- 1Joint Research Unit-Animal-Health-Territories-Risks-Ecosystems (UMR ASTRE), CIRAD, Antananarivo, Madagascar
- 2Joint Research Unit-Animal-Health-Territories-Risks-Ecosystems (UMR ASTRE), CIRAD, Campus International de Baillarguet, Montpellier, France
- 3National Centre for Applied Research in Rural Development- Department of Zootechnical Veterinary and Fish Research (FOFIFA-DRZVP), Antananarivo, Madagascar
- 4Institut Pasteur de Madagascar, Antananarivo, Madagascar
- 5Department of Zoology and Entomology, University of Pretoria, Pretoria, South Africa
Bushmeat consumption and trade plays a relevant role in many tropical countries as a source of protein and income for rural populations. In Madagascar, rural populations depend heavily on natural resources and wildlife as source of income and protein. The bushpig (Potamochoerus larvatus) is the largest mammal available in the island and regularly hunted. However, little is known about the importance and characteristics of this activity and its implication as a potential source of pathogens for both humans and domestic animals. A cross-sectional study was conducted in 2014–2015 in five different regions of rural Madagascar suspected to have significant bushpig populations to (i) quantify and characterize the importance of bushpig hunting, (ii) assess the socioeconomic impact of bushpig trade, (iii) evaluate the potential pathogen transmission between bushpigs, domestic pigs and humans. A total of 77 hunters, 10 butchers and 95 pig farmers were individually interviewed. Hunting seasonality and the perception of local hunters with regards to the dynamics of bushpig populations in the last decade differed between the tropical dry and tropical sub-arid climatic zones. The top reason for hunting bushpigs was crop protection but personal consumption and selling of meat were also common. Hunting efficacy was largely dependent on the technique used. Snares and traps, the most widely used techniques, allowed the majority of hunters to catch from one to 10 bushpigs per year. Limited commercial bushpig trade was observed with only 0.8 bushpig sold in average per year and per hunter, representing a 16 USD income. The average price per kilo sold was USD 0.8 and the average profit received by each butcher/collector after the sale of a carcass was USD 11.9. No perception of disease risks nor precautions were taken to prevent potential pathogen transmission from bushpig to humans or pigs. Most of the hunters (68%) indicated that they had never seen a diseased bushpig. Bushpig hunting in our study areas in Madagascar was basically a small-scale subsistence hunting, very different from commercial bushmeat hunting described in areas of Central Africa or the Amazon Basin. More research is needed to verify the sustainability of bushpig hunting and its potential role in terms of reducing pressure on other endemic wildlife species and transmitting pathogens to humans and pigs.
Introduction
The hunting of wild animal species in developing countries has several implications at different levels: (i) impact on biodiversity conservation, (ii) provision of income and food for rural populations, and (iii) potential impact on the health of domestic animals and humans. The uncontrolled exploitation of this sector jeopardizes the sustainability of wild animal populations (Stoner et al., 2007). The annual consumption of wildlife meat is estimated at 5 million tons in the Congo basin in Africa and 1.3 million tons in the Amazon basin (Nasi et al., 2011). In Asia, few data are available due to the preponderance of informal trade but the supplier countries include Cambodia, Indonesia, Laos, and Vietnam (Secretariat of the Convention on Biological Diversity, 2011). Unsustainable hunting has been blamed since the early 1990s as responsible for contributing to “empty forests” (Redford, 1992) and “empty savannahs” (Lindsey et al., 2013). At the same time, bushmeat represents a significant source of nutrients for many people in developing countries, whose diet is often deficient (Fa et al., 2003). Hunting and selling wildlife is moreover a common way of generating income for a variety of stakeholders, from villagers in rural areas to market sellers in urban areas (van Vliet et al., 2016). Finally, this activity can potentially play an important role in the spread of disease between species (Fa and Brown, 2009). About 60% of emerging infectious disease events in humans are zoonotic and 75% of these originate in wildlife (Jones et al., 2008). Bushmeat processing and consumption can therefore have important consequences for human health through zoonoses and food-borne diseases (Bair-Brake et al., 2014).
Madagascar is known for its rich biodiversity but also for being exposed to high levels of biodiversity loss. Despite a particularly high rate of deforestation, hunting of wild animals remains an important activity to provide meat and income for a share of rural households (Golden, 2009; Jenkins et al., 2011). Malnutrition is indeed a serious problem among rural populations in Madagascar, which is considered one of the most food insecure countries in the world (Economist Intelligence Unit, 2017). It is also one of the countries in the world where people spend most of their cash on food (Economist Intelligence Unit, 2014, 2016). In addition, Madagascar has one of the highest growth rates in the world, and this problem is compounded by climatic hazards and health problems such as malaria, anemia, and high infant mortality rate [WHO (World Health Organization), 2012; Mould et al., 2016; Rice et al., 2016]. As a result, in various rural areas of Madagascar, bushpig meat plays an important part of the household protein diet, together with other species such as the common tenrec, Tenrec ecaudatus (Borgerson et al., 2019). Bushpig (Potamochoerus larvatus) is the largest terrestrial vertebrate since the extinction of the Malagasy megafauna and is thus the most sought-after game in the country. The introduction of this largest ungulate species in Madagascar is suggested to have occurred with the arrival of the first inhabitants from eastern Africa about 2,000 years ago (Lee et al., 2020). Bushpigs are suspected to be present in all types of Malagasy forests, including the most degraded ones, where it is hunted for its meat.
In other countries, wild pigs are considered to represent a real risk for the spread of various pathogens affecting humans and domestic animals (Meng and Lindsay, 2009; Jori et al., 2017a). Wild and feral pigs can be infected with several pathogens that are transmittable to other wildlife, domestic animals, and humans. They carry Brucella suis, Salmonella, Toxoplasma gondii, Trichinella and hepatitis E virus (HEV) (Miller et al., 2017; Brookes et al., 2021). Bushpig in Madagascar are also exposed to African Swine Fever (ASF) which was first reported among domestic pigs in Madagascar in 1998, presumably introduced from Mozambique (Gonzague et al., 2001; Roger et al., 2001; Rousset et al., 2001). A sylvatic cycle of this disease has been demonstrated in many East and Southern African countries, involving Suidae such as warthogs (Phacochoerus sp.) and soft ticks of the Ornithodoros genus (Plowright, 1981; Thomson, 1985; Wilkinson and Pensaert, 1989; Anderson et al., 1998). However, the role of bushpigs in the epidemiology of ASF has been less documented. It is well-established that they may become infected with ASF virus (ASFV), and their ability to transmit the virus to domestic pigs has been demonstrated experimentally. However, the role that bushpig really plays in the dynamics of ASF in natural endemic settings remains to be determined (Anderson et al., 1998; Jori et al., 2013; Ståhl et al., 2014). Two studies have been conducted in Madagascar which found no evidence of the presence of ASFV or its antibodies among bushpig populations (Ravaomanana, 2011; Ramy-Ratiarison, 2014). However, due to their small sample size, no conclusion could be reached on the role of bushpigs in ASF epidemiology. Given the difficulties of capturing a large number of bushpigs for sample collection, we decided to explore the potential circulation of diseases in bushpig populations by inquiring local people living in proximity with nature about potential cases of health incidents or mortality among bushpig populations. We aimed to collect this information through questionnaire surveys of hunters and retailers of bushpigs. This was also a way of characterizing socio-economic aspects of bushpig hunting and trade for which there is currently no data in Madagascar. This work also addresses the determinants that may influence the spread of various pathogens associated with the exploitation of bushpig meat.
The general aim of this study was therefore to understand the importance and characteristics of bushpig hunting in different rural areas of Madagascar (techniques, volume, economic value) and the possible health risks arising from this activity for pig farming (particularly in relation to the epidemiology of ASF) and for humans as consumers of bushpig meat in Madagascar.
Materials and Methods
Study Areas
The study areas were chosen in consultation with veterinarians and wildlife ecologists from the Centre de Coopération Internationale en Recherche Agronomique pour le Développement (CIRAD), the Centre National de la Recherche Appliquée au Développement Rural (FOFIFA), the Veterinary services (DSV) and the Wildlife Conservation Society (WCS) in Madagascar. The study was carried out in areas where bushpigs were known to be present and in areas where their presence was plausible given the abundance of forest cover and the presence of crops. Other criteria that were considered when selecting these study areas included the suspected existence of an interface between bushpigs and domestic pigs at the periphery of protected areas due to the presence of forest. Study areas a priori most favorable for the existence of a bushpig meat trade development were identified as the Moramanga district, the regions of Sofia-Diana, Boeny, Menabe and Atsimo-Andrefana (Figure 1).
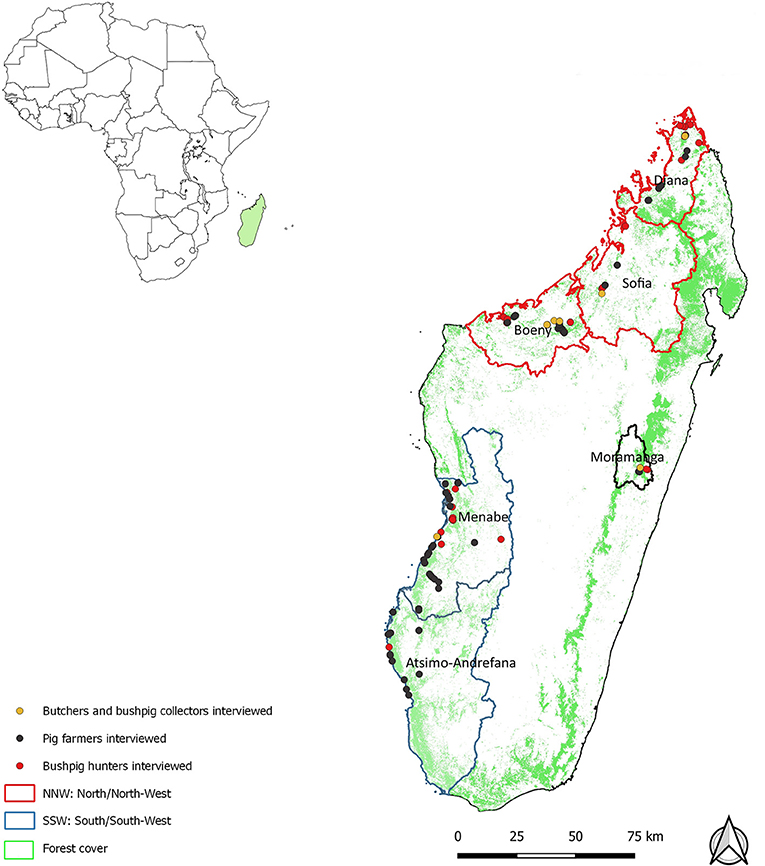
Figure 1. Spatial distribution of stakeholderqs interviewed during a survey on health and socioeconomic aspects of bushpig hunting in Madagascar, 2014–2015.
In the East (Moramanga district), the climate is tropical (hot and humid). Showers fall all year round (average rainfall is 1295 mm) and even during the driest months, rainfalls remain heavy. The elevation is about 900 m and temperatures vary between 11 and 27°C, with an average of 18.7°C.
In the North and Northwest (Boeny and Sofia-Diana regions), the climate is of sub-humid type and characterized by two very distinct seasons, dry from May to October and humid from November to April. Located at an elevation ranging between 0 and 1,000 m, the annual rainfall is between 400 and 1,500 mm and the average annual temperature varies between 24 and 27°C. The vegetation cover is made up of dense rainforests, dry deciduous forests, forests in different stages of degradation, mangroves, and palm savannahs. It has been assumed that this area is a particularly favorable habitat for bushpigs due to the availability of water and food, especially the presence of Strichnos spinosa, a fruit-bearing shrub of the Loganiaceae family much sought after by bushpigs, with a fruiting period from mid-April to mid-August (Andrianjakarivelo, 2003; Rouillé et al., 2014). In the West and Southwest (Menabe and Atsimo-Andrefana regions), the average annual temperature is 25.9°C and the average rainfall is 321 mm. Two types of vegetation can be found including mangroves and dry forests characterized by Didiereaceae, Euphorbia thickets and sclerophyllous clear forests.
Study Respondents
Three types of stakeholders were targeted for our survey. Bushpig hunters and bushpig retailers (including collectors, butchers, and market sellers) were targeted because of their potential knowledge on the health status of bushpigs and on socioeconomic aspects of bushpig trade. Pig farmers were targeted because they can provide information on interactions between pigs and bushpigs which may potentially lead to disease transmission. Inclusion criteria included (i) being an adult; (ii) being either a bushpig hunter, a bushpig retailer or a pig farmer in the study areas; (iii) being willing to answer the questionnaire. The only exclusion criterion was not being able to understand or answer the questionnaire. A sample size was defined for each type of stakeholder and since we had no prior information on the expected prevalence for the different characteristics studied (such as the proportion of hunters who saw ASF signs on bushpigs, or the proportion of income coming from trading bushpig), an expected prevalence of 50% was chosen because this value maximizes the sample size. A degree of precision of 10% and a degree of confidence of 95% (and therefore an alpha risk of error of 5%) were used. Based on discussions with veterinarians and wildlife ecologists of DSV, FOFIFA and WCS, the total population size of stakeholders in the study areas was roughly estimated at 5,000 for pig farmers, 1,000 for bushpig hunters, and 100 for bushpig retailers. The sample size we aimed for was therefore 95 for pig farmers, 88 for bushpig hunters and 49 for bushpig retailers.
Questionnaire Design
Three different questionnaires were designed, one for each type of respondent: bushpig hunters, bushpig retailers and pig farmers (Questionnaire I Questionnaire for pig farmer) (Questionnaire II Questionnaire for bushpig hunter) (Questionnaire III Questionnaire for retailer/butcher). The questionnaires consisted of partially open and closed questions. They were tested in April 2014 to verify the clarity of the questions, to have as complete as possible lists of possible answers for closed questions and to estimate the duration needed to fill in a questionnaire (which was about 30 min). They are available as Supplementary Material.
Questions regarding direct and indirect interactions between bushpigs and domestic pigs were posed to hunters and farmers and the remaining questions asked about suspected ASF outbreaks, husbandry practices (feeding, housing), human behavior (hygiene, disease management) and characteristics of bushpig trade. A direct interaction was defined as the simultaneous presence of bushpigs and domestic pigs sharing the same space at the same time within an area of the size of a football pitch, as used in other similar studies (Jori et al., 2011; Abu Samra et al., 2013). An indirect interaction was defined as the presence of bushpigs and domestic pigs in the same area but at different times (Kukielka et al., 2016). The questionnaire for hunters also included sections on the methods, reasons, areas, and seasons of hunting. For hunters who observed or consumed diseased or dead bushpigs in the wild, we asked them about potential health events (mass mortalities or the observation of macroscopic lesions) in local dialect. To avoid, potential response bias during the surveys, each participant was interviewed individually by an interviewer speaking the local language, under the supervision of a researcher.
Data Collection
Data collection was carried out between May 2014 and December 2015. Each study area was visited at the period of the year considered as optimal in terms of field accessibility (between April and October) and availability of respondents (avoiding periods of religious celebration). Courtesy visits were paid to local administrative authorities (fokontany chiefs) to obtain authorization to work in the area, to identify the names of known local bushpig hunters, bushpig retailers and pig farmers, and to generally facilitate the survey. From this initial convenience sample of identified respondents and in the absence of sampling frames, snowball sampling was used to identify other respondents (Johnson, 2005). Visits were conducted at the home of each respondent to ask them to fill in the questionnaire. Sometimes several visits were necessary for the respondent to be present when the team came. Owing to study budget constraints, a typical field trip lasted five to seven days (excluding transport duration from the capital city Antananarivo to the study area and backward). Within this timeframe, as many visits as possible were conducted and as many questionnaires as possible were filled. Questionnaires were paper printed in French and interviews were conducted in Malagasy.
Ethics
Authorization for the study was granted by the Ministry of Livestock and Animal Protection through the DSV and FOFIFA. Local authorities were informed of the objectives and the modalities of the study prior to its initiation and were asked for permission to conduct the survey on their territory. Bushpig hunters, bushpig retailers and pig farmers were also informed about (i) the objectives of the study and the exclusive use of collected data for research purposes; (ii) the modalities of the studies (types of questions asked, how the interview would be conducted and its duration, how the data would be stored and kept confidential). Their participation to the questionnaire survey was done on a voluntary basis, with the possibility to stop answering questions at any time. Consent of respondents was expressed orally.
Data Analysis
Collected data contained in paper questionnaires was entered in Microsoft Excel® and data entry was cross-checked by a second person. Dynamic cross-tabulations in Excel were used to make a first descriptive analysis of the data. Data were then statistically analyzed using R software version 3.2.2 (R Core Team, 2015). Comparisons were made using univariate tests such as Fisher's test or chi-square test for categorical variables, and Kruskal-Wallis test was used for comparing quantitative variables. The degree of significance retained for the p-value was p < 0.05 for all tests.
Given the low number of hunters interviewed in some of the study areas, comparisons between areas were made by grouping the study areas into distinct climatic zones (Nematchoua et al., 2018): Boeny and Sofia-Diana regions were grouped into a North/North-West (NNW) zone representative of hot tropical climate with dry forest and the Menabe and Atsimo-Andrefana regions were grouped into a South/South-West (SSW) zone representative of sub-arid tropical climate. The eastern zone of Moramanga was not finally considered in the analysis because of its small sample size.
Results
A total of 182 persons were interviewed including 95 pig farmers, 77 hunters and 10 bushpigs meat butchers or retailers (Table 1). The spatial distribution of study areas and interviewed actors is provided in Figure 1.
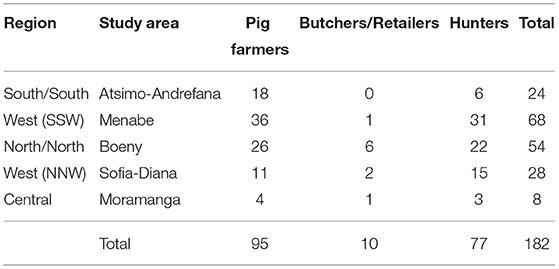
Table 1. Number and type of stakeholders interviewed during a survey on health and socioeconomic aspects of bushpig hunting in Madagascar, 2014−2015.
Characteristics of Interviewed Stakeholders
The majority (57%) of interviewed hunters were between 20 and 40 years old (with an additional 6% under 20 years old and 37% over 40 years old). They had an average of 13 years of experience (minimum 1, median 10, maximum 44) in hunting bushpigs. Almost all (96%) of them were also farmers but only 5% were also pig farmers. The majority of hunters (81%) hunted only bushpigs while 19% also hunted other game: guinea fowl (Numida meleagris), civet (Viverricula indica), and fosa (Cryptoprocta ferox), and more rarely crocodile (Crocodylus niloticus), various species of lemurs, common tenrec (Tenrec ecaudatus), or wild ducks.
Among the 10 retailers interviewed, eight were butchers (also present on the market to sell meat) and two were collectors. For them, bushpig trading was just a side activity to complete their main activity as farmers (four of them), butchers for other types of meat (three of them), or pig collectors (two of them).
The age of pig farmers was evenly distributed with 50% of them being under 40 years old and 50% above 40 years old. Breeding and selling pigs were the main source of income for only 15% of people owning pigs whereas it was an additional source of income for 85% of them (the largest part of income coming from farming). The average number of pigs owned was seven (median 4, maximum 33).
Presence of Bushpigs
The presence of bushpigs around their village was reported by 56% of hunters (42 out of 75 who answered this question) and in 60% (n = 27) of the 45 fokontany in which hunters were interviewed. There was no significant difference in the presence of bushpigs between the two climatic zones. When comparing bushpig populations at the time of the study and 10 years earlier, a large majority of hunters (79%, 22 out of 28 responses) in the SSW zone found that the number of bushpigs had increased compared to 10 years ago. Among other responses, five hunters considered there were about the same number and one considered there were fewer. This trend differed significantly (p = 0.008) with the NNW climatic zone where 45% of the hunters (15/33) hunters found there were more bushpigs than 10 years ago and 54% (18/33) found there were about the same number or fewer. The presence of bushpigs around villages was also reported by 27% of pig farmers (22 out of 83 responses) with no difference between the two climatic zones. Among six farmers who responded to this question five considered bushpigs had increased in recent years, while one farmer considered they had decreased.
Modality and Reason for Bushpig Hunting
The best hunting season was significantly different between the two climatic zones (Table 2). The rainy season was reported as the best season for hunting in the NNW (62%, 23 out of 37 responses), while in the SSW it was rather the dry season (58%, 21 out of 36 responses). Hunting was mainly practiced in rice fields, in forests, or in a combination of both (Table 2). Other possible but more anecdotal hunting sites were savannahs or swamps.
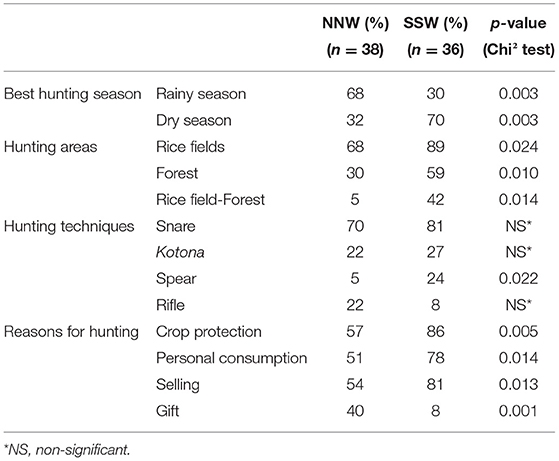
Table 2. Modality and reason for hunting bushpigs during a survey on health and socioeconomic aspects of bushpig hunting in Madagascar, 2014–2015.
In both climatic zones, snares were the most widely used technique for hunting (Table 2). Kotona (Figure 2) (traditional wooden hand-made traps usually baited with food) were also commonly used in both climatic zones whereas spears were more used in the SSW climatic zone. In all regions, when snares were used, a hunter used an average of 7.8 snares per hunting season (minimum 1, median 5, maximum 50). Similarly, when kotona were used, a hunter used an average of 1.9 kotona per hunting season (minimum 1, median 2, maximum 5). There were no significant differences between the hunting techniques used per region, except for the spear, which was more commonly used in the SSW region.
Among the reported reasons for hunting, hunters mentioned crop protection (70%), personal consumption within the household (66%), selling for income (66%), gifts for friends/acquaintances (26%), and passion for hunting (5%). Most frequently, the reasons were a combination of crop protection and/or personal consumption and/or selling for income (68%). When asked to rank the reasons, crop protection was ranked first by 89% of the respondents. The variation among climatic zones in the reasons for hunting is shown in Table 2.
Importance of Bushpig Hunting and Trading
In villages where bushpig hunters were present, there was an average of 3.8 people per village hunting bushpigs (minimum 1, median 3, maximum 20), with no significant difference between the two climatic zones. The number of bushpigs captured per year and per hunter depended on the hunting technique used (Table 3). Using a spear was the least efficient technique whereas using a rifle was the most efficient. Snares and kotonas, the most commonly used techniques, allowed the majority of hunters to capture between one and 10 bushpigs per year.
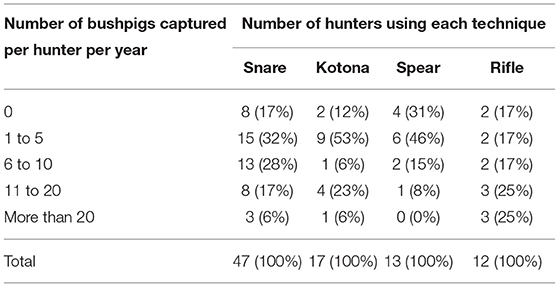
Table 3. Number of bushpigs captured per hunter per year depending on the hunting technique used, during a survey on health and socioeconomic aspects of bushpig hunting in Madagascar, 2014–2015.
The average number of bushpigs sold per hunter in the year preceding the interview was low with an average of 0.8 bushpig/hunter/year. Among the 44 hunters who answered the question, 73% sold none, 20% sold one to five, 5% sold six to 10, and only 2% sold between 11 and 20 bushpigs. There was no significant difference between the two climatic zones (Table 4). Bushpigs sold by hunters were, in majority, sold to other villagers (27 out of 40 responses), to restaurant owners (six responses), to butchers (four responses) or to a mixture of the previous. The maximum distance the hunter had to travel to sell the bushpigs was 20 km.
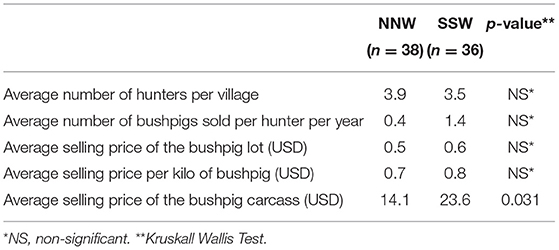
Table 4. Importance and economic contribution of bushpig meat trading during a survey on health and socioeconomic aspects of bushpig hunting in Madagascar, 2014–2015.
Bushpig carcasses were transported by foot (for 89% of hunters), by bicycle (3%), by bus (3%) or with a private car (5%). A majority of hunters (54%) did not take any measure to preserve bushpig meat, whereas others sold the meat immediately (25%) or smoked it (18%). The other methods of preservation, including freezing and glazing, were less common (3%).
Among the seven butchers who provided estimates for the number of bushpigs they sold during the year before the survey, five had sold more than 20 bushpigs and two had sold between six and 10 bushpigs. No comparison between climatic zones could be made for the number of bushpigs sold by butchers due to the low number of catches in the SSW zone.
The average price of the entire bushpig carcass provided by hunters was USD 20.0 (minimum 6.3, median 21.2, maximum 28.3). This price varied significantly between areas (Table 4): when sold per kilo the average price was USD 0.89 per kg (minimum 0.31, median 0.89, maximum 1.88), or in lots with an average price of USD 0.58 per lot (minimum 0.31, median 0.47, maximum 1.10). Those prices did not differ significantly between zones. If we consider that on average a hunter sold 0.8 bushpigs per year, this corresponds to a bushpig meat sales income of 0.8 x 20.0= USD 16.0 per year per hunter. These monetary values are placed in context in the Discussion section.
For butchers/collectors the carcass was bought on average USD 23.3 (minimum 18.8, median 22.0, maximum 28.3). On the butcher's stall, the kilo was sold on average USD 1.22 (minimum 0.94, median 1.10, maximum 1.88). At the end of each sale of a carcass, the average profit received was USD 11.9 (minimum 3.1, median 11.0, maximum 25.1).
Zoonotic Health Risks Linked to Handling and Consuming Bushpig Meat
Two thirds of the hunters (68%) had never observed any bushpig looking diseased or in bad condition. For those who had already observed diseased bushpigs (32%), in half of the descriptions, external signs suggested emaciation and depression. No specific lesions in the carcass were reported except for one hunter who reported a liver with lesions on a hog. In addition, two hunters had already seen bushpigs with white rice grain-like lesions in the meat, compatible with Taenia solium cysticercosis or potential tuberculosis lesions. Among the 77 hunters, 21 (28%) reported sightings of dead bushpigs in the wild.
The large majority of hunters (94%) killed the animal at the time of capture. Viscera could be either left on the hunting grounds (for 67% of hunters), and/or eaten (for 63% of hunters) and/or given to dogs (for 41% of hunters). More rarely, they were buried (11%) or burnt (3%).
Most hunters believed that it was not possible to become infected with any pathogen through the consumption (84% of hunters) or handling (93% of hunters) of bushpig meat bushpig meat. They thought this because they had never seen anyone get sick after eating or handling bushpig meat even without protection.
Health Risks in Relation to Domestic Pig Farming
Among the farmers interviewed, 27% reported observing bushpigs near the village. Bushpigs were reported to approach the village at an average distance of 158 meters (minimum 10, median 100, maximum 500). Direct interaction between domestic pigs and bushpigs coming around the village was reported by 38% (n = 8) of the farmers who answered this question. Equally, 17% of the farmers interviewed, gave parts of their hunting offal to feed their domestic pigs (n = 11).
More than a quarter of the farmers (27%) reported having experienced outbreaks of swine fever in the past or during the survey (n = 25). The average reported mortality rate during those swine fever outbreaks was 74.6% (minimum 20, median 80, maximum 100). However, no hunter or farmer suspected bushpigs being the cause of those mortality events in pigs.
Discussion
This work is the first study aiming to quantify and characterize bushpig hunting in Madagascar, while also identifying its potential socioeconomic and health implications.
The results of our survey suggest that bushpig hunting in Madagascar is not driven by commercial purposes but rather by subsistence needs. Crop protection was the most frequent reason for hunting bushpigs (96% of the hunters we interviewed were also farmers), followed by personal consumption and selling of bushpig meat. There was no organization of the distribution of hunted meat with the involvement of middlemen who will transport the meat to towns, unlike commercial bushmeat value chains observed in other countries (Cowlishaw et al., 2005). This situation can be justified by the small number of bushpigs captured per hunter, a low demand for bushmeat in urban areas and the absence of a road network to transport the game. The low number of bushpigs captured per hunter per year (0.8) (< five for the majority of hunters) is an indicator of low hunting efficiency by traditional methods (snare, spear, kotona), and possibly the lack of availability of more efficient hunting equipment (rifle, ammunition) in remote rural areas. In addition, most rural areas in Madagascar are poorly served by road infrastructure (UNISDR, 2015), forcing hunters to transport captured bushpigs to their village or market by rudimentary methods such as foot or bicycle.
Due to the seasonality of the presence of bushpigs, hunting activity was also seasonal with periods of the year when it was more practiced although hunting could take place all year round. The best reported hunting period was the dry season in the SSW zone and the rainy season in the NNW zone. This divergence is likely related to different harvesting periods for crops and to different periods when more time is available for hunting. Wildlife crop raiding has been linked to crop maturity (Nyirenda, 2011; Payne et al., 2018) and crops in Madagascar (including maize, cassava, banana, beans, sweet potatoes, sugar cane…) are harvested at different periods depending on the climatic zone. In the NNW region, maize, peanuts, sweet potatoes, cassava are ripe during the rainy season, while in the SSW region, sugar cane, rice, lentils, watermelon, and yams (also appreciated by bushpig) reach maturity at the beginning of the dry season. Furthermore, in the SSW zone, where rainfall is lower, people are busier for cultivation during the rainy season and the rest of the year they have fewer activities (Randrianandrianina et al., 2010). Whereas in the NNW, the rainy period coincides with the less busy season and people prefer to have complementary sources of income such as hunting.
In other regions in sub-Saharan Africa, hunting is largely practiced by unemployed young people who seek through this activity an additional source of income but also a certain social value (Fa et al., 2003). In our study, income generated from the trade of bushpig meat was low, with an average of USD 16 per year per hunter. This figure represents only 3% of the average annual income in Madagascar, which is equivalent to USD 520 per year (Sulla and D'Hoore, 2014). Therefore, it is difficult to consider bushmeat hunting and trading as a profitable activity in our study areas and a similar situation was described in the North-East of Madagascar (Golden, 2009). However, this situation can vary depending on the location and socio-economic context and another study reported that the commodity chain for wild meat (including fruit bats, civets, common tenrecs, lemurs and wild pigs) in Madagascar was more formalized than generally thought (Reuter et al., 2016). Our study found that a bushpig carcass was sold for an average of USD 20 whereas another study in western Madagascar in 2008 indicated that a bushpig carcass was sold for USD 29–36 (Randrianandrianina et al., 2010). This difference may be related to a higher proportion of Muslim people in the north-western part of Madagascar, driving swine meat prices down. We found that each butcher earned an average profit of USD 11.9 per bushpig sold and that bushpig meat was half as expensive as pig meat when portion sizes and venue of purchase were accounted for. However, prices of bushmeat are variable and depend on availability of different protein sources and market laws which differ between countries, regions and over time (Damania et al., 2005).
Our study also assessed the health risks associated with hunting and selling bushpigs. Bushpigs seemed to come very close to inhabited areas and have the capacity to share the same space and interact with humans and domestic animals, as observed for wild Suidae in other settings (Jori et al., 2017b). Several hunters reported sightings of dead bushpigs in the wild, which they attributed to fights or old age. During this study, an attempt was made to find out whether hunters had observed abnormal health events among bushpigs (cf. mass mortalities), as potential indicators of African swine fever or Classical swine fever episodes among the bushpig population. An exploration of potential occurrence of diseases events induced by outbreaks of ASF on the Malagasy bushpig was conducted. Nevertheless, our survey did not capture any health event compatible with a suspicion of an infectious outbreaks, even though ASF and CSF are regularly reported in domestic pigs throughout the country (Gonzague et al., 2001; Roger et al., 2001; Rousset et al., 2001). Bushpigs on the African continent are known to be resistant to ASF virus (Anderson et al., 1998). However, considering that Malagasy bushpig populations have been isolated from the African continent and therefore have not had any contact with ASF virus for about 2,000 years. Taking this into account, the possibility of observing massive mortalities of wild pigs after the introduction of the virus in 1998, as it has occurred with other wild pigs species in Europe and Asia (Ewers et al., 2021), could have been a plausible scenario. Nevertheless, hunters in our study did not report observations of animals with signs of swine fever. This seems to confirm the findings of previous surveys in Madagascar which found no evidence of the presence of ASFV in bushpigs and the hypothesis that the Malagasy bushpig is equally resistant to ASF despite being separated from the virus for more than two millennia (Ravaomanana, 2011; Ramy-Ratiarison, 2014). However, further studies with a larger sample size in areas where the virus is circulating in domestic pigs are likely to provide more reliable information on the potential infection rate of ASF virus in an exposed bushpig population.
Another common disease present in the pig population in Madagascar is Classical swine fever. This virus is also reported to affect bushpig populations in experimental conditions and to cause clinical signs similar to those observed in domestic pigs (Gers et al., 2011). Although never described to date, considering the proximity of bushpigs to villages, the chances of bushpigs coming in contact with materials infected with Classical swine fever virus by domestic pigs are not negligible and the potential circulation of pathogens from domestic pigs to bushpigs and vice versa could potentially occur.
Zoonotic diseases are also likely to affect farmers or hunters through contacts with infected pigs at the interface or through the manipulation of carcasses of bushpigs infected with zoonotic pathogens. Most hunters stated that it was impossible to get sick from eating or handling bushpig meat and they did not take any protection during slaughter or meat processing. Pathogen surveys on bushpig populations are very scarce in the scientific literature. The quantity and diversity of pathogens reported to circulate between wild boars, domestic animals, and humans is important and includes brucellosis, tuberculosis, hepatitis E, toxoplasmosis, leptospirosis, and trichinellosis (Meng and Lindsay, 2009; Jori et al., 2017a; Miller et al., 2017). This information suggests that the potential transmission of pathogens from bushpigs to other sympatric species including humans in Africa and Madagascar is likely to be important and deserves further investigation.
Bushpigs were present in all the investigated study areas and their presence was reported in 60% of the villages where the questionnaires were implemented. However, potential differences regarding relative abundance in these zones are not well-known in Madagascar and neither is the biology of bushpig populations. This lack of data makes sustainable management extremely challenging. Although bushpig is an exotic species in Madagascar, it is interesting to note that the presence of this large vertebrate indirectly contributes to the conservation of Malagasy biodiversity, as its hunting diverts poaching pressure from other smaller endemic animals. The biological characteristics of bushpigs (multiple females per family group, 3–4 offspring per litter and at least 1 litter/year) renders them particularly resilient to hunting. This situation is relatively unique as in other countries the largest animals are often priority hunting targets and usually the most prone to extinction (McKinney, 1997). Other species reported as hunted in our survey (apart from bushpig) included species classified as game (such as civet (Viverricula indica), common tenrec (Tenrec ecaudatus), or guinea fowl (Numida meleagris)) and protected species (such as lemurs, fosa (Cryptoprocta ferox) or fruit bats (Pteropus rufus)), which was also evidenced in other studies in Madagascar (Jenkins and Racey, 2008; Jenkins et al., 2011). In order to ensure a sustainable hunting management over the longer term in Madagascar, a preliminary step would need to improve interagency coordination on wildlife law enforcement and provide stronger deterrents such as penalties, for law infringement. The primary obstacles to such reform are not technical, but financial and political. Efforts are needed to raise awareness among the judiciary and law enforcement agencies of the value of wildlife and the threat posed by bushmeat hunting (Lindsey et al., 2013).
It is important to note that our study might have been influenced by some biases such as the representativity of our sample. In our case, random sampling was not a feasible option in the absence of any registry of hunters, retailers, and pig farmers. Therefore, we used snowball sampling instead of random sampling (Johnson, 2005).
In addition, our sample size was a bit lower than our target for hunters and butchers because there were fewer hunters in the field than we had anticipated. More time and resources for field investigations in each study area would likely have allowed us to identify and interview more hunters and butchers. A larger sample size would then have been helpful to increase our statistical power when comparing different regions.
In our sampling design, we chose to cluster our sampled population by profession (hunters, retailers, farmers). We made this choice under the assumption that this category of stakeholders were privileged observers of the natural environment and would provide more reliable information on bushpig related behavior and activities. Therefore, a bias may exist with respect to this choice which might not have captured the observations of other stakeholder categories which could have been equally valuable for our purpose such as crop farmers, field veterinarians, para veterinarians, or communal health workers.
Unlike other contexts where answers to questions about hunting may be biased because wildlife hunting is illegal (Nuno and St. John, 2014), we think answers we collected were truthful since bushpig hunting is not illegal in Madagascar and hunters and retailers did not consider information on bushpig as sensitive.
Future research on quantifying bushpig presence and hunting should include a longitudinal component to establish more clearly seasonal patterns suggested by our results. A year-long monitoring in rural areas where bushpigs are known to be present would also help to quantify bushpig hunting done by urban hunters. These hunters come on an irregular basis but are likely to kill more wild animals since they are richer and better equipped (with rifles and ammunitions).
Our results failed to demonstrate any observed evidence of swine fever occurrence in bushpigs by our respondents. However, this information is insufficient to conclude if diseases such as ASF or CSF are circulating among Malagasy bushpig populations and the role of bushpigs in ASF or CSF epidemiology still needs more research. Collection of biological samples (blood, tissue...) will be necessary to confirm or infirm the presence of ASF and other pathogens in bushpigs in Madagascar (Fredriksson-Ahomaa et al., 2020). Lastly, the use of collars with GPS beacons/camera traps would be interesting to trace possible contacts between domestic pigs and bushpigs around areas where bushpigs are abundant (Jori et al., 2016).
Conclusion
This survey has been useful for highlighting the importance and characteristics of bushpig hunting in Madagascar. It shows that in our five study areas, a commercial bushpig meat value chain does not really exist and that bushpig hunting is a simple subsistence activity for crop protection and protein provision. The hunting pressure seemed to be limited since the most common techniques used (snares and traps) were moderately efficient. Still, bushpig hunting could potentially play a positive role for conservation efforts in Madagascar since it diverts poaching from protected endemic wildlife species. Our results also showed that contacts between domestic and wild pigs are likely to occur in rural areas and that there is the potential for transmission of shared pathogens between bushpigs, domestic pigs and humans through direct and indirect interactions. These results are valid only for the selected study areas and the time during which the study was performed. Indeed, these conditions are likely to vary in other areas or to change after the development of new infrastructures such as access roads. Further research, including the collection of biological samples is needed to assess the burden of different pathogens in bushpig populations and assess the risk of disease circulation and transmission between bushpig populations, and sympatric domestic pigs and humans.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ministry of Livestock and Animal Protection through the DSV (Veterinary services) Centre National de la Recherche Appliquée au Développement Rural (FOFIFA). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
RR, MP, and SM designed and coordinated the study. RR, MP, ER, and RR-R collected the data in the field. SM and RR performed the statistical analysis. RR wrote the manuscript. RR, SM, FJ, and MP conceptualized the thrust and focus of the manuscript. RR, SM, FJ, and MP participated in drafting the manuscript or revising it critically for content. All authors contributed to the article and approved the submitted version.
Funding
Funding for field activities was provided by CIRAD and RR is a recipient of a PhD scholarship under the ASF NIFNAF project (Award # 2019-67015-28981 of the NSF-USDA-NIH Ecology and Evolution of Infectious Disease program).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the staff of the Veterinary Services, FOFIFA, WCS and the Veterinary Department of the University of Antananarivo. We are indebted to the many people who participated in the study, and thank Fridolin Maminiaina, Alain Rakotondravao, Antoine Rouillé, François Roger, Julie Ravaomanana and Vonjy Andrianjakarivelo for helpful comments.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2022.732626/full#supplementary-material
References
Abu Samra, N., Jori, F., Xiao, L., Rikhotso, O., and Thompson, P. N. (2013). Molecular characterization of Cryptosporidium species at the wildlife/livestock interface of the Kruger National Park, South Africa. Comp. Immunol. Microbiol. Infect. Dis. 36, 295–302. doi: 10.1016/j.cimid.2012.07.004
Anderson, E. C., Hutchings, G. H., Mukarati, N., and Wilkinson, P. J. (1998). African swine fever virus infection of the bushpig (Potamochoerus porcus) and its significance in the epidemiology of the disease. Vet. Microbiol. 62, 1–15. doi: 10.1016/S0378-1135(98)00187-4
Andrianjakarivelo, V. (2003). “Artiodactyla:Potamochoerus larvatus, bushpig,” in The Natural History of Madagascar, eds S. M. Goodman and J. P. Benstead (Chicago, IL: The University of Chicago Press), 1365–1367.
Bair-Brake, H., Bell, T., Higgins, A., Bailey, N., Duda, M., and Shapiro, S. (2014). Is that a rodent in your luggage? A mixed method approach to describe bushmeat importation into the United States. Zoonoses Public Health 61, 97–104. doi: 10.1111/zph.12050
Borgerson, C., Razafindrapaoly, B. N., Rajaona, D., Rasolofoniaina, B. J. R., and Golden, C. D. (2019). Food insecurity and the unsustainable hunting of wildlife in a UNESCO world heritage site. Front. Sustain. Food Syst. 3, 99. doi: 10.3389/fsufs.2019.00099
Brookes, V. J., Barrett, T. E., Ward, M. P., Roby, J. A., Hernandez-Jover, M., and Cross, E. M. (2021). A scoping review of African swine fever virus spread between domestic and free-living pigs. Transbound. Emerg. Dis. 68, 2643–2656. doi: 10.22541/au.159413143.35147622
Cowlishaw, G., Mendelson, S., and Rowcliffe, J. M. (2005). Structure and operation of a bushmeat commodity chain in Southwestern Ghana. Conserv. Biol. 19, 139–149. Available online at: http://www.jstor.org/stable/3591017 (accessed May 15, 2021).
Damania, R., Milner-Gulland, E. J., and Crookes, D. J. A. (2005). Bioeconomic analysis of bushmeat hunting. Proc. R. Soc. B Biol. Sci. 272, 259–266. doi: 10.1098/rspb.2004.2945
Economist Intelligence Unit (2014). Global Food Security Index 2014: An Annual Measure of the State of Global Food Security. London: The Economist Intelligence UnitLimited.
Economist Intelligence Unit (2016). Global Food Security Index 2016: An Annual Measure of the State of Global Food Security. London: The Economist Intelligence Unit Limited.
Economist Intelligence Unit (2017). Global Food Security Index 2017: An Annual Measure of the State of Global Food Security. London: The Economist Intelligence Unit Limited.
Ewers, R. M., Nathan, S. K. S. S., and Lee, P. A. K. (2021). African swine fever ravaging Borneo's wild pigs. Nature 593, 37. doi: 10.1038/d41586-021-01189-3
Fa, J. E., and Brown, D. (2009). Impacts of hunting on mammals in African tropical moist forests: a review and synthesis. Mammal Rev. 39, 231–264. doi: 10.1111/j.1365-2907.2009.00149.x
Fa, J. E., Currie, D., and Meeuwig, J. (2003). Bushmeat and food security in the Congo Basin: linkages between wildlife and people's future. Environ Conserv. 30, 71–78. doi: 10.1017/S0376892903000067
Fredriksson-Ahomaa, M., London, L., Skrzypczak, T., Kantala, T., Laamanen, I., and Biström, M. (2020). Foodborne zoonoses common in hunted wild boars. Ecohealth 17, 512–522 doi: 10.1007/s10393-020-01509-5
Gers, S., Vosloo, W., Drew, T., Lubisi, A. B., Pardini, A., and Williams, M. (2011). Experimental infection of common warthogs (Phacochoerus africanus) and bushpigs (Potamochoerus larvatus) with classical swine fever virus II: a comparative histopathological study. Transbound Emerg Dis. 58, 135–144. doi: 10.1111/j.1865-1682.2010.01191.x
Golden, C. D. (2009). Bushmeat hunting and use in the Makira Forest, north-eastern Madagascar: a conservation and livelihoods issue. ORYX. 43, 386–392. doi: 10.1017/S0030605309000131
Gonzague, M., Roger, F., Bastos, A., Burger, C., Randriamparany, T., and Smondack, S. (2001). Isolation of a non-haemadsorbing, non-cytopathic strain of African swine fever virus in Madagascar. Epidemiol. Infect. 126, 453–459. doi: 10.1017/S0950268801005465
Jenkins, B., and Racey, P. A. (2008). Bats as bushmeat in Madagascar. Madagascar Conserv. Dev. 3, 22–30. doi: 10.4314/mcd.v3i1.44132
Jenkins, R. K. B., Keane, A., Rakotoarivelo, A. R., Rakotomboavonjy, V., Randrianandrianina, F. H., and Razafimanahaka, H. J. (2011). Analysis of patterns of bushmeat consumption reveals extensive exploitation of protected species in eastern madagascar. PLoS ONE 6, 27570. doi: 10.1371/journal.pone.0027570
Johnson, T.P. (2005). “Snowball sampling,” in Encyclopedia of Biostatistics (New York, NY: John Wiley and Sons). Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/0470011815.b2a16070 (accessed May 20, 2021).
Jones, K. E., Patel, N. G., Levy, M. A., Storeygard, A., Balk, D., and Gittleman, J. L. (2008). Global trends in emerging infectious diseases. Nature 451, 990–993. doi: 10.1038/nature06536
Jori, F., Brahmbhatt, D., Fosgate, G. T., Thompson, P. N., Budke, C., and Ward, M. P. (2011). A questionnaire-based evaluation of the veterinary cordon fence separating wildlife and livestock along the boundary of the Kruger National Park, South Africa. Prev Vet Med. 100, 210–220. doi: 10.1016/j.prevetmed.2011.03.015
Jori, F., Payne, A., Kock, R., Nava, A., Ståhl, K., and Rossi, S. (2017a). “Disease transmission at the interface between wild and domestic suiform species in the old and new worlds,” in Ecology, Conservation and Management of Wild Pigs and Peccaries, ed M. M. Meijaard (Cambridge: Cambridge University Press), 388–403. doi: 10.1017/9781316941232.037
Jori, F., Payne, A., Stahl, K., Nava, A., and Rossi, S. (2016). “Wild and feral pigs: disease transmission at the interface between wild and domestic pig species in the old and the new world,” in Ecology, Conservation and Management of Wild Pigs and Peccaries, eds M. Melletti and E. Meijaard (Cambridge: Cambridge University Press), 388–403.
Jori, F., Relun, A., Trabucco, B., Charrier, F., Maestrini, O., and Chavernac, D. (2017b). Questionnaire-based assessment of wild boar/domestic pig interactions and implications for disease risk management in Corsica. Front. Vet. Sci. 4, 198. doi: 10.3389/fvets.2017.00198
Jori, F., Vial, L., Penrith, M. L., Pérez-Sánchez, R., Etter, E., and Albina, E. (2013). Review of the sylvatic cycle of African swine fever in sub-Saharan Africa and the Indian ocean. Virus Res. 173, 212–227. doi: 10.1016/j.virusres.2012.10.005
Kukielka, E. A., Jori, F., Martínez-López, B., Chenais, E., Masembe, C., and Chavernac, D. (2016). Wild and domestic pig interactions at the wildlife–livestock interface of Murchison Falls National Park, Uganda, and the Potential Association with African Swine Fever Outbreaks. Front. Vet. Sci. 3, 31. doi: 10.3389/fvets.2016.00031
Lee, C., Day, J., Goodman, S. M., Pedrono, M., Besnard, G., and Frantz, L. (2020). Genetic origins and diversity of bushpigs from Madagascar (Potamochoerus larvatus, family Suidae). Sci. Rep. 10, 20629. doi: 10.1038/s41598-020-77279-5
Lindsey, P. A., Balme, G., Becker, M., Begg, C., Bento, C., and Bocchino, C. (2013). The bushmeat trade in African savannas: impacts, drivers, and possible solutions. Biol. Conserv. 160, 80–96. doi: 10.1016/j.biocon.2012.12.020
McKinney, M. L. (1997). Extinction vulnerability and selectivity: combining ecological and paleontological views. Annu. Rev. Ecol. Syst. 28, 495–516. doi: 10.1146/annurev.ecolsys.28.1.495
Meng, X. J., and Lindsay, D. S. (2009). Wild boars as sources for infectious diseases in livestock and humans. Philos. Trans. R. Soc. B Biol. Sci. 364, 2697–2707. doi: 10.1098/rstb.2009.0086
Miller, R. S., Sweeney, S. J., Slootmaker, C., Grear, D. A., Di Salvo, P. A., Kiser, D., et al. (2017). Cross-species transmission potential between wild pigs, livestock, poultry, wildlife, and humans: implications for disease risk management in North America. Sci. Rep. 7, 1–14. doi: 10.1038/s41598-017-07336-z
Mould, D., Carlson, A., Christofides, N., and Greiner, K. (2016). Socio-Cultural Determinants for the Adoption of Essential Family Practices in Madagascar. Athens, OH: The Institute for the African Child.
Nasi, R., Taber, A., Vliet, N., and Van. (2011). Empty forests, empty stomachs? Bushmeat and livelihoods in the congo and amazon basins. Int For Rev. 13, 355–368. doi: 10.1505/146554811798293872
Nematchoua, M. K., Ricciardi, P., Orosa, J. A., and Buratti, C. (2018). A detailed study of climate change and some vulnerabilities in Indian Ocean: a case of Madagascar island. Sustain Cities Soc. 41, 886–898. doi: 10.1016/j.scs.2018.05.040
Nuno, A., and St. John, F. A. V. (2014). How to ask sensitive questions in conservation: a review of specialized questioning techniques. Biol. Conserv. 189, 5–15. doi: 10.1016/j.biocon.2014.09.047
Nyirenda, V. R. (2011). Wildlife crop depredation in the Luangwa Valley, eastern Zambia. J. Ecol. Nat. Environ. 3, 481–491. doi: 10.5897/JENE11.094
Payne, A., Ogweng, P., Ojok, A., Etter, E., Gilot-Fromont, E., and Masembe, C. (2018). Comparison of three methods to assess the potential for bushpig-domestic pig interactions at the wildlife-livestock interface in Uganda. Front. Vet. Sci. 5:295. doi: 10.3389/fvets.2018.00295
Plowright, W. (1981). “African swine fever,” in Infectious Diseases of Wild Mammals, ed J. Davis, L. Karstad, and D. O. Trainer (Ames: Iowa State University Press), 178–90.
R Core Team. (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at: https://www.r-project.org/
Ramy-Ratiarison, R. (2014). Exploration des maladies du potamochère (Potamochoerus larvatus) transmissibles aux porcs et/ou à l'homme (Master's thesis). University of Antananarivo, Antananarivo, Madagascar.
Randrianandrianina, F. H., Racey, P. A., and Jenkins, R. K. (2010). Hunting and consumption of mammals and birds by people in urban areas of western Madagascar. ORYX 44, 411–415. doi: 10.1017/S003060531000044X
Ravaomanana, J. (2011). La peste porcine africaine à Madagascar : évaluation de l'existence potentielle de compartiments sauvages et de leurs impacts épidémiologiques (PhD thesis). University of Antananarivo, Antananarivo, Madagascar.
Reuter, K. E., Randell, H., Wills, A. R., Janvier, T. E., Belalahy, T. R., and Sewall, B. J. (2016). Capture, movement, trade, and consumption of mammals in Madagascar. PLoS ONE 11, e0150305. doi: 10.1371/journal.pone.0150305
Rice, B. L., Golden, C. D., Anjaranirina, E. J., Botelho, C. M., Volkman, S. K., and Hartl, D. L. (2016). Genetic evidence that the Makira region in northeastern Madagascar is a hotspot of malaria transmission. Malar. J. 15, 596. doi: 10.1186/s12936-016-1644-4
Roger, F., Ratovonjato, J., Vola, P., and Uilenberg, G. (2001). Ornithodoros porcinus ticks, bushpigs, and African swine fever in Madagascar. Exp. Appl. Acarol. 25, 263–269. doi: 10.1023/A:1010687502145
Rouillé, A., Pedrono, M., Rakotomalala, É., Grosbois, V., Ramy-Ratiarison, R., and Roger, F. (2014). Abundance of the Potamochoerus larvatus bush-pig in the savannah zones of North-Western Madagascar and associated epidemiological risks. Bois Forets des Trop. 68, 75–82. doi: 10.19182/bft2014.320.a20546
Rousset, D., Randriamparany, T., Rahantamalala, C. Y. M., Randriamahefa, N., Zeller, H., Rakoto-Andrianarivelo, M., et al. (2001). Introduction de la Peste Porcine Africaine à Madagascar, histoire et leçons d'une émergence [African Swine Fever introduction into Madagascar, history and lessons from an emergence]. Arch Inst Pasteur Madagascar. 67, 31–33.
Secretariat of the Convention on Biological Diversity (2011). Alternatives de moyens de subsistance pour l'utilisation non durable de la viande de brousse. Montréal:SCBD. Available from: http://www.cbd.int (accessed March 28, 2021)
Ståhl, K., Ogweng, P., Okoth, E., Aliro, T., Muhangi, D., LeBlanc, N., et al. (2014). Understanding the dynamics and spread of african swine fever virus at the wildlife-livestock interface: Insights into the potential role of the bushpig Potamochoerus larvatus. Suiform Soundings 3, 24–29. Available online at: https://hdl.handle.net/10568/67750 (accessed March 8, 2021).
Stoner, K. E., Vulinec, K., Wright, S. J., and Peres, C. A. (2007). Hunting and plant community dynamics in tropical forests: a synthesis and future directions. Biotropica 39, 385–392. doi: 10.1111/j.1744-7429.2007.00291.x
Sulla, V., and D'Hoore, A. (2014). Face of Poverty in Madagascar : Poverty, Gender, and Inequality Assessment. Available online at: http://documents.worldbank.org/curated/en/2014/03/19548476/face-poverty-madagascar-poverty-gender-inequality-assessment (accessed June 5, 2021).
Thomson, G. R. (1985). The epidemiology of African swine fever: the role of free-living hosts in Africa. Onderstepoort J. Vet. Res. 52, 201–209.
UNISDR (2015). UNISDR Working Papers on Public Investment Planning and Financing Strategy for Disaster Risk Reduction: Review of Madagascar. Available online at: http://www.preventionweb.net/english/hyogo/gar/2015/en/gar-pdf/UNISDR_Working_Papers_on_Public_Investment_Planning_and_Financing_Strategy_for_Disaster_Risk_Reduction_Review_of_Madagascar.pdf (accessed May 31, 2021).
van Vliet, N., Cornelis, D., Beck, H., Lindsey, P., Nasi, R., LeBel, S., et al. (2016). Meat from the Wild: Extractive Uses of Wildlife and Alternatives for Sustainability. Cham: Springer. doi: 10.1007/978-3-319-27912-1_10
WHO (World Health Organization) (2012). WHO Global Database on Child Growth and Malnutrition. Available online at: https://www.who.int/toolkits/child-growth-standards/standards.513 (accessed August 20, 2021.).
Keywords: bushpig, African swine fever, bushmeat, socioeconomic implications, Potamochoerus larvatus
Citation: Rakotoarivony R, Molia S, Rakotomalala E, Ramy-Ratiarison R, Jori F and Pedrono M (2022) Bushpig (Potamochoerus larvatus) Hunting in Rural Areas of Madagascar and Its Health and Socioeconomic Implications. Front. Conserv. Sci. 3:732626. doi: 10.3389/fcosc.2022.732626
Received: 29 June 2021; Accepted: 11 January 2022;
Published: 03 February 2022.
Edited by:
Om P. Dhungyel, The University of Sydney, AustraliaReviewed by:
Stephanie Mauti, Swiss Tropical and Public Health Institute (Swiss TPH), SwitzerlandLuis Verde, Instituto de Ecología (INECOL), Mexico
Copyright © 2022 Rakotoarivony, Molia, Rakotomalala, Ramy-Ratiarison, Jori and Pedrono. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rianja Rakotoarivony, cmlhbmphcmFrb3RvYXJpdm9ueUBnbWFpbC5jb20=
 Rianja Rakotoarivony
Rianja Rakotoarivony Sophie Molia
Sophie Molia Eric Rakotomalala
Eric Rakotomalala Ranto Ramy-Ratiarison
Ranto Ramy-Ratiarison Ferran Jori
Ferran Jori Miguel Pedrono
Miguel Pedrono