife affected by climate change: a modelling and management approach for polar bears. J. Appl. Ecol. 54, 1534–1543. doi: 10.1111/1365-2664.12864
Richardson, E. S., Davis, C., Stirling, I., Derocher, A. E., Lunn, N. J., and Malenfant, R. M. (2020). Variance in lifetime reproductive success of male polar bears. Behav. Ecol. 31, 1224–1232. doi: 10.1093/beheco/araa074
Rocha, R. C. J., Clapham, P. J., and Ivashchenko, Y. V. (2015). Emptying the oceans: a summary of industrial whaling catches in the 20th century. US Natl. Mar. Fish. Serv. Mar. Fish. Rev. 76, 37–48. doi: 10.7755/MFR.76.4.3
Rode, K. D., Amstrup, S. C., and Regehr, E. V. (2010). Reduced body size and cub recruitment in polar bears associated with sea ice decline. Ecol. Appl. 20, 768–782. doi: 10.1890/08-1036.1
Saveliev, A. A., Cronin, M., Zuur, A. F., Ieno, E. N., Walker, N. J., and Smith, G. M. (2009). “Incorporating temporal correlation in seal abundance data with MCMC,” in Mixed Effects Models and Extensions in Ecology With R, eds A. F. Zuur, E. N. Ieno, N. Walker, A. A. Saveliev and G. M. Smith. (New York, NY: Springer), 503–529.
Schacht, R., Kramer Karen, L., Székely, T., and Kappeler Peter, M. (2017). Adult sex ratios and reproductive strategies: a critical re-examination of sex differences in human and animal societies. Phil. Trans. R. Soc. B Biol. Sci. 372, 20160309. doi: 10.1098/rstb.2016.0309
Schipper, J., Chanson, J. S., Chiozza, F., Cox, N. A., Hoffmann, M., Katariya, V., et al. (2008). The status of the world's land and marine mammals: diversity, threat, and knowledge. Science 322, 225–230. doi: 10.1126/science.1165115
Schliebe, S. L., Evans, T. J., Fischbach, A. S., and Cronin, M. A. (1999). Using genetics to verify sex of harvested polar bears: management implications. Wildl. Soc. Bull. 27, 592–597.
Schweinsburg, R. E., Lee, L. J., and Latour, P. B. (1982). Distribution, movement and abundance of polar bears in Lancaster Sound, Northwest Territories (Canada). Arctic 35, 159–169. doi: 10.14430/arctic2316
Scientific Working Group to the Canada-Greenland Joint Commission on Polar Bear (2016). “Re-assessment of the Baffin Bay and Kane Basin polar bear subpopulations,” in Final Report to the Canada-Greenland Joint Commission on Polar Bear, x + 636p.
Sciullo, L., Thiemann, G. W., Lunn, N. J., and Ferguson, S. H. (2017). Intraspecific and temporal variability in the diet composition of female polar bears in a seasonal sea ice regime. Arctic Science 3, 672–688. doi: 10.1139/as-2017-0004
Scott, R. F., Kenyon, K. W., Buckley, J. L., and Olson, S. T. (1959). “Status and management of the polar bear and Pacific walrus,” in Transactions of the 24th North American Wildlife Conference. Vol. 24, 366–373.
Stapleton, S., Atkinson, S., Hedman, D., and Garshelis, D. (2014). Revisiting Western Hudson Bay: using aerial surveys to update polar bear abundance in a sentinel population. Biol. Conserv. 170, 38–47. doi: 10.1016/j.biocon.2013.12.040
Stapleton, S., Peacock, E., and Garshelis, D. (2016). Aerial surveys suggest long-term stability in the seasonally ice-free Foxe Basin (Nunavut) polar bear population. Mar. Mamm. Sci. 32, 181–201. doi: 10.1111/mms.12251
Stirling, I. (2002). Polar bears and seals in the Eastern Beaufort Sea and Amundsen Gulf: a synthesis of population trends and ecological relationships over three decades. Arctic 55 (Suppl. 1), 59–76. doi: 10.14430/arctic735
Stirling, I., and Andriashek, D. (1992). Terrestrial maternity denning of polar bears in the Eastern Beaufort Sea area. Arctic 45, 363–366. doi: 10.14430/arctic1415
Stirling, I., Andriashek, D., Spencer, C., and Derocher, A. E. (1988). “Assessment of the polar bear population in the Eastern Beaufort Sea,” in Final Report to the Northern Oil and Gas Assessment Program, Canadian Wildlife Service, Edmonton, 81p.
Stirling, I., Calvert, W., and Andriashek, D. (1980). “Population ecology studies of the polar bear in the area of Southeastern Baffin Island,” in Canadian Wildlife Service Occasional Paper 44, 33p.
Stirling, I., Calvert, W., and Andriashek, D. (1984). “Polar bear (Ursus maritimus) ecology and environmental considerations in the Canadian High Arctic,” in Northern Ecology and Resource Management, eds R. H. Olsen and R. Geddes (Edmonton: University of Alberta Press), 201–222.
Stirling, I., and Derocher, A. E. (1993). Possible impacts of climatic warming on polar bears. Arctic 46, 240–245. doi: 10.14430/arctic1348
Stirling, I., Lunn, N. J., and Iacozza, J. (1999). Long-term trends in the population ecology of polar bears in Western Hudson Bay in relation to climate change. Arctic 52, 294–306. doi: 10.14430/arctic935
Stirling, I., McDonald, T. L., Richardson, E. S., Regehr, E. V., and Amstrup, S. C. (2011). Polar bear population status in the northern Beaufort Sea, Canada, 1971-2006. Ecol. Appl. 21, 859–876. doi: 10.1890/10-0849.1
Stirling, I., and Oritsland, N. A. (1995). Relationships between estimates of ringed seal (Phoca hispida) and polar bear (Ursus maritimus) populations in the Canadian Arctic. Can. J. Fish. Aquatic Sci. 52, 2594–2612. doi: 10.1139/f95-849
Stirling, I., and Parkinson, C. L. (2006). Possible effects of climate warming on selected populations of polar bears (Ursus maritimus) in the Canadian Arctic. Arctic 59, 261–275. doi: 10.14430/arctic312
Taylor, M. K., Demaster, D. P., Bunnell, F. L., and Schweinsburg, R. E. (1987). Modeling the sustainable harvest of female polar bears. J. Wildl. Manage. 51, 811–820. doi: 10.2307/3801746
Taylor, M. K., Laake, J., Cluff, H. D., Ramsay, M., and Messier, F. (2002). Managing the risk from hunting for the Viscount Melville Sound polar bear population. Ursus 13, 185–202.
Taylor, M. K., Laake, J., McLoughlin, P. D., Born, E. W., Cluff, H. D., Ferguson, S. H., et al. (2005). Demography and viability of a hunted population of polar bears. Arctic 58, 203–214. doi: 10.14430/arctic411
Taylor, M. K., Laake, J., McLoughlin, P. D., Cluff, H. D., Born, E. W., Rosing-Asvid, A., et al. (2008a). Population parameters and harvest risks for polar bears (Ursus maritimus) of Kane Basin, Canada and Greenland. Polar Biol. 31, 491–499. doi: 10.1007/s00300-007-0375-y
Taylor, M. K., Laake, J., McLoughlin, P. D., Cluff, H. D., and Messier, F. (2006b). Demographic parameters and harvest-explicit population viability analysis for polar bears in M'Clintock Channel, Nunavut, Canada. J. Wildl. Manage. 70, 1667–1673. doi: 10.2193/0022-541X(2006)701667:DPAHPV2.0.CO;2
Taylor, M. K., Laake, J., McLoughlin, P. D., Cluff, H. D., and Messier, F. (2008b). Mark-recapture and stochastic population models for polar bears of the high Arctic. Arctic 61, 143–152. doi: 10.14430/arctic19
Taylor, M. K., Laake, J., McLoughlin, P. D., Cluff, H. D., and Messier, F. (2009). Demography and population viability of polar bears in the Gulf of Boothia, Nunavut. Mar. Mamm. Sci. 25, 778–796. doi: 10.1111/j.1748-7692.2009.00302.x
Taylor, M. K., Lee, J., Laake, J., and McLoughlin, P. D. (2006a). “Estimating population size of polar bears in Foxe Basin, Nunavut, using tetracycline markers,” in Final Wildlife Report No. 1, Department of Environment, Government of Nunavut, Iqaluit, 29p.
Taylor, M. K., McLoughlin, P. D., and Messier, F. (2008c). Sex-selective harvesting of polar bears Ursus maritimus. Wildl. Biol. 14, 52–60. doi: 10.2981/0909-6396(2008)1452:SHOPBU2.0.CO;2
Thiemann, G. W., Iverson, S. J., and Stirling, I. (2008). Polar bear diets and Arctic marine food webs: insights from fatty acid analysis. Ecol. Monogr. 78, 591–613. doi: 10.1890/07-1050.1
Tovey, P. E., and Scott, R. F. (1957). A preliminary report on the status of the polar bear in Alaska. Alaska Sci. Conf 8, 1–11.
Tynan, C. T., and DeMaster, D. P. (1997). Observations and predictions of Arctic climatic change: potential effects on marine mammals. Arctic 50, 308–322. doi: 10.14430/arctic1113
Vajas, P., Calenge, C., Gamelon, M., Girard, F., Melac, O., Chandosne, C., et al. (2021). Catch-effort model used as a management tool in exploited populations: wild boar as a case study. Ecol. Indic. 124, 107442. doi: 10.1016/j.ecolind.2021.107442
Vié, J.-C., Hilton-Taylor, C., and Stuart, S. N. (2009). Wildlife in a Changing World - An Analysis of the 2008 IUCN Red List of Threatened Species. Gland: IUCN.
Vongraven, D., Aars, J., Amstrup, S. C., Atkinson, S. N., Belikov, S. E., Born, E. W., et al. (2012). A circumpolar monitoring framework for polar bears. Ursus Monogr. 5, 1–66. doi: 10.2192/URSUS-D-11-00026.1
Weinbaum, K. Z., Brashares, J. S., Golden, C. D., and Getz, W. M. (2013). Searching for sustainability: are assessments of wildlife harvests behind the times? Ecol. Lett. 16, 99–111. doi: 10.1111/ele.12008
Wenzel, G. W. (2011). Polar bear management, sport hunting and Inuit subsistence at Clyde River, Nunavut. Mar. Policy 35, 457–465. doi: 10.1016/j.marpol.2010.10.020
Wiig, O. (2005). Are polar bears threatened? Science 309, 1814–1815. doi: 10.1126/science.309.5742.1814d
Wiig, Ø., Born, E., and Garner, G. W. (1993). Proceedings of the 11th Working Meeting of the IUCN/SSC Polar Bear Specialist Group 25-27 January 1993. Copenhagen. IUCN.
Wittemyer, G., Northrup, J. M., Blanc, J., Douglas-Hamilton, I., Omondi, P., and Burnham, K. P. (2014). Illegal killing for ivory drives global decline in African elephants. Proc. Natl. Acad. Sci. U. S. A. 111, 13117–13121. doi: 10.1073/pnas.1403984111
Wood, S. N. (2017). Generalized Additive Models: An Introduction With R, 2nd Edn. Boca Raton, FL: Chapman and Hall/CRC.
Keywords: harvest, harvest management, polar bear, sustainability, over-harvest, conservation
Citation: Vongraven D, Derocher AE, Pilfold NW and Yoccoz NG (2022) Polar Bear Harvest Patterns Across the Circumpolar Arctic. Front. Conserv. Sci. 3:836544. doi: 10.3389/fcosc.2022.836544
Received: 15 December 2021; Accepted: 07 April 2022;
Published: 12 May 2022.
Edited by:
William J. McShea, Smithsonian Conservation Biology Institute (SI), United StatesReviewed by:
Sterling Miller, Alaska Department of Fish and Game, United StatesErik Andersen, United States Fish and Wildlife Service (USFWS), United States
Copyright © 2022 Vongraven, Derocher, Pilfold and Yoccoz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dag Vongraven, ZGFnLnZvbmdyYXZlbkBucG9sYXIubm8=
 Dag Vongraven
Dag Vongraven Andrew E. Derocher
Andrew E. Derocher Nicholas W. Pilfold
Nicholas W. Pilfold Nigel G. Yoccoz
Nigel G. Yoccoz