
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Conserv. Sci. , 20 January 2022
Sec. Animal Conservation
Volume 2 - 2021 | https://doi.org/10.3389/fcosc.2021.759038
This article is part of the Research Topic Ladybirds: Conservation, Ecology and Interactions with Other Organisms View all 12 articles
Coccinella quinquepunctata (the five-spot ladybird), was considered extinct in the UK until 1987. Since this time the species is abundant, however, only in very specific habitat in Wales and Scotland. As a result, it is classified as (RDB3) Rare, mainly as a result of its preferred habitat; exposed riverine sediment. This habitat is in a constant state of alteration by natural and anthropogenic means with the quality of the habitat being degraded to the point that specialised invertebrate species, such as C. quinquepunctata, are at risk. In recent years, the rapid spread of the invasive alien Harmonia axyridis (harlequin ladybird) has been linked to a decline in native coccinellid numbers. There is concern that the narrow habitat requirements of C. quinquepunctata, together with the continuing spread of H. axyridis, will result in a decline in the abundance of C. quinquepunctata. Two habitat types (exposed riverine sediment and grassland adjacent to the ERS) along 12 Welsh rivers were surveyed for C. quinquepunctata, H. axyridis, and other coccinellids. When an individual coccinellid was recorded, so too was its elevation from the substrate. Plant species that C. quinquepunctata were observed on and vegetation density on the shingle were assessed in broad categories. Of all recorded coccinellids, 76% were C. quinquepunctata while 7% were H. axyridis. A third of the sites had no records of H. axyridis, while C. quinquepunctata was recorded at all sites. A significantly greater number of C. quinquepunctata were observed within 0.5 m of the exposed riverine sediment rather than higher up on the vegetation. Presence of the invasive plant Himalayan balsam (Impatiens glandulifera) may have a negative effect on C. quinquepunctata, as it directly affects the vegetation growth on expose riverine sediment. These findings indicate that intraguild predation is unlikely to occur given the low abundance of H. axyridis in C. quinquepunctata habitat. However, the unstable nature of exposed riverine sediment, and a combination of threats from invasive alien species indicates that this species is still at risk of sudden decline and requires further monitoring and conservation efforts.
Coccinella quinquepunctata Linnaeus (five-spot ladybird) (Coleoptera: Coccinellidae) is a small conspicuous coccinellid, typically about 5 mm in length and red with black spots. This species is not found in Ireland, whilst in the UK, C. quinquepunctata is only found in limited areas within restricted habitat of unstable river shingle, also known as exposed riverine sediment (ERS), in Wales and Scotland (Roy et al., 2011). Due to very few records since 1913, C. quinquepunctata was considered extinct in the UK until 1987 (Majerus and Fowles, 1988). As a result of the restricted distribution of C. quinquepunctata in the UK, this species falls under the Red Data Book Category 3 (RDB3) Rare. The RDB3 classification is for taxa that are not yet endangered or vulnerable but are at risk due to restrictions in their habitat or geographical area (Hyman, 1992). Upon the rediscovery of C. quinquepunctata in the UK, more information became available regarding vegetation that this species was associated with on the shingle banks. It was also noted that the species was more likely to be observed on low vegetation, not more than 30–45 cm in height (Majerus and Fowles, 1988). In the late 1980s, surveys reported the species to be well-established in west Wales on both the River Ystwyth and Rheidol as well as in south east Wales on River Towy, with reports of up to 50 individuals recorded at some sites (Majerus and Fowles, 1988). Coccinella quinquepunctata was easily found on thistle or dock growing on river shingle along the River Towy and River Severn in 2002 and 2003 (Bates and Sadler, 2004). In Scotland, there were previous records of C. quinquepunctata in the early 1900s (Majerus and Fowles, 1988) and upon the rediscovery in Wales, surveys were subsequently undertaken at previously recorded sites on the River Spey in Scotland. Since then, other sites of suitable habitat have been identified along the River Dee and surveys carried out resulting in further observations of this species in Scotland (Littlewood, 2015).
Climatic conditions in the UK are considered suboptimal for some coccinellids, resulting in the UK being the edge of the acceptable range for several coccinellid species (Brown and Roy, 2015). In central Europe, C. quinquepunctata is not limited to ERS and is found in more generalist habitats such as trees, wild herbaceous vegetation and cereal fields (Honěk et al., 2014; Majerus et al., 2016). Exposed riverine sediment is in a constant state of alteration due to the nature of the river systems and water levels rising and falling regularly (O'Callaghan et al., 2013). Water levels not only rise in terms of depth but water also moves inwards across the shingle to the extent of the terrestrial habitat during high/maximal flow periods. The water level can rise and fall in this way quite quickly (several metres in 30 min), depending on the underlying geology of the upstream river catchment (Baker et al., 2004). As a result, the invertebrate community in these habitats is well-adapted to the unpredictability of these shingle banks (Sadler et al., 2004). Bates and Sadler (2004) have described C. quinquepunctata as having an ERS fidelity grade of 1, like many invertebrates inhabiting ERS. This essentially means that in the UK C. quinquepunctata is dependent on unstable river shingle for at least one stage of its life cycle and is not found in other habitat unless it happens to resemble ERS in some way, for example lakes that have wave action resulting in a sediment similar to ERS (Bates and Sadler, 2004; Sadler et al., 2004). However, due to anthropogenic disturbances such as gravel extraction, livestock access, channel modification and the establishment of invasive alien species (IAS), the quality of ERS habitat is being degraded to the point that specialised invertebrate species are at risk (Hyman, 1992; Hewitt et al., 2010). Impatiens glandulifera (Himalayan balsam) is an invasive alien herbaceous plant that is one of the tallest in the UK reaching 2.5 m in height (Beerling and Perrins, 1993). This IAS outcompetes native plants by blocking light for low-growing plant species (Pyšek and Prach, 1995; Tang et al., 2014). Additionally, I. glandulifera alters the microbial soil community, making it difficult for native plants to take root (Pattison et al., 2016). Furthermore, the annual nature of I. glandulifera and its root structure, work together to de-stabilise the river bank leaving it more susceptible to erosion during flooding (Pyšek and Prach, 1995; Welsh Institute for Sustainable Environments Network, 2014).
Another IAS that may have a negative impact on C. quinquepunctata is Harmonia axyridis Pallas (Coleoptera: Coccinellidae). Harmonia axyridis is now globally established either as a result of its use as biological control of pest aphids and coccids or accidental introduction. Harmonia axyridis is now a threat to native coccinellids and other non-target species (Harmon et al., 2007; Adriaens et al., 2008; Brown et al., 2011a; Losey et al., 2012; Grez et al., 2016; Honěk et al., 2016; Sloggett, 2017), however it is yet to be determined if this IAS is a threat to C. quinquepunctata. Several other coccinellids have also been used as biological control agents, with some spreading to non-target habitats as H. axyridis has, resulting in adverse effects on native species (Evans, 2000; Koch and Galvan, 2008; Roy et al., 2016) and thereby becoming invasive. The decline of Adalia bipunctata Linnaeus (Coleoptera: Coccinellidae) over a broad geographic range in North America became apparent after the invasion of Coccinella septempunctata Linnaeus (Coleoptera: Coccinellidae) and H. axyridis (Harmon et al., 2007). Intraguild predation (IGP) occurs when the competition between two predators of the same prey results in one of those predators preying on the other (Polis et al., 1989). The two main factors affecting the direction of IGP are body size and trophic specialisation, where the biggest and less specialised species are more likely to act as the predator and the smaller and more specialised species become the prey (Polis et al., 1989). If there is a lack of prey for H. axyridis it may turn to intraguild predation and prey upon the eggs and larvae of other coccinellid species (Brown et al., 2011a; Roy et al., 2011). Intraguild predation has frequently been observed in coccinellid species, especially when H. axyridis is present (Pell et al., 2008; Lucas, 2012). In laboratory trials with 11 other coccinellid species, H. axyridis was the dominant predator in the majority of intraguild interactions, including when the IAS was paired with C. quinquepunctata (Ware and Majerus, 2008). In comparison to H. axyridis, C. quinquepunctata larva are smaller and do not possess defensive spines.
Regardless of other threats affecting native coccinellids, H. axyridis is a factor in how coccinellid communities have changed over recent years (Brown and Roy, 2018; Honěk et al., 2019). This is concerning, as a diverse native coccinellid assemblage delivers invaluable services to their habitat by controlling aphids, coccids and other insect herbivores (Sloggett et al., 2008; Grez et al., 2014). It is likely that C. quinquepunctata carries out such a role for the plant community that survives on ERS. If H. axyridis were to become abundant or even dominant in this habitat, it is possible that, together with other invasive threats, this habitat would become irreparably damaged thereby negatively effecting C. quinquepunctata and the wider community of specialised invertebrates (Sadler et al., 2004). Although described as semi-arboreal, H. axyridis has been recorded in a very wide range of habitats in the UK: urban areas and gardens, grassland, arable land and deciduous, and coniferous woodland (Brown et al., 2011b). Additionally, it is not yet clear if H. axyridis can adequately compensate for the role that native coccinellids play in biological control should local extinctions occur (Roy et al., 2012). Research focusing on the impact of an IAS tends to concentrate more on native species that were once abundant and have noticeably declined since the establishment of an IAS, such as Coccinella novemnotata Herbst (Coleoptera: Coccinellidae) in North America (Losey et al., 2012; Tumminello et al., 2015) and A. bipunctata in the UK (Brown and Roy, 2018). Research that investigates specialist or rare coccinellid species that may be at risk of local/national extinction as a result of IAS is uncommon. Although classified as rare and low in abundance, C. quinquepunctata is stable in the UK (Brown and Roy, 2015; Roy et al., 2018), however, this species may be particularly susceptible to negative impacts from H. axyridis through competition for prey and IGP (Roy et al., 2016), if they co-occur. Aside from the short studies above, few details are known about the one of UK's rarest and most specialist coccinellid species. The aim of this study was to discover more about the ecology of C. quinquepunctata and if this nationally rare species may be at risk from H. axyridis. It was expected that C. quinquepunctata would be recorded in low numbers and that H. axyridis would be present in relatively high numbers.
Twelve sites along the Rivers Severn, Towy, Usk, and Wye in Wales were surveyed (Table 1) in 2017 in mid-June, mid-August and late-September. All sites were surveyed at least twice but poor weather conditions resulted in just eight of the sites being surveyed for a third time. Ordnance Survey location was recorded using Garmin GPSmap 60CSx (https://buy.garmin.com/en-GB/GB/p/310). In order to standardise data collection, surveys took place between 10:00 and 16:00 when weather conditions were favourable. As such, data collection was carried out when the temperature was above 14°C, weather conditions were dry and wind speeds were below 5 on the Beaufort scale (Met Office, 2016). Humidity and ambient temperature were recorded using an EasyLog EL-21CFR-2-LCD (https://www.lascarelectronics.com/easylog-el-21cfr-2-lcd). Any gaps in the temperature/humidity data were completed with data sourced from the Met Office.
Sweep-netting was used to survey for coccinellids in vegetation adjacent to the ERS (Figure 1). This method involves the use of a sweep net which is a white canvas bag (46 cm diameter circular aperture) attached to a metal ring on a long pole. One sweep was carried out for 1 m of distance walked. The net contents were checked every 5 m for coccinellids, which were recorded and the net subsequently emptied. Individuals were shaken from the net in the opposite direction to that of further recording to avoid double counting. This was carried out 20 times resulting in 100 m of grassland being surveyed at each site. Sweep-netting this size of area took ~20 min. Coccinella quinquepunctata has adapted to move quickly if disturbed so instead of sweep netting, a direct search was employed to survey the ERS/shingle banks. Direct searching of ERS was carried out by one surveyor (RAF) for 1 h (30 min on one occasion when two surveyors were present). The search was carried out by moving from the water's edge to where the shingle bordered with grassland (became terrestrial in nature) and continued laterally over and back across the shingle (Figure 1). Where each survey started was dependent on time of day to ensure that the researcher's shadow did not disturb any C. quinquepunctata individuals prior to observation or impair detection of individuals. The area of ERS searched varied due to both changeable water levels and varying vegetation density throughout the season. The density of the vegetation on the shingle banks was assessed in broad categories based on percentage cover of the area surveyed: low (0–30%), medium (31–60%), or high (>60%). When C. quinquepunctata was recorded, the distance the individual was from the water's edge was recorded, as was the individual's elevation from the substrate. The vegetation/grassland adjacent to the ERS was relatively low (no more than 1 m high) and included grasses, wildflowers, thistle, bramble, etc. All coccinellids encountered during both sweep-netting and direct searches were recorded. Some coccinellid species were later grouped together to form the category “Other” as there were too few of each species to apply meaningful analysis to. These species were Propylea quattuordecimpunctata Linnaeus (Coleoptera: Coccinellidae), Tytthaspis sedecimpunctata Linnaeus (Coleoptera: Coccinellidae), Psyllobora vigintiduopunctata Linnaeus (Coleoptera: Coccinellidae), and Subcoccinella vigintiquattuorpunctata Linnaeus (Coleoptera: Coccinellidae).

Figure 1. Illustration of a typical site with ERS/shingle bank bordered by grassland/pasture with the start point for direct search highlighted.
The analyses were carried out using R Studio (R Core Team, 2018). The following R packages were used for basic analyses and visualisation of data: dplyr (Wickham et al., 2019), ggfortify (Horikoshi and Tang, 2016; Tang et al., 2016), ggplot2 (Wickham, 2016), ggpubr (Kassambara, 2018). Wilcoxon paired tests were used to compare the abundance of C. quinquepunctata and H. axyridis abundance on ERS and also in the grass habitat. The following R packages were used for regression analyses: fmsb (Nakazawa, 2018), lmtest (Zeileis and Hothorn, 2002), pscl (Zeileis et al., 2008), sandwich (Zeileis, 2004, 2006), lattice and MASS (Venables and Ripley, 2002).
Generalised linear models (GLMs) were utilised to investigate the effects on coccinellid abundance of habitat (ERS or grass), month (Visit–June, August, September), coccinellid diversity (Shannon diversity), and vegetation cover (Cover). Environmental variables (temperature, humidity) were included in the models. When applying a GLM to count data, the results can often be overdispersed. Overdispersion happens for various reasons with the most common being excess zeros in the data (Beckerman et al., 2017). In the case of these data, overdispersion was common and so alternative regression models were applied to the data and a subsequent model selection carried out to determine which was the best fit, if any. The regression models [poisson, negative binomial (NB), zero-inflated poisson (ZIP) model and zero-inflated negative binomial regression (ZINB) model] were applied to the data. The zero-inflated models treat the zeros differently, either as true or false zeros (Zuur et al., 2012). True zeros occur because the habitat is not favoured by the organisms in question, for example, if winters are too harsh. False zeros on the other hand are when an individual was present but not recorded due to survey design or observer error. It is recommended that if a count dataset consists of true and false zeros then zero-inflated regression models should be applied (Zuur et al., 2012). Zero-inflated models can run using a poisson or negative binomial distribution. A zero-inflated model is essentially two models run at the same time, the count model (models the count data) and the binary model (models the zeros). Both parts of the model are fitted simultaneously and are modelled in terms of the explanatory variables (Zuur et al., 2007).
Data from ERS were analysed separately to the grassland data due to differences in sampling method. In the majority of cases, either a zero-inflated negative binomial (ZINB) model or negative binomial regression (NB) model were the best fit for the data, and on occasion the null model was the better fit. Starting with all variables in the model, a step-wise process was used to determine which variables had an impact on the dependent variable. Any variables resulting in a p < 0.2 were removed from the model. All models were compared to the null model, and reduced models compared with the full model. The z-statistic is used in these regression models as the variance is known, unlike in Gaussian models where the variance is estimated resulting in a t-statistic (Zuur et al., 2009). There are several methods to determine which is the best model to choose (e.g., Akaike Information Criterion, Bayesian Information Criterion) where the model with the lowest value is considered the best (Zuur et al., 2009; Beaujean and Morgan, 2016). In this study, log Likelihood, Akaike Information Criterion (AIC), and weighted AIC were utilised, with the weighted AIC being the deciding factor as to which model was the best fit. Temperature and humidity were checked for collinearity with a variance inflation factor (VIF). Neither variables were of concern with a VIF of <1.2 each, and both were incorporated into the regression models.
Shannon diversity was calculated for shingle and grass habitat separately and only for native coccinellid species. Simpson's diversity was not carried out as this measure is not as sensitive to rare species or those recorded in low numbers (Magurran, 2004; Morris et al., 2014) and there are instances in this dataset where there are several species recorded in low numbers. Differences in diversity across site types and season were calculated using t-tests, while ANOVA was used to assess any differences in diversity within the vegetation structure, followed by a post-hoc Tukey, if any significances were apparent. Regression models were applied to determine if native coccinellid diversity had any effect on the abundance of C. quinquepunctata and H. axyridis.
In 2017, nine coccinellid species were recorded at 12 river sites in Wales with 687 individuals being recorded across both the shingle and grass habitat types. Coccinella quinquepunctata was present at all sites, while H. axyridis was only recorded at 7 of the 12 sites surveyed and was only more abundant than C. quinquepunctata at one site (Figure 2). A significantly greater number of C. quinquepunctata were recorded on the ERS habitat in comparison to the grass habitat (z = 6.72, p < 0.0001), however there was no such difference when comparing H. axyridis abundance at both habitat types (Figure 3).
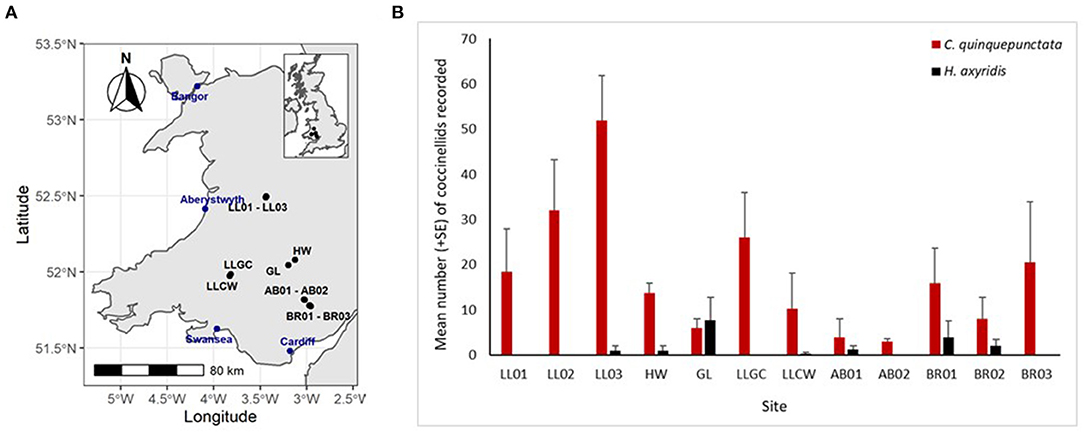
Figure 2. (A,B) Map of survey sites along with mean number (+SE) per site visit of Coccinella quinquepunctata and Harmonia axyridis recorded at each site from both survey methods combined in 2017. Sites: AB01 and AB02, Abergavenny site 1 and 2; BR01, BR02, and BR03, Bryn sites 1, 2, and 3; GL, Glasbury; HW, Hay-on-Wye; LL01, LL02, and LL03, Llandinam sites 1, 2 and 3; LLCW, Cwmgwyn Farm; LLGC, Llandovery.
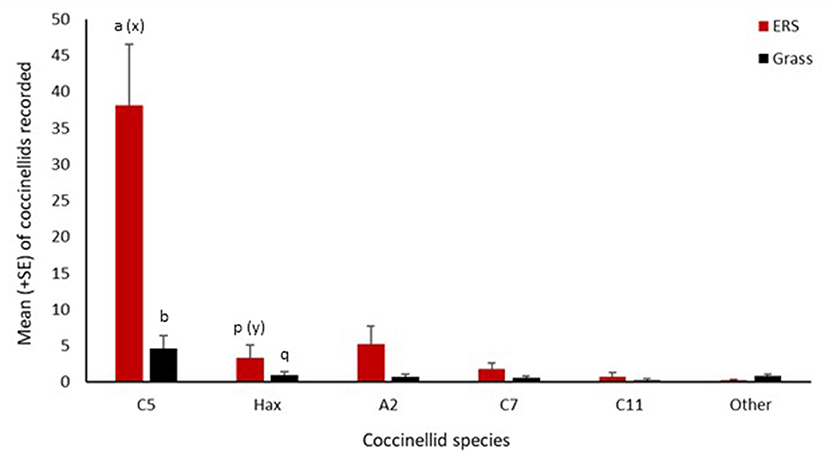
Figure 3. Mean number (+SE) per site visit of coccinellids recorded on ERS and grassland in Wales in 2017. C5, Coccinella quinquepunctata; Hax, Harmonia axyridis; A2, Adalia bipunctata; C7, Coccinella septempunctata; C11, Coccinella undecimpunctata; Other, Propylea quattuordecimpunctata, Psyllobora vigintiduopunctata, Subcoccinella vigintiquattuorpunctata, and Tytthaspis sedecimpunctata. Consecutive letters indicate where significant differences occur.
Six species of coccinellid were observed through direct searching of the ERS habitat. In total, 592 coccinellids were observed by direct search with a large majority (77%) being C. quinquepunctata (Figure 3). The second most abundant coccinellid on ERS was A. bipunctata (10.5%) with H. axyridis (7%) being the third most abundant species. The abundance of C. quinquepunctata was significantly greater than that of H. axyridis on ERS habitat (z = −4.32, p < 0.0001) (Figure 3). Significantly more C. quinquepunctata were recorded in June (z = 2.57, p = 0.01) as opposed to in August and September. Coccinellid diversity had no effect on C. quinquepunctata numbers on shingle, nor did vegetation cover. There was no effect of season or vegetation cover on H. axyridis abundance on ERS. However, the reduced model revealed that H. axyridis abundance was higher when coccinellid diversity was higher (z = 4.71, p < 0.0001) on ERS.
Of the 95 coccinellids recorded in grassland adjacent to the ERS, the majority (58%) again were C. quinquepunctata. This habitat had a higher species richness than the ERS with nine species of coccinellid recorded. This difference was due in part to the presence of specialist coccinellids that are only found in grassland habitat (P. vigintiduopunctata, S. vigintiquattuorpunctata, and T. sedecimpunctata). There were significantly more C. quinquepunctata recorded in grassland than H. axyridis (Z = −2.728, p = 0.02) (Figure 3). In contrast to the ERS habitat, abundance of C. quinquepunctata was higher when coccinellid diversity was higher (z = 2.99, p = 0.002) in the grassland.
Coccinella quinquepunctata was found at a range of distances from the water's edge. Significantly fewer individuals were recorded further from the water's edge at 16–20 and 26–30 m (z = −2.76, p = 0.006 and z = −3.51, p = 0.0004, respectively). The numbers recorded at the other three distances were also lower but not significantly so (Figure 4). Coccinella quinquepunctata was observed at various heights from the shingle substrate but was found more frequently at lower elevations with significantly fewer individuals recorded above 75 cm from the ground (z = −2.85, p = 0.004) (Figure 5).
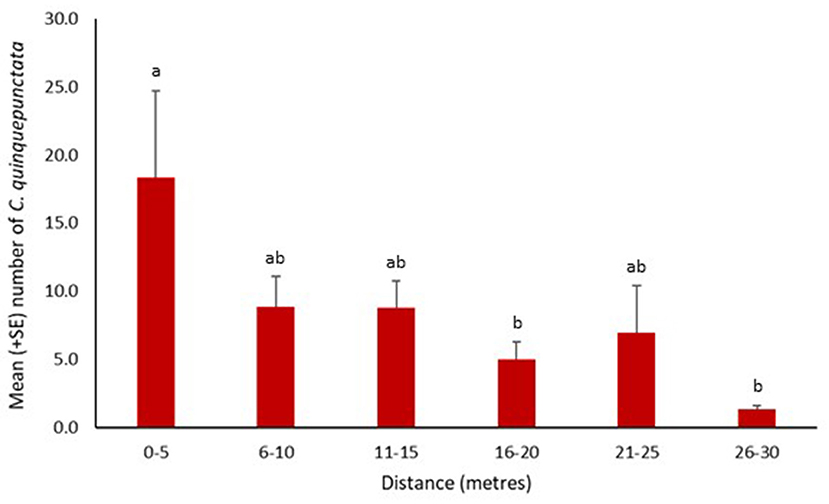
Figure 4. Mean number (+SE) per site of Coccinella quinquepunctata and the distance (in m) from the water's edge they were recorded at in 2017. Consecutive letters indicate where significant differences occur.
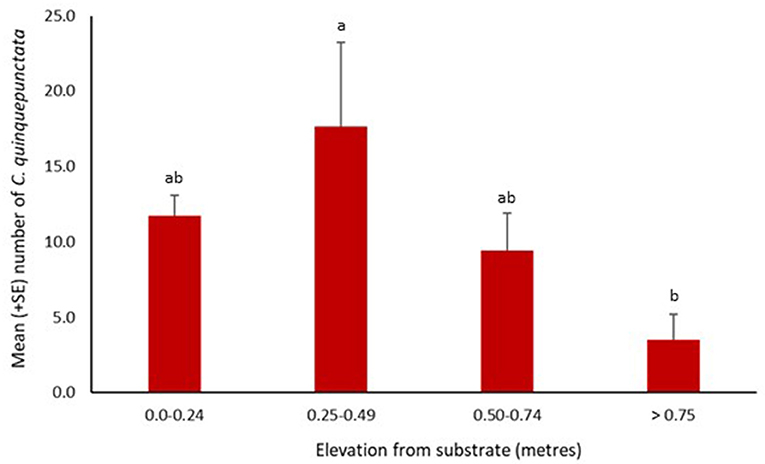
Figure 5. Mean number (+SE) per site of Coccinella quinquepunctata and the distance (in m) from the substrate they were recorded at in 2017. Consecutive letters indicate where significant differences occur.
Coccinella quinquepunctata was recorded in higher numbers than expected during this study and more C. quinquepunctata were observed than H. axyridis on both the ERS habitat and grassland habitat. The generally rural nature of the habitat is likely to be less suitable for H. axyridis (Purse et al., 2014) and consequently a refuge for C. quinquepunctata. The low number of H. axyridis was surprising, however this species has a well-documented preference for urban habitats (Adriaens et al., 2008; Purse et al., 2014; Roy and Brown, 2015; Sloggett, 2017; Viglášová et al., 2017) and in this case, all sites surveyed were in rural areas or on the edge of small rural villages. Urban and other managed habitats are more suitable for H. axyridis by providing secure overwintering sites in buildings (Roy et al., 2011, 2016). Furthermore, the ERS is a unique habitat with sparse vegetation stands where aphid numbers perhaps may be too low to sustain a predator such as H. axyridis. Honěk et al. (2018) reported an increase in H. axyridis numbers when aphid numbers increased but also with an increase in the level of urbanisation. Considering the rural nature of the sites as well as lack of overwintering sites, it is interesting that H. axyridis was recorded at all. Native coccinellid species were present in both habitats and A. bipunctata was present on ERS in greater numbers than was H. axyridis, albeit not significantly so. The overall low number of other coccinellid species recorded further reiterates that ERS is not a particularly suitable habitat for most coccinellids. In the grass habitat the low number of H. axyridis mirrored that of the overall number of coccinellids. Despite the low number of coccinellids in grassland, there was a greater diversity of native coccinellids in this habitat than on the ERS. Even though this was most likely due to the presence of three grass-specialist coccinellid species, the difference was not significant. The time spent searching grass habitat was just less than half the time spent searching on ERS, which partially accounts for the lower numbers recorded. Additionally, it is not possible to directly compare the two sampling techniques. The number of C. quinquepunctata, however, was higher when coccinellid diversity was higher in the grass habitat only. This could be due to a very low number of H. axyridis recorded in the grass habitat. However, it is more likely that coccinellid diversity and abundance was higher where the habitat was less managed or disturbed thereby creating a more suitable habitat for coccinellids (Diepenbrock and Finke, 2013; Grez et al., 2014; Honěk et al., 2014). Further investigation into coccinellid diversity and the heterogeneity/disturbance of the habitat adjacent to ERS would reveal more about the interaction between C. quinquepunctata and other coccinellids as well as the native coccinellid community.
Prior research indicated that H. axyridis could represent a threat for this species (Ware and Majerus, 2008). Thus, it is likely that should IGP occur, it would have a negative effect on C. quinquepunctata. However, the results in this study indicate that habitat separation on ERS may limit the interactions of these two species thereby limiting the opportunities for IGP. In North America, threat in the form of IGP and competition for resources from H. axyridis exacerbated the situation with C. novemnotata, which is found in only a small number of states and where present are in greatly reduced numbers (Tumminello et al., 2015; Ducatti et al., 2017). In Europe, C. quinquepunctata was previously considered an abundant habitat generalist, however, a decline in abundance was evident prior to the arrival of H. axyridis (Honěk et al., 2016). It is thought that changes in land use together with the intensification of agricultural practises has impacted C. quinquepunctata in the Czech Republic (Honěk et al., 2016). The results here also indicate that H. axyridis is not currently impacting C. quinquepunctata negatively, due to very low abundance of the former in the preferred habitat of the latter.
Other threats are very likely to have a negative impact on C. quinquepunctata because of their impact on the ERS. The reason for the RDB3 (Rare) categorisation of this species is due to the habitat where it is found being at risk. There are several threats to this habitat; including invasive plant species, livestock access to shingle banks, gravel extraction and river modification (Fowles, 1988; Bates et al., 2007a; Hewitt et al., 2010). Multiple IAS and various anthropogenic activities together may culminate into drivers of change (Vitousek et al., 1997), and being clear on which factor happens to be the greatest threat will facilitate effective conservation plans for native species (Majerus et al., 2016). More than one invasive alien plant species was identified on or near the shingle habitat (e.g., Japanese knotweed, Fallopia japonica; monkey flower, Erythranthe guttatus), however, the species most likely to have the greatest negative and immediate impact is I. glandulifera (Himalayan balsam). Seven of the 12 sites surveyed here had established stands of I. glandulifera present. This species potentially impacts C. quinquepunctata in two ways. Firstly, I. glandulifera changes the microbial community of the soil which prevents native plant species from taking root (Pattison et al., 2016), thereby homogenising the ERS plant community. During surveys, neither aphids nor any coccinellid species were seen on I. glandulifera plants (pers. obs.). This is not surprising, given that Tanner et al. (2013) reported a reduction in coccinellid numbers on areas invaded by I. glandulifera in comparison to non-invaded areas. Considering the significantly reduced abundance of C. quinquepunctata in the grassland adjacent to the shingle, the potential and inevitable lack of native plant species as a result of the presence of I. glandulifera, potentially leading to insufficient prey for C. quinquepunctata, could see the species become locally extinct in areas where I. glandulifera is not adequately controlled. If this habitat becomes homogenised in terms of vegetation the specialised invertebrate community is likely to be negatively affected (Sadler et al., 2004). Additionally, I. glandulifera de-stabilises the shingle bank as it has shallow roots and the soil around it becomes more fragmented, so when the rivers are in flood, considerably more substrate than usual will be removed. The ERS is in a constant state of flux (Fowles, 1994), however, this increased threat is likely to have an adverse effect not just on C. quinquepunctata but also the many other invertebrates (many of which are also nationally rare) that inhabit the fragile habitat (Sadler et al., 2004). Livestock regularly have access to the ERS for water and also graze on the bank. This is likely to have a negative impact on C. quinquepunctata due to the additional disturbance of the ERS, given this species' reliance on this habitat type. Bates et al. (2007a) determined that trampling by livestock reduced the conservation value of the beetle assemblages on river shingle. However, a small number of sites in this research, that were grazed by sheep during the entire field season, yielded the highest number of observations of C. quinquepunctata. These sites, however, were also clear of I. glandulifera and Day (2015) reported that grazing can be used to help control I. glandulifera successfully. Nevertheless, this IAS can be readily removed by hand and uncontrolled livestock access is more likely to be negative rather than a positive influence for ERS. One of the sites in this study had gravel extracted from it just prior to the final survey. This process resulted in complete removal of the vegetation and a large layer of the shingle bank. This site was the closest site to an urban area and in addition to the gravel extraction, the vegetation was highly managed throughout the entire survey period. Coccinella quinquepunctata was present at the site but in lower numbers than elsewhere. If the vegetation had not been cut back so severely and so frequently, it is possible that a greater number of C. quinquepunctata would have been recorded. This degree of disturbance to the ERS habitat and adjacent grassland mainly as a result of gravel extraction is a serious concern for C. quinquepunctata and other ERS-dwelling invertebrates (Sadler et al., 2004; Bates et al., 2007b). This level of disturbance is especially concerning, considering that after river system modification took place, it was reported that all trace of ERS had disappeared from the midlands and south east of England (O'Callaghan et al., 2013).
In Europe, C. quinquepunctata is in decline, however, in the UK, though it has a very restricted range the species is reported as nationally stable (Roy et al., 2018) and the numbers recorded during this research support that assessment. However, several coccinellid species have declined in recent years (Roy et al., 2018) and it is unlikely that there are other factors driving trends alongside the co-occurrence with H. axyridis. There are numerous threats to ecological communities alongside biological invasions including climate and land-use change. It is clear that the drivers interact and lead to biodiversity change (Harvey, 2015) with different components of each community assemblage reacting differently to these multiple threats (Stewart et al., 2015).
Continued monitoring of C. quinquepunctata (both in Wales and Scotland) is necessary to detect any future changes in the population. In the event of a decline in numbers, the continued monitoring of H. axyridis would further inform researchers if this IAS has started to have an effect on C. quinquepunctata or if a different threat may be having a negative impact. Monitoring will also help determine a more complete distribution in the UK. There does seem to be an edge of range effect in the UK. As a result of its exposure the ERS heats up quickly (Bates et al., 2009) which may be one factor as to why this habitat is preferred by C. quinquepunctata. This would be interesting to explore further in comparison to other habitat preferences for this species throughout Europe. Given that ERS is an important riparian habitat, additional studies into how the specialised invertebrate community contribute to ecosystem function would help bridge the gap between aquatic and terrestrial ecology in the UK. Finally, considering the specialist habitat preference of C. quinquepunctata, and numerous other rare invertebrate species, it would be prudent to designate increased habitat protection status on ERS in order to control livestock access, prevent gravel abstraction, river channel modification, and initiate restoration or enhancement of the habitat where it has been damaged or removed.
It is evident that C. quinquepunctata is thriving in Wales and is relatively unaffected by H. axyridis through IGP or resource competition. The RDB3 Rare categorisation is justified for C. quinquepunctata considering the multiple threats effecting ERS. However, if this unique habitat continues to be degraded, C. quinquepunctata is likely to decline, possibly to the point of extinction in the UK.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
RF, HR, and PB contributed to conception and design of the study. RF carried out field work, performed statistical analysis, and wrote the first draft of the manuscript. All authors contributed to revision of the manuscript and read and approved the submitted version.
RF received a studentship from Anglia Ruskin University to complete this research. HR was supported by the Natural Environment Research Council award number NE/R016429/1 as part of the UK-SCAPE programme Delivering National Capability.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors acknowlege landowners (Wye and Usk Foundation, Plas Dinam Estate, Llanover Estate, Cwmgwyn Farm, Llandovery College, Pontypool Park Estate, Warrington Anglers, and Powys Council) who granted permission of land access to RF to carry out survey work. Thank you to A. Pickwell for assistance during survey work and fine-tuning of survey methods.
Adriaens, T., San Martin y Gomez, G., and Maes, D. (2008). Invasion history, habitat preferences and phenology of the invasive ladybird Harmonia axyridis in Belgium. BioControl 53, 69–88. doi: 10.1007/s10526-007-9137-6
Baker, D. B., Richards, R. P., Loftus, T. T., and Kramer, J. W. (2004). A new flashiness index: characteristics and applications to midwestern rivers and streams. J. Am. Water Resour. Assoc. 40, 503–522. doi: 10.1111/j.1752-1688.2004.tb01046.x
Bates, A., and Sadler, J. (2004). Records of rare and notable species of beetle from exposed riverine sediments (ERS) on the rivers Tywi and Upper Severn. Coleopterist 13, 125–132.
Bates, A. J., Sadler, J. P., and Fowles, A. P. (2007a). Livestock trampling reduces the conservation value of beetle communities on high quality exposed riverine sediments. Biodivers. Conserv. 16, 1491–1509. doi: 10.1007/s10531-006-9028-7
Bates, A. J., Sadler, J. P., Henshall, S. E., and Hannah, D. M. (2009). Ecology and conservation of arthropods of exposed riverine sediments (ERS). Terr. Arthropod Rev. 2, 77–98. doi: 10.1163/187498309X455052
Bates, A. J., Sadler, J. P., Perry, J. N., and Fowles, A. P. (2007b). The microspatial distribution of beetles (Coleoptera) on exposed riverine sediments (ERS). Eur. J. Entomol. 104, 479–487. doi: 10.14411/eje.2007.068
Beaujean, A. A., and Morgan, G. B. (2016). Tutorial on using regression models with count outcomes using R. Pract. Assess. Res. Eval. 21, 1–19. doi: 10.7275/pj8c-h254
Beckerman, A. P., Childs, D. Z., and Petchey, O. L. (2017). Getting started with R: an introduction for biologists, 2nd edition. Oxford: Oxford University Press. doi: 10.1093/acprof:oso/9780198787839.001.0001
Beerling, D. J., and Perrins, J. M. (1993). Impatiens glandulifera Royle (Impatiens roylei Walp.) J. Ecol. 81, 367–382. doi: 10.2307/2261507
Brown, P. M. J., Frost, R., Doberski, J., Sparks, T., Harrington, R., and Roy, H. E. (2011a). Decline in native ladybirds in response to the arrival of Harmonia axyridis: early evidence from England. Ecol. Entomol. 36, 231–240. doi: 10.1111/j.1365-2311.2011.01264.x
Brown, P. M. J., and Roy, H. E. (2015). Reflections on the long-term assessment (Coleoptera: Coccinellidae) populations in the Czech Republic and the United Kingdom. Acta Soc. Zool. Bohem. 79, 19–27.
Brown, P. M. J., and Roy, H. E. (2018). Native ladybird decline caused by the invasive harlequin ladybird Harmonia axyridis: evidence from a long-term field study. Insect Conserv. Divers. 11, 230–239. doi: 10.1111/icad.12266
Brown, P. M. J., Thomas, C. E., Lombaert, E., Jeffries, D. L., Estoup, A., and Lawson Handley, L. (2011b). The global spread of Harmonia axyridis (Coleoptera: Coccinellidae): distribution, dispersal and routes of invasion. Biol. Control 56, 623–641. doi: 10.1007/s10526-011-9379-1
Day, P. (2015). Himalayan Balsam, Impatiens glandulifera. Available online at: http://www.nonnativespecies.org/factsheet/downloadFactsheet.cfm?speciesId=1810 (accessed June 09, 2019).
Diepenbrock, L. M., and Finke, D. L. (2013). Refuge for native lady beetles (Coccinellidae) in perennial grassland habitats. Insect Conserv. Divers. 6, 671–679. doi: 10.1111/icad.12027
Ducatti, R. D. B., Ugine, T. A., and Losey, J. (2017). Interactions of the Asian lady beetle, Harmonia axyridis (Coleoptera: Coccinellidae), and the North American native lady beetle, Coccinella novemnotata (Coleoptera: Coccinellidae): prospects for recovery post-decline. Environ. Entomol. 46, 21–29. doi: 10.1093/ee/nvw153
Evans, E. W. (2000). Morphology of invasion: body size patterns associated with establishment of Coccinella septempunctata (Coleoptera: Coccinellidae) in western North America. Eur. J. Entomol. 97, 469–474. doi: 10.14411/eje.2000.072
Fowles, A. (1994). Invertebrates of Wales: A Review of Important Sites and Species. Peterborough: Joint Nature Conservation Committee.
Fowles, A. P. (1988). An ecological study of the distribution of cursorial invertebrates on polluted riparian shingle (MSc thesis), Aberystwyth University, Aberystwyth.
Grez, A. A., Zaviezo, T., and Gardiner, M. (2014). Local predator composition and landscape affects biological control of aphids in alfalfa fields. Biol. Control 76, 1–9. doi: 10.1016/j.biocontrol.2014.04.005
Grez, A. A., Zaviezo, T., Roy, H. E., Brown, P. M. J., and Bizama, G. (2016). Rapid spread of Harmonia axyridis in Chile and its effects in the local coccinellid biodiversity. Divers. Distrib. 22, 982–994. doi: 10.1111/ddi.12455
Harmon, J. P., Stephens, E., and Losey, J. (2007). The decline of native coccinellids (Coleoptera: Coccinellidae) in the United States and Canada. J. Insect Conserv. 11, 85–94. doi: 10.1007/s10841-006-9021-1
Harvey, J. A. (2015). Conserving host-parasitoid interactions in a warming world. Curr. Opin. Insect. Sci. 12, 79–85. doi: 10.1016/j.cois.2015.09.001
Hewitt, S. M., Parker, J., and Kindemba, V. (2010). ERS Invertebrate Habitat Survey of the Rivers Afon Ystwyth and Afon Rheidol in Ceredigion. Buglife. Available online at: https://cdn.buglife.org.uk/2019/07/ERShabitatsurveysinWales_1.pdf (accessed October 06, 2017).
Honěk, A., Martinkova, Z., Dixon, A. F. G., Roy, H. E., and Pekár, S. (2016). Long-term changes in communities of native coccinellids: population fluctuations and the effect of competition from an invasive non-native species. Insect Conserv. Divers. 9, 202–209. doi: 10.1111/icad.12158
Honěk, A., Martinkova, Z., Kindlmann, P., Ameixa, O. M. C. C., and Dixon, A. F. G. (2014). Long-term trends in the composition of aphidophagous coccinellid communities in Central Europe. Insect Conserv. Divers. 7, 55–63. doi: 10.1111/icad.12032
Honěk, A., Martinková, Z., Roy, H. E., Dixon, A. F. G., Skuhrovec, J., Pekár, S., et al. (2019). Differences in the phenology of Harmonia axyridis (Coleoptera: Coccinellidae) and native coccinellids in Central Europe. Environ. Entomol. 48, 80–87. doi: 10.1093/ee/nvy173
Honěk, A., Martinková, Z., and Štrobach, J. (2018). Effect of aphid abundance and urbanization on the abundance of Harmonia axyridis (Coleoptera: Coccinellidae). Eur. J. Entomol. 115, 703–707. doi: 10.14411/eje.2018.069
Horikoshi, M., and Tang, Y. (2016). ggfortify: Data Visualization Tools for Statistical Analysis Results. Available online at: https://CRAN.R-project.org/package=ggfortify (accessed May 01, 2019).
Hyman, P. S. (1992). UK Nature Conservation Series No. 3: A Review of the Scarce & Threatened Coleoptera of Great Britain (Pt. 1). Peterborough: Joint Nature Conservation Committee.
Kassambara, A. (2018). ggpubr: ‘ggplot2' Based Publication Ready Plots. R package version 0.2. Available online at: https://CRAN.R-project.org/package=ggpubr (accessed May 01, 2019).
Koch, R. L., and Galvan, T. L. (2008). “Bad side of a good beetle: the North American experience with Harmonia axyridis,” in From Biological Control to Invasion: The Ladybird Harmonia axyridis as a Model Species, eds. H.E. Roy and E. Wajnberg (Cham: Springer), 23–35. doi: 10.1007/978-1-4020-6939-0_3
Littlewood, N. A. (2015). Discovery of 5-spot Ladybird Coccinella quinquepunctata (L.) (Col.: Coccinellidae) along the River Dee, Aberdeenshire. Br. J. Entomol. Nat. History 28, 55–57.
Losey, J., Perlman, J., Kopco, J., Ramsey, S., Hesler, L., Evans, E., et al. (2012). Potential causes and consequences of decreased body size in field populations of Coccinella novemnotata. Biol. Control 61, 98–103. doi: 10.1016/j.biocontrol.2011.12.009
Lucas, E. (2012). “Intraguild interactions,” in: Ecology and Behaviour of the Ladybird Beetles (Coccinellidae), ed. I. Hodek (Chichester: Wiley-Blackwell), 343–374. doi: 10.1002/9781118223208.ch7
Majerus, M. E. N., and Fowles, A. P. (1988). The rediscovery of the 5-spot ladybird (Coccinella 5-punctata L.) (Col. Coccinellidae) in Britain. Entomol. Mon. Mag. 125, 177–181.
Majerus, M. E. N., Roy, H. E., and Brown, P. M. J. (2016). A Natural History of Ladybird Beetles. Cambridge: Cambridge University Press. doi: 10.1017/CBO9781316336960
Met Office (2016). Beaufort: National Meteorological Library and Archive Fact sheet 6 – The Beaufort scale. Devon: Met Office.
Morris, E. K., Caruso, T., Buscot, F., Fischer, M., Hancock, C., Maier, T. S., et al. (2014). Choosing and using diversity indices: insights for ecological applications from the German Biodiversity Exploratories. Ecol. Evol. 4, 3514–3524. doi: 10.1002/ece3.1155
Nakazawa, M. (2018). fmsb: Function for Medical Statistics Books With Some Demographic Data. R package version 0.6.3. Available online at: https://CRAN.R-project.org/package=fmsb (accessed October 24, 2018).
O'Callaghan, M. J., Hannah, D. M., Williams, M., and Sadler, J. P. (2013). Exposed riverine sediments (ERS) in England and Wales: distribution, controls and management. Aquat. Conserv. 23, 924–938. doi: 10.1002/aqc.2376
Pattison, Z., Rumble, H., Tanner, R. A., Jin, L., and Gange, A. C. (2016). Positive plant-soil feedbacks o the invasive Impatiens glandulifera and their effects on above-ground microbial communities. Weed Res. 56, 198–207. doi: 10.1111/wre.12200
Pell, J. K., Baverstock, J., Roy, H. E., Ware, R. L., and Majerus, M. E. N. (2008). “Intraguild predation involving Harmonia axyridis: a review of current knowledge and future perspectives,” in From Biological Control to Invasion: The Ladybird Harmonia axyridis as a Model Species, eds. H. E. Roy and E. Wajnberg (Dordrecht: Springer), 147–168. doi: 10.1007/978-1-4020-6939-0_10
Polis, G. A., Myers, C. A., and Holt, R. D. (1989). The ecology and evolution of intraguild predation: potential competitors that eat each other. Annu. Rev. Ecol. Evol. Syst. 20, 297–330. doi: 10.1146/annurev.es.20.110189.001501
Purse, B. V., Comont, R., Butler, A., Brown, P. M. J., Kessel, C., and Roy, H. E. (2014). Landscape and climate determine patterns of spread for all colour morphs of the alien ladybird Harmonia axyridis. J. Biogeogr. 42, 575–588. doi: 10.1111/jbi.12423
Pyšek, P., and Prach, K. (1995). Invasion dynamics of Impatiens glandulifera – a century of spreading reconstructed. Biol. Conserv. 7, 41–48. doi: 10.1016/0006-3207(95)00013-T
R Core Team (2018). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/ (accessed November 08, 2018).
Roy, H. E., Adriaens, T., Isaac, N. J. B., Kenis, M., Onkelinx, T., San Martin, G., et al. (2012). Invasive alien predator causes rapid declines of native European ladybirds. Divers. Distrib. 18, 717–725. doi: 10.1111/j.1472-4642.2012.00883.x
Roy, H. E., and Brown, P. M. J. (2015). Ten years of invasion Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) in Britain. Ecol. Entomol. 40, 336–348. doi: 10.1111/een.12203
Roy, H. E., Brown, P. M. J., Adriaens, T., Berkvens, N., Borges, I., Clusella-Trullas, S., et al. (2016). The harlequin ladybird, Harmonia axyridis: global perspectives on invasion history and ecology. Biol. Invasions 18, 997–1044. doi: 10.1007/s10530-016-1077-6
Roy, H. E., Brown, P. M. J., Frost, R., and Poland, R. (2011). Ladybirds (Coccinellidae) of Britain and Ireland: An Atlas of the Ladybird of Britain, Ireland, the Isle of Man and the Channel Islands. Shrewsbury: FSC Publications.
Roy, H. E., Groom, Q., Adriaens, T., Agnello, G., Antic, M., Archambeau, A., et al. (2018). Increasing understanding of alien species through citizen science (Alien-CSI). Res. Ideas Outcomes 4:e31412. doi: 10.3897/rio.4.e31412
Sadler, J. P., Bell, D., and Fowles, A. (2004). The hydroecological controls and conservation value of beetles on exposed riverine sediments in England and Wales. Biol. Conserv. 118, 41–56. doi: 10.1016/j.biocon.2003.07.007
Sloggett, J. J. (2017). Harmonia axyridis (Coleoptera: Coccinellidae): smelling the rat in native ladybird declines. Eur. J. Entomol. 114, 455–461. doi: 10.14411/eje.2017.058
Sloggett, J. J., Zeilstra, I., and Obrycki, J. J. (2008). Patch residence by aphidophagous ladybird beetles: do specialists stay longer? Biol. Control 47, 199–206. doi: 10.1016/j.biocontrol.2008.08.003
Stewart, A. J. A., Bantock, T. M., Beckmann, B. C., Botham, M. S., Hubble, D., and Roy, D. B. (2015). The role of ecological interactions in determining species ranges and range changes. Biol. J. Linn. Soc. 115, 647–663. doi: 10.1111/bij.12543
Tang, Y., Horikoshi, M., and Li, W. (2016). ggfortify: unified interface to visualize statistical result of popular R packages. R J. 8, 478–489. doi: 10.32614/RJ-2016-060
Tanner, R. A., Jin, L., Shaw, R., Murphy, S. T., and Gange, A. C. (2014). An ecological comparison of Impatiens glandulifera Royle in the native and introduced range. Plant Ecol. 215, 833–843. doi: 10.1007/s11258-014-0335-x
Tanner, R. A., Varia, S., Eschen, R., Wood, S., Murphy, S. T., and Gange, A. C. (2013). Impacts of an invasive non-native annual weed, Impatiens glandulifera, on above- and below-ground invertebrate communities in the United Kingdom. PLoS ONE 8:e67271. doi: 10.1371/journal.pone.0067271
Tumminello, G., Ugine, T. A., and Losey, J. E. (2015). Intraguild interactions of native and introduced coccinellids: the decline of a flagship species. Environ. Entomol. 44, 64–72. doi: 10.1093/ee/nvu010
Venables, W. N., and Ripley, B. D. (2002). Modern Applied Statistics With S, 4th Edition. New York, NY: Springer. doi: 10.1007/978-0-387-21706-2
Viglášová, S., Nedvěd, O., Zach, P., Julfan, J., Parák, M., Honěk, A., et al. (2017). Species assemblages of ladybirds including the harlequin ladybird Harmonia axyridis: a comparison at large spatial scale in urban habitats. BioControl 62, 409–421. doi: 10.1007/s10526-017-9793-0
Vitousek, P. M., D'Anotnio, C. M., Loope, L. L., Rejmánek, M., and Westbrooks, R. (1997). Introduced species: a significant component of human-caused global change. N. Z. J. Ecol. 21, 1–16.
Ware, R. L., and Majerus, M. E. N. (2008). Intraguild predation of immature stages of British and Japanese coccinellids by the invasive ladybird Harmonia axyridis. BioControl 53, 169–188. doi: 10.1007/s10526-007-9135-8
Welsh Institute for Sustainable Environments Network (2014). Distribution and Management of the Invasive Species Himalayan Balsam (Impatiens glandulifera) at Penellergare Valley Woods. Swansea: Swansea University.
Wickham, H. (2016). ggplot2: Elegant Graphics for Data Analysis. New York, NY: Springer-Verlag. doi: 10.1007/978-3-319-24277-4
Wickham, H., Francois, R., Henry, L., and Muller, K. (2019). dplyr: a grammar of data manipulation. R package version 0.8.0.1. Avaialble online at: https://CRAN.R-project.org/package=dplyr (accessed May 16, 2019).
Zeileis, A. (2004). Econometric computing with HC and HAC covariance matric estimators. J. Stat. Softw. 11, 1–17. doi: 10.18637/jss.v011.i10
Zeileis, A. (2006). Object-oriented computation of sandwich estimators. J. Stat. Softw. 16, 1–16. doi: 10.18637/jss.v016.i09
Zeileis, A., and Hothorn, T. (2002). Diagnostic checking in regression relationships. R News 2, 7–10. Available online at: https://CRAN.R-project.org/doc/Rnews/ (accessed May 16, 2019).
Zeileis, A., Kleiber, C., and Jackman, S. (2008). Regression models for count data in R. J. Stat. Softw. 27, 1–25. doi: 10.18637/jss.v027.i08
Zuur, A. F., Ieno, E. N., and Smith, G. M. (2007). Analysing Ecological Data. New York, NY: Springer-Verlag. doi: 10.1007/978-0-387-45972-1
Zuur, A. F., Ieno, E. N., Walker, N. J., Saveliev, A. A., and Smith, G. M. (2009). Mixed Effects Models and Extensions in Ecology With R. New York, NY: Springer-Verlag. doi: 10.1007/978-0-387-87458-6
Keywords: 5-spot ladybird, Coccinella quinquepunctata, exposed riverine sediment, Harmonia axyridis, invasive alien species, unstable river shingle
Citation: Farrow RA, Roy HE and Brown PMJ (2022) The Rare Five-Spot Ladybird Coccinella quinquepunctata (Coleoptera: Coccinellidae) Surviving in an Unstable Habitat. Front. Conserv. Sci. 2:759038. doi: 10.3389/fcosc.2021.759038
Received: 15 August 2021; Accepted: 02 December 2021;
Published: 20 January 2022.
Edited by:
Danny Haelewaters, Ghent University, BelgiumCopyright © 2022 Farrow, Roy and Brown. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachel A. Farrow, cmFjaGVsYWZhcnJvd0BnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.