
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Conserv. Sci., 09 August 2022
Sec. Animal Conservation
Volume 3 - 2022 | https://doi.org/10.3389/fcosc.2022.759046
This article is part of the Research TopicLadybirds: Conservation, Ecology and Interactions with Other OrganismsView all 12 articles
The invasive alien species Harmonia axyridis (Coleoptera: Coccinellidae) was first observed in the UK in 2004. Previous studies have demonstrated the adverse effects on other species of H. axyridis during its early stages of establishment. However, habitat factors are important in determining distribution and population trends of ladybirds. Whilst the abundance of H. axyridis is well known in the UK within urban and other managed habitats, much less is known about its abundance in the wider countryside. Here we present the results of surveys from rural woodland habitats to assess whether or not H. axyridis dominates coccinellid communities in these rural habitats. Additionally, we explored the relationship between coccinellid and aphid abundance within these habitats. All field sites were in Cambridgeshire or Suffolk, East Anglia, UK and were surveyed between May and October 2016 and 2017. Three deciduous sites and three coniferous sites were included in the study. Surveys were conducted using a standardised approach involving sweep-netting within grass margins and tree beating to sample ladybirds from trees. Three distinct vegetation structures or layers were surveyed within both the coniferous and deciduous sites; tree, shrub and herb layer. All captured coccinellids were identified to species-level. Seventeen species of coccinellid and over 1300 individuals were recorded during the study period from two distinct site types (deciduous, coniferous). Species richness was lower at deciduous sites (n = 12) in comparison to coniferous (n = 16) sites. The coccinellid community also did not appear to be dominated by H. axyridis at rural sites, in contrast to urban areas. Deciduous woodland appeared to be a lesser preferred habitat of H. axyridis than coniferous woodland. Additionally, there was a distinct difference in the coccinellid community in relation to vegetation structure (across the tree, shrub and herb layers) between coniferous and deciduous sites. Our results indicate that there appear to be distinct native coccinellid communities at deciduous and coniferous sites. We discuss the way in which rural woodlands could act as a refuge for some native coccinellids.
The natural world is changing rapidly with increased human activity having dramatic consequences for biodiversity and ecosystems (IPBES, 2019). The last 50 years has seen a rapid escalation in the movement of animals and plants, locally and globally, often resulting in species establishing in habitats where they would not otherwise naturally occur (Blackburn et al., 2014; Lucy et al., 2016). The number of species being introduced to regions beyond their natural range has risen steadily over the last two centuries, with increasing trade and travel but also linked to other drivers of environmental change that facilitate biological invasions such as land and sea use change, climate change and pollution (Seebens et al., 2017; IPBES, 2019). Change in invertebrate biodiversity, including the addition of invasive alien species within ecological networks, is a major concern globally (Didham et al., 2005; Mikanowski, 2017). When introduced to a new region, generalist alien species are more likely to become invasive than specialist species; this can result in native specialist species being outcompeted and thereby leading to functional homogenisation (Clavel et al., 2011). It can be difficult to ascertain the effect that the presence of an invasive alien species (IAS) may have on ecosystem function, however in recent years, there has been evidence of the effects on the invaded ecosystem and community (Simberloff et al., 2013) due to invasive alien plants (Liao et al., 2007) and invasive alien aquatic invertebrates (Crawford et al., 2006; Constable and Birkby, 2016; Mathers et al., 2016).
Many coccinellid species provide ecosystem services in the form of pest control (Roy et al., 2012; Honěk et al., 2017). While there have been notable successes of introducing coccinellids for biological control (Dixon et al., 1997; Fowler, 2004), a number of introduced species (e.g. Harmonia axyridis, Coccinella septempunctata) have become established beyond their release sites and have subsequently had negative effects on native coccinellid species (Evans, 2000; Adriaens et al., 2008; Brown et al., 2011a; Roy et al., 2016; Sloggett, 2017); hence considered as IAS. These IAS are often studied in urban (Brown et al., 2011a; Viglášová et al., 2017) or agricultural habitats (Bianchi et al., 2007; Grez et al., 2008; Grez et al., 2014a), however less is known of the ecology of these species in rural habitats1.
In any habitat, a small number of dominant coccinellid species (between two and four) are expected to comprise around 90% of the community (Honěk, 2012). Selyemová et al. (2007) reported a diverse coccinellid community in rural coniferous woodland that was dominated by four species, however, H. axyridis was not established in the region at the time. When investigating overwintering coccinellids in coniferous woodland, Holecová et al. (2018) reported that H. axyridis was not the most abundant coccinellid. In the UK, just as H. axyridis was establishing, Brown et al. (2011a) reported that H. axyridis was largely absent from coniferous woodland. Furthermore, Purse et al. (2014) predicted coniferous woodland could be a refuge for native coccinellids because climate models suggest these habitats are suboptimal for H. axyridis. Vegetation structure of a habitat can also influence coccinellid assemblages. Grassland has been shown to be a refuge for native coccinellid species with very few invasive alien coccinellids recorded in this habitat (Diepenbrock and Finke, 2013). Rural woodland generally consists of a range of tree species and areas of wild herbs/grassland. In Michigan (USA) coccinellid species richness was higher when the habitat was more complex and contained a range of vegetation structures from deciduous trees to grassland and crops (Colunga-Garcia et al., 1997). When non-crop vegetation was added to an agricultural habitat, coccinellid abundance increased (Woltz and Landis, 2014) and intraguild predation between a native coccinellid and H. axyridis decreased (Amaral et al., 2015). Additionally, trees and grassland tend to have a more diverse coccinellid community than crops (Honěk, 2012). Beginning to understand how and why certain habitats are used by particular coccinellid species would be beneficial to understanding the relationship between H. axyridis and native specialist coccinellid species (Sloggett and Majerus, 2000a). Competition for food resources from H. axyridis is one of the reasons why native coccinellids may be negatively affected (Brown et al., 2011a) and so monitoring aphid abundance adds another dimension to studies of coccinellid community dynamics.
Harmonia axyridis is an intraguild predator; i.e. it preys upon other aphid natural enemies including the eggs and larvae of other coccinellid species (Roy et al., 2011; Brown et al., 2011a). It has been suggested that an ongoing increase of H. axyridis numbers may lead to the extinction of some coccinellid species locally (Harmon et al., 2007; Adriaens et al., 2010; Comont et al., 2014; Honěk et al., 2016). Harmon et al. (2007) highlighted the decline of A. bipunctata over a broad geographic range after the invasion of C. septempunctata and H. axyridis in the North America. The dramatic decline of Coccinella novemnotata in North America has also been attributed to a combination of pressures exerted by both C. septempunctata and H. axyridis (Losey et al., 2012b; Tumminello et al., 2015; Ducatti et al., 2017). In Europe there has been a decline in A. bipunctata (Belgium & UK), Adalia decempunctata and Calvia quattuordecimguttata (Czech Republic) since the arrival and establishment of H. axyridis (Brown et al., 2011a; Roy et al., 2012; Honěk et al., 2016; Brown and Roy, 2018). In the UK, distribution of H. axyridis is well known within urban and other anthropogenic habitats and Labrie et al. (2008) reported H. axyridis surviving very cold winters only where people dwell, as this species prefers to over-winter in anthropogenic structures. Additionally, Brown et al. (2011b) reported that H. axyridis tended to oviposit and feed at sites that have human structures nearby. However, much less is known on detailed habitat use of H. axyridis in the wider countryside (Brown et al., 2011a; Brown and Roy, 2018).
Considering the aforementioned studies together with the documented declines of native coccinellids in urban areas strongly correlated with the presence of H. axyridis in the UK (Roy et al., 2012), it is important to understand how native coccinellids are faring in rural areas (Viglášová et al., 2017). The aim of this study was to explore the relationship between the invasive alien H. axyridis and native coccinellid species in rural habitats.
All sites were in Cambridgeshire or Suffolk and were identified based on the presence of native tree species. During the 2016 field season, four deciduous sites (Brampton Wood, Monk’s Wood, Raveley Wood and Wistow Wood) and two coniferous sites (two sites at King’s Forest, a much larger woodland at approximately 23km2) were sampled. Wistow Wood was removed as a site for 2017 as there were not sufficient tree numbers or grassland habitat to complete full surveys. An additional coniferous site at King’s Forest was added for surveying in 2017. Thus, during the 2017 field season three deciduous woodlands (Brampton Wood, Monk’s Wood and Raveley Wood) and three coniferous woodlands (three sites at King’s Forest) were surveyed (Table 1). Site locations can be found in Supplementary Material Figure S1. Grid references were recorded using a Garmin GPSmap 60CSx. Surveys took place from the beginning of May to the end of October incorporating two seasons: summer (May, June, July) and autumn (August, September, October). In order to standardise data collection sampling took place between 10:00 and 16:00 when weather conditions were favourable, i.e. when the temperature was greater than 14°C, conditions were dry and wind speeds were below 5 on the Beaufort scale (Jones et al., 2006). Some surveys were carried out when the temperature was below 14°C, however in such instances there was at least 60% sun. Humidity and ambient temperature were recorded using an EasyLog EL-21CFR-2-LCD.
Three vegetation layers were selected for data collection; tree, shrub and herb layer. These layers encompass the key vegetation types found within a woodland and collectively contain the majority of UK ladybird species (Roy et al., 2013). The tree and shrub species selected for surveying were all native to the UK. Additionally, the number of individuals of each tree/shrub species was sufficient to allow regular visits during the sampling season at the respective woodland sites and avoid over-sampling. The herb layer (grassland/grass layer) comprised low vegetation including grasses, wildflowers, thistle, bramble, saplings etc. Vegetation height in the grass margins did not exceed one metre in height. The shrub layer (intermediate layer) comprised shrubs (Hall et al., 2004) and immature trees, with the sampled plants being no higher than three metres. The species selected for data collection were hazel (Corylus avellana) and hawthorn (Crataegus monogyna) in deciduous woodland and immature Scots Pine (Pinus sylvestris) and birch (Betula pendula) in coniferous woodland. The tree layer (mature layer) consisted of trees that were over three metres high with the target species being oak (Quercus robur) and field maple (Acer campestre) in deciduous woodland and mature Scots pine (Pinus sylvestris) and silver birch (Betula pendula) in coniferous woodland. The tree and shrub layer are on occasion referred to collectively as woodland and the herb layer referred to as grassland.
Sweep-netting was used to sample coccinellids in the herb layer. This method involved the use of a sweep net which comprised of a white canvas bag (46cm in diameter) attached to a metal ring on a large pole. One sweep was carried out for one metre of distance walked with 100 metres of grassland being surveyed at deciduous and coniferous woodland sites only. Sweeping this area took approximately 25 minutes. An estimate of the percentage plant cover of the grass margin was determined by eye at each sampling point. Tree beating was used to collect ladybirds from the tree and shrub layers. This method involved using a stick (approximately 1.5 metres in length) to sharply tap tree branches whereby insects fell onto a large white beating tray (110cm x 86cm) (Roy et al., 2013). Three individual branches on each tree were sampled by tapping each branch three times in quick succession. Depending on accessibility, each survey was carried out around the full circumference of the tree. Ten trees of each species in both the intermediate and mature layers were surveyed in deciduous and coniferous woodland. Surveying ten trees within one gradient took approximately 25 minutes. All captured coccinellids were identified to species level in the field. Larvae in the early stages of development, especially first and second instar, are very difficult to identify to species level in the field and so where there was uncertainty the term ‘Early Stage Larva’ (ESL) was used. Additionally, third instar Harmonia spp. larvae are included in the ESL group due to the similarity between H. axyridis and H. quadripunctata at this life stage. Just over six percent of larval records were not identifiable due to their early stage (ESL) (Table S1) and were excluded from analyses. The number of aphids (adult and immature) captured during sweeping/tree beating were also recorded. Due to potentially very large numbers being present these numbers were estimated in increments of 5 (for example, 1, 5, 10, 15, 20, 25, etc.). Aphids (Aphidoidea) were identified to superfamily.
Analyses was carried out using R Studio (R Core Team, 2019) except for canonical correspondence analysis which was carried out in PAleontological Statistics (PAST) Version 3.23 (Hammer et al., 2001). The following R packages were used for basic analyses and visualisation of data: dplyr (Wickham et al., 2019), ggfortify (Horikoshi and Tang, 2016; Tang et al., 2016), ggplot2 (Wickham, 2016), ggpubr (Kassambara, 2018). For multivariate analyses three packages were used: Hotelling (Curran, 2018), lattice (Sarkar, 2008) and vegan (Oksanen et al., 2019). The remaining packages used for regression analyses were: fmsb (Nakazawa, 2018), lmtest (Zeileis and Hothorn, 2002), pscl (Zeileis et al., 2008), sandwich, (Zeileis, 2004; Zeileis, 2006), lattice and MASS (Venables and Ripley, 2002). Wilcoxon paired tests were used to compare abundances of native coccinellids and H. axyridis at the same locations, e.g. deciduous woodland. Spearman’s correlation was utilised to investigate any association between both H. axyridis and native coccinellid abundance and that of aphids.
Generalised linear models (GLM) were utilised to investigate the effects of site type (deciduous, coniferous), vegetation structure (tree, shrub, herb) and season (summer, autumn) on coccinellid and aphid abundance. Data from tree and shrub layer were analysed together under the collective term ‘woodlands’ when investigating site type while the grassland data was analysed separately due to differences in sampling method. Environmental variables (temperature, humidity) were included in the models. We assessed overdispersion commonly associated with count data and accordingly used log Likelihood, Akaike Information Criterion (AIC) and weighted AIC, with the weighted AIC being the deciding factor as to which model was the best fit. In the majority of cases, either a zero-inflated negative binomial (ZINB) model or negative binomial regression (NB) model were the best fit for the data, and on occasion the null model was the better fit. Temperature and humidity were checked for collinearity with a variance inflation factor (VIF). Neither variables were of concern with a VIF of < 1.2 each, and both were incorporated into the regression models. Table 2 presents which model was the best fit for explaining the effects of the variables on H. axyridis, native coccinellid and aphid abundance in rural woodlands and rural grasslands. Model comparisons can be found in Supplementary Material, Tables S3–S19.
Shannon Diversity was calculated for rural sites only and for native coccinellid species only. Simpson’s diversity was not carried out as rare species or those recorded in low numbers are not given the same consideration by the index as more abundant species by this measure (Magurran, 2004; Morris et al., 2014). Differences in diversity across site types and season were calculated using t-tests while ANOVA was used to assess any differences in diversity within the vegetation structure followed by a post-hoc Tukey if any significances were apparent. Regression models were run to determine if native coccinellid diversity had any effect on the abundance of H. axyridis and native coccinellids.
Canonical Correspondence Analysis (CCA) detects patterns of variation in a given community that can be explained by environmental data. The analysis focuses on beta-diversity (how dissimilar sites are) instead of alpha diversity (diversity of a site) (Zuur et al., 2007). This method of multivariate analysis generates an ordination diagram where a given species point is at the weighted average or centroid of the sites where it was recorded (ter Braak and Verdonschot, 1995). The qualitative environmental variables (site type and vegetation layer) are illustrated by a point that is the centroid of site points belonging to that group, for example the weighted average of the tree layer where the weight is the total abundance of the tree layer (ter Braak and Verdonschot, 1995). This analysis was used to investigate the relationship that two variables had on the coccinellid assemblage; site type (deciduous or coniferous) and vegetation layer (tree, shrub or herb). The coccinellid data were fourth root transformed to remove any effect of highly abundant species (Chessman, 2003; Pickwell, 2012). Interpretation of the resulting ordination is based on the eigenvalues, statistical significance determined by Monte Carlo permutation test and ecological interpretability (ter Braak and Verdonschot, 1995). In this case, the biplot rule (described below) was applied as the eigenvalues were less than 0.4 and this rule is more informative than the centroid rule when eigenvalues are low (ter Braak and Verdonschot, 1995). Firstly, the direction of maximum change in the relative abundance of a species (e.g. species X or Y) was determined by drawing a line from Species X to the origin. Subsequently the sites were then projected onto the arrow for Species X, illustrating the share each site (site A or B) has in the total abundance of each species (ter Braak and Verdonschot, 1995; Zuur et al., 2007). To interpret how a species relates to an environmental variable, imagine the variable line (e.g. ‘Type’) is extended in the opposite direction for the same distance, forming an axis of its own. Each species can be projected perpendicular to the axis, indicating the species relationship with that variable (Zuur et al., 2007). The combination of regression models, the Shannon diversity index and the ordination analysis yielded a detailed representation of coccinellid assemblages.
Seventeen species of coccinellid totalling 1,330 individuals were recorded during the study period across three different vegetation gradients (tree, shrub & herb layer) from deciduous and coniferous woodland. Just eight of these coccinellid species were recorded in the herb layer in comparison to 16 species on trees & shrubs in woodland (see Supplementary Material Tables S1, S2). Five species (Myzia oblongoguttata, Myrrha octodecimguttata, Scymnus suturalis, Subcoccinella vigintiquattuorpunctata and Tytthaspis sedecimpunctata) were recorded in coniferous woodland only, while one species (Psyllobora vigintiduopunctata) was recorded in deciduous woodland only. In grassland, four coccinellid species (Exochomus quadripustulatus, S. suturalis, S. vigintiquattuorpunctata and T. sedecimpunctata) were recorded at coniferous sites only. Inclusive of all three vegetation layers, species richness was lower at deciduous (n = 12) than coniferous (n = 16) sites.
Rural woodland site type (deciduous and coniferous) was analysed separately. Native coccinellid abundance was significantly greater than that of H. axyridis in deciduous woodland (median = 2 and 0 respectively, Z = -5.43, p < 0.001, r = 0.60) and coniferous woodland (median = 3.5 and 1 respectively, Z = -4.15, p < 0.001, r = 0.50) (Figure 1). The binary model of the ZINB revealed that the likelihood of recording H. axyridis was higher in the summer rather than autumn across rural woodlands combined (z = -3.011, p = 0.003). The only variable that affected H. axyridis abundance was vegetation layer in both deciduous only and coniferous only woodland, with a greater number recorded in the tree layer (z = 2.65, p = 0.008 and z = 2.82, p = 0.005 respectively) (Figure 1). The abundance of H. axyridis was higher during the summer (z = 4.78, p < 0.001) in deciduous woodland with no effect of season apparent in coniferous woodland. In addition to the results from the logistic model, the binary model explained in greater detail what the zeros represented in these data, with the likelihood of recording H. axyridis being significantly higher in coniferous woodland in comparison to deciduous woodland (z = 3.67, p = 0.0002). In rural woodland, deciduous sites had a significantly lower number of native coccinellids than did coniferous sites (z = -3.16, p = 0.002) (Figure 1). In deciduous woodland, vegetation layer had no effect on native coccinellid abundance, however, abundance was significantly higher in the tree layer of coniferous woodland as opposed to the shrub layer (z = 2.67, p = 0.008) (Figure 1). Season did not influence the abundance of native coccinellids in deciduous only or coniferous only woodland.
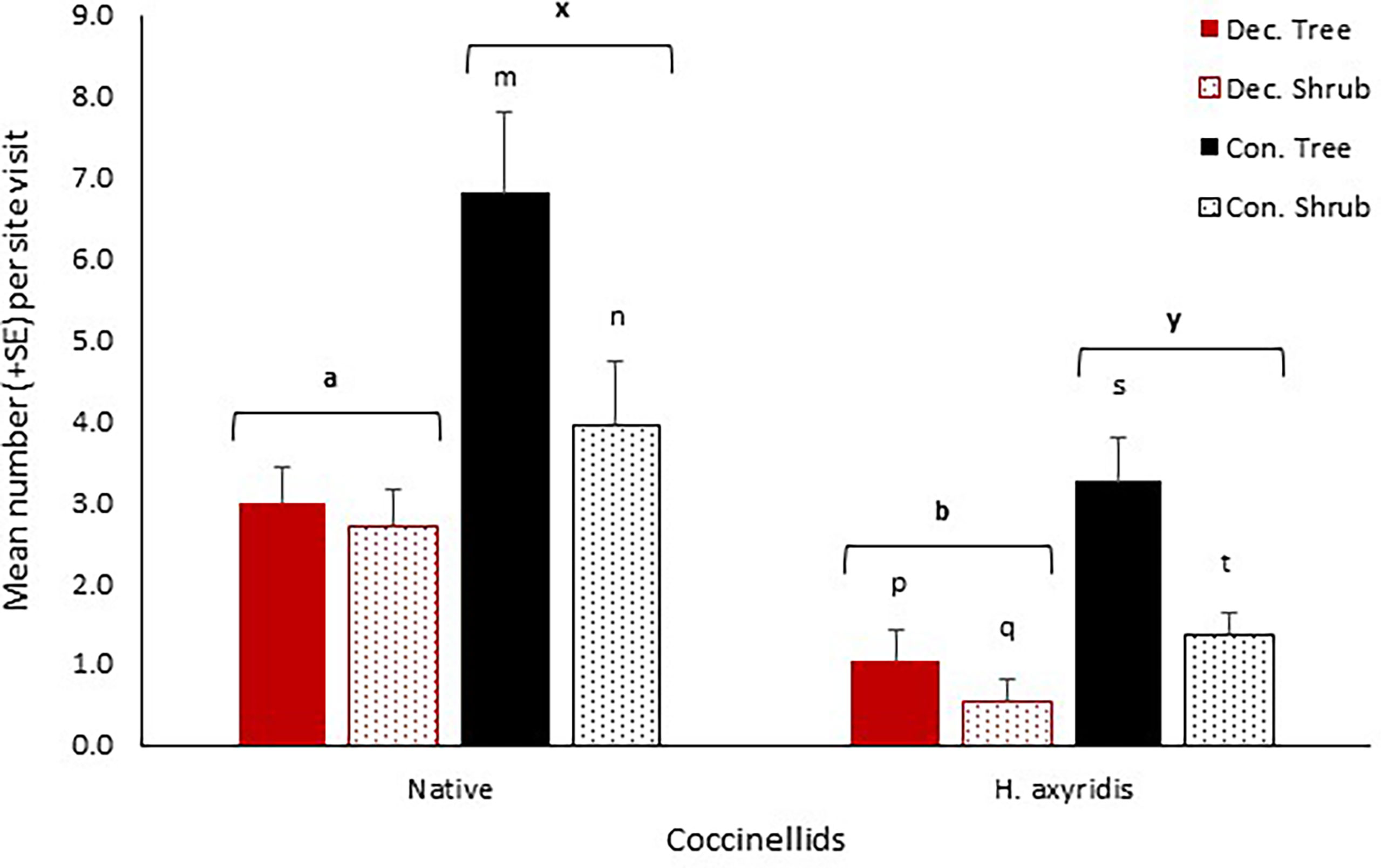
Figure 1 Mean number (+SE) per site visit of coccinellids recorded in woodland at deciduous-only and coniferous-only sites in Cambridgeshire and Suffolk. Native = all native coccinellids recorded; Dec. = Deciduous; Con. = Coniferous; Tree = Tree layer; Shrub = Shrub layer. Square brackets indicate the grouping of the Tree and Shrub layer for the respective deciduous and coniferous sites. Consecutive letters indicate where significant differences occur.
Eight coccinellid species totalling 405 individuals were recorded in the grassland habitat. Two of the species, S. vigintiquattuorpunctata and T. sedecimpunctata were predominantly confined to grassland (with only two occurrences of S. vigintiquattuorpunctata on trees). Very few H. axyridis were recorded in the herb layer (n = 12) and as a result it was not possible to apply any statistical analysis. Significantly fewer native coccinellids were recorded in grassland within deciduous habitat (z = -3.08, p = 0.002) as indicated by the reduced negative binomial model (Figure 2) than within grassland in coniferous habitats. In coniferous woodland, native coccinellids were significantly more abundant during summer rather than autumn (z = 3.23, p = 0.001). The null model was the best fit to investigate coccinellid abundance in grassland at deciduous sites revealing no effect of season on native coccinellid abundance.
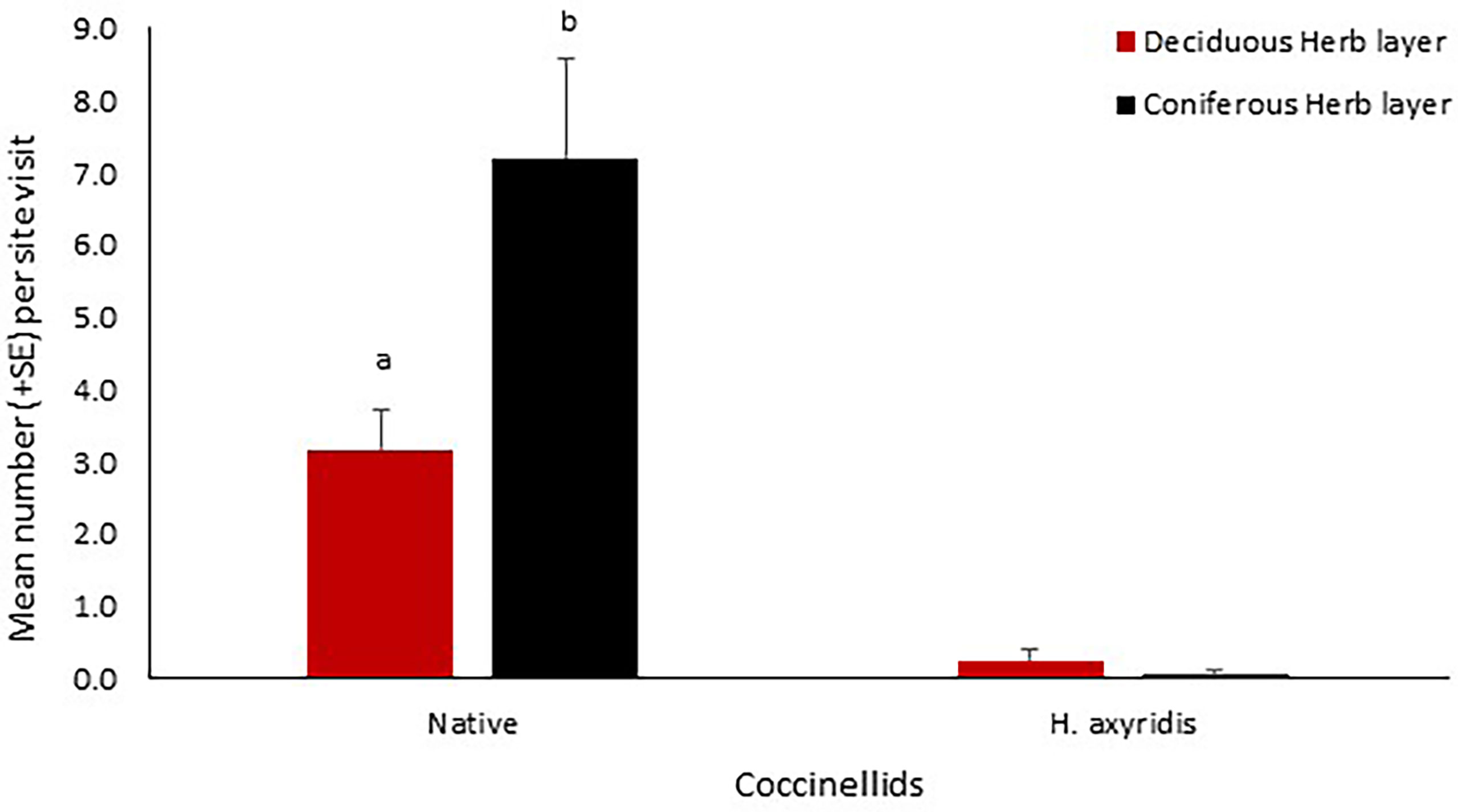
Figure 2 Mean number (+SE) per site visit of coccinellids recorded in the grass layer at deciduous and coniferous sites in Cambridgeshire and Suffolk. Native = all native coccinellids recorded. Consecutive letters indicate where significant differences occur.
When considered as an entire habitat, coniferous sites hosted a significantly higher native coccinellid diversity (t = 5.83, p < 0.001) than deciduous woodlands (Figure 3). In deciduous woodland sites, native coccinellid diversity varied significantly (one-way ANOVA: F = 4.35, p = 0.015) with the tree layer having greater diversity than the grass layer (p = 0.01) as revealed by a post-hoc Tukey test. Coniferous sites also exhibited differences between the different vegetation structures (F = 9.24, p <0.0002) with a significantly lower diversity in both the shrub layer and grass layer (p = 0.0005 & p = 0.001 respectively; Figure 3) in comparison to tree layer. There was no effect of seasonality on native coccinellid diversity in deciduous woodland, however native coccinellid diversity in coniferous woodlands was higher during the summer (t = -2.23, p = 0.02). The count part of the ZINB model revealed that native coccinellid diversity did not affect H. axyridis abundance, however, the binary model indicated that the probability of recording H. axyridis was significantly lower when native coccinellid diversity was higher (z = -2.37, p = 0.01). As expected, native coccinellid abundance was higher when native coccinellid diversity was higher (z = 5.6, p < 0.001).
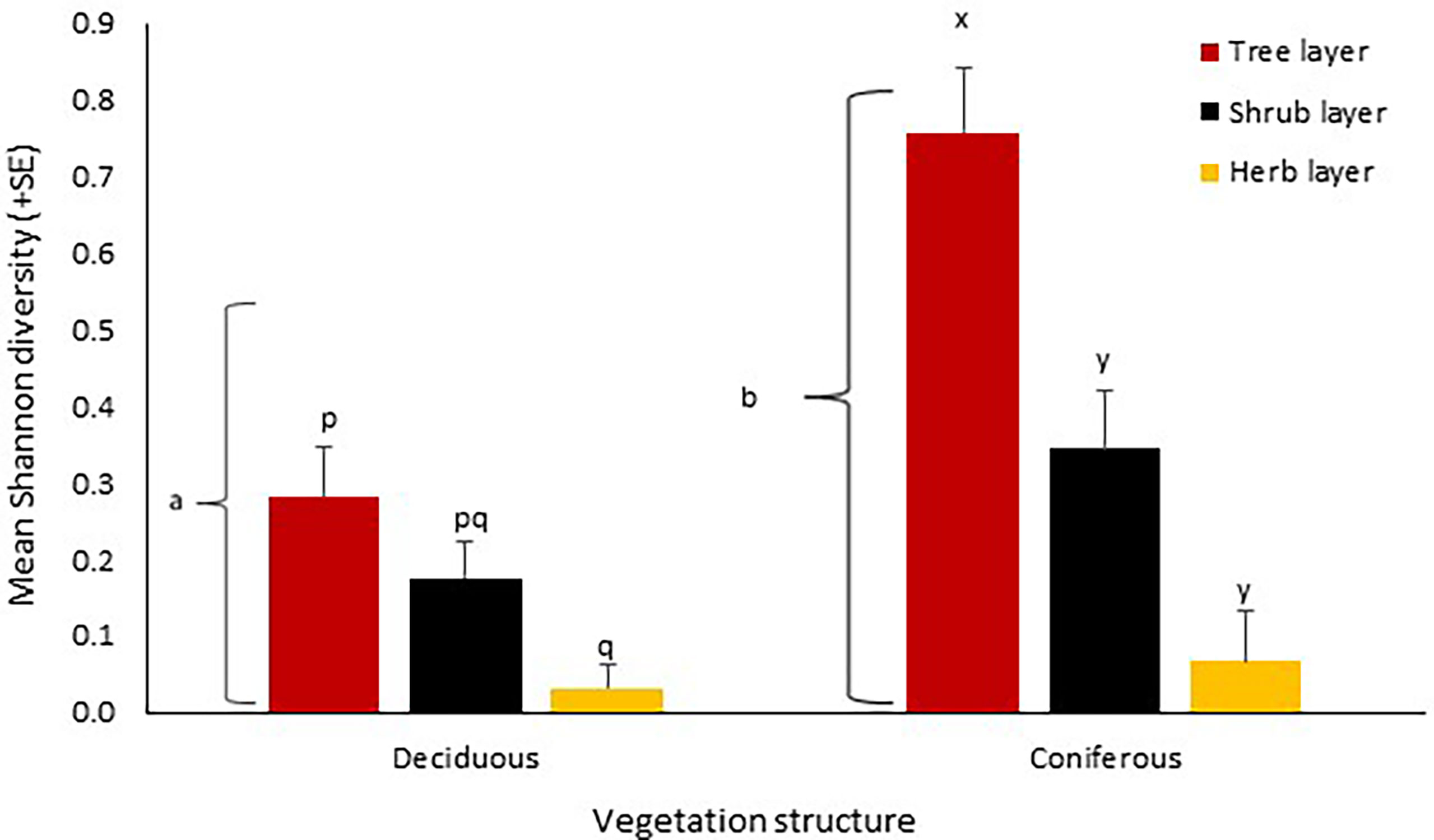
Figure 3 Mean Shannon diversity (+SE) of native coccinellid species at deciduous and coniferous sites and at different vegetation layers across all sites in Cambridgeshire and Suffolk. Consecutive letters indicate where significant differences occur. Letters on brackets represent differences between deciduous and coniferous sites collectively.
The coccinellid assemblage is represented by an ordination plot (Figure 4) which is interpreted below by starting with the environmental variables, Type and Layer. Focusing firstly on the ‘Type’ axis, there is a clear difference in the coccinellid communities that are associated with coniferous only and deciduous only sites. Some species are positively associated with coniferous (M. oblongoguttata, M. octodecimguttata, Scymnus suturalis., H. quadripunctata) and deciduous (Halyzia sedecimguttata, A. decempunctata, P. quattuordecimpunctata) sites while other species are more generalist and are associated with both sites in varying abundances (H. axyridis, E. quadripustulatus, C. septempunctata) (Figure 4). The ‘Layer’ axis also reveals that certain species are associated with particular vegetation layers and others are quite generalist in their habitat preferences. Habitat generalist species appear to aggregate along the centre of the ‘Layer’ axis (H. axyridis, E. quadripustulatus, Scymnus suturalis, P. quattuordecimpunctata) while the herb layer has a distinct coccinellid assemblage (P. vigintiduopunctata, S. vigintiquattuorpunctata, T. sedecimpunctata) (Figure 4).
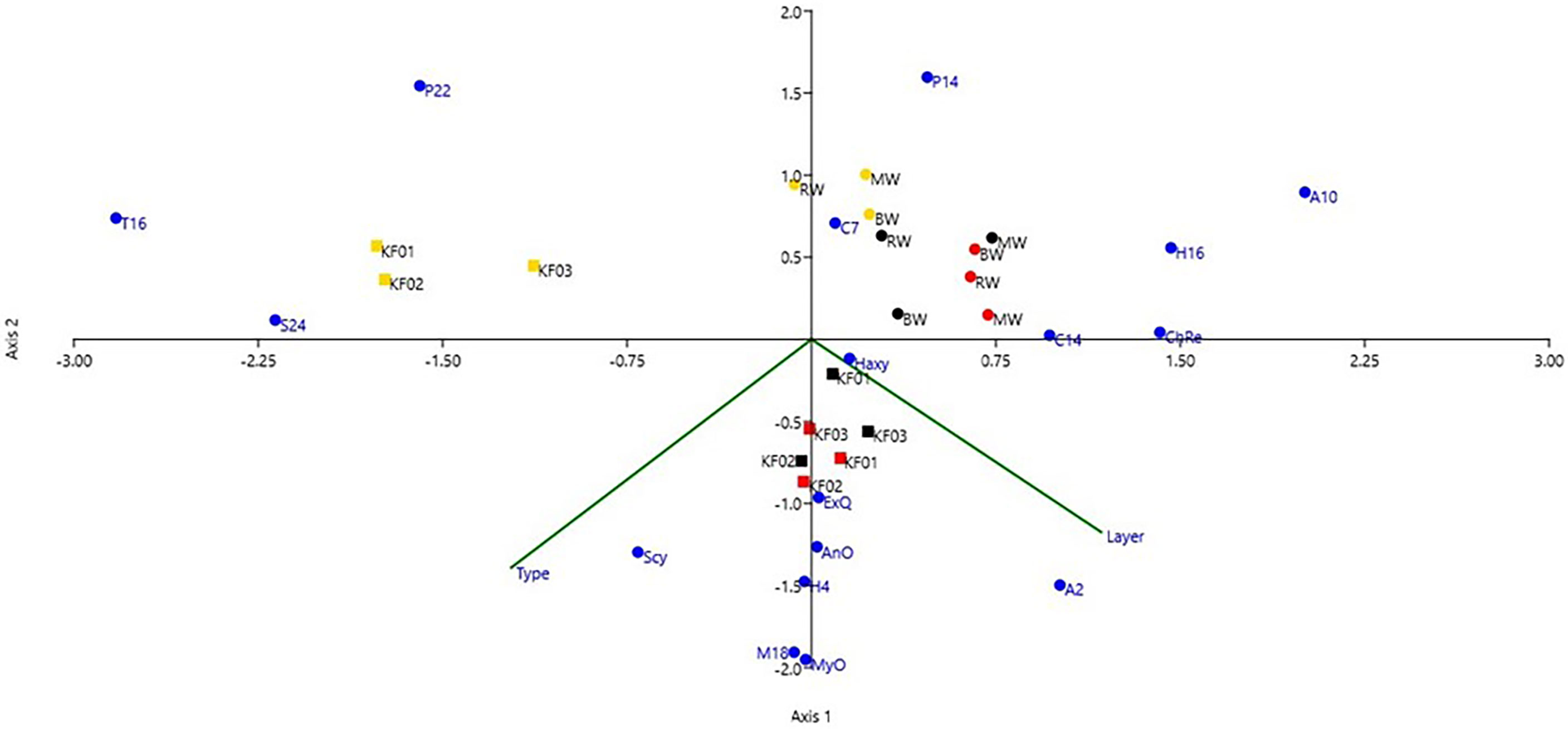
Figure 4 Species-conditional CCA triplot based on a canonical correspondence analysis of the coccinellid and environmental data recorded at rural sites in Cambridgeshire and Suffolk. Environmental vectors are amplified by a factor of two. Axis 1 explained 99.3% of the variation in the taxon-environmental structure and axis 2 explained 0.7% of the variation (Eigenvalues were 0.3505 and 0.0024 respectively); Type = coniferous sites (KF01, KF02, KF03 = Kings Forest site 1, 2 and 3) and deciduous sites (BW = Brampton Wood, MW = Monk’s Wood, RW = Raveley Wood); Layer = tree, shrub and herb layer (coloured in red, black and gold respectively); coniferous sites are indicated by filled squares, deciduous sites by filled dots and coccinellid species by blue dots; coccinellid species = (A2, Adalia bipunctata; A10, Adalia decempunctata; AnO, Anatis ocellata; C7, Coccinella septempunctata; C14, Calvia quattuordecimguttata; ChRe, Chilocorus renipustulatus; ExQ, Exochomus quadripustulatus; H4, Harmonia quadripunctata; H16, Halyzia sedecimguttata; Hax, Harmonia axyridis; M18, Myrrha octodecimguttata; MyO, Myzia oblongoguttata; P14, Propylea quatuordecimpunctata; P22, Psyllobora vigintiduopunctata; S24, Subcoccinella vigintiquatuorpunctata; Scy, Scymnus suturalis; T16, Tytthaspis sedecimpunctata).
Several species show a preference for the tree layer over the shrub layer in both coniferous (A. bipunctata, M. oblongoguttata, M. octodecimguttata, H. quadripunctata, Anatis ocellata) and deciduous sites (Chilocorus renipustulatus, Calvia quattuordecimguttata, H. sedecimguttata, A. decempunctata) (Figure 4). The herb layer at coniferous sites is distinct from the other coniferous vegetation layers and as expected is more similar to the deciduous herb layer (Figure 4). There was no difference in species diversity between the shrub and tree layer at deciduous sites and from the CCA plot (Figure 4) it becomes apparent that coccinellids use both vegetation structures with little variation between them, particularly when comparing the herb layer. For example, C. septempunctata (C7) is associated with both the herb and shrub layer, but with a greater abundance associated with the herb layer and a lower abundance associated with the tree layer. The tree and shrub layer at coniferous sites host similar coccinellid assemblages to each other. For example, E. quadripustulatus (ExQ) was associated across all coniferous sites for both the tree and shrub layer yet has a greater association with the tree layer. Two coccinellid species (T. sedecimpunctata & S. vigintiquattuorpunctata) dominated the herb layer at coniferous sites that were not associated with any other site type or vegetation layer.
Harmonia axyridis appears as a generalist in the ordination diagram, being situated close to the origin and almost halfway on both variable axes. This species, however, was more positively associated with coniferous sites and with the shrub layer (KF01, KF03, KF02 & BW), while H. axyridis was negatively associated with the herb layer at both site types. Associations with certain native coccinellid species were evident, however these species were not as abundant as H. axyridis. Both E. quadripustulatus (ExQ) and A. bipunctata (A2) have a similar association with coniferous sites as H. axyridis, however A. bipunctata is positively associated with the tree layer, unlike E. quadripustulatus which seemed to utilise both the tree and shrub layer (Figure 4).
Across all site types (deciduous, coniferous) and vegetation structures (tree, shrub and herb layers) a total of 10,683 aphids (prey) were recorded. Woodland type had an effect on aphid abundance with significantly lower abundance at deciduous sites (z = -3.34, p = 0.0008; Figure 5). Vegetation structure had no effect on aphid abundance at either deciduous or coniferous woodland sites. Harmonia axyridis abundance was positively associated with aphid abundance at coniferous-only woodland while native coccinellid abundance was negatively associated with aphid abundance at deciduous-only woodland (Table 3). There was a negative association between aphid and native coccinellid abundance in deciduous grassland (Table 3).
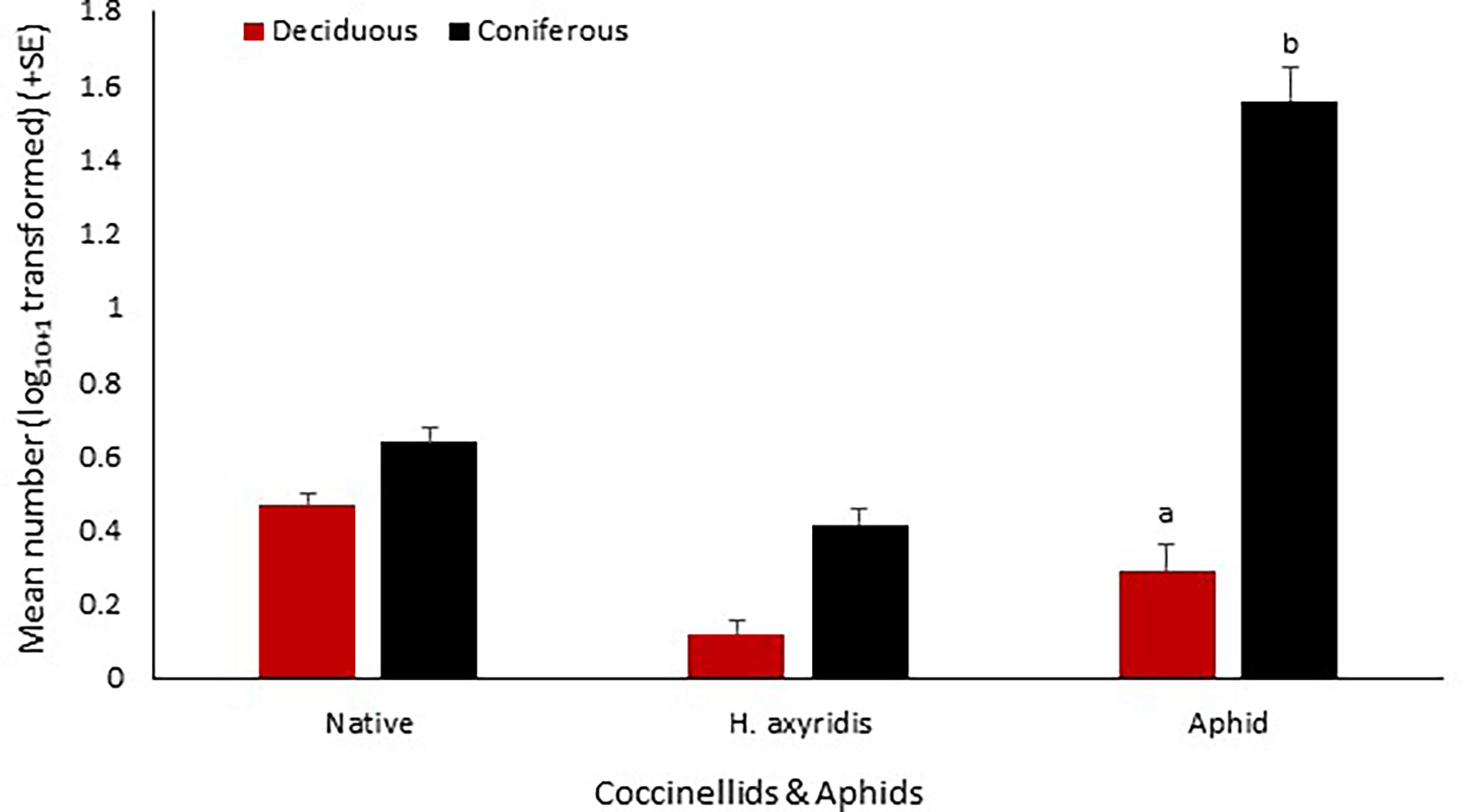
Figure 5 Mean number (log10+1 transformed) (+SE) per site visit of H. axyridis and native coccinellids recorded in relation to records of Aphids in East Anglia. Native = native coccinellids recorded. Consecutive letters indicate where significant differences occur.
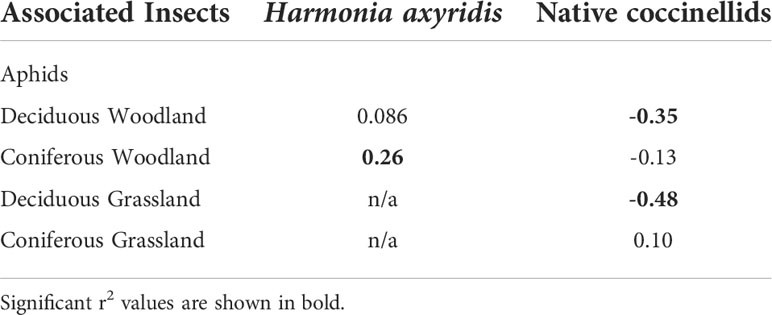
Table 3 Significance of Spearman correlations for coccinellid abundance with aphids recorded from trees at different site types.
In this study, native coccinellids were more abundant than H. axyridis in both coniferous and deciduous woodland. This contrasts with studies on coccinellid assemblages in urban habitats which are dominated by H. axyridis (Brown et al., 2011a; Viglášová et al., 2017). When comparing deciduous and coniferous woodland habitats, the coniferous sites hosted a higher number of native coccinellids than did deciduous sites. Previous studies have suggested that coniferous habitat may act as a barrier to the continuing establishment of H. axyridis, thereby providing a refuge for native coccinellids from H. axyridis (Brown et al., 2011a; Purse et al., 2014), but our results do not support this. It is possible that H. axyridis has adapted to conditions found at coniferous sites in the UK and can overwinter successfully within them. Harmonia axyridis is highly phenotypically plastic and can adapt to available habitats, prey efficiently (Majerus et al., 2006) and climatic extremes (Sloggett and Majerus, 2000b).
When investigating overwintering coccinellid assemblages, Holecová et al. (2018) found that even though H. axyridis was one of the most abundant species on Scots Pine, the majority of the time, either E. quadripustulatus or C. septempunctata made up a larger proportion of overwintering coccinellids, being similar to that observed in this study. Coniferous woodlands experience less extreme temperature variation than deciduous woodlands (Ferrez et al., 2011) and greater overwintering success as a result could explain the increased abundance of native coccinellids at these sites. Additionally, considering the preference H. axyridis has for more sheltered overwintering sites, it is possible that the large area of coniferous plantation in this study provided sufficient shelter to enable this species to overwinter successfully. With a shortage of knowledge on coccinellid assemblages at overwintering sites (Pendleton and Pendleton, 1997-2019; Hodek, 2012; Holecová et al., 2018) investigating the overwintering coccinellid assemblages in coniferous woodland would provide further knowledge of coccinellid behaviour and importantly how climate change may influence coccinellid assemblages in the future. Another feature of the coccinellid life cycle that may be impacted by climate change is diapause. As the onset of diapause varies among coccinellid species (Hodek, 2012) the coccinellid community can vary. Studies on coccinellid diapause tend to focus on one of a small number of species and very little is known in relation to how diapause may impact coccinellid assemblages. Exploring the influence of diapause would lead to a greater understanding of the coccinellid community.
There was a distinct difference in the coccinellid community in relation to vegetation structure (tree, shrub and herb layer) between coniferous and deciduous sites. Within these individual site types, vegetation structure affected both the abundance and distribution of different species. The tree layer in both deciduous and coniferous woodland supported the greatest coccinellid diversity and in both cases differed to the herb layer. At urban sites, Viglášová et al. (2017) found that coccinellid diversity also differed across the different vegetation types that were surveyed, with higher species diversity in trees in comparison to nettle stands. The herb layer in this current study also hosted a different coccinellid community to that of the tree and shrub layer, likely due to the very different food sources available in the herb layer (mildew, plant material, different aphids). Similar findings were reported by Viglášová et al. (2017) for the coccinellid species observed on nettle stands. The differences reported in this current research relate to both site types with a unique coccinellid assemblage at both coniferous and deciduous sites. Grassland specialists dominated the herb layer at coniferous sites (e.g. T. sedecimpunctata & S. vigintiquattuorpunctata), while generalist coccinellids, such C. septempunctata dominated at deciduous sites. Interestingly, Viglášová et al. (2017) reported seasonal differences in how C. septempunctata used different vegetation structures, with greater numbers in nettles in the summer, and higher abundance on trees later in the year. No such seasonal effect was evident within our study, however, C. septempunctata did make use of the different vegetation layers as previously illustrated.
The abundance of H. axyridis, when present, was not influenced by native coccinellid diversity, however, when native coccinellid diversity was higher, the probability of occurrence of H. axyridis was lower. In an agricultural habitat, Grez et al. (2021) reported native coccinellid diversity to be negatively associated with non-native coccinellid abundance. It is widely documented that H. axyridis is often the most abundant coccinellid at urban sites, however this was not the case for rural woodland and grassland habitats in this study. Adalia bipunctata is reported to have high niche overlap with H. axyridis (Sloggett, 2008), however, the extent of co-occurrence between H. axyridis and A. bipunctata is likely to vary between habitats (urban/rural, tree/grass). Recently, Gardiner et al. (2021) reported native coccinellid diversity to be positively associated with forested habitat, while being negatively associated with urbanised habitats.
A negative relationship was observed between native coccinellid and aphid abundance at deciduous sites but at coniferous sites H. axyridis and aphid abundance were positively correlated. The majority of coccinellids recorded at deciduous sites were C. septempunctata, which is a species that is known to tolerate areas with low aphid density (Honěk, 1985). The third and fourth most frequently observed coccinellids at deciduous sites were P. quattuordecimpunctata and A. decempunctata, both of which are also tolerant of low aphid abundance (Honěk, 1985). The relationship between coccinellid abundance and that of aphids, however, is not an easy one to tease apart, in part due to the dynamic nature of both aphid and coccinellid populations. Vandereycken et al. (2013) reported a positive relationship between aphids and coccinellids in a range of crop habitats. Conversely, when investigating coccinellids in urban areas, Viglášová et al. (2017) found the relationship between common coccinellid species and aphid abundance to be non-linear with coccinellid abundance increasing with that of aphids, however when aphid abundance became very high, coccinellid abundance decreased. Furthermore, Brown et al. (2011a) and Brown and Roy (2018) did not find any correlation between H. axyridis or aphidophagous coccinellids and aphid abundance. A network ecology approach may reveal more about the complex relationship between coccinellids and aphids.
The decline of native coccinellids is not solely a consequence of the arrival of H. axyridis and indeed some studies show that native species were in decline prior to its arrival (e.g. A. bipunctata & C. quinquepunctata in Czech Republic, Honěk et al., 2016). Climate change, land use change, intensification of agricultural practices (Honěk et al., 2016) and increased anthropogenic disturbance (Brown and Roy, 2018) may all have contributed to the decline of these species alongside the arrival of H. axyridis. There are suggestions that the initial decline of native species will reverse and that the invasive alien and native populations may stabilise and co-exist (Hentley et al., 2016). Research by Honěk et al. (2016) illustrates just how important long-term population studies are in having baseline data prior to the establishment of an IAS but also in determining how native coccinellid abundance can fluctuate over several years. Long-term studies in a range of habitats are needed to reveal a more complete picture on native coccinellid communities and how they change in the presence of IAS and other drivers of environmental change. Other process may be influencing the differences we observed between coniferous and deciduous sites, perhaps connectivity or adjacent land use or a combination of the two. Including these aspects in future work would enhance our knowledge of coccinellid communities and contribute to informing conservation action.
Coccinellid communities are not often the sole focus of studies and information on their structure tends to come as an add-on to other works (Honěk, 2012). More research needs to be initiated to investigate the coccinellid community as a whole and not just focus on individual species. In this study H. axyridis was less abundant than native coccinellids as a group within both rural woodland and rural grassland. A distinct native coccinellid assemblage was present within all three vegetation layers (tree, shrub and herb layer) sampled. Moving this research further forward, it would be interesting to explore how the coccinellid community varies with seasonality.
With increasing pressures from multiple drivers acting at varying temporal and spatial scales (Bonebrake et al., 2019), it is important to continue research into the dynamics of native coccinellid communities to inform appropriate conservation action. There is increasing evidence that the composition of landscapes can determine community composition (Gardiner et al., 2009), indeed Grez et al., 2014b demonstrated the importance of heterogeneous landscapes for increasing coccinellid diversity and abundance within agricultural systems. Indeed, conserving insect diversity, particularly as the pressure from global environmental change increases, will depend on improving spatial and temporal heterogeneity, including maintaining unique habitats and ensuring functional connectivity, to create landscape mosaics (Samways et al., 2020) that benefits insects including coccinellids. We have demonstrated the importance of different woodland habitats in supporting diverse coccinellid communities. Considering the complex relationship between aphids and generalist coccinellids it is important to further understand the importance of coccinellid diversity, how the communities exist in different landscape contexts and their role in ecosystem functioning.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
RAF, HER, PMJB contributed to conception and design of the study. RAF carried out field work and performed statistical analysis. RAF wrote the first draft of the manuscript. All authors contributed to revision of the manuscript, read and approved the submitted version.
RAF received a studentship from Anglia Ruskin University to complete this research. HER was supported by the Natural Environment Research Council award number NE/R016429/1 as part of the UK-SCAPE programme Delivering National Capability.
Thank you to the following who granted land access for RAF to undertake surveys: Bedfordshire Cambridgeshire Northamptonshire Wildlife Trust; Forestry Commission; Natural England. Thank you to A. Pickwell, I. Bradley & M. Farrow for assistance during survey work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2022.759046/full#supplementary-material
Adriaens T., San Martin G., Hautier L., Branquart E., Maes D. (2010). Toward a noah’s ark for native ladybirds in Belgium? Benefits Risks Exotic Biol. Control Agents 58, 1–3.
Adriaens T., San Martin y Gomez G., Maes D. (2008). Invasion history, habitat preferences and phenology of the invasive ladybird Harmonia axyridis in Belgium. BioControl 53, 69–88. doi: 10.1007/s10526-007-9137-6
Amaral D. S. S. L., Venson M., Perez A. L., Schmidt J. M., Harwood J. D. (2015). Coccinellid interactions mediated by vegetation heterogeneity. Entomol. Exp. Appl. 156, 160–169. doi: 10.1111/eea.12319
BCN (2019) The wildlife trusts Bedfordshire Cambridgeshire Northamptonshire: Cambridgeshire nature reserves. Available at: https://www.wildlifebcn.org/nature-reserves/cambridgeshire-nature-reserves (Accessed 15 August 2019).
Bianchi F. J. J. A., Honěk A., van der Werf W. (2007). Changes in agricultural land use can explain population decline in a ladybeetle species in the Czech Republic: evidence from a process-based spatially explicit model. Landsc. Ecol. 22, 1541–1554
Blackburn T. M., Essl F., Evans T., Hulme P. E., Jeschke J. M., Kühn I., et al. (2014). A unified classification of alien species based on the magnitude of their environmental impacts. PloS Biol. 12, e1001850. doi: 10.1371/journal.pbio.1001850
Bonebrake T. C., Guo F., Dingle C., Baker D. M., Kitching R. L., Ashton L. A. (2019). Integrating proximal and horizon threats to biodiversity for conservation. Trends Ecol. Evol. 34, 781–788. doi: 10.1016/j.tree.2019.04.001
Brown P. M. J., Frost R., Doberski J., Sparks T., Harrington R., Roy H. E. (2011a). Decline in native ladybirds in response to the arrival of Harmonia axyridis: early evidence from England. Ecol. Entomol. 36, 231–240. doi: 10.1111/j.1365-2311.2011.01264.x
Brown P. M. J., Roy H. E. (2018). Native ladybird decline caused by the invasive harlequin ladybird Harmonia axyridis: evidence from a long-term field study. Insect Conserv. Divers. 11, 230–239. doi: 10.1111/icad.12266
Brown P. M. J., Thomas C. E., Lombaert E., Jeffries D. L., Estoup A., Lawson Handley L. (2011b). The global spread of Harmonia axyridis (Coleoptera: Coccinellidae): distribution, dispersal and routes of invasion. Biol. Control 56, 623–641. doi: 10.1007/s10526-011-9379-1
Chessman B. C. (2003). New sensitivity grades for Australian river macroinvertebrates. Mar. Freshw. Res. 54, 95–103. doi: 10.1071/MF02114
Clavel J., Julliard R., Devictor V. (2011). Worldwide decline of specialist species: toward a global functional homogenization? Front. Ecol. Environ. 9, 222–228. doi: 10.1890/080216
Colunga-Garcia M., Gage S. H., Landis D. A. (1997). Response of an assemblage of coccinellidae (Coleoptera) to a diverse agricultural landscape. Environ. Entomol. 26, 797–804. doi: 10.1093/ee/26.4.797
Comont R. F., Roy H. E., Harrington R., Shortall C. R., Purse B. V. (2014). Ecological correlates of local extinction and colonisation in the British ladybird beetles (Coleoptera: Coccinellidae). Biol. Invasions 16, 14805–11817. doi: 10.1007/s10530-013-0628-3
Constable D., Birkby N. J. (2016). The impact of the invasive amphipod Dikerogammarus haemobaphes on leaf litter processing in UK rivers. Aquatic Ecol. 50, 273–281.
Crawford L., Yeomans W., Adams C. E. (2006). The impact of introduced signal crayfish Pacifastacus leniusculus on stream invertebrate communities. Aquat. Conserv.: Mar. Freshw. Ecosyst. 16, 611–621.
Curran J. (2018) Hotelling: Hotelling’s T^2 test and variants. r package version 1.0-5. Available at: https://CRAN.R-project.org/package=Hotelling (Accessed 19 September 2018).
Didham R. K., Tylianakis J. M., Hutchison M. A., Ewers R. M., Gemmell N. J. (2005). Are invasive species the drivers of ecological change? Trends Ecol. Evol. 20, 470–474. doi: 10.1016/j.tree.2005.07.006
Diepenbrock L. M., Finke D. L. (2013). Refuge for native lady beetles (Coccinellidae) in perennial grassland habitats. Insect Conserv. Divers. 6, 671–679. doi: 10.1111/icad.12027
Dixon A. F. G., Hemptinne J. -L., Kindlmann P. (1997). Effectiveness of ladybirds as biological control agents: patterns and processes. Entomphaga 42, 71–83.
Ducatti R. D. B., Ugine T. A., Losey J. (2017). Interactions of the Asian lady beetle, Harmonia axyridis (Coleoptera: Coccinellidae), and the north American native lady beetle, Coccinella novemnotata (Coleoptera: Coccinellidae): prospects for recovery post-decline. Environ. Entomol. 46, 21–29. doi: 10.1093/ee/nvw153
Evans E. W. (2000). Morphology of invasion: body size patterns associated with establishment of Coccinella septempunctata (Coleoptera: Coccinellidae) in western north America. Eur. J. Entomol. 97, 469–474. doi: 10.14411/eje.2000.072
Ferrez J., Davison A. C., Rebetez M. (2011). Extreme temperature analysis under forest cover compared to open field. Agric. For. Meteorol. 151, 992–1001. doi: 10.1016/j.agrformet.2011.03.005
Fowler S. V. (2004). Biological control of an exotic scale, Orthezia insignis Browne (Homoptera: Ortheziidae) saves the endemic gumwood tree, Commidendrum robustum (Toxb.) DC. (Asteraceae) on the island of St. Helena. Biol. Control 29, 367–374.
Gardiner M. M., Landis D. A., Gratton C., Schmidt N., O’Neal M., Mueller E., et al. (2009). Landscape composition influences patterns of native and exotic lady beetle abundance. Divers. Distrib. 15, 554–564. doi: 10.1111/j.1472-4642.2009.00563.x
Gardiner M. M., Perry K. I., Riley C. B., Turo K. J., Delgado de la flor Y. A., Sivakoff F. S. (2021). Community science data suggests that urbanization and forest habitat loss threaten aphidophagous native lady beetles. Ecol. Evol. 11, 2761–2774. doi: 10.1002/ece3.7229
Grez A. A., Zaviezo T., Casanoves F., Oberti R., Pliscoff P. (2021). The positive association between natural vegetation, native coccinellids and functional diversity of aphidophagous coccinellid communities in alfalfa. Insect Conserv. Divers. 14, 464–475. doi: 10.1111/icad.12473
Grez A. A., Zaviezo T., Diaz S., Camousseigt B., Cortes G. (2008). Effects of habitat loss and fragmentation on the abundance and species richness of aphidophagous beetles and aphids in experimental alfalfa landscapes. Eur. J. Entomol. 105, 411–420. doi: 10.14411/eje.2008.052
Grez A. A., Zaviezo T., Gardiner M. (2014a). Local predator composition and landscape affects biological control of aphids in alfalfa fields. Biol. Control 76, 1–9. doi: 10.1016/j.biocontrol.2014.04.005
Grez A. A., Zaviezo T., Hernández J., Rodríguez-San Pedro A., Acuña P. (2014b). The heterogeneity and composition of agricultural landscapes influence native and exotic coccinellids in alfalfa fields. Agric. For. Entomol. 16, 382–390. doi: 10.1111/afe.12068
Hall J. E., Kirby K. J., Whitbread A. M. (2004) National Vegetation Classification: field guide to woodland. Peterborough: Joint Nature Conservation Committee.
Hammer Ø., Harper D. A. T., Ryan P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 4, 1–9.
Harmon J. P., Stephens E., Losey J. (2007). The decline of native coccinellids (Coleoptera: Coccinellidae) in the united states and Canada. J. Insect Conserv. 11, 85–94. doi: 10.1007/s10841-006-9021-1
Hentley W. T., Vanbergen A. J., Beckerman A. P., Brien M. N., Hails R. S., Jones T. H., et al. (2016). Antagonistic interactions between an invasive alien and a native coccinellid species may promote coexistence. J. Anim. Ecol. 85, 1087–1097. doi: 10.1111/1365-2656.12519
Hodek I. (2012). ““Diapause/dormancy,”,” in Ecology and behaviour of the ladybird beetles (Coccinellidae). Ed. Hodek I. (Chichester, West Sussex: Wiley-Blackwell), 275–342.
Holecová M., Zach P., Hollá K., Šebestová M., Klesniaková M., Šestáková A., et al. (2018). Overwintering of ladybirds (Coleoptera: Coccinellidae) on scots pine in central Europe. Eur. J. Entomol. 115, 658–667. doi: 10.14411/eje.2018.065
Honěk A. (1985). Habitat preferences of aphidophagous coccinellids. Entomophaga 30, 253–264. doi: 10.1007/BF02372226
Honěk A. (2012). “Distribution and habitats,” in Ecology and behaviour of the ladybird beetles (Coccinellidae). Ed. Hodek I. (Chichester, West Sussex: Wiley-Blackwell), 110–140.
Honěk A., Dixon A. F. G., Soares A. O., Skuhrovec J., Martinkova Z. (2017). Spatial and temporal changes in the abundance and composition of ladybird (Coleoptera: Coccinellidae) communities. Curr. Opin. Insect. Sci. 20, 61–67. doi: 10.1016/j.cois.2017.04.001
Honěk A., Martinkova Z., Dixon A. F. G., Roy H. E., Pekár S. (2016). Long-term changes in communities of native coccinellids: population fluctuations and the effect of competition from an invasive non-native species. Insect Conserv. Divers. 9, 202–209. doi: 10.1111/icad.12158
Horikoshi M., Tang Y. (2016) Ggfortify: data visualization tools for statistical analysis results. Available at: https://CRAN.R-project.org/package=ggfortify (Accessed 01 May 2019).
IPBES (2019). Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the intergovernmental science-policy platform on biodiversity and ecosystem services. Eds. Díaz S., Settele J., Brondízio E. S., Ngo H. T., Guèze M., Agard J., et al (Bonn, Germany: IPBES secretariat). Available at: https://ipbes.net/sites/default/files/inline/files/ipbes_global_assessment_report_summary_for_policymakers.pdf.
Janssen P., Fuhr M., Cateau E., Nusillard B., Bouget C. (2017). Forest continuity acts congruently with stand maturity in structuring the functional composition of saproxylic. doi: 10.1016/j.biocon.2016.11.021
Jones J. C., Reynolds J. D., Raffaelli D. (2006). “Environmental variables,” in Ecological census techniques: a handbook. Ed. Sutherland W. J. (Cambridge: Cambridge University Press), 370–407.
Kassambara A. (2018) Ggpubr: ‘ggplot2’ based publication ready plots. r package version 0.2. Available at: https://CRAN.R-project.org/package=ggpubr (Accessed 01 May 2019).
Labrie G., Coderre D., Lucas E. (2008). Overwintering strategy of multicolored Asian lady beetle (Coleoptera: Coccinellidae): cold-free space as a factor of invasive success. Ann. Entomol. Soc Am. 101, 860–866. doi: 10.1093/aesa/101.5.860
Liao C., Luo Y., Jiang L., Zhou X., Wu X., Fang C., et al. (2007). Invasion of Spartina alterniflora enhanced ecosystem carbon and nitrogen stocks in the Yangtze estuary, China. Ecosystems 10, 1351–1361. doi: 10.1007/s10021-007-9103-2
Losey J., Perlman J., Kopco J., Ramsey S., Hesler L., Evans E., et al. (2012b). Potential causes and consequences of decreased body size in field populations of Coccinella novemnotata. biol. Control 61, 98–103. doi: 10.1016/j.biocontrol.2011.12.009
Lucy F. E., Roy H., Simpson A., Carlton J. T., Hanson J. M., Magellan K., et al. (2016). INVASIVESNET towards an international association for open knowledge on invasive alien species. Manage. Biol. Invasions 7, 131–139. doi: 10.3391/mbi.2016.7.2.01
Majerus M. E. N., Strawson V., Roy H. E. (2006). The potential impacts of the arrival of the harlequin ladybird, Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) in Britain. Ecol. Entomol. 31, 207–215. doi: 10.1111/j.1365-2311.2006.00734.x
Mathers K. L., Chadd R. P., Dunbar M. J., Extence C. A., Reeds J., Rice S. P., et al. (2016). The long-term effects of invasive signal crayfish (Pacifastacus leniusculus) on instream macroinvertebrate communities. Sci. Total Environ. 556, 207–218. doi: 10.1016/j.scitotenv.2016.01.215
Mikanowski J. (2017) A different dimension of loss: inside the great insect die-off. the guardian. Available at: https://www.theguardian.com/environment/2017/dec/14/a-different-dimension-of-loss-great-insect-die-off-sixth-extinction (Accessed 14 December, 2017).
Morris E. K., Caruso T., Buscot F., Fischer M., Hancock C., Maier T. S., et al. (2014). Choosing and using diversity indices: insights for ecological applications from the German biodiversity exploratories. Ecol. Evol. 4, 3514–3524. doi: 10.1002/ece3.1155
Nakazawa M. (2018) Fmsb: function for medical statistics books with some demographic data. r package version 0.6.3. Available at: https://CRAN.R-project.org/package=fmsb (Accessed 24 October 2018).
Oksanen J., Blanchet F. G., Friendly M., Kindt R., Legendre P., McGlinn D., et al. (2019) Vegan: community ecology package. r package version2.5-4. Available at: https://CRAN.R-project.org/package=vegan (Accessed 01 May 2019).
Pendleton T., Pendleton D. (2019) Known ladybird over-wintering sites 1997-2019. Available at: http://www.eakringbirds.com/eakringbirds3/ladybirdswintering.htm (Accessed 13 November 2019).
Pickwell A. (2012). “Development of a novel invertebrate indexing tool for the determination of salinity in aquatic inland drainage channels,” in MPhil (Lincoln: University of Lincoln).
Purse B. V., Comont R., Butler A., Brown P. M. J., Kessel C., Roy H. E. (2014). Landscape and climate determine patterns of spread for all colour morphs of the alien ladybird Harmonia axyridis. J. Biogeogr. 42, 575–588. doi: 10.1111/jbi.12423
R Core Team (2019). R: a language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org.
Roy H. E., Adriaens T., Isaac N. J. B., Kenis M., Onkelinx T., San Martin G., et al. (2012). Invasive alien predator causes rapid declines of native European ladybirds. Divers. Distrib. 18, 717–725. doi: 10.1111/j.1472-4642.2012.00883.x
Roy H. E., Brown P. M. J., Adriaens T., Berkvens N., Borges I., Clusella-Trullas S., et al. (2016). The harlequin ladybird, Harmonia axyridis: global perspectives on invasion history and ecology. Biol. Invasions 18, 997–1044. doi: 10.1007/s10530-016-1077-6
Roy H. E., Brown P. M. J., Comont R. F., Poland R. L., Sloggett J. J. (2013). Ladybirds (Second edition. Exeter: Pelagic Publishing).
Roy H. E., Brown P. M. J., Frost R., Poland R. (2011). Ladybirds (Coccinellidae) of Britain and Ireland: an atlas of the ladybird of Britain, Ireland, the isle of man and the channel islands (Shrewsbury. FSC Publications).
Samways M. J., Sarton P. S., Birkhofer K., Chichorro F., Deacon C., Fartmann T., et al. (2020). Solution for humanity on how to conserve insects. Biol. Conserv. 242, 108427. doi: 10.1016/j.biocon.2020.108427
Seebens H., Blackburn T. M., Dyer E. E., Genovesi P., Hulme P. E., Jeschke J. M., et al. (2017). No saturation in the accumulation of alien species worldwide. Nat. Commun. 8, 14435. doi: 10.1038/ncomms14435 [accessed 07 February 2018
Selyemová D., Zach P., Némethová D., Kulfan J., Úradník M., Holecová M., et al. (2007). Assemblage structure and altitudinal distribution of lady beetles (Coleoptera, coccinellidae) in the mountain spruce forests of pol’ana mountains, the West carpathians. Biologia 62, 610–616. doi: 10.2478/s11756-007-0120-6
Simberloff D., Martin J.-L., Genovesi P., Maris V., Wardle D. A., Aronson J., et al. (2013). Impacts of biological invasions: what’s what and the way forward. Trends Ecol. Evol. 28, 58–66. doi: 10.1016/j.tree.2012.07.013
Sloggett J. J. (2017). Harmonia axyridis (Coleoptera: Coccinellidae): smelling the rat in native ladybird declines. Eur. J. Entomol. 114, 455–461. doi: 10.14411/eje.2017.058
Sloggett J. J., Majerus M. E. N. (2000a). Habitat preference and diet in the predatory coccinellidae (Coleoptera): an evolutionary perspective. Biol. J. Linn. Soc 70, 63–88. doi: 10.1111/j.1095-8312.2000.tb00201.x
Sloggett J. J., Majerus M. E. N. (2000b). Aphid-mediated coexistence of ladybirds (Coleoptera: Coccinellidae) and the wood ant Formica rufa: seasonal effects, interspecific variability and the evolution of a coccinellid myrmecophile. Oikos 89, 345–359. doi: 10.1034/j.1600-0706.2000.890216.x
Sloggett J. J., Zeilstra I., Obrycki J. J. (2008). Patch residence by aphidophagous ladybird beetles: do specialists stay longer? Biol. Control 47, 199–206. doi: 10.1016/j.biocontrol.2008.08.003
Tang Y., Horikoshi M., Li W. (2016). Ggfortify: unified interface to visualize statistical result of popular r packages. R. J., Vol. 8.2. 478–489.
ter Braak C. J. F., Verdonschot P. F. M. (1995). Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat. Sci. 57, 255–289. doi: 10.1007/BF00877430
Tumminello G., Ugine T. A., Losey J. E. (2015). Intraguild interactions of native and introduced coccinellids: the decline of a flagship species. Environ. Entomol. 44, 64–72. doi: 10.1093/ee/nvu010
Vandereycken A., Durieux D., Joie E., Sloggett J. J., Haubruge E., Verheggen F. J. (2013). Is the multicolored Asian ladybeetle, Harmonia axyridis, the most abundance natural enemy to aphids in agroecosystems? J. Insect Sci. 13. doi: 10.1673/031.013.15801
Venables W. N., Ripley B. D. (2002). Modern applied statistics with s, 4th edition (New York: Springer).
Viglášová S., Nedvěd O., Zach P., Julfan J., Parák M., Honěk A., et al. (2017). Species assemblages of ladybirds including the harlequin ladybird Harmonia axyridis: a comparison at large spatial scale in urban habitats. BioControl 62, 409–421. doi: 10.1007/s10526-017-9793-0
Wickham H., Francois R., Henry L., Muller K. (2019) Dplyr: a grammar of data manipulation. r package version 0.8.0.1. Available at: https://CRAN.R-project.org/package=dplyr (Accessed 16 May 2019).
Woltz J. M., Landis D. A. (2014). Coccinellid response to landscape composition and configuration. Agric. For. Entomol. 16, 341–349. doi: 10.1111/afe.12064
Zeileis A. (2004). Econometric computing with HC and HAC covariance matric estimators. J. Stat. Software 11, 1–17. doi: 10.18637/jss.v011.i10 [accessed 01 May 2019
Zeileis A. (2006). Object-oriented computation of sandwich estimators. J. Stat. Software 16, 1–16. doi: 10.18637/jss.v016.i09 [accessed 01 May 2019
Zeileis A., Hothorn T. (2002)Diagnostic checking in regression relationships. In: R news. Available at: https://CRAN.R-project.org/doc/Rnews/ (Accessed 16 May 2019).
Zeileis A., Kleiber C., Jackman S. (2008)Regression models for count data in r. In: J. stat. softw. Available at: http://www.jstatsoft.org/v27/i08 (Accessed 20 August 2018).
Keywords: biological invasions, Coccinellidae, Harmonia axyridis, invasive alien species, ladybirds, non-native species, rural habitat, woodland
Citation: Farrow RA, Roy HE and Brown PMJ (2022) Ladybird communities in rural woodlands: Does an invader dominate? Front. Conserv. Sci. 3:759046. doi: 10.3389/fcosc.2022.759046
Received: 15 August 2021; Accepted: 08 July 2022;
Published: 09 August 2022.
Edited by:
Danny Haelewaters, Ghent University, BelgiumReviewed by:
Junfeng Tang, China West Normal University, ChinaCopyright © 2022 Farrow, Roy and Brown. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachel A. Farrow, cmFjaGVsYWZhcnJvd0BnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.